Abstract
Defects in HLA class I antigen processing machinery (APM) component expression often have a negative impact on the clinical course of tumors and on the response to T cell-based immunotherapy. Since only scant information is available about the frequency and clinical significance of HLA class I APM component abnormalities in prostate cancer, the APM component expression pattern was analyzed in 59 primary prostate carcinoma, adjacent normal tissues, as well as in prostate carcinoma cell lines. The IFN-γ inducible proteasome subunits LMP2 and LMP7, TAP1, TAP2, calnexin, calreticulin, ERp57, and tapasin are strongly expressed in the cytoplasm of normal prostate cells, whereas HLA class I heavy chain (HC) and β2-microglobulin are expressed on the cell surface. Most of the APM components were downregulated in a substantial number of prostate cancers. With the exception of HLA class I HC, TAP2 and ERp57 not detectable in about 0.5% of tumor lesions, all other APM components were not detected in at least 21% of lesions analyzed. These APM component defects were associated with a higher Gleason grade of tumors and an early disease recurrence. Prostate carcinoma cell lines also exhibit a heterogeneous, but reduced constitutive APM component expression pattern associated with lack or reduced HLA class I surface antigens, which could be upregulated by IFN-γ. Our results suggest that HLA class I APM component abnormalities are mainly due to regulatory mechanisms, play a role in the clinical course of prostate cancer and on the outcome of T cell-based immunotherapies.
Keywords: Antigen processing machinery, HLA class I antigens, Immune escape, Prostate cancer
Introduction
Prostate cancer (PC), a complex heterogeneous disease with a variable clinical course, is the most common cancer in the United States and in Western countries. It accounts for 33% of cancer cases and about 9% of cancer-related deaths among men [1, 2]. Initially most PC patients can be effectively treated with surgery, radiation, and androgen deprivation, but a large percentage of men require additional therapy due to the high frequency of recurrence of the disease. Due to its low growth rate coupled with a significantly variable presentation it is difficult or even impossible to conclusively determine, which treatment modality is the best for a patient. Currently ongoing clinical trials provide promise for the implementation of immunotherapy for the treatment of PC [3] and a number of immunotherapeutic strategies alone or in combination with radiation or chemotherapy have been explored [4, 5]. They target the prostate-specific antigen (PSA), other prostate-associated antigens or block the coinhibitory receptor CTLA4 in order to enhance PC antigen-specific immune responses [3, 6–11]. These studies demonstrated that immune-based therapies are safe, feasible, and exhibit some clinical benefit in PC patients. There exists evidence for improved responses at earlier disease stages. Activated effector CD8+ T cells, but also tumor antigen (TA)-specific T cells could be induced, although it still has to be defined, whether these PC antigen-specific immune responses have clinical relevance [6, 9, 12–14].
The recently described positive outcome of a clinical trial with T cell-based immunotherapy in PC has stimulated interest in the characterization of the antigen processing machinery (APM) component expression in PC lesions, since this machinery plays a crucial role in the generation and expression of the trimeric HLA class I surface antigen complex on tumor cells. This complex, which mediates the interaction of tumor cells with HLA class I antigen-restricted, TA-specific cytotoxic T lymphocytes (CTL), is generated through several steps with the participation of different molecules. Specifically, 9–11 amino acid long peptides are derived from the cleavage of mostly, although non-exclusively, endogenous cytosolic and nuclear proteins by the constitutive and interferon (IFN)-γ inducible proteasome subunits as well as by other aminopeptidases [15]. Peptides are then translocated via the heterodimeric transporter associated with antigen processing (TAP) complex from the cytosol into the endoplasmic reticulum (ER), where they are loaded with the help of the chaperones calnexin, calreticulin, ERp57, and tapasin (tpn) onto β2-microglobulin (β2-m)-HLA class I heavy chain (HC) complexes. The assembled trimeric HLA class I-peptide complex is released from the peptide-loading complex and transported via the trans-Golgi apparatus to the cell surface for presentation to CD8+ CTL [16].
Defects in APM component expression have been described in several types of malignant diseases [reviewed by 17–20]. They appear to have clinical relevance, since they can be associated in some tumors with tumor progression, metastases formation as well as poor patients’ survival. To the best of our knowledge, only limited information is available about the MHC class I APM component expression in murine and human PC cells and its impact on immune responses [21–24]. Murine primary and metastatic prostate cancer cell lines express MHC class I surface antigens despite the lack of detectable LMP2, LMP7, TAP1, and TAP2 transcription; IFN-γ treatment induced these four APM components and enhanced MHC class I surface expression [22]. The TAP defect in the murine metastatic PC cell line is caused by impaired initiation of TAP1 transcription [24]. In particular, Sanda et al. [21] determined the constitutive and IFN-γ-induced transcription of TAP2, β2-m and HLA class I HC as well as the HLA class I surface antigen expression in five human PC cell lines. Lack of TAP2 expression in one metastatic human prostate cancer cell line resulted in loss of HLA class I antigen expression, which could be restored by IFN-γ. Two of the five analyzed PC cell lines did not express HLA class I antigens due to defects in their assembly or impaired histone acetylation [21] thereby escaping from T cell recognition. Similar defects were found in surgically removed PC lesions. Immunohistochemical staining of benign hyperplastic, primary and/or metastatic PC lesions with HLA class I- and/or β2-m-specific monoclonal antibodies (mAb) demonstrated a downregulation of these molecules in a high percentage of PC lesions [25]. To the best of our knowledge, the expression of HLA class I APM components in human PC lesions has not yet been investigated in detail. Since this information contributes to our understanding of the molecular mechanisms underlying defects in the presentation of PC antigen-derived peptides to CTL, we have analyzed in this study the expression of HLA class I APM components in 59 formalin-fixed, paraffin-embedded primary PC lesions. Furthermore, we have analyzed the modulation of APM component expression by IFN-γ in four established human PC cell lines with a phenotype similar to that found in surgically removed lesions to determine whether the defects observed are caused by regulatory rather than structural abnormalities. Finally, to assess the clinical significance of our findings, we have correlated the results of immunohistochemical staining with the histopathological characteristics of the lesions and with disease progression.
Materials and methods
Patients and tissue samples
The tissue samples analyzed included 59 archival formalin-fixed, paraffin-embedded primary PC lesions and corresponding normal prostate tissues from patients with a mean age of 63 years (49–75). All patients had been treated with an endoscopic extra peritoneal radical prostatectomy [26] and were R0-resected. The tumors analyzed were diagnosed according to the WHO classification of tumors [1] and staged according to the tumor-node-metastases system [27]. The mean follow-up was 71 months (6–144). The clinico-pathological characteristics of the PC lesions are summarized in Table 1. Informed consent for this study was obtained from all patients.
Table 1.
Characteristics of the patients and tumors analyzed
| Stage | n |
| pT1-3a | 38 |
| pT3b-3c | 17 |
| n.a. | 4 |
| Grade | |
| 1 | 1 |
| 2 | 41 |
| 3 | 13 |
| n.a. | 4 |
| Gleason score | |
| <7 | 26 |
| ≥7 | 30 |
| n.a. | 3 |
n number of samples analyzed, n.a. not analyzed
A tissue microarray (TMA) containing 59 1.5 mm large tissue cores was constructed as described [28, 29]. The TMA included 19 cases with PSA recurrence defined as detectable serum PSA (≥0.2 ng/ml) within 3 years following radical prostatectomy and 37 cases without PSA recurrence within 5 years. The two groups of patients did not significantly differ in their clinico-pathological characteristics. No data concerning recurrence were available for three patients.
Cell lines and cytokine treatment
The human PC cell lines LNCaP, 22RVI, PC3, and DU145, all purchased from American Tissue Culture Collection (ATCC), Manassas, VA, were cultured in phenol red-free RPMI1640 medium (PAA Laboratories, Pasching, Austria) supplemented with 2 mM l-glutamine (Cambrex Bio Science, Verviers, Belgium), 7.5 mM HEPES (CC pro, Oberdorla, Germany), 10% fetal calf serum (FCS) (Invitrogen, Carlsbad, CA), 100 μg/ml penicillin and 100 μg/ml streptomycin (PAA Laboratories).
Cytokines
Human recombinant IFN-γ and tumor necrosis factor (TNF)-α was purchased from Pan Biotech, Aidenbach, Germany.
Monoclonal and polyclonal antibodies
The mouse monoclonal antibody (mAb) HC-10, which recognizes β2-m-free HLA-A3, -A10, -A28, -A29, -A30, -A31, -A32, -A33, and -B (excluding -B5702, -B5804, and -B73) HC [30, 31]; the β2-m-specific mAb L368 [32]; the low molecular weight polypeptide (LMP) 2-specific mAb SY-1 [32], the LMP7-specific mAb HB2 [33]; the TAP1-specific mAb NOB-1 [34], the TAP2-specific mAb NOB-2 [34], the calnexin-specific mAb TO-5 [35]; the calreticulin-specific mAb TO-11 [35]; the ERp57-specific mAb TO-2 [35] and the tpn-specific mAb TO-3 [36] were developed and characterized as described. All the above-mentioned mAb are IgG1 except mAb HC-10, which is an IgG2a. mAb were purified from ascitic fluid by sequential precipitation with ammonium sulfate and caprylic acid [36]. The purity of the mAb preparations was assessed by SDS-PAGE. The activity of the mAb preparations was monitored by testing lymphoid cell lysates in Western blot. The combined mouse/rabbit “Envision and Dual Link System HRP detection” was purchased from Dako (Hamburg, Germany). The FITC-labeled HLA class I-specific mAb B9.12.5 and the FITC-labeled lgG2a mAb used for flow cytometric analyses were purchased from Beckman Coulter (Krefeld, Germany).
Semi-quantitative and quantitative RT-PCR analysis
Total cellular RNA was extracted from cells using the RNeasy Mini Kit (Qiagen, Hilden, Germany) followed by digestion with DNase I (Invitrogen) according to the manufacturer’s instructions. cDNA was synthesized from 500 ng of total RNA using the RevertAidTM H Minus First Strand cDNA Synthesis Kit (Fermentas, St. Leon-Rot, Germany) as described in the manufacturer’s instructions.
The target-specific primers and conditions used for semi-quantitative and quantitative RT-PCR are summarized in Table 2. Semi-quantitative RT-PCR was performed as recently described [37]. Comparative quantification of gene expression was performed by real-time PCR on a Rotor Gene 2000 (Corbett Research, Sydney, Australia) using the quantitative Platinum® SYBR green qPCR SuperMix-UDG (Invitrogen). Amplifications were carried out by an initial hold at 50°C for 2 min followed by denaturation at 95°C for 2 min. After 40 cycles with denaturation at 95°C for 15 s and annealing between 58°C and 60°C for 30 s the melting steps were performed starting at 60°C rising up to 99°C with 1°C at each step. The melting curve analysis was provided at the end of each run to control PCR specificity. Results of the qRT-PCR data are presented as relative mRNA expression quantified with the Rotor Gene analysis software and normalized to β-actin transcription levels.
Table 2.
Nucleotide sequences of APM component primers and conditions used for RT-PCR
| Gene | Name | Sequence | Annealing temperature (°C) | Cycles |
|---|---|---|---|---|
| huCalnexin | HCln-5 | tgt gag tca gct cct gga tg | 60 | 28 |
| HCln-3 | gac cac agc tcc aaa cca at | |||
| huCalreticulin | hCrt-5 | tct cag ttc cgg caa gtt ct | 59 | 30 |
| hCrt-3 | tct gag tct ccg tgc atg tc | |||
| huLMP10 | hLMP10-5 | ggg ctt ctc ctt cga gaa ct | 60 | 31 |
| hLMP10-3 | cag ccc cac agc agt aga tt | |||
| huLMP2 | hLMP2-5 | tgc tgc atc cac ata acc at | 60 | 29 |
| hLMP2-3 | tgt gca ctc tct ggt tca gc | |||
| huLMP7 | hLMP7-5 | tct gcg tca tca gca aga ac | 60 | 26 |
| hLMP7-3 | gcc att cag gaa gtg tcc at | |||
| huTAP1 | hTAP1-5 | gga atc tct ggc aaa gtc ca | 60 | 27 |
| hTAP1-3 | tgg gtg aac tgc atc tgg ta | |||
| huTAP2 | hTAP2-5 | cca aga cgt ctc ctt tgc at | 60 | 30 |
| hTAP2-3 | ttc atc cag cag cac ctg tc | |||
| huTapasin | htpn-5 | tgg gta agg gac atc tgc tc | 60 | 34 |
| htpn-3 | acc tgt cct tgc agg tat gg | |||
| huβ2 M | hβ2M-5 | ctc gcg cta ctc tct ctt | 60 | 25 |
| hβ2M-3 | aag accagt cct tgc tga | |||
| huβ-actin | HβActin-5 | tcc tgt ggc atc cac gaa act | 58 | 31 |
| HβAactin-3 | gaa gca ttt gcg gtg gac gat |
Immunohistochemistry
Immunohistochemical staining of formalin-fixed, paraffin-embedded tissues was performed as recently described [38]. After staining, all slides were reviewed by one surgical pathologist (AH) without knowledge of the clinical data. Results were scored as negative (0), heterogeneous (1) and positive (2), when the percentage of stained tumor cells was <25, between 25 and 75, and >75, respectively. Adjacent normal prostate epithelium served as a control. In addition, the staining intensity was scored as 0 (negative), 1 (weak), 2 (moderate) and 3 (strong). The results were then analyzed according to Armes et al. [39]: a combined score was derived by adding the two scores, i.e. % of stained tumor cells and staining intensity. Combined scores of “0” were designed as negative, “1–2” as weak expression, “3–4” as moderate expression, and “5” as strong expression. Negative controls were performed by omitting primary antibodies.
Flow cytometry
Cells were stained with a FITC-labeled HLA class I-specific mAb (Beckman Coulter) using a FITC-labeled lgG2a mAb (Beckman Coulter) as control [40]. Stained cells were analyzed with a fluorescence activated cell sorter (Beckmann & Coulter, Krefeld, Germany). The results are expressed as mean fluorescence intensity (MFI) + SD of the MFI values obtained in three independent experiments.
Statistical analysis
The results of immunohistochemical staining were correlated with the histopathological characteristics of the PC lesions and with the clinical characteristics of the patients utilizing χ2-test (Fisher exact test, two-sided). p values <0.05 were considered to be significant. Statistical analyses were performed utilizing the Statistical Package for the Social Sciences (SPSS) version 13.0 (SPSS, Chicago, IL, USA).
Results
HLA class I APM component expression in normal prostate tissue
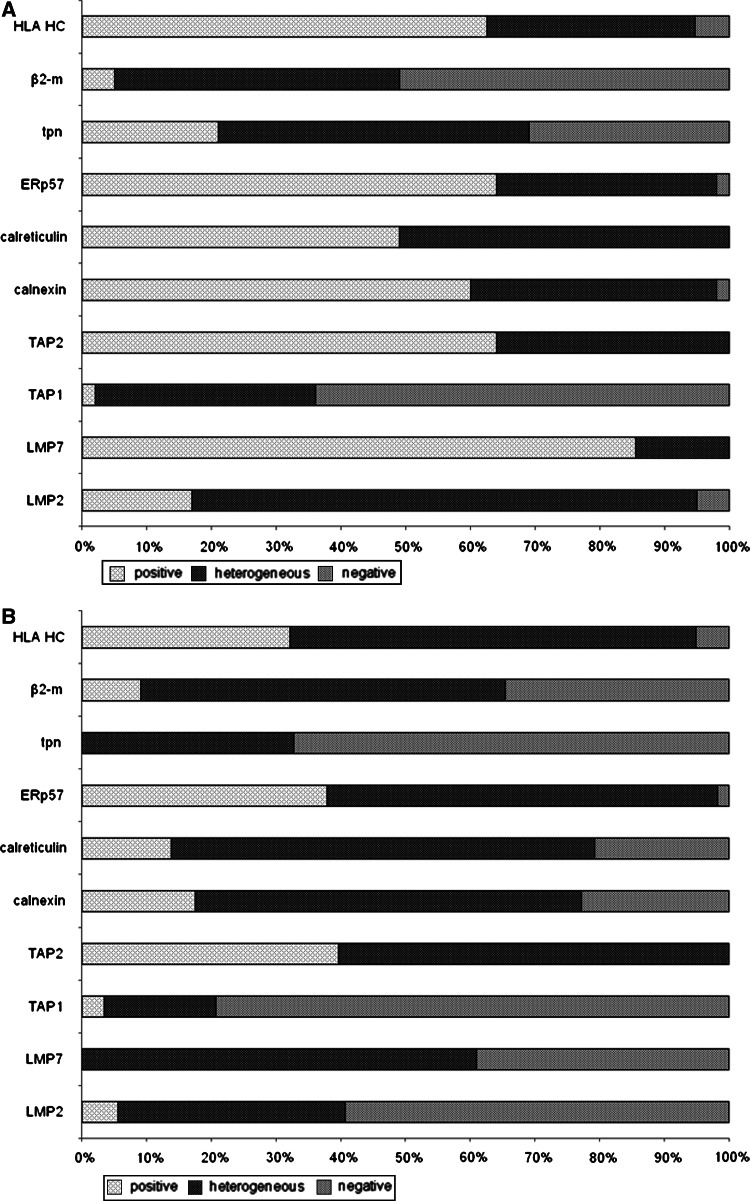
A TMA comprising 59 PC specimens and corresponding normal prostate tissue was stained with LMP2-, LMP7-, TAP1-, TAP2-, calnexin-, calreticulin-, ERp57-, tpn-, HLA class I HC-, and β2-m-specific mAb. With the exception of the TAP1-specific mAb displaying a weak staining, all the other mAb displayed a strong positive staining of normal prostate tissue sections with a focal pattern, but with a distinct density and frequency ranging between 50 and 100% (Fig. 1). These findings confirmed the results previously obtained by immunostaining smaller series of normal prostate epithelium with anti-HLA class I-specific antibodies [41–43].
Fig. 1.
Heterogeneous expression pattern of the HLA class I APM components analyzed in corresponding normal prostate epithelium and PC lesions. a Prostate epithelium. b PC lesions. The data are expressed as diagram using the scoring system described in “Materials and methods”
Differential expression of HLA class I APM components in PC lesions
Immunohistochemical staining with the HLA class I HC-specific mAb HC10 and the β2-m-specific mAb L368 resulted in a cytoplasmic/membraneous staining of 95 and 65% of the 59 PC lesions analyzed, respectively (Table 3). A strong cytoplasmic staining of the PC lesions analyzed with LMP2-, LMP7-, TAP1-, TAP2-, tpn-, calnexin-, calreticulin, and ERp57-specific mAbs was found, but the percentage of stained tumor cells varied with a maximum reaching 39.7%, whereas a moderate to weak cytoplasmic staining of these lesions was detected with a percentage of stained tumor cells ranging between 17.2 and 65.5% in 52 to 59 PC lesions analyzed (Table 3, Figs. 1, 2). Seven HLA class I APM components were coordinately downregulated in 7% and at least five components coordinately lost in 54% of the PC lesions. As measured by staining intensity the expression level of TAP2 (p = 0.016), LMP2, LMP7, calnexin, calreticulin, tpn (p < 0.001), and ERp57 (p = 0.002) was significantly lower in PC lesions than in normal prostate tissues.
Table 3.
Heterogeneous expression of HLA class I APM components in PC
| Frequency of staining (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LMP2 | LMP7 | TAP1 | TAP2 | Calnexin | Calreticulin | ERp57 | tpn | β2-m | HLA HC | |
| No. of cases analyzed | 54 | 59 | 58 | 58 | 57 | 58 | 58 | 52 | 55 | 59 |
| Positive | 5.6 | 0.0 | 3.5 | 39.7 | 17.5 | 13.8 | 37.9 | 0.0 | 9.1 | 32.2 |
| Heterogeneous | 35.2 | 61.0 | 17.2 | 60.3 | 59.7 | 65.5 | 60.4 | 32.7 | 56.4 | 62.7 |
| Negative | 59.3 | 39.0 | 79.3 | 0.0 | 22.8 | 20.7 | 1.7 | 67.3 | 34.5 | 5.1 |
Immunohistochemistry and scoring were performed as described in “Materials and methods”
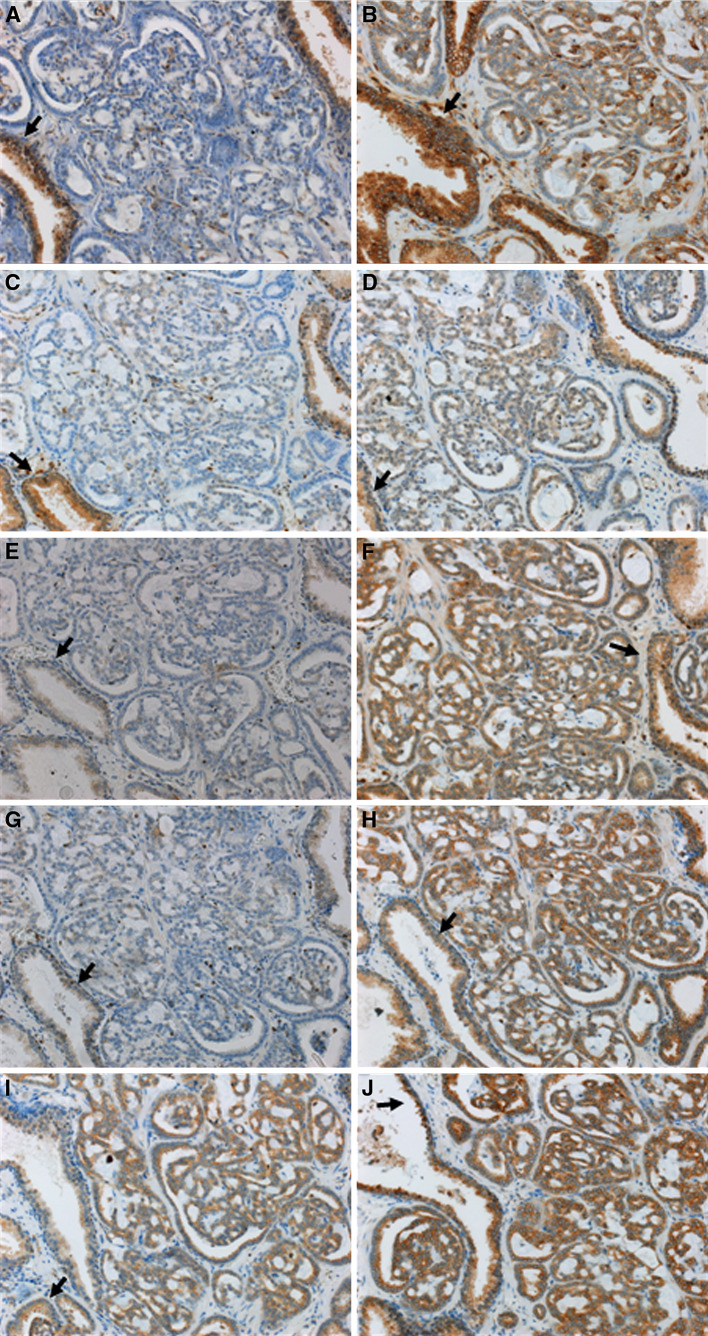
Fig. 2.
Immunohistochemical staining of PC lesions and surrounding normal prostate tissues with HLA class I APM component-specific mAb. Representative staining results of a prostate carcinoma (pT3cG2, Gleason 7 (3 + 4), no recurrence) are shown. Magnification: ×200. Staining of the normal prostate gland from this PC patient is marked (arrow) for each antibody. a β2-m loss in PC; b Marked HLA class I HC downregulation in PC; c LMP2 loss in PC; d LMP7 downregulation in PC; e weak TAP1 expression in normal prostate gland and TAP1 loss in PC; f positive staining for TAP2 in normal prostate gland and PC; g tpn downregulation in PC; h comparable high calnexin expression in normal prostate gland and PC; i positive staining for calreticulin in normal prostate gland and PC; j strong expression of ERp57 in normal prostate gland and PC
Association of HLA class I APM deficiencies with tumor characteristics and disease recurrence
To determine whether HLA class I APM component downregulation or loss is associated with disease progression, the immunohistochemical data obtained from the analysis of 59 primary PC lesions were correlated with tumor grading, staging, as well as PSA recurrence. Complete loss or weak expression of calnexin was associated with an early recurrence (p = 0.003) and an aggressive tumor phenotype (Gleason score ≥7, p = 0.002). In addition, lack of cytoplasmic and membranous staining by the mAb HC-10 was correlated with early tumor recurrence (p = 0.03). Finally, a synchronous downregulation of at least seven APM components was found in 41% of PC patients with early recurrence, but in only 24% of the patients without recurrence.
Heterogeneous constitutive and IFN-γ-inducible HLA class I APM component expression in PC cell lines
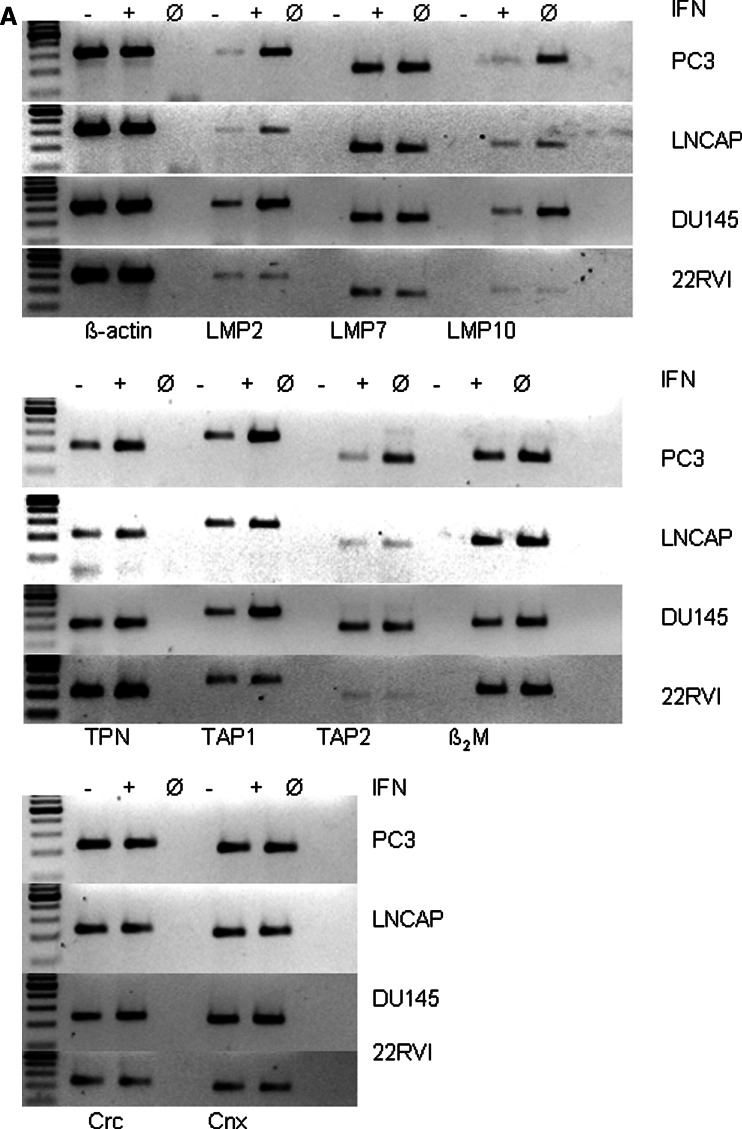
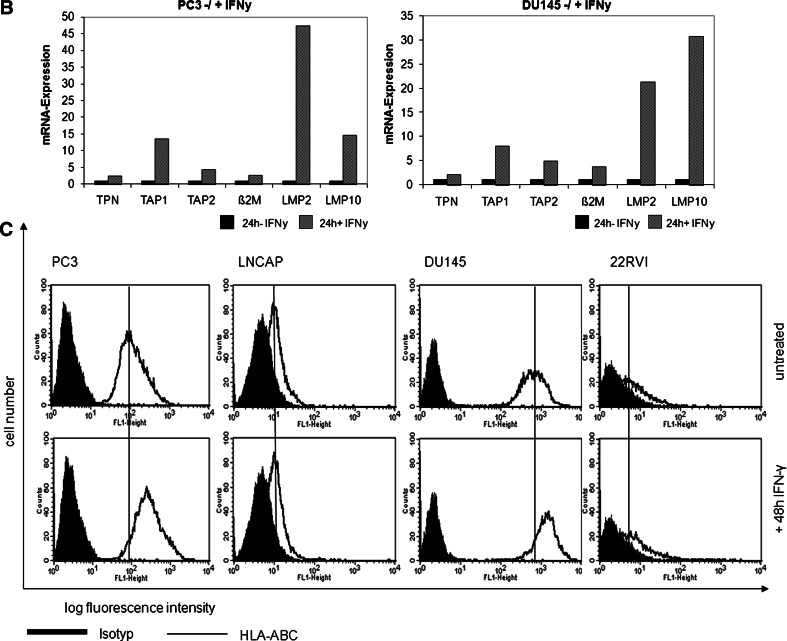
To get insight into the mechanisms underlying HLA class I APM abnormalities in PC lesions, four PC cell lines (LNCaP, PC3, 22RVI, and DU145) were analyzed for their constitutive and IFN-γ-inducible HLA class I APM component and HLA class I surface antigen expression. As representatively shown in Fig. 3a, the level of HLA class I APM component mRNA was in general low in the PC cell lines analyzed, although the extent of downregulation varied among the APM components. In particular, the mRNA level of TAP2 was lower in LNCaP and 22RVI cells than in PC3 and DU145 cells. As shown by qRT-PCR in PC3 and DU145 cells, the expression of most APM molecules was upregulated by IFN-γ (Fig. 3b), whereas LNCaP and 22RVI cells were not IFN-γ responsive; this change was associated with an increased HLA class I surface antigen expression in PC3 and DU145 cells, but not in the two other PC cell lines (Fig. 3c). The low APM component expression was accompanied by the lack or low expression of HLA class I antigens on all PC cell lines (Fig. 3c). These data suggest that the reduced HLA class I APM component expression in PC3 and DU145 cells was mainly due to their dysregulation rather than structural alterations of the APM molecules. In contrast neither APM components nor HLA class I antigens were upregulated by incubating LNCaP and 22RVI cells with IFN-γ (Fig. 3a). The resistance of both PC cell lines to IFN-γ treatment was not due to impaired expression of the IFN-γ receptors (data not shown). The impaired restoration of APM component expression by IFN-γ in LNCaP and 22RVI cells points to defects in the IFN signal transduction pathway in these cell lines since 10 ng/ml TNF-α was able to enhance APM component expression (data not shown).
Fig. 3.
Constitutive and IFN-γ-inducible HLA class I APM component expression by PC cell lines. The four PC cell lines PC3, LNCaP, DU145, and 22RVI were cultured in the presence and absence of IFN-γ (100 U/ml) for 24 h before RT-PCR (a), qRT-PCR (b) and for 48 h before flow cytometry (c) was performed, as described in “Material and methods”
Discussion
Immunohistochemical staining of a TMA comprising 59 primary PC lesions and autologous normal tissues has shown downregulation of all the HLA class I APM components analyzed, although with a different frequency in tumor lesions when compared to normal prostate tissues. A key finding of this study was the frequent loss or downregulation of calnexin and/or tpn, which appears to be an independent prognostic marker of tumor recurrence (Fig. 1). In contrast, HLA class I HC expression levels were less affected in primary PC lesions; the frequency of downregulation described in this manuscript is markedly lower than that reported by other authors [25, 41] in a large number of PC lesions and that reported in other tumor types, e.g. melanoma and colon carcinoma [19, 20, 44, 45]. In contrast a high frequency of loss or downregulation of LMP2, TAP1, and tpn as well as of β2-m was demonstrated in PC lesions when compared to normal prostate tissue. The frequency of LMP2, TAP1, and tpn downregulation is higher than that found in lung, colorectal, hepatocellular, cervical, and renal cell carcinoma (RCC) [19, 20, 38, 46]. In parallel to the findings in head and neck squamous cell carcinoma, cervical carcinoma, esophageal carcinoma, breast carcinoma, and melanoma the impaired TAP expression in PC cancer lesions is associated with tumor grading, staging, and time to recurrence [47–50]. In this context it is noteworthy that the HC10 antibody used for immunohistochemical analysis of the TMA only recognizes free HLA class I HC, but not HLA class I surface expression. This is based on the fact that the TMA represents paraffin-embedded tissues and therefore the use of the W6/32 mAb recognizing HLA class I surface expression was not possible. Thus, a positive staining with this antibody need not necessarily indicate HLA class I surface antigen expression. Based on this limitation samples might be classified as positive exhibiting haplotype-, locus-, or allele-specific HLA losses. This might explain the difference in the frequency of HLA class I abnormalities in prostate cancer, which has been reported to occur in approximately 80% PC lesions [41]. However, APM defects have been shown to be directly associated with reduced or impaired HLA class I surface expression, since the generation and supply of antigenic peptides is not sufficient for proper HLA class I antigen presentation.
In the present study we show a correlation of the HLA class I APM defects with clinical parameters in PC. The disease recurrence as well as biological aggressiveness of the tumor determined by high Gleason scores was associated with loss and/or downregulation of selected HLA class I APM components, e.g. calnexin and tpn. If independently confirmed in a larger number of patients, these data argue in favor of the possibility that the evaluation of the structural integrity and expression pattern of these molecules might be helpful to optimize the selection of PC patients to be treated with T cell-based immunotherapy.
The molecular events causing HLA class I APM component abnormalities could either reflect structural alterations in these molecules, in particular haplotype, locus, or allele-specific loss of HLA class I HC, mutations or deletions in β2-m, defects in the IFN signal transduction pathway, or deregulation of APM components occurring at the transcriptional, posttranscriptional, or epigenetic level. Deregulation of APM components and the IFN-γ signal transduction cascade is suggested by our results analyzing the constitutive and IFN-γ-inducible APM component and HLA class I surface antigen expression in the PC cell lines LNCaP, 22RVI, PC3, and DU145. The PC3 and DU145 cells express higher levels of APM components than the LNCaP and 22RVI. Furthermore, in PC3 and DU145 cells, but not in LNCaP and 22RVI cells, the impaired APM component and HLA class I antigen surface expression was upregulated by IFN-γ (Fig. 3b). However, the resistance of LNCaP and 22RVI to IFN-γ was not due to defects in the IFN-γ receptor (data not shown). It is noteworthy, that also IFN-α and IFN-β does not upregulate HLA class I APM components in these cells, whereas TNF-α treatment caused an increased HLA class I surface antigen expression. Thus, the reduced APM component expression in LNCaP and 22RVI cells appears not to be caused by structural alterations, but might be due to their dysregulation. However, we could not exclude epigenetic mechanisms, since HLA class I downregulation in LNCaP cells has been demonstrated to be associated with an altered acetylation status of the β2-m promoter, which could be corrected by trichostatin A [25].
The LMP2 and LMP7 expression in normal prostate tissues is a primarily unexpected finding since these immunoproteasome subunits are generally only expressed in cells upon IFN-γ treatment. This finding might reflect either a tissue-specific regulation controlled by the microenvironment or undetected pathological processes. Whatever the mechanism, LMP2 and LMP7 appear to be coordinately downregulated in prostate cancer cells. In addition, LMP2 and TAP1 loss or downregulation in prostate cancer lesions is coregulated in about 17 and 59% of cases, respectively, due to their regulation by the bi-directional LMP2/TAP1 promoter [51]. So far, the expression of the chaperones calnexin and calrecticulin has been analyzed only in a few types of human tumors. In cervical cancer, calnexin and calreticulin expression was not impaired [50], whereas in laryngeal carcinoma both chaperones were downregulated in 25% of the lesions analyzed. In contrast, a frequent downregulation or loss of calnexin and calreticulin was found in PC lesions (Fig. 1b). Recently it has been described that ERp57 expression constitutes a negative prognostic marker in some neoplasia including gastric cancer [52]. Furthermore the inability of calreticulin to be presented on the cell surface may lead in tumor cells to elicit a proper immune response [53, 54], which might be also associated with chemotherapy resistance [55]. These data suggest that in particular calreticulin is involved in HLA class I antigen stabilization and assembly and thus may be essential for HLA class I surface antigen expression. Thus, deficiencies in the function of chaperones might affect the efficacy of peptide loading and presentation onto HLA class I molecules, but their role in PC development and progression has still to be defined.
Acknowledgments
We would like to thank Rudolf Jung for excellent technical support and Claudia Stoerr and Sylvi Magdeburg for the excellent secretarial help in preparing the manuscript. This work is supported by a grant of the Deutsche Forschungsgemeinschaft SE-581/10-1, 2 (BS) and by PHS grants RO1CA110249 awarded by the National Cancer Institute, DHHS and DOD grant 7-01-0096 (SF).
Abbreviations
- APM
Antigen processing machinery
- AR
Androgen receptor
- ATCC
American tissue culture collection
- β2-m
β2-Microglobulin
- CTL
Cytotoxic T lymphocyte
- FCS
Fetal calf serum
- HC
Heavy chain
- IFN
Interferon
- LMP
Low molecular weight protein
- mAb
Monoclonal antibody
- MHC
Major histocompatibility complex
- PC
Prostate carcinoma
- PSA
Prostate-specific antigen
- RCC
Renal cell carcinoma
- TA
Tumor antigen
- TAP
Transporter associated with antigen processing
- tpn
Tapasin
- TMA
Tissue microarray
- TNF
Tumor necrosis factor
References
- 1.Epstein JI, Algabe F, Allsbrook WC, Bastacky S, et al. Tumours of the prostate. In: Eble JN, Sauter G, Epstein JI, Sesterhenn IA, et al., editors. World Health Organization, classification of tumours. Pathology and genetic. Tumours of the urinary system and male genital organs. Lyon: IARC; 2004. pp. 159–216. [Google Scholar]
- 2.Thompson TC. Growth factors and oncogenes in prostate cancer. Cancer cells. 1990;2:345–354. [PubMed] [Google Scholar]
- 3.Risk M, Corman JM. The role of immunotherapy in prostate cancer: an overview of current approaches in development. Rev Urol. 2009;11:16–27. [PMC free article] [PubMed] [Google Scholar]
- 4.Chakraborty NG, Stevens RL, Mehrotra S, Laska E, Taxel P, Sporn JR, Schauer P, Albertsen PC. Recognition of PSA-derived peptide antigens by T cells from prostate cancer patients without any prior stimulation. Cancer Immunol Immunother. 2003;52:497–505. doi: 10.1007/s00262-003-0377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang YS, Tsang YW, Chi CH, Cahng CC, Chu RM, Chi KH. Synergistic anti-tumor effect of combination radio- and immunotherapy by electro-gene therapy plus intra-tumor injection of dendritic cells. Cancer Lett. 2008;266:275–285. doi: 10.1016/j.canlet.2008.02.063. [DOI] [PubMed] [Google Scholar]
- 6.Kiessling A, Füssel S, Wehner R, Bachmann M, Wirth MP, Rieber EP, Schmitz M. Advances in specific immunotherapy for prostate cancer. Eur Urol. 2008;53:694–708. doi: 10.1016/j.eururo.2007.11.043. [DOI] [PubMed] [Google Scholar]
- 7.Olson WC, Heston WD, Rajasekaran AK. Clinical trials of cancer therapies targeting prostate-specific membrane antigen. Rev Recent Clin Trials. 2007;2:182–190. doi: 10.2174/157488707781662724. [DOI] [PubMed] [Google Scholar]
- 8.Small EJ, Tchekmedyian NS, Rini BI, Fong L, Lowy I, Allison JP. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res. 2007;13:1810–1815. doi: 10.1158/1078-0432.CCR-06-2318. [DOI] [PubMed] [Google Scholar]
- 9.Fong L, Kwek SS, O’Brien S, Kavanagh B, McNeel DG, Weinberg V, Lin AM, Rosenberg J, Ryan CJ, Rini BI, Small EJ. Potentiating endogenous antitumor immunity to prostate cancer through combination immunotherapy with CTLA4 blockade and GM-CSF. Cancer Res. 2009;69:609–615. doi: 10.1158/0008-5472.CAN-08-3529. [DOI] [PubMed] [Google Scholar]
- 10.Theoret MR, Arlen PM, Pazdur M, Dahut WL, Schlom J, Gulley JL. Phase I trail of an enhanced prostate-specific antigen-based vaccine and anti-CTLA-4 antibody in patients with metastatic androgen-independent prostate cancer. Clin Genitourin Cancer. 2007;5:347–350. doi: 10.3816/CGC.2007.n.017. [DOI] [PubMed] [Google Scholar]
- 11.Slovin SF. Pitfalls or promise in prostate cancer immunotherapy which is winning? Cancer J. 2008;14:26–34. doi: 10.1097/PPO.0b013e318161bffa. [DOI] [PubMed] [Google Scholar]
- 12.Burch PA, Breen JK, Buckner JC, Gastineau DA, Kaur JA, Laus RL, Padley DJ, Peshwa MV, Pitot HC, Richardson RL, Smits BJ, Sopapan P, Strang G, Valone FH, Vuk-Pavlović S. Priming tissue-specific cellular immunity in a phase I trial of autologous dendritic cells for prostate cancer. Clin Cancer Res. 2000;6:2175–2182. [PubMed] [Google Scholar]
- 13.Eder JP, Kantoff PW, Roper K, Xu GX, Bubley GJ, Boyden J, Gritz L, Mazzara G, Oh WK, Arlen P, Tsang KY, Panicali D, Schlom J, Kufe DW. A phase I trial of a recombinant vaccina virus expressing prostate-specific antigen in advanced prostate cancer. Clin Cancer Res. 2000;6:1632–1638. [PubMed] [Google Scholar]
- 14.Simons JW, Mikhak B, Chang JF, DeMarzo AM, Carducci MA, Lim M, Weber CE, Baccala AA, Goemann MA, Clift SM, Ando DG, Levitsky HI, Cohen LK, Sanda MG, Mulligan RC, Partin AW, Carter HB, Piantadosi S, Marshall FF, Nelson WG. Induction of immunity to prostate cancer antigens: results of a clinical trial of vaccination with irradiated autologous prostate tumor cell engineered to secrete granulocyte-macrophage colony-stimulating factor using ex vivo gene transfer. Cancer Res. 2001;59:5160–5168. [PubMed] [Google Scholar]
- 15.Hammer GE, Kanaseki T, Shastri N. The final touches make perfect the peptide-MHC class I repertoire. Immunity. 2007;26:397–406. doi: 10.1016/j.immuni.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Jensen PE. Recent advances in antigen processing and presentation. Nat Immunol. 2007;8:1041–1048. doi: 10.1038/ni1516. [DOI] [PubMed] [Google Scholar]
- 17.Aptsiauri N, Cabrera T, Mendez R, Garcia-Lora A, Ruiz-Cabello F, Garrido F. Role of altered expression of HLA class I molecules in cancer progression. Adv Exp Med Biol. 2007;601:123–131. doi: 10.1007/978-0-387-72005-0_13. [DOI] [PubMed] [Google Scholar]
- 18.Cabrera T, Maleno I, Collado A, Lopez Nevot MA, Tait BD, Garrido F. Analysis of HLA class I alterations in tumors: choosing a strategy based on known patterns of underlying molecular mechanisms. Tissue Antigens. 2007;1:264–268. doi: 10.1111/j.1399-0039.2006.00777.x. [DOI] [PubMed] [Google Scholar]
- 19.Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human solid tumor from T cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74:181–273. doi: 10.1016/S0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- 20.Seliger B, Ritz U, Ferrone S. Molecular mechanisms of HLA class I antigen abnormalities following viral infection and transformation. Int J Cancer. 2006;118:129–138. doi: 10.1002/ijc.21312. [DOI] [PubMed] [Google Scholar]
- 21.Sanda MG, Restifo NP, Walsh JC, Kawakami Y, Nelson WG, Pardoll DM, Simons JW. Molecular characterization of defective antigen processing in human prostate cancer. J Natl Cancer Inst. 1995;87:280–285. doi: 10.1093/jnci/87.4.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee HM, Timme TL, Thompson TC. Resistance to lysis by cytotoxic T cells: a dominant effect in metastatic mouse prostate cancer cells. Cancer Res. 2000;60:1927–1933. [PubMed] [Google Scholar]
- 23.Sharpe JC, Abel PD, Gilbertson JA, Brawn P, Foster CS. Modulated expression of human leukocyte antigen class I and class II determinants in hyperplastic and malignant human prostatic epithelium. Br J Urol. 1994;74:609–616. doi: 10.1111/j.1464-410X.1994.tb09193.x. [DOI] [PubMed] [Google Scholar]
- 24.Setiadi AF, David MD, Chen SS, Hiscott J, Jefferies WA. Identification of mechanisms underlying transporter associated with antigen processing deficiency in metastatic murine carcinomas. Cancer Res. 2005;65:7485–7492. doi: 10.1158/0008-5472.CAN-03-3734. [DOI] [PubMed] [Google Scholar]
- 25.Kitamura H, Torigoe T, Asanuma H, Honma I, Sato N, Tsukamoto T. Down-regulation of HLA class I antigens in prostate cancer tissues and up-regulation by histone deacetylase inhibition. J Urol. 2007;178:692–696. doi: 10.1016/j.juro.2007.03.109. [DOI] [PubMed] [Google Scholar]
- 26.Stolzenburg JU, Rabenalt R, Do M, Kallidonis P, Liatsikos EN. Endoscopic extraperitoneal radical prostatectomy: the University of Leipzig experience of 2000 cases. J Endourol. 2008;22:2319–2325. doi: 10.1089/end.2008.9714. [DOI] [PubMed] [Google Scholar]
- 27.Sobin LH, Wittekind C. TNM classification of malignant tumours. New York: Wiley-Liss; 1997. pp. 172–175. [Google Scholar]
- 28.Kasper G, Weiser AA, Rump A, Sparbier K, Dahl E, Hartmann A, Wild P, Schwidetzky U, Castaños-Vélez E, Lehmann K. Expression levels of putative zinc transporter LIV-1 are associated with a better outcome of breast cancer patients. Int J Cancer. 2005;117:961–973. doi: 10.1002/ijc.21235. [DOI] [PubMed] [Google Scholar]
- 29.van Oers JM, Wild PJ, Burger M, Denzinger S, Stoehr R, Rosskopf E, Hofstaedter F, Steyerberg EW, Klinkhammer-Schalke M, Zwarthoff EC, van der Kwast TH, Hartmann A. FGFR3 mutations and normal CK20 staining pattern define low-grade noninvasive urothelial bladder tumors. Eur Urol. 2007;52:760–768. doi: 10.1016/j.eururo.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Stam NJ, Spits H, Ploegh HL. Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J Immunol. 1986;137:2299–2306. [PubMed] [Google Scholar]
- 31.Perosa F, Luccarelli G, Prete M, Favoino E, Ferrone S, Dammacco F. Beta 2-microglobulin-free HLA class I heavy chain epitope mimicry by monoclonal antibody HC-10-specific peptide. J Immunol. 2003;171:1918–1926. doi: 10.4049/jimmunol.171.4.1918. [DOI] [PubMed] [Google Scholar]
- 32.Lampson LA, Fisher CA, Whelan JP. Striking paucity of HLA-A, B, C and beta 2-microglobulin on human neuroblastoma cell lines. J Immunol. 1983;30:2471–2478. [PubMed] [Google Scholar]
- 33.Bandoh N, Ogino T, Cho HS, Hur SY, Shen J, Wang X, Kato S, Miyokawa N, Harabuchi Y, Ferrone S. Development and characterization of human constitutive proteasome and immunoproteasome subunit-specific monoclonal antibodies. Tissue Antigens. 2005;66:185–194. doi: 10.1111/j.1399-0039.2005.00462.x. [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Campoli M, Cho HS, Ogino T, Bandoh N, Shen J, Hur SY, Kageshita T, Ferrone S. A method to generate antigen-specific mAb capable of staining formalin-fixed, paraffin-embedded tissue sections. J. Immunol. Methods. 2005;299:139–151. doi: 10.1016/j.jim.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Ogino T, Wang X, Kato S, Miyokawa N, Harabuchi Y, Ferrone S. Endoplasmic reticulum chaperone-specific monoclonal antibodies for flow cytometry and immunohistochemical staining. Tissue Antigens. 2003;62:382–393. doi: 10.1034/j.1399-0039.2003.00114.x. [DOI] [PubMed] [Google Scholar]
- 36.Temponi M, Kageshita T, Perosa F, Ono R, Okada H, Ferrone S. Purification of murine IgG monoclonal antibodies by precipitation with caprylic acid: comparison with other methods of purification. Hybridoma. 1989;8:85–95. doi: 10.1089/hyb.1989.8.85. [DOI] [PubMed] [Google Scholar]
- 37.Norell H, Carlsten M, Ohlum T, Malmberg KJ, Masucci G, Schedvins K, Altermann W, Handke D, Atkins D, Seliger B, Kiessling R. Frequent loss of HLA-A2 expression in metastasizing ovarian carcinomas associated with genomic haplotype loss and HLA-A2-restricted HER-2/neu-specific immunity. Cancer Res. 2006;66:6387–6394. doi: 10.1158/0008-5472.CAN-06-0029. [DOI] [PubMed] [Google Scholar]
- 38.Atkins D, Ferrone S, Schmahl GE, Störkel S, Seliger B. Down-regulation of HLA class I antigen processing molecules: an immune escape mechanism of renal cell carcinoma? J Urol. 2004;171:885–889. doi: 10.1097/01.ju.0000094807.95420.fe. [DOI] [PubMed] [Google Scholar]
- 39.Armes JE, Trute L, White D, Southey MC, Hammet F, Tesoriero A, Hutchins AM, Dite GS, McCredie MR, Giles GG, Hopper JL, Venter DJ. Distinct molecular pathogeneses of early-onset breast cancers in BRCA1 and BRCA2 mutation carriers: a population-based-study. Cancer Res. 1999;59:2011–2017. [PubMed] [Google Scholar]
- 40.Seliger B, Atkins D, Bock M, Ritz U, Ferrone S, Huber C, Störkel S. Characterization of human lymphocyte antigen class I antigen-processing machinery defects in renal cell carcinoma lesions with special emphasis on transporter-associated with antigen-processing down-regulation. Clin Cancer Res. 2003;95:1721–1727. [PubMed] [Google Scholar]
- 41.Blandes RA, Keating PJ, McWilliams LJ, George NJR, Stern PL. Loss of HLA class I expression in prostate cancer: implications for immunotherapy. Urology. 1995;46:681–687. doi: 10.1016/S0090-4295(99)80301-X. [DOI] [PubMed] [Google Scholar]
- 42.Bander NH, Yao D, Liu H, Chen YT, Steiner M, Zuccaro W, Moy P. MHC class I and II expression in prostate carcinoma and modulation by interferon-alpha and -gamma. Prostate. 1997;33:233–239. doi: 10.1002/(SICI)1097-0045(19971201)33:4<233::AID-PROS2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 43.Zhang H, Melamed J, Wei P, Cox K, Frankel W, Bahnson RR, Robinson N, Pyka R, Liu Y, Zheng P. Concordant down-regulation of proto-oncogene PML and major histocompatibility antigen HLA class I expression in high-grade prostate cancer. Cancer Immun. 2003;3:2. [PubMed] [Google Scholar]
- 44.Chang CC, Ogino T, Mullins DW, Oliver JL, Yamshchikov GV, Bandoh N, Slingluff CL, Jr, Ferrone S. Defective human leukocyte antigen class I-associated antigen presentation caused by a novel beta2-microglobulin loss-of-function in melanoma cells. J Biol Chem. 2006;281:18763–18773. doi: 10.1074/jbc.M511525200. [DOI] [PubMed] [Google Scholar]
- 45.Momburg F, Koch S. Selective loss of beta 2-microglobulin mRNA in human colon carcinoma. J Exp Med. 1989;169:309–314. doi: 10.1084/jem.169.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vermeulen CF, Jordanova ES, ter Haar NT, Kolkman-Uljee SM, de Miranda NF, Ferrone S, Peters AA, Fleuren GJ. Expression and genetic analysis of transporter associated with antigen processing in cervical carcinoma. Gynecol Oncol. 2007;105:593–599. doi: 10.1016/j.ygyno.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 47.Kageshita T, Hirai S, Ono T, Hicklin DJ, Ferrone S. Down-regulation of HLA class I antigen-processing molecules in malignant melanoma: association with disease progression. Am J Pathol. 1999;154:745–754. doi: 10.1016/S0002-9440(10)65321-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lou Y, Vitalis TZ, Basha G, Cai B, Chen SS, Choi KB, Jeffries AP, Elliott WM, Atkins D, Seliger B, Jefferies WA. Restoration of the expression of transporters associated with antigen processing in lung carcinoma increases tumor-specific immune responses and survival. Cancer Res. 2005;65:7926–7933. doi: 10.1158/0008-5472.CAN-04-3977. [DOI] [PubMed] [Google Scholar]
- 49.Meissner M, Reichert TE, Kunkel M, Gooding W, Whiteside TL, Ferrone S, Seliger B. Defects in the human leucocyte antigen class I antigen-processing machinery in head and neck squamous cell carcinoma: association with clinical outcome. Clinical Cancer Res. 2005;11:2552–2560. doi: 10.1158/1078-0432.CCR-04-2146. [DOI] [PubMed] [Google Scholar]
- 50.Mehta AM, Jordanova ES, Kenter GG, Ferrone S, Fleuren GJ. Association of antigen processing machinery and HLA class I defects with clinicopathological outcome in cervical carcinoma. Cancer Immunol Immunother. 2008;57:197–206. doi: 10.1007/s00262-007-0362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wright KL, White LC, Kelly A, Beck S, Trowsdale J, Ting JP. Coordinate regulation of the human TAP1 and LMP2 genes from a shared bidirectional promoter. J Exp Med. 1995;181:1459–1471. doi: 10.1084/jem.181.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leys CM, Nomura S, LaFleur BJ, Ferrone S, Kaminishi M, Montgomery E, Goldenring JR. Expression and prognostic significance of prothymosin-alpha and ERp57 in human gastric cancer. Surgery. 2007;141:41–50. doi: 10.1016/j.surg.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 53.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, Métivier D, Larochette N, van Endert P, Ciccosanti F, Piacentini M, Zitvogel L, Kroemer G. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 54.Panaretakis T, Joza N, Modjtahedi N, Tesniere A, Vitale I, Durchschlag M, Fimia GM, Kepp O, Piacentini M, Froehlich KU, van Endert P, Zitvogel L, Madeo F, Kroemer G. The co-translocation of ERp57 and calreticulin determines the immunogenicity of cell death. Cell Death Differ. 2008;15:1499–1509. doi: 10.1038/cdd.2008.67. [DOI] [PubMed] [Google Scholar]
- 55.Clarke C, Smyth MJ. Calreticulin exposure increases cancer immunogenicity. Nat Biotechnol. 2007;25:192–193. doi: 10.1038/nbt0207-192. [DOI] [PubMed] [Google Scholar]