Abstract
Most aspects of human physiology and behavior exhibit 24-h rhythms driven by a master circadian clock in the brain, which synchronizes peripheral clocks. Lung function and ventilation are subject to circadian regulation and exhibit circadian oscillations. Sleep disruption, which causes circadian disruption, is common in those with chronic lung disease, and in the general population; however, little is known about the effect on the lung of circadian disruption. We tested the hypothesis circadian disruption alters expression of clock genes in the lung and that this is associated with altered lung mechanics. Female and male mice were maintained on a 12:12-h light/dark cycle (control) or exposed for 4 wk to a shifting light regimen mimicking chronic jet lag (CJL). Airway resistance (Rn), tissue damping (G), and tissue elastance (H) did not differ between control and CJL females. Rn at positive end-expiratory pressure (PEEP) of 2 and 3 cmH2O was lower in CJL males compared with controls. G, H, and G/H did not differ between CJL and control males. Among CJL females, expression of clock genes, Bmal1 and Rev-erb alpha, was decreased; expression of their repressors, Per2 and Cry 2, was increased. Among CJL males, expression of Clock was decreased; Per 2 and Rev-erb alpha expression was increased. We conclude circadian disruption alters lung mechanics and clock gene expression and does so in a sexually dimorphic manner.
Keywords: clock, corticosterone, impedance, jet lag, body weight
the lung, as most organs, is subject to circadian regulation (53). Lung functional residual capacity (52), lung resistance (16), and peak expiratory flow (29) exhibit circadian oscillations. Individuals with asthma (23), chronic obstructive pulmonary disease (COPD) (1), and sleep apnea (7) experience circadian oscillation of symptoms. More than 75% of people with COPD report nighttime symptoms, including sleep disturbances (45), which cause circadian disruption (42). As our societal life and work styles have changed, disruption of our environmental clock has become more common and is thought to be a contributing factor to many diseases, including depression (55), cancer (50), coronary heart disease (27), metabolic syndrome (15), and to higher overall mortality (36).
Circadian rhythms are driven by a master clock in the suprachiasmatic nucleus (SCN) that synchronizes numerous subsidiary clocks in peripheral tissues (4). In both locations, central and peripheral, circadian clocks comprise interlocking transcriptional and translational feedback loops, which culminate in the rhythmic expression and activity of a set of core clock genes in each organ. In mammals, CLOCK and BMAL1 drive the rhythmic expression of three Period genes and two cryptochrome genes. In turn, their protein products, PER and CRY, translocate back to the nucleus, where they downregulate the transcriptional activity of their own inducers, Bmal1 and Clock. An additional loop includes REV-ERBα, which regulates Bmal1 and Clock transcription (44). Then, this molecular clock regulates downstream clock-controlled genes controlling cell function. The lung has its own clock; Clara cells express PER2 and CLOCK and are responsible for local circadian rhythmicity (17).
The master clock in the SCN through its anatomical connection with the preautonomic motor neurons in the paraventricular nucleus of the hypothalamus transmits signals to the parasympathetic and sympathetic nervous system enforcing its endogenous rhythmicity on the rest of the body (6). The lung is innervated by both the sympathetic and parasympathetic nervous system. Besides its direct effect on bronchial smooth muscle cells, the autonomous nervous system also affects lung inflammation (25), and submucosal bronchial gland secretion (22), which, if dysregulation occur, can alter the lung (22).
Moreover, many facets of the immune system are also subject to circadian regulation; in rodents and humans, various immune cell populations and cytokines have a circadian rhythm (8, 28), and macrophages exhibit robust circadian regulation in gene expression (33). Susceptibility to a lethal dose of bacterial endotoxin exhibits a diurnal rhythm (39) that is increased by circadian disruption (8). Circadian disruption alters the immune system (9, 38), and dysregulation of the immune system occurs in common chronic lung diseases such as asthma, COPD, and fibroproliferative lung diseases (30, 46).
When mice are exposed to a shift of the light-dark pattern, their circadian changes of physical activity are indicators their central circadian clock has been disrupted (11). Because changes of autonomous nervous signaling and the innate immune system could alter the lung, we tested the hypothesis that circadian disruption would affect lung mechanical function and clock gene expression in the lung.
MATERIALS AND METHODS
Animals.
Experiments were performed on 2- to 3-mo-old C57BL6J female and male mice (Jackson Laboratory, Bar Harbor, ME). Mice were housed in the Department of Comparative Medicine (Georgetown University) at an ambient temperature of 20–26°C, four or five per cage, with overhead fluorescent lights of an intensity ranging from 200 to 400 lx at the level of the top of the cage (31). They were maintained on a 12:12-h light/dark (LD12:12) light schedule and allowed Rodent Chow 5001 and tap water ad libitum. All procedures were approved by the Georgetown University Animal Care and Use Committee and comply with the National Institutes of Health guidelines.
Chronic jet lag.
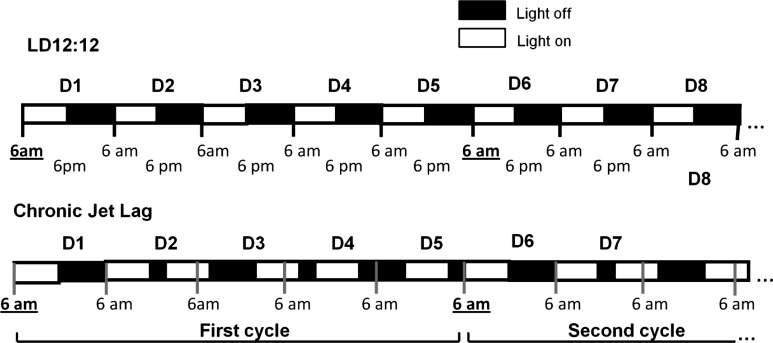
The mice were synchronized to standard lighting conditions of LD12:12, i.e., lights on at 6 AM and lights off at 6 PM, until they entered the study. Zeitgeber time 0 (ZT 0) was defined as the clock time the light was turned on (6 AM). Then mice were assigned to either remain in this lighting regimen or to undergo experimental chronic jet lag (CJL). To produce chronic jet lag we subjected mice to serial 8-h advances of the light/dark cycle every 2 days for 4 wk (Fig. 1) (11, 12). Every day of “jet lag” was preceded and followed by a 12-h light, 12-h dark day. Using this protocol, ZT recurs at the same clock time on the first day of the 5-day cycle in both groups. To avoid the direct effect of light on lung function, corticosterone concentration, and gene expression, mice were placed in darkness during the third day of the fourth cycle (the light was not turned on at the beginning of the third day) and studied on the first day of the next cycle. This continuous darkness for 2 days eliminates any masking effect of light on the endogenous circadian pattern (40). Because all the parameters we studied are subject to circadian oscillation, we studied all animals on the first day of a 5-day cycle; on that day of the chronic jet lag schedule, controls and CJL animals are at the same clock time; both groups had the light turned on at 6 AM, which means they were living on the same Zeitgeber time (time since the light had been turned on). All animals were anesthetized on the first day of the fifth cycle between ZT 3 and ZT 3:30 to obtain blood for corticosterone measurements and to obtain lung tissue for measurement of gene expression. Lung mechanics were measured between ZT 5 and ZT 8 in a second group of animals. Each mouse was weighed at the beginning of the study, then weekly.
Fig. 1.
Chronic jet lag lighting schedule. Mice from the control group remained in standard lighting conditions of 12:12-h light/dark (LD12:12), with light on (empty square) from 6 AM (Zeitgeber time 0) to 6 PM (Zeitgeber time 12). Zeitgeber time 0 is defined at the clock time the light was turned on (6 AM). Chronic jet lag consisted of serial 8-h advances of light-dark cycle every two days for 4 wk. This protocol is set so that, in both groups, ZT recurs at the same time clock on the first day of a 5-day cycle.
Animal preparation.
After having subjected them to 4 wk of either a 12:12-h light/dark cycle or the light-dark regimen to induce CJL, mice were anesthetized by the intraperitoneal injection of xylazine (∼15 mg/kg) and ketamine (∼50 mg/kg), under red light conditions (10 lx) to avoid bright-light exposure, which could induce clock gene expression. After achieving a surgical level of anesthesia (failure to withdraw from a toe pinch), we performed a tracheotomy, inserted an 18-gauge metal cannula into the trachea, and secured it with thread. The mice were then placed in a supine position on a heating mat and connected via the tracheal cannula to a computer-controlled small animal ventilator (FlexiVent, Scireq Montréal, Quebec, Canada). During the time on the ventilator, including measurements of lung mechanics, the level of anesthesia was checked (loss of pedal withdrawal reflex) and additional anesthetic [xylazine (5 mg/kg) plus ketamine (20 mg/kg)] was given, if needed, to maintain loss of pedal withdrawal reflex and prevent spontaneous breathing. Mice were ventilated at 150 breaths/min with room air at a tidal volume (Vt) of 10 ml/kg body mass at a positive end-expiratory pressure (PEEP) of ∼2.5 cmH2O.
Measurements of lung mechanical properties.
Measurements of impedance were performed using the forced oscillation technique provided by the FlexiVent (Scireq Montréal) against three different levels of PEEP (0–3 cmH2O) applied in the following order: 3, 2, and 0 cm H2O to map the lung volume dependence of the lung mechanical parameters (20, 21). Measurement of lung mechanics began with 30 s of mechanical ventilation (Vt 10 ml/kg, 150 breaths/min) at the PEEP level to be studied. Volume history was then standardized by inflating the lung, three times to a pressure at the airway opening of 25 cmH2O. Fifteen seconds after these lung inflations, ventilation was suspended, and the animals were allowed to expire passively to the functional residual capacity at the applied PEEP. An 8-s volume perturbation was then applied to the airway opening by the Flexivent, after which Vt was immediately resumed. The Flexivent software provides a computer-generated volume signal made up of 19 mutually primed sinusoids ranging from 0.25 to 19.5 Hz that was applied to the airway opening. The software fits a four-parameter model to the impedance data to obtain airway resistance (Rn), inertance (I), and the constant-phase parameters of tissue damping (G) and tissue elastance (H) (26). Rn indicates the resistance of the conducting airways. G and H reflect energy dissipation and energy stored, respectively, within the respiratory tissues. Tissue hysteresivity (eta) was calculated as G/H (13). The FlexiVent software corrects the values of Rn and I for the tracheal cannula resistance and inertance. Because most of the inertance is residing in the cannula, the value of I was negligible and not reported. After recording the impedance measurements, ventilation was stopped and we waited for spontaneous ventilation to resume thereby demonstrating the mice were alive throughout the experiment; results from mice that did not resume spontaneous breathing are not included in the data presented in this report.
Measurement of lung volume.
After measurement of lung mechanics, lungs were fixed at a transpulmonary pressure of 20 cmH2O. This was achieved by instilling formalin through the canula already in place. The trachea was then ligated, the lungs excised from the chest, and fixation was continued overnight at room temperature. Lung volume was measured by volume displacement (49).
Reverse transcription-PCR.
Mice never mechanically ventilated were anesthetized with the intraperitoneal injection of xylazine (∼10 mg/kg) plus ketamine (∼75 mg/kg). We injected 2,000 units of heparin intraperitoneally at the time we injected the anesthetic to prevent blood from clotting in pulmonary vessels. We excised the left atrium and perfused the pulmonary vasculature with ice-cold phosphate buffered 0.15 M saline. Then the lungs were excised, snap-frozen in liquid nitrogen, and stored at −80°C. Total RNA was extracted using TRIzol (Invitrogen) and further purified using RNeasy (Qiagen). The RNA concentration of each sample was determined from the absorbance at 260 nm, and 0.125 mg was reverse transcribed to cDNA using the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA).
Quantitative PCR was used to measure the relative amount of mRNA for Clock, Bmal1, Per1, Per2, Cry2, and Rev-erbα. Following the reverse-transcription step, cDNA samples were amplified in the ABI prism 7300 sequence detection system (Applied Biosystems) in triplicate, using TaqMan Gene expression assays and Master mix (Applied Biosystems). Levels of gene expression in each sample were determined with the comparative C(T) method (51). The expression of the different clock-related gene transcripts was normalized to 18S, which we used as an endogenous control. The amplification conditions were as follows: 50°C for 2 min, 95°C for 10 min, 40 cycles at 95°C for 0.5 min, and 60°C for 1 min.
Plasma corticosterone.
Before excising the lungs, blood was drawn from mice never mechanically ventilated and immediately centrifuged at 9,000 g at 4°C for 10 min. Plasma was stored at −80°C. Corticosterone concentration was determined by a HPLC-tandem mass spectrometry (MS/MS) (24).
Statistical analysis.
All statistics were analyzed using GraphPad Prism version 5.04 for Windows (GraphPad Software, San Diego, CA). For each parameter measured or calculated from measurements, the values for individual animals were averaged per experimental group and the standard deviation of the group mean was calculated. The statistical significance of the difference between control and CJL mice was obtained by an unpaired two-tailed t-test (i.e., each parameter of respiratory mechanics at each PEEP level and each clock gene expression). A repeated-measures ANOVA was used for the analysis of temporal changes in weight in all mice. An unpaired two-tailed t-test analysis was used for comparisons of the weekly weight gain between control and CJL mice. For comparisons of one measurement, to more than one other measurement, the Bonferroni adjustment was used to determine statistical significance. We used the Dixon's Q-test to detect outliers (48) (a single outlier, a Rev-erb α male, was not used).
RESULTS
Weight changes of male and female mice.
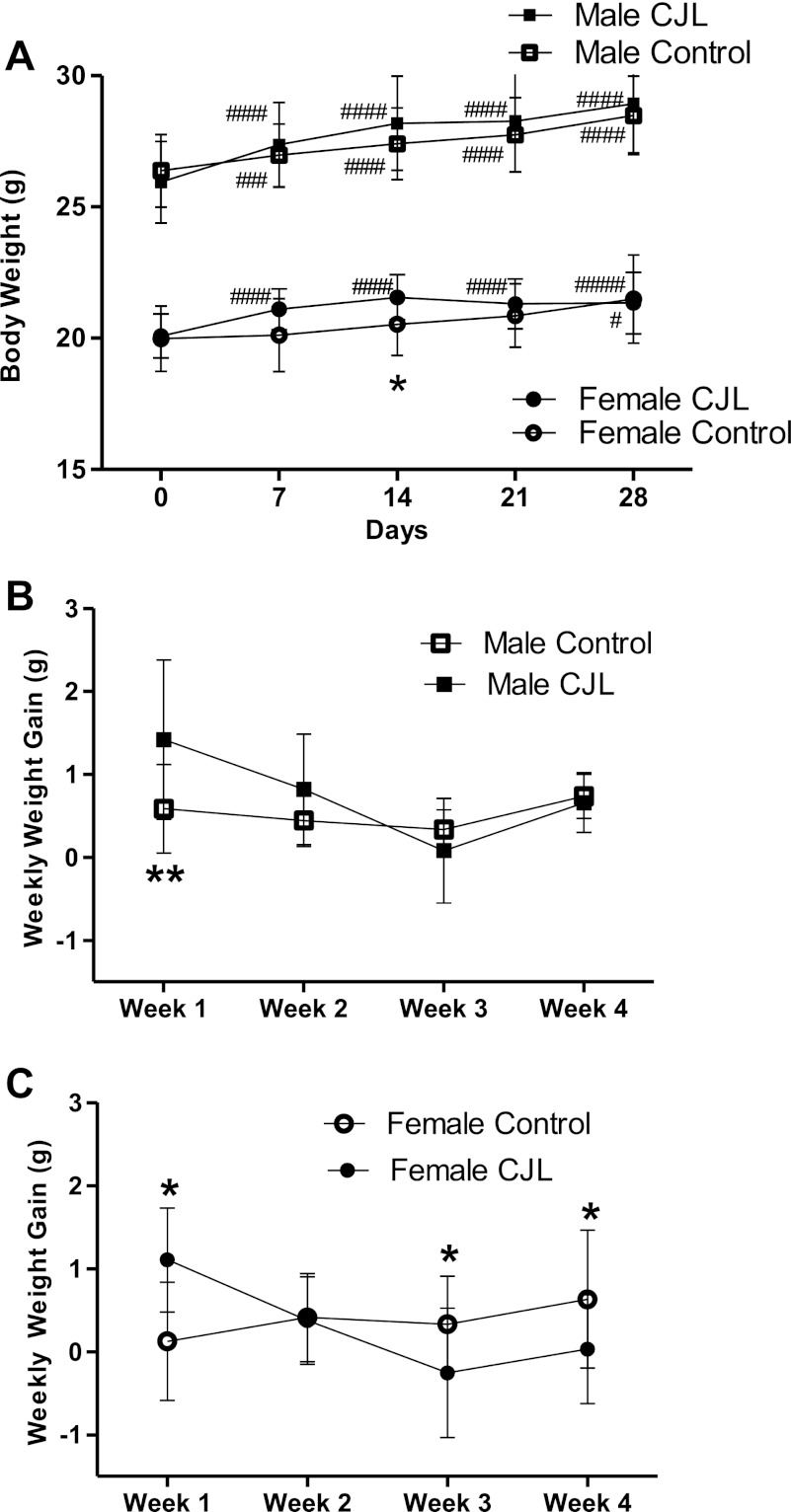
Among control males, body weight increased weekly compared with baseline (Fig. 2, A and B). This increase did not differ to a statistically significant degree between weeks. Males exposed to chronic jet lag (CJL males) increased weight during the first week more than controls (P = 0.008), then exhibited the same weight gain as male controls for the next 3 wk (Fig. 2B).
Fig. 2.
Body weight and weekly weight gain during 4 wk of chronic jet lag. Weight (A) and weekly weight gain in males (B) and females (C) during the 4-wk experiment when female (n = 15) and male (n = 14) C57BL6 mice are exposed to LD12:12 (control group) or chronic jet lag (CJL) (females, n = 15; males, n = 15). Data are expressed as group means ± 1 standard deviation of the mean (SD). Statistical difference between controls and CJL: *0.01 < P < 0.05; **0.005 < P < 0.01. A repeated-measures ANOVA was used for comparisons between times in the same group with Bonferroni correction made for more than two times. Significant change when compared with baseline: # (#, 0.01 < P < 0.05; ##, 0.005 < P < 0.01; ###, 0. 0001 < P < 0.005; ####, P < 0.00001).
Control females increased body weight compared with their baseline weight (Fig. 2, A and C). Among CJL females, weight gain during the first week was higher compared with controls of the same sex (Fig. 2C). Weight gain among CJL females was less than control females at the third (P = 0.027) and fourth week (P = 0.037), and in fact, CJL females lost weight during the third week (−0.3 ± 0.8 g) as, during the same week, control females gained weight (+0.3 ± 0.6 g) (Fig. 2C).
Lung mechanics and lung volume of female and male mice exposed to chronic jet lag.
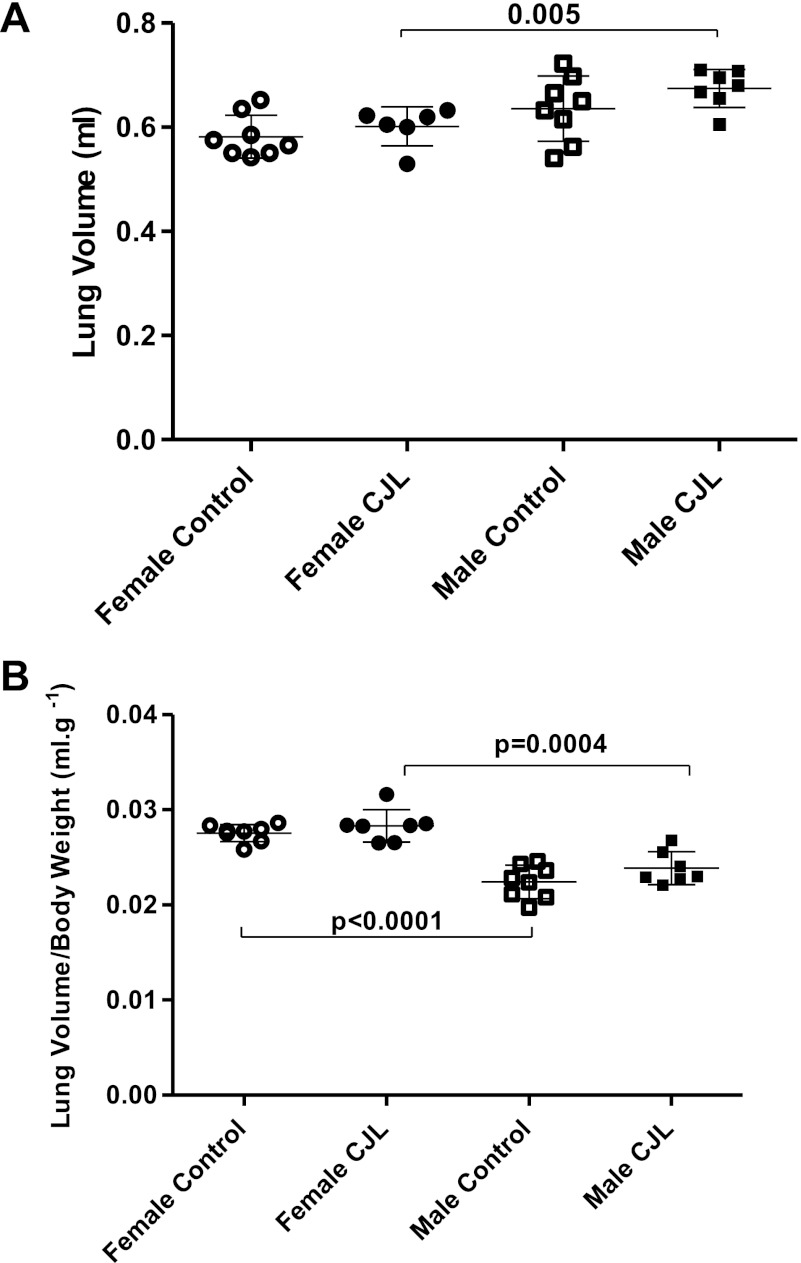
Lung volume was greater in CJL males compared with CJL females (Fig. 3). The difference of lung volume between control males and control females did not achieve statistical significance (P = 0.061). Lung volume-to-body weight ratio was higher in female controls and female CJL compared with male controls (P < 0.0001) and male CJL (P = 0.0004), respectively. After 4 wk of either the usual light regimen (LD12:12) or the shifting light regimen that causes chronic jet lag (11), total lung volumes did not differ between the control and the experimental group in either sex.
Fig. 3.
Lung volume (A) and lung volume-to-body weight ratio (B) in female and male C57BL6J mice controls (females, n = 7; males, n = 6), and those exposed to CJL (females, n = 7; males, n = 8). Data are expressed as individual values and group means ± 1 SD.
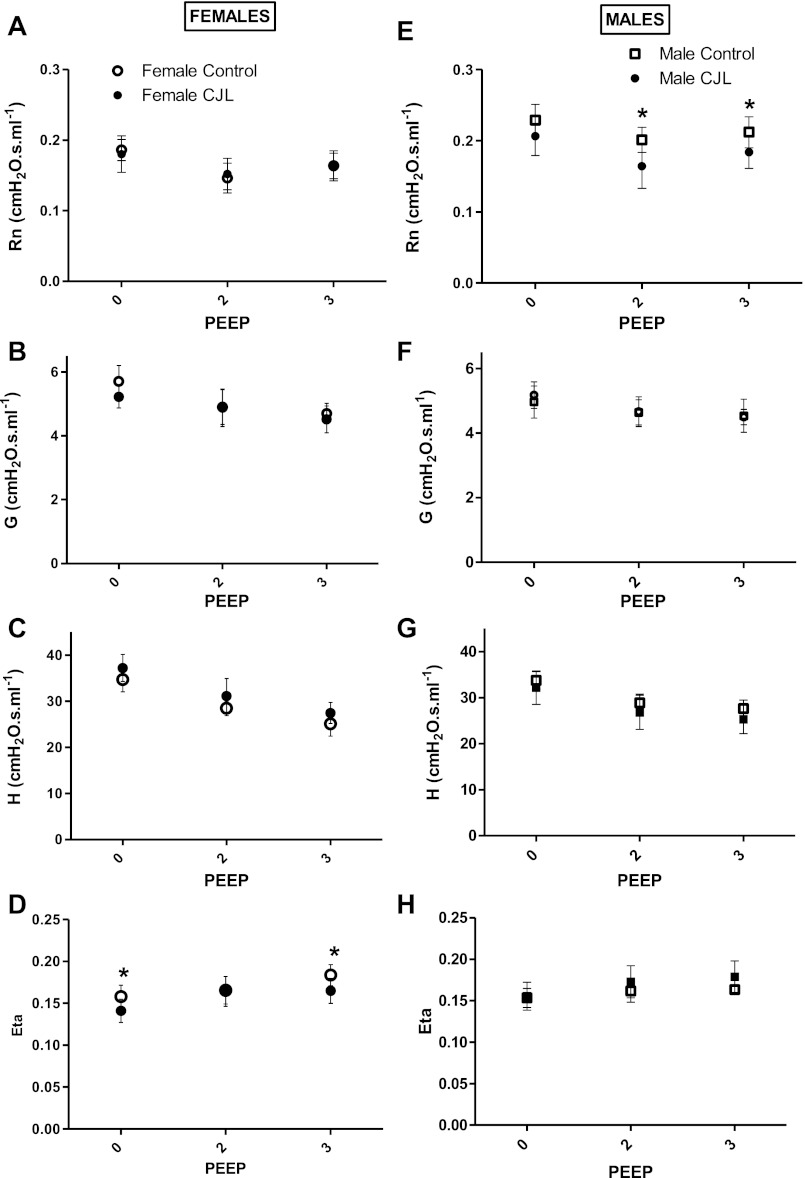
Lung mechanics were measured at three different levels of PEEP. The parameters of the constant-phase model were fitted to respiratory system impedance values of female and male mice controls and those exposed to chronic jet lag (Fig. 4).
Fig. 4.
Respiratory mechanics (parameters of the constant-phase model fitted to respiratory system impedance) of female (n = 7) and male (n = 6) mice, controls and those exposed to chronic jet lag (females, n = 7; males, n = 8). Females (left column): airway resistance, Rn (A); tissue damping, G (B); tissue elastance, H (C); and hysteresivity, Eta (D). Males (right column): airway resistance, Rn (E); tissue damping, G (F); tissue elastance, H (G); and hysteresivity, Eta (H). Data are expressed as group means ± 1 SD. *Statistical difference between controls and CJL (P < 0.05) at each positive end-expiratory pressure (PEEP) level (0, 2, 3 cmH2O).
Among females (Fig. 4, A–D), airway resistance, Rn, did not differ between the two groups at any PEEP (Fig. 4A). Tissue damping, G, and tissue elastance, H, decreased progressively from PEEP 0 to PEEP 3, without any difference between groups (Fig. 4, B and C). At PEEP 0 and PEEP 3, tissue hysteresivity, eta, was lower among female CJL mice compared with female controls (Fig. 4D).
Among males, airway resistance, Rn, at PEEP 2 and 3 was lower in CJL mice compared with controls (Fig. 4E). However, tissue damping, G, tissue elastance, H, and tissue hysteresivity, eta, did not differ between the two groups of males at any level of PEEP.
Clock gene expression in the lung.
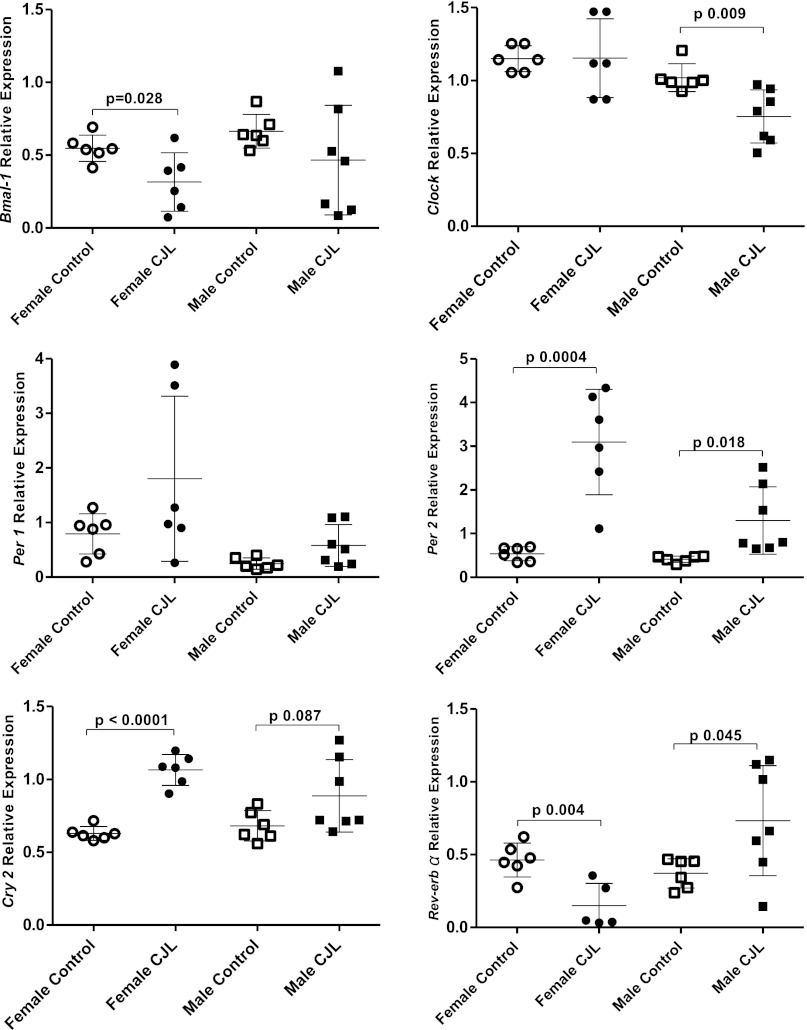
In CJL females, Bmal1 gene expression was lower than in controls, but Clock gene expression did not differ between groups (Fig. 5). Males displayed the opposite pattern; Bmal1 gene expression did not differ between groups, but Clock gene expression was lower in CJL males than in control males. Per1 gene expression did not differ among females or among males. However, Per 2 expression was higher in female and male mice exposed to chronic jet lag compared with same-sex controls. Cry2 gene expression was higher in CJL females, but not in CJL males (P = 0.087), than in controls of the same sex.
Fig. 5.
Clock gene expression in the lung of female (n = 6) and male (n = 7) C57BL6 mice exposed to chronic jet lag and controls (females, n = 6; males, n = 6). Bmal1, Clock, Per1, Per2, Cry2, and Rev-erbα gene expression normalized to 18S. Data are expressed as individual values and group means ± 1 SD.
The orphan nuclear receptor REV-ERBα participates in a second loop of circadian regulation repressing Bmal1 gene expression (44). Rev-erbα gene expression in the lung was increased among CJL males (P = 0.042) and decreased among CJL females compared with same sex controls (Fig. 6).
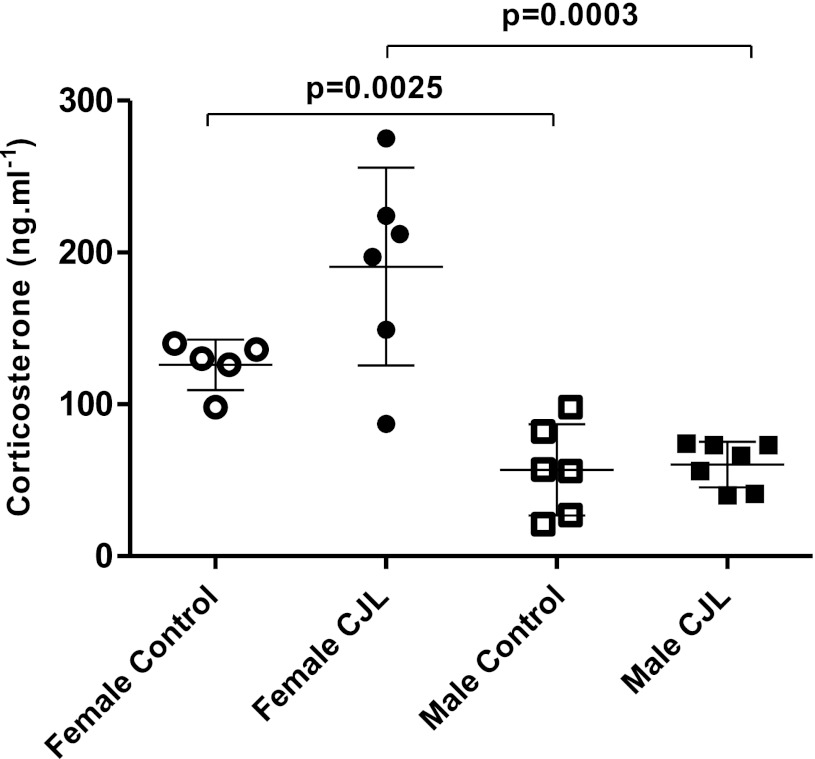
Fig. 6.
Serum corticosterone concentrations in female (n = 6) and male (n = 6) controls and those exposed to chronic jet lag (females, n = 6; males, n = 7). Data are expressed as individual values and group means ± 1 SD.
Bmal1, Clock, Per1, Per2 and Cry2 and Rev-erbα each exhibited a much broader distribution of values among CJL than among control mice as shown by the larger coefficient of variation among CJL mice compared with controls (Table 1).
Table 1.
Coefficient of variation of clock gene expression
| Female Control | Female CJL | Male Control | Male CJL | |
|---|---|---|---|---|
| Bmal1 | 0.17 | 0.63 | 0.17 | 0.81 |
| Clock | 0.08 | 0.23 | 0.09 | 0.24 |
| Cry 2 | 0.08 | 0.10 | 0.15 | 0.28 |
| Per 2 | 0.29 | 0.39 | 0.17 | 0.59 |
| Rev-erbα | 0.25 | 1.03 | 0.27 | 0.51 |
Coefficient of variation of gene expression for Bmal1, Clock, Cry2, Per2, and Rev-Erbα in female and male mice controls and those exposed to chronic jet lag (CJL).
Plasma corticosterone.
To determine if the light regimen used to produce chronic jet lag altered a component of the hypothalamic-pituitary-adrenal axis, which can alter clock gene expression (32) and lung function (37), we measured serum corticosterone (Fig. 6). Corticosterone was higher in control and CJL females than in control and CJL males, respectively. There was no difference between control and CJL males. The mean value of corticosterone was higher among CJL females than control females, but the P value for the difference (P = 0.06) missed the accepted level of statistical significance. There was a much wider distribution of the corticosterone blood levels among CJL females (coefficient of variation 0.34) compared with control females (coefficient of variation 0.13). We did not observe this pattern among CJL males.
DISCUSSION
We tested the hypothesis that alteration of the circadian rhythm, produced by shifting the light-dark schedule of mice, would alter the lung circadian clock gene expression and lung mechanics. Thus our work pertains to two situations: one lived by shift workers, night workers, and those exposed to chronic jet lag (military, pilots, flight attendants, health care providers); and one experienced by many suffering from sleep disturbances secondary to chronic lung disease. Numerous models of circadian disruption have been studied (2, 34, 57). We chose the 8-h shifting CJL regimen (Fig. 1) because it disrupts the circadian rhythm of physical activity in mice (11). Further, the advanced light schedule we used is more disruptive to the organism's circadian rhythm than a delayed light schedule and the effect of its disruption on the organism is more severe (10). With a closed pattern of chronic jet lag (6 h phase advanced every 7 days), mice do not experience sleep loss, nor are hormonal or behavioral measures of stress increased (9). This indicates disruption of the endogenous biological clock, rather than sleep loss, is the basis for the changes we found in lung mechanics and clock gene expression in the lung.
During the 4 wk of exposure to the shifting light pattern, weight gain fluctuated differently between males and females. The weight gain fluctuations are consistent with the clear links between sleep, circadian rhythms, clock genes, and metabolic function (19). In fact, many mutant mice lines bearing genetic lesions of clock, or clock-related genes, exhibit abnormal changes of weight and anomalies of lipid and glucose metabolism (56, 58).
To determine if circadian disruption affects the mechanical properties of the respiratory system, we measured lung volume and input impedance (Zrs) of the respiratory system. Lung volume and mechanics were measured in all animals at the same clock time of the day between ZT 5 and ZT 8. As expected, lung volume/body weight was significantly different between female and male controls (41), but exposure to the shifting light pattern did not affect lung volume among females and males when compared with their respective controls. As the lung volume (functional residual capacity) was not measured during the recording of the lung impedance, we established a specific airway pressure set by the PEEP. This functional residual capacity can depend on the previous ventilation history and the viscoelastic proprieties of the lung. Therefore, prior to each recording, ventilation history was standardized to minimize differences between animals and measurements. Rn provides the overall resistance of the conducting airways (54). CJL decreases Rn among male mice by 20% (at the physiological PEEP of 2 cmH2O). The effect of CJL on lung mechanics may appear modest. However, CJL was applied for only 1 mo, which is less than 3% of a mouse life span (for a life expectancy of 36 mo in a C57BL/6J). Humans subjected to circadian disruption, whether due to disease, e.g., COPD, or occupation (pilots), are subject to circadian disruption for 30% or more of their life. The decrease of Rn did not occur in females exposed to CJL. The basis for this effect on Rn in males is not clear, nor is the basis for the absence of this effect of Rn in females clear. Circadian signals are conveyed from the master circadian clock in the hypothalamus to peripheral organs via two neural routes: central sympathetic and central parasympathetic innervations. In C57BL6 mice, the parasympathetic nervous system transmits signals from the suprachiasmatic nucleus to the lung and regulates mucin secretion and clock gene protein oscillation (3). C57BL6 mice, under the protocol of chronic jet lag we used, lose their circadian rhythm of physical activity, demonstrating their central circadian clock has been disrupted (11). Therefore, dysregulation of the central clock could have affected the signaling from the suprachiasmatic nucleus to the preautonomic motoneurons of the hypothalamus and disrupted the balance between the parasympathetic and sympathetic nervous system (35). Thus the decreased Rn could be related to diminished smooth muscle airway tone due to decreased cholinergic activity, increased sympathetic activity, or both. Besides its effect on bronchial smooth muscle cells, the parasympathetic nervous system also affects lung inflammation (25), and submucosal bronchial gland secretion (22) that could have been responsible for the effect of chronic jet lag on lung mechanics (5, 14). Rn is decreased among CJL males at PEEP 2 and 3 but not in CJL females. This sexual dimorphism may be related to the sexual dimorphism of adrenergic and muscarinic receptor density described in the lung of 129SVJ/C57Bl6 line mice (43). Unmanipulated female mice have a higher density of α1-adrenergic receptors than males, but males have twice the density of muscarinic receptor as females (43). The higher density of muscarinic receptors in males could make them more susceptible to any change in the cholinergic activity in the lung (43).
The differences observed in lung mechanics among females are small and sparse; Rn, G, and H did not differ between the two groups of females. Eta is decreased among females exposed to CJL at PEEP 0 and 3. Besides being statistically significant, the biological significance of this decrease seems small.
As with lung function, we also found sexual dimorphism of clock gene expression in the lungs of mice exposed to chronic jet lag. We did not measure expression of clock genes over 24 h, which would have allowed identification of the presence of rhythm differences, i.e., mesor, amplitude, and phase of expression of those genes (47). However, use of the CJL model in which controls and CJL mice are at the same clock time every first day (Fig. 1) of a 5-day cycle allowed us to test our hypothesis that we disrupted gene expression in the lung (11, 12). Based on the differences in clock gene expression between CJL and controls, we conclude the light regimen we used altered the clock gene expression in the lungs.
Rev-erbα gene expression is also sexually dimorphic; its expression is downregulated in CJL females and upregulated in CJL males. REV-ERBα oscillations act as an additional stabilizing loop within the clock and act with the main core loop to maintain the precision of the circadian oscillation (44). In the lung of CJL exposed animals, Clock is downregulated in males, Bmal1 in females, and their repressor Per2 and Cry2 are upregulated when compared with same-sex control animals. Thus we have demonstrated that every limb of the core loop has been disrupted. Moreover, REV-ERBα directly modulates lipid, glucose, bile acid metabolism, adipogenesis, the inflammatory reaction, and the innate immunity (18, 59). Because of the multiple direct roles of REV-ERBα on metabolism and inflammation, the sexually dimorphic dysregulation of REV-ERBα could have directly contributed to differences in weight gain and lung mechanics in the model of circadian disruption we used.
In conclusion, a change of light schedule regimen that disrupts the organism's circadian rhythm as occurs in chronic jet lag, and may occur because of sleep disturbances in chronic lung diseases, alters lung mechanics, and clock gene expression in the lung in a sexually dimorphic manner.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant HL-020366-33.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: H.H. and D.M. conception and design of research; H.H., S.J.S., and D.M. performed experiments; H.H. and D.M. analyzed data; H.H. and D.M. interpreted results of experiments; H.H. prepared figures; H.H. drafted manuscript; H.H. and D.M. edited and revised manuscript; S.J.S. and D.M. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank E. Filipski, PhD, INSERM U776, Hôpital Paul Brousse, Villejuif F-94807, for helpful discussions regarding the light regimen of the chronic jet lag. We thank G. Massaro, MD, Lung Regeneration Laboratory, Department of Pediatrics, Georgetown University School of Medicine, for critically reading the manuscript, and Zofia Opalka for technical assistance.
REFERENCES
- 1. Agusti A, Hedner J, Marin JM, Barbe F, Cazzola M, Rennard S. Night-time symptoms: a forgotten dimension of COPD. Eur Respir Rev 20: 183–194, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arble DM, Ramsey KM, Bass J, Turek FW. Circadian disruption and metabolic disease: findings from animal models. Best Pract Res Clin Endocrinol Metab 24: 785–800, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bando H, Nishio T, van der Horst GT, Masubuchi S, Hisa Y, Okamura H. Vagal regulation of respiratory clocks in mice. J Neurosci 27: 4359–4365, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bechtold DA, Gibbs JE, Loudon AS. Circadian dysfunction in disease. Trends Pharmacol Sci 31: 191–198, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Brennan S, Hall GL, Horak F, Moeller A, Pitrez PM, Franzmann A, Turner S, de Klerk N, Franklin P, Winfield KR, Balding E, Stick SM, Sly PD. Correlation of forced oscillation technique in preschool children with cystic fibrosis with pulmonary inflammation. Thorax 60: 159–163, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buijs RM, van Eden CG, Goncharuk VD, Kalsbeek A. The biological clock tunes the organs of the body: timing by hormones and the autonomic nervous system. J Endocrinol 177: 17–26, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Burioka N, Koyanagi S, Endo M, Takata M, Fukuoka Y, Miyata M, Takeda K, Chikumi H, Ohdo S, Shimizu E. Clock gene dysfunction in patients with obstructive sleep apnoea syndrome. Eur Respir J 32: 105–112, 2008 [DOI] [PubMed] [Google Scholar]
- 8. Castanon-Cervantes O, Wu M, Ehlen JC, Paul K, Gamble KL, Johnson RL, Besing RC, Menaker M, Gewirtz AT, Davidson AJ. Dysregulation of inflammatory responses by chronic circadian disruption. J Immunol 185: 5796–5805, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Castanon-Cervantes O, Wu M, Ehlen JC, Paul K, Gamble KL, Johnson RL, Besing RC, Menaker M, Gewirtz AT, Davidson AJ. Dysregulation of inflammatory responses by chronic circadian disruption. J Immunol 185: 5796–5805, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davidson AJ, Sellix MT, Daniel J, Yamazaki S, Menaker M, Block GD. Chronic jet-lag increases mortality in aged mice. Curr Biol 16: R914–R916, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Filipski E, Delaunay F, King VM, Wu MW, Claustrat B, Grechez-Cassiau A, Guettier C, Hastings MH, Francis L. Effects of chronic jet lag on tumor progression in mice. Cancer Res 64: 7879–7885, 2004 [DOI] [PubMed] [Google Scholar]
- 12. Filipski E, Subramanian P, Carriere J, Guettier C, Barbason H, Levi F. Circadian disruption accelerates liver carcinogenesis in mice. Mutat Res 680: 95–105, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Fredberg JJ, Stamenovic D. On the imperfect elasticity of lung tissue. J Appl Physiol 67: 2408–2419, 1989 [DOI] [PubMed] [Google Scholar]
- 14. Gangell CL, Horak F, Jr, Patterson HJ, Sly PD, Stick SM, Hall GL. Respiratory impedance in children with cystic fibrosis using forced oscillations in clinic. Eur Respir J 30: 892–897, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Garaulet M, Madrid JA. Chronobiology, genetics and metabolic syndrome. Curr Opin Lipidol 20: 127–134, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Gaultier C, Reinberg A, Girard F. Circadian rhythms in lung resistance and dynamic lung compliance of healthy children. Effects of two bronchodilators. Respir Physiol 31: 169–182, 1977 [DOI] [PubMed] [Google Scholar]
- 17. Gibbs JE, Beesley S, Plumb J, Singh D, Farrow S, Ray DW, Loudon AS. Circadian timing in the lung: a specific role for bronchiolar epithelial cells. Endocrinology 150: 268–276, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gibbs JE, Blaikley J, Beesley S, Matthews L, Simpson KD, Boyce SH, Farrow SN, Else KJ, Singh D, Ray DW, Loudon AS. The nuclear receptor REV-ERBalpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci USA 109: 582–587, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gimble JM, Bray MS, Young A; Pennington Symposium Circadian biology and sleep: missing links in obesity and metabolism? Obes Rev 10, Suppl 2: 1–5, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Gomes RF, Shardonofsky F, Eidelman DH, Bates JH. Respiratory mechanics and lung development in the rat from early age to adulthood. J Appl Physiol 90: 1631–1638, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Gomes RF, Shen X, Ramchandani R, Tepper RS, Bates JH. Comparative respiratory system mechanics in rodents. J Appl Physiol 89: 908–916, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Gosens R, Zaagsma J, Meurs H, Halayko AJ. Muscarinic receptor signaling in the pathophysiology of asthma and COPD. Respir Res 7: 73, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Greenberg H, Cohen RI. Nocturnal asthma. Curr Opin Pulm Med 18: 56–62, 2012 [DOI] [PubMed] [Google Scholar]
- 24. Guo T, Taylor RL, Singh RJ, Soldin SJ. Simultaneous determination of 12 steroids by isotope dilution liquid chromatography-photospray ionization tandem mass spectrometry. Clin Chim Acta 372: 76–82, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Gwilt CR, Donnelly LE, Rogers DF. The non-neuronal cholinergic system in the airways: an unappreciated regulatory role in pulmonary inflammation? Pharmacol Ther 115: 208–222, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Hantos Z, Adamicza A, Govaerts E, Daroczy B. Mechanical impedances of lungs and chest wall in the cat. J Appl Physiol 73: 427–433, 1992 [DOI] [PubMed] [Google Scholar]
- 27. Haupt CM, Alte D, Dorr M, Robinson DM, Felix SB, John U, Volzke H. The relation of exposure to shift work with atherosclerosis and myocardial infarction in a general population. Atherosclerosis 201: 205–211, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Haus E, Smolensky MH. Biologic rhythms in the immune system. Chronobiol Int 16: 581–622, 1999 [DOI] [PubMed] [Google Scholar]
- 29. Hetzel MR, Clark TJ. Comparison of normal and asthmatic circadian rhythms in peak expiratory flow rate. Thorax 35: 732–738, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Holgate ST. Pathogenesis of asthma. Clin Exp Allergy 38: 872–897, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Institute of Laboratory Animal Research, Commission on Life Sciences, National Research Council Guide for the Care and Use of Laboratory Animals. Washington, DC: The National Academies Press, 2011 [Google Scholar]
- 32. Kalsbeek A, van der Spek R, Lei J, Endert E, Buijs RM, Fliers E. Circadian rhythms in the hypothalamo-pituitary-adrenal (HPA) axis. Mol Cell Endocrinol 349: 20–29, 2012 [DOI] [PubMed] [Google Scholar]
- 33. Keller M, Mazuch J, Abraham U, Eom GD, Herzog ED, Volk HD, Kramer A, Maier B. A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci USA 106: 21407–21412, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kiessling S, Eichele G, Oster H. Adrenal glucocorticoids have a key role in circadian resynchronization in a mouse model of jet lag. J Clin Invest 120: 2600–2609, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kishi T, Sunagawa K. Experimental “jet lag” causes sympathoexcitation via oxidative stress through AT(1) receptor in the brainstem. Conf Proc IEEE Eng Med Biol Soc 2011: 1969–1972, 2011 [DOI] [PubMed] [Google Scholar]
- 36. Knutsson A, Hammar N, Karlsson B. Shift workers' mortality scrutinized. Chronobiol Int 21: 1049–1053, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Landstra AM, Postma DS, Boezen HM, van Aalderen WM. Role of serum cortisol levels in children with asthma. Am J Respir Crit Care Med 165: 708–712, 2002 [DOI] [PubMed] [Google Scholar]
- 38. Logan RW, Sarkar DK. Circadian nature of immune function. Mol Cell Endocrinol 349: 82–90, 2012 [DOI] [PubMed] [Google Scholar]
- 39. Marpegan L, Leone MJ, Katz ME, Sobrero PM, Bekinstein TA, Golombek DA. Diurnal variation in endotoxin-induced mortality in mice: correlation with proinflammatory factors. Chronobiol Int 26: 1430–1442, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Marques MD, Waterhouse JM. Masking and the evolution of circadian rhythmicity. Chronobiol Int 11: 146–155, 1994 [DOI] [PubMed] [Google Scholar]
- 41. Massaro D, Massaro GD. Estrogen receptor regulation of pulmonary alveolar dimensions: alveolar sexual dimorphism in mice. Am J Physiol Lung Cell Mol Physiol 290: L866–L870, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev 12: 197–210, 2008 [DOI] [PubMed] [Google Scholar]
- 43. Novakova M, Kvetnansky R, Myslivecek J. Sexual dimorphism in stress-induced changes in adrenergic and muscarinic receptor densities in the lung of wild type and corticotropin-releasing hormone-knockout mice. Stress 13: 22–35, 2010 [DOI] [PubMed] [Google Scholar]
- 44. Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110: 251–260, 2002 [DOI] [PubMed] [Google Scholar]
- 45. Price D, Small M, Milligan G. The prevalence and impact of nighttime symptoms in COPD patients: results from a cross sectional study in five Europeans countries. In: Proceedings of the IV World Asthma and COPD Forum, Paris, France, 2011 [Google Scholar]
- 46. Provinciali M, Cardelli M, Marchegiani F. Inflammation, chronic obstructive pulmonary disease and aging. Curr Opin Pulm Med 17, Suppl 1: S3–S10, 2011 [DOI] [PubMed] [Google Scholar]
- 47. Refinetti R. (editor). Circadian Physiology. Boca Raton, FL: Taylor and Francis, 2006, p. 667 [Google Scholar]
- 48. Rorabacher DB. Statistical treatment for rejection of deviant values: critical values of Dixon's “Q” parameter and related subrange ratios at the 95% confidence level. Anal Chem 63: 139, 1991 [Google Scholar]
- 49. Scherle W. A simple method for volumetry of organs in quantitative stereology. Mikroskopie 26: 57–60, 1970 [PubMed] [Google Scholar]
- 50. Schernhammer ES, Laden F, Speizer FE, Willett WC, Hunter DJ, Kawachi I, Fuchs CS, Colditz GA. Night-shift work and risk of colorectal cancer in the nurses' health study. J Natl Cancer Inst 95: 825–828, 2003 [DOI] [PubMed] [Google Scholar]
- 51. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3: 1101–1108, 2008 [DOI] [PubMed] [Google Scholar]
- 52. Shibata H, Horie O, Sugita M. Studies on the deviation of short-term FRC measurements with He closed-circuit method and on diurnal variation of FRC. Rinsho Byori 38: 463–467, 1990 [PubMed] [Google Scholar]
- 53. Spengler CM, Shea SA. Endogenous circadian rhythm of pulmonary function in healthy humans. Am J Respir Crit Care Med 162: 1038–1046, 2000 [DOI] [PubMed] [Google Scholar]
- 54. Tomioka S, Bates JH, Irvin CG. Airway and tissue mechanics in a murine model of asthma: alveolar capsule vs. forced oscillations. J Appl Physiol 93: 263–270, 2002 [DOI] [PubMed] [Google Scholar]
- 55. Turek FW. From circadian rhythms to clock genes in depression. Int Clin Psychopharmacol 22, Suppl 2: S1–S8, 2007 [DOI] [PubMed] [Google Scholar]
- 56. Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308: 1043–1045, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science 288: 682–685, 2000 [DOI] [PubMed] [Google Scholar]
- 58. Yang S, Liu A, Weidenhammer A, Cooksey RC, McClain D, Kim MK, Aguilera G, Abel ED, Chung JH. The role of mPer2 clock gene in glucocorticoid and feeding rhythms. Endocrinology 150: 2153–2160, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yin L, Wu N, Lazar MA. Nuclear receptor Rev-erbalpha: a heme receptor that coordinates circadian rhythm and metabolism. Nucl Recept Signal 8: e001, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]