Abstract
Oligonucleotides (ONs) are an emerging class of drugs being developed for the treatment of a wide variety of diseases including the treatment of respiratory diseases by the inhalation route. As a class, their toxicity on human lungs has not been fully characterized, and predictive toxicity biomarkers have not been identified. To that end, identification of sensitive methods and biomarkers that can detect toxicity in humans before any long term and/or irreversible side effects occur would be helpful. In light of the public's greater interests, the Inhalation Subcommittee of the Oligonucleotide Safety Working Group (OSWG) held expert panel discussions focusing on the potential toxicity of inhaled ONs and assessing the strengths and weaknesses of different monitoring techniques for use during the clinical evaluation of inhaled ON candidates. This white paper summarizes the key discussions and captures the panelists' perspectives and recommendations which, we propose, could be used as a framework to guide both industry and regulatory scientists in future clinical research to characterize and monitor the short and long term lung response to inhaled ONs.
Introduction
As with any inhaled drug, and especially for a novel drug class such as oligonucleotides (ONs), pre-clinical and clinical safety assessment is challenging. With this in mind, a panel of internationally recognized lung experts from academic clinical laboratories, as well as industry-employed clinicians, with experience in drug development and pulmonary toxicology was convened by the Oligonucleotide Safety Working Group's (OSWG) Inhalation Subcommittee. The panel was asked whether the toxicities observed in pre-clinical animal studies would be anticipated in humans, to identify the strengths and weaknesses of current methods for detecting such effects in clinical trials and to make recommendations for human safety risk management of inhaled ONs.
There is a diversity of structures, chemistries, and mechanisms of action for ON therapeutics, but most of the members of this drug class can be categorized on the basis of whether or not they target mRNA or proteins. Antisense oligonucleotides (ASO), short-interfering RNA (siRNA), antagomirs, microRNA mimetics, and DNAzymes are part of the RNA-targeting group, while immunostimulatory sequences (ISS), aptamers, and decoys are members of the protein-targeting group. The toxicology and pharmacokinetics of ONs following systemic administration (e.g., intravenous and subcutaneous routes of administration) used in a variety of indications have been well characterized, and a large body of information is available through the regulatory databases and the broad scientific literature (Levin et al., 1998; LEVIN, 1999; Levin et al., 2001; Sazani et al., 2010). For example, the mechanisms of toxicity for RNA-targeting ONs can be subdivided into hybridization-independent and hybridization-dependent effects. Hybridization-independent toxicities are due to interactions between the ON drug and proteins, which are unrelated to Watson and Crick base pairing to RNA. Hybridization-dependent toxicities arise because the ON hybridizes to cellular RNA using the normal base-pairing principles which can lead to side effects associated with inhibition of the intended target (referred to as exaggerated pharmacology) or inhibition of unintended RNA targets (referred to as off-target effects). The majority of toxicities observed for ASOs and siRNAs tested to date fall into the hybridization-independent category and are believed to mainly result from the ON's chemistry or the composition of the delivery system (Levin et al., 2001). For example, the most common modification used in ON compounds, phosphorothioate (PS) linkages (and other related backbone alterations) typically strengthen the polyanionic character of the molecule and render it more reactive. This, in addition to the greater tissue persistence, translates into more pronounced non-specific effects, such that systemic administration of PS ASOs result in various forms of toxicity largely unrelated to the mechanism of action (i.e., hybridization-independent). The most frequent and well studied hybridization-independent effects associated with PS ON administration are immune related, and the tendency to stimulate pro-inflammatory reactions, principally occurring in tissues containing the highest concentration of ONs (KRIEG, 2000; Levin et al., 2001).
Some pharmaceutical companies and investigators have explored alternative dosing routes. Currently the only 2 approved ON products are for local administration to the eye (CROOKE, 1998; Ng and Adamis, 2005). As for local delivery to the lungs, there is a relatively small amount of information on efficacy, deposition, and tolerability by this route. Furthermore, the Division of Pulmonary, Allergy, and Rheumatology Products at the US Food and Drug Administration (FDA) has reviewed only six ON drug candidates with representatives of the ASO, siRNA, and ISS types through investigational new drug (IND) applications or pre-IND meetings (DIA Conference, 2010), thus limiting the regulatory database of information available to this division.
Based on published information (Templin et al., 2000; Ali et al., 2001; Guimond et al., 2008) in rodent and primate species and the personal experiences of the Inhalation Subcommittee members, a list has been assembled of key findings that have been observed in non-clinical animal toxicity studies of inhaled ONs, which are generally limited to the respiratory tract and lung, the tissue of major accumulation.
With the understanding that the type and/or severity of findings may differ among ON subclasses (e.g. different backbone chemistries, duration of administration, and target species, certain types of changes in the lungs have been commonly observed). A summary of these key findings, which are typically dose related and reversible upon termination of treatment and which primarily occur at high toxicological doses is listed below.
Alveolar macrophage “accumulation” (i.e., reflecting increased numbers and prominence upon light microscopy);
Interstitial macrophages and mononuclear cell infiltration and accumulation in the lung parenchyma, more than the upper airway tissues and trans-bronchial lymph nodes;
Occasional observations of hemorrhage, possibly secondary to tissue inflammation; and
Fibroplasia and metaplasia in the lung or associated tissues (e.g., trachea, lymph nodes), usually with relatively pronounced inflammation.
One of the primary challenges in advancing these molecules into clinical trials is the observation of such findings and whether the findings represent safety concerns for humans. In addition, although toxicity has not been reported in normal subjects to date, there may be increased susceptibility in patients with diseased lungs, for example, due to impaired epithelial barrier function. However, there have been no reports of increased lung inflammation in patients following inhalation of ONs (Ball et al., 2003; Gauvreau et al., 2006; DeVincenzo et al., 2008; Gauvreau et al., 2008; DeVincenzo et al., 2010). However, the inhaled doses have been low and the clinical trials have been of relatively short duration (i.e., less than 1 month); thus, the effects of prolonged exposure to ONs to human lungs remain undetermined. However, an important concern is that the techniques for monitoring lung toxicity may be insensitive to detect early clinical changes similar to those seen in animals. Although similar histopathological changes have been observed in other target organs (e.g., liver and kidney) when ONs are delivered via other routes of administration (e.g., parenteral), these other tissues can be monitored with increasingly sensitive biomarkers able to detect even earlier functional perturbation, whereas the technologies to monitor subtle pathologic changes in the lungs are less advanced.
This position paper addresses these issues and summarizes the panel discussion and outlines the consensus points and recommendations from the experts and the members of the Inhalation Subcommittee.
The Inhalation Oligonucleotide Subcommittee.
Following the April 2007 Drug Information Association (DIA) meeting on oligonucleotide therapeutics in Bethesda, Maryland, the Oligonucleotide Safety Working Group (OSWG) was set up with representatives from both industry and regulatory authorities. Several subcommittees have been formed to deal with genotoxicity, off-target effects, immunostimulation, exaggerated pharmacology, safety pharmacology, reproductive toxicity, and carcinogenicity. The Inhalation Oligonucleotide Subcommittee, formed in 2009, has been discussing the main toxicology issues/challenges relating to the non-clinical development of inhaled oligonucleotides.
Panel Discussion Highlights
The panel first addressed whether inhaled ON-induced lung findings observed in animals were likely to be observed in humans. The panel concluded that it should be expected that ON drugs are similar in this respect to traditional inhaled small molecule drugs with respect to potential toxicity resulting from the intended and unintended pharmacological action of the drug and chemistry class effects. In this respect, as with toxicities observed with other therapeutics during pre-clinical animal safety assessment, these toxicities could manifest in normal and especially in diseased patients. It is accepted that the intrinsic DNA/RNA-like property of ONs might trigger an inherent innate response. The pro-inflammatory properties of ONs are arguably one of the primary concerns of safety assessment of this drug class. Thus, specific ON chemistries and indications may warrant specific risk assessment monitoring techniques and/or readouts in humans as with different small molecule drug classes.
One common toxicological pre-clinical finding of inhaled ONs is the accumulation of alveolar and interstitial macrophages within the lungs of animals. This is particularly observable histologically because their intra-cytoplasmic vacuoles contain large amounts of basophilic granular material, which is believed to reflect ON uptake by the macrophage. This is consistent with the fact that oligonucleotides stain blue with hematoxylin and eosin (Levin et al., 1998). This macrophage staining, with no or little evidence of activation, nor other inflammatory cell accumulation, would typically be considered a non-adverse adaptive mechanism in an otherwise healthy animal lung (Lewis et al., 2002; Mosser and Edwards, 2008; Brasey et al., 2011). Whether this finding might be considered adverse—particularly in patients with established lung disease—is not known, because this response also shows reversibility upon termination lends credence to the response being non adverse, whereas fibrosis and metaplasia, described at high inhaled ON doses in animals, showed only partial reversibility in the recovery time allotted (Guimond et al., 2008; FDA personal communication, 2011). Reversibility, even if incomplete for some findings, could be observed in as little as 2 to 4 weeks. As there are no reports of chronic toxicology studies, the complete reversibility of findings or the potential for delayed toxicity is unclear.
The lung histological changes observed in pre-clinical animal studies of inhaled drugs are not directly monitorable and indirect techniques may not be sensitive enough to detect such effects in humans. Thus, a more specific or targeted approach for measuring the potential inflammatory response is recommended to monitor patients; however, there are currently no established/validated biomarkers for early pulmonary toxicity that precede clinically important pulmonary lesions such as airway disease, alveolitis, interstitial fibrosis, or malignancy. With these potential limitations, our clinical expert panel summarized several useful clinical monitoring techniques in humans (Table 1), which have previously been employed to monitor the progression of lung diseases and the potential toxicity of non-ON drugs. The panelists initially commented on these standard tests and then discussed additional or less proven tests for monitoring potential lung toxicity.
Table 1.
List of Clinical Monitoring techniques
| Procedure | Advantages | Potential limitations |
|---|---|---|
| STANDARD TESTING | ||
| Pulmonary functional testing | • Non-invasive | • Sensitivity |
| • Cost | • Indirect measurements of inflammation | |
| • Standardized procedure | ||
| • Availability | ||
| • Longitudinal assessment possible | ||
| Blood biomarkers | • Non-invasive | • Correlation with lung inflammation unknown |
| • Cost | ||
| • Availability | ||
| • Longitudinal assessment possible | ||
| • Cellular/biochemical readouts of inflammation | ||
| ADDITIONAL TESTS1 | ||
| Exhaled gases and breath condensates | • Non-invasive | • Correlation with inflammation not clear |
| • Cost | ||
| • Availability | • Predicted value references not available | |
| • Standardized procedure | ||
| • Longitudinal assessment possible | • High variability | |
| Sputum induction | • Non-invasive | • Sample yield |
| • Cost | • Reproducibility | |
| • Longitudinal assessment possible | • Correlation with tissue unknown | |
| • Cellular/biochemical readouts of inflammation | ||
| Imaging modalities | • Correlation with tissue changes | • Availability |
| • Cost | ||
| • Sensitivity | ||
| • Safety/ethical concerns | ||
| Bronchoalveolar lavage | • Standardized procedure | • Moderately invasive |
| • Cost | • Correlation with tissue unknown | |
| • Reproducible | • Nature of cell infiltrate may vary between diseases | |
| • Longitudinal assessment possible | ||
| • Cellular/biochemical readouts of inflammation | ||
| Endo- and trans-bronchial biopsies | • Standardized procedure | • Invasive |
| • Tissue/cellular/biochemical readouts of inflammation | • Focal analysis/sampling error | |
The tests in this category are reviewed from less invasive methods to more invasive procedures.
Standard Testing
Pulmonary function testing
Spirometry is a routine, non-invasive procedure that is employed in the clinic to assess pulmonary function. Spirometry is a relatively inexpensive, reproducible, readily available test in most clinics and hospitals. The test measures lung volumes [e.g., forced expiratory volume (FEV1), FEV6, forced vital capacity (FVC), FEV1/FVC] and flows [e.g., forced expiratory flow (FEF25–75), peak expiratory flow (PEF), maximum inspiratory pressure (MIP) and maximum expiratory pressure (MEP)] during forced expiration from total lung capacity. Deviation from established reference data can indicate airway or parenchymal disease. In addition, more specialized pulmonary function laboratories can also collect measurements indicating lung parenchymal and vascular abnormalities [e.g., static lung volumes of various sub compartments, lung diffusion capacity (DLCO)], the adequacy of ventilation (tidal volume and frequency of breathing, 6-minute walk test, maximal oxygen consumption), disturbed lung mechanics (resistance and compliance) and the non-uniformity of gas distribution (gas washout). Moreover, blood gases can be measured that ultimately indicate the adequacy of ventilation.
The procedures and equipment to perform these tests and training of personnel have been standardized, but multi-center variability during clinical trials may still pose a challenge for some of the measurements (e.g. DLCO). Pulmonary function testing can be performed in adults and children (usually of 5 years and older) at most stages of disease. Some of these measures such as the diffusion of a gas across the pulmonary epithelium may be disease or tissue specific, while others (e.g., FEV1) can be used in a broader range of indications. For example, gas diffusion (DLCO) has proven valuable in establishing disease severity and in defining prognosis in idiopathic pulmonary fibrosis where a decline in DLCO over time suggests progression of the disease (Martinez and Flaherty, 2006). DLCO measurements have been shown to reveal vascular changes or diffusion impairments caused by fibrosis or damaged pulmonary epithelium in the absence of significant airway function change (HSIA, 2002).
Although many of these tests are routinely employed to assess the severity of lung disease and disease progression, their sensitivity for the assessment of early development of toxicity to inhaled medication needs to be established and remains under investigation. Preliminary information has been gathered by employing these tests to monitor pulmonary effects of inhaled drugs (e.g., Pulmozyme®, Exubera®) and have permitted the recognition of early evidence of small but significant changes. For example, FEF25–75 appears to be a sensitive measure of small airway function but is less reproducible and more variable than measures such as FVC and FEV1. It may be affected before FEV1, so serial measurements may act as an early warning sign of small airway disease (Drewek et al., 2009). It has been postulated that without changes in any of the physiologic measurements, the pro-inflammatory toxicity of an inhaled medication may be minimal and irrelevant (Veen et al., 2000). The nitrogen washout test can detect early small-airways disease and may be considered to be more sensitive than conventional lung function testing (KING, 2011). One way to improve the sensitivity of inhaled medication testing is to examine its effects on the pulmonary response to challenge, by methacholine, hyper- or hypo-tonic saline, allergen, exercise, or eucapnic hyperpnea of cool, dry air. These commonly employed tests for assessing allergic airway diseases may be applicable to other indications, especially if airway hyperreactivity were to become suspected as a potential toxicity of ON and can be particularly helpful longitudinally.
Blood biomarkers
The measurement of hematological and biochemical markers in blood can be reliably performed with minimally invasive procedures and equipment that is cost effective and readily available in almost all clinical settings. Repeated sampling is feasible from patients of all ages and at all stages of disease. In addition to the standard blood gas, electrolyte, and clinical pathology measures, measurement of certain biochemical markers such as C-reactive protein, interleukin (IL)-6, IL-8, and MCP-1 may reflect the systemic inflammatory manifestations or the downstream sequelae of pulmonary inflammation. However, the relationship between the levels of such biomarkers in the blood and lung inflammation or lung toxicity caused by medications has not been clearly established.
Additional Tests
Exhaled gases
Fractional exhaled nitric oxide (FeNO) is a simple non-invasive procedure for monitoring pulmonary and systemic NO production. FeNO is elevated in atopic asthma and is thought to reflect eosinophilic inflammation of the airways. However, its role in diseases other than asthma remains questionable. In particular in cystic fibrosis, the levels of FeNO may even be reduced, despite the underlying inflammation, compared to normal subjects. Some reports have shown that FeNO is increased in chronic obstructive pulmonary disease (COPD) patients suggesting a correlation with neutrophilic inflammation (Hillas et al., 2009).
Techniques have been standardized using relatively inexpensive, commercially available equipment that can be deployed rapidly and require minimal staff training. These measurements can be frequently repeated, are not burdensome to patients, and may be applied to children and patients at all stages of disease. Exhaled gas shows promise in the differential diagnosis of airway diseases as well as in the longitudinal monitoring of therapy. Despite the complexity of NO exchange dynamics, the partitioning of exhaled NO from proximal airways, as opposed to distal airspaces, is possible using measurements obtained at different flow rates and has been successfully used in distinguishing inflammation due to alveolitis from that of the bronchial compartment (Lehtimaki et al., 2001). Numerous factors that influence FeNO values (e.g., medication, age, diet) may be difficult to define, and correlation of changes with airway histological changes has not been firmly established.
Other exhaled gases such as carbon monoxide, hydrocarbons or 8-isoprostanes might be potentially useful non-invasive biomarkers of airway inflammation and oxidative stress (Zhang et al., 2010), but further research is needed to validate the use of these measures as sensitive indicators of lung injury.
Exhaled breath condensates
Analysis of condensates of exhaled air, such as for hydrogen peroxide, is a possible approach to monitoring volatile and non-volatile compounds as biomarkers of lung disease. The collection procedure differs from that for exhaled gases. Now standardized, there is growing evidence that abnormalities in exhaled breath condensate composition may reflect biochemical changes of airway lining fluid. The procedure is safer and more convenient than bronchoalveolar lavage (BAL) discussed below. However, interpretation of condensate data are complicated by uncertainty regarding the source of condensate solutes (and potential contamination from the gastrointestinal tract), the dilution effect, and by the high variability of the values obtained. Further standardization is therefore needed, for example, with regard to a defined flow rate during exhalation.
Sputum induction
Sputum induction is a well-tolerated, relatively non-invasive method (especially for spontaneous sputum) that can be repeated multiple times (more frequently than BAL) over the course of a study allowing for serial assessments (Vlachos-Mayer et al., 2000). Although induced sputum is generally considered to monitor airway inflammation, in healthy people, normal sputum consist of approximately 60% alveolar macrophages and therefore can be regarded as sampling more distal lung sites (Belda et al., 2000). Accordingly, it can be used to assess lung parenchymal disease such as cancer and infections (Kelly et al., 2000b). Sputum allows collection of biosamples (cells and solutes) from the subglottic airways and is used in pulmonary clinics as a diagnostic procedure in asthma, COPD, and infectious diseases (e.g., tuberculosis). The same markers of inflammation as those described for BAL samples can be measured from sputum samples, but the correlation between the values measured in sputum and those measured in BAL or in the lung tissue is not straightforward and does not appear to be the same for different cellular and protein markers (Kelly et al., 2000a; Kelly et al., 2002; Saha et al., 2009; Doe et al., 2010).
Induced sputum also permits assessment of the pharmacologic activity (inflammatory cell influx and protein markers of inflammation similar to BAL) of drugs in development for respiratory diseases, particularly for the evaluation of anti-inflammatory drugs such as inhaled corticosteroids (Kelly et al., 2006).
The main drawbacks to sputum analysis are its admixture with secretions from the upper airway, the relatively low yield of sputum from some subjects or in patients with certain diseases such as cystic fibrosis, the uncertainty over the consistency/reproducibility of induced sputum findings for a particular patient, and the need for standardization of a technically demanding procedure among centers. Standardized protocols for effective collection and analysis and growing experience with the procedure has led to it becoming more popular and widely practiced (Kelly et al., 2003). Although a cheaper procedure than BAL, proper training of clinical and laboratory staff and close monitoring required for quality/reproducibility data reduce the cost savings.
Imaging modalities
Computed tomography (CT) of the lung and other imaging techniques [e.g., fluorodeoxyglucose-positron emission tomography (FDG-PET) and CT/PET, X-rays] have been used to evaluate the effects of drugs in various lung diseases including asthma, COPD, pulmonary fibrosis, hypersensitivity pneumonitis, and cystic fibrosis. There are generally high costs associated with these procedures unless a limited number of imaging slices are captured. In addition, the safety and/or ethical concerns of the effects of radiation exposure have limited its use in pulmonary drug development. High resolution CT is very sensitive to small scale changes and can be used to quantitatively assess changes in the alveolar, interstitial and airway compartments as well as in the mediastinum and even to quantitatively assess airway remodeling. Detection of lung parenchymal changes by CT correlates with histological signs of lung pathology (Attili et al., 2008; VERSCHAKELEN, 2010) but is dependent on the type of tissue response. For example, the detection of lung inflammation is less sensitive than detection of fibrosis (Nagatani et al., 2011). The relationships between imaging analysis and biomarkers of inflammation and repair in blood, BAL, sputum, and exhaled breath condensate need to be established.
Bronchoalveolar lavage
Bronchoalveolar lavage (BAL) is generally a well tolerated but moderately invasive technique that allows collection of biosamples (cells and solutes) from the lower airways and alveolar space. The application of BAL is widespread and has been used as a standard clinical research tool in a wide range of indications (e.g., asthma, COPD, pulmonary hypertension, idiopathic pulmonary fibrosis, cystic fibrosis, respiratory tract infections, and adult respiratory distress syndrome) (Task Force, 1990). Drug developers have used the procedure for monitoring potential adverse (e.g., inhaled insulin) (Liu et al., 2008) and beneficial effects (e.g., inhaled corticosteroids) (Berry et al., 2007) on pulmonary inflammation following chronic inhaled drugs administration.
While generally safe with minor side effects, the procedure requires administration of various additional pharmacological agents (anti-anxiety, anti-secretory, analgesics, or even general anesthesia), depending on age and disease severity. BAL provides an opportunity for longitudinal data acquisition on the cellular response (inflammatory cell influx) and protein markers of inflammation or activation status. Many biological markers of inflammation can therefore be simultaneously analyzed from BAL samples, including differential white blood cell counts, reactive nitrogen species (e.g., peroxynitrite), reactive oxygen species (e.g., superoxide) (Verhoeven et al., 2000), pro-inflammatory cytokines (e.g. TNF-α, IL-1, IL-2, IL-6, and IL-23), markers of macrophage activation (e.g., metalloproteases), markers of pulmonary capillary permeability (e.g., albumin), markers of tissue remodeling (e.g., fibrinogen, collagen, elastin) and markers of excess mucus (mucin, MUC5AC, Gob5). Careful consideration may be needed when interpreting BAL differential cell counts, as in certain pulmonary disease states, clear differences in the inflammatory cell infiltrate and tissue biopsy samples have been observed. For example, in cystic fibrosis, neutrophils predominantly accumulate in the lumen (and are thus observed in BAL), while lymphocytes generally remain in the interstitium and are absent from the BAL. Because of potential qualitative differences in cellularity within the interstitial and airway compartments, and of course the influence of a background disease state, cytokine profiles may also differ, making it important to obtain baseline values (Blanc et al., 1991).
Endo- and trans-bronchial biopsies
Bronchoscopically obtained biopsies require an invasive procedures under local or even general anesthesia. It can be performed in children and adults and allows the collection of specimens using brush, needle, or forceps from the airway wall or pulmonary interstitium to evaluate for inflammation, infection, fibrosis, or neoplasia. Although an established procedure, trans-bronchial biopsies may have higher morbidity compared to endo-bronchial biopsies. In addition to the assessment of cellular and structural changes, levels of pro-inflammatory mediators or markers of remodeling can be measured from tissue sample homogenates or aspirated fluid. However, because of the difficulty in sampling from the same location within the tissue, they mainly represent snap-shots and the opportunity for serial assessments may be limited. Due to the potential for sampling error (false negative), several biopsy samples (4–6) are required, which may still be unrepresentative of the general situation in the airways or lungs (Kelly et al., 2010). Similarly, bronchoscopic biopsies may have limited utility in instances where focal pathology is present. In patients with severe disease, it may not be possible to collect samples by bronchoscopy, as there is an increased risk of complications. Finally, there is limited experience using results obtained from biopsies for regulatory submission.
Despite these challenges, this is the only method that allows the identification of histological changes within the lung tissue similar to those observed in pre-clinical studies in animals. Therefore, when used concomitantly with other investigative procedures such as BAL or sputum, it may provide useful additional information to supplement less invasive methods for monitoring lung toxicity of ONs without missing subtle tissue changes.
Consensus points and recommendations
The clinical expert panel was reassured that most of the findings observed in animals following inhaled ON exposure share some commonalities with other inhaled drugs. First, similar histopathological findings reflecting pro-inflammatory effects have been seen with other inhaled drug classes. For example, mild to moderate alveolitis was observed in rats after one month dosing of recombinant human deoxyribonuclease (rhDNase) via the inhalation route. This lesion was characterized principally by inflammatory cell accumulation in alveolar spaces, a perivascular/interstitial inflammatory cell infiltrate, and proliferation of type-2 alveolar lining cells. Following 26 weeks of inhalation treatment in cynomolgus monkeys, a similar immune response of increased perivascular lymphocytic cuffing, peribronchial lymphoid hyperplasia, terminal airway-related bronchiolitis/alveolitis with eosinophilic infiltrates, and increased siderophages was observed in the lungs of treated animals. The findings partially resolved following a treatment free period. It has been suggested that an immune response to the rhDNase protein may have contributed to the changes observed in monkey (GREEN, 1994).
Second, most of the ON findings observed at relevant doses have been reversible upon termination of exposure; thus, if such changes occurred in humans, termination of exposure might allow regression of lung inflammation. An example where conventional physiological tests have been able to sensitively detect abnormalities and reversibility of pulmonary effects is with inhaled insulin. Insulin is one of many proteins that have been delivered systemically via inhalation (BRAIN, 2007). For the first approved insulin formulation (Exubera®), fully reversible changes in FEV1 and DLCO appeared within 1–3 weeks of initiation of therapy and returned to normal within 12 weeks of cessation of therapy (Liu et al., 2008). Interestingly, no significant effects were found on lung BAL fluid cellular composition or protein concentration.
On addressing the issue of the potential toxicity of inhaled ONs, the expert panel concurred with the Inhalation Subcommittee that current understanding of the pre-clinical effects of inhaled ONs in the lung is not well understood. The panel recommended that the risk assessment for inhaled ONs be improved by understanding the generalizability of findings between different subtypes of ONs and by evaluating the consequences of these inhaled drugs with chronic exposures to determine if tolerance, or lesion progression, occurs. Obtaining a better understanding of the mechanism of particulate uptake by phagocytic cells and the resultant activation state engendered by ingestion of ONs was also endorsed.
Regarding monitoring pulmonary inflammation or the consequence of the inflammatory response, the methods that were discussed included the majority of the generally known techniques used for the clinical assessment of lung toxicity. The panel noted that despite different systemic toxicological and tolerability profiles of various ONs, the spectrum of potential toxic effects observed in pre-clinical toxicology studies was relatively similar to that observed with other inhaled drugs and therefore no additional precaution was warranted. Available methods, such as those discussed above, should be considered for monitoring for specific pulmonary toxicity, safety, and tolerability. Selection of one or more of these methods for the characterization of pulmonary safety and their incorporation during development needs to be performed on a case-by-case basis. This recommendation challenges the presumption that lung toxicity cannot be monitored. Indeed, the panel believes that monitoring techniques are available, have adequate levels of sensitivity, and, therefore, could be used to detect lung inflammation or its consequence. As with any diagnostic test, there is the possibility that something may be missed or even that the most appropriate test may not be performed. However, the panel believes that if appropriate tests of lung function, inflammation, and structure (as described above) are examined and the results are normal, then the absence of pulmonary toxicities would support human safety.
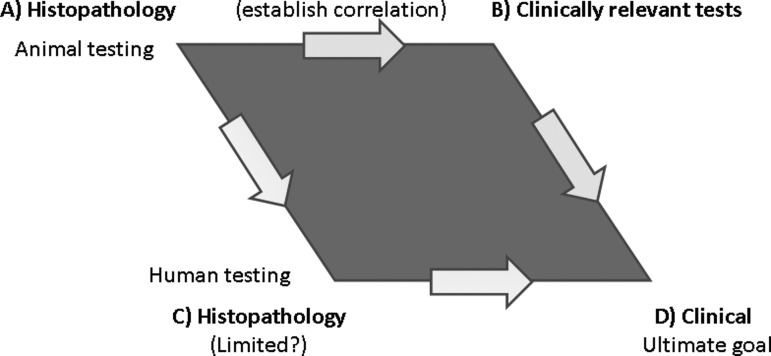
Verification of this conclusion is however needed. It was suggested that some of the clinical tests that are done in humans (e.g., BAL, pulmonary function, and possibly imaging) be done in animals to correlate with histopathology to help assess the sensitivity of these tests for subsequent clinical application (Fig. 1). Structure-function relationships of lung disease have been amply demonstrated, in particular using various rodent models (Costa et al., 1986). Similarly, the use of human lung biopsy tissue to help validate some of the more promising, less invasive clinical techniques, such as sputum induction, exhaled gases or breath condensates collection, and measurement of pulmonary function, was also encouraged. However, it was pointed out that BAL, induced sputum, and biopsies sample different compartments (complementary) of the lung, and therefore, correlation between the results of these methods may be difficult.
FIG. 1.
Pulmonary safety testing of oligonucleotides. The incorporation of clinical tests during the pre-clinical and clinical stage of development of inhaled oligonucleotides should establish a clear correlation with histopathology, assess the sensitivity of the tests, and ultimately confirm their utility for the detection of lung lesions in human.
Conclusion
In summary, pharmacokinetic and pharmacodynamic properties of inhaled ONs in animals support the lung as the likely target organ in humans. At present, the data do not suggest that inhaling ONs presents any more, or less, risk of toxicity than has been demonstrated with other novel classes of drugs. Thus, the panel concluded that pre-clinical testing for inhaled ONs should follow current best practice as applied to the assessment of other inhaled drug classes. A plan to adequately test their pre-clinical and then clinical safety, in discussion with the respective regulatory authorities, needs to be developed. As various inhaled ON drug candidates advance through development, unexpected toxicity may be encountered, which will help guide future evaluations. Although the reversible nature of the lung lesions is reassuring, it will be important to understand the evolution and resolution of these lesion over different patterns and durations of administration. The tests reviewed above appear to be appropriately sensitive to assess the safety of this class of drugs under these various condition in patients. The choice of these tests will depend on the disease of concern, the results from toxicology studies, and input from regulatory scientists.
Author Disclosure Statement
NF and RS were employees of and are currently consulting for Topigen Pharmaceuticals (part of the Pharmaxis Group), PMR is currently consulting for Topigen Pharmaceuticals (part of the Pharmaxis Group), JDP and RM are employed by GlaxoSmithKline R&D Ltd., HG holds shares in Sterna Biologicals and was employed by this company at the time of the preparation of this manuscript, JC is a consultant for Access BIO. HR is a consultant of Novartis, Boehringer Ingelheim, and Sterna Biologicals and has received research funding from Sterna Biologicals. The position paper from the Inhalation Oligonucleotide Subcommittee of the Oligonucleotides Safety Working Group does not reflect the position or opinions of the working group members from the United States Food and Drug Administration, nor that of the organization they represent.
References
- ALI S. LEONARD S.A. KUKOLY C.A. JAMES METZGER W. WOOLES W.R. MCGINTY J.F. TANAKA M. SANDRASAGRA A. NYCE J.W. Absorption, distribution, metabolism, and excretion of a respirable antisense oligonucleotide for asthma. Am. J. Respir. Crit. Care Med. 2001;163:989–993. doi: 10.1164/ajrccm.163.4.9907078. [DOI] [PubMed] [Google Scholar]
- ATTILI A.K. KAZEROONI E.A. GROSS B.H. FLAHERTY K.R. MYERS J.L. MARTINEZ F.J. Smoking-related interstitial lung disease: radiologic-clinical-pathologic correlation1. Radiographics. 2008;28:1383–1396. doi: 10.1148/rg.285075223. [DOI] [PubMed] [Google Scholar]
- BALL H. SANDRASAGRA A. TANG L. VAN SCOTT M. WILD J. NYCE J. Clinical potential of respirable antisense oligonucleotides (RASONs) in asthma. Am. J. Pharmacogenomics. 2003;3:97–106. doi: 10.2165/00129785-200303020-00003. [DOI] [PubMed] [Google Scholar]
- BELDA J. LEIGH R. PARAMESWARAN K. O'BYRNE P.M. SEARS M.R. HARGREAVE F.E. Induced sputum cell counts in healthy adults. Am. J. Respir. Crit. Care Med. 2000;161:475–478. doi: 10.1164/ajrccm.161.2.9903097. [DOI] [PubMed] [Google Scholar]
- BERRY M. MORGAN A. SHAW D.E. PARKER D. GREEN R. BRIGHTLING C. BRADDING P. WARDLAW A.J. PAVORD I.D. Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax. 2007;62:1043–1049. doi: 10.1136/thx.2006.073429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLANC P.D. WONG H. BERNSTEIN M.S. BOUSHEY H.A. An experimental human model of metal fume fever. Ann. Intern. Med. 1991;114:930–936. doi: 10.7326/0003-4819-114-11-930. [DOI] [PubMed] [Google Scholar]
- BRAIN J.D. Inhalation, deposition, and fate of insulin and other therapeutic proteins. Diabetes Technol. Ther. 2007;9:S4–S15. doi: 10.1089/dia.2007.0228. [DOI] [PubMed] [Google Scholar]
- BRASEY A. IGUE R. DJEMAME L. SÉGUIN S. RENZI P.M. FERRARI N. SEGUIN R. The effect of in vitro exposure to antisense oligonucleotides on macrophage morphology and function. J. Nucleic Acids Investig. 2011;2:e12. [Google Scholar]
- COSTA D.L. KUTZMAN R.S. LEHMANN J.R. DREW R.T. Altered lung function and structure in the rat after subchronic exposure to acrolein. Am. Rev. Respir. Dis. 1986;133:286–291. doi: 10.1164/arrd.1986.133.2.286. [DOI] [PubMed] [Google Scholar]
- CROOKE S.T. Vitravene: another piece in the mosaic. Antisense Nucleic Acid Drug Dev. 1998;8:7–8. doi: 10.1089/oli.1.1998.8.vii. [DOI] [PubMed] [Google Scholar]
- DEVINCENZO J. LAMBKIN-WILLIAMS R. WILKINSON T. CEHELSKY J. NOCHUR S. WALSH E. MEYERS R. GOLLOB J. VAISHNAW A. A randomized, double-blind, placebo-controlled study of an RNAi-based therapy directed against respiratory syncytial virus. Proc. Natl. Acad. Sci. U. S. A. 2010;107:8800–8805. doi: 10.1073/pnas.0912186107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEVINCENZO J. CEHELSKY J.E. ALVAREZ R. ELBASHIR S. HARBORTH J. TOUDJARSKA I. NECHEV L. MURUGAIAH V. VLIET A.V. VAISHNAW A.K. MEYERS R. Evaluation of the safety, tolerability and pharmacokinetics of ALN-RSV01, a novel RNAi antiviral therapeutic directed against respiratory syncytial virus (RSV) Antiviral Res. 2008;77:225–231. doi: 10.1016/j.antiviral.2007.11.009. [DOI] [PubMed] [Google Scholar]
- DIA/FDA/HEALTH CANADA/AAPS/OTS. 3rd Oligonucleotide based therapeutics conference–where regulators and industry partner to advance oligonucleotide science together; Mar 23–25;2010 ; Bethesda MD. 2010. [Google Scholar]
- DOE C. BAFADHEL M. SIDDIQUI S. DESAI D. MISTRY V. RUGMAN P. MCCORMICK M. WOODS J. MAY R., et al. Expression of the T helper 17-associated cytokines IL-17A and IL-17F in asthma and COPD. Chest. 2010;138:1140–1147. doi: 10.1378/chest.09-3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DREWEK R. GARBER E. STANCLIK S. SIMPSON P. NUGENT M. GERSHAN W. The FEF25–75 and its decline as a predictor of methacholine responsiveness in children. J. Asthma. 2009;46:375–381. doi: 10.1080/02770900802492079. [DOI] [PubMed] [Google Scholar]
- GAUVREAU G.M. HESSEL E.M. BOULET L.-P. COFFMAN R.L. O'BYRNE P.M. Immunostimulatory sequences regulate interferon-inducible genes but not allergic airway responses. Am. J. Respir. Crit. Care Med. 2006;174:15–20. doi: 10.1164/rccm.200601-057OC. [DOI] [PubMed] [Google Scholar]
- GAUVREAU G.M. BOULET L.P. COCKCROFT D.W. BAATJES A. COTE J. DESCHESNES F. DAVIS B. STRINICH T. HOWIE K., et al. Antisense therapy against CCR3 and the common beta chain attenuates allergen-induced eosinophilic responses. Am. J. Respir. Crit. Care Med. 2008;177:952–958. doi: 10.1164/rccm.200708-1251OC. [DOI] [PubMed] [Google Scholar]
- GREEN J.D. Pharmaco-toxicological expert report pulmozyme rhDNase Genentech. Inc. Hum. Exp. Toxicol. 1994;13:S1–S42. [PubMed] [Google Scholar]
- GUIMOND A. VIAU E. AUBE P. RENZI P.M. PAQUET L. FERRARI N. Advantageous toxicity profile of inhaled antisense oligonucleotides following chronic dosing in non-human primates. Pulm. Pharmacol. Ther. 2008;21:845–854. doi: 10.1016/j.pupt.2008.08.001. [DOI] [PubMed] [Google Scholar]
- HILLAS G. LOUKIDES S. KOSTIKAS K. BAKAKOS P. Biomarkers obtained by non-invasive methods in patients with COPD: where do we stand, what do we expect? Curr. Med. Chem. 2009;16:2824–2838. doi: 10.2174/092986709788803178. [DOI] [PubMed] [Google Scholar]
- HSIA C.C. Recruitment of lung diffusing capacity. Chest. 2002;122:1774–1783. doi: 10.1378/chest.122.5.1774. [DOI] [PubMed] [Google Scholar]
- KELLY M.M. LEIGH R. HARGREAVE F. Validation of assays for inflammatory mediators in sputum. Eur. Respir. J. 2000a;16:1208–1209. doi: 10.1034/j.1399-3003.2000.16f31.x. [DOI] [PubMed] [Google Scholar]
- KELLY M.M. HARGREAVE F.E. COX G. A method to preserve sputum for delayed examination. Eur. Respir. J. 2003;22:996–1000. doi: 10.1183/09031936.03.00036603. [DOI] [PubMed] [Google Scholar]
- KELLY M.M. LEIGH R. MCKENZIE R. KAMADA D. RAMSDALE E.H. HARGREAVE F.E. Induced sputum examination: diagnosis of pulmonary involvement in Fabry's disease. Thorax. 2000b;55:720–721. doi: 10.1136/thorax.55.8.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLY M.M. LEIGH R. JAYARAM L. GOLDSMITH C.H. PARAMESWARAN K. HARGREAVE F.E. Eosinophilic bronchitis in asthma: a model for establishing dose-response and relative potency of inhaled corticosteroids. J. Allergy Clin. Immunol. 2006;117:989–994. doi: 10.1016/j.jaci.2006.01.045. [DOI] [PubMed] [Google Scholar]
- KELLY M.M. KEATINGS V. LEIGH R. PETERSON C. SHUTE J. VENGE P. DJUKANOVIĆ R. Analysis of fluid phase mediators. Eur. Respir. J. 2002;37:24s–39s. [PubMed] [Google Scholar]
- KELLY M.M. O'CONNOR T.M. LEIGH R. OTIS J. GWOZD C. GAUVREAU G.M. GAULDIE J. O'BYRNE P.M. Effects of budesonide and formoterol on allergen-induced airway responses, inflammation, and airway remodeling in asthma. J. Allergy Clin. Immunol. 2010;125:349–356.e13. doi: 10.1016/j.jaci.2009.09.011. [DOI] [PubMed] [Google Scholar]
- KING G.G. Cutting edge technologies in respiratory research: lung function testing. Respirology. 2011;16:883–890. doi: 10.1111/j.1440-1843.2011.02013.x. [DOI] [PubMed] [Google Scholar]
- KRIEG A. The role of CpG motifs in innate immunity. Curr. Opin. Immunol. 2000;12:35–43. doi: 10.1016/s0952-7915(99)00048-5. [DOI] [PubMed] [Google Scholar]
- LEHTIMAKI L. KANKAANRANTA H. SAARELAINEN S. HAHTOLA P. JARVENPAA R. KOIVULA T. TURJANMAA V. MOILANEN E. Extended exhaled NO measurement differentiates between alveolar and bronchial inflammation. Am. J. Respir. Crit. Care Med. 2001;163:1557–1561. doi: 10.1164/ajrccm.163.7.2010171. [DOI] [PubMed] [Google Scholar]
- LEVIN A.A. A review of the issues in the pharmacokinetics and toxicology of phosphorothioate antisense oligonucleotides. Biochim. Biophys. Acta. 1999;1489:69–84. doi: 10.1016/s0167-4781(99)00140-2. [DOI] [PubMed] [Google Scholar]
- LEVIN A.A. HENRY S.P. MONTEITH D.K. TEMPLIN M. Toxicity of antisense oligonucleotides. In: Crooke S.T., editor. Antisense Drug Technology: Principles, Strategies, and Applications. Marcel Dekker, Inc.; New York: 2001. pp. 201–267. [Google Scholar]
- LEVIN A.A. MONTEITH D.K. LEEDS J.M. NICKLIN P.L. GEARY R.S. BUTLER M. TEMPLIN M.V. HENRY S.P. Toxicity of oligonucleotide therapeutic agents. In: Born G.V.R, et al., editors. Handbook of Experimental Pharmacology. Springer-Verlag; New York: 1998. pp. 169–215. [Google Scholar]
- LEWIS R.W. BILLINGTON R. DEBRYUNE E. GAMER A. LANG B. CARPANINI F. Recognition of adverse and nonadverse effects in toxicity studies. Toxicol. Pathol. 2002;30:66–74. doi: 10.1080/01926230252824725. [DOI] [PubMed] [Google Scholar]
- LIU M.C. RIESE R.J. VAN GUNDY K. NORWOOD P. SULLIVAN B.E. SCHWARTZ P.F. TEETER J.G. Effects of inhaled human insulin on airway lining fluid composition in adults with diabetes. Eur. Resp. J. 2008;32:180–188. doi: 10.1183/09031936.00129907. [DOI] [PubMed] [Google Scholar]
- MARTINEZ F.J. FLAHERTY K. Pulmonary function testing in idiopathic interstitial pneumonias. Proc. Am. Thorac. Soc. 2006;3:315–321. doi: 10.1513/pats.200602-022TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOSSER D.M. EDWARDS J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGATANI Y. NITTA N. OTANI H. MUKAISHO K. SONODA A. NITTA-SEKO A. TAKAHASHI M. MURATA K. Quantitative measurement of bleomycin-induced lung fibrosis in rabbits using sequential in vivo regional analysis and high-resolution computed tomography: correlation with pathologic findings. Acad. Radiol. 2011;18:672–681. doi: 10.1016/j.acra.2011.01.010. [DOI] [PubMed] [Google Scholar]
- NG E.W. ADAMIS A.P. Targeting angiogenesis, the underlying disorder in neovascular age-related macular degeneration. Can. J. Ophthalmol. 2005;40:352–368. doi: 10.1016/S0008-4182(05)80078-X. [DOI] [PubMed] [Google Scholar]
- SAHA S. DOE C. MISTRY V. SIDDIQUI S. PARKER D. SLEEMAN M. COHEN E. S. BRIGHTLING C.E. Granulocyte–macrophage colony-stimulating factor expression in induced sputum and bronchial mucosa in asthma and COPD. Thorax. 2009;64:671–676. doi: 10.1136/thx.2008.108290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAZANI P. WELLER D.L. SHREWSBURY S.B. Safety pharmacology and genotoxicity evaluation of AVI-4658. Int. J. Toxicol. 2010;29:143–156. doi: 10.1177/1091581809359206. [DOI] [PubMed] [Google Scholar]
- TASK FORCE, ESOP. Clinical guidelines and indications for bronchoalveolar lavage (BAL): report of the European Society of Pneumology task group on BAL. Eur. Respir. J. 1990;3:937–976. [PubMed] [Google Scholar]
- TEMPLIN M. LEVIN A. GRAHAM M. ABERG P. AXELSSON B. BUTLER M. GEARY R. BENNETT C. Pharmacokinetic and toxicity profile of a phosphorothioate oligonucleotide following inhalation delivery to lung in mice. Antisense Nucleic Acid Drug Dev. 2000;10:359–368. doi: 10.1089/oli.1.2000.10.359. [DOI] [PubMed] [Google Scholar]
- VEEN J.C. BEEKMAN A.J. BEL E. H. Sterk P.J. Recurrent exacerbations in severe asthma are associated with enhanced airway closure during stable episodes. Am. J. Respir. Crit. Care Med. 2000;161:1902–1906. doi: 10.1164/ajrccm.161.6.9906075. in ’t. [DOI] [PubMed] [Google Scholar]
- VERHOEVEN G.T. WIJKHUIJS A.J. HOOIJKAAS H. HOOGSTEDEN H.C. SLUITER W. Effect of an inhaled glucocorticoid on reactive oxygen species production by bronchoalveolar lavage cells from smoking COPD patients. Mediators Inflamm. 2000;9:109–113. doi: 10.1080/096293500411578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERSCHAKELEN J.A. The role of high-resolution computed tomography in the work-up of interstitial lung disease. Curr. Opin. Pulm. Med. 2010;16:503–510. doi: 10.1097/MCP.0b013e32833cc997. [DOI] [PubMed] [Google Scholar]
- VLACHOS-MAYER H. LEIGH R. SHARON R. HUSSACK P. HARGREAVE F. Success and safety of sputum induction in the clinical setting. Eur. Resp. J. 2000;16:997–1000. doi: 10.1183/09031936.00.16599700. [DOI] [PubMed] [Google Scholar]
- ZHANG J. YAO X. YU R. BAI J. SUN Y. HUANG M. ADCOCK I. BARNES P. Exhaled carbon monoxide in asthmatics: a meta-analysis. Resp. Res. 2010;11:50. doi: 10.1186/1465-9921-11-50. [DOI] [PMC free article] [PubMed] [Google Scholar]