Abstract
Previous data have suggested that insulin-resistant skeletal muscle may exhibit a diminished ability to undergo hypertrophy and that this result may be mediated, at least in part, from decrements in mammalian target of rapamycin (mTOR) signaling (Katta A, Kundla S, Kakarla SK, Wu M, Fannin J, Paturi S, Liu H, Addagarla HS, Blough ER. Am J Physiol Regul Integr Comp Physiol 299: R1666–R1675, 2010). Herein, we attempt to extend these observations by determining if this attenuation in muscle growth is associated with alterations in AMP-activated protein kinase (AMPK) signaling, an upstream mediator of mTOR, and changes in the activation of dsRNA-dependent protein kinase (PKR), which functions as an inhibitor of protein synthesis and potential mediator of protein degradation. Compared with that observed in lean Zucker (LZ) rats, the phosphorylation of AMPKα at Thr172 was higher after 3 wk of overload in the insulin-resistant obese Zucker (OZ) soleus (P < 0.05). This change in AMPKα phosphorylation was accompanied by increases in the amount of phosphorylated PKR (Thr446), elevations in the PKR-dependent phosphorylation of eukaryotic initiation factor (eIF)-2α (Ser51), augmented p38 MAP kinase (Thr180/Tyr182) phosphorylation, and increases in the amount of protein ubiquitination (P < 0.05). Taken together, these results suggest that the diminished hypertrophic response we observe in the OZ rat may be mediated, at least in part, by the hyperactivation of AMPK- and PKR-related signaling.
Keywords: obese Zucker rat, skeletal muscle, hypertrophy
the obese Zucker (fa/fa) rat (OZ) is commonly used as an animal model for the investigation of metabolic syndrome given its proclivity to exhibit severe skeletal muscle insulin resistance, hyperglycemia, and hyperlipidemia (40). Previous work from our laboratory using the OZ rat has demonstrated that insulin resistance is associated with a diminished ability of skeletal muscle to undergo hypertrophy that may be caused, at least in part, by decrements in activation of the mammalian target of rapamycin (mTOR) (19, 23). Why insulin resistance may be associated with differences in the regulation of mTOR signaling is currently unclear.
Recent studies have reported that the 5′-AMP-activated protein kinase (AMPK) may function as a master sensor of cellular energy balance and as a negative regulator of protein synthesis (3, 22). The AMPK is a heterotrimeric complex consisting of one catalytic subunit (α) and two noncatalytic regulatory (β and γ) subunits, whose activation is controlled through phosphorylation of the α subunit at Thr172 (34). It has been shown that AMPK may inhibit protein synthesis through its ability to suppress mTOR activation (2–4, 24, 37). The factors controlling the activity of AMPK have not been fully elucidated; however, it is thought that protein tumor suppressor LKB1 kinase (28, 29, 36), transforming growth factor-β-activated kinase-1 (TAK1), a member of the mitogen-activated protein (MAP) kinase family (20, 38), and Ca2+/calmodulin-dependent protein kinase kinase (CaMKKβ) (30, 39) may be involved.
In addition to AMPK signaling, other recent in vitro and in vivo studies have suggested that the dsRNA-dependent protein kinase (PKR) can also play a role in the inhibition of protein synthesis (7, 10, 25, 26). PKR when activated phosphorylates eukaryotic initiation factor (eIF)-2α at Ser-51 (7), which in turn leads to translational inhibition and presumably a decrease in protein synthesis (15). In addition to its potential effect on protein synthesis, PKR may also have a hand in controlling the ubiquitin proteolytic pathway through the activation of p38 MAP kinase (8, 27). How insulin resistance or its associated comorbidities may affect the regulation of PKR during muscle hypertrophy has to our knowledge not been investigated. Using the same animals and tissues used in our previous work (19), we hypothesized that the attenuated hypertrophic response we observe in the OZ soleus after increased mechanical loading is associated with alterations in the regulation of the AMPK and PKR.
MATERIALS AND METHODS
Materials.
Primary antibodies against AMPKα (no. 2532), AMPKα1 (no. 2795), AMPKα2 (no. 2757), AMPKβ1/2 (no. 150), phospho-AMPKα (Thr172) (no. 2535), LKB1 (no. 3050), phospho-LKB1 (Ser428) (no. 3482), TAK1 (no. 4505), phospho-TAK1 (Thr184/187) (no. 4531), eIF2α (no. 9722), phospho-eIF2α (Ser51) (no. 3597), glycogen synthase kinase (GSK)-3β (no. 9315), phospho-GSK-3β (Ser9) (no. 9336), p38 MAP kinase (no. 9212), phospho-p38 MAP kinase (Thr180/Tyr182) (no. 9216), ubiquitin (no. 3936), glyceraldehyde 3-phosphate dehydrogenase (GAPDH, no. 2118), and secondary antibodies conjugated with horseradish peroxidase (HRP) [anti-rabbit (no. 7074) or anti-mouse (no. 7076)] were purchased from Cell Signaling Technology (Beverly, MA). PKR (sc-708), phospho-PKR (Thr446) (sc-101783), and CamKK beta (sc-50341) were from Santa Cruz Biotechnology (Santa Cruz, CA). The Laemmli 2× sample buffer was from Sigma-Aldrich (St. Louis, MO). Pierce Tissue Protein Extraction Reagent (T-PER), Pierce 660nm protein assay reagent (no. 22660), GE Healthcare Amersham ECL Western Blotting Detection Reagents (RPN2106), and Advance Western Blotting Detection Kit (RPN2135) were from Thermo Fisher Scientific (Rockford, IL). The PAGEr Gold Precast gels (10%) were from purchased from Lonza (Rockland, ME).
Animal care.
All procedures were performed as outlined in the “Guide for the Care and Use of Laboratory Animals” as approved by the Council of the American Physiological Society and the institutional animal use review board of Marshall University. The animals and tissues used in this study have been previously examined (19). Young male LZ (n = 12) and OZ (n = 12) rats were obtained from the Charles River Laboratories. All animals were 12 wk of age at completion of this study. Rats were housed two per cage in an AAALAC-approved vivarium. Housing conditions consisted of a 12:12-h dark-light cycle, and the temperature was maintained at 22° ± 2°C. Animals were provided food and water ad libitum and allowed to recover from shipment for at least 2 wk before experimentation. During this time the animals were carefully observed and weighed weekly to ensure none exhibited signs of failure to thrive, such as precipitous weight loss, disinterest in the environment, or unexpected gait alterations.
Synergist ablation procedure.
Unilateral overload of the soleus muscle for 1 and 3 wk was achieved through the surgical ablation of the medial and the proximal two-thirds of the lateral head of the gastrocnemius (1). The unilateral ablation model allows within-animal comparisons, thus eliminating bias due to systemic factors. Rats were anesthetized with a ketamine-xylazine (4:1) cocktail (50 mg/kg ip), and the distal two-thirds of the gastrocnemius muscle were surgically removed from the left hindlimb as previously described (1). A sham (control) operation was performed on the right hindlimb. The sham procedure consisted of an incision through the skin, followed by blunt isolation of the Achilles tendon and gastrocnemius muscle prior to closure. Animals were active immediately after recovering from anesthesia and were checked twice daily during the 7-day postoperative period. No signs of infection or other complications were observed postoperatively.
Tissue collection.
Soleus muscles were collected 7 days (n = 6 LZ-7 and n = 6 OZ-7) or 21 days (n = 6 LZ-21 and n = 6 OZ-21) after the synergist ablation procedure. Animals were 12 wk old at the time of tissue collection. Rats were anesthetized with a ketamine-xylazine (4:1) cocktail (50 mg/kg ip) and supplemented as necessary for reflexive response. Soleus muscles from both legs were quickly removed, trimmed of excess connective tissue, weighed on an analytical balance, frozen in liquid nitrogen, and stored at −80°C until further analysis.
Tissue protein extraction.
Muscles were homogenized in a Pierce Tissue Protein Extraction Reagent (T-PER) (10 ml/g tissue; Rockford, IL) that contained protease inhibitors (P8340, Sigma-Aldrich, St. Louis, MO) and phosphatase inhibitors (P5726, Sigma-Aldrich). After incubation on ice for 30 min, the homogenate was collected by centrifuging at 12,000 g for 5 min at 4°C. The protein concentration of homogenates was determined via the Bradford method (Fisher Scientific, Rockford, IL). Homogenate samples were boiled in Laemmli 2× sample buffer (Sigma-Aldrich) for 5 min prior to SDS-PAGE.
SDS-PAGE and immunoblotting.
Forty micrograms of total protein from each sample was separated on a 10% PAGEr Gold Precast gel (Lonza, Rockland, ME) and then transferred to a nitrocellulose membrane. Visual verification of transfer and equal protein loading among lanes was accomplished by Ponceau S staining of the membranes. Immunodetection of antigens was performed as described previously (17, 18). Briefly, membranes were blocked for 1 h at room temperature in blocking buffer [5% nonfat dry milk in TBS-T (20 mM Tris-base, 150 mM NaCl, 0.05% Tween-20), pH 7.6], serially washed in TBS-T at room temperature, then incubated overnight at 4°C in primary antibody buffer (5% BSA in TBS-T, pH 7.6, primary antibody diluted 1:1,000), followed by washing in TBS-T (3 × 5 min each), and incubation with HRP-conjugated secondary antibody [anti-rabbit (no. 7074) or anti-mouse (no. 7076), Cell Signaling Technology, Danvers, MA] in blocking buffer for 1 h. After removal of the secondary antibody, membranes were washed (3 × 5 min each) in TBS-T and protein bands visualized on reaction with ECL reagent (Amersham ECL Western Blotting reagent RPN 2106, GE Healthcare Bio-Sciences, Piscataway, NJ). Target protein levels were quantified by AlphaEaseFC image analysis software (Alpha Innotech, San Leandro, CA) and normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
Statistical analysis.
Results are presented as means ± SE. Data were analyzed using the Sigma Stat 3.5 statistical program. The effects of insulin resistance on protein phosphorylation were analyzed using a two-way ANOVA followed by Student-Newman-Keuls post hoc testing where appropriate. Values of P < 0.05 were considered to be statistically significant.
RESULTS
Diminished hypertrophic response seen in the OZ rat following muscle overload is characterized by hyperphosphorylation of AMP-activated protein kinase (AMPK).
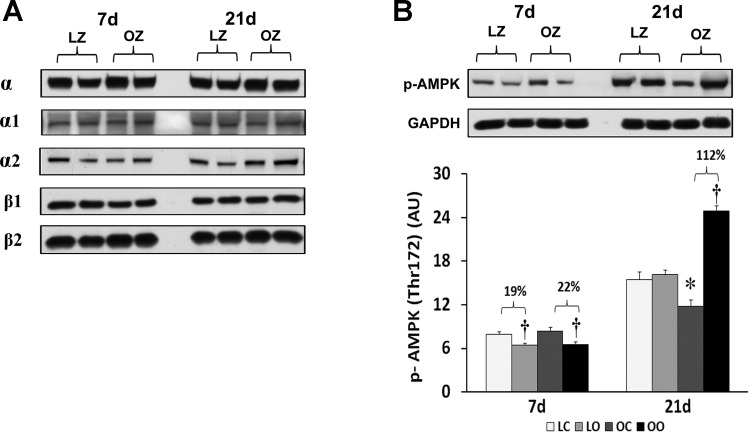
As outlined previously, the OZ rat exhibited a significantly higher body weight than the LZ at both 7 and 14 days of observation (464 ± 12 vs. 302 ± 3 mg at 7 days; P < 0.05; 460 ± 26 vs. 289 ± 10 mg at 21 days; P < 0.05) and soleus muscle wet weights were significantly lower in the OZ compared with the LZ (128 ± 11 vs. 150 ± 6 mg; at 7 days; P < 0.05; 128.0 ± 6.5 vs. 141.0 ± 8.3 mg at 21 days; P < 0.05) (19). The hypertrophic response, as determined by calculating the muscle weight-to-body weight ratio, in the OZ was similar to that observed in the LZ rats at 7 days (LZ hypertrophy 39.9% vs. OZ hypertrophy 35.9%) but was less than that seen in the LZ rats at 21 days (LZ hypertrophy 35.3% vs. OZ hypertrophy 16.6%) following 21 days overload (19). To examine the effect of insulin resistance or associated comorbidities on the regulation of AMPK with overload, we compared the protein content of different AMPK isoforms and phosphorylation of AMPKα at Thr172. Muscle loading did not alter the expression of AMPK isoforms (α1, α2, β1, and β2) in either the LZ or OZ animals (Fig. 1). The phosphorylation of AMPKα at Thr172 was diminished in both LZ and OZ animals after 7 days of increased loading, while it was significantly increased in OZ rats after 21 days of overload (P < 0.05; Fig. 1).
Fig. 1.
AMP-activated protein kinase (AMPK) subunit protein content (A) and immunoblot analysis of the AMPKα at Thr172 in control and overloaded soleus muscles of lean Zucker (LZ) and obese Zucker (OZ) (B). Data are expressed as integrated optical density (IOD) × band area and expressed in arbitrary units relative to the amount of GAPDH; n = 6. †Significantly different from contralateral control muscle; *significantly different from lean control value (P < 0.05). 7d, 7 days of loading; 21d, 21 days of loading; lean control; LO, lean overload; OC, obese control; OO, obese overload.
Effect of overload on different potential upstream regulators of AMPK signaling in the soleus muscle of LZ and OZ rats.
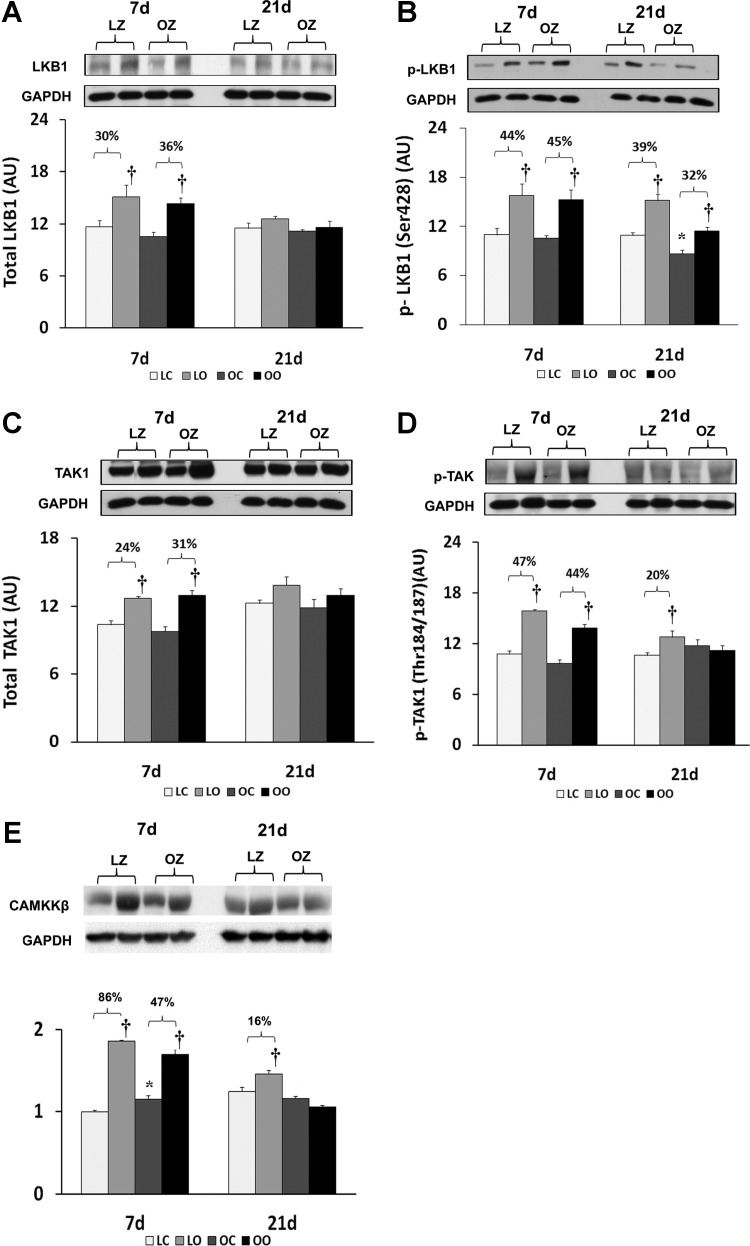
To examine the effect of insulin resistance on the activation of different potential upstream regulators of AMPK with overload, we compared the regulation of LKB1, TAK1, and CaMKK between control and overloaded muscles. The protein expression for LKB1 was increased by muscle overload in the LZ and OZ animals at 7 days and the phosphorylation of LKB1 at Ser428 was increased by muscle overload in both LZ and OZ animals at both 7 and 21 days (P < 0.05; Fig. 2, A and B). The amount of TAK1 protein was increased by muscle overload in the LZ and OZ animals at 7 days and the amount of phosphorylated TAK1 at Thr 184/187 was increased at 7 and 21 days of overload in the LZ rats while it was elevated only after 7 days of overload in the OZ rats (P < 0.05; Fig. 2, C and D). The amount of CaMKKβ protein was increased at 7 and 21 days of overload in the LZ rats and at 7 days of overload in the OZ rats (P < 0.05; Fig. 2E).
Fig. 2.
Immunoblot analysis of the different upstream regulators of AMPK, LKB1 protein content (A) and phosphorylation at Ser428 (B), TAK1 protein content (C) and phosphorylation at Thr286 (D), and CaM kinase kinase β protein content (E) in control and overloaded soleus muscles of LZ and OZ. Data are expressed as IOD × band area and expressed in arbitrary units relative to the amount of GAPDH; n = 6. †Significantly different from contralateral control muscle; *significantly different from lean control value (P < 0.05).
Diminished hypertrophic response seen in the OZ rat with 21 days overload is associated with the activation of PKR-dependent signaling.
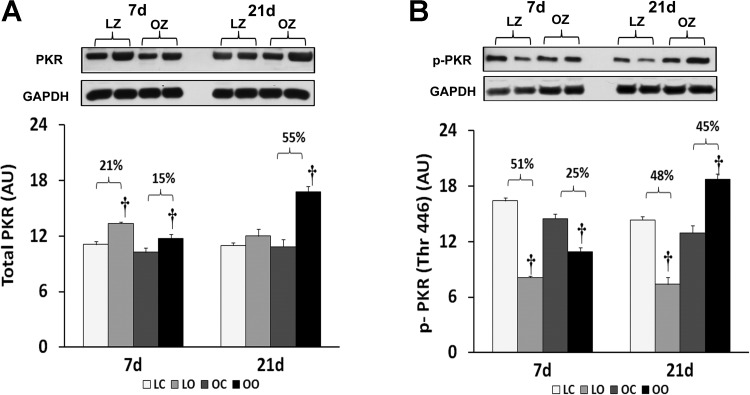
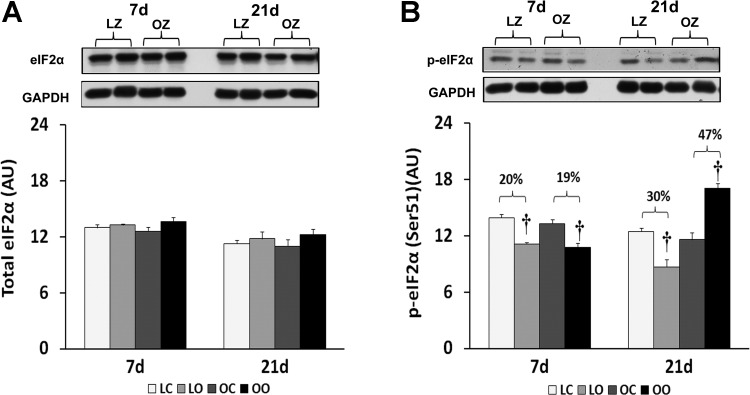
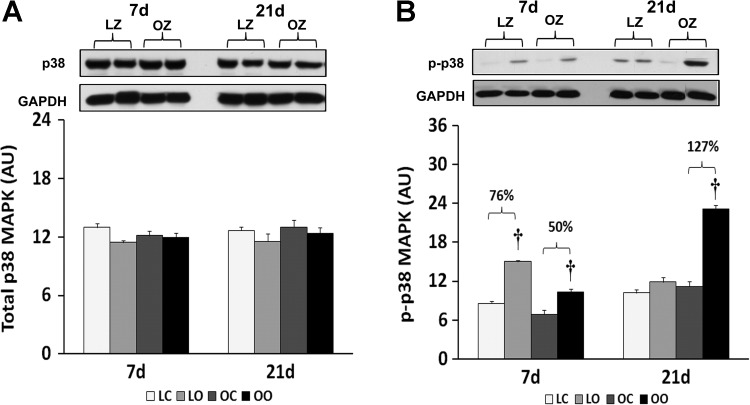
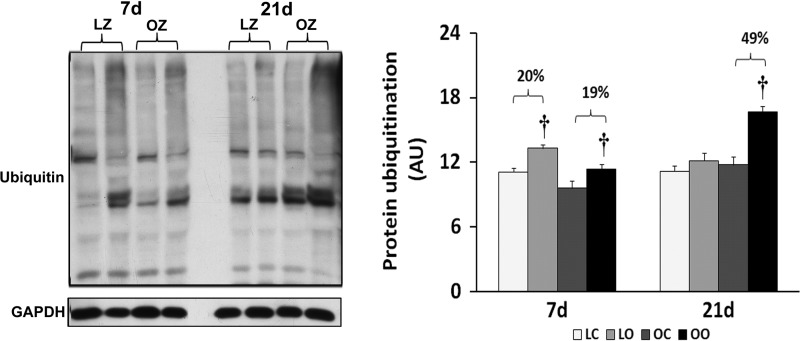
To examine the effect of insulin resistance or associated comorbidities on the activation of PKR-dependent signaling with overload, we compared the protein content and phosphorylation of PKR, eIF2α, and p38 MAP kinase between control and overloaded muscles. The total amount of PKR protein in overloaded muscles was elevated relative to the contralateral control after 7 days of overload in the LZ rats and after 7 and 21 days of overload in the OZ rats (P < 0.05; Fig. 3). Conversely, the phosphorylation of PKR at Thr446 was decreased at both 7 and 21 days of overload in the LZ rats while it was decreased after 7 days of overload and significantly elevated after 21 days overload in the OZ rats (P < 0.05; Fig. 3). Similar to our findings for PKR, the amount of phosphorylated eIF2α at Ser51 was decreased at both 7 and 21 days of overload in the LZ rats while it was decreased after 7 days of overload but significantly elevated after 21 days overload in the OZ rats (P < 0.05; Fig. 4). Muscle loading did not alter the expression of eIF2α and p38 MAP kinase in either the LZ or OZ animals (Figs. 4 and 5). The phosphorylation levels of p38 MAP kinase at Thr180/Tyr182 in overloaded muscles were elevated relative to the contralateral control after 7 days of overload in the LZ rats and after 7 and 21 days of overload in the OZ rats (P < 0.05; Fig. 5). The ubiquitinated protein levels were increased in both LZ and OZ rats after 7 days and significantly elevated only in the OZ rats after 21 days overload (P < 0.05; Fig. 6).
Fig. 3.
Immunoblot analysis of the dsRNA-dependent protein kinase (PKR) protein content (A) and phosphorylation at Thr446 (B) in control and loaded soleus muscles of LZ and OZ. Data are expressed as IOD × band area and expressed in arbitrary units relative to the amount of GAPDH; n = 6. †Significantly different from contralateral control muscle, P < 0.05.
Fig. 4.
Immunoblot analysis of the eukaryotic initiation factor (eIF)-2α protein content (A) and phosphorylation at Ser51 (B) in control and loaded soleus muscles of LZ and OZ. Data are expressed as IOD × band area and expressed in arbitrary units relative to the amount of GAPDH; n = 6. †Significantly different from contralateral control muscle, P < 0.05.
Fig. 5.
Immunoblot analysis of the p38 MAP kinase protein content (A) and phosphorylation at Thr180/Tyr182 (B) in control and loaded soleus muscles of LZ and OZ. Data are expressed as IOD × band area and expressed in arbitrary units relative to the amount of GAPDH; n = 6. †Significantly different from contralateral control muscle, P < 0.05.
Fig. 6.
Immunoblot analysis of the ubiquitinated protein levels in control and loaded soleus muscles of LZ and OZ rats. Data are expressed as IOD × band area and expressed in arbitrary units relative to the amount of GAPDH; n = 6. †Significantly different from contralateral control muscle, P < 0.05.
Effect of overload on regulation of GSK-3β in the soleus muscle of LZ and OZ rats.
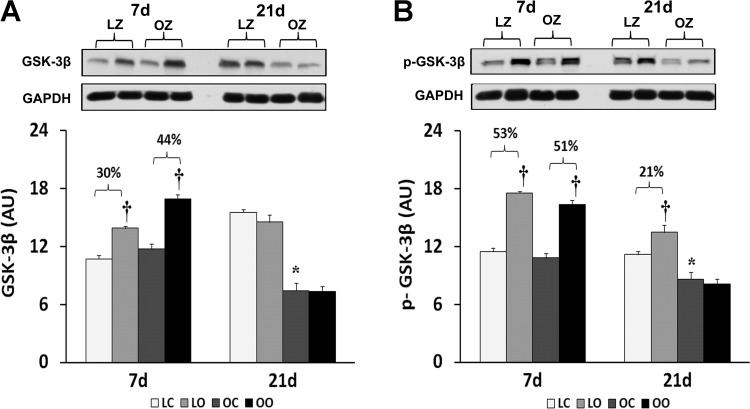
Given the role of GSK-3β in suppression of protein synthesis through its ability to inhibit the translational initiation complex (16), we next examined the regulation of GSK-3β with overload. The muscle content and phosphorylation of GSK-3β on Ser9 was increased by muscle overload in the LZ and OZ animals at 7 days. Conversely, after 21 days of overload the phosphorylation of GSK-3β on Ser9 was elevated only in LZ animals (P < 0.05; Fig. 7).
Fig. 7.
Immunoblot analysis of the glycogen synthase kinase (GSK)-3β protein content (A) and phosphorylation at Ser9 (B) in control and loaded soleus muscles of LZ and OZ. Data are expressed as IOD × band area and expressed in arbitrary units relative to the amount of GAPDH; n = 6. †Significantly different from contralateral control muscle; *significantly different from lean control value (P < 0.05).
DISCUSSION
The phosphorylation of AMPK at Thr172 is thought to lead to an inhibition of protein synthesis and has been found to be negatively correlated with the degree of muscle hypertrophy in the overloaded rat (33). Here we found that the phosphorylation of Thr172 appears to be similar after 1 wk of overload in the LZ and OZ animals but that it is significantly higher in the overloaded OZ rat compared with LZ rat after 3 wk of overload (Fig. 1). These latter data are consistent with our previous report demonstrating a decrease in the ability of the OZ to undergo muscle hypertrophy following overload (23). In addition, because the AMPK is thought to inhibit the activity of mTOR (3, 37), the increased AMPK phosphorylation (activation) observed in the OZ animals may help to explain why mTOR signaling may be decreased in the OZ soleus following muscle overload.
The mechanism(s) responsible for the elevation in AMPK phosphorylation during muscle overload in the OZ rat is currently unknown but does not appear to be related to changes in the amount or phosphorylation of its upstream activators, LKB1 (13, 36), TAK1 (21), or CaMKK (14, 35) (Fig. 2). Specifically, we failed to find any change in the phosphorylation of LKB1 (Ser428) that would be suggestive of an increased ability of LKB1 to activate AMPK (31). Similarly, we failed to find changes in the phosphorylation of TAK1 or in the expression of CaMKK that would be predictive for the increased AMPK phosphorylation we observed in the OZ animals (Fig. 2). Whether the differences in AMPK phosphorylation we see between rat models is due to differences in adenine nucleotide levels (5), alterations in the amount of phosphatase(s) capable of acting upon AMPK, or some other mechanism is currently unclear and requires further investigation.
Recent studies have suggested that the phosphorylation (activation) of PKR may be involved in the depression of protein synthesis, via phosphorylating eIF2α at Ser51, which may reduce the rate of translational initiation, while it may also cause an increase in protein degradation through a p38 MAP kinase-dependent mechanism (7, 11). Here we demonstrate that the level of PKR phosphorylation (activation) appears to be regulated similarly after 1 wk of overload in both LZ and OZ rats but that it is significantly higher in the overloaded OZ after 3 wk of overload (Fig. 3). Whether this difference is PKR activation is directly responsible for the attenuated hypertrophy we see in the OZ soleus is currently unclear and requires further experimentation.
To examine how changes in the amount of activated PKR might influence the activation of molecules thought to be important in regulating protein synthesis, we next examined the phosphorylation of eIF2α. It has been hypothesized that PKR activation is associated with increases in the phosphorylation of eIF2α at Ser51, which in turn results in the inactivation of eIF2α and the inhibition of translational initiation (9). The degree of eIF2α phosphorylation at Ser51 was significantly higher in overloaded OZ rats compared with LZ rats after 3 wk of overload (Fig. 4). In addition to eIF2α, the GSK-3β has also been proposed to negatively regulate cellular growth as the inhibition of this protein by protein phosphorylation is considered an important mechanism of hypertrophy in cardiac tissue (12). Consistent with our findings regarding the attenuated hypertrophic response, we found no increase in phosphorylation of GSK-3β at Ser9 in the OZ rats after 3 wk of overload (Fig. 7).
To further explore the potential effects of PKR activation on protein degradation, we next examined the phosphorylation of p38 MAP kinase. It has been postulated that PKR activation can lead to the phosphorylation of p38 MAP kinase, which, in turn, has been associated with the activation of the ubiquitin-proteasome pathway (6, 25). Consistent with our PKR data, the phosphorylation of p38 MAP kinase (Thr180/Tyr182) was significantly higher in the overloaded OZ rat compared with LZ rat after 3 wk of overload (Fig. 5). To investigate the potential downstream effects of elevated p38 MAP kinase activation, we next measured the degree of protein ubiquitination. Ubiquitin is a conserved polypeptide that when covalently linked to cellular proteins acts to target that protein for degradation by the 26S proteosome (32). As expected, we found that the level of ubiquitination was much higher in OZ muscle compared with LZ muscle after 3 wk overload (Fig. 6).
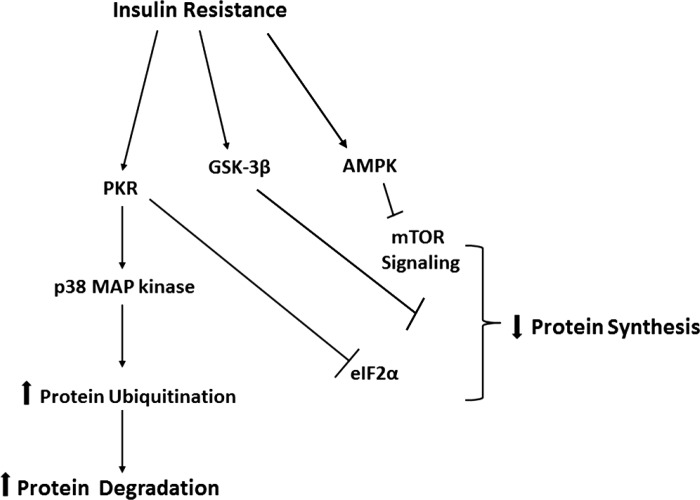
When considered in the context of our previous data showing that the diminished muscle hypertrophy in the OZ rat soleus following increased muscle loading may be associated with decrements in the activation of mTOR (19), the results of the present study suggest that this finding may be due to alterations in the phosphorylation (activation) of AMPK, PKR, eIF2α, and p38 MAP kinase phosphorylation. With the exception of the latter, each of these changes would be predicted to decrease protein synthesis while the increased activation of p38 MAP kinase, and the increase in protein ubiquitination occurring in parallel to this finding, would be thought to lead to increases in the amount of protein degradation (Fig. 8). Although our experimental design precludes the investigation of cause and effect, this study nonetheless provides new insight into why insulin resistance or its associated comorbidities may be associated with differences in the ability of skeletal muscle to adapt following an overload stimulus.
Fig. 8.
Schematic representation of possible mechanism(s) for attenuation of hypertrophy in obese insulin-resistant skeletal muscle.
GRANTS
This study was supported by National Institute on Aging Grant AG-027103-1 to E. R. Blough.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.K. and E.R.B. conception and design of research; A.K., S.K.K., N.D.M., S.K., M.K., and S.K.N. performed experiments; A.K., S.K.K., N.D.M., M.W., S.K., M.K., and S.K.N. analyzed data; A.K., M.W., and E.R.B. interpreted results of experiments; A.K. and N.D.M. prepared figures; A.K. and E.R.B. drafted manuscript; A.K., N.D.M., and E.R.B. edited and revised manuscript; A.K. and E.R.B. approved final version of manuscript.
REFERENCES
- 1. Blough ER, Linderman JK. Lack of skeletal muscle hypertrophy in very aged male Fischer 344 × Brown Norway rats. J Appl Physiol 88: 1265–1270, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3: 1014–1019, 2001 [DOI] [PubMed] [Google Scholar]
- 3. Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem 277: 23977–23980, 2002 [DOI] [PubMed] [Google Scholar]
- 4. Bolster DR, Kubica N, Crozier SJ, Williamson DL, Farrell PA, Kimball SR, Jefferson LS. Immediate response of mammalian target of rapamycin (mTOR)-mediated signalling following acute resistance exercise in rat skeletal muscle. J Physiol 553: 213–220, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carling D, Aguan K, Woods A, Verhoeven AJ, Beri RK, Brennan CH, Sidebottom C, Davison MD, Scott J. Mammalian AMP-activated protein kinase is homologous to yeast and plant protein kinases involved in the regulation of carbon metabolism. J Biol Chem 269: 11442–11448, 1994 [PubMed] [Google Scholar]
- 6. Eley HL, Russell ST, Tisdale MJ. Attenuation of depression of muscle protein synthesis induced by lipopolysaccharide, tumor necrosis factor, and angiotensin II by beta-hydroxy-beta-methylbutyrate. Am J Physiol Endocrinol Metab 295: E1409–E1416, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Eley HL, Russell ST, Tisdale MJ. Mechanism of activation of dsRNA-dependent protein kinase (PKR) in muscle atrophy. Cell Signal 22: 783–790 [DOI] [PubMed] [Google Scholar]
- 8. Eley HL, Russell ST, Tisdale MJ. Mechanism of attenuation of muscle protein degradation induced by tumor necrosis factor-alpha and angiotensin II by beta-hydroxy-beta-methylbutyrate. Am J Physiol Endocrinol Metab 295: E1417–E1426, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Eley HL, Skipworth RJ, Deans DA, Fearon KC, Tisdale MJ. Increased expression of phosphorylated forms of RNA-dependent protein kinase and eukaryotic initiation factor 2alpha may signal skeletal muscle atrophy in weight-losing cancer patients. Br J Cancer 98: 443–449, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eley HL, Tisdale MJ. Skeletal muscle atrophy, a link between depression of protein synthesis and increase in degradation. J Biol Chem 282: 7087–7097, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Gil J, Esteban M. Induction of apoptosis by the dsRNA-dependent protein kinase (PKR): mechanism of action. Apoptosis 5: 107–114, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Haq S, Choukroun G, Kang ZB, Ranu H, Matsui T, Rosenzweig A, Molkentin JD, Alessandrini A, Woodgett J, Hajjar R, Michael A, Force T. Glycogen synthase kinase-3beta is a negative regulator of cardiomyocyte hypertrophy. J Cell Biol 151: 117–130, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol 2: 28, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab 2: 9–19, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Holcik M, Sonenberg N. Translational control in stress and apoptosis. Nat Rev Mol Cell Biol 6: 318–327, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Jefferson LS, Fabian JR, Kimball SR. Glycogen synthase kinase-3 is the predominant insulin-regulated eukaryotic initiation factor 2B kinase in skeletal muscle. Int J Biochem Cell Biol 31: 191–200, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Katta A, Kakarla S, Wu M, Paturi S, Gadde MK, Arvapalli R, Kolli M, Rice KM, Blough ER. Altered regulation of contraction-induced Akt/mTOR/p70S6k pathway signaling in skeletal muscle of the obese Zucker rat. Exp Diabetes Res 2009: 384683, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Katta A, Karkala SK, Wu M, Meduru S, Desai DH, Rice KM, Blough ER. Lean and obese Zucker rats exhibit different patterns of p70s6 kinase regulation in the tibialis anterior muscle in response to high-force muscle contraction. Muscle Nerve 39: 503–511, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Katta A, Kundla S, Kakarla SK, Wu M, Fannin J, Paturi S, Liu H, Addagarla HS, Blough ER. Impaired overload-induced hypertrophy is associated with diminished mTOR signaling in insulin-resistant skeletal muscle of the obese Zucker rat. Am J Physiol Regul Integr Comp Physiol 299: R1666–R1675, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marette A. The AMPK signaling cascade in metabolic regulation: view from the chair. Int J Obes (Lond) 32, Suppl 4: S3–S6, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Momcilovic M, Hong SP, Carlson M. Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J Biol Chem 281: 25336–25343, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Nader GA, Hornberger TA, Esser KA. Translational control: implications for skeletal muscle hypertrophy. Clin Orthop Relat Res S178–S187, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Paturi S, Gutta AK, Kakarla SK, Katta A, Arnold EC, Wu M, Rice KM, Blough ER. Impaired overload-induced hypertrophy in Obese Zucker rat slow-twitch skeletal muscle. J Appl Physiol 108: 7–13, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Reynolds THt, Bodine SC, Lawrence JC., Jr Control of Ser2448 phosphorylation in the mammalian target of rapamycin by insulin and skeletal muscle load. J Biol Chem 277: 17657–17662, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Russell ST, Rajani S, Dhadda RS, Tisdale MJ. Mechanism of induction of muscle protein loss by hyperglycaemia. Exp Cell Res 315: 16–25, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Russell ST, Siren PM, Siren MJ, Tisdale MJ. Mechanism of attenuation of protein loss in murine C2C12 myotubes by d-myo-inositol 1,2,6-triphosphate. Exp Cell Res 316: 286–295 [DOI] [PubMed] [Google Scholar]
- 27. Russell ST, Tisdale MJ. Mechanism of attenuation by beta-hydroxy-beta-methylbutyrate of muscle protein degradation induced by lipopolysaccharide. Mol Cell Biochem 330: 171–179, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Sakamoto K, McCarthy A, Smith D, Green KA, Grahame Hardie D, Ashworth A, Alessi DR. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J 24: 1810–1820, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci USA 101: 3329–3335, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shen QW, Zhu MJ, Tong J, Ren J, Du M. Ca2+/calmodulin-dependent protein kinase kinase is involved in AMP-activated protein kinase activation by alpha-lipoic acid in C2C12 myotubes. Am J Physiol Cell Physiol 293: C1395–C1403, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Song P, Xie Z, Wu Y, Xu J, Dong Y, Zou MH. Protein kinase Czeta-dependent LKB1 serine 428 phosphorylation increases LKB1 nucleus export and apoptosis in endothelial cells. J Biol Chem 283: 12446–12455, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32. Su V, Lau AF. Ubiquitin-like and ubiquitin-associated domain proteins: significance in proteasomal degradation. Cell Mol Life Sci 66: 2819–2833, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thomson DM, Gordon SE. Diminished overload-induced hypertrophy in aged fast-twitch skeletal muscle is associated with AMPK hyperphosphorylation. J Appl Physiol 98: 557–564, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Witczak CA, Sharoff CG, Goodyear LJ. AMP-activated protein kinase in skeletal muscle: from structure and localization to its role as a master regulator of cellular metabolism. Cell Mol Life Sci 65: 3737–3755, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab 2: 21–33, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol 13: 2004–2008, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell 124: 471–484, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Xie M, Zhang D, Dyck JR, Li Y, Zhang H, Morishima M, Mann DL, Taffet GE, Baldini A, Khoury DS, Schneider MD. A pivotal role for endogenous TGF-beta-activated kinase-1 in the LKB1/AMP-activated protein kinase energy-sensor pathway. Proc Natl Acad Sci USA 103: 17378–17383, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yamauchi M, Kambe F, Cao X, Lu X, Kozaki Y, Oiso Y, Seo H. Thyroid hormone activates adenosine 5′-monophosphate-activated protein kinase via intracellular calcium mobilization and activation of calcium/calmodulin-dependent protein kinase kinase-beta. Mol Endocrinol 22: 893–903, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zucker LM. Fat mobilization in vitro and in vivo in the genetically obese Zucker rat “fatty”. J Lipid Res 13: 234–243, 1972 [PubMed] [Google Scholar]