Abstract
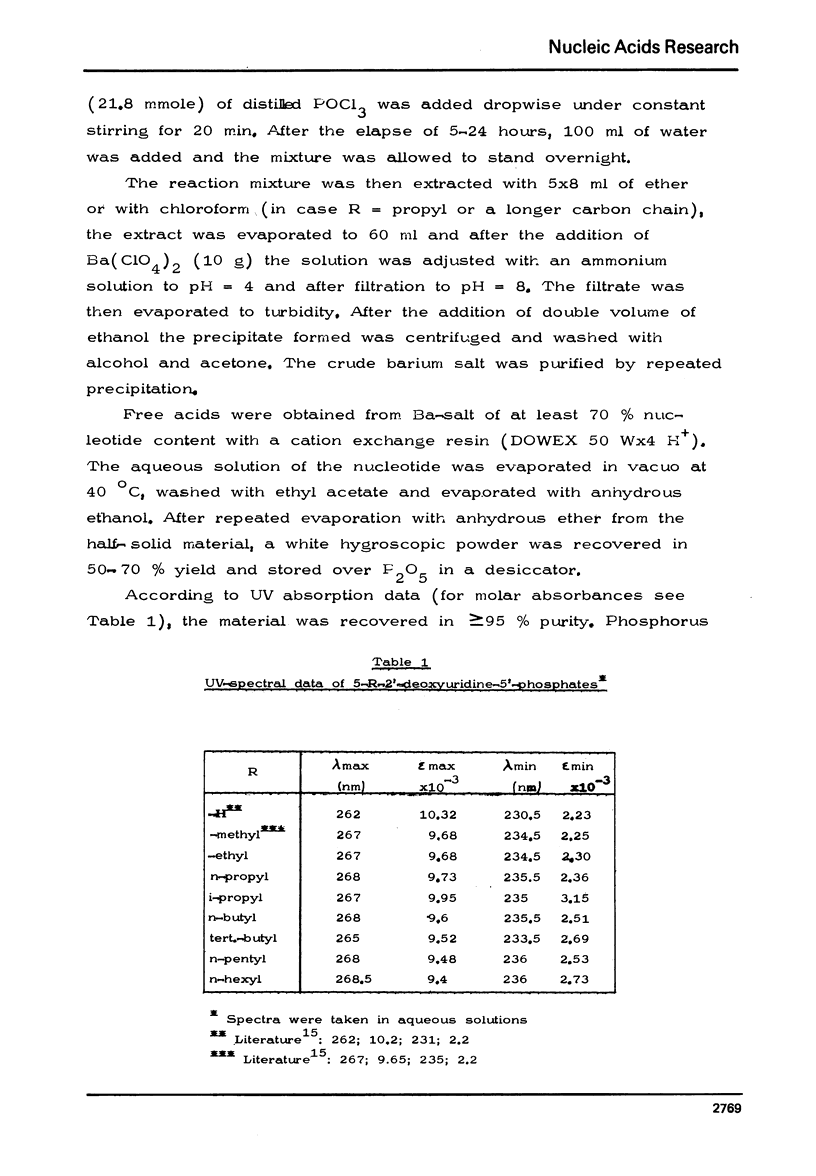
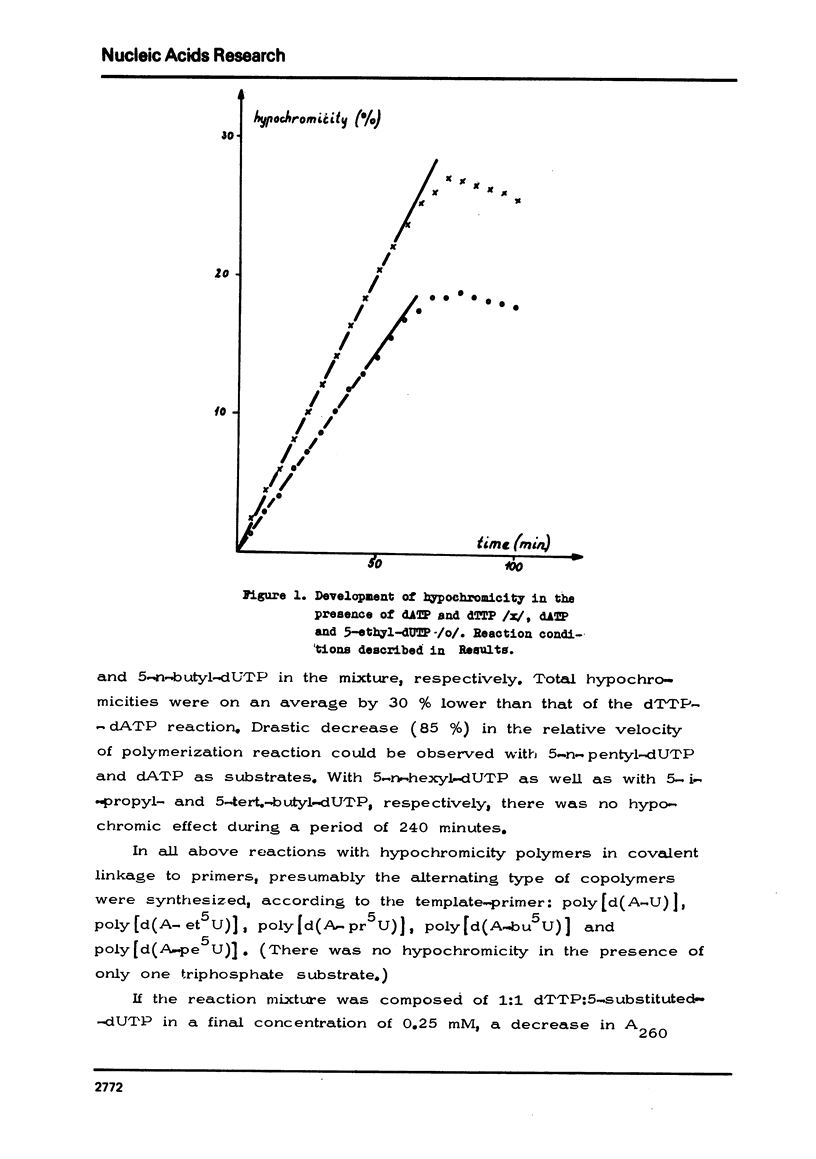
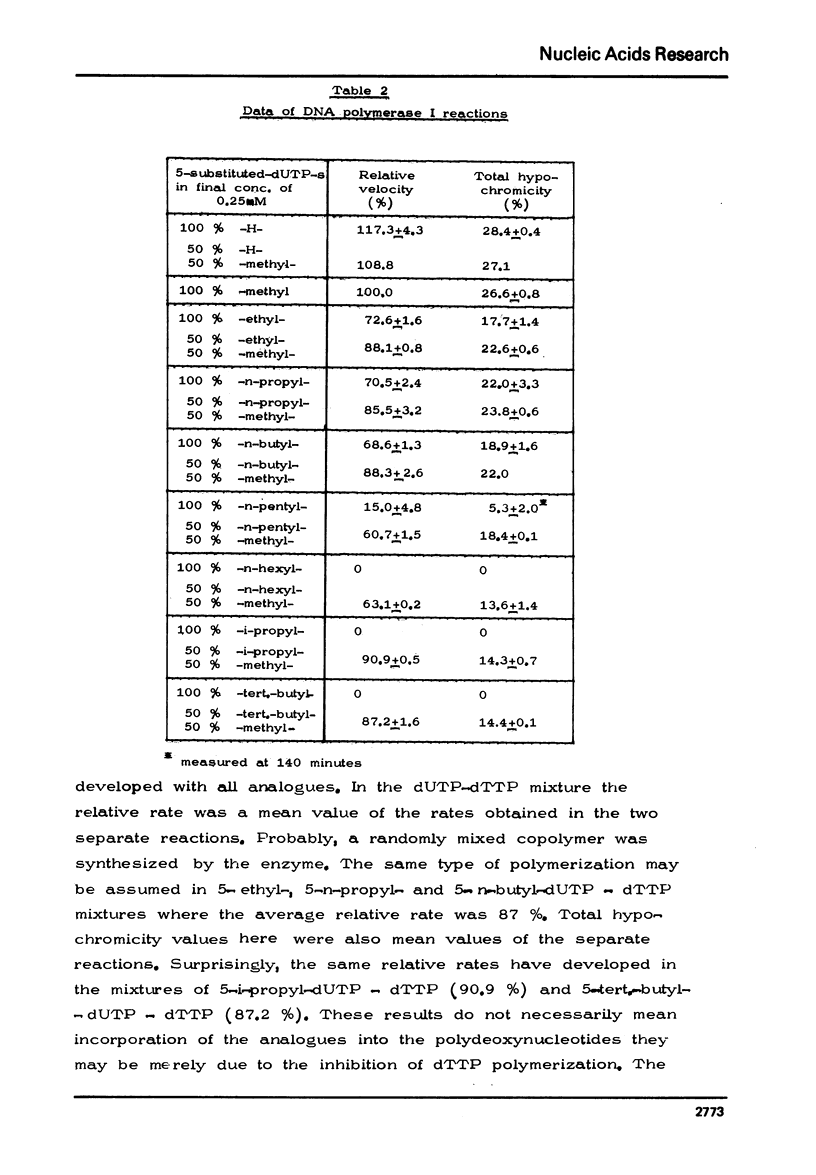
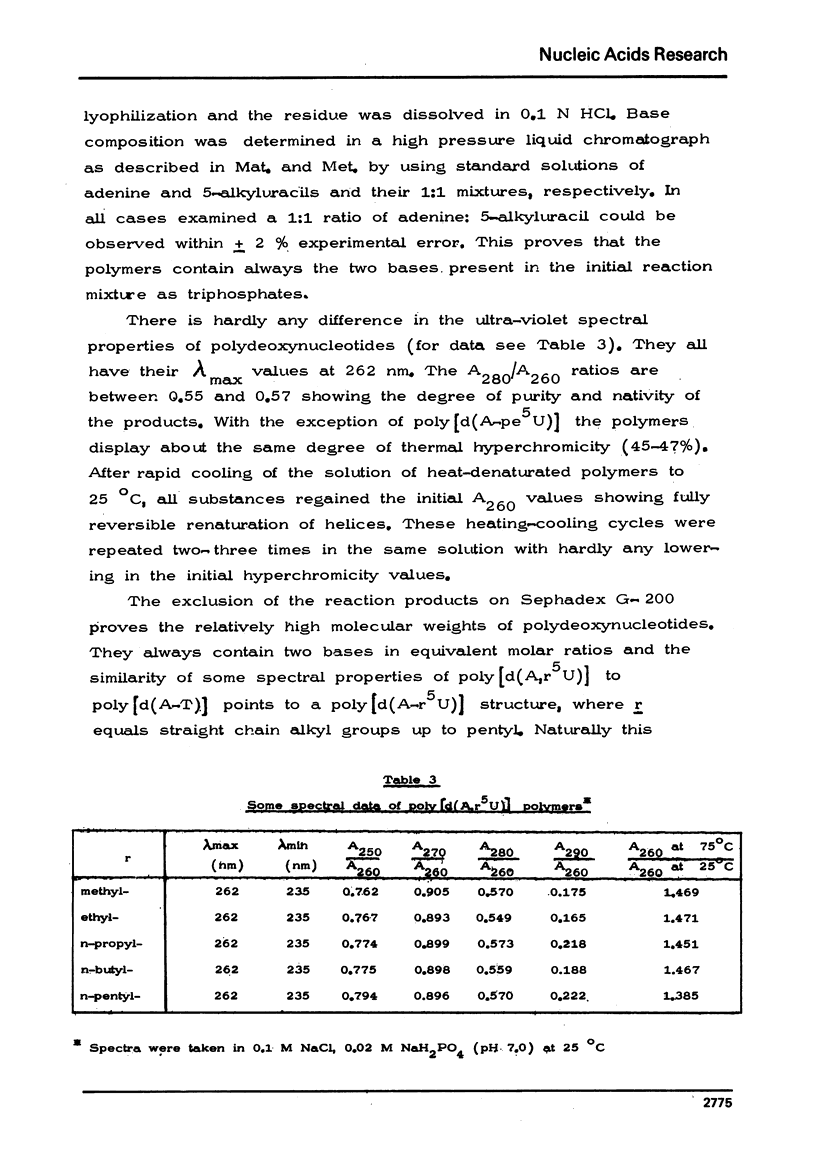
The chemical synthesis of 5-alkyl-dUTP-s and their participation as substrates in poly[d(A-6)] primed polymerization reactions with dATP by E. coli DNA polymerase I enzyme has been described. In comparison with dTTP, at saturating substrate concentrations, the rate of hypochromic effect was found to be 17.3% higher for dUTP and was lower by 27.4% for 5-ethyl-dUTP, 29.5% for 5-n-propyl-dUTP, 31.4% for 5-n-butyl-dUTP and by 85.0% for 5-n-pentyl-dUTP. No hypochromic effect could be observed, however, with 5-iso-propyl-, 5-tert.butyl- and 5-n-hexyl-dUTP-s. Polydeoxynucleotides have also been isolated from the reaction mixture and some of their structural properties determined.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. J. The estimation of phosphorus. Biochem J. 1940 Jun;34(6):858–865. doi: 10.1042/bj0340858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd J. F., Wells R. D. Effect of incubation conditions on the nucleotide sequence of DNA products of unprimed DNA polymerase reactions. J Mol Biol. 1970 Nov 14;53(3):435–459. doi: 10.1016/0022-2836(70)90076-8. [DOI] [PubMed] [Google Scholar]
- Cheng Y. C., Domin B. A., Sharma R. A., Bobek M. Antiviral action and cellular toxicity of four thymidine analogues: 5-ethyl-,5-vinyl-, 5-propyl-, and 5-allyl-2'- deoxyuridine. Antimicrob Agents Chemother. 1976 Jul;10(1):119–122. doi: 10.1128/aac.10.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., Livingston D. C., Ward D. C. The synthesis and enzymatic polymerization of nucleotides containing mercury: potential tools for nucleic acid sequencing and structural analysis. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2238–2242. doi: 10.1073/pnas.70.8.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., Ward D. C. Mercurated polynucleotides: new probes for hybridization and selective polymer fractionation. Biochemistry. 1975 Jun 3;14(11):2458–2469. doi: 10.1021/bi00682a028. [DOI] [PubMed] [Google Scholar]
- Dorman D. E., Roberts J. D. Nuclear magnetic resonance spectroscopy: 13C spectra of some common nucleotides. Proc Natl Acad Sci U S A. 1970 Jan;65(1):19–26. doi: 10.1073/pnas.65.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOX J. J., SHUGAR D. Spectrophotometric studies of nucleic acid derivatives and related compounds as a function of pH. II. Natural and synthetic pyrimidine nucleosides. Biochim Biophys Acta. 1952 Oct;9(4):369–384. doi: 10.1016/0006-3002(52)90181-9. [DOI] [PubMed] [Google Scholar]
- INMAN R. B., BALDWIN R. L. HELIX--RANDOM COIL TRANSITIONS IN DNA HOMOPOLYMER PAIRS. J Mol Biol. 1964 Apr;8:452–469. doi: 10.1016/s0022-2836(64)80003-6. [DOI] [PubMed] [Google Scholar]
- Kropinski A. M., Bose R. J., Warren R. A. 5-(4-Aminobutylaminomethyl)uracil, an unusual pyrimidine from the deoxyribonucleic acid of bacteriophage phiW-14. Biochemistry. 1973 Jan 2;12(1):151–157. doi: 10.1021/bi00725a025. [DOI] [PubMed] [Google Scholar]
- Marmur J., Brandon C., Neubort S., Ehrlich M., Mandel M., Konvicka J. Unique properties of nucleic acid from Bacillus subtilis phage SP-15. Nat New Biol. 1972 Sep 20;239(90):68–70. doi: 10.1038/newbio239068a0. [DOI] [PubMed] [Google Scholar]
- McClure W. R., Jovin T. M. The steady state kinetic parameters and non-processivity of Escherichia coli deoxyribonucleic acid polymerase I. J Biol Chem. 1975 Jun 10;250(11):4073–4080. [PubMed] [Google Scholar]
- Piechowska M., Shugar D. Replacement of 5-methyluracil (thymine) by 5-ethyluracil in bacterial DNA. Biochem Biophys Res Commun. 1965 Sep 22;20(6):768–773. doi: 10.1016/0006-291x(65)90084-7. [DOI] [PubMed] [Google Scholar]
- Rae P. M. 5-Hydroxymethyluracil in the DNA of a dinoflagellate. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1141–1145. doi: 10.1073/pnas.70.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHACHMAN H. K., ADLER J., RADDING C. M., LEHMAN I. R., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. VII. Synthesis of a polymer of deoxyadenylate and deoxythymidylate. J Biol Chem. 1960 Nov;235:3242–3249. [PubMed] [Google Scholar]
- Swierkowska K. M., Jasińska J. K., Steffen J. A. 5-Ethyl-2'-deoxyuridine: evidence for incorporation into DNA and evaluation of biological properties in lymphocyte cultures grown under conditions of amethopterine-imposed thymidine deficiency. Biochem Pharmacol. 1973 Jan 1;22(1):85–93. doi: 10.1016/0006-2952(73)90257-8. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI I., MARMUR J. Replacement of thymidylic acid by deoxyuridylic acid in the deoxyribonucleic acid of a transducing phage for Bacillus subtilis. Nature. 1963 Feb 23;197:794–795. doi: 10.1038/197794a0. [DOI] [PubMed] [Google Scholar]


