Abstract
Purpose
Defects in the antigen processing machinery (APM) may provide tumor cells with a mechanism to escape immune recognition. The purpose of this study is to determine the clinical significance of APM component down-regulation and tumor-infiltrating T cells in ovarian carcinoma.
Experimental Design
After institutional review board approval, tumor samples from 150 patients with invasive epithelial ovarian cancers were examined for TAP1, TAP2, tapasin, HLA class I heavy chain (HLA-HC), β2 microglobulin, and T-cell (CD3+ and CD8+) tumor infiltration using immunohistochemistry.
Results
The majority of tumors had either heterogeneous or positive expression of TAP1, TAP2, HLA-HC, and β2 microglobulin (66.7%, 73.3%, 70.7%, and 63.3%, respectively), except tapasin for which 58% of the tumors lacked expression. Furthermore, 67% and 88% of the lesions possessed intratumoral and peritumoral CD3+ or CD8+ cells, respectively. The majority of APM component expression examined was significantly associated with both intratumoral and peritumoral T-cell infiltration (P < 0.05). The expression of APM components and the presence of intratumoral T-cell infiltrates were significantly associated with improved survival (all P ≤ 0.01); however, peritumoral T-cell infiltrates did not significantly affect survival (P = 0.33). APM component down-regulation (P < 0.001), lack of intratumoral T-cell infiltrates (P = 0.03), and suboptimal cytoreduction (P < 0.001) were independent prognostic markers for death from ovarian carcinoma.
Conclusion
The negative effectof APM component down-regulation by itself and in combination with absent intratumoral T-cell infiltration on the survival of patients with ovarian carcinoma implies a role for immune escape in addition to immunosurveillance in the clinical course of disease.
With the highest mortality of all cancers of the female reproductive tract, ovarian carcinoma will claim an estimated 15,280 lives in the United States in 2007 (1). Surgical debulking and chemotherapy with paclitaxel and carboplatin remain the standard of care; however, most patients will eventually develop drug resistance and succumb to this disease (2). These clinical findings have emphasized the need to develop novel therapeutic strategies that take advantage of an improved understanding of relevant events within the tumor microenvironment that affect patient survival.
T-cell–based immunotherapy has attracted much attention in recent years because immunotherapeutic strategies have been convincingly shown to control tumor growth in animal models (3). Furthermore, the identification of human tumor antigens has provided well-defined moieties to immunize patients with malignant diseases and to monitor tumor antigen–specific immune responses in immunized patients. Contrary to expectations, however, clinical responses have been observed in only a minority of the immunized patients and no correlation has been found between induction of a T-cell immune response and clinical response (4, 5). These disappointing clinical results have stimulated interest in defining the mechanisms by which tumor cells escape from the host's immune recognition and destruction. Several escape mechanisms have been identified in various types of tumors. Among them are abnormalities in the expression and/or function of antigen processing machinery (APM) components and/or HLA class I antigen subunits, which lead to defects in the expression of HLA class I antigen-tumor antigen–derived peptide complexes (6–9). These complexes mediate the recognition of tumor cells by HLA class I antigen restricted, tumor antigen–specific CTLs. APM plays a critical role in the processing and presentation of tumor antigens for recognition of tumor cells by cytotoxic T cells. The frequency of APM down-regulation has been shown to be higher in metastasis than in primary lesion. In some malignancies, it is significantly associated with higher grade, aggressive histology, and abnormal DNA content (10, 11). Furthermore, APM component down-regulation in tumor lesions is associated with reduced patient survival in certain carcinomas (12–14).
The downstream effect of intact APM is the activation of cytotoxic T cells. Tumor-infiltrating T cells have been shown to confer a survival advantage in several cancers, including ovarian, breast, and colorectal carcinomas (15–18). However, to our knowledge, the clinical significance of APM components in the context of tumor-infiltrating T cells in ovarian carcinoma is not known. The purpose of our study is to determine the frequency of APM component down-regulation and its effect on T-cell infiltration in the tumor microenvironment as it relates to patient survival in ovarian carcinoma.
Materials and Methods
Patient population
Institutional review board approval was obtained and 150 archived primary invasive ovarian epithelial cancer samples collected between 1988 to 2006 were gathered from the established institutional tumor bank. The average follow-up period was 22.2 mo (range 1–110 mo). All women whose samples were included had undergone initial cytoreductive surgery, and 92% subsequently went on to receive adjuvant chemotherapy with carboplatin and paclitaxel. We excluded samples from patients with metastases to the ovaries from other organs, synchronous gynecologic primary malignancies, borderline tumors, previous cancer diagnosis, regular use of systemic steroid medication in the month before tumor debulking, and presence of a comorbid condition with known effects on the immune system such as autoimmune diseases.
Immunohistochemistry
Paraffin-embedded tissue samples were cut to 4- to 5-μm thickness and were fixed on glass slides. Tissue sections were dewaxed on a hot plate set at 60°C for 30 min, dewaxed in xylene, and rehydrated in successively dilute solutions of ethanol. Antigen retrieval was done by immersing tissue slides in citrate buffer (pH 6.0) and then microwaving them for 10 min. After rinsing with TBS, we treated the samples with 30% hydrogen peroxide solution to block endogenous peroxidase activity. To minimize nonspecific antibody binding, we incubated the tissue with blocking serum consisting of 10% normal horse serum at room temperature for 30 min.
Next, we applied the murine generated primary antibodies [TAP1-specific monoclonal antibody NOB-1, TAP2-specific monoclonal antibody NOB-2, tapasin-specific monoclonal antibody TO-3, HLA class I heavy chain (HLA-HC)– specific monoclonal antibody HC-10, and β2 microglobulin–specific monoclonal antibody L368 were developed and previously described in refs. 19–23] at a dilution of 1:100 for 60 min using the blocking serum as the diluent. The samples were incubated with horse anti-mouse biotinylated secondary antibodies (ABC Vector Labs) for 30 min at room temperature to enhance binding and signaling and then exposed to streptavidin–horseradish peroxidase for an additional 30 min at room temperature. Binding signal was visualized after incubation with 3,3′-diaminobenzidine (Phoenix Biotechnologies) and counterstained with Gil's no. 3 hematoxylin (Sigma Chemicals Co.). Negative controls were done by omitting the primary antibody (14).
We used similar deparaffinization and antigen retrieval conditions for CD3+ and CD8+ T-cell immunostaining. The primary antibodies, provided in culture supernatant configuration in 1-mL vials (Dako), were diluted at 1:100 in 10% fish gelatin for optimal amount of antibody and incubated with the tumor samples at room temperature for 60 min. Signal visualization was achieved using the EnVision + Dual Link System (Dako) with subsequent reaction in 3,3′-diaminobenzidine and counterstained with Gil's no. 3 hematoxylin. For negative controls, the primary antibody was omitted, whereas human thymus was used as a positive control.
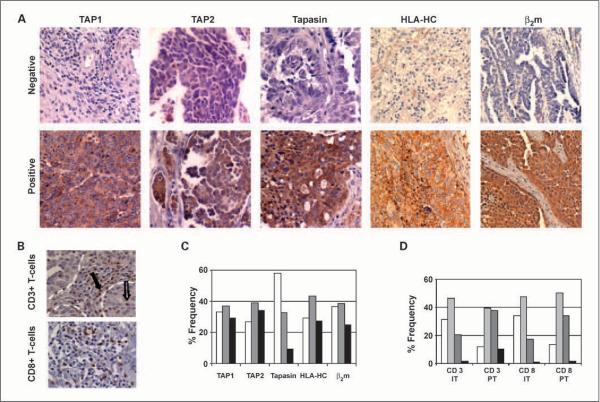
A board-certified pathologist who was blinded to the patients' clinical outcome scored the stained slides. To avoid variability, our scoring was done using the criteria established by the HLA and Cancer Component of the 12th International Histocompatibility Workshop (24). Briefly, tumors were scored as negative if the percentage of the tumor cells stained in an entire lesion was <25%, and positive if >75% or heterogeneous if staining pattern was intermediate (Fig. 1A). Scoring for T-cell presence was categorized based on the number of T cells per high-power field: 0 (0 T cells), 1+ (≤5 T cells), 2+ (6–19 T cells), or 3+ (≥20 T cells; Fig. 1B; ref. 15). T-cell hotspots in and around tumor lesion were identified and 15 high-power fields were counted.
Fig. 1.
Representative staining pattern of APM components and T cells. A, staining patterns for negative versus positive (including heterogeneous) immunostaining in epithelial ovarian carcinoma. Original magnification, ×100. B, immunohistochemical staining patterns for positive CD3+ and CD8+ T cells. Solid arrow, CD3+ T cell in the peritumoral tissue; open arrow, intratumoral CD3+ T cell. Borders of tumor and stroma tissues are demarcated by white dotted lines. Original magnification, ×200. C, distribution of staining patterns for the various APM components in epithelial ovarian cancers. D, distribution of staining patterns for the CD3+/CD8+ T cells. IT, intratumoral; PT, peritumoral.
Clinicopathologic data
Patient charts were reviewed for clinical and pathologic information, including International Federation of Gynecologists and Obstetricians stage, presence or absence of ascites, residual disease after tumor cytoreductive surgery, operative findings, time to recurrence from diagnosis, and demise. All patients were surgically staged by International Federation of Gynecologists and Obstetricians standards. Optimal cytoreduction was defined as <1 cm of residual disease following surgery. At time of diagnosis, the pathology of all patients with cancer was reviewed by a gynecologic pathologist. The status of each patient was recorded as alive without disease, alive with disease, dead of disease, or dead of other causes. Furthermore, disease-specific survival was defined as the interval from the date of diagnosis to the date of death from ovarian cancer or the date of last follow-up. Patients who died from causes other than ovarian cancer were censored.
Statistical analyses
The χ2 test was used to determine the association between binary categories of APM component expression (negative versus heterogeneous or positive) and traditional clinical variables. The Wilcoxon rank sum test was used to determine significance with continuous clinical variables such as age and preoperative CA125 levels. Survival time was estimated using the Kaplan-Meier product limit method. The two-sided log-rank test was used to detect the difference in survival. Univariate associations between number of deaths and potential prognostic factors were studied by examining the Cox proportional hazard model. Wald χ2 P values were used to calculate univariate statistical significance, and 95% confidence intervals (95% CI) were estimated.
Finally, survival was explored in a multivariate setting using a proportional hazard regression model in a stepwise selection method to derive independent prognostic factors for death due to disease. An initial model with all prognostic factors of interest was explored, whereas a second model using stepwise selection was created to determine the remaining variables that influenced survival time. The entry criterion for this second model was α ≤ 0.25, and the staying requirement was α ± 0.10. However, because the goal of this study was to examine the significance of CD3+/CD8+ T cells, our final multivariate analysis also included peritumoral CD3+/CD8+ T cells in the model. Furthermore, we analyzed the number of APM components as a continuous variable in this model, and to justify this choice, a Martingale residual plot was generated to examine the effects of additional APM component expression on survival time. A linear risk ratio was determined, indicating that survival is lengthened with increasing number of APM components expressed. Therefore, the number of APM component was studied as a continuous variable in this multivariate model. P values <0.05 were considered significant in all analyses. All of the statistical analyses were done using SAS version 9.0 (SAS Institute).
Results
Patient characteristics
Our study collection comprised samples from 150 women with primary epithelial ovarian carcinoma who underwent surgical tumor cytoreduction. The median age of the patients at time of diagnosis was 61 years (range 28–89 years), and the median preoperative CA125 level was 2,937.7 units/mL (range 21–77,413units/mL). Ninety-one percent and 92% of the patients had high-stage and high-grade cancers, respectively (Table 1). Specifically, 9 patients had stage I disease, 4 had stage II, 107 had stage III, and 30 had stage IV ovarian cancers. In terms of histologic grade, 12 patients had low-grade disease, and 138 had high-grade tumors (33 had grade 2, and 105 had grade 3). Nonserous histologies included 14 patients with endometrioid subtype, 8 with mucinous, and the remainder included other histologies like transitional or clear cells. Ascites was detected in 77% of patients and 60% underwent an optimal cytoreductive surgery.
Table 1.
Patient characteristics
| Clinical variables | n (% Total) |
|---|---|
| Stage | |
| Low (I and II) | 13 (8.7) |
| High (III and IV) | 137 (91.3) |
| Grade | |
| Low (1) | 12 (8.0) |
| High (2 and 3) | 138 (92.0) |
| Histology | |
| Nonserous | 32 (21.3) |
| Serous | 118 (78.7) |
| Ascites | |
| Absent | 34 (22.7) |
| Present | 116 (77.3) |
| Cytoreduction | |
| Optimal | 90 (60) |
| Suboptimal | 60 (40) |
| Lymph nodes | |
| Negative | 23 (15.5) |
| Positive | 25 (16.9) |
| Not done | 102 (67.6) |
| Distant metastasis | |
| Absent | 120 (80.0) |
| Present | 30 (20.0) |
Clinical significance of APM component defects
The majority of the tumor samples had heterogeneous or positive staining for TAP1, TAP2, HLA-HC, and β2 microglobulin (66.7%, 73.3%, 70.7%, and 63.3%, respectively). However, 58% of the samples were negative for tapasin expression (Fig. 1C). Next, we examined the association of APM component expression with traditional clinical variables and against other individual APM components (Table 2). TAP1 expression, scored as heterogeneous or positive, was highly associated with low-stage disease (P = 0.008), low-grade disease (P = 0.011), and negative lymph nodes (P = 0.006). TAP2 expression, similarly scored, was associated with low-grade disease (P = 0.029) and negative lymph nodes (P = 0.021) whereas tapasin expression was significantly associated with younger age, low-stage, low-grade disease, and negative lymph nodes (P = 0.035, 0.001, 0.003, and 0.045, respectively). There was no association between HLA-HC expression and these clinical variables. β2 Microglobulin expression was associated with low-grade disease (P = 0.035) and absence of distant metastasis (P = 0.011).
Table 2.
Association between traditional clinical variables and APM/T-cell factors
| Clinical variables | Positive expression |
||||||
|---|---|---|---|---|---|---|---|
| TAP1 | TAP2 | Tapasin | HLA-HC | β2m | IT CD3+/CD8+ T cells | PT CD3+/CD8+ T cells | |
| Age | NS | NS | 0.035 | NS | NS | NS | NS |
| Low stage | 0.008 | NS | 0.001 | NS | NS | NS | 0.001 |
| Low grade | 0.011 | 0.029 | 0.003 | NS | 0.035 | NS | NS |
| Serous histology | NS | NS | NS | NS | NS | NS | NS |
| Preoperative CA125 | NS | NS | NS | NS | NS | NS | 0.014 |
| Ascites (−) | NS | NS | NS | NS | NS | NS | NS |
| Optimal cytoreduction | NS | NS | NS | NS | NS | NS | NS |
| Lymph nodes (−) | 0.006 | 0.021 | 0.045 | NS | NS | NS | 0.018 |
| Distant metastasis (−) | NS | NS | NS | NS | 0.011 | NS | NS |
| Positive expression | |||||||
| TAP1 | ** | <0.001 | <0.001 | NS | <0.001 | NS | 0.018 |
| TAP2 | <0.001 | ** | <0.001 | <0.001 | <0.001 | 0.003 | 0.001 |
| Tapasin | <0.001 | <0.001 | ** | 0.007 | <0.001 | 0.007 | NS |
| HLA-HC | NS | <0.001 | 0.007 | ** | <0.001 | 0.005 | <0.001 |
| β2m | <0.001 | <0.001 | <0.001 | <0.001 | ** | 0.001 | 0.011 |
| IT CD3+/CD8+ T cells | NS | 0.003 | 0.007 | 0.005 | 0.001 | ** | <0.001 |
| PT CD3+/CD8+ T cells | 0.018 | 0.001 | NS | <0.001 | 0.011 | <0.001 | ** |
NOTE: χ2 Test was used for categorical data and the Wilcoxon rank sum test was used for continuous variables.
Abbreviations: NS, not significant; β2m, β2 microglobulin; IT, intratumoral; PT, peritumoral;
NA, not applicable.
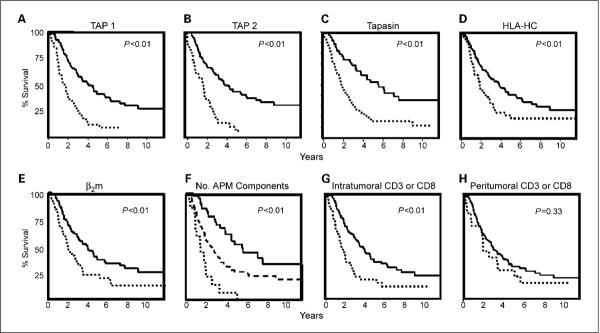
Based on the association of APM component expression with several clinical features, we next identified its potential effects on patient survival using the Kaplan-Meier method. For each individual APM component analyzed, patients with tumors of either heterogeneous or positive score had a significantly longer survival than those with negative expression (all P < 0.01; Fig. 2A–E). Specifically, patients with intact expression of TAP1 had an overall survival of 4.08 years versus 1.75 years for patients who lacked TAP1 expression [hazard ratio (HR), 0.38; 95% CI, 0.25–0.57]. A similar survival benefit also accrued to patients whose tumor samples expressed TAP2: Their median survival was 3.97 years versus 1.78 years for their negative expression counterparts (HR, 0.32; 95% CI, 0.21–0.49). This advantageous trend is carried on for patients with intact expression of tapasin, HLA-HC, or β2 microglobulin; in fact, those with positive individual expressions were 0.39 (95% CI, 0.26–0.60), 0.57 (95% CI, 0.38–0.85), or 0.54 (95% CI, 0.36–0.81) times less likely to die from their disease, respectively. Patients with positive expression of tapasin lived a median of 5.75 years whereas those with defects in tapasin expression lived 1.98 years; those with intact HLA-HC survived 3.92 years versus 1.92 years for those with defects in HLA-HC and β2 microglobulin, 3.95 years versus 1.92 years, all P < 0.01. Patients with tumors lacking all APM components had approximately half as long survival (median survival of 1.44 years versus 2.58 years; P < 0.01) compared with those with one to four positive markers (Table 3). Patients with all five markers positive in the tumor lived almost four times as long (median survival 5.67 years; P < 0.01) and were 4.74 times less likely to die from their disease (Fig. 2F).
Fig. 2.
Kaplan Meier survival curves for ovarian carcinoma patients based on the presence (solid lines) or absence (dotted lines) of APM components or tumoral T cells. A, TAP1 (HR, 0.38; 95% CI, 0.25–0.57), median survival of 4.08 y for patients with intact expression versus 1.75 y for patients with absent expression; B, TAP2 (HR, 0.32; 95% CI, 0.21–0.49), median survival of 3.97 y versus 1.78 y. C, tapasin (HR, 0.39; 95% CI, 0.26–0.81), median survival of 5.75 y versus 1.98 y. D, HLA-HC (HR, 0.57; 95% CI, 0.38–0.85), median survival of 3.92 y versus 1.92 y. E, β2 microglobulin (β2m; HR, 0.54; 95% CI, 0.36–0.81), median survival of 3.95 y versus 1.92 y. F, response by number of APM components expressed: solid line, presence of all five APM components; dashed line, one to four components intact (HR, 1.95; 95% CI, 1.21–3.14); dotted line, complete absence of all five components (HR, 4.74; 95% CI, 2.40–9.36). Median survival of 1.44 y (none) versus 2.58 y (1–4 APM component expressed) versus 5.67 y (all five APM components expressed). G, intratumoral CD3+ or CD8+ T cells (HR, 2.04; 95% CI, 1.35–3.07), median survival 1.67 y versus 3.79 y. H, peritumoral CD3+ or CD8+ T cells (HR, 1.32; 95% CI, 0.75–2.33), median survival of 2 y versus 3.02 y.
Table 3.
Univariate survival analysis
| Variables | No. patients (no. death) | Median survival (y) | HR (95% CI) | P |
|---|---|---|---|---|
| APM component markers | ||||
| All (−) | 15 (14) | 1.44 | 4.74 (2.40–9.36) | <0.01 |
| 1–4 (+/−) or (+) | 93 (65) | 2.58 | 1.95 (1.21–3.14) | <0.01 |
| All (+/−) or (+) | 42 (23) | 5.67 | ||
| IT CD3+/CD8+ T cells | ||||
| Absent | 44 (35) | 1.67 | 2.04 (1.35–3.07) | <0.01 |
| Present | 106 (67) | 3.79 | ||
| PT CD3+/CD8+ T cells | ||||
| Absent | 17 (14) | 2.00 | 1.32 (0.75–2.33) | 0.33 |
| Present | 133 (88) | 3.02 | ||
| Stage | ||||
| High | 137 (100) | 2.67 | 10.26 (2.52–41.81) | <0.01 |
| Low | 13 (2) | NR | ||
| Grade | ||||
| High | 138 (97) | 2.81 | 3.25 (1.31–8.06) | 0.01 |
| Low | 12 (5) | NR | ||
| Histology | ||||
| Serous | 118 (82) | 2.81 | 1.66 (1.01–2.71) | 0.05 |
| Nonserous | 32 (20) | 5.10 | ||
| Cytoreduction | ||||
| Suboptimal | 60 (54) | 1.54 | 2.93 (1.97–4.37) | <0.01 |
| Optimal | 90 (47) | 4.89 |
Abbreviations: (+/−), heterogeneous; NR, not reached.
Our initial analyses included patients with low-grade tumors. Because low-grade tumors often have a different clinical course than their higher-grade counterparts, we also did analyses focusing only on patients with high-grade ovarian cancers. A similar trend was noted with number of positive APM markers, presence of intratumoral CD3+/CD8+ T cells, stage, and level of cytoreduction being significant effectors of survival in univariate analyses. Similarly, in patients presenting with advanced disseminated disease (stages III and IV), this trend continues to hold. In addition, the presence of peritumoral CD3+/CD8+ T cells had a significant effect on survival: Patients with peritumoral CD3+/CD8+ T cells had a median survival of 2.83 years versus patients without who had a median survival of 1.85 years (P = 0.01; Table 4).
Table 4.
Univariate survival analysis for patients with stage III and IV ovarian cancers
| Variables | No. patients (no. death) | Median survival (y) | HR (95% CI) | P |
|---|---|---|---|---|
| APM component markers | ||||
| All (−) | 15 (14) | 1.44 | 4.12 (2.06–8.23) | <0.01 |
| 1–4 (+/−) or (+) | 87 (64) | 2.29 | 1.92 (1.17–3.15) | 0.01 |
| All (+/−) or (+) | 35 (22) | 4.58 | ||
| IT CD3+/CD8+ T cells | ||||
| Absent | 40 (33) | 1.52 | 2.11 (1.39–3.22) | <0.01 |
| Present | 97 (67) | 3.37 | ||
| PT CD3+/CD8+ T cells | ||||
| Absent | 12 (12) | 1.85 | 2.26 (1.23–4.16) | 0.01 |
| Present | 125 (88) | 2.83 | ||
| Grade | ||||
| High | 131 (96) | 2.58 | 1.82 (0.66–4.97) | 0.25 |
| Low | 6 (4) | 5.39 | ||
| Histology | ||||
| Serous | 112 (81) | 2.58 | 1.34 (0.81–2.23) | 0.25 |
| Nonserous | 25 (19) | 3.42 | ||
| Cytoreduction | ||||
| Suboptimal | 60 (54) | 1.54 | 2.43 (1.63–3.64) | <0.01 |
| Optimal | 77 (46) | 3.37 |
Significance of infiltrating tumoral T cells
Given the important role of infiltrating tumoral T cells as a downstream effect of an intact APM mechanism, we examined intratumoral and peritumoral (in the stromal tissues that surround and support the tumor cells) CD3+ and CD8+ T cells. CD3+ T cells transmit the activation signal to cytotoxic T cells upon antigen recognition, and CD8+ T cells activate coreceptors specific to class I MHC molecules (25). Because our ultimate interest is the activation of T cells, CD3+ and CD8+ T cells were analyzed together with distinction based on their location: intratumoral versus peritumoral. Analysis of tumor-infiltrating T cells revealed that collectively, the majority of patients showed some degree of intratumoral and peritumoral T-cell infiltration. Specifically, 67% and 88% of tumors contained at least 5 CD3+/CD8+ T cells (i.e., staining score of 1+), respectively (Fig. 1D). The presence of peritumoral CD3+/CD8+ T cells was significantly associated with low stage (P = 0.001), lower levels of preoperative CA125 (P = 0.014), and negative lymph nodes (P = 0.018). The presence of either intratumoral or peritumoral T cells was highly associated with positive expression of most of the APM components analyzed (Table 2). In addition, intratumoral T-cell infiltration significantly affected patient survival (Fig. 2G) whereas peritumoral T-cell infiltration did not (Fig. 2H). Patients with complete absence of tumor-infiltrating T cells were 2.04 times more likely to die from their disease (95% CI, 1.35–3.07) than those with one or more T cells (P < 0.01; median survival 1.67 years versus 3.79 years).
Based on the significant associations noted between APM component expression, T-cell infiltration, and survival, we examined the effects of these variables in the context of traditional prognostic factors (Table 3). As expected, traditional clinical variables such as low-stage, low-grade, and suboptimal cytoreduction were significantly associated with longer survival (P < 0.01; P = 0.01; P < 0.01, respectively) in univariate analyses. Even after adjusting for stage, grade, serous histology, and cytoreduction in a multivariate survival analysis, the number of positive APM components proved to be an independent prognostic factor for survival (P < 0.001; Table 5). Initial analysis by the Martingale residual plot established a linear relationship between increasing number of positive APM components and longer survival. Therefore, this factor was examined as a continuous variable. Furthermore, lack of intratumoral CD3+/CD8+ T cells also emerged as a significant independent poor prognostic factor for death from ovarian carcinoma (P = 0.03) as was suboptimal cytoreduction (P < 0.001). When similar analyses were done for patients with advanced-stage ovarian carcinoma, these trends hold with the number of APM markers and suboptimal cytoreduction being independent prognostic factors for survival. Although peritumoral presence of CD3+/CD8+ T cells was a significant survival factor in the univariate analyses limited to patients with advanced-stage cancer, it did not emerge as a significant factor in multivariate analyses.
Table 5.
Multivariate survival analysis
| Clinical variables | HR (95% CI) | P |
|---|---|---|
| No. APM markers | 0.71 (0.61–0.83) | <0.001 |
| Absent IT CD3+/CD8+ T cells | 1.76 (1.06–2.92) | 0.030 |
| Absent PT CD3+/CD8+ T cells | 0.72 (0.35–1.48) | NS |
| High stage | 3.64 (0.79–16.83) | NS |
| High grade | 0.92 (0.34–2.49) | NS |
| Serous histology | 1.32 (0.79–2.22) | NS |
| Suboptimal cytoreduction | 3.22 (2.07–5.02) | <0.001 |
Discussion
The key finding from this study is that the number of positive APM component expression (TAP1, TAP2, tapasin, HLA-HC, and β2 microglobulin) is an independent prognostic factor for death from disease in patients with ovarian carcinoma. Furthermore, the complete lack of tumor-infiltrating T cells conveys unfavorable prognosis after adjusting for traditional clinical factors such as stage, grade, histology, and cytoreduction. The presence of any one of the various APM components expressed was highly associated with each other and with positive intratumoral T cells, thereby suggesting an integral and group effect.
CTLs play an active role in the recognition and destruction of tumor cells. Its activation and regulation involve a complicated network of proteins, starting with HLA class I molecule binding to tumor antigen–derived peptides. This is a multistep process such that down-regulation of any protein along the pathway may provide a mechanism of tumor evasion from the immune system. The presentation of processed tumor antigens requires transportation via TAP1 and TAP2 into the endoplasmic reticulum, and subsequent assembly with HLA-HC and β2 microglobulin with the aid of chaperone proteins such as tapasin (26). Ultimately, the complex dissociates from the chaperone proteins and translocates to the cellular surface through the Golgi pathway (10, 26). The interaction of tumor antigen–derived peptides with T-cell receptors initiates a cascade of events leading to recognition and activation of CTL (27, 28). It is, therefore, conceivable that due to tumoral inherent genetic instability, any down-regulation of the APM component could lead to ineffectual or absent T-cell response, providing a mechanism of immune escape (29).
Our findings show that intact expression of APM components is strongly associated with longer survival of ovarian carcinoma patients. Specifically, there seems to be an incremental survival advantage with an increasing number of intact APM components. This is a novel finding that supports the notion that APM exerts a collective effort for tumor antigen presentation and recruitment of CTL.
Down-regulation of APM components, such as TAP1, TAP2, and tapasin, has been found to be associated with failure of CTL recognition in squamous cell carcinoma of the head and neck (30). Moreover, APM component down-regulation was associated with a significant decrease in overall survival in patients with cancer from this disease site (12). In both renal cell and colorectal carcinomas, APM component defects were found to be a prevalent phenomenon and associated with K-Ras mutations in the latter, offering additional mechanistic support for a possible relationship between immune system dysfunction and transformation of a proto-oncogene (31, 32). However, before our study, examination of patient survival as a function of increasing presence of APM component had not been addressed.
Tumor antigen presentation via APM induces CTL; therefore, examination of tumor-infiltrating T cells provides a specific measurement of immune response. Zhang and colleagues concluded that tumor-infiltrating T cells may be a marker of antitumor response mechanism; furthermore, the absence of intratumoral T cells is associated with higher levels of vascular endothelial growth factor, a known poor prognostic factor in ovarian carcinoma (15, 33). In colorectal cancer, the presence of T cells within tumors was associated with beneficial markers such as absent vascular emboli, lymphatic invasion, and perineural invasion (34). Similarly, we have determined that intratumoral infiltration of T cells significantly and independently affects prolonged patient survival in ovarian carcinoma. Additionally, our data indicate that although T cells in the surrounding host stromal tissue are associated with low-stage disease, lower preoperative CA125 levels, and negative lymph nodes, peritumoral CD3+/CD8+ T cells do not significantly contribute to survival, suggesting that the critical step remains in the interaction between tumor antigen presentation and T-cell activation within the actual tumor microenvironment. In addition, when our analyses were restricted to patients with advanced-stage disease (stages III and IV), peritumoral presence of CD3+/CD8+ T cells seemed to have a significant effect on patient survival. However, this factor was not significant in multivariate analyses.
Previous studies regarding APM component down-regulation and its effect on cancer patient survival have shown varying results. These inconsistencies highlight the complex network of the immune system with its positive and negative regulators (35, 36). Although our report is a documentation of the clinical effects of APM component down-regulation and tumor-infiltrating T cells, there are other known factors such as IFNγ, reported to induce APM activity and restore T-cell recognition, or natural killer cells, another key element in immunosurveillance, that may merit further consideration in future immunotherapeutic trials (12, 37).
In summary, APM component down-regulation and subsequent lack of intratumoral T cells are independent prognostic factors for death from disease in patients with ovarian carcinoma. Immune escape occurs in a substantial proportion of patients with ovarian cancers and is predictive of poor clinical outcome. Methods to overcome this challenge may improve the efficacy of T-cell immunotherapeutic strategies.
Acknowledgments
We thank Dr. David Lubaroff for helpful input and discussion regarding this work, Donna Reynolds for her assistance with immunohistochemistry, and Walter Pagel for his suggestions during the writing of the manuscript.
Grant support: National Cancer Institute grants CA110793 and CA109298, The Marcus Foundation, and The University of Texas M. D. Anderson Cancer Center Specialized Programs of Research Excellence in ovarian cancer grant 2P50CA083639-06A1.
Footnotes
Disclosure of Potential Conflicts of Interest No potential conflicts of interest were disclosed.
References
- 1.Cancer Facts and Figures 2007. American Cancer Society; Atlanta (GA): 2007. [Google Scholar]
- 2.Agarwal R, Kaye SB. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat Rev Cancer. 2003;3:502–16. doi: 10.1038/nrc1123. [DOI] [PubMed] [Google Scholar]
- 3.Palena C, Abrams SI, Schlom J, Hodge JW. Cancer vaccines: preclinical studies and novel strategies. Adv Cancer Res. 2006;95:115–45. doi: 10.1016/S0065-230X(06)95004-0. [DOI] [PubMed] [Google Scholar]
- 4.Steinman RM, Mellman I. Immunotherapy: bewitched, bothered, and bewildered no more. Science. 2004;305:197–200. doi: 10.1126/science.1099688. [DOI] [PubMed] [Google Scholar]
- 5.Blattman JN, Greenberg PD. Cancer immunotherapy: a treatment for the masses. Science. 2004;305:200–5. doi: 10.1126/science.1100369. [DOI] [PubMed] [Google Scholar]
- 6.Chang CC, Campoli M, Ferrone S. HLA class I antigen expression in malignant cells: why does it not always correlate with CTL-mediated lysis? Curr Opin Immunol. 2004;16:644–50. doi: 10.1016/j.coi.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74:181–273. doi: 10.1016/s0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- 8.Marincola FM, Wang E, Herlyn M, Seliger B, Ferrone S. Tumors as elusive targets of T-cell-based active immunotherapy. Trends Immunol. 2003;24:335–42. doi: 10.1016/s1471-4906(03)00116-9. [DOI] [PubMed] [Google Scholar]
- 9.Pawelec G. Tumour escape: antitumour effectors too much of a good thing? Cancer Immunol Immunother. 2004;53:262–74. doi: 10.1007/s00262-003-0469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hicklin DJ, Marincola FM, Ferrone S. HLA class I antigen downregulation in human cancers: T-cell immunotherapy revives an old story. Mol Med Today. 1999;5:178–86. doi: 10.1016/s1357-4310(99)01451-3. [DOI] [PubMed] [Google Scholar]
- 11.Campoli M, Chang CC, Ferrone S. HLA class I antigen loss, tumor immune escape and immune selection. Vaccine. 2002;20(Suppl 4):A40–5. doi: 10.1016/s0264-410x(02)00386-9. [DOI] [PubMed] [Google Scholar]
- 12.Meissner M, Reichert TE, Kunkel M, et al. Defects in the human leukocyte antigen class I antigen processing machinery in head and neck squamous cell carcinoma: association with clinical outcome. Clin Cancer Res. 2005;11:2552–60. doi: 10.1158/1078-0432.CCR-04-2146. [DOI] [PubMed] [Google Scholar]
- 13.Kageshita T, Hirai S, Ono T, Hicklin DJ, Ferrone S. Down-regulation of HLA class I antigen-processing molecules in malignant melanoma: association with disease progression. Am J Pathol. 1999;154:745–54. doi: 10.1016/S0002-9440(10)65321-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogino T, Shigyo H, Ishii H, et al. HLA class I antigen down-regulation in primary laryngeal squamous cell carcinoma lesions as a poor prognostic marker. Cancer Res. 2006;66:9281–9. doi: 10.1158/0008-5472.CAN-06-0488. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–13. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 16.Marrogi AJ, Munshi A, Merogi AJ, et al. Study of tumor infiltrating lymphocytes and transforming growth factor-β as prognostic factors in breast carcinoma. Int J Cancer. 1997;74:492–501. doi: 10.1002/(sici)1097-0215(19971021)74:5<492::aid-ijc3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 17.Naito Y, Saito K, Shiiba K, et al. CD8+ Tcells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58:3491–4. [PubMed] [Google Scholar]
- 18.Nakano O, Sato M, Naito Y, et al. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res. 2001;61:5132–6. [PubMed] [Google Scholar]
- 19.Lampson LA, Fisher CA, Whelan JP. Striking paucity of HLA-A, B, C and β2-microglobulin on human neuroblastoma cell lines. J Immunol. 1983;130:2471–8. [PubMed] [Google Scholar]
- 20.Ogino T, Wang X, Kato S, Miyokawa N, Harabuchi Y, Ferrone S. Endoplasmic reticulum chaperone-specific monoclonal antibodies for flow cytometry and immunohistochemical staining. Tissue Antigens. 2003;62:385–93. doi: 10.1034/j.1399-0039.2003.00114.x. [DOI] [PubMed] [Google Scholar]
- 21.Perosa F, Luccarelli G, Prete M, Favoino E, Ferrone S, Dammacco F. β2-microglobulin-free HLA class I heavy chain epitope mimicry by monoclonal antibody HC-10-specific peptide. J Immunol. 2003;171:1918–26. doi: 10.4049/jimmunol.171.4.1918. [DOI] [PubMed] [Google Scholar]
- 22.Stam NJ, Spits H, Ploegh HL. Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J Immunol. 1986;137:2299–306. [PubMed] [Google Scholar]
- 23.Wang X, Campoli M, Cho HS, et al. A method to generate antigen-specific mAb capable of staining formalin-fixed, paraffin-embedded tissue sections. J Immunol Methods. 2005;299:139–51. doi: 10.1016/j.jim.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Genetic diversity of HLA: functional and medical implications. Hum Immunol; 12th International Histocompatibility Conference; Paris, France. June 9–12, 1996; 1996. pp. 1–184. abstracts. [PubMed] [Google Scholar]
- 25.Garcia KC. Molecular interactions between extracellular components of the T-cell receptor signaling complex. Immunol Rev. 1999;172:73–85. doi: 10.1111/j.1600-065x.1999.tb01357.x. [DOI] [PubMed] [Google Scholar]
- 26.Pamer E, Cresswell P. Mechanisms of MHC class I-restricted antigen processing. Annu Rev Immunol. 1998;16:323–58. doi: 10.1146/annurev.immunol.16.1.323. [DOI] [PubMed] [Google Scholar]
- 27.Bentley GA, Mariuzza RA. The structure of the T cell antigen receptor. Annu Rev Immunol. 1996;14:563–90. doi: 10.1146/annurev.immunol.14.1.563. [DOI] [PubMed] [Google Scholar]
- 28.Johnsen AK, Templeton DJ, Sy M, Harding CV. Deficiency of transporter for antigen presentation (TAP) in tumor cells allows evasion of immune surveillance and increases tumorigenesis. J Immunol. 1999;163:4224–31. [PubMed] [Google Scholar]
- 29.Facoetti A, Nano R, Zelini P, et al. Human leukocyte antigen and antigen processing machinery component defects in astrocytic tumors. Clin Cancer Res. 2005;11:8304–11. doi: 10.1158/1078-0432.CCR-04-2588. [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Albaitero A, Nayak JV, Ogino T, et al. Role of antigen-processing machinery in the in vitro resistance of squamous cell carcinoma of the head and neck cells to recognition by CTL. J Immunol. 2006;176:3402–9. doi: 10.4049/jimmunol.176.6.3402. [DOI] [PubMed] [Google Scholar]
- 31.Seliger B, Atkins D, Bock M, et al. Characterization of human lymphocyte antigen class I antigen-processing machinery defects in renal cell carcinoma lesions with special emphasis on transporter-associated with antigen-processing down-regulation. Clin Cancer Res. 2003;9:1721–7. [PubMed] [Google Scholar]
- 32.Atkins D, Breuckmann A, Schmahl GE, et al. MHC class I antigen processing pathway defects, ras mutations and disease stage in colorectal carcinoma. Int J Cancer. 2004;109:265–73. doi: 10.1002/ijc.11681. [DOI] [PubMed] [Google Scholar]
- 33.Cooper BC, Ritchie JM, Broghammer CL, et al. Preoperative serum vascular endothelial growth factor levels: significance in ovarian cancer. Clin Cancer Res. 2002;8:3193–7. [PubMed] [Google Scholar]
- 34.Pages F, Berger A, Camus M, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–66. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 35.Ramnath N, Tan D, Li Q, et al. Is downregulation of MHC class I antigen expression in human non-small cell lung cancer associated with prolonged survival? Cancer Immunol Immunother. 2006;55:891–9. doi: 10.1007/s00262-005-0085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vitale M, Pelusi G, Taroni B, et al. HLA class I antigen down-regulation in primary ovary carcinoma lesions: association with disease stage. Clin Cancer Res. 2005;11:67–72. [PubMed] [Google Scholar]
- 37.Raffaghello L, Prigione I, Bocca P, et al. Multiple defects of the antigen-processing machinery components in human neuroblastoma: immunotherapeutic implications. Oncogene. 2005;24:4634–44. doi: 10.1038/sj.onc.1208594. [DOI] [PubMed] [Google Scholar]