Summary
Chondroitin sulfate proteoglycan 4 (CSPG4), a transmembrane proteoglycan originally identified as a highly immunogenic tumor antigen on the surface of melanoma cells, is associated with melanoma tumor formation and poor prognosis in certain melanomas and several other tumor types. The complex mechanisms by which CSPG4 affects melanoma progression have started to be defined, in particular the association with other cell surface proteins and receptor tyrosine kinases (RTKs) and its central role in modulating the function of these proteins. CSPG4 is essential to the growth of melanoma tumors through its modulation of integrin function and enhanced growth factor receptor-regulated pathways including sustained activation of ERK 1,2. This activation of integrin, RTK, and ERK 1,2 function by CSPG4 modulates numerous aspects of tumor progression. CSPG4 expression has further been correlated to resistance of melanoma to conventional chemotherapeutics. This review outlines recent advances in our understanding of CSPG4-associated cell signaling, describing the central role it plays in melanoma tumor cell growth, motility, and survival, and explores how modifying CSPG4 function and protein–protein interactions may provide us with novel combinatorial therapies for the treatment of advanced melanoma.
Keywords: CSPG4, melanoma chondroitin sulfateproteoglycan, NG2, HMW-MAA, melanoma, therapeutics
There has been much progress in our understanding of the biology of melanoma progression and metastasis in recent years. Despite this progress, metastatic melanoma remains a significant clinical problem, representing one of the most untreatable cancers to date. While advances in the early detection and surgical removal of primary lesions have reduced mortality resulting from malignant melanoma, metastatic disease still is responsible for most tumor relapse. The development of specific inhibitors of mutant active BRAF (BRAFV600E) and the use of immune-modulating CTLA4 antibodies have provided encouraging therapeutic outcomes, but as these benefits are temporary, there remains a critical need to develop targeted therapeutics to manage progressive disease (Blank et al., 2011; Smalley, 2010). Human chondroitin sulfate proteoglycan 4 (CSPG4), originally referred to as high molecular weight-melanoma-associated antigen (HMW-MAA) or melanoma chondroitin sulfate proteoglycan (MCSP), was first identified 30 yr ago on human melanoma cells (Wilson et al., 1981). Parallel investigations at that time identified the rat ortholog of CSPG4 termed nerve/glial antigen 2 (NG2) (Stallcup, 2002). CSPG4 and NG2 are highly conserved, and many of the important concepts regarding the significance and functions of CSPG4 are based on studies from both orthologs. However, it needs to be emphasized that complete structural/functional comparisons between each ortholog are still in process, and thus, we have attempted to specify which ortholog has contributed to these concepts. Therefore, as these studies are still incomplete, we will refer to CSPG4 in studies that utilized or investigated the human ortholog, and NG2 in studies that have looked at the function of the rat or mouse orthologs. The notation CSPG4/NG2 in this review is intended to demonstrate areas where these studies overlap. Additional discussion of the complexities associated with the functions of CSPG4/NG2 has been summarized in excellent reviews (Campoli et al., 2010; Couchman, 2010; Stallcup, 2002; Stallcup and Huang, 2008; Trotter et al., 2010). While not the focus of this review, heparan sulfate glycosaminoglycans have also been implicated in melanoma metastasis, for example, as a result of their ability to impact on Wnt5A signal transduction (O’Connell et al., 2009). Furthermore, other core proteins for cell surface proteoglycans (e.g., syndecans and CD44) have also been studied extensively in several model systems, and they share many of properties and functions of CSPG4. The reader is therefore referred to several excellent reviews on these types of cell surface proteoglycans (Beauvais and Rapraeger, 2004; Bourguignon, 2001; Couchman, 2010; Sanderson and Borset, 2002; Simpson and Lokeshwar, 2008; Toole and Slomiany, 2008; Turley et al., 2002).
Both CSPG4 and NG2 are cell surface type I transmembrane proteins that are covalently modified with CS glycosaminoglycan. As with other proteoglycans, decoration of CSPG4 with CS modification occurs in the brefeldin-A-resistant trans-Golgi compartment (Spiro et al., 1991). Both orthologs have unique and complex mechanisms associated with their signaling functions, indicating that they most likely play a central role in linking multiple oncogenic pathways required for malignant progression. CSPG4 expression in radial growth phase (RGP) human melanomas facilitates migration, protease activation, and epithelial to mesenchymal transition (EMT), indicating that it may be very important in primary tumors for facilitating progression from a radial to vertical growth phase phenotype (Yang et al., 2009). CSPG4 and NG2 facilitate sustained, high-level activation of key survival and growth pathways, in particular integrin-regulated focal adhesion kinase (FAK), ERK 1,2, and PI3K/AKT pathways. Such studies implicate CSPG4 /NG2 as important in facilitating the growth and survival of malignant melanoma (Chekenya et al., 2008; Yang et al., 2004) and most importantly link activation of survival and growth pathways to the intracellular signaling capability of integrins as well as constitutive activation of ERK 1,2. Therefore, this cell surface proteoglycan may be a central factor in controlling the consequences of microenvironment on melanoma progression, and thus, its therapeutic potential is likely to be considerable for delaying progression and/or recurrence in patients with melanoma.
CSPG4 expression in normal and neoplastic tissues
CSPG4 and Ng2 are expressed in a number of normal tissues throughout development, suggesting an important role in the development or homeostasis of adult tissues (Campoli et al., 2010; Stallcup, 2002). NG2 is implicated in the development of vascular tissue, as it is expressed by angiogenesis-associated pericytes (both normal and pathologic) and mice lacking Ng2 have defective vasculature (Huang et al., 2010; Schlingemann et al., 1990). In addition to a role of NG2 in angiogenesis, the expression of CSPG4 and Ng2 in several pluripotent progenitor cell populations also indicates a role for CSPG4 in tissue development and stem cell niche maintenance. CSPG4 is detected in stem-like cells associated with the interfollicular epidermis, where it regulates the position and motility of these progenitor cells in their niche (Ghali et al., 2004; Legg et al., 2003). Ng2 expression in the central nervous system has been linked to cell populations that can give rise to oligodendrocytes as well as protoplasmic astrocytes and neurons in vivo (Trotter et al., 2010). CSPG4-positive stem cells in the epidermis are important for the renewal of epithelial keratinocytes; loss of these cells is associated with aging of the skin. CSPG4/NG2 is also expressed in both fetal and adult articular chondrocytes (Midwood and Salter, 1998), bone marrow mesenchymal cells (Kozanoglu et al., 2009), and smooth muscle cells (Grako and Stallcup, 1995; Grako et al., 1999; Ozerdem et al., 2001). CSPG4/Ng2-expressing pluripotent stem and progenitor cells often lose expression of the proteoglycan as they undergo terminal differentiation; however, this is not always the case (Campoli et al., 2010). CSPG4 has been demonstrated to be expressed on melanocytes, although at levels lower than what is seen on most melanomas (Campoli et al., 2010; Medic et al., 2011; Tsujisaki et al., 1987). Altogether, these data implicate CSPG4 in the maintenance and differentiation of progenitor/stem cell populations in the development of a variety of adult tissues. While the role of CSPG4/NG2 in homeostasis is only partially understood, it is noteworthy that embryonic deletion of this gene in mice is not lethal and to date immune-based therapies against this target show no obvious deleterious side effects.
In addition to its role in melanoma, CSPG4 is associated with the progression of other cancers including oligodendrocytomas, gliomas, triple-negative breast carcinomas, and squamous cell carcinoma. This review, however, will focus on the role of CSPG4 in malignant melanoma. The link between CSPG4 and melanoma progression was first appreciated as a result of its widespread expression in the majority (70% or greater) of superficial spreading and nodular human melanomas (Campoli et al., 2010). In superficial spreading and/or nodular melanoma, the core CSPG4 protein is expressed at multiple stages of melanoma progression and is even detected prior to tumor initiation in melanocytes within nevi. In these subtypes of melanoma, CSPG4 is not considered a prognostic factor, because it is expressed prior to the initiation of tumor formation and, indeed, has been detected on melanocytes in vitro. Although not considered a prognostic factor in superficial spreading or nodular melanoma, some studies have suggested that it may be negatively prognostic in acral lentiginous melanomas (Kageshita et al., 1994; Nishi et al., 2010). Despite its general lack of prognostic significance in melanoma, however, numerous studies in vitro and in vivo using melanoma cell lines in which CSPG4 expression is altered have directly linked the proteoglycan core protein to the development of several phenotypic traits required for tumor progression (Campoli et al., 2010; Wang et al., 2010b). For example, expression of CSPG4 in human RGP melanoma cells promotes their anchorage-independent growth and increased motility in vitro (Iida et al., 1992, 1995; Yang et al., 2004, 2009). Development of these phenotypic traits requires the intact core protein, because expression of truncated constructs lacking the cytoplasmic domain fails to enhance growth or motility. Importantly, subcutaneous tumor growth of these RGP melanomas also requires the intact core protein, emphasizing the functional importance of the cytoplasmic domain in intracellular signaling and tumor formation. In addition, enforced expression of this proteoglycan in mouse B16 melanomas enhances experimental metastasis in mice, again illustrating the potential importance of this protein in metastasis formation (Burg et al., 1998). Thus, although not prognostic, the data consistently point to an important role for CSPG4 in melanoma progression. One explanation for this discrepancy is that CSPG4 is multifunctional, which allows it to participate in and facilitate the activation of multiple oncogenic signaling pathways that are dynamically altered during multiple stages of malignant progression.
Although it is not the focus of this review, the expression of CSPG4 in a number of non-melanoma tumors is of particular note, suggesting a more widespread role for CSPG4 in the progression of multiple tumor types. It is detected readily in tumors of neuroectodermal origin including oligodendroglioma and glioblastomas (Stallcup and Huang, 2008). CSPG4 expression is detected in a subset of childhood acute lymphoblastic leukemia and acute myeloid leukemia, where its expression correlates with a poorer prognosis, particularly for those patients demonstrating 11q23 translocations (Hilden et al., 1997; Petrovici et al., 2010). CSPG4 is also detected in renal cell carcinomas, chondrosarcomas, and pancreatic cell carcinomas, although studies are needed to identify potential tumor-related functions in those tumors (Campoli et al., 2010; Wang et al., 2010b). More recently, CSPG4 has been identified in triple-negative breast cancer and squamous cell carcinoma of the head and neck, where it has been associated with highly tumorigenic ‘cancer stem cell’ subpopulations (Wang et al., 2010a,b).
Structural/functional domains of CSPG4
Both CSPG4 and NG2 are single-pass type I transmembrane proteins expressed as either a approximately 250-kd glycoprotein or a approximately 450-kd proteoglycan (Figure 1). Thus, both appear to be ‘part-time’ proteoglycans, meaning that both chondroitin sulfate glycosaminoglycan (CS)–modified and unmodified core protein are expressed in the same population of tumor cells. CSPG4 appears to be largely decorated with chondroitin-4 sulfate chains (CS) in human melanomas, although how or whether tumor-associated changes in the sulfation patterns (e.g., chondroitin-6 sulfation) occur is currently unknown (Iida et al., 2007). The CS chain influences the cell surface distribution of CSPG4, suggesting that it is important for focusing the core protein / proteoglycan into different microdomains in the plasma membrane (Stallcup and Dahlin-Huppe, 2001). CS modification also facilitates an interaction of CSPG4 with α4β1 integrin and fibronectin. CSPG4-linked CS also regulates the activation of proMMP2 by transmembrane matrix metalloproteinases (e.g., MT3-MMP). In those studies, it was shown that chondroitin 4 sulfate was critical for activating proteases, suggesting that the sulfation pattern of CS is important for this function of CSPG4 (Iida et al., 1998, 2007). Whether cells can actively regulate CS modification of the core protein is not known, although it is noteworthy that a mechanism has been identified that regulates CS extension (or lack thereof) on specific GAG acceptor sites on core proteins (Manzi et al., 1995). Thus, it is possible that one mechanism by which tumor cells might regulate CSPG4 /NG2 function is by selectively modifying GAG acceptor sites on the core protein, which could influence its localization to specific membrane microdomains.
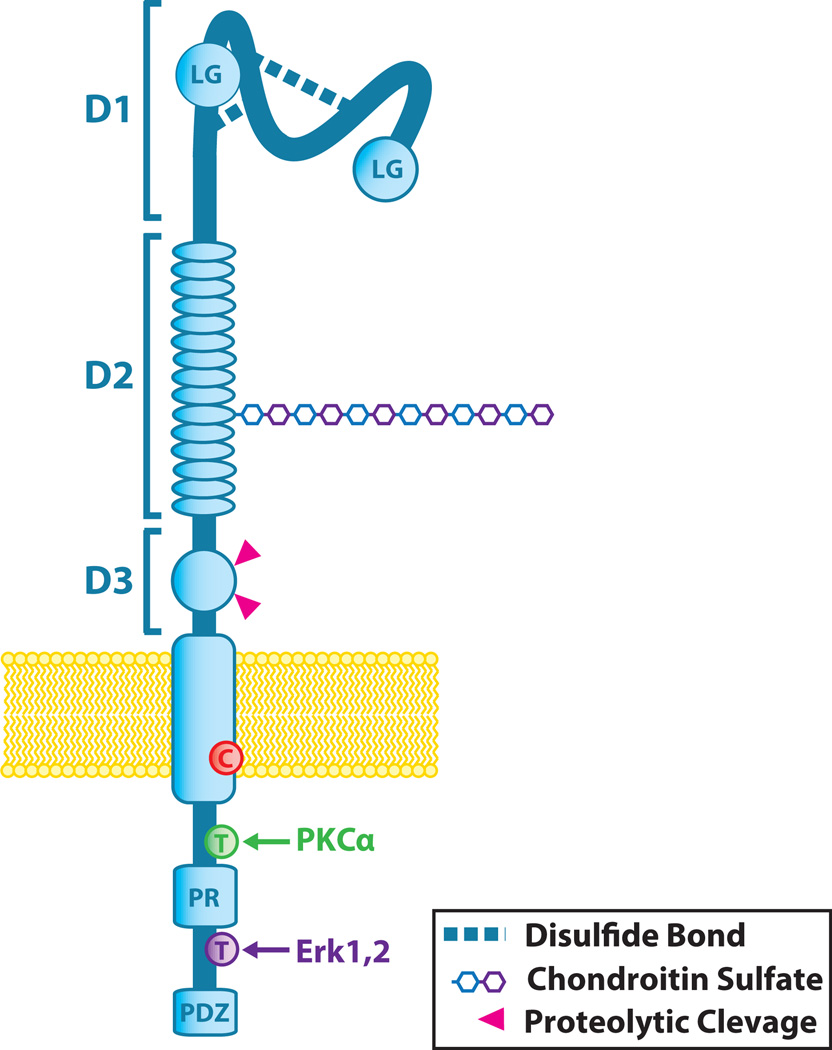
Figure 1.
Chondroitin sulfate proteoglycan 4 (CSPG4)/NG2 Structure. CSPG4/NG2 is composed of three major structural components: the extracellular domain, the transmembrane region, and the cytoplasmic C-terminal domain (CTD). The extracellular domain contains an N-terminal globular subdomain (D1) consisting of laminin G-type regions (LG) and disulfide bonds. The D2 subdomain consists of 15 CSPG repeats and, in NG2, a single chondroitin sulfate glycosaminoglycan (CS-GAG) chain. This region of the core protein is known to bind certain soluble growth factor ligands, and the CS-GAG is responsible for CSPG4 binding to integrin and matrix metalloproteinases (MMPs). CS modification is also associated with distinct membrane distribution patterns of CSPG4/NG2 on the cell surface. CSPG4/NG2 can be expressed with or without CS modification. Proximal to the plasma membrane, the D3 globular subdomain contains sites for N-linked carbohydrate modification, binding sites for lectins (e.g., galectin 3), and proteolytic cleavage by MMPs or other proteases. The transmembrane region of CSPG4 contains a cysteine residue (C) at position 2230 that may play a role in CSPG4 membrane localization, although this is yet to be evaluated. The CTD contains tyrosine residues (T) that serve as phosphoacceptor sites for PKCa and ERK 1,2 (CSPG4 residues 2252 and 2310, respectively). The proline-rich region (PR) may comprise a non-canonical SH3 protein interaction domain, and the C-terminus contains a 4 residue PDZ domain-binding motif (PDZ) that is responsible for interactions with various PDZ domain-containing binding partners.
The CSPG4 (as with NG2) core protein consists of three main structural domains (Figure 1): a large extracellular domain, a 25-amino acid transmembrane region, and a short, 75-amino acid cytoplasmic domain (Stallcup, 2002). While the crystal structure of the core protein has yet to be resolved, the full-length primary sequence of the proteoglycan encodes a number of known putative structural/functional motifs. The extracellular domain consists of three subdomains termed D1-3 (Figure 1) (Campoli et al., 2010; Stallcup, 2002). The D1 subdomain is a globular domain containing two laminin G-type domains near the N-terminus, which may be involved in ligand binding, and a number of disulfide linkages that are important for maintaining tertiary protein structure. The D2 subdomain is made up of a series of 15 ‘CSPG repeat’ motifs, some of which, in NG2, bind directly to collagens V and VI. The D2 subdomain also contains a number of potential acceptor sites for CS chain modification although site-directed mutagenesis of NG2 demonstrates that not all of these are utilized and not all core proteins expressed are exported with a CS chain. The membrane proximal globular subdomain D3 contains carbohydrate modifications that have the potential to bind to galectin 3 or other lectins (e.g., p-selectin) as well as α3β1 integrin (Cooney et al., 2011; Fukushi et al., 2004). This portion of the extracellular domain also contains a number of putative proteolytic cleavage sites. Fragmentation and release of CSPG4 has been documented, and these fragments are detectable in sera from both normal patients and those with malignant disease (Campoli et al., 2010). The transmembrane domain of both orthologs contains one notable cysteine residue that may be involved in localization within the membrane, although this remains speculative.
The cytoplasmic domain of CSPG4 (and NG2) contains several structural features that are critical for the function of the proteoglycan (Figure 1). The carboxyl terminal 4 residues of the proteoglycan comprise a PDZ domain binding motif that binds to the PDZ domain of scaffold proteins such as syntenin, MUPP1, and GRIP1(NG2) (Barritt et al., 2000; Chatterjee et al., 2008; Stegmuller et al., 2002). The CSPG4 cytoplasmic domain also contains multiple potential threonine phosphoacceptor sites, including two (T2256/T2314 in NG2 and T2252/T2310 in CSPG4) phosphorylated by PCKa and ERK 1,2, respectively (Makagiansar et al., 2007). Sequence analysis has also identified a putative D-domain docking site for ERK 1,2, and our laboratory has demonstrated that ERK 1,2 binds to the cytoplasmic domain of CSPG4. Finally, the proline-rich region present in the cytoplasmic domain may facilitate additional protein–protein interactions.
CSPG4 is a co-receptor
Chondroitin sulfate proteoglycan 4 does not exhibit any known catalytic activity, which is similar to other transmembrane core proteoglycans such as the syndecans and CD44 as well as cell surface adhesion receptors such as integrins (Beauvais and Rapraeger, 2004; Bourguignon, 2001; Couchman, 2010; Sanderson and Borset, 2002; Simpson and Lokeshwar, 2008; Toole and Slomiany, 2008; Turley et al., 2002). There is evidence that these cell surface proteoglycans participate in signal transduction both as co-receptors in partnership with receptors containing intrinsic receptor tyrosine kinase (RTK) activity and as a result of their ability to associate directly and indirectly with such cytoplasmic kinases as FAK and ERK 1,2. For example, the NG2 core protein binds to both bFGF and PDGFaa, an interaction that presents these growth factors to their cognate receptors (Nishiyama et al., 1996; Stallcup, 2002; Stallcup and Huang, 2008). CSPG4 and NG2 both interact with a number of extracellular matrix (ECM) components, including collagen types II, V, and VI, laminin, tenascin, and fibronectin. These results suggest that this proteoglycan may also link ECM components within the tumor microenvironment to growth factor receptor and integrin-regulated signaling pathways (Campoli et al., 2010; Stallcup, 2002; Wang et al., 2010b). Indeed, early studies using monoclonal antibodies against the CSPG4 core protein reduced both adhesion-induced cell spreading (Harper and Reisfeld, 1983) and anchorage-independent growth of melanoma cells, while expression of CSPG4 in CSPG4-null human melanoma cells leads to enhanced spreading on fibronectin (Iida et al., 1992; Yang et al., 2004). Decoration of CSPG4 with CS is necessary for both integrin activation and direct binding and activation of MMP complexes on the cell surface, implicating CS in regulating migration and invasion (Iida et al., 2007). Recent studies have also linked CSPG4 and NG2 to enhanced activation of integrin-related signal transduction pathways, which may be key for its importance in tumor progression (Chekenya et al., 2008; Yang et al., 2004, 2009). Collectively, these studies implicate CSPG4 in integrin-controlled cellular response.
Mechanisms of signal transduction by CSPG4 and NG2
Further studies on the signaling mechanisms of CSPG4 and NG2 indicate that it activates two types of signaling pathways: RTK signaling through the MAPK cascade and integrin signaling through (FAK) activation (Figure 2). Activation of these pathways by CSPG4 /NG2 leads to regulation of a number of cellular functions that drive tumorigenesis, including cytoskeletal reorganization, adhesion, migration, EMT, growth, survival, and chemoresistance. As CSPG4 does not have intrinsic catalytic activity, we propose that it functions as a type of scaffold protein. Thus, CSPG4 functions to affix signaling molecules in close proximity to one another to form a ‘progression-associated signaling complex’ that facilitates an increased duration and/or intensity of RTK and FAK transduction pathways (Figure 2). We thus have hypothesized that these enhanced signaling functions provided by CSPG4 result in a selective growth and survival advantage when adhesion molecules and growth factors are limiting (Yang et al., 2004).
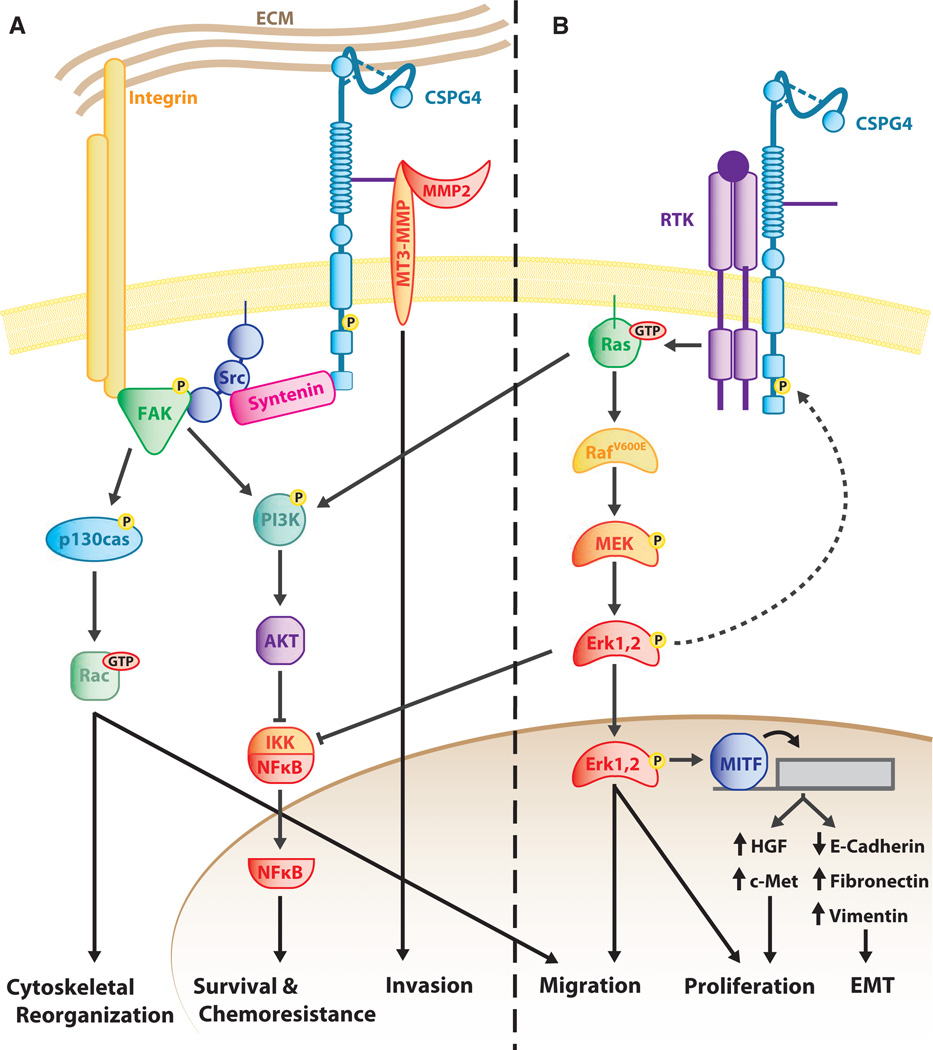
Figure 2.
Chondroitin sulfate proteoglycan 4 (CSPG4) Signaling Pathways. CSPG4 functions to activate two major overlapping but distinct signaling cascades: integrin/focal adhesion kinase (FAK) signaling (A) and MAPK pathway signaling (B). Through these two branches, CSPG4 ultimately promotes tumor progression through a variety of cellular functions. (A) CSPG4 influences integrin function and signaling pathways. CSPG4 promotes Src-FAK complexing through its interaction with the scaffold protein Syntenin. This leads to FAK activation by Src, prompting a number of signaling cascades including FAK–integrin–extracellular matrix (ECM) complex assembly, activation of Rac through p130cas, and activation of the PI3K/AKT/NFκB signaling cascade; activation of these pathways mediate the effects of CSPG4 on cytoskeletal reorganization, survival, chemoresistance, and migration. Activation of MMPs by CSPG4 via direct binding to the CS chain leads to local invasion of cancer cells. CSPG4/NG2 can also enhance survival as a result of its constitutive activation of integrin-related signals. (B) CSPG4 promotes MAPK signaling through receptor tyrosine (RTK)-dependent and independent mechanisms. In human melanomas, CSPG4 impacts activation of the ERK 1,2 pathway, likely by impacting on the growth factor-induced activation of RTKs. Note the expression of BRAFV600E in this pathway, which results in the constitutive activation of this kinase (see text). In human melanoma cells expressing this BRAF-activating mutation, constitutive activation of the ERK 1,2 pathway requires the presence of CSPG4. There are several possible downstream oncogenic targets of the ERK 1,2 pathway, including MITF and activated c-Met, which are implicated in epithelial to mesenchymal transition. Inhibition of IKK by ERK 1,2 downstream of CSPG4 could also lead to cell survival and chemoresistance.
CSPG4 activation of integrins and effects on adhesion, motility, and survival
Chondroitin sulfate proteoglycan 4/NG2 interactions with integrins lead to the activation of downstream adhesion-related signaling pathways that contribute to malignant progression (Figure 2A). Integrin α4β1 (and likely other β1 integrin heterodimers) can be super-activated in response to an ECM ligand (fibronectin) when activated in the presence of CSPG4, which results in a high level of activated FAK compared to activation of integrin alone (Yang et al., 2004). Antibody clustered CSPG4 co-precipitates with a complex of the small GTPase CDC42, ACK-1, and p130CAS in human melanoma. These factors are implicated in filopodia formation and integrin-mediated cytoskeletal reorganization (Eisenmann et al., 1999), consistent with studies demonstrating that CSPG4 localizes to cell surface microspikes (the in vitro equivalent of filopodia) (Garrigues et al., 1986). Similar studies have shown RAC- and p130CAS-mediated cell spreading in Ng2 transfected human astrocytoma cells (Majumdar et al., 2003). Soluble NG2 released from tumor cells or tumor-associated pericytes can stimulate endothelial cell migration in the tumor microenvironment by interacting with galectin 3 and α3β1 integrin on the endothelial surface (Fukushi et al., 2004; Wen et al., 2006). Expression of a full-length CSPG4 in RGP human melanoma cells lacking endogenous CSPG4 expression results in significantly enhanced integrinmediated spreading and FAK activation (Yang et al., 2004). As CSPG4 can activate MMP complexes on melanoma cell surfaces, the collective results implicate CSPG4 as an important contributing factor to localized invasion at the leading edge of invasive primary tumors. Finally, NG2-mediated activation of integrin facilitates enhanced survival of human glioblastoma cells when they were exposed to clinically used chemotherapeutic agents by enhancing sustained activation of AKT via PI3K (Chekenya et al., 2008).
Multiple structural features of CSPG4/NG2 have been linked to integrin-mediated cell adhesion and survival. Both the carboxyl terminal PDZ motif-binding domain and PKCa phosphorylation site within the C-terminal cytoplasmic domain of CSPG4/NG2 are important for modulating cell migration. Phosphorylation at T2256 in NG2 by PKCa is important for the localization of proteoglycan to lamellipodia at the leading edge of cells, where it co-distributes with β1 integrin (Makagiansar et al., 2007). Inhibiting phosphorylation at this site, either by pharmacologic blockade of PKCa or by mutation of the phosphoacceptor site, inhibits migration of NG2-transfected U251 human astrocytoma cells. In rat OPGs, binding of NG2 to the PDZ domain containing protein syntenin was demonstrated to be important for integrin-mediated migration of these cells (Chatterjee et al., 2008). Syntenin is an important adaptor molecule for mediating tumor cell migration in a number of human tumor types including breast and gastric cancers. In melanoma, enforced expression of syntenin results in NFjB activation via a FAK-dependent mechanism, which is critical for melanoma migration (Boukerche et al., 2008, 2010). Furthermore, melanoma cells plated on fibronectin activate FAK via a PKCα/syntenin-related mechanism, where syntenin is upregulated by PKCα activation in response to plating on fibronectin (Hwangbo et al., 2010). Melanoma cells expressing a truncated CSPG4, which lacks the cytoplasmic domain, are migration deficient compared to cells expressing an intact CSPG4 (Yang et al., 2009). Taken together, these data suggest a model of outside-in signaling resulting from the coupling of integrins with CSPG4/NG2 forming a complex that controls the organization of signaling modules near the plasma membrane (Figure 2A).
CSPG4 in the activation of MAPK-mediated growth and survival
In addition to its control of integrin signaling, CSPG4/NG2 ligand complexes also impact on RTK pathways (Figure 2B). Early studies demonstrated that NG2 impacts the RAS-RAF-MEK-ERK 1,2 pathway by binding to and presenting growth factors (e.g., bFGF and PDGFaa) to their cognate transmembrane RTK (Nishiyama et al., 1996). This capability may have particular consequences to melanoma progression because approximately 60% of human cutaneous melanomas express a constitutively active mutant BRAF (BRAFV600E). Although this mutation contributes to a high level of constitutive ERK 1,2 phosphorylation, expression of BRAFV600E alone is not sufficient to sustain high constitutive activation of ERK 1,2. Studies in multiple melanoma cell lines expressing mutant BRAFV600E retain a requirement for exogenous growth factor stimulation to maximally activate the ERK 1,2 pathway (Satyamoorthy et al., 2003). These results resemble growth factor requirements for sustaining ERK 1,2 activation through mutant active Ras and suggest a complexity of pathway regulation that is not yet fully understood (Gysin et al., 2011). Recent studies also show that BRAFV600E requires expression of a full-length CSPG4 expression sustained and maximal ERK 1,2 activation (Yang et al., 2009). Studies utilizing RNA interference or specific monoclonal antibodies that inhibit CSPG4 function in melanoma cells expressing BRAFV600E also show an inhibition in constitutive ERK 1,2 activation (Wang et al., 2010a; Yang et al., 2009). Furthermore, these same CSPG4 antibodies also enhance the effects of BRAFV600E-specific inhibitors in glioblastoma multiforme and melanoma cells, implying that CSPG4-mediated activation of ERK 1,2 may also occur through a BRAF-independent mechanism (Yu et al., 2011). Pharmacologic inhibition of ERK 1,2 using MEK 1 inhibitors abrogates the growth and motility-promoting effects of CSPG4 in BRAFV600E-expressing cells (Yang et al., 2009). Furthermore, stable expression of a constitutively active MEK1 can bypass the need for CSPG4 expression in stabilizing high levels of ERK 1,2 activation and can lead to ERK 1,2-mediated growth and motility in the absence of the proteoglycan (Yang et al., 2004). Collectively, these data support a model where CSPG4 functions as a membrane scaffold to facilitate the formation of complexes that stabilize both RTKs and integrins. Such complexes would result in enhanced growth factor presentation and increased ECM signaling efficiency (Figure 2B), theoretically providing one mechanism for cells to have a selective advantage over cells that lack CSPG4. Furthermore, because CSPG4 acts at a key interface between the tumor microenvironment and a highly penetrant oncogenic mutation in melanoma, it may prove to be an important therapeutic target in combination with BRAFV600E inhibitors for more effective control of this cancer.
CSPG4 promotes epithelial to mesenchymal transition (EMT) in radial growth phase tumor cells
Epithelial to mesenchymal transition-like changes are well recognized to occur in many primary tumors in experimental tumor models and to signal a transition to a more malignant phenotype. Although the pathological EMT associated with cancer progression does not fully replicate a developmental EMT, it does involve many of the same molecular events including increased motility/invasion and the acquisition of a mesenchymal signature, for example, E-cadherin loss, acquisition of mesenchymal cadherins (N-cadherin and CDH11), and increased expression of vimentin and fibronectin (Thiery, 2002). CSPG4 expression in primary melanoma RGP cells leads to a morphologic change consistent with EMT in which the cells appear less differentiated and more mesenchymal (Yang et al., 2009). Constitutive expression of CSPG4 also leads to alterations in gene transcription resulting in increased expression of fibronectin and vimentin as well as expression and activation of MET (Figure 2B) because of increased expression of HGF and loss of E-cadherin, the latter of which is related to increased expression of HGF by CSPG4-expressing cells. Loss of MET expression or function limits CSPG4-mediated increases in growth and motility (Yang et al., 2009). Furthermore, the melanocyte lineage-specific transcription factor MITF, which targets the c-Met locus, is induced by CSPG4 through constitutive activation of ERK 1,2 (Figure 2B) (Yang et al., 2009).
CSPG4 as a therapeutic target in malignant melanoma
While CSPG4 is not an oncogene per se, its expression directly or indirectly enhances activation of multiple signaling pathways associated with oncogenic transformation. As the proteoglycan is multifunctional, it can interact with distinct key oncogenic pathways that may change dynamically during progression. This may be one explanation for the high proportion of human melanomas that retain CSPG4 expression, even at the stage of metastatic lesions. Pathways that are impacted by CSPG4 include survival (PI3K, AKT, and NFκB), adhesion (FAK and integrin function), and growth/motility (RTK and downstream pathways including ERK 1,2) (Figure 2). It is therefore not surprising that numerous reports have implicated CSPG4 as a potential therapeutic target for the treatment of malignant melanoma and other tumors (Blank et al., 2011; Erfurt et al., 2009; Maciag et al., 2008; Murray et al., 2004; Schmidt et al., 2011; Schrappe et al., 1992; Wang et al., 2010a,b).
Several studies in patients or preclinical mouse models have focused on treating CSPG4 as an immune target for the treatment of melanomas. Analyses of clinical trials focused on evaluating the safety and potential efficacy of CSPG4 antibodies: targeting toxin conjugates or anti-idiotype antibodies used to actively generate CSPG4 antibodies in patients (Campoli et al., 2010). Patients treated with anti-idiotypic antibodies demonstrate a statistically significant increase in survival when segregated according to the presence of serum anti-CSPG4 antibodies generated following treatment. Anti-anti-idiotypic antibodies mimicking CSPG4 have been shown to induce HLA class 1-restricted CSPG4-specific CTL (Murray et al., 2004), suggesting that T-cell based immunotherapy targeted to CSPG4 may also be a viable option for the treatment of melanoma. In support of this hypothesis, a recent animal study showed efficacy for adoptively transferred CTLs engineered to express antibodies against CD20 and CSPG4 antigens in the treatment of CSPG4-expressing melanomas both blocked growth and promoted regression of CSPG4-expressing tumors (Schmidt et al., 2011). Although CSPG4-specific CD4+ T cells have been detected in the circulation of both healthy subjects and melanoma patients, stimulation of an anti-CSPG4 immune response is not apparently associated with autoimmunity (Campoli et al., 2010). Collectively, these results support the use of CSPG4 as a target in tumor immunotherapy with a minimum of side effects.
Analyses of experimental tumor models have also shown that anti-CSPG4 monoclonal antibody (mAb) administered to human melanoma or basal breast tumor xenografts inhibit their growth and metastasis (Wang et al., 2010a). These particular studies, which are performed in immunocompromised animals, also demonstrated that these anti-CSPG4 mAb blocked CSPG4-regulated signaling pathways. Similarly, the direct injection of lentivirus encoding a CSPG4 shRNA into human melanoma tumor xenografts caused tumor regression, indicating that lowering CSPG4 expression levels also limits tumor growth and/or survival, although the mechanisms for these effects were not defined (Wang et al., 2011). These studies did demonstrate, however, that the effect of targeting CSPG4 expression with viral delivery of shRNA or treating the tumors with injection of mAb resulted in reduced blood vessel formation in human melanoma xenografts (Maciag et al., 2008; Schlingemann et al., 1990). As vascular pericytes express CSPG4, the anti-CSPG4 mAb and shRNA may block neoangiogenesis by limiting pericyte growth or viability. This notion is supported by studies in the Ng2 knockout mouse that show a reduction in pathological angiogenesis that correlates with a loss of proliferation of vascular pericytes (Ozerdem and Stallcup, 2004). This effect is similar to the consequences of genetic deletion of Ng2 in mouse glioma susceptibility models that also lack tumor-associated pericytes (Huang et al., 2010). Soluble NG2 shed by tumor cells or pericytes has also been shown to stimulate motility in endothelial cells involved in angiogenesis through binding of α3β1 integrin and galectin-3, effects that may be abrogated by antibody against NG2 (Fukushi et al., 2004). Therefore, targeting CSPG4 could have direct effects on tumor cell signaling and also on tumor–stromal cell interactions that promote tumor growth and angiogenesis (Campoli et al., 2010; Stallcup and Huang, 2008).
Some evidence suggests that CSPG4 may also influence drug resistance. Chemoresistance is a major factor in the successful treatment of melanoma and other cancers. Although tumors in many patients initially respond to therapy, resistance to treatment develops over time and cancer progresses. The development of drug resistance in melanoma appears to be particularly linked to the use of single-target inhibitors, such as those that target BRAFV600E. CSPG4 expression is associated with multidrug resistance in glioblastoma and melanoma tumor experimental models, and this is mediated by its association with integrin-induced activation of PI3K pathways (Chekenya et al., 2008). The ability of anti-CSPG4 mAb to prolong the growth inhibitory effects of PLX4032, a BRAFV600E inhibitor, on melanoma cell lines in culture provides direct evidence for a role of this proteoglycan in promoting chemoresistance (Yu et al., 2011). Taken as a whole, these data provide a rationale for targeting CSPG4 as an adjuvant therapy for melanoma and other select tumors using either an immune-based therapeutic approach or development of CSPG4 small molecule inhibitors.
Future questions
Over the past 30 yr, much work has gone into documenting CSPG4 expression in diverse human tumors and increasing an understanding of its function in tumor cell biology. While progress has been made in determining its function, there is relatively little information of how CSPG4 expression is regulated. One intriguing report demonstrated that CSPG4 expression in human melanoma cells is epigenetically regulated, involving changes in promoter methylation, although specific mechanisms involved in CSPG4 locus regulation remain to be identified (Luo et al., 2006). CSPG4 expression is detected in the majority of human melanomas; however, the expression pattern and function in melanoma and many other tumor types is still being documented and requires further study. To date, the effect of CSPG4 on signaling has been associated with its ability to localize with other receptors in specific membrane domains, consistent with the presence of phosphoacceptor sites on the cytoplasmic domain and extracellular CS modification of the core protein, both of which are known to influence distribution of proteins to plasma membrane microdomains. This targeting can modify its participation in progression-associated signaling complexes with a resulting change in the phenotype of tumor cells at different stages of progression. As CSPG4 is a co-receptor for integrins and growth factor receptors in these membrane microdomains, understanding the molecular mechanisms associated with aberrant expression of CSPG4 and identifying the structural motifs that facilitate both its interactions with binding partners and stimulation of specific signaling pathways could yield new therapeutic targets that could be exploited using a combinatorial approach. Such treatments could be used in conjunction with immunotherapy or small molecule inhibitors as a first-line therapy or could be used to help manage chemoresistant melanomas in patients in whom disease progressed.
Acknowledgements
This work was supported by NIH grant RO1 CA092222 (JM), the Canadian Breast Cancer Foundation (ET). The authors are also grateful for partial support from the Allen-Pardee Chair in Cancer Biology (Leo T. Furcht).
References
- Barritt DS, Pearn MT, Zisch AH, Lee SS, Javier RT, Pasquale EB, Stallcup WB. The multi-PDZ domain protein MUPP1 is a cytoplasmic ligand for the membrane-spanning proteoglycan NG2. J. Cell. Biochem. 2000;79:213–224. doi: 10.1002/1097-4644(20001101)79:2<213::aid-jcb50>3.0.co;2-g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauvais DM, Rapraeger AC. Syndecans in tumor cell adhesion and signaling. Reprod. Biol. Endocrinol. 2004;2:3. doi: 10.1186/1477-7827-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank CU, Hooijkaas AI, Haanen JB, Schumacher TN. Combination of targeted therapy and immunotherapy in melanoma. Cancer Immunol. Immunother. 2011;60:1359–1371. doi: 10.1007/s00262-011-1079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukerche H, Su ZZ, Prevot C, Sarkar D, Fisher PB. mda-9/Syntenin promotes metastasis in human melanoma cells by activating c-Src. Proc. Natl. Acad. Sci. U S A. 2008;105:15914–15919. doi: 10.1073/pnas.0808171105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukerche H, Aissaoui H, Prevost C, Hirbec H, Das SK, Su ZZ, Sarkar D, Fisher PB. Src kinase activation is mandatory for MDA-9/syntenin-mediated activation of nuclear factor-kappaB. Oncogene. 2010;29:3054–3066. doi: 10.1038/onc.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon LY. CD44-mediated oncogenic signaling and cytoskeleton activation during mammary tumor progression. J. Mammary Gland Biol. Neoplasia. 2001;6:287–297. doi: 10.1023/a:1011371523994. [DOI] [PubMed] [Google Scholar]
- Burg MA, Grako KA, Stallcup WB. Expression of the NG2 proteoglycan enhances the growth and metastatic properties of melanoma cells. J. Cell. Physiol. 1998;177:299–312. doi: 10.1002/(SICI)1097-4652(199811)177:2<299::AID-JCP12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Campoli M, Ferrone S, Wang X. Functional and clinical relevance of chondroitin sulfate proteoglycan 4. Adv. Cancer Res. 2010;109:73–121. doi: 10.1016/B978-0-12-380890-5.00003-X. [DOI] [PubMed] [Google Scholar]
- Chatterjee N, Stegmuller J, Schatzle P, Karram K, Koroll M, Werner HB, Nave KA, Trotter J. Interaction of syntenin-1 and the NG2 proteoglycan in migratory oligodendrocyte precursor cells. J. Biol. Chem. 2008;283:8310–8317. doi: 10.1074/jbc.M706074200. [DOI] [PubMed] [Google Scholar]
- Chekenya M, Krakstad C, Svendsen A, et al. The progenitor cell marker NG2 /MPG promotes chemoresistance by activation of integrin-dependent PI3K/Akt signaling. Oncogene. 2008;27:5182–5194. doi: 10.1038/onc.2008.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney CA, Jousheghany F, Yao-Borengasser A, et al. Chondroitin sulfates play a major role in breast cancer metastasis: a role for CSPG4 and CHST11 gene expression in forming surface P-selectin ligands in aggressive breast cancer cells. Breast Cancer Res. 2011;13:R58. doi: 10.1186/bcr2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couchman JR. Transmembrane signaling proteoglycans. Annu. Rev. Cell Dev. Biol. 2010;26:89–114. doi: 10.1146/annurev-cellbio-100109-104126. [DOI] [PubMed] [Google Scholar]
- Eisenmann KM, Mccarthy JB, Simpson MA, Keely PJ, Guan JL, Tachibana K, Lim L, Manser E, Furcht LT, Iida J. Melanoma chondroitin sulphate proteoglycan regulates cell spreading through Cdc42, Ack-1 and p130cas. Nat. Cell Biol. 1999;1:507–513. doi: 10.1038/70302. [DOI] [PubMed] [Google Scholar]
- Erfurt C, Muller E, Emmerling S, Klotz C, Hertl M, Schuler G, Schultz ES. Melanoma-associated chondroitin sulphate proteoglycan as a new target antigen for CD4+ T cells in melanoma patients. Int. J. Cancer. 2009;124:2341–2346. doi: 10.1002/ijc.24235. [DOI] [PubMed] [Google Scholar]
- Fukushi J, Makagiansar IT, Stallcup WB. NG2 proteoglycan promotes endothelial cell motility and angiogenesis via engagement of galectin-3 and alpha3beta1 integrin. Mol. Biol. Cell. 2004;15:3580–3590. doi: 10.1091/mbc.E04-03-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrigues HJ, Lark MW, Lara S, Hellstrom I, Hellstrom KE, Wight TN. The melanoma proteoglycan: restricted expression on microspikes, a specific microdomain of the cell surface. J. Cell Biol. 1986;103:1699–1710. doi: 10.1083/jcb.103.5.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghali L, Wong ST, Tidman N, Quinn A, Philpott MP, Leigh IM. Epidermal and hair follicle progenitor cells express melanoma-associated chondroitin sulfate proteoglycan core protein. J. Invest. Dermatol. 2004;122:433–442. doi: 10.1046/j.0022-202X.2004.22207.x. [DOI] [PubMed] [Google Scholar]
- Grako KA, Stallcup WB. Participation of the NG2 proteoglycan in rat aortic smooth muscle cell responses to platelet-derived growth factor. Exp. Cell Res. 1995;221:231–240. doi: 10.1006/excr.1995.1371. [DOI] [PubMed] [Google Scholar]
- Grako KA, Ochiya T, Barritt D, Nishiyama A, Stallcup WB. PDGF (alpha)-receptor is unresponsive to PDGF-AA in aortic smooth muscle cells from the NG2 knockout mouse. J. Cell Sci. 1999;112(Pt 6):905–915. doi: 10.1242/jcs.112.6.905. [DOI] [PubMed] [Google Scholar]
- Gysin S, Salt M, Young A, Mccormick F. Therapeutic strategies for targeting ras proteins. Genes Cancer. 2011;2:359–372. doi: 10.1177/1947601911412376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JR, Reisfeld RA. Inhibition of anchorage-independent growth of human melanoma cells by a monoclonal antibody to a chondroitin sulfate proteoglycan. J. Natl Cancer Inst. 1983;71:259–263. [PubMed] [Google Scholar]
- Hilden JM, Smith FO, Frestedt JL, et al. MLL gene rearrangement, cytogenetic 11q23 abnormalities, and expression of the NG2 molecule in infant acute myeloid leukemia. Blood. 1997;89:3801–3805. [PubMed] [Google Scholar]
- Huang FJ, You WK, Bonaldo P, Seyfried TN, Pasquale EB, Stallcup WB. Pericyte deficiencies lead to aberrant tumor vascularizaton in the brain of the NG2 null mouse. Dev. Biol. 2010;344:1035–1046. doi: 10.1016/j.ydbio.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwangbo C, Kim J, Lee JJ, Lee JH. Activation of the integrin effector kinase focal adhesion kinase in cancer cells is regulated by crosstalk between protein kinase C alpha and the PDZ adapter protein mda-9/Syntenin. Cancer Res. 2010;70:1645–1655. doi: 10.1158/0008-5472.CAN-09-2447. [DOI] [PubMed] [Google Scholar]
- Iida J, Skubitz AP, Furcht LT, Wayner EA, Mccarthy JB. Coordinate role for cell surface chondroitin sulfate proteoglycan and alpha 4 beta 1 integrin in mediating melanoma cell adhesion to fibronectin. J. Cell Biol. 1992;118:431–444. doi: 10.1083/jcb.118.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida J, Meijne AM, Spiro RC, Roos E, Furcht LT, Mccarthy JB. Spreading and focal contact formation of human melanoma cells in response to the stimulation of both melanoma-associated proteoglycan (NG2) and alpha 4 beta 1 integrin. Cancer Res. 1995;55:2177–2185. [PubMed] [Google Scholar]
- Iida J, Meijne AM, Oegema TR, Jr, Yednock TA, Kovach NL, Furcht LT, Mccarthy JB. A role of chondroitin sulfate glycosaminoglycan binding site in alpha4beta1 integrin- mediated melanoma cell adhesion. J. Biol. Chem. 1998;273:5955–5962. doi: 10.1074/jbc.273.10.5955. [DOI] [PubMed] [Google Scholar]
- Iida J, Wilhelmson KL, Ng J, Lee P, Morrison C, Tam E, Overall CM, Mccarthy JB. Cell surface chondroitin sulfate glycosaminoglycan in melanoma: role in the activation of pro-MMP-2 (pro-gelatinase A) Biochem. J. 2007;403:553–563. doi: 10.1042/BJ20061176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageshita T, Kimura T, Yoshi A, Hirai S, Ono T, Ferrone S. Antigenic profile of mucosal melanoma lesions. Int. J. Cancer. 1994;56:370–374. doi: 10.1002/ijc.2910560313. [DOI] [PubMed] [Google Scholar]
- Kozanoglu I, Boga C, Ozdogu H, Sozer O, Maytalman E, Yazici AC, Sahin FI. Human bone marrow mesenchymal cells express NG2: possible increase in discriminative ability of flow cytometry during mesenchymal stromal cell identification. Cytotherapy. 2009;11:527–533. doi: 10.1080/14653240902923153. [DOI] [PubMed] [Google Scholar]
- Legg J, Jensen UB, Broad S, Leigh I, Watt FM. Role of melanoma chondroitin sulphate proteoglycan in patterning stem cells in human interfollicular epidermis. Development. 2003;130:6049–6063. doi: 10.1242/dev.00837. [DOI] [PubMed] [Google Scholar]
- Luo W, Wang X, Kageshita T, Wakasugi S, Karpf AR, Ferrone S. Regulation of high molecular weight-melanoma associated antigen (HMW-MAA) gene expression by promoter DNA methylation in human melanoma cells. Oncogene. 2006;25:2873–2884. doi: 10.1038/sj.onc.1209319. [DOI] [PubMed] [Google Scholar]
- Maciag PC, Seavey MM, Pan ZK, Ferrone S, Paterson Y. Cancer immunotherapy targeting the high molecular weight melanoma-associated antigen protein results in a broad antitumor response and reduction of pericytes in the tumor vasculature. Cancer Res. 2008;68:8066–8075. doi: 10.1158/0008-5472.CAN-08-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar M, Vuori K, Stallcup WB. Engagement of the NG2 proteoglycan triggers cell spreading via rac and p130cas. Cell. Signal. 2003;15:79–84. doi: 10.1016/s0898-6568(02)00045-1. [DOI] [PubMed] [Google Scholar]
- Makagiansar IT, Williams S, Mustelin T, Stallcup WB. Differential phosphorylation of NG2 proteoglycan by ERK and PKCalpha helps balance cell proliferation and migration. J. Cell Biol. 2007;178:155–165. doi: 10.1083/jcb.200612084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzi A, Salimath PV, Spiro RC, Keifer PA, Freeze HH. Identification of a novel glycosaminoglycan core-like molecule. I. 500 MHz 1H NMR analysis using a nano-NMR probe indicates the presence of a terminal alpha-GalNAc residue capping 4-methylumbelliferyl-beta-D-xylosides. J. Biol. Chem. 1995;270:9154–9163. doi: 10.1074/jbc.270.16.9154. [DOI] [PubMed] [Google Scholar]
- Medic S, Rizos H, Ziman M. Differential PAX3 functions in normal skin melanocytes and melanoma cells. Biochem. Biophys. Res. Commun. 2011;411:832–837. doi: 10.1016/j.bbrc.2011.07.053. [DOI] [PubMed] [Google Scholar]
- Midwood KS, Salter DM. Expression of NG2/human melanoma proteoglycan in human adult articular chondrocytes. Osteoarthritis Cartilage. 1998;6:297–305. doi: 10.1053/joca.1998.0128. [DOI] [PubMed] [Google Scholar]
- Murray JL, Gillogly M, Kawano K, Efferson CL, Lee JE, Ross M, Wang X, Ferrone S, Ioannides CG. Fine specificity of high molecular weight-melanoma-associated antigen- specific cytotoxic T lymphocytes elicited by anti-idiotypic monoclonal antibodies in patients with melanoma. Cancer Res. 2004;64:5481–5488. doi: 10.1158/0008-5472.CAN-04-0517. [DOI] [PubMed] [Google Scholar]
- Nishi H, Inoue Y, Kageshita T, Takata M, Ihn H. The expression of human high molecular weight melanoma-associated antigen in acral lentiginous melanoma. Biosci. Trends. 2010;4:86–89. [PubMed] [Google Scholar]
- Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB. Interaction between NG2 proteoglycan and PDGF alphareceptor on O2A progenitor cells is required for optimal response to PDGF. J. Neurosci. Res. 1996;43:315–330. doi: 10.1002/(SICI)1097-4547(19960201)43:3<315::AID-JNR6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- O’Connell MP, Fiori JL, Kershner EK, Frank BP, Indig FE, Taub DD, Hoek KS, Weeraratna AT. Heparan sulfate proteoglycan modulation of Wnt5A signal transduction in metastatic melanoma cells. J. Biol. Chem. 2009;284:28704–28712. doi: 10.1074/jbc.M109.028498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozerdem U, Grako KA, Dahlin-Huppe K, Monosov E, Stallcup WB. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev. Dyn. 2001;222:218–227. doi: 10.1002/dvdy.1200. [DOI] [PubMed] [Google Scholar]
- Ozerdem U, Stallcup WB. Pathological angiogenesis is reduced by targeting pericytes via the NG2 proteoglycan. Angiogenesis. 2004;7:269–276. doi: 10.1007/s10456-004-4182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovici K, Graf M, Hecht K, Reif S, Pfister K, Schmetzer H. Use of NG2 (7.1) in AML as a tumor marker and its association with a poor prognosis. Cancer Genomics Proteomics. 2010;7:173–180. [PubMed] [Google Scholar]
- Sanderson RD, Borset M. Syndecan-1 in B lymphoid malignancies. Ann. Hematol. 2002;81:125–135. doi: 10.1007/s00277-002-0437-8. [DOI] [PubMed] [Google Scholar]
- Satyamoorthy K, Li G, Gerrero MR, Brose MS, Volpe P, Weber BL, Van Belle P, Elder DE, Herlyn M. Constitutive mitogen-activated protein kinase activation in melanoma is mediated by both BRAF mutations and autocrine growth factor stimulation. Cancer Res. 2003;63:756–759. [PubMed] [Google Scholar]
- Schlingemann RO, Rietveld FJ, De Waal RM, Ferrone S, Ruiter DJ. Expression of the high molecular weight melanoma-associated antigen by pericytes during angiogenesis in tumors and in healing wounds. Am. J. Pathol. 1990;136:1393–1405. [PMC free article] [PubMed] [Google Scholar]
- Schmidt P, Kopecky C, Hombach A, Zigrino P, Mauch C, Abken H. Eradication of melanomas by targeted elimination of a minor subset of tumor cells. Proc. Natl. Acad. Sci. U S A. 2011;108:2474–2479. doi: 10.1073/pnas.1009069108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrappe M, Bumol TF, Apelgren LD, Briggs SL, Koppel GA, Markowitz DD, Mueller BM, Reisfeld RA. Long-term growth suppression of human glioma xenografts by chemoimmunoconjugates of 4-desacetylvinblastine-3-carboxyhydrazide and monoclonal antibody 9.2.27. Cancer Res. 1992;52:3838–3844. [PubMed] [Google Scholar]
- Simpson MA, Lokeshwar VB. Hyaluronan and hyaluronidase in genitourinary tumors. Front. Biosci. 2008;13:5664–5680. doi: 10.2741/3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalley KS. PLX-4032, a small-molecule B-Raf inhibitor for the potential treatment of malignant melanoma. Curr. Opin. Investig. Drugs. 2010;11:699–706. [PubMed] [Google Scholar]
- Spiro RC, Freeze HH, Sampath D, Garcia JA. Uncoupling of chondroitin sulfate glycosaminoglycan synthesis by brefeldin A. J. Cell Biol. 1991;115:1463–1473. doi: 10.1083/jcb.115.5.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallcup WB. The NG2 proteoglycan: past insights and future prospects. J. Neurocytol. 2002;31:423–435. doi: 10.1023/a:1025731428581. [DOI] [PubMed] [Google Scholar]
- Stallcup WB, Dahlin-Huppe K. Chondroitin sulfate and cytoplasmic domain-dependent membrane targeting of the NG2 proteoglycan promotes retraction fiber formation and cell polarization. J. Cell Sci. 2001;114:2315–2325. doi: 10.1242/jcs.114.12.2315. [DOI] [PubMed] [Google Scholar]
- Stallcup WB, Huang FJ. A role for the NG2 proteoglycan in glioma progression. Cell Adh. Migr. 2008;2:192–201. doi: 10.4161/cam.2.3.6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmuller J, Schneider S, Hellwig A, Garwood J, Trotter J. AN2, the mouse homologue of NG2, is a surface antigen on glial precursor cells implicated in control of cell migration. J. Neurocytol. 2002;31:497–505. doi: 10.1023/a:1025743731306. [DOI] [PubMed] [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- Toole BP, Slomiany MG. Hyaluronan: a constitutive regulator of chemoresistance and malignancy in cancer cells. Semin. Cancer Biol. 2008;18:244–250. doi: 10.1016/j.semcancer.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter J, Karram K, Nishiyama A. NG2 cells: properties, progeny and origin. Brain Res. Rev. 2010;63:72–82. doi: 10.1016/j.brainresrev.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujisaki M, Igarashi M, Sakaguchi K, Eisinger M, Herlyn M, Ferrone S. Immunochemical and functional analysis of HLA class II antigens induced by recombinant immune interferon on normal epidermal melanocytes. J. Immunol. 1987;138:1310–1316. [PubMed] [Google Scholar]
- Turley EA, Noble PW, Bourguignon LY. Signaling properties of hyaluronan receptors. J. Biol. Chem. 2002;277:4589–4592. doi: 10.1074/jbc.R100038200. [DOI] [PubMed] [Google Scholar]
- Wang X, Osada T, Wang Y, et al. CSPG4 protein as a new target for the antibody-based immunotherapy of triple-negative breast cancer. J. Natl Cancer Inst. 2010a;102:1496–1512. doi: 10.1093/jnci/djq343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang Y, Yu L, et al. CSPG4 in cancer: multiple roles. Curr. Mol. Med. 2010b;10:419–429. doi: 10.2174/156652410791316977. [DOI] [PubMed] [Google Scholar]
- Wang J, Svendsen A, Kmiecik J, et al. Targeting the NG2/CSPG4 proteoglycan retards tumour growth and angiogenesis in preclinical models of GBM and melanoma. PLoS One. 2011;6:e23062. doi: 10.1371/journal.pone.0023062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y, Makagiansar IT, Fukushi J, Liu FT, Fukuda MN, Stallcup WB. Molecular basis of interaction between NG2 proteoglycan and galectin-3. J. Cell. Biochem. 2006;98:115–127. doi: 10.1002/jcb.20768. [DOI] [PubMed] [Google Scholar]
- Wilson BS, Imai K, Natali PG, Ferrone S. Distribution and molecular characterization of a cell-surface and a cytoplasmic antigen detectable in human melanoma cells with monoclonal antibodies. Int. J. Cancer. 1981;28:293–300. doi: 10.1002/ijc.2910280307. [DOI] [PubMed] [Google Scholar]
- Yang J, Price MA, Neudauer CL, Wilson C, Ferrone S, Xia H, Iida J, Simpson MA, Mccarthy JB. Melanoma chondroitin sulfate proteoglycan enhances FAK and ERK activation by distinct mechanisms. J. Cell Biol. 2004;165:881–891. doi: 10.1083/jcb.200403174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Price MA, Li GY, Bar-Eli M, Salgia R, Jagedeeswaran R, Carlson JH, Ferrone S, Turley EA, Mccarthy JB. Melanoma proteoglycan modifies gene expression to stimulate tumor cell motility, growth, and epithelial-to-mesenchymal transition. Cancer Res. 2009;69:7538–7547. doi: 10.1158/0008-5472.CAN-08-4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Favoino E, Wang Y, Ma Y, Deng X, Wang X. The CSPG4-specific monoclonal antibody enhances and prolongs the effects of the BRAF inhibitor in melanoma cells. Immunol. Res. 2011;50:294–302. doi: 10.1007/s12026-011-8232-z. [DOI] [PubMed] [Google Scholar]