This article examines the role of cytokinin in ovule patterning, finding that cytokinin acts through the auxin efflux facilitator PIN1 and requires the transcription factors BEL1 and SPL/NZZ for proper patterning of the ovule.
Abstract
Hormones, such as auxin and cytokinin, are involved in the complex molecular network that regulates the coordinated development of plant organs. Genes controlling ovule patterning have been identified and studied in detail; however, the roles of auxin and cytokinin in ovule development are largely unknown. Here we show that key cytokinin pathway genes, such as isopentenyltransferase and cytokinin receptors, are expressed during ovule development. Also, in a cre1-12 ahk2-2 ahk3-3 triple mutant with severely reduced cytokinin perception, expression of the auxin efflux facilitator PIN-FORMED 1 (PIN1) was severely reduced. In sporocyteless/nozzle (spl/nzz) mutants, which show a similar phenotype to the cre1-12 ahk2-2 ahk3-3 triple mutant, PIN1 expression is also reduced. Treatment with the exogenous cytokinin N6-benzylaminopurine also altered both auxin distribution and patterning of the ovule; this process required the homeodomain transcription factor BELL1 (BEL1). Thus, this article shows that cytokinin regulates ovule development through the regulation of PIN1. Furthermore, the transcription factors BEL1 and SPL/NZZ, previously described as key regulators of ovule development, are needed for the auxin and cytokinin signaling pathways for the correct patterning of the ovule.
INTRODUCTION
The plant hormone cytokinin acts in concert with auxin, and the different accumulation of these two hormones is known to be important for the development of plant organs (Skoog and Miller, 1957).
Despite increasing evidence for the importance of hormonal networks in the regulation of plant development, the role of auxin and cytokinin in ovule patterning is still unknown. There is evidence that both hormones play important functions in ovule primordia formation and female fertility. Plants with reduced cytokinin production or perception show a drastic reduction in ovule numbers and female fertility (Werner et al., 2003; Hutchison et al., 2006; Miyawaki et al., 2006; Riefler et al., 2006; Kinoshita-Tsujimura and Kakimoto, 2011). CYTOKININ INDEPENDENT1 (CKI1) is known to be involved in cytokinin signaling, and the cki1 mutant shows female gametophyte defects (Kakimoto, 1996; Pischke et al., 2002). When the amount of cytokinin increases, like in the ckx3 ckx5 double mutant, the number of ovule primordia increases, confirming a clear correlation between cytokinin levels and ovule numbers (Bartrina et al., 2011).
Effects on ovule development have also been reported in plants treated with auxin efflux inhibitors, which develop a naked placenta (Nemhauser et al., 2000). Furthermore, female gametophyte cell identity seems to be compromised when the expression of auxin synthesis or auxin response genes are modified (Pagnussat et al., 2007). Although the role of hormones in ovule formation has been understudied, the genetic network controlling ovule development has been investigated for many years, and several key factors have been identified and characterized (reviewed in Colombo et al., 2008). Among them, BELL1 (BEL1), a homeodomain transcription factor, has been reported to be one of the major factors controlling ovule pattering, in particular determining identity and development of the integuments. In the bel1 mutant, ovules develop a single integument-like structure, which expresses carpel-specific genes (Robinson-Beers et al., 1992; Reiser et al., 1995; Brambilla et al., 2007). It has been reported that the right balance between BEL1 and the MADS domain transcription factors AGAMOUS (AG) and SEEDSTICK (STK) is needed for the correct determination of integument identity (Brambilla et al., 2007). Another important factor regulating ovule pattering is SPOROCYTELESS/NOZZLE (SPL/NZZ), which is required for the development of the megasporocyte, from which the female gametophyte develops (Schiefthaler et al., 1999; Yang et al., 1999). Furthermore, SPL together with BEL1 has been shown to control chalaza development, because, in the bel1 spl double mutant, the ovules develop as finger-like structures without integuments (Balasubramanian and Schneitz, 2002).
Here we analyze the role of cytokinin in ovule development and show that an increase in cytokinin levels influences ovule patterning. These phenotypes are a consequence of a change in PIN-FORMED 1 expression. PIN1 is one of the best-studied auxin efflux facilitators, and recently it has been reported that, at least in roots, cytokinin negatively controls secondary root formation by regulating PIN1 expression and consequently changing the auxin pattern along the root (Laplaze et al., 2007; Dello Ioio et al., 2008; Ruzicka et al., 2009). The link between PIN1 expression and cytokinin was further evidenced by the fact that in plants defective for the cytokinin receptors ARABIDOPSIS HISTIDINE KINASE4/CYTOKININ RESPONSE1 (AHK4/CRE1), AHK2, and AHK3, the expression of PIN1 was compromised.
The data we present here show an important role for the transcription factors BEL1 and SPL in the cytokinin-dependent regulation of PIN1, which is important for the correct development of the chalaza region in the ovule.
RESULTS
Analysis of the Cytokinin Pathway during Ovule Development
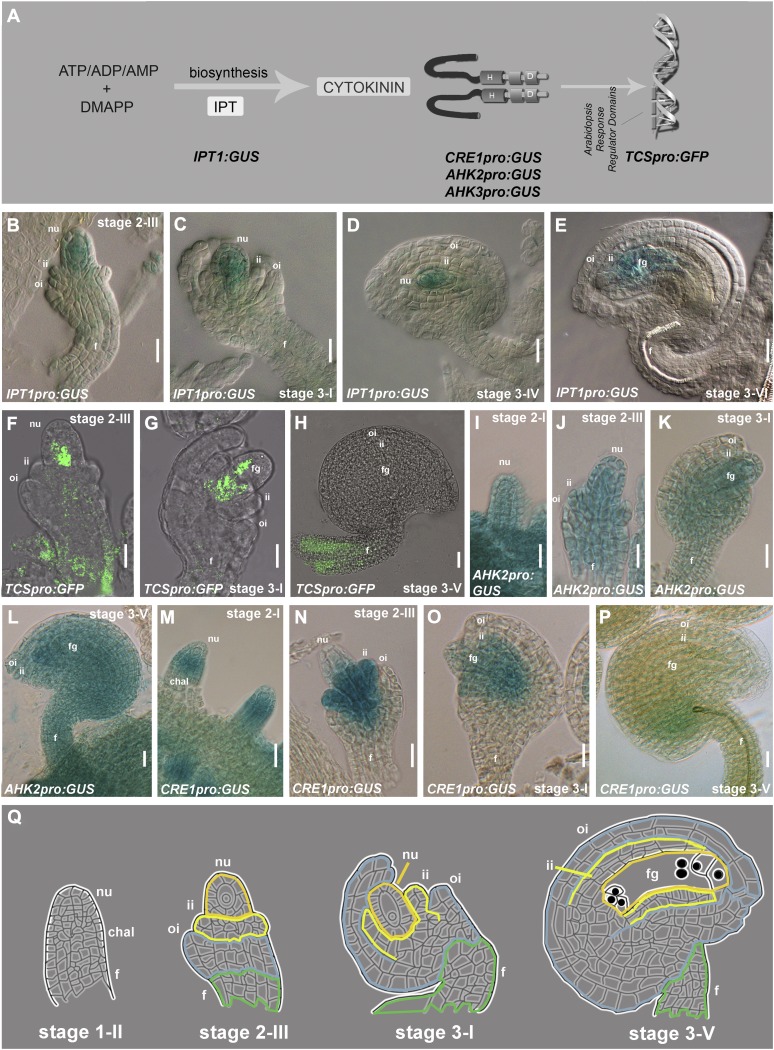
Recent studies indicated the involvement of auxin in controlling ovule development, including the formation of the megagametophyte (Benková et al., 2003; Pagnussat et al., 2009). However, so far little is known about the role of other plant hormones, such as cytokinin, in this process. A first step to investigate the possible role of cytokinin in ovule development was the analysis of the expression of genes involved in the cytokinin signaling pathway (Figure 1A). Among these, the genes encoding isopentenyltransferases (IPT), which are the principal enzymes responsible for cytokinin synthesis, were selected (Kakimoto, 2001; Sun et al., 2003). Previously, it has been reported that Arabidopsis thaliana IPT1 is the only isopentenyltransferase–encoding gene that is expressed in ovules (Miyawaki et al., 2004). We have analyzed in detail IPT1 expression using 20 pistils at different stages of development from eight IPT1pro:β-glucuronidase (GUS) plants (Miyawaki et al., 2004). GUS expression was observed in all these plants in the whole ovule starting from stage 2-III (Figure 1B). During the following stages, GUS activity was detected in the funiculus and in the developing female gametophyte (Figures 1C to 1E).
Figure 1.
Analysis of the Cytokinin Pathway during Ovule Development.
Ovule stages as in Schneitz et al. (1995).
(A) Schematic representation of the cytokinin pathway and the genes analyzed in this article.
(B) to (E) GUS expression in IPT1pro:GUS ovules from stage 2-III to stage 3-VI.
(F) to (H) GFP expression in TCSpro:GFP ovules from stage 2-III to ovule stage 3-V.
(I) to (L) GUS expression in AHK2pro:GUS ovules from stage 1-I to stage 3-V.
(M) to (P) GUS expression CRE1pro:GUS ovules from stage 1-I to stage 3-V.
(Q) Scheme of ovule development from stage 1-II to stage 3-V.
chal, chalaza; f, funiculus; fg, female gametophyte; ii, inner integument; oi, outer integument; n, nucellus.
Bars = 20 μm.
To detect the cytokinin signaling output (Figure 1A), we analyzed ovules at different stages of development in eight Arabidopsis plants (20 pistils each) containing the TCSpro:green fluorescent protein (GFP) construct. TCS is a synthetic promoter, containing the B-type Arabidopsis response regulator binding motifs and the minimal 35S promoter (Müller and Sheen, 2008). The GFP signal was detected in the basal part of the nucellus and in the funiculus starting from stage 2-III (Figures 1F and 1G). At stage 3-V, the GFP signal was drastically reduced and was hardly visible except for the funiculus, where GFP expression remained detectable (Figure 1H).
The receptors AHK2, AHK3, and AHK4/CRE1 are important components of the cytokinin signaling pathway and are needed for cytokinin signal transduction (Figure 1A). These proteins are known to interact with cytokinins to start the multistep two-component signaling pathway (Inoue et al., 2001). To study the expression pattern of these three genes during ovule development, we analyzed transgenic plants containing the CRE1pro:GUS, AHK2pro:GUS, and AHK3pro:GUS constructs (Nishimura et al., 2004). All three GUS lines showed activity in developing ovules. GUS expression driven by the AHK2 regulatory region was observed during all stages of ovule development, starting from the early primordia stage (Figure 1I) until the ovule reached maturity (stage 3-V; Figures 1J to 1L). The same GUS activity was observed in AHK3pro:GUS lines (see Supplemental Figure 1 online). Transgenic plants containing the CRE1pro:GUS construct showed GUS expression in the chalaza region of the developing ovule primordia (Figure 1M). Subsequently, the CRE1 promoter maintains its activity in the chalaza and in the inner integuments until stage 3-I of ovule development (Figures 1N to 1O). After stage 3-I, the GUS signal drastically decreased (Figure 1P).
This analysis showed that important components of the cytokinin pathway are expressed during ovule development.
Cytokinin Perception Is Required for PIN1 Expression in Ovules
Because important genes for the cytokinin signaling pathway are expressed during Arabidopsis ovule development, we were interested to investigate the role of cytokinin during this process. Therefore, we analyzed the ovules of the cre1-12 ahk2-2 ahk3-3 triple mutant, which is considered to have a dramatic reduction in cytokinin responses, including cytokinin primary-response gene induction (Higuchi et al., 2004).
As reported previously, the single and double mutants do not present a phenotype at the level of the ovule (Kinoshita-Tsujimura and Kakimoto, 2011), whereas the cre1-12 ahk2-2 ahk3-3 triple mutant showed defects in the formation of the female gametophyte, which arrested at stage FG1-FG2 (Figure 2B) (Higuchi et al., 2004). We analyzed two pistils of five cre1-12 ahk2-2 ahk3-3 triple mutant plants and noticed a severe reduction in ovule number with respect to the wild type (see Supplemental Table 1 and Supplemental Figure 2 online). Furthermore, 10% of these ovules (50 out of 530) developed as finger-like structures (Figure 2C); in wild-type plants, this phenotypic defect was never observed.
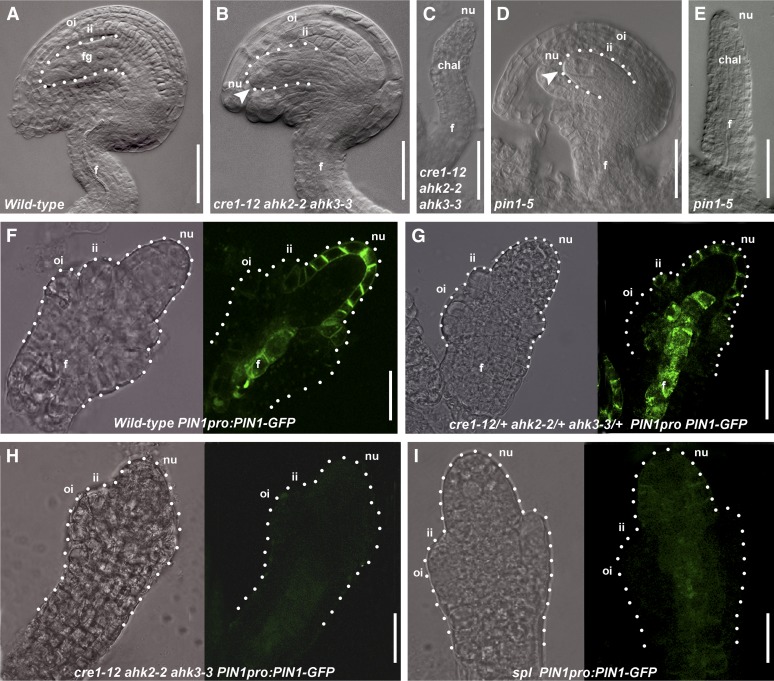
Figure 2.
Role of Cytokinin in Ovule Development.
(A) Wild-type ovule at stage 3-V. The dotted line indicates the female gametophyte.
(B) cre1-12 ahk2-2 ahk3-3 ovule at stage 3-V. The female gametophyte arrested at stage FG1 (arrowhead).
(C) cre1-12 ahk2-2 ahk3-3 finger-like structure.
(D) pin1-5 ovule at stage 3-V. The female gametophyte arrested at stage FG1 (arrowhead).
(E) pin1-5 finger-like structure.
(F) Wild-type ovule expressing PIN1pro:PIN1-GFP.
(G) cre1-12/+ ahk2-2/+ ahk3-3/+ ovule expressing PIN1pro:PIN1-GFP.
(H) cre1-12 ahk2-2 ahk3-3 triple mutant ovule expressing PIN1pro:PIN1-GFP.
(I) spl ovule expressing PIN1pro:PIN1-GFP.
(F) to (I) Pictures taken using the bright field (left) and the dark field (right). The dotted line shows the ovule profile.
chal, chalaza; f, funiculus; fg, female gametophyte; ii, inner integument; nu, nucellus; oi, outer integument.
Bars = 20 μm.
The cre1-12 ahk2-2 ahk3-3 triple mutant phenotype is very similar, if not identical, to the weak pin1-5 mutant phenotype. It is important to note that the weak pin1-5 mutant does develop flowers with ovule-bearing carpels (Bennett et al., 1996; Sohlberg et al., 2006). We analyzed in detail ovule development in the pin1-5 mutant and observed a reduction in ovule number with respect to the wild type (see Supplemental Table 1 and Supplemental Figure 2 online). Furthermore, in this mutant, 10% of the ovules (17 out of 184 analyzed) developed as finger-like structures (Figure 2E). A few ovules developed normally (37 out of 184 analyzed), whereas most of them (130 out of 184) (Figure 2D) showed an arrest in gametophyte development at stage FG1.
It has been reported that cytokinin regulates PIN1 expression in roots (Dello Ioio et al., 2008; Ruzicka et al., 2009); thus, we investigated whether cytokinin controls PIN1 expression in ovules as well and whether this regulation can explain the similarity in ovule phenotype between pin1-5 and the cre1-12 ahk2-2 ahk3-3 triple mutant. Therefore, we crossed the PIN1pro:PIN1-GFP marker line with the cre1-12 ahk2-2 ahk3-3/AHK3 mutant. 8 (F3) plants with the PIN1pro:PIN1-GFP construct in the cre1-12 ahk2-2 ahk3-3 triple mutant background were analyzed by confocal microscopy. Two cre1-12/CRE1 ahk2-2/AHK2 ahk3-3/AHK3 plants containing PIN1pro:PIN1-GFP identified in the F1 generation were used as a control.
We examined the ovules of 10 pistils in each of the two cre1-12/CRE1 ahk2-2/AHK2 ahk3-3/AHK3 plants (Figure 2G), showing that PIN1-GFP is expressed in the funiculus, in the nucellus, and in the inner integument primordium at stage 2-III as in wild-type ovules (Benková et al., 2003) (Figure 2F). In PIN1pro:PIN1-GFP cre1-12 ahk2-2 ahk3-3 plants, PIN1-GFP was undetectable in the ovule of the 10 pistils of each of the 10 plants analyzed (Figure 2H). This strongly suggests that cytokinin is indeed important for the correct activation of PIN1 expression in ovules.
The Transcription Factor SPL Is Required for PIN1 Expression
To identify putative targets of the cytokinin signaling pathway that could be involved in the regulation of PIN1 expression, ovule phenotypes of the cre1-12 ahk2-2 ahk3-3 triple mutant were compared with those of previously described mutants. Among them, the spl/nzz mutant captured our attention.
SPL is a gene encoding a putative transcription factor (Yang et al., 1999), which is expressed throughout the ovule during its development (Schiefthaler et al., 1999; Balasubramanian and Schneitz, 2000; Ito et al., 2004; Sieber et al., 2004). Although spl single mutant ovules have normal integuments, they do not develop the megaspore mother cell (only 5% of the ovules at stage 2-III showed a megaspore mother cell) (Balasubramanian and Schneitz, 2000).
To analyze the spl-1 mutant in more detail, we crossed PIN1pro:PIN1-GFP and DR5rev-pro:GFP reporter lines with plants heterozygous for the spl-1 mutation. Analysis of GFP expression in homozygous spl-1 mutant plants showed that the GFP signal driven by the PIN1 promoter in the nucellus, the inner integument and the funiculus was very weak (Figure 2I) when compared with spl-1/SPL control plants (see Supplemental Figure 2 online) that segregated from the same F2 population.
Furthermore, DR5rev-pro:GFP spl-1 plants did not show a GFP signal at stage 2-III, although at early stages (stage 1-II) of development, the GFP signal was detected in fewer ovules (53 out of 494 ovules analyzed) (see Supplemental Figure 2 online). By contrast, the GFP signal was clearly visible in all ovules of spl-1/SPL control plants (see Supplemental Figure 2 online).
Taken together, these results suggest that in ovules SPL seems to be required for PIN1 expression.
SPL Is Required for Cytokinin-Induced PIN1 Expression in Ovules
Because our results showed that PIN1 expression in ovules was dependent on the cytokinin signaling pathway, we analyzed the effects of an increase in cytokinin levels in ovules by treating Arabidopsis flowers with the exogenous cytokinin N6-benzylaminopurine (BAP). BAP treatment has already been successfully used for flower meristem studies (Venglat and Sawhney, 1996; D’Aloia et al., 2011).
First, to investigate the effects of BAP application on cytokinin pathway activity, 10 transgenic plants containing the TCSpro:GFP construct (Müller and Sheen, 2008) were treated with BAP, and the ovules were analyzed 1 d after treatment. As a control, five TCSpro:GFP plants were treated with water only as a mock control. One d after the BAP treatment, the plants showed a general increase in the GFP signal in ovules, in particular at the level of the chalaza, suggesting a good penetrance of BAP (see Supplemental Figure 3 online). However, 1 d after the BAP treatment, the ovules still looked normal from a morphological point of view (see Supplemental Figure 3 online). Interestingly, the BAP treatment resulted in the formation of new primordia (in average 20 ± 3 primordia in each of the 20 pistils that were analyzed) positioned among the ovules formed before the treatment (see Supplemental Figure 3 online). We have verified the identity of these new primordia by treating two STKpro:GUS plants with BAP. The ovule-specific STK promoter (Kooiker et al., 2005) was shown to be active in these new primordia, indicating that these primordia have ovule identity (see Supplemental Figure 3 online).
An increase in ovule number was also reported in the cytokinin oxidase ckx5 ckx6 double mutant, which has increased endogenous cytokinin levels caused by absence of these oxidases (Bartrina et al., 2011).
To study the effect of increased levels of cytokinin on the regulation of PIN1 expression, flowers of PIN1pro:PIN1-GFP lines were treated with BAP, and GFP expression in the ovules was analyzed by confocal microscopy.
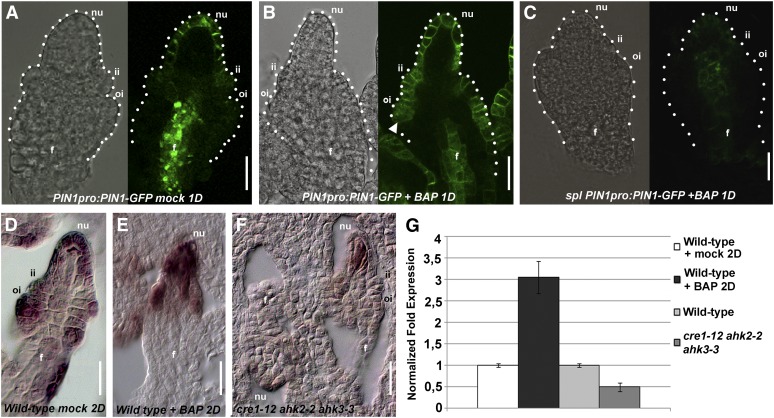
In PIN1pro:PIN1-GFP plants 1 d after BAP treatment, the PIN1-GFP signal was present in 293 ovules out of 300 analyzed not restricted to the nucellus, the inner integument, and the inner region of the funiculus as was observed in the mock-treated control plants (300 ovules analyzed; Figure 3A) but was also detected in the outer integument and in the epidermal layer of the funiculus (Figure 3B). This suggests that cytokinin is able to trigger ectopic PIN1 expression. We also treated 10 plants having the PIN1pro:PIN1-GFP construct in the spl-1 mutant background and found that, in ovules of 10 pistils for each of the 10 plants that were analyzed, the PIN1-GFP signal was not induced by the BAP treatment (Figure 3C). This observation further strengthened our hypothesis that SPL is needed for PIN1 expression. Furthermore, these data also suggest that cytokinin induced the expression of SPL. To investigate this in more detail, we studied the expression of SPL by in situ hybridization analysis using ovules treated with BAP and cre1-12 ahk2-2 ahk3-3 triple mutant ovules. As shown in Figure 3D, SPL is expressed in ovule tissues at stage 2-III, as was reported previously (Balasubramanian and Schneitz, 2000; Sieber et al., 2004). In BAP-treated plants, the SPL expression seemed to increase (Figure 3E), whereas in the cre1-12 ahk2-2 ahk3-3 triple mutant, SPL transcripts were drastically reduced and only detectable in the nucellus (Figure 3F). To quantify the changes in SPL expression in BAP-treated pistils and in cre1-12 ahk2-2 ahk3-3 triple mutant pistils, we performed real-time PCR analysis (Figure 3G). In BAP-treated pistils, SPL was upregulated with respect to mock-treated plants, whereas it was downregulated in cre1-12 ahk2-2 ahk3-3 triple mutant ovules (Figure 3G), confirming the in situ hybridization analysis.
Figure 3.
Analysis of BAP-Treated Ovules.
(A) Wild-type ovule at stage 2-III mock-treated, expressing PIN1pro:PIN1-GFP 1 d after treatment (1D).
(B) Wild-type ovule at stage 2-III BAP-treated, expressing PIN1pro:PIN1-GFP 1 d after treatment. Arrow indicates ectopic PIN1 expression.
(C) spl ovule at stage 2-III BAP-treated, expressing PIN1pro:PIN1-GFP 1 d after treatment.
(D) In situ hybridization with SPL/NZZ probe, on wild-type mock-treated ovule (2D, 2 d after treatment).
(E) In situ hybridization with SPL/NZZ probe on wild-type BAP-treated ovule 2 d after treatment.
(F) In situ hybridization with SPL/NZZ probe on cre1-12 ahk2-2 ahk3-3 triple mutant ovule.
(G) Quantitative SPL/NZZ expression analysis in wild-type BAP-treated plants and cre1-12 ahk2-2 ahk3-3 triple mutant flowers by real-time RT-PCR.
(A) to (C) Pictures were taken using bright field (left) and dark field (right). The dotted line shows the ovule’s profile.
f, funiculus; fg, female gametophyte; ii, inner integument; nu, nucellus; oi, outer integument.
Bars = 20 μm.
High Cytokinin Levels Modify Ovule Patterning
Interestingly, 2 d after BAP treatment, all ovules at stage 2-III developed instead of two integuments a single structure (which we named Cytokinin-Induced Structure [CK-IS]) (Figure 4A).
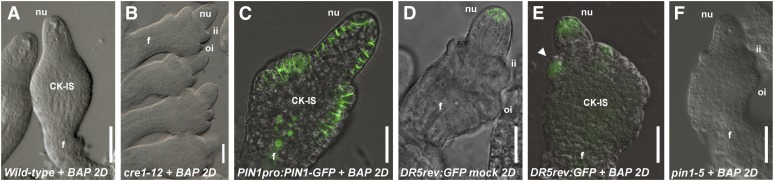
Figure 4.
Effect on Ovule Development after 2 d of BAP Treatment.
(A) Wild-type ovule 2 d (2D) after BAP treatment.
(B) cre1-12 ovule 2 d after BAP treatment.
(C) Wild-type ovule of PIN1pro:PIN1-GFP plants 2 d after BAP treatment.
(D) and (E) DR5rev-pro:GFP ovule 2 d after the mock treatment (D) or BAP treatment (E). Arrow indicates ectopic DR5rev:GFP signal in the CK-IS.
(F) pin1-5 ovule 2 d after BAP treatment.
f, funiculus; ii, inner integument; nu, nucellus; oi, outer integument.
Bars = 20 μm.
The cytokinin receptors CRE1, AHK2, and AHK3 are important for ovule development, as reported here and by Higuchi et al. (2004). To understand whether the observed BAP-induced ovule phenotype was mediated by these three cytokinin receptors, five plants for each cre1-12, ahk2-2, and ahk3-3 single mutant were treated with BAP. After 2 d, the effect of the BAP treatment was evaluated in terms of the number of ovules that formed the CK-IS instead of developing two integuments, like in mock-treated control plants (see Supplemental Table 2 online). Interestingly, only cre1-12 mutant plants treated with BAP developed two integuments, whereas in the other single mutants, integument development was affected (Figure 4B), suggesting that the CRE1 receptor has a major role in the response to cytokinin in the chalaza region.
The analysis of the PIN1pro:PIN1-GFP plants treated with BAP showed that, 2 d after BAP treatment, PIN1-GFP expression was observed in the epidermal layer of the CK-IS that developed from the chalaza (Figure 4C). Considering that the PIN1-GFP ectopic expression was seen before CK-IS formation (Figure 3B), this suggests that the phenotype of the BAP-treated ovules is a consequence of ectopic PIN1-GFP expression.
To monitor the effects of the ectopic PIN1 expression on the formation of auxin maxima, the same BAP treatment experiments were done using plants containing the GFP reporter gene driven by the auxin-induced DR5 promoter construct. In two mock-treated DR5rev-pro:GFP control plants, the GFP signal was detected in the nucellus of all 30 ovules that we analyzed (Figure 4D), confirming the auxin pattern that was reported by Benková et al. (2003). In BAP-treated DR5rev-pro:GFP plants, the GFP signal was also detected inside the CK-IS structures that developed from the chalaza (Figure 4E). These auxin maxima in the CK-IS structures are in agreement with the observed PIN1-GFP localization in BAP-treated plants (Figure 4C).
Interestingly, pin1-5 mutant plants were insensitive to the BAP treatment (Figure 4G); the ovules developed integuments as in pin1-5 mock-treated plants. These results show that high cytokinin levels resulted in a deregulation of PIN1 expression, causing severe defects in ovule development. All together, these results corroborate the hypothesis that the role of cytokinin in ovule development is mediated by the PIN1-dependent auxin distribution.
The Homeodomain Transcription Factor BEL1 Is Involved in PIN1 Regulation
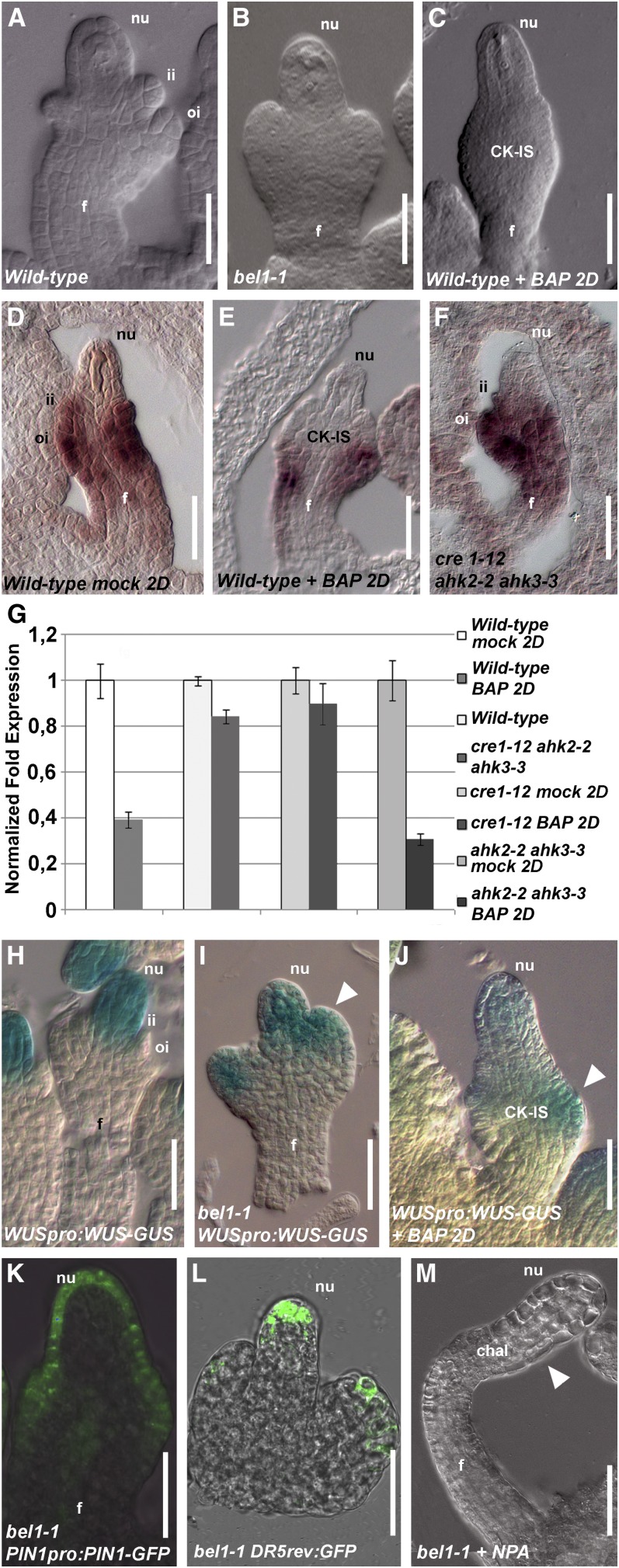
It has been reported that one of the major players in chalaza development is the homeodomain transcription factor BEL1 (Robinson-Beers et al., 1992; Reiser et al., 1995). BEL1 is expressed in the chalaza of ovules starting from stage 1-II of development. The bel1-1 mutant shows interesting similarities with the ovule phenotype obtained by BAP treatment, because in bel1-1, the two integuments (Figure 5A) are replaced by a single structure (Figure 5B) that resembles the CK-IS structure we observed in the BAP-treated plants (Figure 5C). Moreover, it has been published that, in the bel1-1 mutant, this structure is at later developmental stages converted into a carpel-like structure (Robinson-Beers et al., 1992; Brambilla et al., 2007), as has been reported to happen after BAP treatment (Venglat and Sawhney, 1996).
Figure 5.
BEL1 Expression Is Regulated by Cytokinin.
(A) Wild-type ovule, stage 2-III.
(B) bel1-1 ovule, stage 2-III.
(C) Wild-type ovule, stage 2-III, 2 d (2D) after BAP treatment.
(D) In situ hybridization on wild-type ovule with BEL1 probe.
(E) In situ hybridization on wild-type ovule treated with BAP using the BEL1 probe 2 d after treatment.
(F) In situ hybridization on cre1-12 ahk2-2 ahk3-3 triple mutant ovule with BEL1 probe.
(G) Quantitative BEL1 expression analysis by real-time RT-PCR. Wild-type mock-treated or BAP-treated 2 d after treatment, wild-type and cre1-12 ahk2-2 ahk3-3 triple mutant flowers, cre1-12 single mutant, and ahk2-2 ahk3-3 double mutant 2 d after mock treatment or BAP treatment.
(H) to (J) WUSpro:WUS-GUS activity in wild-type ovule (H), bel1-1 ovule (I), and in a wild-type ovule 2 d after BAP treatment (J). The ovules are at stage 2-III/3-I. The white arrowhead indicates ectopic WUSpro:WUS-GUS expression in the aberrant structures of the ovules ([I] to [J]).
(K) PIN1pro:PIN1-GFP in bel1-1 ovule.
(L) DR5rev-pro:GFP in bel1-1 ovule. The ovule is at stage 2-III.
(M) bel1-1 ovule treated with NPA. The arrowhead indicates the region where the bel1-1 structure is formed.
chal, chalaza; f, funiculus; ii, inner integument; nu, nucellus; oi, outer integument.
Bars = 20 μm.
These data suggest a possible interaction between BEL1 and cytokinin signaling in the ovule. To understand whether cytokinin controls BEL1 expression, we performed in situ hybridization using wild-type ovules mock-treated (control plants) or treated with BAP. In control plants, BEL1 expression was observed in the chalaza and in the developing integuments, which is similar to wild-type plants (Figure 5D). In BAP-treated plants, BEL1 expression was restricted to a small group of cells at the basal part of the CK-IS (Figure 5E). Furthermore, BEL1 was expressed similar to the wild type in cre1-12 ahk2-2 ahk3-3 triple mutant ovules (Figure 5F). To quantify BEL1 expression, we performed real-time PCR, confirming that in BAP-treated plants, BEL1 was downregulated, whereas in cre1-12 ahk2-2 ahk3-3 triple mutant ovules, BEL1 was expressed similar to the wild type (Figure 5G). We also quantified BEL expression in the BAP-treated cre1-12 mutant and in the ahk2-2 ahk3-3 double mutant (Figure 5G), which confirmed that the cytokinin regulation of BEL1 expression in the chalaza is mediated by the CRE receptor.
These data are consistent with the observed phenotypes and suggest that cytokinin might control BEL1 expression. To corroborate this conclusion, we analyzed the regulation of WUSCHEL (WUS) expression. WUS is expressed in the nucellus (Figure 5H; Gross-Hardt et al., 2002), but in the bel1-1 mutant, WUS is ectopically expressed in the chalaza (Figure 5I; Brambilla et al., 2007). Based on this observation, it has been suggested previously that BEL1 negatively regulates WUS expression (Brambilla et al., 2007).
We analyzed the ovules (two pistils for each of three BAP-treated plants) (Figure 5J) containing a WUSpro:WUS-GUS construct and showed that GUS in these plants is ectopically expressed in the chalaza, as observed in bel1-1 ovules (Figure 5I), supporting the observed downregulation of BEL1 expression after BAP treatment.
To understand the role of BEL1 in PIN1 regulation in ovules, PIN1pro:PIN1-GFP and DR5rev-pro:GFP constructs were introduced in the bel1-1 mutant background.
As shown in Figure 5K, in the bel1-1 mutant, the PIN1-GFP expression profile was similar to the profile that was observed in the PIN1pro:PIN1-GFP plants treated with BAP (Figures 3B and 4C), suggesting that BEL1 is important for the correct expression of PIN1.
Because BEL1 expression was deregulated on application of exogenous cytokinin, we were curious whether correct auxin fluxes were dependent on BEL1 activity. This would suggest that the bel1-1 mutant phenotype is caused by changes in auxin fluxes, as we showed for the BAP-treated plants (Figure 5L). To investigate this, we treated bel1-1 mutant plants with the auxin transport inhibitor N-1-naphthylphthalamic acid (NPA). Analysis of these plants showed that after 2 d of treatment, finger-like ovules were obtained (Figure 5M), suggesting that formation of the abnormal structures in the bel1-1 mutant is mediated, as in BAP-treated plants, by PIN1 ectopic expression.
In conclusion, we found that cytokinin is involved in ovule development by modulating auxin fluxes through the control of PIN1 expression. Furthermore, our data suggest that the transcription factors NZZ/SPL and BEL1 play an important role in this hormonal network in ovules.
DISCUSSION
Regulation of PIN1 Expression Requires SPL and BEL1 in Ovules
To integrate the known molecular network controlling ovule patterning with the hormonal regulation of this process, we have selected well-characterized transcription factor mutants with ovule phenotypes that resemble those obtained by the increase in cytokinin levels or mutations in cytokinin receptors.
SPL/NZZ is a transcription factor expressed throughout the ovule and is needed for correct nucellus development and together with BEL1 for chalaza formation (Schiefthaler et al., 1999; Yang et al., 1999; Balasubramanian and Schneitz, 2002).
Previously it was suggested that SPL is involved in auxin homeostasis (Li et al., 2008). The activation-tagged mutant spl-D showed an auxin-related defective phenotype, such as reduced apical dominance and a reduced number of lateral roots. Furthermore, the ARF, YUC2, and YUC6 genes were downregulated in this mutant (Li et al., 2008).
We found that in the spl mutant, PIN1 expression was compromised, suggesting that SPL is important for PIN1 expression. Interestingly, an increase in exogenous cytokinin levels in the spl mutant background did not result in a change in PIN1 expression, whereas in wild-type flowers treated with cytokinin (BAP), PIN1 expression was strongly increased. This clearly indicates that for cytokinin-mediated PIN1 expression, the SPL function is required (Figure 6). All together, these findings attribute to SPL a master role in auxin-dependent ovule developmental processes.
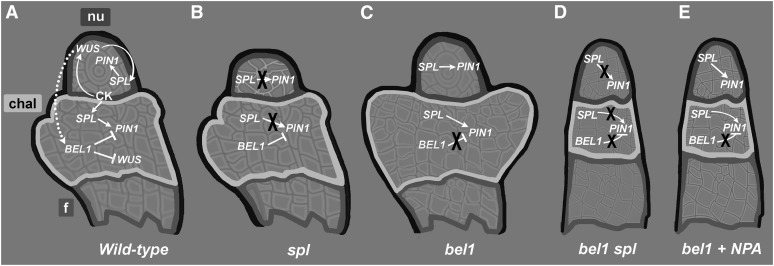
Figure 6.
BEL1 and SPL Integrate Hormonal Signaling in Ovules.
(A) In wild-type ovules, cytokinin activates WUS, which promotes the expression of SPL in the nucellus (nu) (Sieber et al., 2004) and BEL1 in the chalaza (chal). PIN1 is activated in the nucellus by SPL and repressed in the chalaza by BEL1, which in turn represses WUS (Brambilla et al., 2007). f, funiculus.
(B) In spl mutant ovules, PIN1 is not expressed, leading to a premature block of female gametophyte development and phenocopies the pin1-5 mutant.
(C) In the bel1 mutant, PIN1 is upregulated and is also expressed in the chalaza region, where normally it is not present.
(D) In finger-like bel1 spl double mutant ovules, PIN1 is not expressed in the ovule.
(E) The application of exogenous NPA to the bel1 mutant triggers the formation of finger-like ovules, because the inhibition of the auxin flux by NPA treatment avoids the formation of the aberrant structures typical for bel1 ovules.
As mentioned previously, Balasubramanian and Schneitz (2002) proposed that BEL1 works together with NZZ/SPL for the proper formation of the chalaza, because in nzz/spl bel1 double mutant ovules, no chalaza structures developed, and finger-shaped organs formed instead. The mechanism behind the redundant function of these two different transcription factors involved in ovule development remained unclear. Our findings suggest a scenario in which the bel1 phenotype is caused by an ectopic expression of PIN1 and that NZZ/SPL is essential for PIN1 expression in the ovules. We therefore propose that the transcription factor SPL is necessary for the ectopic expression of PIN1 in the bel1 mutant. If the NZZ/SPL function is missing in the bel1 mutant, the ectopic expression of PIN1 is not possible, and for this reason a bel1 nzz/spl double mutant phenotype is similar to the bel1 mutant treated with the auxin flux inhibitor NPA (Figure 6).
Similarities in the WUS Regulatory Networks in Ovules and the Shoot Apical Meristem
The bel1 nzz/spl double mutant phenotype is similar to the phenotype previously described for wus mutant ovules (Gross-Hardt et al., 2002). In the bel1 mutant, WUS is ectopically expressed in the chalaza (Brambilla et al., 2007). Confirming this, WUS ectopic expression was also observed in wild-type plants treated with exogenous cytokinin (BAP), showing that the downregulation of BEL1 caused by the increase of cytokinin levels caused the same effect on WUS expression (Figure 6).
Interestingly, regulation of WUS expression in ovules seems to be similar to the regulation of this gene in the shoot apical meristem. For instance, in the shoot apical meristem, cytokinin is important for WUS expression (Gordon et al., 2009), and we have shown that SPL/NZZ might be involved in cytokinin-mediated WUS expression (Figure 6).
WUS and WUSCHEL RELATED HOMEOBOX are known to act in a non–cell-autonomous manner for the maintenance of stem cells both in shoot apical and root apical meristems (Brand et al., 2000; Schoof et al., 2000). This stem cell maintenance depends on a negative feedback loop between WUS and CLAVATA3 (CLV3) (Brand et al., 2000; Schoof et al., 2000). In ovules, WUS is expressed in the nucellus (Figure 6) and plays an important role in the chalaza, promoting integument development (Gross-Hardt et al., 2002). Furthermore, WUS might promote in a non–cell-autonomous manner the expression of BEL1 in the chalaza, which as already proposed, negatively regulates WUS expression (Brambilla et al., 2007).
METHODS
Plant Material and Growth Conditions
Arabidopsis thaliana (ecotype Columbia) plants were grown at 22°C under long-day (16-h light/8-h dark) conditions. The Arabidopsis lines that were obtained from the European Arabidopsis Stock Centre collection are spl-1 (N6586), bel1-1 (N3090). TCSpro (two-component-output-sensor):GFP, AHK2pro:GUS, AHK3pro:GUS, CRE1/AHK4pro:GUS, DR5rev-pro:GFP, PIN1pro:PIN1:GFP, cre1-12, ahk2-2, and ahk3-3 seeds were provided by Jiří Friml (Ghent University). WUSpro:WUS-GUS seeds were provided by Thomas Laux (University of Freiburg). IPT1pro:GUS seeds were provided by Tatsuo Kakimoto (Osaka University).
The observed ovule phenotypes were consistent in the F2, F3, and F4 segregating populations and the backcross population, which was made to introduce a reporter gene construct. This indicates that the observed phenotypic effects are not caused by differences in the ecotype background of our mutants.
The cre1-12 ahk2-2 ahk3-3 PIN1pro:PIN1-GFP lines were obtained by crossing PIN1pro:PIN1-GFP plants with cre1-12 ahk2-2 ahk3-3/AHK3 plants. F3 cre1-12 ahk2-2 ahk3-3 plants homozygous for PIN1pro:PIN1-GFP were selected. The PIN1pro:PIN1-GFP plants were crossed with the spl/SPL mutant. F3 spl/nzz plants homozygous for PIN1pro:PIN1-GFP were selected, and GFP expression was analyzed in the root as positive control (Benková et al., 2003).
Genotyping
To genotype for the spl allele, the following primers were used: SPL-F (5′-GGCGAGATCCGGACAGAGAC) and SPL-R (5′-AGAAGCGTTAAACATTTGAGGATT) and Ds primers DS 3-3A (5′-TCGTTTCCGTCCCGCAAGT) or DS 5 to 3A (5′-CGGTCGGTACGGGATTTTCC). The bel1-1 allele contains a C-to-T transition at nucleotide 497, which introduces a BsaAI restriction site. The bel1-1 allele was identified by BsaAI digestion of PCR products amplified with the primers 5′-GAGAG ACATGGCAAGAGATCAG and 5′-GAGCATGGAGAGCAACTTGG. To identify the presence of the T-DNA encoding PIN1pro:PIN1-GFP, the following primers were used: PIN1-RP (5′-CCAGTACGTGGAGAGGGAAG) and GFP-LP (5′-GAAAGTAGTGACAAGTGTTGGC).
BAP Treatment
BAP was obtained from Sigma-Aldrich and was used at a concentration of 10−3 M. Plants were treated once with 30 μL of a BAP solution or a solution of distilled water for mock-treated controls (both in 0.05% Tween 20). Solutions were applied directly onto the inflorescences, and then the plants were covered with a plastic transparent bag for 1 d. NPA was used at a concentration of 1 μM and was applied as described for the BAP treatment.
Microscopy
To analyze ovule development, flowers at different developmental stages were cleared and analyzed as described previously (Brambilla et al., 2007).
All GUS assays were performed overnight as described previously (Liljegren et al., 2000) or with a different clearing method according to Jones-Rhoades et al. (2007). Samples were incubated in clearing solution, dissected, and observed using a Zeiss Axiophot D1 microscope equipped with differential interference contrast optics. Images were captured on an Axiocam MRc5 camera (Zeiss) using the Axiovision program (version 4.1)
For confocal laser scanning microscopy, dissected ovules were mounted in water and observed with a SP2 Leica confocal microscope and SPE Leica confocal with a 488-nm argon laser line for excitation of GFP fluorescence. Emissions were detected between 505 and 580 nm. Using a 63× water-immersion objective (numerical aperture = 1.25, pinhole), confocal scans were performed with the pinhole at 1 airy unit.
In Situ Hybridization and Real-Time PCR
In situ hybridization was performed as described by Dreni et al. (2011). The SPL/NZZ and BEL1 specific antisense probes were amplified according to Balasubramanian and Schneitz (2000).
For expression analysis, total RNA was extracted using NucleoSpin RNA Plant KIT (Macherrey-Nagel) and was then subjected to reverse transcription using the ImProm-II Reverse Transcription System (Promega). The cDNAs were standardized relative to UBIQUITIN10 (UBI10), ACTIN8 (ACT8), PROTEIN PHOSPHATASE 2A SUBUNIT A3 (PP2A [At1g13320]) transcripts, and gene expression analysis was performed using the iQ5 Multi Color Real-Time PCR detection system (Bio-Rad) with a SYBR Green PCR Master Mix (Bio-Rad). Baseline and threshold levels were set according to the manufacturer's instructions.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: STK (At4G09960), BEL1 (At5G41410), SPL/NZZ (At4G27330), WUS (At2G17950), PIN1 (At1G73590), AHK2 (At5G35750), AHK3 (At1G27320), AHK4/CRE1 (At2G01830), IPT1 (At1G68460), CKX5 (At1g75450), and CKX6 (At3g63440).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. GUS Expression in AHK3pro:GUS Ovules from Stage 1-II to Stage 3-IV.
Supplemental Figure 2. Ovules of cre1-12 ahk2-2 ahk3-3 and pin1-5 Mutants; PIN1pro:PIN1-GFP and DR5rev-pro:GFP Analyses in spl-1 and spl-1/SPL Plants.
Supplemental Figure 3. Ovule Development after BAP Treatment.
Supplemental Table 1. Ovule Number in the cre1-12 ahk2-2 ahk3-3 and pin1-5 Mutants.
Supplemental Table 2. The Effect of BAP Treatment on the Cytokinin Receptor Mutants.
Supplementary Material
Acknowledgments
We thank Tatsuo Kakimoto for providing the IPT1pro:GUS line, Agnieszka Bielach for technical help, and Simona Masiero and Martin M. Kater for their comments and critical reading of the article.
This research was supported by Fondazione Cariplo and by a Starting Independent Research grant from the European Research Council (ERC-2007-Stg-207362-HCPO to E.B.) and the project CZ.1.07/2.3.00/20.0043 (to the Central European Institute of Technology).
AUTHOR CONTRIBUTIONS
S.B., E.B., and L.C. designed the research. S.B. and S.S. performed the experiments. All authors analyzed and discussed the data and the article. S.B. and L.C. wrote the article.
Glossary
- GUS
β-glucuronidase
- GFP
green fluorescent protein
- BAP
N6-benzylaminopurine
- CK-IS
cytokinin-induced structure
- NPA
naphthylphthalamic acid
References
- Balasubramanian S., Schneitz K. (2000). NOZZLE regulates proximal-distal pattern formation, cell proliferation and early sporogenesis during ovule formation in Arabidopsis thaliana. Development 127: 4227–4238 [DOI] [PubMed] [Google Scholar]
- Balasubramanian S., Schneitz K. (2002). NOZZLE links proximal-distal and adaxial-abaxial pattern formation during ovule development in Arabidopsis thaliana. Development 129: 4291–4300 [DOI] [PubMed] [Google Scholar]
- Bartrina I., Otto E., Strnad M., Werner T., Schmülling T. (2011). Cytokinin regulates the activity of reproductive meristems, flower organ size, ovule formation, and thus seed yield in Arabidopsis thaliana. Plant Cell 23: 69–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benková E., Michniewicz M., Sauer M., Teichmann T., Seifertová D., Jürgens G., Friml J. (2003). Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602 [DOI] [PubMed] [Google Scholar]
- Bennett M.J., Marchant A., Green H.G., May S.T., Ward S.P., Millner P.A., Walker A.R., Schulz B., Feldmann K.A. (1996). Arabidopsis AUX1 gene: A permease-like regulator of root gravitropism. Science 273: 948–950 [DOI] [PubMed] [Google Scholar]
- Brambilla V., Battaglia R., Colombo M., Masiero S., Bencivenga S., Kater M.M., Colombo L. (2007). Genetic and molecular interactions between BELL1 and MADS box factors support ovule development in Arabidopsis. Plant Cell 19: 2544–2556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand U., Fletcher J.C., Hobe M., Meyerowitz E.M., Simon R. (2000). Dependence of stem cell fate in Arabidopsis on a feedback loop regulated by CLV3 activity. Science 289: 617–619 [DOI] [PubMed] [Google Scholar]
- Colombo L., Battaglia R., Kater M.M. (2008). Arabidopsis ovule development and its evolutionary conservation. Trends Plant Sci. 13: 444–450 [DOI] [PubMed] [Google Scholar]
- D’Aloia M., Bonhomme D., Bouché F., Tamseddak K., Ormenese S., Torti S., Coupland G., Périlleux C. (2011). Cytokinin promotes flowering of Arabidopsis via transcriptional activation of the FT paralogue TSF. Plant J. 65: 972–979 [DOI] [PubMed] [Google Scholar]
- Dello Ioio R., Nakamura K., Moubayidin L., Perilli S., Taniguchi M., Morita M.T., Aoyama T., Costantino P., Sabatini S. (2008). A genetic framework for the control of cell division and differentiation in the root meristem. Science 322: 1380–1384 [DOI] [PubMed] [Google Scholar]
- Dreni L., Pilatone A., Yun D., Erreni S., Pajoro A., Caporali E., Zhang D., Kater M.M. (2011). Functional analysis of all AGAMOUS subfamily members in rice reveals their roles in reproductive organ identity determination and meristem determinacy. Plant Cell 23: 2850–2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S.P., Chickarmane V.S., Ohno C., Meyerowitz E.M. (2009). Multiple feedback loops through cytokinin signaling control stem cell number within the Arabidopsis shoot meristem. Proc. Natl. Acad. Sci. USA 106: 16529–16534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross-Hardt R., Lenhard M., Laux T. (2002). WUSCHEL signaling functions in interregional communication during Arabidopsis ovule development. Genes Dev. 16: 1129–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M., et al. (2004). In planta functions of the Arabidopsis cytokinin receptor family. Proc. Natl. Acad. Sci. USA 101: 8821–8826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison C.E., Li J., Argueso C., Gonzalez M., Lee E., Lewis M.W., Maxwell B.B., Perdue T.D., Schaller G.E., Alonso J.M., Ecker J.R., Kieber J.J. (2006). The Arabidopsis histidine phosphotransfer proteins are redundant positive regulators of cytokinin signaling. Plant Cell 18: 3073–3087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue T., Higuchi M., Hashimoto Y., Seki M., Kobayashi M., Kato T., Tabata S., Shinozaki K., Kakimoto T. (2001). Identification of CRE1 as a cytokinin receptor from Arabidopsis. Nature 409: 1060–1063 [DOI] [PubMed] [Google Scholar]
- Ito T., Wellmer F., Yu H., Das P., Ito N., Alves-Ferreira M., Riechmann J.L., Meyerowitz E.M. (2004). The homeotic protein AGAMOUS controls microsporogenesis by regulation of SPOROCYTELESS. Nature 430: 356–360 [DOI] [PubMed] [Google Scholar]
- Kakimoto T. (1996). CKI1, a histidine kinase homolog implicated in cytokinin signal transduction. Science 274: 982–985 [DOI] [PubMed] [Google Scholar]
- Kakimoto T. (2001). Identification of plant cytokinin biosynthetic enzymes as dimethylallyl diphosphate:ATP/ADP isopentenyltransferases. Plant Cell Physiol. 42: 677–685 [DOI] [PubMed] [Google Scholar]
- Kinoshita-Tsujimura K., Kakimoto T. (2011). Cytokinin receptors in sporophytes are essential for male and female functions in Arabidopsis thaliana. Plant Signal. Behav. 6: 66–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooiker M., Airoldi C.A., Losa A., Manzotti P.S., Finzi L., Kater M.M., Colombo L. (2005). BASIC PENTACYSTEINE1, a GA binding protein that induces conformational changes in the regulatory region of the homeotic Arabidopsis gene SEEDSTICK. Plant Cell 17: 722–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Rhoades M.W., Borevitz J.O., Preuss D. (2007). Genome-wide expression profiling of the Arabidopsis female gametophyte identifies families of small, secreted proteins. PLoS Genet. 3: 1848–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplaze L., et al. (2007). Cytokinins act directly on lateral root founder cells to inhibit root initiation. Plant Cell 19: 3889–3900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.-C., Qin G.-J., Tsuge T., Hou X.-H., Ding M.-Y., Aoyama T., Oka A., Chen Z., Gu H., Zhao Y., Qu L.-J. (2008). SPOROCYTELESS modulates YUCCA expression to regulate the development of lateral organs in Arabidopsis. New Phytol. 179: 751–764 [DOI] [PubMed] [Google Scholar]
- Liljegren S.J., Ditta G.S., Eshed Y., Savidge B., Bowman J.L., Yanofsky M.F. (2000). SHATTERPROOF MADS-box genes control seed dispersal in Arabidopsis. Nature 404: 766–770 [DOI] [PubMed] [Google Scholar]
- Miyawaki K., Matsumoto-Kitano M., Kakimoto T. (2004). Expression of cytokinin biosynthetic isopentenyltransferase genes in Arabidopsis: Tissue specificity and regulation by auxin, cytokinin, and nitrate. Plant J. 37: 128–138 [DOI] [PubMed] [Google Scholar]
- Miyawaki K., Tarkowski P., Matsumoto-Kitano M., Kato T., Sato S., Tarkowska D., Tabata S., Sandberg G., Kakimoto T. (2006). Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. Proc. Natl. Acad. Sci. USA 103: 16598–16603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller B., Sheen J. (2008). Cytokinin and auxin interaction in root stem-cell specification during early embryogenesis. Nature 453: 1094–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemhauser J.L., Feldman L.J., Zambryski P.C. (2000). Auxin and ETTIN in Arabidopsis gynoecium morphogenesis. Development 127: 3877–3888 [DOI] [PubMed] [Google Scholar]
- Nishimura C., Ohashi Y., Sato S., Kato T., Tabata S., Ueguchi C. (2004). Histidine kinase homologs that act as cytokinin receptors possess overlapping functions in the regulation of shoot and root growth in Arabidopsis. Plant Cell 16: 1365–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnussat G.C., Alandete-Saez M., Bowman J.L., Sundaresan V. (2009). Auxin-dependent patterning and gamete specification in the Arabidopsis female gametophyte. Science 324: 1684–1689 [DOI] [PubMed] [Google Scholar]
- Pagnussat G.C., Yu H.J., Sundaresan V. (2007). Cell-fate switch of synergid to egg cell in Arabidopsis eostre mutant embryo sacs arises from misexpression of the BEL1-like homeodomain gene BLH1. Plant Cell 19: 3578–3592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pischke M.S., Jones L.G., Otsuga D., Fernandez D.E., Drews G.N., Sussman M.R. (2002). An Arabidopsis histidine kinase is essential for megagametogenesis. Proc. Natl. Acad. Sci. USA 99: 15800–15805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser L., Modrusan Z., Margossian L., Samach A., Ohad N., Haughn G.W., Fischer R.L. (1995). The BELL1 gene encodes a homeodomain protein involved in pattern formation in the Arabidopsis ovule primordium. Cell 83: 735–742 [DOI] [PubMed] [Google Scholar]
- Riefler M., Novak O., Strnad M., Schmülling T. (2006). Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell 18: 40–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson-Beers K., Pruitt R.E., Gasser C.S. (1992). Ovule development in wild-type Arabidopsis and two female-sterile mutants. Plant Cell 4: 1237–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzicka K., Simásková M., Duclercq J., Petrásek J., Zazímalová E., Simon S., Friml J., Van Montagu M.C., Benková E. (2009). Cytokinin regulates root meristem activity via modulation of the polar auxin transport. Proc. Natl. Acad. Sci. USA 106: 4284–4289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefthaler U., Balasubramanian S., Sieber P., Chevalier D., Wisman E., Schneitz K. (1999). Molecular analysis of NOZZLE, a gene involved in pattern formation and early sporogenesis during sex organ development in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 96: 11664–11669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneitz K., Hülskamp M., Pruitt R.E. (1995). Wild-type ovule development in Arabidopsis thaliana: A light microscope study of cleared whole-mount tissue. Plant J. 7: 731–749 [Google Scholar]
- Schoof H., Lenhard M., Haecker A., Mayer K.F., Jürgens G., Laux T. (2000). The stem cell population of Arabidopsis shoot meristems in maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100: 635–644 [DOI] [PubMed] [Google Scholar]
- Sieber P., Gheyselinck J., Gross-Hardt R., Laux T., Grossniklaus U., Schneitz K. (2004). Pattern formation during early ovule development in Arabidopsis thaliana. Dev. Biol. 273: 321–334 [DOI] [PubMed] [Google Scholar]
- Skoog F., Miller C.O. (1957). Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp. Soc. Exp. Biol. 11: 118–130 [PubMed] [Google Scholar]
- Sohlberg J.J., Myrenås M., Kuusk S., Lagercrantz U., Kowalczyk M., Sandberg G., Sundberg E. (2006). STY1 regulates auxin homeostasis and affects apical-basal patterning of the Arabidopsis gynoecium. Plant J. 47: 112–123 [DOI] [PubMed] [Google Scholar]
- Sun J.Q., Niu Q.W., Tarkowski P., Zheng B.L., Tarkowska D., Sandberg G., Chua N.H., Zuo J.R. (2003). The Arabidopsis AtIPT8/PGA22 gene encodes an isopentenyl transferase that is involved in de novo cytokinin biosynthesis. Plant Physiol. 131: 167–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venglat S.P., Sawhney V.K. (1996). Benzylaminopurine induces phenocopies of floral meristem and organ identity mutants in wild-type Arabidopsis plants. Planta 198: 480–487 [DOI] [PubMed] [Google Scholar]
- Werner T., Hanus J., Holub J., Schmülling T., Van Onckelen H., Strnad M. (2003). New cytokinin metabolites in IPT transgenic Arabidopsis thaliana plants. Physiol. Plant. 118: 127–137 [DOI] [PubMed] [Google Scholar]
- Yang W.C., Ye D., Xu J., Sundaresan V. (1999). The SPOROCYTELESS gene of Arabidopsis is required for initiation of sporogenesis and encodes a novel nuclear protein. Genes Dev. 13: 2108–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.