This work demonstrates that photosystem I, not photosystem II, is susceptible to photodamage under natural fluctuations in light intensity in Arabidopsis thaliana. PGR5 is an indispensable protein for protection of photosystem I, particularly in young plants. PGR5-mediated protection takes place via regulation of linear electron transfer, rather than via cyclic electron transfer.
Abstract
In nature, plants are challenged by constantly changing light conditions. To reveal the molecular mechanisms behind acclimation to sometimes drastic and frequent changes in light intensity, we grew Arabidopsis thaliana under fluctuating light conditions, in which the low light periods were repeatedly interrupted with high light peaks. Such conditions had only marginal effect on photosystem II but induced damage to photosystem I (PSI), the damage being most severe during the early developmental stages. We showed that PROTON GRADIENT REGULATION5 (PGR5)–dependent regulation of electron transfer and proton motive force is crucial for protection of PSI against photodamage, which occurred particularly during the high light phases of fluctuating light cycles. Contrary to PGR5, the NAD(P)H dehydrogenase complex, which mediates cyclic electron flow around PSI, did not contribute to acclimation of the photosynthetic apparatus, particularly PSI, to rapidly changing light intensities. Likewise, the Arabidopsis pgr5 mutant exhibited a significantly higher mortality rate compared with the wild type under outdoor field conditions. This shows not only that regulation of PSI under natural growth conditions is crucial but also the importance of PGR5 in PSI protection.
INTRODUCTION
During the past few years, the structure of the major thylakoid membrane protein complexes has been resolved at atomic resolution, and their roles in primary electron transfer and photophosphorylation are fairly well characterized. On the other hand, elucidation of the dynamic light acclimation and regulation of the photosynthetic apparatus and individual pigment protein complexes upon changes in environmental conditions has remained more elusive, particularly when the physiological roles of the alternative electron transfer pathways around photosystem I (PSI) are concerned.
Higher plants have evolved a number of mechanisms to cope with changes in light conditions. These mechanisms can be divided into short- and long-term responses, which take place in time scales from seconds and minutes up to several days, respectively (Bailey et al., 2004; Dietzel and Pfannschmidt, 2008). Traditionally, the short-term responses have been classified to include: (1) the dissipation of excess light energy as heat (quenching of the excess energy), which is a component of nonphotochemical quenching (NPQ) (Horton et al., 1996; Müller et al., 2001); and (2) the state transitions, in which the STATE TRANSITION7 (STN7) kinase catalyzes the phosphorylation of the light-harvesting complex II (LHCII), which then transfers excitation energy not only to photosystem II (PSII) but also to PSI (Allen and Forsberg, 2001). Long-term light responses, on the contrary, arise from changes in gene expression, altering both the amount and stoichiometry of photosynthetic proteins and protein complexes (Pfannschmidt, 2003; Wagner et al., 2008). Other light acclimation responses include the chloroplast and leaf movements and alterations in the degree of granal stacking (Anderson and Aro, 1994) as well as in the thickness of the cuticle and the trichome layers.
A vast majority of research concerning photosynthetic acclimation has focused on linear electron transfer (LET), which involves both photosystems. On the contrary, cyclic electron transfer (CET) involves only one photosystem and occurs around either PSII or PSI (Shikanai, 2007; Bondarava et al., 2010; Johnson, 2011; Shinopoulos and Brudvig, 2012). Despite active research, the molecular mechanisms and biological functions of CETs are still largely unknown. The chloroplastic NAD(P)H dehydrogenase (NDH)-like complex is capable of reducing the intersystem electron transfer chain (ETC) independently of PSII (Peng et al., 2011) and has a significant role in electron transfer in the bundle sheath cells of C4 plants (Majeran et al., 2008). Cyanobacteria thylakoid membranes also have the NDH-like complex, albeit more simple in structure than that in the chloroplasts. This complex functions in PSI CET as well as in acquisition of inorganic carbon (Battchikova et al., 2011). Nevertheless, the ndh mutants of Arabidopsis thaliana show little visible phenotype (Shikanai, 2007). This has led to an assumption that the antimycin-sensitive ferredoxin (Fd)-mediated pathway, not the NDH-mediated pathway, is largely responsible for PSI CET in C3 plants (Nandha et al., 2007; Shikanai, 2007). The exact protein components catalyzing the Fd-mediated CET are still unknown. A complex comprised of the PROTON GRADIENT REGULATION5 (PGR5) and PGR5-LIKE PHOTOSYNTHETIC PHENOTYPE1 (PGRL1) proteins has been suggested to mediate electron transfer via the Fd-mediated CET (Munekage et al., 2002, 2004; DalCorso et al., 2008). On the other hand, based on measurements indicating that the rate of PSI CET in the pgr5 mutant is only slightly affected, it has also been suggested that the PGR5 protein has a regulative role in partitioning the electrons between LET and CET (Nandha et al., 2007; Joliot and Johnson 2011). In addition, it was recently shown that phosphorylation of the PGRL1 protein by the STN8 kinase ensures optimal rate of electron transfer at the onset of illumination (Reiland et al., 2011).
Accumulating evidence on the molecular dynamics of the photosynthetic apparatus has prompted us to design and perform light acclimation experiments that take into consideration the natural short-term variations in plant light environment. For instance, instead of using different quality of light favoring the excitation of PSII or PSI to induce state transitions, our approach has been to induce or inhibit the phosphorylation of the LHCII proteins using low and high intensity of white light, respectively (Rintamäki et al., 2000; Tikkanen et al., 2011). In our fluctuating light conditions, the plants were exposed to low light intensity for 5 min, followed by a high light illumination pulse for 1 min, after which the cycle was being repeated for the entire photoperiod (Tikkanen et al., 2010). Such fluctuations in light intensity were faster than reactions required for reversible phosphorylation of the LHCII proteins, and accordingly, the steady state level of LHCII protein phosphorylation was maintained at a low light level. Nevertheless, the repeating fluctuations between low and high illumination phases expose the leaves to opposite, rapid, and mixed signals and thus challenge the light acclimation capacity of the photosynthetic machinery.
Growth analyses under fluctuating light conditions of several Arabidopsis mutants defective in regulatory proteins of the thylakoid membrane have revealed a retarded growth phenotype for the Arabidopsis stn7 mutant, which lacks the Ser/Thr protein kinase STN7, and a complete lethality of the pgr5 mutant (Tikkanen et al., 2010). However, the molecular basis behind such a lethality of the pgr5 mutant has not been resolved. Here, we show that the damage of PSI centers under fluctuating light induces seedling lethality of the pgr5 mutants. Fluctuating light-enhanced seedling lethality of pgr5 is demonstrated to occur both under artificial light conditions in growth chambers and under naturally fluctuating light in the field. Mature pgr5 leaves likewise suffer from fluctuating light with PSI as the primary target of photodamage, whereas the PSII centers remain active. We demonstrate that the primary role of PGR5 in protection of PSI against photodamage due to fluctuating light results from its function in regulation of LET.
RESULTS
PGR5 Is Essential for Growth Both under Fluctuating Light and in Natural Conditions
To investigate the molecular mechanisms behind dynamic light acclimation, the wild-type and the pgr5 mutant plants were grown in the growth chambers under constant light and under fluctuating light, provided by an electronically controlled shading system, as well as in a natural light environment in the field site (Table 1). Both young (cotyledons and the first true leaves) and mature leaves were analyzed to address the role of the developmental stage in the light acclimation capacity of the wild-type and pgr5 plants (Table 1).
Table 1. Summary of the Experimental Conditions Used to Analyze the Light Acclimation Capacity of Wild-Type and pgr5 Mutant Plants.
| Growth Condition | Light Intensity | Plant Developmental Stage | Figure |
|---|---|---|---|
| Constant light | Low light (50 µmol photons m−2 s−1) | Mature leaves (from 4- to 5-week-old rosettes) | 2 |
| Moderate growth light (120 µmol photons m−2 s−1) | Mature leaves (from 4- to 5-week-old rosettes) | 2, 5–8 | |
| Growth light (200 µmol photons m−2 s−1) | Young (cotyledons and the first true leaves) | 1–4 | |
| High light (500 µmol photons m−2 s−1) | Mature leaves (from 4- to 5-week-old rosettes) | 2 | |
| Fluctuating light | Fluctuating light | Young (cotyledons and the first true leaves) | 1–4 |
| 5 min low light (50 µmol photons m−2 s−1), 1 min high light (500 µmol photons m−2 s−1) | Mature leaves after 5 weeks of pregrowth under constant growth light | 8 | |
| Mildly fluctuating light 5 min low light (50 µmol photons m−2 s−1), 1 min high light (350 μmol photons m−2 s−1) | Mature leaves (from 4- to 5-week-old rosettes) | 2, 5 | |
| Field experiment | Natural outdoor conditions in Northern Sweden, with light intensity fluctuating irregularly due to clouds and shading by nearby vegetation (Külheim et al., 2002; Frenkel et al., 2008) | 6-week-old mature leaves (4 weeks outside) | 1 |
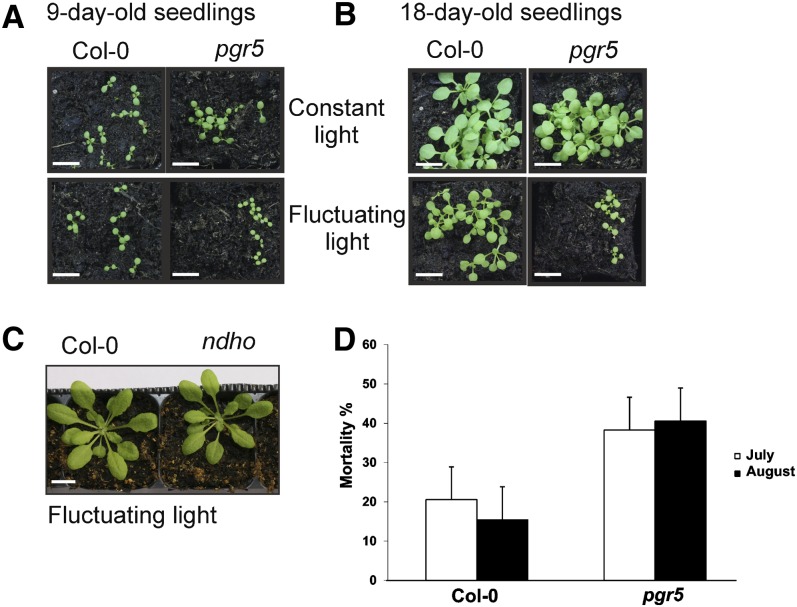
Under constant light conditions, the pgr5 mutant did not show any distinct growth phenotype compared with the wild type, whereas a distinct visible phenotype of pgr5 was evident upon growth under fluctuating light conditions (Figure 1A). Under fluctuating light, the seedlings developed nearly normally until the cotyledon stage, but thereafter a strong growth defect appeared. Nine-day-old seedlings of the pgr5 mutant grown under standard fluctuating light were clearly smaller than the wild-type seedlings (Figure 1A). Confocal microscopy images from the mesophyll tissues of 9-d-old seedlings revealed smaller cells in pgr5 grown under fluctuating light compared with the wild type, likely explaining the retarded growth (see Supplemental Figure 1 online). No difference in the size of the pgr5 and wild-type cells was observed in seedlings grown under constant light. After 18 d growth under fluctuating light, the pgr5 mutant still remained at the two- or four-leaf state, whereas the wild-type seedlings continued growing (Figure 1B). Accordingly, the fresh weight of the pgr5 mutant seedlings (1.23 ± 0.35 mg, mean ± sd) was much lower compared with the wild type (7.22 ± 1.68 mg). At this point, the mutant seedlings started gradually wilting. It is worth noting that the growth of the wild type was also clearly retarded under fluctuating light compared with constant light, with roughly similar amount of photons received during the photoperiod (Figure 1B).
Figure 1.
Growth of the Wild Type, pgr5, and ndho under Constant and Fluctuating Light Conditions and in the Field Site.
(A) and (B) Phenotypes of the wild type and pgr5 grown under constant or fluctuating light. The wild-type and pgr5 seedlings were grown for 9 d (A) or 18 d (B) under constant light (200 µmol photons m−2 s−1) and under fluctuating light with 5 min of low light (50 µmol photons m−2 s−1) and 1 min of high light (500 µmol photons m−2 s−1) repeated during the entire photoperiod. Col-0, Columbia-0. Bars = 1 cm.
(C) Four-week-old rosettes of the wild type and the ndho mutant grown under fluctuating light. Bar = 1 cm.
(D) Mortality rates of the wild-type and the pgr5 mutant plants in two field experiments. Graph shows estimated marginal means ± 95% confidence limits from the mixed-model analysis of variance (n = 175).
Finally, the growth was tested by sowing the seeds of the wild type and pgr5 on Murashige and Skoog (MS) plates supplemented with 0, 1, or 2% Suc. Under these experimental conditions, growth on 2% Suc rescued the pgr5 mutant phenotype under fluctuating light, whereas gradual bleaching and wilting were still evident with 1% Suc or when Suc was omitted (see Supplemental Figure 2A online). However, the pgr5 seedlings that were first grown under constant light (120 µmol photons m−2 s−1) for 2 weeks on MS plates supplemented with 2% Suc and then shifted to fluctuating light, first bleached and eventually died during 3 weeks in fluctuating light (see Supplemental Figure 2B online). Indeed, the external Suc failed to rescue the growth of pgr5 under fluctuating light conditions in the absence of acclimated growth (see Supplemental Figure 2A online) under such conditions.
Importantly, growth experiments with the ndho mutant plants, which lack the NDHO protein, and therefore also are deficient in functional NDH-like complex (Ifuku et al., 2011), showed no difference in phenotype compared with the wild type regardless of whether the plants were grown under fluctuating light or under constant light of different intensities (Figure 1C).
The fluctuating light conditions of our experimental design were designed to expose the plants to variable and changing light conditions, thus mimicking natural outdoor light environments, while being strictly controlled. To compare the artificial fluctuating light conditions applied here to natural outdoor conditions, the growth experiments with the wild type and pgr5 were repeated also under the field conditions. The field experiments were performed twice during the summer 2011 (July and August), allowing us to address also the possible effect of nonrepresentative weather conditions on the survival of pgr5. As shown in Figure 1D, the pgr5 mutants had roughly 2 (in July) to 3 (in August) times higher mortality rates than the wild-type plants under field conditions, the difference being statistically significant (F1,68 34.534, P < 0.001). This difference was parallel in both July and August experiments as indicated by the nonsignificant interaction term: genotype × time P = 0.312. Taken together, it is evident that PGR5 is essential for proper growth of Arabidopsis under natural conditions. This is likely due to a reduced capacity of the pgr5 mutants to cope with fluctuations in light intensity.
pgr5 Plants Grown under Fluctuating Light Are Specifically Depleted in PSI Proteins
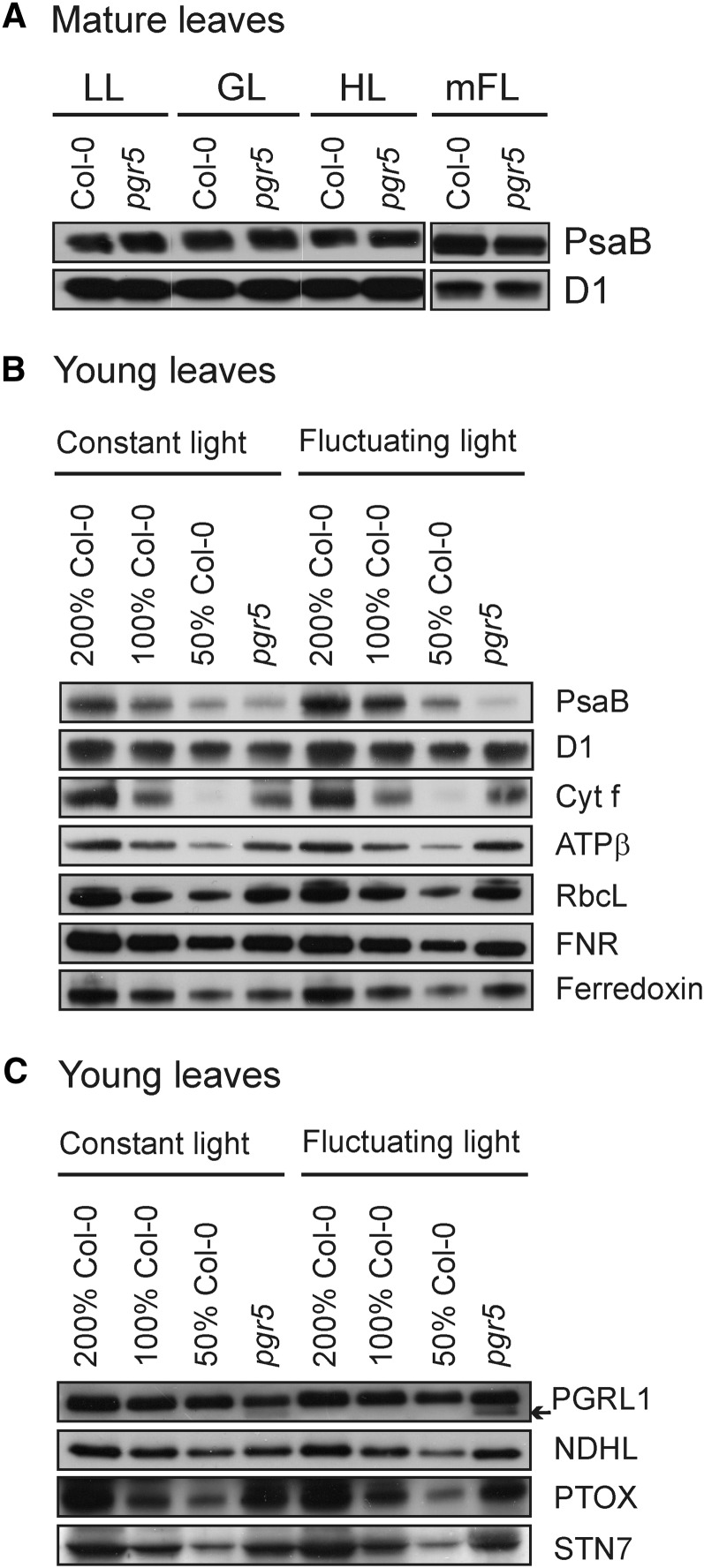
Under constant growth light, the pgr5 mutant exhibited no distinct growth phenotype (Figure 1A) and, as demonstrated in Figure 2A, the mature leaves also accumulated both photosystems normally, deduced from the equal amounts of the D1 and PsaB proteins present in the pgr5 and wild-type plants grown under constant low light, moderate growth light, or high light. On the contrary, under fluctuating growth light, the pgr5 mutants eventually died (Figure 1B).
Figure 2.
Immunoblots Demonstrating the Relative Amounts of Chloroplast Proteins between the Wild Type and the pgr5 Mutant.
(A) Immunoblots of the PsaB and D1 proteins from mature leaves of the wild type and pgr5. Plants were grown under constant low light (50 µmol photons m−2 s−1) (LL), moderate growth light (120 µmol photons m−2 s−1) (GL), high light (500 µmol photons m−2 s−1) (HL), or mildly fluctuating light (mFL) with 5 min of low (50 µmol photons m−2 s−1) and 1 min of moderate high (350 µmol photons m−2 s−1) light repeating for the entire photoperiod. Col-0, Columbia-0.
(B) and (C) The amounts of the chloroplast proteins representing the major photosynthetic protein complexes (B) and alternative electron transfer routes (C) in the wild type and pgr5. The seedlings were grown for 9 d under constant (200 µmol photons m−2 s−1) or under fluctuating light with 5 min of low light (50 µmol photons m−2 s−1) and 1 min of high light (500 µmol photons m−2 s−1) repeating during the entire photoperiod. Gels were loaded on equal protein basis for the wild type (100%) and pgr5. For each protein, a representative example of at least three biological replications is shown, including the dilution series (50 to 200%) made with the wild-type Col-0 leaf extracts.
To address the reason behind the lethality of the pgr5 seedlings under strongly fluctuating light, we harvested the leaf material from the wild-type and pgr5 seedlings well prior to any signs of the death of the pgr5 mutant (i.e., after growth for 9 d under constant and fluctuating light) and compared the chloroplast protein contents between pgr5 and the wild type. Immunoblotting revealed no distinct differences between the pgr5 mutant and wild-type plants in the amounts of the D1, cytochrome f (Cyt f), and RbcL proteins representing the PSII core, the Cyt b6f complex, and ribulose-1,5-bisphosphate carboxylase/oxygenase, respectively (Figure 2B). Under constant light, the amount of the D1 protein in the pgr5 seedlings was 87% ± 6% and under fluctuating light 96% ± 6% of the wild-type level, whereas the respective values for Cyt f in pgr5 were 110% ± 8% and 98% ± 23% and those for RbcL were 115% ± 22% and 104% ± 17% (under constant and fluctuating light, respectively). By contrast, the PSI core protein PsaB was strongly depleted in the pgr5 mutant compared with the wild type and particularly so when the plants were grown under fluctuating light conditions. The amount of PsaB in pgr5 was 53% ± 23% of the wild-type level under constant light and only 36% ± 16% under fluctuating light. This result was even more striking when considering the fact that the acclimation strategy of the wild-type plants to fluctuating light conditions involved a marked increase (149% ± 24%) in the amount of the PsaB protein (Figure 2B).
The amounts of the PSI electron acceptors Fd and Fd-NAD(P)H-oxidoreductase (FNR) were also analyzed but no marked differences between the wild type and pgr5 grown either under constant or fluctuating light were observed. Compared with the wild type, the pgr5 mutant contained 85% ± 13% of Fd under constant light and 99% ± 21% under fluctuating light, whereas the level of FNR was 94% ± 1% under constant light and 86% ± 10% under fluctuating light (Figure 2B). The amount of the ATPβ protein, representing the ATP synthase, was elevated (142% ± 32.5%) in the pgr5 mutant compared with the wild type, but only when the seedlings were grown under fluctuating light (Figure 2B).
Several other regulatory thylakoid membrane proteins, related to alternative electron transfer routes (PGRL1, NDH-LIKE COMPLEX SUBUNIT L [NDHL], and plastid terminal oxidase [PTOX]) and to state transitions (STN7) (Figure 2C) were also quantified. The pgr5 mutant seedlings accumulated considerable amounts of the PGRL1 protein, particularly under fluctuating light (pgr5: 86% ± 11% of the wild-type PGRL1 level). The PGRL1 antibody also recognized another band of slightly lower molecular mass from the pgr5 mutants (Figure 2C, marked with arrow), which might represent a degradation product of the PGRL1 protein. The NDHL protein, representing the NDH-like complex, and the STN7 kinase were present at approximately similar amounts in the pgr5 mutant and wild type both under fluctuating and constant growth light conditions (Figure 2C). The amount of the PTOX was slightly upregulated in the pgr5 mutant, both under constant (110% ± 13% of the wild-type level in pgr5) and fluctuating growth light conditions (109% ± 11% of the wild-type level in pgr5) (Figure 2C).
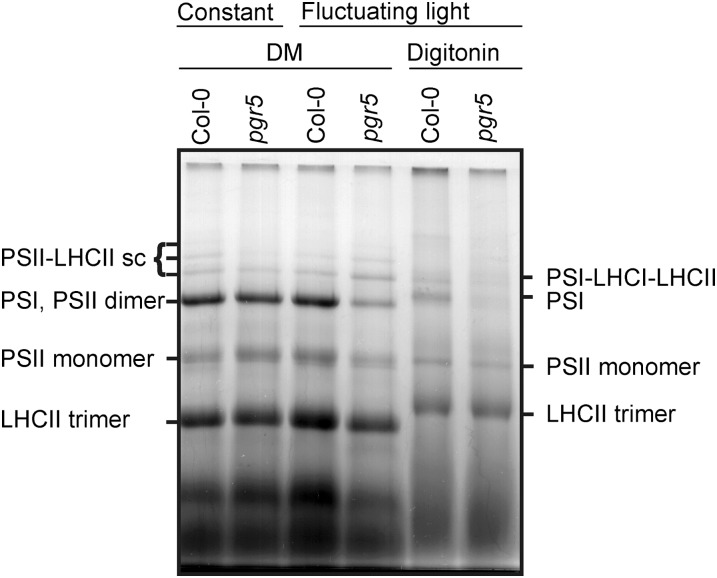
To address whether the thylakoid proteins were properly assembled into protein complexes, the composition of the thylakoid membrane protein complexes from the 9-d-old pgr5 and wild-type leaves developed both under fluctuating and constant light conditions was analyzed using one-dimensional large-pore blue-native (lpBN) PAGE (Figure 3) (Järvi et al., 2011). The amounts of the PSI thylakoid membrane protein complexes were strongly reduced in pgr5 compared with the wild type (Figure 3). When n-dodecyl β-d-maltoside (DM) was used for thylakoid solubilization, the migration of PSI and the PSII dimer overlapped, yet the intensity of this particular band was clearly lowered in the pgr5 mutant when grown under fluctuating light. Solubilization of the thylakoid membrane with digitonin, which avoids overlapping of PSII and PSI upon lpBN PAGE, clearly revealed the low amount of PSI complexes in the pgr5 seedlings (Figure 3). Likewise, the state transition supercomplex, composed of PSI, LHCI, and LHCII (Pesaresi et al., 2009), was present in much lower amount in the young pgr5 leaves than in the wild type, both grown under fluctuating light (Figure 3).
Figure 3.
Composition of the Thylakoid Membrane Protein Complexes in 9-d-Old Leaves of the Wild Type and pgr5.
Plants were grown either under constant (200 µmol photons m−2 s−1) or fluctuating light (5 min of low light, 50 µmol photons m−2 s−1, and 1 min of high light, 500 µmol photons m−2 s−1, repeating during the photoperiod). Thylakoids (55 µg protein) were solubilized with 1% DM or 1% digitonin, and the thylakoid membrane protein complexes were separated with the lpBN PAGE. The protein complexes consisting of the PSII and PSI complexes, together with various combinations of LHCs, were identified based on Järvi et al. (2011). sc, supercomplex. Col-0, Columbia-0.
pgr5 Plants Have High Antioxidant Capacity for Scavenging Reactive Oxygen Species
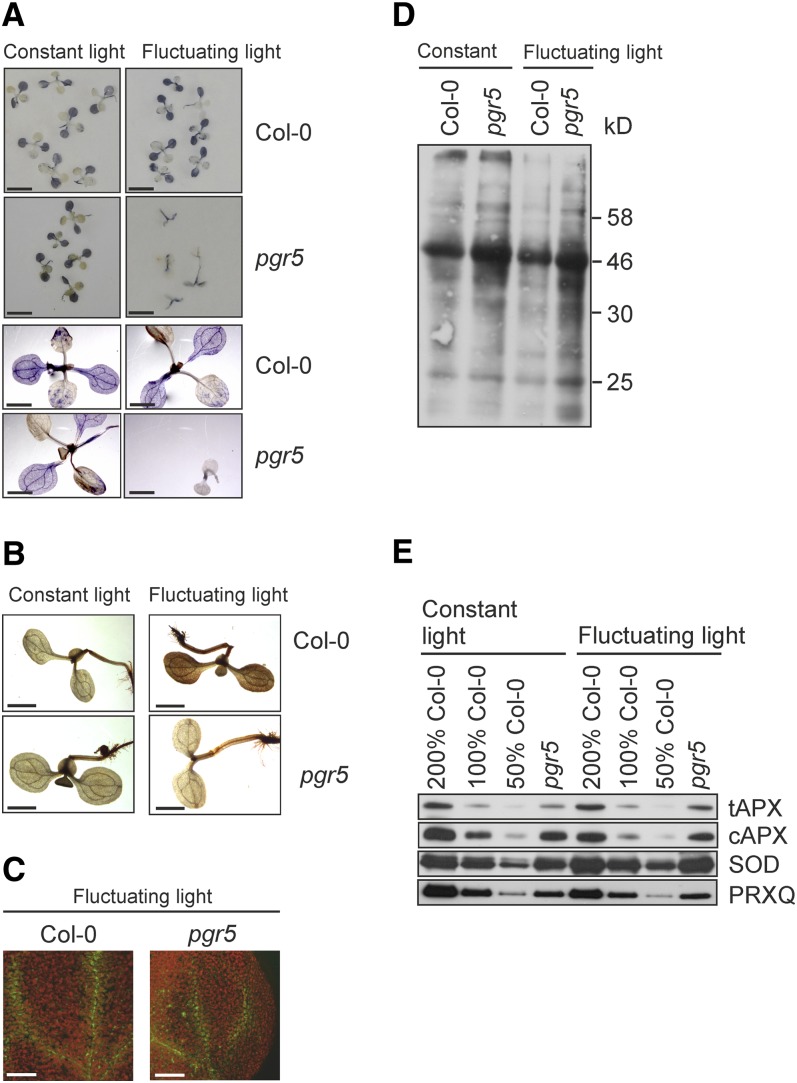
Histochemical staining with nitroblue tetrazolium (NBT) indicated reduced levels of foliar superoxide (O2−) in 9-d-old pgr5 mutants grown under fluctuating light compared with the wild-type seedlings or the pgr5 mutant grown under constant light (Figure 4A). Likewise, staining with 3,3-diaminobenzidine (DAB) revealed that pgr5 mutant seedlings accumulate less hydrogen peroxide (H2O2) under fluctuating light compared with wild-type plants (Figure 4B). Moreover, detection of singlet oxygen (1O2) (Flors et al., 2006) revealed comparable levels of singlet oxygen in the vascular tissues of wild-type and the pgr5 leaves (Figure 4C).
Figure 4.
Characterization of Oxidative Stress in the Wild-Type and pgr5 Seedlings.
Plants were grown for 9 d under constant light (200 µmol photons m−2 s−1) or under fluctuating light conditions with 5 min of low light (50 µmol photons m−2 s−1) and 1 min of high light (500 µmol photons m−2 s−1) repeating during the photoperiod. Col-0, Columbia-0.
(A) Photographs and stereomicrographs depicting foliar O2−, appearing as blue following staining of seedlings with NBT. The seedlings were incubated in NBT for 2 h in darkness and then illuminated under constant or fluctuating light for 30 min. Bars = 5 mm for the top photographs and 2 mm for the close-up stereomicrographs at the bottom.
(B) Stereomicrographs depicting foliar H2O2 (seen as brown precipitate) by staining with the DAB method. The seedlings were incubated in the DAB stain overnight and thereafter exposed to constant or fluctuating light for 30 min. Bars = 2 mm.
(C) Confocal microscopy images of 1O2 from wild-type and pgr5 seedlings grown under fluctuating light. Seedlings were incubated for 30 min in Singlet Oxygen Sensor Green reagent; thereafter, 1O2 was visualized by excitation at 488 nm and emission at 535 to 590 nm. Chlorophyll autofluorescence (red) was excited at 488 nm and detected at 650 to 710 nm. Bars = 100 µm.
(D) Carbonylation of leaf proteins detected with the OxyBlot protein oxidation detection kit.
(E) Immunoblots of tAPX and cAPX, Cu/Zn SOD, and PRXQ antioxidant enzymes. Representative examples from at least three biological replications are shown with the dilution series from the wild-type Columbia-0 leaf extracts.
However, oxidative damage was evident from results assessing the degree of protein carbonylation in leaf total protein extracts and demonstrated stronger oxidation of proteins in the pgr5 seedlings than in the wild type (Figure 4D). Furthermore, immunoanalysis of the leaf total protein extracts revealed elevated contents of Cu/Zn superoxide dismutase (SOD) in the pgr5 mutant seedlings than in the wild type both under constant (170% ± 53%) and fluctuating light (177% ± 61%) (Figure 4E). Similarly, the levels of the thylakoid-bound (tAPX) and cytosolic ascorbate peroxidase (cAPX) increased in the pgr5 mutant. The levels of tAPX and cAPX in pgr5 compared with the wild type were 129% ± 29% and 143% ± 80% under constant light and 152% ± 85% and 143% ± 80% under fluctuating light, respectively. The steady state level of the peroxiredoxin Q (PRXQ) is known to be modulated in response to pathogen attack in plants, which typically also involves accumulation of reactive oxygen species (ROS) (Kiba et al., 2005; Trotta et al., 2011). However, the level of PRXQ did not markedly differ between the wild type and pgr5 (Figure 4E), as the pgr5 seedlings contained 106% ± 54% of wild-type PRXQ level under constant light and 88% ± 22% under fluctuating light.
The pgr5 Mutant Shows Unbalanced Redox Behavior
Due to difficulties in obtaining enough leaf material for functional measurements from the pgr5 plants with a clear growth phenotype under standard fluctuating light, we grew the wild-type and the pgr5 plants also under mildly fluctuating light, with the cycles of 5 min low light and 1 min of moderate high light following each other (for light intensities, see Table 1). This light cycle partially rescued the growth of the pgr5 mutant under fluctuating light and thus allowed also the mutant plants to reach a full rosette state. The amount of the PsaB protein in these pgr5 mutant plants was slightly reduced (64% ± 29%, mean ± sd), whereas the D1 protein was present at levels comparable to wild-type thylakoids (Figure 2A, mFL).
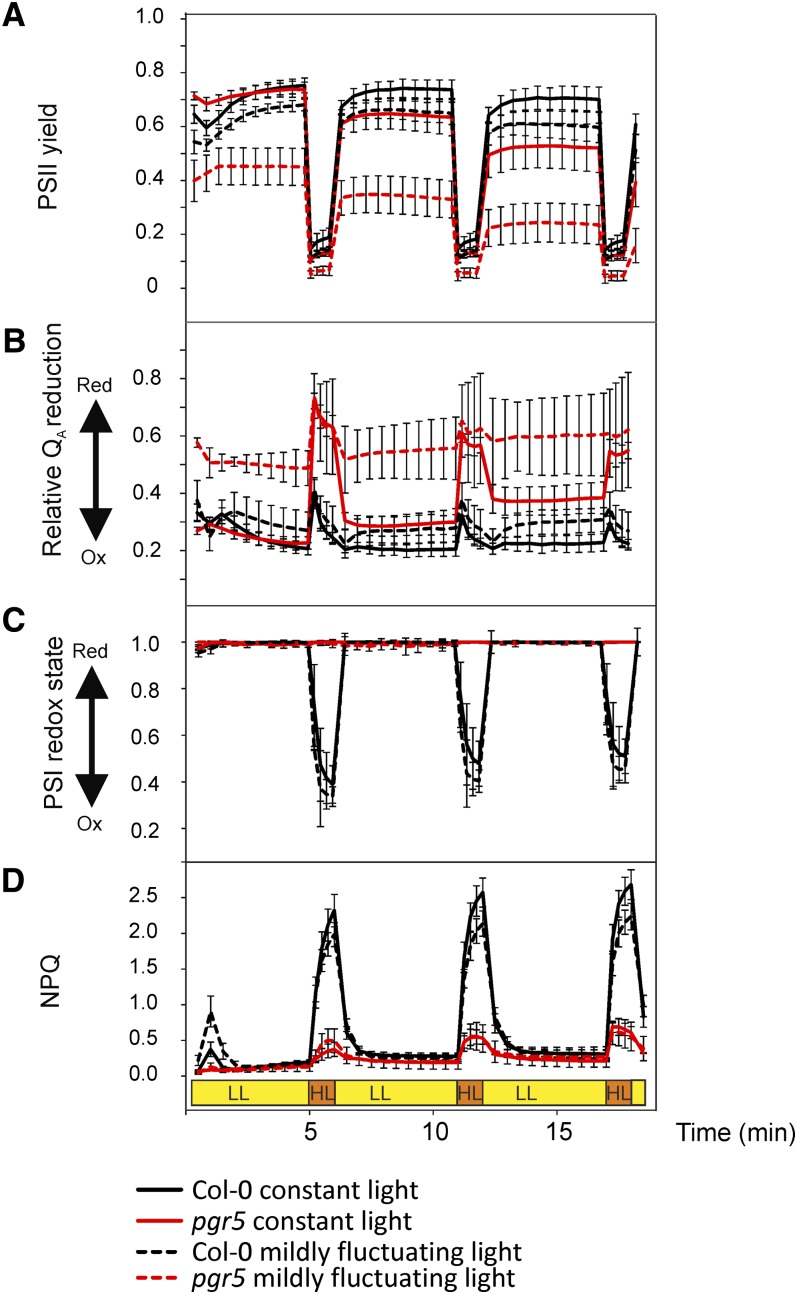
To study the impact of the PGR5 protein on photosynthetic electron transfer reactions, the PSII yield, the redox state of QA (F′/Fm parameter) and that of PSI (P700 oxidation) were next evaluated (Figure 5). 1-qL or 1-qP were not selected as parameters to describe the QA redox state because the fluctuating light experiments present non–steady state conditions in the high light phases. Consequently, we decided to use the raw signal of the chlorophyll a fluorescence yield as an indicator of the QA redox state (i.e., the fluorescence yield values under actinic light [F′] normalized to the maximal fluorescence Fm [parameter F′/Fm]). Although it is known that F′/Fm is not linearly correlated to [QA−] due to the antenna connectivity, it is nevertheless the best parameter for estimation of changes in the reduction level of QA in non–steady state conditions. Therefore, F′/Fm was denoted as “relative QA reduction.” The dynamics of NPQ was also recorded (Figure 5D). It is important to note that although the measurements were performed on leaves from the wild-type and pgr5 plants grown under constant and mildly fluctuating light, the actinic light during the measurements was adjusted to mimic the strongly fluctuating light illumination that provoked the pgr5 lethal phenotype (5 min of 50 µmol photons m2 s−1 and 1 min of 500 µmol photons m−2 s−1).
Figure 5.
Photosynthetic Parameters of Mature Wild-Type and pgr5 Plants Measured under Illumination Mimicking the Fluctuating Growth Light Condition.
The plants were grown either under constant moderate light (120 µmol photons m−2 s−1) or under mildly fluctuating light with 5 min of low light (50 µmol photons m−2 s−1) and 1 min of moderate high light (350 µmol photons m−2 s−1) repeating for the entire photoperiod. Col-0, Columbia-0.
(A) to (D) Actinic light that mimics the fluctuating light (5 min of low, 58 µmol photons m−2 s−1 light, depicted as yellow, and 1 min of high, 533 µmol photons m−2 s−1 light, depicted as orange) was applied on detached leaves. The values represent means ± sd, n = 4 to 6.
(A) Photochemical quantum yield of PSII.
(B) The relative QA reduction level, measured by the chlorophyll a fluorescence parameter F′/Fm.
(C) The PSI redox state.
(D) Induction of NPQ. HL, high light (orange); LL, low light (yellow).
In pgr5 plants grown under constant and mildly fluctuating light, the PSII yield decreased stepwise at transfer to low light after every high light pulse, a phenomenon not present in the wild type (Figure 5A). Opposite to the PSII yield, the QA reduction level of the pgr5 plants increased during the low light phases (Figure 5B). Since a steady state condition was reached during the low light phase, the QA reduction level was indicative also of the plastoquinone (PQ) redox state. During high light periods, the pgr5 plants showed lower PSII yield and a much higher QA reduction compared with the wild type, regardless of the growth condition (Figure 5B).
PSI redox behavior also differed between pgr5 and the wild type. During the low actinic light phases, PSI was fully reduced in all plants (Figure 5C). Upon the subsequent high light pulse, PSI became highly oxidized in both constant light and mildly fluctuating light-grown wild-type plants. On the contrary, in the pgr5 plants (both from constant and mildly fluctuating growth light), PSI remained fully reduced independently of the actinic light cycles (Figure 5C). We also tested the dynamics of P700 redox state upon fluctuating light using white actinic light and by omitting the saturating pulse (SP) from the beginning. This application mimics the natural outdoor light fluctuations even better and led to a similar conclusion: The wild-type plants are capable of oxidizing P700 during the high light phase, whereas P700 of the pgr5 mutants remained highly reduced (see Supplemental Figure 3 online).
Exactly opposite behavior to that of PSI oxidoreduction was observed when the formation of NPQ was monitored: High actinic light efficiently induced NPQ in wild-type plants, regardless of whether the plants were grown under constant or mildly fluctuating light, whereas the pgr5 mutants were not capable of inducing NPQ during the high light phases (Figure 5D). Taken together, the results in Figure 5 indicate that during the high light phases, the photosynthetic ETC of the pgr5 plants accumulates reducing power that cannot be dissipated during the subsequent low light phases, thus progressively decreasing the PSII yield and increasing the reduction level of the PQ pool.
It is worth noting that before the first high light phase, the pgr5 plants grown under constant light exhibited rather similar PSII yield and QA reduction state as the wild-type plants, whereas in the pgr5 mutants grown under mildly fluctuating light, these parameters were altered already prior to the measurements under strongly fluctuating actinic light (Figures 5A and 5B). This suggests that in the pgr5 plants grown under constant light, the disturbances in the PSII yield and the QA reduction state were induced mainly by the high light phases during the measurement under actinic strongly fluctuating light. On the contrary, in the pgr5 mutants grown under mildly fluctuating light, the photosynthetic machinery was largely disturbed (low PSII yield and high QA reduction state) already before starting the experiment under strongly fluctuating actinic light. The redox state of PSI as well as the induction of NPQ, on the other hand, behaved similarly during measurements under strongly fluctuating actinic light in both the constant light– and mildly fluctuating light–grown pgr5 mutants (Figures 5C and 5D).
To analyze in more detail the light energy–dependent increase in the PQ redox state under the low light phases of fluctuating actinic light, the cumulative increase in the F′/Fm during five fluctuating light cycles [Δ(F′/Fm)] was plotted against the intensity of the high light pulse (see Supplemental Figure 4 online). The actinic fluctuating light was applied as follows: The intensity of the 5-min low-light period (50 µmol photons m−2 s−1) was kept similar to that in fluctuating growth light, whereas the intensity of the 1-min high-light pulses was in different measurements increased from 0 and 50 µmol photons m−2 s−1 to ∼2000 µmol photons m−2 s−1. Until the intensity of high light pulses reached 500 µmol photons m−2 s−1, the wild type was capable of maintaining low and stable reduction level of the PQ pool. With further increase in the intensity of the high light pulses, the F′/Fm parameter started gradually increasing also in the wild type. On the contrary, in the pgr5 mutant, the increase of the PQ pool redox state started at ∼100 µmol photons m−2 s−1 (i.e., at the light intensity that is only twice that of the low light phase in the applied fluctuating light cycle). Upon further increase in the intensity of the high light pulse, the Δ(F′/Fm) value of the pgr5 mutant increased drastically, reaching a plateau at ∼1000 µmol photons m−2 s−1 (see Supplemental Figure 4A online). The PQ redox state increase during the low light phases (steady state condition) was calculated also as a Δ(1-qL) parameter, which led to the same conclusion (see Supplemental Figure 4B online).
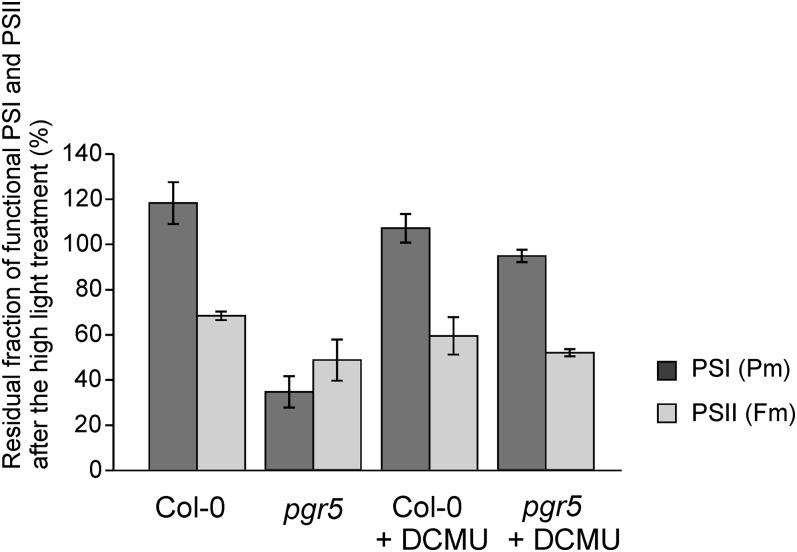
Inhibition of PSI in pgr5 by High Light Pulses Can Be Largely Prevented by Blocking LET
The sensitivity of the two photosystems to photoinhibition in pgr5 was further investigated by the following experiments. The Fm and Pm parameters, as indicators of functional intactness of PSII and PSI, respectively, were first measured on leaf discs from the wild-type and pgr5 plants directly from constant growth light conditions (with 20 min incubation in darkness to relax NPQ). The same leaves were then exposed to high light (1200 µmol photons m−2 s−1) for 15 min in the absence (control values) or presence of DCMU, followed by remeasurement of the Fm and Pm parameters. As shown in Figure 6, after the 15-min high light treatment, the Pm values of pgr5 decreased to roughly one-third (35% of the control value) of that measured at growth light, confirming the extreme susceptibility of PSI to photoinhibition in pgr5 upon shift to high light. Importantly, when the electron transfer from PSII was blocked with DCMU, the damage to PSI was largely avoided also in pgr5 (Figure 6).
Figure 6.
Photoinhibition of PSI and PSII in Mature Wild-Type and pgr5 Plants by a High Light Treatment in the Presence and Absence of DCMU.
Following the high light treatment of 1200 µmol photons m−2 s−1 for 15 min, the functionality of PSI and PSII reaction centers was determined by the Pm and Fm parameters, respectively, measured from leaf discs (n = 3) after 20 min dark incubation and expressed as percentage of the initial values. Plants were grown under constant moderate light. DCMU (80 µM in 330 mM sorbitol) was infiltrated by a syringe. The values represent means ± sd from three independent measurements. Col-0, Columbia-0.
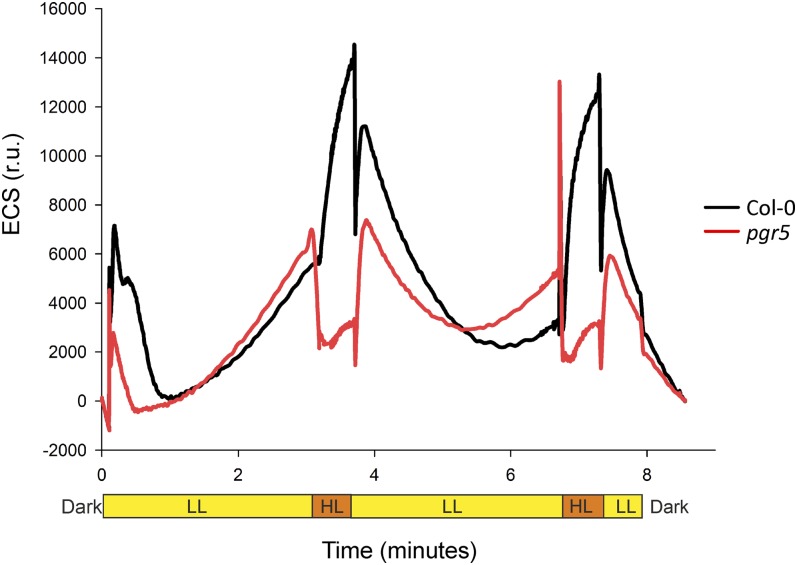
The Proton Motive Force of the pgr5 Mutant Is Disrupted Only during High Light Peaks
Electrochromic shift (ECS) of carotenoids was used to monitor changes in proton motive force (pmf) (Takizawa et al., 2007) during fluctuations between low light (50 µmol photons m−2 s−1) and high light (500 µmol photons m−2 s−1) phases. Under low light phases, the wild type and pgr5 showed similar behavior in generating pmf (Figure 7). Upon shift from low light to high light, the pmf of the wild type increased dramatically. By contrast, the pgr5 mutant lost the pmf generated under low illumination phases nearly completely when plants were shifted to the high light phase.
Figure 7.
Generation of pmf, Detected as ECS of Carotenoids, upon the Shifts of Mature Wild-Type and pgr5 Plants between Low and High Intensities of Light.
ECS of carotenoids was monitored spectrophotometrically during fluctuations between low light (50 µmol photons m−2 s−1, LL, yellow) and high light (500 µmol photons m−2 s−1, HL, orange) phases. r.u., relative units.
Shifting Mature Plants from Constant to Fluctuating Light Damages the PSI of pgr5, but in a Nonlethal Manner
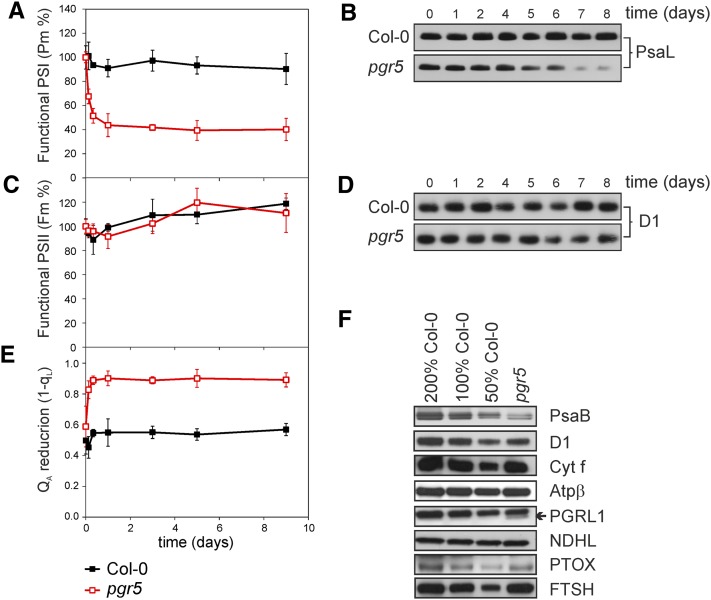
It was next investigated whether the mature plants that already have successfully assembled their thylakoid membrane protein complexes upon growth under constant light conditions are more tolerant to fluctuating light than the young seedlings. The pgr5 and wild-type plants were grown under constant light of 120 µmol photons m−2 s−1 till a full rosette stage (5 weeks) and then transferred to fluctuating light conditions (5 min of low light, 50 µmol photons m−2 s−1, and 1 min of high light, 500 µmol photons m−2 s−1) for 9 d. During this time, the functionality of PSI (Pm) and PSII (Fm) was recorded together with the quantification of the PsaL and the D1 proteins (representing PSII and PSI, respectively) (Figure 8). The steady state QA reduction level (1-qL) was likewise recorded to estimate the effect of the fluctuating light treatment on the redox balance between PSII and PSI.
Figure 8.
Structural and Functional Changes in the Photosynthetic Machinery of Mature Wild-Type and pgr5 Plants upon Shift from Constant Light to Fluctuating Light for 9 d.
Wild-type and pgr5 plants were grown under constant light (120 µmol photons m−2 s−1) for 5 weeks (time point 0) and then transferred to fluctuating light during the photoperiod with 5 min of low light (50 µmol photons m−2 s−1) and 1 min of high light (500 µmol photons m−2 s−1) for 9 d.
(A) and (B) The relative changes in the functionality (A) and the amount of PSI detected by immunoblots of the PsaL protein (B). The values represent means ± sd, n = 3. Col-0, Columbia-0.
(C) to (E) Changes in functionality of PSII (C), the amount of PSII based on the amount of the D1 protein (D), and in the QA reduction level (1-qL) during 9-d growth of mature leaves under fluctuating light (E). The fluorescence measurements were performed using intact leaves after 20 min dark incubation. The values represent means ± sd, n = 3.
(F) Immunoblots of thylakoid membrane proteins 9 d after the transfer to fluctuating light. Representative examples from at least three biological replications are shown with the dilution series from wild-type Columbia-0 leaf extracts (50 to 200%).
A shift from constant light to fluctuating light induced a drastic and fast decrease in the Pm parameter of the pgr5 plants (Figure 8A). Indeed, already after the first day under fluctuating light, the Pm value of the pgr5 mutant was only about half of that in the beginning of the day. Thereafter, no further decrease in the Pm value occurred. The PSI protein content (PsaL), on the contrary, remained stable several days, and only after 4 to 5 d following the shift of the pgr5 mutants to fluctuating light the PSI protein content started to decrease (Figure 8B). In wild-type plants, the shift to fluctuating light did not markedly affect the functionality or the amount of PSI. PSII function, by contrast, was not affected by the shift to fluctuating light, in either wild-type or pgr5 plants (Figures 8C and 8D). Nevertheless, the QA redox state rapidly increased in the pgr5 mutant, but not in the wild type, during the first hours of the day the plants were shifted to fluctuating light and remained high thereafter (Figure 8E).
Nine days after the transfer of mature wild-type and pgr5 plants from constant to fluctuating light, the levels of thylakoid membrane proteins were assessed by immunoblotting (Figure 8F). The amount of the PSI core complex protein PsaB was significantly lower (40% ± 18%) in pgr5 compared with the wild type, similar to the PsaL protein shown in Figure 8B. By contrast, the levels of PSII and the ATP synthase were similar in mature pgr5 and wild-type plants, deduced from the amounts of the D1 and ATPβ proteins (107% ± 37% of D1 and 100% ± 4% of ATPβ in pgr5 compared with the wild type) (Figure 8F). Transfer to fluctuating light somewhat increased the level of the Cyt b6f complex; the amount of the Cyt f protein in mature pgr5 was 136% ± 36% of the wild-type level. Similar to young seedlings, also the mature pgr5 plants contained substantial amounts of the PGRL1 protein (70% ± 16% of the wild-type level). This is noticeable because it has been shown that the PGR5 and the PGRL1 proteins interact and that the presence of the PGR5 increases the stability of PGRL1 (DalCorso et al., 2008). The levels of the NDHL and PTOX proteins were approximately similar between the wild type and pgr5, as the pgr5 plants contained 93% ± 22% NDHL and 95% ± 9% of PTOX compared with the wild type. However, the amount of the FTSH protease after transfer to fluctuating light was found to be higher in pgr5 (168% ± 71%) compared with the wild type (Figure 8F).
It is worth stressing that, whereas the functionality and the protein content of PSI in pgr5 were drastically lowered upon shift of plants from constant light to fluctuating light, this did not lead to lethality. Indeed, the mature pgr5 plants survived for weeks under fluctuating light and eventually flowered (see Supplemental Figure 5 online).
DISCUSSION
Experimental plant biologists often grow the research material under well-controlled steady state conditions in growth chambers or greenhouses, keeping the environmental parameters, such as temperature, humidity and, perhaps most importantly, the light intensity at constant level during the photoperiod. Such experiments are important to dissect the specific molecular responses to well-defined environmental cues. Nevertheless, they also conceal a wide variety of regulatory and acclimation mechanisms crucial for plants to cope with constantly changing environmental conditions in their natural habitats.
Here, we addressed the plant acclimation strategies to fluctuating light conditions and indicate a crucial role for the PGR5 protein. The PGR5 protein is often connected with the Fd-dependent CET around PSI (Munekage et al., 2002, 2004; Nandha et al., 2007; DalCorso et al., 2008; Joliot and Johnson 2011), and its importance was first realized when Toshiharu Shikanai and his coworkers produced the double mutant pgr5 crr2-2 (Munekage et al., 2004) lacking both putative cyclic PSI routes, the Fd- and the NDH-dependent route, respectively. The double mutant has stunted growth phenotype and its photosynthesis is severely impaired (Munekage et al., 2004). Moreover, it has a lower amount of the PSI core protein PsaA compared with the wild type (Okegawa et al., 2010). Absence of the growth phenotype from the single pgr5 and crr2-2 mutants made the authors suggest at least partial redundancy between the two putative PSI CET pathways. Nevertheless, our results indicating that the Arabidopsis single mutant pgr5 is lethal when grown under fluctuating light (Tikkanen et al., 2010) pointed to a unique function of the PGR5 protein in enabling plant acclimation to fluctuating light. Moreover, as demonstrated in this study, the role of PGR5 appears to be regulation of LET rather than PSI CET.
Damage to PSI as Plant Develop under Fluctuating Light Is Avoided via PGR5
Analysis of the thylakoid membrane protein complexes from young seedlings developed under fluctuating light revealed a conspicuous decrease in the amount of PSI complexes in the pgr5 mutant compared with the wild type, whereas the level of the PSII complexes was unaffected (Figure 3). Further protein analysis from both young and mature (Figures 2 and 8) leaves confirmed that PSI is the primary target of the damage caused by fluctuating light in the absence of PGR5. The extent in the reduction of the PSI pool is dependent on the developmental stage of the plant, the young leaves being more susceptible to damage upon exposure to fluctuating light. Presumably, there is a particular susceptibility of PSI to photodamage in fast-growing tissues, in which the maximal amount of energy should be allocated to growth rather than to the fine-tuning of the photosynthetic apparatus and the PSI/PSII ratio. Indeed, it has been reported that the PSI levels in mature leaves are generally highly constant, whereas in younger leaves there are more light-dependent variations in the amount of PSI (Schöttler et al., 2011).
PSI has generally been regarded as a stable photosystem compared with PSII, in which the D1 protein turns over in the light intensity–dependent manner (Aro et al., 1993). On the other hand, it is well known that PSI lacks efficient repair, making the recovery of PSI from photoinhibition extremely slow (Kudoh and Sonoike, 2002). For this reason, the damage to PSI has been considered to be practically irreversible (Scheller and Haldrup, 2005; Sonoike, 2011). The selective photoinhibition of PSI has been considered to take place very seldom in vivo (Sonoike, 2011) and has been previously reported to occur only in certain plant species, such as chilling-sensitive cucumber (Cucumis sativus) or potato (Solanum tuberosum; Sonoike and Terashima, 1994), or under specific environmental conditions, such as a combination of low temperature and weak light (Havaux and Devaud, 1994; Sonoike et al., 1995; Sonoike, 2011). Furthermore, it has been suggested that when the photodamaged PSI proteins are degraded, the chlorophyll molecules of PsaA and PsaB may cause secondary damage (Sonoike, 2011). In line with this, oxidative modifications of the leaf total proteome (Figure 4D) were typical for the pgr5 mutant under fluctuating light and may also influence the accumulation of the FTSH protease (Figure 8F).
Considering the irreversibility as well as the deleterious secondary effects of PSI photodamage, it is obvious that photoinhibition of PSI should be avoided by efficient protective mechanisms. For this purpose, chloroplasts have three important mechanisms: (1) regulation of LET (Figure 6), (Joliot and Johnson 2011); (2) CET, which has been considered to act as a safety valve (Munekage et al., 2002, 2004; Rumeau et al., 2007; Johnson, 2011; and references therein), but paradoxically directs electrons from the PSI acceptor side to the already highly reduced PQ pool; and (3) the stromal ROS scavenging enzyme systems, such as SOD and APX (Asada, 1999).
In contrast with earlier knowledge, we demonstrate here that PSI is potentially very susceptible to light, but the PGR5 protein in chloroplasts has evolved to ensure the maintenance of PSI even under strongly fluctuating light intensity. It is noteworthy that the pgr5 mutant is perfectly capable of accumulating PSI under constant high light conditions (Figure 2A) despite the fact that PGR5 was previously suggested to have a role in protection of PSI under high light conditions (Munekage et al., 2002). It is conceivable that in the course of long-term acclimation to constant high light, the pgr5 mutant plants are capable of establishing cellular homeostasis, which probably involves several molecular adjustments not only in chloroplasts but possibly also in mitochondria and/or other cell compartments. Indeed, the high intensity of light as such is not as challenging for plant acclimation as are the fluctuations in the light intensity, which lead to the damage of PSI centers. In the absence of a PSI repair cycle, the fluctuating light conditions have a potential to markedly reduce the PSI pool (Figures 2, 3, and 8B) and to induce drastic imbalances in the redox state of the ETC (Figure 5) and to the transthylakoid pmf (Figure 7), finally resulting in lethality, as demonstrated by the young seedlings of the pgr5 mutant (Figure 1).
However, remarkable protection provided by the PGR5 protein against PSI photodamage in the wild type under fluctuating light is not inclusive. This can be deduced from increased accumulation of foliar H2O2 (Figure 4B) and from enhanced production of the major ROS scavenging enzyme SOD (Figure 4E) in the wild type, providing evidence that the PSI complex even in the wild type is to some extent sensitized to photodamage under fluctuating light. Even though the wild-type plants are capable of slowing down LET upon shift to high light (Figure 6) or to fluctuating light (Figure 8), the wild-type plants grown under fluctuating light have further circumvented the problem of PSI photodamage by increasing the relative amount of PSI centers in the thylakoid membrane (Figure 2B). The pgr5 mutant, on the contrary, fails in such a strategy, most likely because of strongly accelerated damage and degradation of the PSI proteins, vastly exceeding the poor capacity for PSI repair.
Molecular Mechanisms behind PSI Photodamage under Fluctuating Light
To investigate the molecular mechanism(s) behind the damage of PSI under fluctuating light, we measured the photochemical parameters from mature wild-type and pgr5 leaves. Functional analyses revealed that in pgr5 (grown both under constant and mildly fluctuating light), the PSI centers remain fully reduced under the high light phases, whereas in the wild type, the PSI complex becomes normally oxidized upon the high light pulse (Figure 5C). This implies that the photosynthetic machinery in the wild type is capable of decreasing the electron flow to PSI during the high light phase, which seems to be essential to prevent the overreduction of PSI and further to keep the reduction level of the entire intersystem ETC low enough during the subsequent low light period. Indeed, it was shown decades ago that there exists a photosynthetic control that inhibits LET (Rumberg and Siggel, 1969; Bendall, 1982; Harbinson et al., 1990; Kramer et al., 1999) by the means of changes in lumenal pH via a so far uncharacterized mechanism(s). In contrast with the wild type, the pgr5 mutant (both constant- and mildly fluctuating light-grown plants) fails in such a fundamental regulation.
PSI can be experimentally oxidized by two different ways. Far-red light strongly favors the excitation of PSI and slows down the activity of PSII, leading to PSI oxidation. PSI becomes oxidized also upon a shift to high light. As the increased quantity of white light does not affect the relative excitations of PSII and PSI (Tikkanen et al., 2010), the oxidation of PSI upon a shift of wild-type plants to high light must be based on a mechanism that slows down the electron transfer from PSII to PSI. Consequently, our data suggest that the primary role of the PGR5 protein concerns the regulation of LET, instead of only being a component of the Fd-dependent CET. Indeed, the high light–induced damage to PSI in pgr5 can be prevented by blocking the intersystem electron transfer from PSII to PSI by DCMU (Figure 6). Also, it was recently shown that the formation of ΔpH via regulation of Cyt b6f, in which PGR5 has an essential role, is indispensable for photoprotection of PSI (Joliot and Johnson, 2011). This photosynthetic control of electron transfer, which protects PSI from overreduction and oxidative damage, is clearly missing from the pgr5 mutant, leading to increased vulnerability of PSI under high light pulses (Figures 5C and 6). Since PSI in the pgr5 mutant is not under the protection of the photosynthetic control of the ETC, the high light pulses are likely to provoke damage to the PSI iron-sulfur centers (Sonoike et al., 1995; Sonoike, 2011).
Besides having highly reduced PSI, the pgr5 mutant showed a stepwise increase of the QA redox state upon every low light phase of the light intensity cycles (Figure 5B). This indicates that during every high light phase, the ETC of pgr5 accumulates excess reducing power, which the system is not capable of dissipating. Likewise, the analysis of the light energy–dependent increase in the QA redox state indicated that in pgr5 the F′/Fm parameter increased more drastically with increasing intensity of the high light pulses than in the wild type (see Supplemental Figure 4 online). Taken together, such a hyperreduction under low light of the entire intersystem ETC is likely causing a strong reducing burst to the acceptor side of PSI upon the following shift to high light, eventually leading to photodamage.
In addition to the uncontrolled electron transfer, the pgr mutant is incapable of controlling the transthylakoid pmf under the high light peaks of fluctuating light (Figure 7). In other words, everything flows in pgr5. The defects in pmf indicate that the pgr5 mutant loses its capability to produce ATP under high light phases. An interesting comparison to pgr5 is provided by the ATP synthase mutant (Rott et al., 2011). ATPase mutants behave in many ways exactly opposite to the pgr5 mutant: They exhibit (1) increased NPQ and (2) pmf, which lead to overacidification of the thylakoid lumen, and most importantly, they (3) strongly inhibit the LET. As a consequence, the tobacco (Nicotiana tabacum) ATP synthase mutants were shown to exaggerate their photosynthetic control (Rott et al., 2011), whereas the pgr5 mutant has lost this control. Indeed, this comparison and our results on the pmf parameter in pgr5 (Figure 7) hint to a possibility that the PGR5 protein bears a yet uncharacterized role in regulation of the ATP synthase as well. This hypothesis is further supported by the observation that the content of the ATPβ protein, representing the ATP synthase, was markedly increased in young pgr5 leaves grown under fluctuating light (Figure 2B). It is conceivable that the pgr5 mutants try to compensate their leaky ATP synthases by increasing the amount of the enzyme.
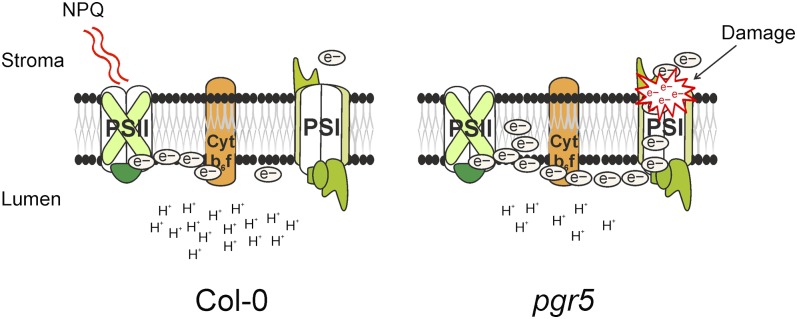
Based on functional characterization of the pgr5 mutant (Figures 5 to 8), the most devastating step in fluctuating light is the shift from low to high light. To summarize the mechanism behind the role of PGR5 in protection of PSI against photodamage in that particular phase, the following model is proposed (Figure 9). In wild-type plants, a shift to high light induces transient lumenal protonation, which downregulates the Cyt b6f complex (and possibly also the ATP synthase) and, thus, the electron flow from PSII to PSI complex. By contrast, the pgr5 mutant is not able to protonate the lumen and therefore fails in downregulation of the Cyt b6f complex, which in turn results in overreduction of the entire intersystem ETC. For this reason, upon the high light pulse, an excess of electrons strikes out to PSI and causes overreduction (Figure 5C) and eventually an irreversible damage of the PSI acceptor side (Tsuyama and Kobayashi, 2009). Incapability of pgr5 to induce NPQ (Figure 5D) possibly somewhat reinforces the PSI injury due to the fact that the pgr5 mutant cannot downregulate PSII via heat dissipation of the excess excitation energy.
Figure 9.
Role of PGR5 in Protection of PSI from Photodamage upon Shift to High Light.
The PGR5 protein is required for formation of a proton gradient across the thylakoid membrane upon increase in the light intensity, occurring by a still uncharacterized mechanism. Rapid generation of a proton gradient upon increase in light intensity is a prerequisite to slow down the electron transfer through the Cyt b6f complex, which is required for the protection of PSI. Following an increase in light intensity, the pgr5 mutant fails to slow the speed of electron transfer through Cyt b6f. This leads to overreduction of PSI electron donors relative to the capacity of the PSI electron acceptors. This uncontrolled burst of reducing equivalents damages the iron-sulfur clusters of PSI. The absence of the NPQ induction might further aggravate the reducing pressure on PSI because PSII continues feeding the electrons to PSI electron donors.
Earlier reports have linked the PGR5 protein to Fd-dependent CET (Munekage et al., 2002, 2008). Nevertheless, it was recently shown that the pgr5 mutant is capable of performing Fd-dependent CET (Avenson et al., 2005; Nandha et al., 2007). Based on our results and on the fact that the proteins/protein complexes required for the Fd-dependent electron transfer pathway have not yet been identified in higher plants, we suggest the published pgr5 data to be reevaluated to indicate (1) which part of these data could be explained by the lack of the ΔpH-dependent regulation of the Cyt b6f complex or the ATP synthase and accompanying pleiotropic effects and (2) which part could result from the lack of the Fd-dependent CET.
Hierarchy among Mechanisms Facilitating the Acclimation to Fluctuating Light
It is clear that under fluctuating light the lack of PGR5 is not compensated for by upregulation of the NDH-dependent CET (Figures 2C and 8F) and, in fact, even the complete absence of the NDH complex (ndho mutant) does not induce a fluctuating light phenotype (Figure 1C). Likewise, PTOX does not seem crucial under fluctuating light (Figures 2C and 8F). Moreover, even though the accumulation of SOD and APX (Figure 4E) reduced the production of ROS in pgr5 plants under fluctuating light (Figures 4A to 4C), the elevated capacity of the antioxidant scavenging systems in pgr5 is clearly not effective enough to protect PSI against fluctuating light-induced stress.
None of the well-known regulatory mechanisms of stress avoidance was found to play as decisive a role in acclimation and survival strategy of the seedlings under fluctuating light as does the PGR5 protein. Indeed, there seems to be a strict dissection in mechanisms responsible for low/high light acclimation and those responsible for acclimation to fluctuating light. So far, only two other dynamic regulation mechanisms, the PsbS-dependent NPQ and the STN7-based phosphorylation of the LHCII proteins, have been reported to play a role in acclimation to short-term fluctuations in light intensity (Külheim et al., 2002; Tikkanen and Aro, 2012). However, npq4 has no phenotype and stn7 has only a stunted growth phenotype under fluctuating light (Tikkanen et al., 2010) and thus is not comparable in severity to the lethal phenotype of the pgr5 seedlings. Moreover, although the stn7 and npq4 mutants have been shown to exhibit lower fitness under field conditions, they did not show any visible growth defects in the field experiment (Frenkel et al., 2007). The pgr5 mutant, on the contrary, has significantly higher mortality rate under field conditions compared with the wild type (Figure 1D). Although the growth under natural field site is likely to expose plants to a broad variety of environmental stresses, it is most likely that in line with the growth chamber experiments, the variations in the light intensity are responsible for the strongly increased mortality rate of the pgr5 mutant in the field conditions. Taken together, our data emphasize the importance of the PGR5 protein as a crucial acclimation mechanism to growth conditions with strong fluctuations in the light intensity.
Conclusions
From the light acclimation experiments performed under fluctuating light conditions, the following important conclusions can be made: (1) Fluctuating growth light induces damage particularly to the PSI complex leaving the PSII complex mostly undamaged; (2) the PGR5 protein is essential in photoprotection of PSI; (3) the primary role of PGR5 lies on the regulation of LET; and (4) photoprotection of PSI by PGR5 under fluctuating light is most crucial upon early developmental stage of Arabidopsis and thus is essential for the survival of plants under fluctuating light. Moreover, although PSI has been considered to be very stable compared with PSII, it is evident that (5) under natural conditions the occurrence of PSI photodamage is much more frequent than has been previously anticipated. The correlation between results obtained from fluctuating light experiments in growth chambers with those from the field experiments demonstrates that (6) our experimental design using fluctuating light is a useful experimental tool to mimic natural outdoor light conditions.
METHODS
Plant Material and Growth Conditions
Arabidopsis thaliana ecotype Columbia wild type and the pgr5 (Munekage et al., 2002) and ndho (SALK_068922) mutants (Rumeau et al., 2005) were used for the experiments. For the pgr5 mutant, a wild-type Columbia type gl1 (Munekage et al., 2002) was used as a reference, since similar to pgr5, it does not possess trichomes. The experiments were performed either with cotyledon leaves detached from 9-d-old seedlings or with mature leaves detached from 4- to 5-week-old rosettes.
All plant material was grown under the photoperiod of 8 h light/16 h dark. OSRAM PowerStar HQIT 400/D Metal Halide lamps were used as a light source. The summary of the light conditions and plant material is presented in Table 1. The young seedlings were grown either (1) under constant light (200 µmol photons m−2 s−1) or (2) under fluctuating light, in which an electronically controlled shading system was used to expose the plants to low light (50 µmol photons m−2 s−1) for 5 min and subsequently to high light (500 µmol photons m−2 s−1) for 1 min (Tikkanen et al., 2010). These cycles were repeated during the entire photoperiod. In addition, the wild-type and pgr5 seedlings were also grown aseptically in (3) MS medium for 2 weeks (Murashige and Skoog, 1962) without sugar or supplemented with 1% (w/v) and 2% (w/v) Suc. Mature plants were either grown under (4) mildly fluctuating light with 5 min of low light (50 µmol photons m−2 s−1) and 1 min of moderate high light (350 µmol photons m−2 s−1) repeated during the entire photoperiod, or (5) they were first grown for 5 weeks under constant light (200 µmol photons m−2 s−1) and thereafter transferred to fluctuating light. In addition, for specific experiments, plants were grown under constant (6) low light (50 µmol photons m−2 s−1), (7) moderate growth light (120 µmol photons m−2 s−1), or (8) high light (500 µmol photons m−2 s−1).
Isolation of Thylakoid and Total Protein
Thylakoid membranes were isolated as described by Suorsa et al. (2004) and the leaf total proteins as described by Kangasjärvi et al. (2008). The chlorophyll content was measured according to Porra et al. (1989) and the protein content according to Lowry et al. (1951).
Separation and Detection of Thylakoid Proteins and Protein Complexes
For one-dimensional separation of the thylakoid membrane or leaf total proteins, SDS-PAGE (15% polyacrylamide, 6 M urea) was used (Laemmli, 1970). After electrophoresis, the proteins were electroblotted to a polyvinylidene difluoride membrane (Millipore) and blocked with 5% milk (nonfat dry milk; Bio-Rad). Immunoblotting using enhanced chemiluminescence detection was performed according to Kangasjärvi et al. (2008). The Cyt f antibody was kindly provided by L. Zhang, the STN7 kinase antibody by R. Barbato, the PTOX antibody by M. Kuntz, the PGRL1 antibody by D. Leister, the FNR, PsaL, and PsaD antibodies by H. Vibe Scheller, the FTSH antibody by T. Ogura, and the NDHL antibody by T. Shikanai. The antibodies against APX, ATPβ, Fd, PsaB, PRXQ, RbcL, and the Cu/Zn SOD were purchased from Agrisera. The oxidative modifications of proteins were studied using the OxyBlot protein oxidation detection kit (Chemicon International) according to the manufacturer’s instructions. The immunoblots were quantified with a FluorChem 8000 image analyzer (Alpha Innotech Corporation, ProteinSimple).
To separate the thylakoid membrane protein complexes, isolated thylakoids were solubilized either with DM (Sigma-Aldrich) or with digitonin (Calbiochem) and separated in lpBN PAGE as described (Järvi et al., 2011).
In Vivo Measurements of PSII and PSI Photosynthetic Parameters
The Dual-PAM-100 (Heinz Walz) was used for the simultaneous measurement of PSII and PSI photosynthetic parameters, based on chlorophyll a fluorescence and P700 oxidation signal (difference of intensities of 875 and 830 nm pulse-modulated measuring light reaching the photodetector) (Klüghammer and Schreiber, 2008), respectively. The actinic light was red (635-nm wavelength). The measuring light (460-nm wavelength) intensity was 5 µmol photons m−2 s−1. The QA redox state was estimated by the parameter 1-qL (Kramer et al., 2004). The relative QA redox state in non–steady state conditions was measured as F′/Fm, where F′ is the fluorescence yield under actinic light and Fm is the maximal fluorescence from a dark adapted leaf during a SP (6000 µmol photons m−2 s−1 for 300 ms). NPQ was measured as (Fm − Fm′)/Fm′, where Fm′ is the maximal fluorescence yield upon SP from illuminated leaf (Bilger and Björkman, 1990). The PSII photochemical yield was measured as Fm′ − F′/Fm′ (Genty et al., 1989). The PSI redox state was determined by the P700 oxidation signal upon application of a SP (Klüghammer and Schreiber, 1994, 2008). The amounts of functional PSI and PSII reaction centers were estimated by the Pm and Fm parameters, respectively. The leaves were incubated in darkness for 20 min prior to measurements. Pm was determined according to Klüghammer and Schreiber (2008). Both the Pm and Fm values were normalized according to the chlorophyll content (Pm and Fm per microgram of chlorophyll). The high light (1200 µmol photons m−2 s−1) treatments were performed on leaf discs (9-mm diameter). The DCMU solution (80 µM in 330 mM sorbitol) was infiltrated by a syringe.
The redox state of P700 was also measured with PAM 101/103 chlorophyll fluorometer (Heinz Walz) connected to an ED-P700DW emitter-detector unit (see Supplemental Figure 3 online). Leaves were illuminated with white light (50 µmol photons m−2 s−1 or 500 µmol photons m−2 s−1) (KL-1500 lamp; Walz), and the absorbance was recorded at 810 and 860 nm. For methodological reasons, the duration of low light phases was 3 min and that of the high light phase was 1 min.
Measurement of the ECS
The pmf was assessed by measuring the carotenoid ECS substantially as described (Theg and Tom, 2011). After dark incubation (5 min), the leaves were illuminated with repeating periods of white light (3 min of low light, 50 µmol photons m−2 s−1, followed by 1 min of high light, 500 µmol photons m−2 s−1). Absorbance change at 520 nm was monitored with the JTS-10 spectrophotometer (Biologic Science Instruments).
Histochemical Staining for H2O2 and O2−
Foliar H2O2 accumulation was detected with the DAB method (Sigma-Aldrich) (Thordal-Christensen et al., 1997). Detached seedlings or mature leaves were incubated in the DAB solution for 2 h (seedlings) or overnight (mature leaves) in darkness substantially as described (Woo et al., 2011), after which accumulation of H2O2 was studied under the constant or fluctuating growth light conditions, as described above, for 30 min. For visualization of O2−, seedlings were floated on 0.1% NBT (Sigma-Aldrich) for 2 h and then illuminated under constant or fluctuating light for 30 min. Finally, the seedlings or leaves were cleared in 96% (v/v) ethanol. Stereomicrographs were taken with a Zeiss Lumar V12 stereomicroscope.
Visualization of 1O2 and Leaf Tissues by Confocal Laser Scanning Microscopy
Confocal laser scanning microscopy images of the 9-d-old wild-type and pgr5 seedlings were obtained with an inverted confocal laser scanning microscope (Zeiss LSM510 META) with ×10/0.30 or ×20/0.50 water objectives. For detection of singlet oxygen, seedlings were incubated for 30 min in singlet oxygen sensor green reagent (Molecular Probes) dissolved and diluted according to the manufacturer’s instructions. For imaging of 1O2, fluorescence was excited at 488 nm with an argon diode laser and detected with a 535- to 590-nm passing emission filter. Leaf cells were imaged by chlorophyll autofluorescence, which was excited at 488 nm, and detected with a 650- to 710-nm passing emission filter. Maximal projections of the sequential confocal images were created with the Zeiss LSM Image Browser version 3.5.0.376.
Field Experiments
The field experiment was conducted at the experimental garden of Umeå University in Northern Sweden essentially as described previously (Külheim et al., 2002; Frenkel et al., 2007, 2008). In short, the pgr5 and wild-type plants were first pregrown for 2 weeks in a climate chamber under a low light intensity of 50 µmol photons m−2 s−1; thereafter, five wild-type and five pgr5 plants were planted to a bigger pot. The pots were transferred to the field and randomized within the two big trays (35 plants per tray). Altogether, 175 wild-type and 175 pgr5 plants were studied. The experiment was conducted twice, with the first experiment between the 22nd of July and the 4th of August, and the following one between the 8th of August and the 25th of August, making the final number of sampled plant 350 per genotype. At the end of the experiment, the survival percentage was counted (survivors/5) × 100.
The survival/mortality data were analyzed by applying mixed-model analysis of variance (SPSS package, version 16.0). Mortality percentage was treated as a dependent variable. Fixed factors included genotype (pgr versus the wild type) and batch (July versus August) and their interaction term, genotype × batch. Since survival/mortality for the pgr5 mutant and the wild-type plants growing in a same stand is not independent, we treated the growing stand as a random factor in the mixed models.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL database or the Arabidopsis Genome Initiative database under the following accession numbers: PGR5 (locus AT2G05620, GenBank NP_565327.1), NDHO (locus AT1G74880, GenBank NP_565093.1), PsaB (locus ATCG00340, GenBank NP_051058), cyt f (locus ATCG00540, GenBank NP_051072), ATP β (locus ATCG00480, GenBank NP_051066.1), RBCL (locus ATCG00490, GenBank NP_051067.1), PGRL1A (locus AT4G22890, GenBank NP_849422.1), PGRL1B (locus AT4G11960, GenBank NP_192933.2), NDHL (locus AT1G70760, GenBank NP_177233.1), PTOX (locus AT4G22260, GenBank NP_567658.1), STN7 (locus AT1G68830, GenBank NP_564946.1), tAPX (locus AT1G77490, GenBank NP_177873.1), sAPX (locus AT4G08390, GenBank NP_192579.1), SOD (locus AT2G28190, GenBank NP_565666.1), PRXQ (locus AT3G26060, GenBank NP_189235.1), PSAL (AT4G12800, GenBank NP_193016.1). Germplasm used as follows: pgr5 (Munekage et al., 2002), gl1 (Munekage et al., 2002), and ndho T-DNA insertion mutant (SALK_068922; Rumeau et al., 2005).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Confocal Microscopy Images of Mesophyll Cells from 9-d-Old Wild-Type and pgr5 Seedlings.
Supplemental Figure 2. Wild-Type and pgr5 Seedlings on MS Plates.
Supplemental Figure 3. The PSI Redox State in Wild-Type and pgr5 Plants under Illumination with Fluctuating White Actinic Light.
Supplemental Figure 4. Energy-Dependent Increase of the Plastoquinone Reduction Level in Low Light Phases of Fluctuating Light in Wild-Type and pgr5 Plants Grown under Constant Moderate Light.
Supplementary Material
Acknowledgments
This research was supported by the Academy of Finland (Projects 118637 to E.-M.A., 138703 to M.S., and 218157 and 130595 for S.K.), the European FP7 SOLAR-H2 Program (Contract 212508), the European Union FP7 Marie Curie Initial Training Network (Projects 215174 to E.-M.A. and PITN-GA-2009-238017 to S. Jansson), the Finnish Doctoral Program in Plant Science, the Swedish Research Council (Vetenskapsrådet), and the Swedish Research Council for Environment, Agricultural Sciences, and Spatial Planning (Formas). We thank Toshiharu Shikanai and Dominique Rumeau for providing the pgr5, wild-type Colombia type gl1, and ndho seeds, respectively. We thank The Cell Imaging Core of the Turku Center for Biotechnology for the microscopy facilities. We also thank Kurt Ståhle for constructing the growth chambers with fluctuating light, Julia Rönnberg for assistance in the field experiment, Mika Keränen for helping with the figures, Petri Suorsa for helping with the statistical analyses, and Grzegorz Konert, Moona Rahikainen, and Anna Lepistö for helping with photography.
AUTHOR CONTRIBUTIONS
M.S., S. Järvi, M.G., M.N., M.P., M.T., S.K., S. Jansson, and E.-M.A. designed the research. M.S., S.J., M.G., M.N., M.R., M.P., M.T., S.K., and V.P. performed the research. M.S., S. Järvi, M.G., M.N., M.P., M.T., S.K., S. Jansson, and E.-M.A. analyzed the data and wrote the article.
Glossary
- PSI
photosystem I
- NPQ
nonphotochemical quenching
- LET
linear electron transfer
- CET
cyclic electron transfer
- PSII
photosystem II
- NDH
NAD(P)H dehydrogenase
- Fd
ferredoxin
- MS
Murashige and Skoog
- FNR
Fd-NAD(P)H-oxidoreductase
- PTOX
plastid terminal oxidase
- lpBN
large-pore blue-native
- DM
n-dodecyl β-d-maltoside
- NBT
nitroblue tetrazolium
- O2−
superoxide
- DAB
3,3-diaminobenzidine
- H2O2
hydrogen peroxide
- 1O2
singlet oxygen
- SOD
superoxide dismutase
- PRXQ
peroxiredoxin Q
- ROS
reactive oxygen species
- PQ
plastoquinone
- ECS
electrochromic shift
- pmf
proton motive force
- SP
saturating pulse
References
- Allen J.F., Forsberg J. (2001). Molecular recognition in thylakoid structure and function. Trends Plant Sci. 6: 317–326 [DOI] [PubMed] [Google Scholar]
- Anderson J.M., Aro E.M. (1994). Grana stacking and protection of photosystem-II in thylakoid membranes of higher-plant leaves under sustained high irradiance - An hypothesis. Photosynth. Res. 41: 315–326 [DOI] [PubMed] [Google Scholar]
- Aro E.M., Virgin I., Andersson B. (1993). Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim. Biophys. Acta 1143: 113–134 [DOI] [PubMed] [Google Scholar]
- Asada K. (1999). The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50: 601–639 [DOI] [PubMed] [Google Scholar]
- Avenson T.J., Cruz J.A., Kanazawa A., Kramer D.M. (2005). Regulating the proton budget of higher plant photosynthesis. Proc. Natl. Acad. Sci. USA 102: 9709–9713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey S., Horton P., Walters R.G. (2004). Acclimation of Arabidopsis thaliana to the light environment: The relationship between photosynthetic function and chloroplast composition. Planta 218: 793–802 [DOI] [PubMed] [Google Scholar]
- Battchikova N., Eisenhut M., Aro E.M. (2011). Cyanobacterial NDH-1 complexes: Novel insights and remaining puzzles. Biochim. Biophys. Acta 1807: 935–944 [DOI] [PubMed] [Google Scholar]
- Bendall D.S. (1982). Photosynthetic cytochromes of oxygenic organisms. Biochim. Biophys. Acta 683: 119–151 [Google Scholar]
- Bilger W., Björkman O. (1990). Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbency changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth. Res. 25: 173–185 [DOI] [PubMed] [Google Scholar]
- Bondarava N., Gross C.M., Mubarakshina M., Golecki J.R., Johnson G.N., Krieger-Liszkay A. (2010). Putative function of cytochrome b559 as a plastoquinol oxidase. Physiol. Plant. 138: 463–473 [DOI] [PubMed] [Google Scholar]
- DalCorso G., Pesaresi P., Masiero S., Aseeva E., Schünemann D., Finazzi G., Joliot P., Barbato R., Leister D. (2008). A complex containing PGRL1 and PGR5 is involved in the switch between linear and cyclic electron flow in Arabidopsis. Cell 132: 273–285 [DOI] [PubMed] [Google Scholar]
- Dietzel L., Pfannschmidt T. (2008). Photosynthetic acclimation to light gradients in plant stands comes out of shade. Plant Signal. Behav. 3: 1116–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flors C., Fryer M.J., Waring J., Reeder B., Bechtold U., Mullineaux P.M., Nonell S., Wilson M.T., Baker N.R. (2006). Imaging the production of singlet oxygen in vivo using a new fluorescent sensor, Singlet Oxygen Sensor Green. J. Exp. Bot. 57: 1725–1734 [DOI] [PubMed] [Google Scholar]
- Frenkel M., Bellafiore S., Rochaix J., Jansson S. (2007). Hierarchy amongst photosynthetic acclimation responses for plant fitness. Physiol. Plant. 129: 455–459 [Google Scholar]
- Frenkel M., Jänkänpää H.J., Moen J., Jansson S. (2008). An illustrated gardener’s guide to transgenic Arabidopsis field experiments. New Phytol. 180: 545–555 [DOI] [PubMed] [Google Scholar]
- Genty B., Briantais J.M., Baker N.R. (1989). The relationship between the quantum yield of photosynthetic electron-transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 990: 87–92 [Google Scholar]
- Harbinson J., Genty B., Foyer C.H. (1990). Relationship between photosynthetic electron transport and stromal enzyme activity in pea leaves. Plant Physiol. 94: 545–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaux M., Devaud A. (1994). Photoinhibition of photosynthesis in chilled potato leaves is not correlated with a loss of photosystem II activity - Preferential inactivation of photosystem I. Photosynth. Res. 40: 75–92 [DOI] [PubMed] [Google Scholar]
- Horton P., Ruban A.V., Walters R.G. (1996). Regulation of light harvesting in green plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47: 655–684 [DOI] [PubMed] [Google Scholar]
- Ifuku K., Endo T., Shikanai T., Aro E.M. (2011). Structure of the chloroplast NADH dehydrogenase-like complex: Nomenclature for nuclear-encoded subunits. Plant Cell Physiol. 52: 1560–1568 [DOI] [PubMed] [Google Scholar]
- Johnson G.N. (2011). Physiology of PSI cyclic electron transport in higher plants. Biochim. Biophys. Acta 1807: 384–389 [DOI] [PubMed] [Google Scholar]
- Joliot P., Johnson G.N. (2011). Regulation of cyclic and linear electron flow in higher plants. Proc. Natl. Acad. Sci. USA 108: 13317–13322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järvi S., Suorsa M., Paakkarinen V., Aro E.M. (2011). Optimized native gel systems for separation of thylakoid protein complexes: Novel super- and mega-complexes. Biochem. J. 439: 207–214 [DOI] [PubMed] [Google Scholar]
- Kangasjärvi S., Lepistö A., Hännikäinen K., Piippo M., Luomala E.M., Aro E.M., Rintamäki E. (2008). Diverse roles for chloroplast stromal and thylakoid-bound ascorbate peroxidases in plant stress responses. Biochem. J. 412: 275–285 [DOI] [PubMed] [Google Scholar]
- Kiba A., Nishihara M., Tsukatani N., Nakatsuka T., Kato Y., Yamamura S. (2005). A peroxiredoxin Q homolog from gentians is involved in both resistance against fungal disease and oxidative stress. Plant Cell Physiol. 46: 1007–1015 [DOI] [PubMed] [Google Scholar]
- Klüghammer C., Schreiber U. (1994). An improved method, using saturating light-pulses, for the determination of photosystem-I quantum yield via P700+-absorbancy changes at 830 nm. Planta 192: 261–268 [Google Scholar]
- Klüghammer C., Schreiber U. (2008). PAM Application Notes (PAN). 1: 11–14 at http://www.walz.com
- Kramer D.M., Johnson G.N., Kiirats O., Edwards G.E. (2004). New fluorescence parameters for the determination of q(a) redox state and excitation energy fluxes. Photosynth. Res. 79: 209–218 [DOI] [PubMed] [Google Scholar]
- Kramer D.M., Sacksteder C.A., Cruz J.A. (1999). How acidic is the lumen? Photosynth. Res. 60: 151–163 [Google Scholar]
- Kudoh H., Sonoike K. (2002). Irreversible damage to photosystem I by chilling in the light: Cause of the degradation of chlorophyll after returning to normal growth temperature. Planta 215: 541–548 [DOI] [PubMed] [Google Scholar]
- Külheim C., Ågren J., Jansson S. (2002). Rapid regulation of light harvesting and plant fitness in the field. Science 297: 91–93 [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. (1951). Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193: 265–275 [PubMed] [Google Scholar]
- Majeran W., Zybailov B., Ytterberg A.J., Dunsmore J., Sun Q., van Wijk K.J. (2008). Consequences of C4 differentiation for chloroplast membrane proteomes in maize mesophyll and bundle sheath cells. Mol. Cell. Proteomics 7: 1609–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller P., Li X.P., Niyogi K.K. (2001). Non-photochemical quenching. A response to excess light energy. Plant Physiol. 125: 1558–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munekage Y., Hashimoto M., Miyake C., Tomizawa K., Endo T., Tasaka M., Shikanai T. (2004). Cyclic electron flow around photosystem I is essential for photosynthesis. Nature 429: 579–582 [DOI] [PubMed] [Google Scholar]
- Munekage Y., Hojo M., Meurer J., Endo T., Tasaka M., Shikanai T. (2002). PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell 110: 361–371 [DOI] [PubMed] [Google Scholar]
- Munekage Y.N., Genty B., Peltier G. (2008). Effect of PGR5 impairment on photosynthesis and growth in Arabidopsis thaliana. Plant Cell Physiol. 49: 1688–1698 [DOI] [PubMed] [Google Scholar]
- Murashige T., Skoog F. (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 15: 473–497 [Google Scholar]
- Nandha B., Finazzi G., Joliot P., Hald S., Johnson G.N. (2007). The role of PGR5 in the redox poising of photosynthetic electron transport. Biochim. Biophys. Acta 1767: 1252–1259 [DOI] [PubMed] [Google Scholar]
- Okegawa Y., Kobayashi Y., Shikanai T. (2010). Physiological links among alternative electron transport pathways that reduce and oxidize plastoquinone in Arabidopsis. Plant J. 63: 458–468 [DOI] [PubMed] [Google Scholar]
- Peng L., Yamamoto H., Shikanai T. (2011). Structure and biogenesis of the chloroplast NAD(P)H dehydrogenase complex. Biochim. Biophys. Acta 1807: 945–953 [DOI] [PubMed] [Google Scholar]
- Pesaresi P., Hertle A., Pribil M., Kleine T., Wagner R., Strissel H., Ihnatowicz A., Bonardi V., Scharfenberg M., Schneider A., Pfannschmidt T., Leister D. (2009). Arabidopsis STN7 kinase provides a link between short- and long-term photosynthetic acclimation. Plant Cell 21: 2402–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfannschmidt T. (2003). Chloroplast redox signals: How photosynthesis controls its own genes. Trends Plant Sci. 8: 33–41 [DOI] [PubMed] [Google Scholar]
- Porra R.J., Thompson W.A., Kriedemann P.E. (1989). Determination of accurate extinction coefficients and simultaneous-equations for assaying chlorophyll-a and chlorophyll-b extracted with 4 different solvents - Verification of the concentration of chlorophyll standards by atomic-absorption spectroscopy. Biochim. Biophys. Acta 975: 384–394 [Google Scholar]
- Reiland S., Finazzi G., Endler A., Willig A., Baerenfaller K., Grossmann J., Gerrits B., Rutishauser D., Gruissem W., Rochaix J.D., Baginsky S. (2011). Comparative phosphoproteome profiling reveals a function of the STN8 kinase in fine-tuning of cyclic electron flow (CEF). Proc. Natl. Acad. Sci. USA 108: 12955–12960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rintamäki E., Martinsuo P., Pursiheimo S., Aro E.M. (2000). Cooperative regulation of light-harvesting complex II phosphorylation via the plastoquinol and ferredoxin-thioredoxin system in chloroplasts. Proc. Natl. Acad. Sci. USA 97: 11644–11649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rott M., Martins N.F., Thiele W., Lein W., Bock R., Kramer D.M., Schöttler M.A. (2011). ATP synthase repression in tobacco restricts photosynthetic electron transport, CO2 assimilation, and plant growth by overacidification of the thylakoid lumen. Plant Cell 23: 304–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumberg B., Siggel U. (1969). pH changes in the inner phase of the thylakoids during photosynthesis. Naturwissenschaften 56: 130–132 [DOI] [PubMed] [Google Scholar]
- Rumeau D., Bécuwe-Linka N., Beyly A., Louwagie M., Garin J., Peltier G. (2005). New subunits NDH-M, -N, and -O, encoded by nuclear genes, are essential for plastid Ndh complex functioning in higher plants. Plant Cell 17: 219–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumeau D., Peltier G., Cournac L. (2007). Chlororespiration and cyclic electron flow around PSI during photosynthesis and plant stress response. Plant Cell Environ. 30: 1041–1051 [DOI] [PubMed] [Google Scholar]
- Scheller H.V., Haldrup A. (2005). Photoinhibition of photosystem I. Planta 221: 5–8 [DOI] [PubMed] [Google Scholar]
- Schöttler M.A., Albus C.A., Bock R. (2011). Photosystem I: Its biogenesis and function in higher plants. J. Plant Physiol. 168: 1452–1461 [DOI] [PubMed] [Google Scholar]
- Shikanai T. (2007). Cyclic electron transport around photosystem I: Genetic approaches. Annu. Rev. Plant Biol. 58: 199–217 [DOI] [PubMed] [Google Scholar]
- Shinopoulos K.E., Brudvig G.W. (2012). Cytochrome b₅₅₉ and cyclic electron transfer within photosystem II. Biochim. Biophys. Acta 1817: 66–75 [DOI] [PubMed] [Google Scholar]
- Sonoike K. (2011). Photoinhibition of photosystem I. Physiol. Plant. 142: 56–64 [DOI] [PubMed] [Google Scholar]
- Sonoike K., Terashima I. (1994). Mechanism of photosystem-i photoinhibition in leaves of Cucumis sativus L. Planta 194: 287–293 [Google Scholar]
- Sonoike K., Terashima I., Iwaki M., Itoh S. (1995). Destruction of photosystem I iron-sulfur centers in leaves of Cucumis sativus L. by weak illumination at chilling temperatures. FEBS Lett. 362: 235–238 [DOI] [PubMed] [Google Scholar]
- Suorsa M., Regel R.E., Paakkarinen V., Battchikova N., Herrmann R.G., Aro E.M. (2004). Protein assembly of photosystem II and accumulation of subcomplexes in the absence of low molecular mass subunits PsbL and PsbJ. Eur. J. Biochem. 271: 96–107 [DOI] [PubMed] [Google Scholar]
- Takizawa K., Cruz J.A., Kanazawa A., Kramer D.M. (2007). The thylakoid proton motive force in vivo. Quantitative, non-invasive probes, energetics, and regulatory consequences of light-induced pmf. Biochim. Biophys. Acta 1767: 1233–1244 [DOI] [PubMed] [Google Scholar]
- Theg S.M., Tom C. (2011). Measurement of the ΔpH and electric field developed across Arabidopsis thylakoids in the light. Methods Mol. Biol. 775: 327–341 [DOI] [PubMed] [Google Scholar]
- Thordal-Christensen H., Zhang Z., Wei Y., Collinge D.B. (1997). Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley powdery mildew interaction. Plant J. 11: 1187–1194 [Google Scholar]
- Tikkanen M., Aro E.M. (2012). Thylakoid protein phosphorylation in dynamic regulation of photosystem II in higher plants. Biochim. Biophys. Acta 1817: 232–238 [DOI] [PubMed] [Google Scholar]
- Tikkanen M., Grieco M., Aro E.M. (2011). Novel insights into plant light-harvesting complex II phosphorylation and ‘state transitions’. Trends Plant Sci. 16: 126–131 [DOI] [PubMed] [Google Scholar]
- Tikkanen M., Grieco M., Kangasjärvi S., Aro E.M. (2010). Thylakoid protein phosphorylation in higher plant chloroplasts optimizes electron transfer under fluctuating light. Plant Physiol. 152: 723–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta A., et al. (2011). Regulatory subunit B’gamma of protein phosphatase 2A prevents unnecessary defense reactions under low light in Arabidopsis. Plant Physiol. 156: 1464–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuyama M., Kobayashi Y. (2009). Reduction of the primary donor P700 of photosystem I during steady-state photosynthesis under low light in Arabidopsis. Photosynth. Res. 99: 37–47 [DOI] [PubMed] [Google Scholar]
- Wagner R., Dietzel L., Bräutigam K., Fischer W., Pfannschmidt T. (2008). The long-term response to fluctuating light quality is an important and distinct light acclimation mechanism that supports survival of Arabidopsis thaliana under low light conditions. Planta 228: 573–587 [DOI] [PubMed] [Google Scholar]
- Woo N., Gordon M., Graham S., Rossel J., Badger M.R., Pogson B.J. (2011). A mutation in the purine biosynthetic enzyme ATASE2 impacts high light signalling and acclimation responses in green and chlorotic sectors of Arabidopsis leaves. Funct. Plant Biol. 38: 401–419 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.