Abstract
The safety profile of HibMenCY was compared with licensed Hib conjugate vaccines in a pooled analysis that included more than 8,500 subjects who were administered a four-dose series of HibMenCY or commercially available Hib vaccines at 2, 4, 6 and 12–15 mo of age in two primary vaccination and two fourth dose phase 3 studies. In all studies, HibMenCY or Hib vaccine was co-administered with age-appropriate, routinely recommended vaccines. In one primary and one fourth dose study (n = 4180), local and general symptoms were solicited using diary cards for 4 d after each dose. Serious adverse events (SAEs) and the occurrence of adverse events (AEs) indicating new onset of chronic disease (NOCD), rash, and conditions prompting Emergency Room (ER) visits were reported from dose 1 until 6 mo after dose 4. The incidences of solicited local and general symptoms were similar following HibMenCY and commercially available Hib vaccines. For some solicited symptoms (pain at the injection site and irritability), rates were lower in the HibMenCY group compared with the Hib control group (p value < 0.05). There were no statistically significant differences between groups in the incidences of SAEs, NOCDs, rash, or AEs leading to ER visits, with the exceptions of anemia and viral gastroenteritis, which occurred significantly less frequently in those receiving HibMenCY than those receiving commercially available Hib vaccines. In this pooled safety analysis, the safety profile of HibMenCY was similar to the safety profile of licensed monovalent Hib vaccines, despite the addition of meningococcal antigens.
These studies are registered at www.clinicaltrials.gov NCT00345579 (primary vaccination study), NCT00345683 (fourth dose vaccination study) and NCT00289783 (primary and fourth dose vaccination studies)
Keywords: Neisseria meningitidis, Haemophilus influenzae type b, adverse drug event, vaccine
Until recently there has been no licensed meningococcal conjugate vaccine available for infants in the United States (US), even though the highest incidence of meningococcal disease is observed in this age group.1 In April 2011, a meningococcal serogroups A, C, W-135, and Y diphtheria conjugate vaccine was approved for vaccination at 9 mo and 12 mo of age.2 However, initiating vaccination of infants at 9 mo of age misses the peak incidence of disease in infants, which occurs under 6 mo of age.1
The US vaccination schedule has recommended routine vaccination of infants with Haemophilus influenzae type b (Hib) polysaccharide-protein conjugate vaccines since the early 1990s. Widespread use has shown that Hib vaccines have acceptable safety profiles.3 In the completed phase 2 and phase 3 development program, the investigational Haemophilus influenzae type b–Neisseria meningitidis serogroups C and Y tetanus toxoid conjugate vaccine [HibMenCY, GlaxoSmithKline (GSK) Biologicals, Belgium], had a clinically acceptable safety profile, which was comparable to licensed monovalent Hib vaccines and a monovalent MenC conjugate vaccine licensed outside the US.4-8
Significant adverse events (AEs) associated with a new vaccine may only be detectable in a sufficiently large sample size. Therefore, safety data from the entire phase 3 HibMenCY development program were pooled, allowing evaluation of the occurrence of common and uncommon AEs in > 8,500 recipients who received at least one dose of HibMenCY or Hib control vaccine. The objectives of the pooled analysis were to describe the HibMenCY safety profile in comparison to a licensed monovalent Hib conjugate vaccine; to identify events that may require further investigation in appropriately designed and powered follow-up safety studies; and to specifically evaluate the occurrence of AEs affecting the nervous system.
The following analysis summarizes the occurrence of AEs after vaccination with four consecutive doses of HibMenCY compared with four consecutive doses of Hib vaccine by pooling data from two large phase 3 single-blind, randomized and controlled studies, each with a primary vaccination (dose 1, 2 and 3) phase and a fourth dose vaccination phase. Each phase was a technically distinct clinical study [primary/fourth dose studies A/B8 (registered under a single designation NCT00289783) and primary/fourth dose studies C/D (registered under the separate designations NCT00345579 and NCT00345683); www.clinicaltrials.gov].
Healthy infants were randomized (3:1) to receive HibMenCY or Hib (ActHIB®, Sanofi Pasteur) at 2, 4 and 6 mo of age, and HibMenCY or Hib (PedvaxHIB®, Merck and Co., Inc.) at 12–15 mo of age. HibMenCY does not contain any adjuvants or preservatives. PedvaxHIB® (Merck and Co.) was used for the fourth dose at the request of the US Food and Drug Administration.8 HibMenCY and Hib vaccines were co-administered with routinely recommended infant and toddler vaccines, which included combined diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated poliovirus vaccine (DTaP-HepB-IPV; Pediarix™/Infanrix® penta, GSK Biologicals) and 7-valent pneumococcal conjugate vaccine (PCV7, Prevnar™/Prevenar™, Pfizer) at 2, 4, and 6 mo of age; and MMR and varicella (VAR) vaccines (M-M-RII® and Varivax®, respectively, Merck and Co.) at 12–15 mo of age. In total, six different clinical HibMenCY vaccine lots, manufactured at industrial scale (~11,000 vials per batch), were administered during the studies. All vaccines were administered intramuscularly except for the MMR and VAR vaccines which were administered subcutaneously.
Study A/B was conducted at 91 centers in the US, Australia and Mexico between February 2006 and July 2008. Details of the study design and immunogenicity and safety results are described elsewhere.8 Study C/D was conducted in 61 centers in the US and Mexico between September 2006 and November 2008. Thirty-six centers participated in all studies.
The studies were conducted according to Good Clinical Practices (GCP) and the Declaration of Helsinki. Study documents were reviewed and approved by appropriate local (and/or national) institutional review boards. Subjects were enrolled after obtaining written informed consent from parents/guardians. After enrolment in the primary phase, randomization was performed using a central, web-based system. In each study, the randomization algorithm included a minimization procedure to ensure balanced allocation between groups at individual centers.
Due to differences in the appearance of the HibMenCY and comparator vaccines, parents/guardians of subjects in all studies were blinded with regard to whether their child received study vaccine or control, but study personnel were not. Outside of Australia, parents remained blinded until the end of the extended safety follow-up that lasted until 6 mo after dose 4. Parents of subjects enrolled in Australia remained blinded until completion of the blood draw post-dose 4 but were un-blinded for the extended safety follow-up. This was done because subjects in the control group in Australia were offered a dose of monovalent MenC conjugate vaccine after completion of the four-dose study vaccination series in order to comply with the Australian vaccination schedule.9 Compliance with the Australian vaccination schedule effectively unblinded the parents/guardians of the subjects, since they knew that if the child received an additional vaccination after study participation, the child was randomized to the control group.
In study A/B, but not C/D, local (injection site pain, redness, and swelling) and general symptoms (fever ≥ 38.0°C, drowsiness, irritability, and loss of appetite) were solicited using paper diary cards for 4 d (Day 0–3) after each dose. The parents/guardians of the subjects recorded the incidence and intensity (grade 1, 2, or 3 according to pre-defined criteria), which were transcribed by study center personnel onto an electronic case report form. All other (unsolicited) symptoms reported within 31 d after each vaccination were also recorded.
In all studies, serious adverse events (SAE) and the occurrence of new onset of chronic disease (NOCD), rash (potentially indicative of autoimmune or allergic diseases), and conditions prompting emergency room (ER) visits were reported from the day of the first vaccination until 6 mo after dose 4 at 12–15 mo of age. An SAE was defined as any untoward medical occurrence that resulted in death, was life-threatening, resulted in persistent or significant disability/incapacity, required in-patient hospitalization or prolongation of an existing hospitalization, or any important medical event that might have jeopardized the subject or might have required intervention to prevent one of the other outcomes listed above. These events were collected retrospectively using a standardized telephone script. Verbatim terms were coded using the Medical Dictionary for Regulatory Activities (MedDRA).
The studies were designed with the objective of pre-specified pooling of safety data to increase the power to detect the incidence of infrequent but serious AEs. The study design, study vaccines and concomitant vaccines, vaccination schedule and study population in terms of age at enrolment, and inclusion/exclusion criteria, were similar in the primary vaccination and fourth dose studies. Although the primary and fourth dose studies were technically distinct, the results were analyzed as a full 4-dose series.
For a given subject and the analysis of solicited symptom within 4 d post-vaccination, completely missing or non-evaluable measurements were not replaced. Subjects who had documented the presence of a symptom without having recorded any daily measurement were assigned to the lowest intensity category (i.e., grade 1) while the ones without documentation were excluded. Less than 1% of subjects did not indicate a daily intensity value for a reported solicited symptom.
For analysis of all other unsolicited AEs, subjects who did not report an event were considered as subjects without the event.
An exploratory safety analysis was conducted on the total vaccinated cohort (TVC), which included all subjects who received at least one dose of study vaccine. In order to take into account the country effect while comparing the groups using exact statistical tests, differences between the HibMenCY group and the Hib group were evaluated in terms of relative risks (RR). The common RR across countries, its 95% confidence interval (CI) and the corresponding 2-sided p value were based on the exact conditional likelihood approach adjusted for the country effect. In addition, a Breslow and Day (B&D) test for homogeneity between countries was performed. p value less than 0.05 were used as an indicator that a difference between groups/countries might exist. Since p values were not adjusted for multiplicity of endpoints and do not necessarily denote a clinically relevant difference, statistically significant findings should be interpreted with caution.
This pooled trial size was sufficient to detect a tripling in the occurrence of any AE with a frequency of 0.3% or more, with more than 80% power.
Statistical analyses used SAS® software version 9.1 (Copyright® 2002–2003; SAS Institute Inc.) with exact tests and B&D test obtained from Proc StatXact 7.0, Cytel.
There were a total of 8872 infants enrolled in the pooled data set of the two primary vaccination studies. Due to GCP violations and protocol non-compliance despite intervention from the study Sponsor, data from a single center in the US that participated in all four studies (301 subjects) were eliminated from the analyses. Thus, a total of 8,571 subjects contributed to the safety analysis (6,414 in the HibMenCY group and 2,157 in the Hib group). A supplementary analysis that included subjects from the excluded site showed that this site had no impact on the conclusions of the final analysis. All SAEs reported at that center were summarized and separately listed. No additional SAE terms were reported (data not shown).
A total of 8,571 subjects were enrolled in the primary vaccination studies A and C: 4,101 were enrolled in the US, 3,866 were enrolled in Mexico and 604 were enrolled in Australia (94.8% of these subjects received all three primary vaccination doses). This was the pooled data set for analysis of common solicited symptoms, SAEs, NOCDs, rashes, and AEs resulting in ER visits. A further 7,712 subjects received a fourth dose of study vaccine.
The demographic profile of the two treatment groups of subjects in the pooled data set was comparable with respect to mean age, gender and racial distribution (data not shown). The mean age of 8,571 subjects at the time of the first vaccine dose was 61.0 d (range 37–116 d), and 51.5% of the subjects were male. The predominant races were Hispanic (46.6%) and Caucasian (43.7%). African heritage/African American heritage was represented by 4.5% of subjects.
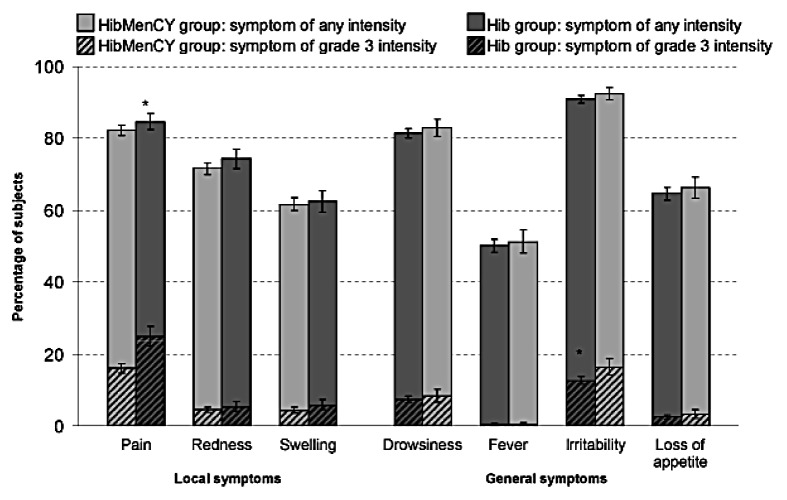
The percentages of subjects reporting any solicited symptom, or grade 3 solicited symptoms, were at least similar in HibMenCY recipients than in control vaccine recipients (Fig. 1). Fever > 40.0°C, was reported by 0.4% of subjects in both groups. Some statistically significant differences were noted between groups in the incidences of solicited local and general symptoms per subject during the 4-dose series. For all statistical differences, rates were lower in the HibMenCY group than the Hib control group (Fig. 1).
Figure 1.
Percentage of subjects reporting each solicited adverse event within the 4-d post-vaccination period after each dose, during the four-dose series (total vaccinated cohort, studies A/B). Grade 3, prevented normal activity; except for pain, cries when limb is moved/spontaneously painful; fever, > 40.0°C; and for loss of appetite, not eating at all; redness and swelling, > 30 mm. Local symptoms refers to symptoms occurring at any injection site. *p < 0.05 for the 2-sided exact stratified test conditional to number of cases, taking into account the country effect.
No statistically significant differences (p values < 0.05) between groups were observed in terms of unsolicited AEs of any intensity occurring in at least 5% of subjects (Table 1). The only statistically significant differences were observed for AEs with an incidence in both groups of < 5%. All statistically significant differences in the incidences of unsolicited AEs were attributable to lower frequency of the events in the HibMenCY group compared with the Hib control group, with the exception of constipation, which was higher in the HibMenCY group (Table 1).
Table 1. Unsolicited symptoms reported by at least 5% of subjects in either group, and unsolicited symptoms for which a statistically significant difference between groups was observed, within 31 d after each dose over the four-dose vaccination series (total vaccinated cohort, studies A/B only).
| |
HibMenCY n = 3136 |
Hib n = 1044 |
Relative Risk |
|
|---|---|---|---|---|
| Symptom | n (%) | n (%) | [95% CI] | p value |
| At least one symptom |
2033 (64.8) |
669 (64.1) |
1.01 [0.93; 1.11] |
0.82 |
| Upper respiratory tract infection |
635 (20.2) |
209 (20.0) |
1.01 [0.86; 1.19] |
0.92 |
| Otitis media |
433 (13.8) |
140 (13.4) |
1.03 [0.85; 1.25] |
0.81 |
| Pyrexia |
325 (10.4) |
124 (11.9) |
0.87 [0.71; 1.08] |
0.22 |
| Diarrhea |
256 (8.2) |
84 (8.0) |
1.01 [0.79; 1.31] |
0.97 |
| Vomiting |
252 (8.0) |
93 (8.9) |
0.90 [0.71; 1.16] |
0.43 |
| Teething |
248 (7.9) |
87 (8.3) |
0.95 [0.74; 1.23] |
0.72 |
| Rash |
203 (6.5) |
72 (6.9) |
0.94 [0.71; 1.25] |
0.69 |
| Cough |
208 (6.6) |
69 (6.6) |
1.00 [0.76; 1.34] |
1.00 |
| Irritability |
196 (6.3) |
59 (5.7) |
1.11 [0.82; 1.51] |
0.55 |
| Rhinorrhoea |
189 (6.0) |
63 (6.0) |
1.00 [0.75; 1.35] |
1.00 |
| Nasal congestion |
162 (5.2) |
60 (5.7) |
0.90 [0.66; 1.23] |
0.52 |
| Constipation |
102 (3.3) |
21 (2.0) |
1.62 [1.00; 2.72] |
0.05* |
| Insomnia |
23 (0.7) |
16 (1.5) |
0.48 [0.24; 0.97] |
0.04 |
| Allergic rhinitis |
17 (0.5) |
13 (1.2) |
0.44 [0.20; 0.97] |
0.04 |
| Croup |
31 (1.0) |
21 (2.0) |
0.49 [0.27; 0.90] |
0.02 |
| Crying | 6 (0.2) | 7 (0.7) | 0.29 [0.08; 0.99] | 0.05* |
N, total number of subjects in A/B only, with at least one administered dose; n (%), number (percentage) of subjects reporting the symptoms at least once. There were two preferred terms with p values for the Breslow and Day test that were significant, indicating a difference in relative reporting patterns between the different countries: application site hematoma (reported by 0.1% in the HibMenCY group and 0.3% in the Hib group, p = 0.03) and cough (p = 0.01), p values < 0.05 are highlighted in bold. *p < 0.05.
There were no statistically significant differences across countries detected from the B&D test with respect to the overall incidence per subject of solicited AEs reported during the 4-dose series, with the exception of grade 3 injection site pain (p = 0.04). This finding is due to relatively fewer HibMenCY vaccinees as compared with Hib vaccinees from the US reporting grade 3 pain than in the other countries [as measured by RR (HibMenCY group over Hib group): RR = 0.53 for the US, 0.76 for Australia and 0.79 for Mexico].
At least one SAE was reported by 6% of subjects in each group (Table 2). For most SAEs, the rate in each MedDRA Preferred Term category was ≤ 0.1% in both treatment groups (data not shown). SAEs (and other AEs of interest) occurring at a rate of 0.3% or more are given in Table 2. SAEs occurring in ≥ 0.1% but < 0.3% of subjects are given in Supplementary Table. Viral infection, febrile convulsion and urinary tract infection were the most frequently reported SAEs. No statistically significant differences were detected between HibMenCY and Hib groups for any individual MedDRA preferred term.
Table 2. Relative risk of adverse events (with an incidence of at least 0.3% in either group, or where p < 0.05), reported from Dose 1 until 6 mo post-dose 4 (total vaccinated cohort, all studies).
| Symptom (Preferred term) |
HibMenCY n = 6414 |
Hib n = 2157 |
Relative Risk |
p value |
|---|---|---|---|---|
| |
n (%) |
n (%) |
[95% CI] |
|
| SAEs | ||||
| At least one |
388 (6.0) |
130 (6.0) |
1.00 [0.82; 1.23] |
1.00 |
| Dehydration |
29 (0.5) |
9 (0.4) |
1.09 [0.50; 2.62] |
0.99 |
| Gastroenteritis |
84 (1.3) |
29 (1.3) |
0.97 [0.63; 1.53] |
0.96 |
| Pneumonia |
21 (0.3) |
6 (0.3) |
1.18 [0.46; 3.57] |
0.92 |
| Respiratory syncytial virus bronchiolitis |
19 (0.3) |
3 (0.1) |
2.15 [0.63; 11.33] |
0.31 |
| Bronchiolitis |
65 (1.0) |
15 (0.7) |
1.46 [0.82; 2.75] |
0.23 |
| Bronchopneumonia | 20 (0.3) | 12 (0.6) | 0.56 [0.26; 1.25] | 0.16 |
| New onset of chronic disease | ||||
|---|---|---|---|---|
| At least one symptom |
341 (5.3) |
120 (5.6) |
0.96 [0.78; 1.19] |
0.74 |
| Drug hypersensitivity |
26 (0.4) |
8 (0.4) |
1.10 [0.48; 2.81] |
1.00 |
| Asthma |
52 (0.8) |
20 (0.9) |
0.88 [0.52; 1.56] |
0.71 |
| Gastro-esophageal reflux disease |
24 (0.4) |
6 (0.3) |
1.36 [0.54; 4.06] |
0.67 |
| Food allergy |
29 (0.5) |
7 (0.3) |
1.40 [0.60; 3.78] |
0.56 |
| Allergic rhinitis |
23 (0.4) |
5 (0.2) |
1.56 [0.58; 5.26] |
0.50 |
| Eczema |
126 (2.0) |
36 (1.7) |
1.18 [0.81; 1.76] |
0.44 |
| Bronchial hyper-reactivity |
23 (0.4) |
12 (0.6) |
0.65 [0.31; 1.43] |
0.30 |
| Milk allergy |
13 (0.2) |
8 (0.4) |
0.55 [0.21; 1.53] |
0.27 |
| Anemia | 0 (0.0) | 3 (0.1) | 0.00 [0.00; 0.82] | 0.03 |
| Rash | ||||
|---|---|---|---|---|
| At least one symptom |
1209 (18.8) |
416 (19.3) |
0.98 [0.88; 1.10] |
0.74 |
| Diaper dermatitis |
184 (2.9) |
63 (2.9) |
0.99 [0.74; 1.33] |
0.97 |
| Eczema |
254 (4.0) |
87 (4.0) |
0.99 [0.77; 1.27] |
0.96 |
| Generalized rash |
30 (0.5) |
11 (0.5) |
0.92 [0.45; 2.03] |
0.93 |
| Contact dermatitis |
23 (0.4) |
9 (0.4) |
0.86 [0.39; 2.12] |
0.84 |
| Erythema |
24 (0.4) |
10 (0.5) |
0.81 [0.37; 1.90] |
0.70 |
| Erythematous rash |
24 (0.4) |
10 (0.5) |
0.81 [0.37; 1.91] |
0.70 |
| Dermatitis |
46 (0.7) |
18 (0.8) |
0.86 [0.49; 1.58] |
0.69 |
| Urticaria |
92 (1.4) |
35 (1.6) |
0.89 [0.60; 1.35] |
0.61 |
| Atopic dermatitis |
99 (1.5) |
29 (1.3) |
1.15 [0.75; 1.81] |
0.58 |
| Macular rash |
22 (0.3) |
5 (0.2) |
1.49 [0.55; 5.03] |
0.58 |
| Rash |
517 (8.1) |
185 (8.6) |
0.94 [0.79; 1.12] |
0.50 |
| Seborrheic dermatitis |
32 (0.5) |
14 (0.6) |
0.77 [0.40; 1.56] |
0.50 |
| Papular rash | 19 (0.3) | 11 (0.5) | 0.59 [0.27; 1.36] | 0.23 |
| AEs resulting in ER visit | ||||
|---|---|---|---|---|
| At least one symptom |
613 (9.6) |
215 (10.0) |
0.96 [0.82; 1.13] |
0.67 |
| Febrile convulsion |
22 (0.3) |
8 (0.4) |
0.93 [0.40; 2.42] |
1.00 |
| Upper respiratory tract infection |
68 (1.1) |
22 (1.0) |
1.05 [0.64; 1.78] |
0.96 |
| Diarrhea |
18 (0.3) |
5 (0.2) |
1.21 [0.43; 4.19] |
0.92 |
| Pneumonia |
31 (0.5) |
12 (0.6) |
0.88 [0.44; 1.87] |
0.81 |
| Rash |
19 (0.3) |
8 (0.4) |
0.80 [0.33; 2.11] |
0.73 |
| Viral infection |
41 (0.6) |
16 (0.7) |
0.87 [0.48; 1.66] |
0.72 |
| Bronchiolitis |
63 (1.0) |
18 (0.8) |
1.18 [0.69; 2.12] |
0.64 |
| Vomiting |
28 (0.4) |
12 (0.6) |
0.79 [0.39; 1.71] |
0.60 |
| Otitis media |
95 (1.5) |
27 (1.3) |
1.19 [0.77; 1.90] |
0.49 |
| Pyrexia |
76 (1.2) |
21 (1.0) |
1.23 [0.75; 2.09] |
0.48 |
| Head injury |
21 (0.3) |
4 (0.2) |
1.78 [0.60; 7.12] |
0.41 |
| Gastroenteritis |
53 (0.8) |
23 (1.1) |
0.78 [0.47; 1.33] |
0.37 |
| Infectious croup |
25 (0.4) |
13 (0.6) |
0.65 [0.32; 1.39] |
0.28 |
| Dehydration |
20 (0.3) |
3 (0.1) |
2.26 [0.67; 11.88] |
0.26 |
| Viral Gastroenteritis | 13 (0.2) | 0 (0.0) | INF [1.03: INF] | 0.05* |
At least one symptom, at least one symptom experienced (regardless of the MedDRA Preferred term); N, number of subjects with at least one administered dose; n (%), number (percentage) of subjects reporting the symptom at least once; 95% CI, 95% confidence interval for relative risk (exact stratified conditional to total number of cases); p value, 2-sided exact stratified text conditional to number of cases; INF, infinity. p values < 0.05 are highlighted in bold. *p < 0.05
The occurrence of nervous system disorders associated with MMR and VAR vaccines and symptoms possibly indicating meningococcal disease were specifically assessed. SAEs affecting the nervous system were reported in 26 (0.40%) of the 6414 subjects in the HibMenCY group and 10 (0.46%) of the 2157 subjects in the Hib group. None of these events were considered by the investigators as related to vaccination. The 95% CI on the RR between the HibMenCY group and the control Hib group included “1” for all individual events (Table 3).
Table 3. Relative risk of serious adverse events affecting the nervous system, reported from Dose 1 until 6 mo post-dose 4 (total vaccinated cohort, all studies).
| Symptom (Preferred term) |
HibMenCY n = 6414 |
Hib n = 2157 |
Relative Risk |
p value |
|---|---|---|---|---|
| n (%) | n (%) | [95% CI] | ||
| At least one nervous system disorder |
26 (0.4) |
10 (0.5) |
- |
- |
| Ataxia |
1 (0.0) |
1 (0.0) |
0.33 [0.00; 26.18] |
0.88 |
| Cerebellar ataxia |
1 (0.0) |
0 (0.0) |
INF [0.01; INF] |
1.00 |
| Convulsion |
5 (0.1) |
2 (0.1) |
0.84 [0.14; 8.84] |
1.00 |
| Depressed level of consciousness |
0 (0.0) |
1 (0.0) |
0.00 [0.00; 13.23] |
0.51 |
| Epilepsy |
1 (0.0) |
0 (0.0) |
INF [0.01; INF] |
1.00 |
| Febrile convulsion |
14 (0.2) |
5 (0.2) |
0.94 [0.32; 3.33] |
1.00 |
| Hemorrhage intracranial |
1 (0.0) |
0 (0.0) |
INF [0.01; INF] |
1.00 |
| Hypotonia |
1 (0.0) |
0 (0.0) |
INF [0.01; INF] |
1.00 |
| Infantile spasms |
1 (0.0) |
0 (0.0) |
INF [0.01; INF] |
1.00 |
| Nystagmus | 1 (0.0) | 1 (0.0) | 0.33 [0.00; 26.18] | 0.88 |
At least one symptom, at least one symptom experienced (regardless of the MedDRA Preferred term); N, number of subjects with at least one administered dose; n (%), number (percentage) of subjects reporting the symptom at least once; 95% CI, 95% confidence interval for relative risk (exact stratified conditional to total number of cases); p value, 2-sided exact stratified test conditional to number of cases; INF, infinity.
There were 6 cases of sudden infant death syndrome (4 cases, 0.06%, in the HibMenCY group and 2 cases, 0.09%, in the Hib group, p = 0.94). One case occurred 10 d after Dose 1 (HibMenCY group), the remaining cases in both groups occurred between 22 and 42 d after dose 1.
During the study period, the new onset of a chronic disease was reported by 5.3% of subjects in the HibMenCY group and by 5.6% of subjects in the Hib group (Table 2).
Eczema was the most frequently reported new onset chronic disease in both groups (2.0% of subjects in the HibMenCY group and 1.7% of subjects in the Hib group). No statistically significant differences were detected between groups with the exception of anemia (p-value = 0.03), which was reported more frequently in the Hib group than the HibMenCY group (Table 2).
Any rash (regardless of characterization) was reported by 18.8% of subjects in the HibMenCY group and 19.3% of subjects in the Hib group during the follow-up period (Table 2). The most frequently reported rash event in both groups was unspecified rash (8.1% of subjects in the HibMenCY group and 8.6% of subjects in the Hib group). No statistically significant differences were detected between HibMenCY and Hib groups when comparing individual rash terms, including rash syndromes associated with meningococcal disease or MMR vaccines, namely petechiae, purpura and urticaria.
Adverse events resulting in ER visits were reported by 9.6% of subjects in the HibMenCY group and 10.0% of subjects in the Hib group during the follow-up period (Table 2). Otitis media (1.5% in the HibMenCY group and 1.3% in the Hib group), pyrexia (1.2% in the HibMenCY group and 1.0% in the Hib group) and upper respiratory tract infection (1.1% in the HibMenCY group and 1.0% in the Hib group) were the most frequently reported AEs resulting in an ER visit. Only one statistically significant difference was detected: viral gastroenteritis was reported more frequently in HibMenCY vaccine recipients than in those that received Hib vaccine (0.2% vs. 0.0%, respectively: Table 2).
There were no statistically significant differences in the reporting pattern of HibMenCY compared with Hib across countries with respect to the overall incidence of SAEs and the occurrence of specific AEs indicating new onset of chronic illness, rash, and conditions prompting ER visits over the four-dose vaccination series. This was also true when considering specific preferred terms; with the exception of “pyrexia” that resulted in an ER visit (from the B&D test). Pyrexia leading to an ER visit was reported more frequently in the HibMenCY group compared with the Hib group (as measured by RR) in the US (RR = 1.5), whereas the reverse trend was noted in Australia (RR = 0.25). There were no reports of pyrexia leading to an ER visit in Mexico.
In the phase 3 clinical development program which involved more than 8,500 infants, HibMenCY had a reactogenicity and safety profile similar to that of licensed Hib and MenC vaccines. This pooled analysis of safety from similarly designed primary and fourth dose vaccination studies describes the safety profile of a 4-dose vaccination schedule with HibMenCY compared with licensed Hib vaccines in 8,571 infants, of whom 6,414 received four HibMenCY doses. The results support the observation that HibMenCY has a safety profile comparable to licensed monovalent Hib vaccines,4,5,7 which have a demonstrated record of safety during their use over the past 20 y.3
Adverse events were recorded for a period of approximately 18 mo in each child. The results showed very few statistically significant differences between HibMenCY and Hib groups in the incidence of common unsolicited AEs (within 31 d after each dose), SAEs, rash, NOCD, or AEs leading to ER visits (all within 6 mo after the last vaccination). All statistically significant differences were the result of a lower reported rate in the HibMenCY group as compared with the Hib group, with the exceptions of constipation of any severity as an unsolicited symptom and viral gastroenteritis leading to an ER visit (Table 2). It is noteworthy that no statistically significant differences between groups were observed in the incidences of SAEs due to gastroenteritis (unspecified), viral gastroenteritis or dehydration, or in gastroenteritis (unspecified), diarrhea, vomiting, dehydration or vial illnesses leading to ER visits (Table 2), suggesting that the isolated finding of increased viral gastroenteritis in the HibMenCY group may have occurred by chance. Multiple statistical comparisons performed may lead to positive associations due solely to chance. Thus the results of these exploratory comparisons need to be interpreted cautiously.
The results of the B&D test for the multiple exploratory analyses per country suggest that in the three countries that participated in the phase 3 clinical program, comparisons between the HibMenCY group and the Hib group yielded similar conclusions. No statistically significant B&D tests were found either overall or by MedDRA preferred term for SAEs, rashes, new onset of chronic diseases, or AEs leading to ER visits from dose 1 until 6 mo post dose 4, except for the occurrence of pyrexia as an AE resulting in an ER visit (but not fever overall). This finding may have been chance-related, given the large number of comparisons performed. The homogeneity in reporting rates across countries is reassuring because it suggests that despite the ethnic differences and the differences in medical systems for the subject populations in the three countries, the general patterns of relative reporting of AEs in the HibMenCY group compared with the Hib group are comparable. Thus, the HibMenCY safety database can be considered as a single pooled entity for the purpose of signal detection.
Potential limitations of the study include possible limitations of study power of the B&D test to detect differences against countries for AEs with low event rates. In addition, the study personnel were not blinded as to treatment group, which could have biased the assessment of causality and intensity of the unsolicited events, but the bias is more likely to have worked against the HibMenCY vaccine, since the physicians assigning causality were aware that HibMenCY is an investigational vaccine which includes additional antigens as compared with licensed Hib vaccines. Finally, the study was not powered for any given between-group comparison, which means that definitive conclusions about the RR of a given AE term between the treatment groups cannot be drawn.
Evaluation of vaccine safety is optimally assessed in large, randomized, controlled clinical trials such as the present analysis, which, unlike post-marketing studies, limit the potential for detection and reporting biases, and allow comprehensive documentation of AEs. Within the limits of this study, no safety signal was identified as necessitating further investigation in subsequent studies, specifically powered and designed for this purpose. Nevertheless, the complete safety profile of a new vaccine is only achieved after extensive post-licensure experience.
HibMenCY had an acceptable clinical safety profile in both phase 2 and 3 studies. In this analysis of safety in a large cohort of children, the comparability of the safety profile of HibMenCY to licensed Hib vaccine was confirmed, despite the addition of meningococcal antigens. These data suggest that HibMenCY, if licensed, could be safely introduced into the US pediatric vaccination schedule with no increased risk to young infants as compared with licensed Hib vaccines.
Trademarks. ActHIB is a registered trademark of sanofi pasteur. PedvaxHIB is a registered trademark of Merck and Co., Inc. MMRII and Varivax are registered trademarks of Merck and Co. Pediarix and Infanrix are registered trademarks of the GlaxoSmithKline Group of Companies. Prevnar and Prevenar are registered trademarks of Pfizer.
Sources of support. GlaxoSmithKline (GSK) Biologicals was the funding source and was involved in all stages of the study conduct and analysis. GSK Biologicals also funded all costs associated with the development and the publishing of the present manuscript. All authors have contributed to the interpretation of the data, the writing and reviewing of the paper and approved the submitted version of the paper. The corresponding author had full access to the data and was responsible for submission of the publication.
Supplementary Material
Acknowledgments
The authors thank co-investigators and study co-ordinators:
In Australia: Doctors Helen Marshall, Peter Richmond, Michael Nissen, Jodie McVernon, and study coordinators Marita Kefford, Susan Lee and Jennifer Kent.
In Mexico: Doctors Tania Espinosa Sierra, Renatta Pacheco Garcia and Norma Dominguez Valois.
In the US for study A/B: Doctors Gerald Bader, Mark Blatter, Dean A Blumberg, Joseph Elser, Earl Ruffin Franklin, Fernando Guerra, James Hedrick, Michael Husseman, Farha Khan, Michael Leonardi, Steven Manson, Gary S. Marshall, Sharon Nachman, Zack Sanders, Julie Shepard, Lawrence D. Sher, Hernani Soberano, Alawia Suliman, Christine Turley, Lisa Turner, Meera Varman, Emmanuel Walter, and Leonard Weiner.
In the US for study C/D: Doctors Blaise L. Congeni, Matthew Cox, John Fling, Stephen Fries, Michael Green, Edward Goldblatt, Dan Henry, Richard Hines, William Hitchcock, Michael Husseman, William Johnston, Karen Kamachi, Michael Leonardi, Joseph Ley, Keith Ramsey, Zack Sanders, Michael Simon, Hernani Soberano, Malcolm Sperling, Bradley J. Sullivan, Steven Thompson, Lisa Turner, Ellen Wald, Leonard Weiner, and study coordinators Jodie Devers and Theresa Lane.
The authors specially thank the volunteers and their parents/guardians who participated in the study, the study nurses and other staff members without whom this study would not have been possible. The authors are also grateful to collaborators from GSK: Dr Dominique Boutriau and Dr Leonard Friedland for critical evaluation of the studies; Dr Pascal Lestrate for Lab testing, Karine Muller and Anne Sumbul for performing the statistical analyses, GSK study managers Valérie Sengers, Heather Santiago, and Catherine Streeton; GSK Australia Clinical Operations Team for coordination and contacts with investigators: Maja Galic, Jacob Ryan, Audra Wilson and Felicity King. The authors also thank Dr Joanne Wolter (independent medical writer) for assistance in writing the initial manuscript draft and Dr Tatjana Mijatovic and Wouter Houthoofd (both XPE Pharma and Science) for editorial assistance and manuscript coordination on behalf of GSK.
Glossary
Abbreviations:
- AE
adverse event
- B&D
Breslow and Day
- CI
confidence interval
- DTaP–HepB–IPV
diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated poliovirus vaccine
- ER
emergency room
- Hib
Haemophilus influenzae type b
- HibMenCY
Haemophilus influenzae type b and Neisseria meningitidis serogroups C and Y tetanus toxoid conjugate vaccine
- FDA
Food and Drug Administration
- ACIP
Advisory Committee on Immunization Practices
- MedDRA
Medical Dictionary for Regulatory Activities
- MenC
monovalent meningococcal serogroup C conjugate vaccine
- MMR
measles-mumps-rubella vaccine
- PCV7
7-valent pneumococcal conjugate vaccine
- RR
relative risk
- SAE
serious adverse event
- TT
tetanus toxoid
- VAR
varicella vaccine
Disclosure of Potential Conflicts of Interest
S.R. is a principal investigator in studies funded by GSK and has received travel support from GSK for attendance at investigator meetings.
K.B. is a principal investigator in studies funded by GSK, Pfizer, Novartis and MedImmune. She has also received honoraria from sanofi pasteur for lectures and consulting services, and from GSK for consulting services.
T.N. is a principal investigator in studies funded by GSK, has received travel support from GSK to scientific meetings for presentation of scientific data and an honorarium for membership of an independent DSMB for an unrelated vaccine.
N.P.R. is a principal investigator on clinical trials funded by GSK, Pfizer and Bristol, and has received honoraria for board membership on Tibotec.
C.A.D. is a principal investigator on clinical trials funded by GSK.
M.A.R.W. declares no conflict of interest.
C.C., E.A., N.M. and J.M.M. are employees of GSK Biologicals. C.C., N.M. and J.M.M. report ownership of stock options.
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/18752
References
- 1.Cohn AC, MacNeil JR, Harrison LH, Hatcher C, Theodore J, Schmidt M, et al. Changes in Neisseria meningitidis disease epidemiology in the United States, 1998-2007: implications for prevention of meningococcal disease. Clin Infect Dis. 2010;50:184–91. doi: 10.1086/649209. [DOI] [PubMed] [Google Scholar]
- 2.FDA - Menactra approval letter April 22, 2011. http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm252511.htm. Accessed June 8, 2011.
- 3.WHO position paper on Haemophilus influenzae type b conjugate vaccines. Wkly Epidemiol Rec. 2006;81:445–52. [PubMed] [Google Scholar]
- 4.Marchant CD, Miller JM, Marshall GS, Blatter M, Aris E, Friedland LR, et al. Randomized trial to assess immunogenicity and safety of Haemophilus influenzae type b and Neisseria meningitidis serogroups C and Y-tetanus toxoid conjugate vaccine in infants. Pediatr Infect Dis J. 2010;29:48–52. doi: 10.1097/INF.0b013e3181c3ce88. [DOI] [PubMed] [Google Scholar]
- 5.Marshall GS, Marchant CD, Blatter M, Aris E, Boutriau D, Poolman JT, et al. Immune response and one-year antibody persistence after a fourth dose of a novel Haemophilus influenzae type b and Neisseria meningitidis serogroups C and Y-tetanus toxoid conjugate vaccine (HibMenCY) at 12 to 15 months of age. Pediatr Infect Dis J. 2010;29:469–71. doi: 10.1097/INF.0b013e3181cdd379. [DOI] [PubMed] [Google Scholar]
- 6.Nolan T, Lambert S, Roberton D, Marshall H, Richmond P, Streeton C, et al. A novel combined Haemophilus influenzae type b-Neisseria meningitidis serogroups C and Y-tetanus-toxoid conjugate vaccine is immunogenic and induces immune memory when co-administered with DTPa-HBV-IPV and conjugate pneumococcal vaccines in infants. Vaccine. 2007;25:8487–99. doi: 10.1016/j.vaccine.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 7.Nolan T, Richmond P, Marshall H, McVernon J, Alexander K, Mesaros N, et al. Immunogenicity and safety of an investigational combined Haemophilus influenzae type B-Neisseria meningitidis serogroups C and Y-tetanus toxoid conjugate vaccine. Pediatr Infect Dis J. 2011;30:190–6. doi: 10.1097/INF.0b013e3181fcb2bf. [DOI] [PubMed] [Google Scholar]
- 8.Bryant KA, Marshall GS, Marchant CD, Pavia-Ruiz N, Nolan T, Rinderknecht S, et al. Immunogenicity and Safety of H influenzae Type b-N meningitidis C/Y Conjugate Vaccine in Infants. Pediatrics. 2011;127:e1375–85. doi: 10.1542/peds.2009-2992. [DOI] [PubMed] [Google Scholar]
- 9.Australian Government Department of Health and Ageing, National Health and Medical Research Council. The Australian Immunisation Handbook 9th Edition 2008. http://www.health.gov.au/internet/immunise/publishing.nsf/content/handbook-home. Accessed August 9, 2011.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.