Abstract
The programmed death 1 (PD-1) receptor is a negative regulator of activated T cells and is up-regulated on exhausted virus-specific CD8+ T cells in chronically infected mice and humans. Programmed death ligand 1 (PD-L1) is expressed by multiple tumors, and its interaction with PD-1 resulted in tumor escape in experimental models. To investigate the role of PD-1 in impairing spontaneous tumor Ag-specific CD8+ T cells in melanoma patients, we have examined the effect of PD-1 expression on ex vivo detectable CD8+ T cells specific to the tumor Ag NY-ESO-1. In contrast to EBV, influenza, or Melan-A/MART-1-specific CD8+ T cells, NY-ESO-1-specific CD8+ T cells up-regulated PD-1 expression. PD-1 up-regulation on spontaneous NY-ESO-1-specific CD8+ T cells occurs along with T cell activation and is not directly associated with an inability to produce cytokines. Importantly, blockade of the PD-1/PD-L1 pathway in combination with prolonged Ag stimulation with PD-L1+ APCs or melanoma cells augmented the number of cytokine-producing, proliferating, and total NY-ESO-1-specific CD8+ T cells. Collectively, our findings support the role of PD-1 as a regulator of NY-ESO-1-specific CD8+ T cell expansion in the context of chronic Ag stimulation. They further support the use of PD-1/PD-L1 pathway blockade in cancer patients to partially restore NY-ESO-1-specific CD8+ T cell numbers and functions, increasing the likelihood of tumor regression.
Human melanomas express Ags recognized by autologous T lymphocytes (1). Among these Ags, cancer-germline Ags (CGAs)3 are expressed by tumors of many different histological types, including melanoma, but not by normal tissues, except testis. Because germ cells in testis do not express HLA molecules on their surface (2), CGAs represent strictly tumor-specific T cell targets (1). In contrast to the large majority of CGAs identified to date, NY-ESO-1 stimulates spontaneous cellular and humoral responses in patients with NY-ESO-1-expressing tumors (3, 4). Spontaneous immune responses to NY-ESO-1 are detectable only in patients with advanced NY-ESO-1-expressing cancer and appear to decrease with tumor regression, following therapy or surgery (5). Therefore, understanding the failure of spontaneous NY-ESO-1-specific T cell responses to promote the immunological clearance of NY-ESO-1-expressing tumors appears critical for the design of novel therapeutic interventions aimed at overcoming the tumor evasion of host immune responses.
Among the numerous mechanisms of tumor-induced immuno-suppression that contribute to the resistance of tumors to CTL responses (6), a number of experimental studies in animals (7, 8) and in vitro (9) have suggested the role of programmed death 1 (PD-1)/programmed death ligand 1 (PD-L1) interactions in inhibiting the effector functions of tumor-specific CD8+ T cells (7, 8). PD-1 is a receptor expressed by activated T and B cells (10–13), which binds to two known ligands: PD-L1 (B7-H1) (11, 14) and programmed death ligand 2 (PD-L2; B7-DC) (15, 16). PD-1 negatively regulates T cell functions through the engagement of PD-L1, which is expressed by a wide variety of tissues (11, 13, 14). PD-L1 is also expressed by human tumors, including melanoma, either constitutively or after treatment with IFN-γ (7, 8). In chronically infected mice, lymphocytic choriomeningitis virus-specific CD8+ T cells exhibit a diminished capability to produce cytokines, lyse infected cells, and proliferate in a progressive and hierarchical fashion (17). These “exhausted” T cells have been shown to up-regulate PD-1, and blockade of the PD-1/PD-L1 pathway led to increased cytokine production and proliferation, resulting in a significant reduction of the viral load (18). Recent studies have further extended these observations made in murine models to humans chronically infected with HIV (19–21), hepatitis B virus (22) or hepatitis C virus (23, 24) with partial restoration of cytokine production (IFN-γ and TNF-α, but not IL-2) and proliferative capacities.
To further define the role of PD-1/PD-L1 interactions in impairing spontaneous CD8+ T cell responses to melanoma, we have investigated PD-1 expression on ex vivo detectable CD8+ T cells specific to NY-ESO-1 in patients with advanced melanoma. Our findings show the up-regulation of PD-1 expression on NY-ESO-1-specific CD8+ T cells and demonstrate that blockade of the PD-1/PD-L1 pathway augments the number of NY-ESO-1-specific CD8+ T cells producing cytokines and proliferating in response to prolonged stimulation with cognate Ag.
Materials and Methods
Study subjects and cell lines
Blood samples were obtained under the University of Pittsburgh Cancer Institute (UPCI) Institutional Review Board-approved protocols 96-099 and 00-079 from 17 HLA-A2+ patients with NY-ESO-1-expressing stage IV melanoma. NY-ESO-1 expression by patients’ tumors was assessed by RT-PCR and/or immunohistochemistry. All patients had serum NY-ESO-1-specific Abs detected by ELISA assays. Frequencies of NY-ESO-1-specific CD8+ T cells were assessed ex vivo by flow cytometry using PE-labeled HLA-A2/NY-ESO-1157–165 tetramers. Nine patients who exhibited spontaneous NY-ESO-1-specific CD8+ T cell responses were included in the study. The percentages of NY-ESO-1-specific CD8+ T cells isolated from patients’ PBMCs ranged from 0.010% to 2.4% of total CD8+ T cells. PBMCs used in this study were obtained from patients with no prior immunotherapy. The HLA-A2+NY-ESO-1+ UPCI-MEL 285.1 melanoma cell line used in this study was derived from metastatic lesions of one melanoma patient, UPCI-MEL 285, at the University of Pittsburgh Cancer Institute.
Phenotypic analysis
CD8+ lymphocytes were purified from PBMCs of patients using MACS Column Technology (Miltenyi Biotec) and incubated with PE-labeled HLA-A2/NY-ESO-1157–165, HLA-A2/influenza (Flu)-M58 – 66, HLA-A2/EBV-BMLF1280 –288, or HLA-A2/MART-126 –35 tetramers. The purity of CD8+ T cells was always >95%. Tetramers were provided by the Ludwig Cancer Institute for Cancer Research, Lausanne branch. As controls, we did not observe HLA-A2/NY-ESO-1157–165 and HLA-A2/MART-126 –35 tetramer staining of CD4+ T cells purified from PBMCs of melanoma patients (data not shown). The minimum percentage of Ag-specific CD8+ T cells detected ex vivo in patients using these tetramers was 0.010% of total CD8+ T cells. Next, cells were stained with PD-1-FITC or IgG1-FITC (BD Pharmingen), CD8-energy-coupled dye (ECD), and CD3-PE-Cy5 (Beckman Coulter) conjugated Abs. A violet amine reactive dye (Invitrogen) was used to assess viability of cells.
Alternatively, after tetramer labeling, cells were stained with the following conjugated Abs and reagents: PD-1-FITC, CD8-ECD, CD8-allophycocyanin (BD Pharmingen), CD45RA-ECD, CD45RO-ECD, HLA-DR-ECD, streptavidin-ECD, CD38-PE-Cy5 (Beckman Coulter), CCR7-PE-Cy7, CD28-PerCP-Cy5.5, CD57-biotin (BD Biosciences), and CD27-allophycocyanin-Cy7 (eBioscience). Two million events were collected during flow cytometric analysis on a FACSAria machine (BD Biosciences) and analyzed using FlowJo software (Tree Star). Additionally, 2 million PBMCs from patients were incubated for 6 days with or without peptide NY-ESO-1157–165 (10 μg/ml) before HLA-A2/NY-ESO-1157–165 tetramer and PD-1 staining of CD8+ T cells.
To assess PD-L1 expression on APCs, PBMCs of melanoma patients with spontaneous CD8+ T cell response against NY-ESO-1 were stained ex vivo with CD14-ECD (Beckman Coulter), CD3-Alexa 700, PD-L1-PE, or IgG-PE (BD Pharmingen) conjugated Abs before flow cytometric analysis. To assess PD-L1 expression on melanoma cells, 2 million CFSE-labeled HLA-A2+NY-ESO-1+ UPCI-MEL 285.1 melanoma cells were incubated for 2 days with 2 million CD8+ T cells isolated from PBMCs of one HLA-A2+ melanoma patient (MP3) with spontaneous NY-ESO-1157–165-specific CD8+ T cell response. Culture medium was supplemented with 50 IU/ml rhIL-2 (PeproTech) and 0.5 μg/ml anti-CD28/anti-CD49d Abs (BD Biosciences). Melanoma cells were then stained with PD-L1-PE or IgG-PE conjugated Abs and analyzed by flow cytometry. A violet amine reactive dye was used to assess viability of cells.
Intracellular cytokine staining assay
For in vitro stimulation (IVS) assays, 5 million PBMCs were incubated for 6 days in culture medium containing 50 IU/ml rhIL-2 (PeproTech) with or without peptide NY-ESO-1157–165, EBV BMLF1280 –288, or MART-126 –35 (10 μg/ml) in the presence of either anti-PD-1 (AF 1086; R&D Systems), anti-PD-L1, anti-PD-L2 (16-5983 and 16-5888, respectively; eBioscience), or isotype control (BD Biosciences) Abs (10 μg/ml). On day 6, cells were restimulated for 6 h with the same peptide (10 μg/ml) with or without anti-PD-L1 or isotype control Abs (10 μg/ml). Alternatively, 2 million purified CD8+ T cells were incubated for 6 days with 2 million irradiated HLA-A2+NY-ESO-1+ UPCI-MEL 285.1 melanoma cells in the presence of anti-PD-1, anti-PD-L1, or an isotype control Ab (10 μg/ml). Culture medium was supplemented with 50 IU/ml rhIL-2 and 0.5 μg/ml anti-CD28/anti-CD49d Abs. On day 6, cells were restimulated with 2 million UPCI-MEL 285.1 melanoma cells for 6 h.
After 1 h of incubation, brefeldin A (Sigma-Aldrich) was added into the culture medium (10 μg/ml). Cells were then stained with PE-labeled tetramers, CD14-ECD, CD19-ECD, CD56-biotin, streptavidin-ECD (Beckman Coulter), CD8-PE-Cy7 (BD Biosciences), and CD3-PE-Cy5.5 (Invitrogen) conjugated Abs. Intracellular staining was performed with IFN-γ-FITC, IL-2-allophycocyanin (Miltenyi Biotec), and TNF-α-Alexa 700 (BD Pharmingen) Abs. In ex vivo cytokine production assays, 1 million purified CD8+ T cells were incubated for 6 h with peptide-pulsed (10 μg/ml) non-CD3 autologous cells in the presence or absence of anti-PD-1, anti-PD-L1, anti-PD-L2, or isotype control Abs (10 μg/ml) before tetramer and intracellular cytokine staining. Alternatively, CD8+ T cells were incubated for 6 h with PMA (1 μg/ml) and ionomycin (0.25 μg/ml) (Sigma-Aldrich) before staining.
CFSE proliferation assay
Five million CFSE-labeled PBMCs were incubated for 6 days in culture medium containing 50 IU/ml rhIl-2 with or without peptide (10 μg/ml) in the presence of either anti-PD-1, anti-PD-L1, anti-PD-L2, or isotype control Abs (10 μg/ml). On day 6, cells were stained with PE-labeled tetramers, CD14-ECD, CD19-ECD, CD56-biotin, streptavidin-ECD, CD8-PE-Cy7, and CD3-PE-Cy5.5 conjugated Abs. One million events per sample were acquired and analyzed by flow cytometry.
Statistical analysis
Statistical hypotheses were tested with the Wilcoxon signed rank test (for paired results from the same patient) and the Wilcoxon Mann-Whitney U test (for comparisons between patients and healthy donors) using SAS version 9.1. Tests were two-sided and considered significant for p values of <0.05. Because rank tests are not sensitive to the actual values in a comparison, only to their ranks, differing sets of values can produce identical p values.
Results
PD-1 expression is up-regulated on NY-ESO-1-specific CD8+ T cells
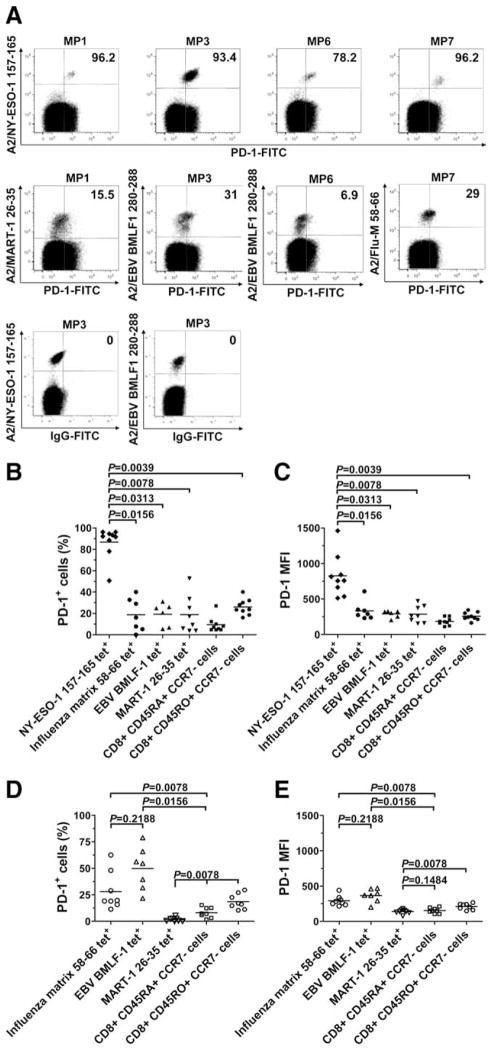
We investigated PD-1 expression on the surface of spontaneous ex vivo detectable NY-ESO-1-, Flu-, EBV-, and MART-1-specific CD8+ T cells isolated from PBMCs of nine HLA-A*0201+ (HLA-A2+) stage IV melanoma patients and eight HLA-A2+ healthy donors (frequencies of NY-ESO-1-, Flu-, EBV-, and MART-1-specific CD8+ T cells are reported in Table I). Our results showed that PD-1 is highly expressed on spontaneous NY-ESO-1-specific CD8+ T cells with a mean percentage of PD-1-expressing (PD-1+) cells of 86.9% (Fig. 1, A and B). Significantly higher PD-1 expression was consistently observed for NY-ESO-1-specific CD8+ T cells than for Flu- (p = 0.0156) and EBV-(p = 0.0313) specific CD8+ T cells in the same subjects. The levels of PD-1 expression by NY-ESO-1-specific CD8+ T cells isolated from PBMCs of the melanoma patients were significantly higher than those of total effector memory (CD45RO+CCR7−) CD8+ T cells (p = 0.0039). Of note, the mean percentage of PD-1+ cells among tetramer− (tet−) CD8+ T cells was 7% (data not shown). In eight of the nine melanoma patients, spontaneous CD8+ T cells directed against the tumor Ag MART-1 were detectable ex vivo, and PD-1 expression of MART-1-specific CD8+ T cells was significantly lower than that of NY-ESO-1-specific CD8+ T cells (p = 0.0078). Similar observations were made when analyzing PD-1 expression in terms of mean fluorescence intensity (MFI) (Fig. 1C). Healthy donors exhibited no detectable NY-ESO-1-specific CD8+ T cell response and no significant difference with melanoma patients in terms of PD-1 expression by Flu-specific CD8+ T cells (p = 0.9497), total effector (CD45RA+CCR7−), and effector memory (CD45RO+CCR7−) CD8+ T cells (p = 0.8335 and p = 0.0925, respectively) (Fig. 1, D and E). Notably, the percentages of EBV-specific and MART-1-specific CD8+ T cells expressing PD-1 were, respectively, significantly higher (p = 0.0082) and lower (p = 0.0019) for healthy donors than for melanoma patients.
Table I.
Ex vivo percentages of spontaneous NY-ESO-1157–165, Flu-M58 – 66, EBV-BMLF1280 –288, and MART-126 –35 tet+CD8+ T cells in melanoma patients and healthy donors included in this studya
| Subjects | Percentage of Ex Vivo tet+CD8+ T Cells/Total CD8+ T cells
|
|||
|---|---|---|---|---|
| NY-ESO-1157–165 | Flu-M58–66 | EBV-BMLF1280–288 | MART-126–35 | |
| Melanoma patients | ||||
| MP1 | 0.016 | 0.091 | 0.060 | 0.020 |
| MP2 | 0.011 | 0.056 | ND | 0.030 |
| MP3 | 2.400 | ND | 0.630 | 0.031 |
| MP4 | 0.021 | 0.017 | 0.033 | 0.410 |
| MP5 | 0.010 | 0.560 | 0.340 | 0.031 |
| MP6 | 0.069 | ND | 0.640 | 0.034 |
| MP7 | 0.026 | 0.027 | ND | 0.011 |
| MP8 | 0.041 | 0.450 | ND | ND |
| MP9 | 0.056 | 0.022 | 2.160 | 0.023 |
| Median | 0.026 | 0.056 | 0.485 | 0.031 |
| Healthy donors | ||||
| HD1 | ND | 0.026 | 0.075 | 0.030 |
| HD2 | ND | 0.013 | 0.041 | 0.014 |
| HD3 | ND | 0.012 | ND | 0.120 |
| HD4 | ND | 0.110 | 0.640 | 0.044 |
| HD5 | ND | 0.021 | 0.100 | 0.046 |
| HD6 | ND | 0.014 | 0.062 | 0.037 |
| HD7 | ND | 0.640 | 0.024 | 0.052 |
| HD8 | ND | 0.026 | 0.010 | 0.066 |
| Median | NA | 0.024 | 0.062 | 0.045 |
ND indicates not detectable; NA, not applicable.
FIGURE 1.
PD-1 expression is up-regulated on NY-ESO-1-specific CD8+ T cells. A, Representative dot plots from different melanoma patients (MP) showing PD-1 expression on HLA-A2 (A2)/NY-ESO-1157–165 tet+, A2/EBV-BMLF1280–288, A2/Flu-M58–66, and A2/MART-126 –35 tet+CD8+ T cells (upper panels). Representative examples of NY-ESO-1 tet+ and EBV tet+CD8+ T cells stained with a FITC-labeled isotype IgG control Ab are also displayed and were used to establish the threshold for identifying PD-1+ cells (lower panel). Values indicate the percentage of CD8+ T cells expressing PD-1 among tet+ cells. B–E, Pooled data showing the percentage and MFI of PD-1 expression on NY-ESO-1-, Flu-, EBV-, and MART-1-specific as well as total effector (CD45RA+CCR7−) and effector memory (CD45RO+CCR7−) CD8+ T cells from nine melanoma patients (B and C) and eight healthy donors (D and E). Horizontal bars depict the mean percentage and MFI of PD-1 expression on tet+CD8+ T cells. The p values were calculated using the Wilcoxon signed rank test.
Collectively, our results showed that PD-1 expression on tumor-induced NY-ESO-1-specific CD8+ T cells in advanced melanoma patients is significantly up-regulated compared with Flu-, EBV-, and MART-1-specific CD8+ T cells.
PD-1 up-regulation on spontaneous NY-ESO-1-specific CD8+ T cells occurs along with T cell activation and is not directly associated with an inability to produce cytokines
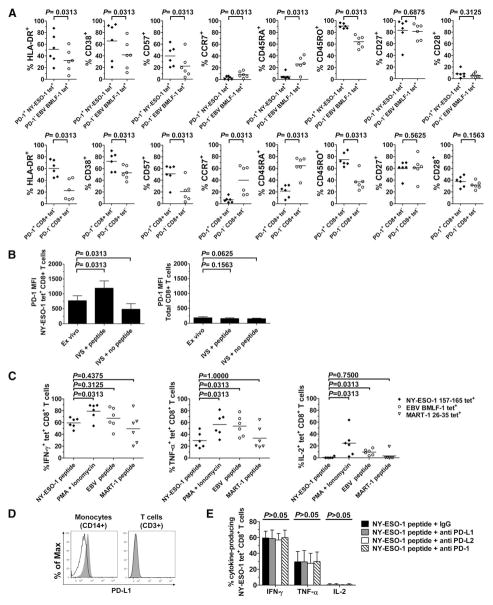
To investigate the potential association between NY-ESO-1-specific CD8+ T cell differentiation/activation status and PD-1 expression, we have compared the percentages of CD8+ T cells from PBMCs of six stage IV melanoma patients that express one of the following markers—HLA-DR, CD38, CD57, CD45RA, CD45RO, CCR7, CD27, and CD28—among PD-1+NY-ESO-1 tet+CD8+ T cells (which represent the large majority of NY-ESO-1-specific CD8+ T cells) and PD-1− EBV tet+CD8+ T cells (which represent the large majority of EBV-specific CD8+ T cells). The expression of HLA-DR, CD38, and CD57 on PD-1+ NY-ESO-1-specific CD8+ T cells was significantly higher than on PD-1− EBV-specific CD8+ T cells (p = 0.0313) (Fig. 2A, upper panel), suggesting that PD-1 up-regulation occurs along with T cell activation. We also found that PD-1+ NY-ESO-1-specific CD8+ T cells expressed lower levels of CD45RA and CCR7 and higher levels of CD45RO than did PD-1− EBV-specific CD8+ T cells (p = 0.0313), whereas both T cell populations expressed high levels of CD27 and low levels of CD28 (p = 0.6875 and p = 0.3125, respectively). Notably, these differences between PD-1+ and PD-1− cells were also observed among the tet−CD8+ T cells (Fig. 2A, lower panel).
FIGURE 2.
Activation, maturational, and functional status of NY-ESO-1-specific CD8+ T cells. A, Pooled data from melanoma patients (n = 6) showing expression of HLA-DR, CD38, CD57, CCR7, CD45RA, CD45RO, CD27, and CD28 on PD-1+ NY-ESO-1-specific and PD-1− EBV-specific CD8+ T cells (upper panel) as well as on PD-1+ and PD-1− tet−CD8+ T cells (lower panel). Horizontal bars depict the mean percentage of cells that express the corresponding marker. B, Pooled data showing the mean MFI and SD of PD-1 expression by A2/NY-ESO-1157–165 tet+ (left panel) and total (right panel) CD8+ T cells assessed either ex vivo or after 6-day IVS with or without cognate peptide (n = 6). C, Pooled data showing percentages of Ag-specific tet+CD8+ T cells that produce IFN-γ, TNF-α, and IL-2 ex vivo in the presence of cognate peptide-pulsed APCs or PMA plus ionomycin (n = 6). Horizontal bars depict the mean percentage of tet+CD8+ T cells that produce cytokines. D, Histograms showing ex vivo expression of PD-L1 by monocytes isolated from PBMCs of one representative melanoma patient. Cells were stained either with anti-PD-L1 Abs (gray) or an isotype control Ab (black line) and analyzed by flow cytometry. As controls, T cells (CD3+ cells) do not express PD-L1. E, Summary data for melanoma patients (n = 6) showing the mean percentage and SD of A2/NY-ESO-1157–165 tet+CD8+ T cells that produce IFN-γ, TNF-α, and IL-2 ex vivo after stimulation with cognate peptide-pulsed APCs and with Abs against PD-L1, PD-L2, PD-1, or an isotype control (IgG). The p values were calculated using the Wilcoxon signed rank test.
To determine whether the levels of PD-1 expression on NY-ESO-1-specific CD8+ T cells are affected by Ag-specific stimulation, we have evaluated PD-1 expression on NY-ESO-1-specific CD8+ T cells from six melanoma patients after a 6-day IVS with or without cognate peptide (Fig. 2B). As compared with ex vivo levels, PD-1 expression on NY-ESO-1-specific CD8+ T cells, but not on total CD8+ T cells, was up-regulated after IVS with cognate peptide and down-regulated after IVS without peptide. Collectively, our findings suggest that PD-1 up-regulation on NY-ESO-1-specific CD8+ T cells is associated with a higher level of T cell activation in the presence of cognate Ag.
We next compared the ability of Ag-specific CD8+ T cells to produce cytokines upon short ex vivo stimulation with cognate peptide-pulsed APCs (6 h). Our findings in six melanoma patients showed that spontaneous NY-ESO-1-specific CD8+ T cells produced IFN-γ in their large majority, TNF-α for a minority of cells, and no IL-2. Following stimulation with PMA plus ionomycin, we observed a significant increase in the numbers of NY-ESO-1-specific CD8+ T cells that produced IFN-γ, TNF-α, and IL-2 (Fig. 2C). MART-1-specific CD8+ T cells, which express low levels of PD-1 by a large majority, exhibited the same cytokine profile as NY-ESO-1-specific CD8+ T cells after peptide stimulation, whereas EBV-specific CD8+ T cells, also expressing low levels of PD-1, produced significantly higher levels of TNF-α and IL-2, albeit similar amounts of IFN-γ.
In agreement with previous studies (25), we have observed that monocytes (CD14+ cells) present in PBMCs express PD-L1 (Fig. 2D). Next, we measured the percentages of cytokine-producing NY-ESO-1-specific CD8+ T cells stimulated ex vivo with cognate peptide-pulsed APCs in the presence of Abs against either PD-1, its ligands PD-L1 or PD-L2, or an isotype control (Fig. 2E). We observed no significant effect of PD-1 pathway manipulation upon IFN-γ, TNF-α, and IL-2 production by NY-ESO-1-specific CD8+ T cells (p > 0.05). Collectively, our findings suggest that PD-1 expression does not directly affect the capacity of NY-ESO-1-specific CD8+ T cells to produce cytokines upon stimulation with cognate Ag.
Blockade of the PD-1/PD-L1 pathway significantly increases frequency of cytokine-producing NY-ESO-1-specific CD8+ T cells
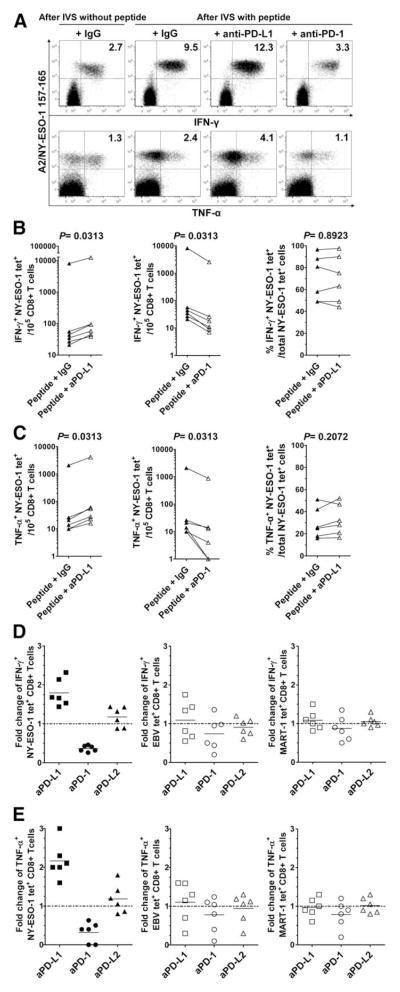
We next assessed the impact of PD-1 expression on cytokine production by NY-ESO-1-specific CD8+ T cells after a multiday IVS with peptide-pulsed APCs. PBMCs from six melanoma patients with spontaneous NY-ESO-1-, EBV-, and MART-1-specific CD8+ T cell responses were stimulated for 6 days either with NY-ESO-1 peptide, EBV peptide, or MART-1 peptide in the presence of Abs against PD-1, PD-L1, PD-L2, or an isotype control, before evaluating cytokine production in response to cognate peptide. As a control, PBMCs were also incubated for 6 days without cognate peptide. We observed a significant increase in the numbers of NY-ESO-1-specific CD8+ T cells that produced IFN-γ and TNF-α in the presence of cognate peptide and anti-PD-L1 Ab, but not anti-PD-L2 (data not shown), as compared with peptide and isotype control (p = 0.0313; Fig. 3A and Fig. 3, B and C, left panels), resulting in a 1.8-fold and 2.2-fold change in the numbers of IFN-γ- and TNF-α-producing NY-ESO-1-specific CD8+ T cells, respectively (Fig. 3, D and E, left panels). Cross-linking of PD-1 receptor by anti-PD-1 Ab resulted in a significant decrease in the number of IFN-γ- and TNF-α-producing NY-ESO-1-specific CD8+ T cells (p = 0.0313; Fig. 3A and Fig. 3, B and C, center panels; 0.4-fold and 0.3-fold change, respectively). Notably, the number of NY-ESO-1-specific CD8+ T cells that produced IL-2 was extremely low for all patients (<0.01% of NY-ESO-1 tet+CD8+ T cells), with or without PD-1/PD-L1 pathway blockade (data not shown). In agreement with our data showing that PD-1 expression does not directly affect the capacity of NY-ESO-1-specific CD8+ T cells to produce cytokines, PD-1/PD-L1 blockade in multiday assay did not significantly increase the percentages of NY-ESO-1 tet+CD8+ T cells that produced cytokines among total NY-ESO-1 tet+CD8+ T cells (Fig. 3, B and C, right panels). Consistent with their lower levels of PD-1 expression, we did not observe any significant influence of PD-1 pathway manipulation on cytokine production by EBV-specific and MART-1-specific CD8+ T cells (Fig. 3, D and E, center and right panels). Additionally, we observed that anti-PD-L1 Ab had no additional effect on cytokine production by NY-ESO-1-specific CD8+ T cells when added after IVS, before intracellular cytokine staining (Fig. 4), although these cells exhibited higher levels of PD-1 expression (Fig. 2B).
FIGURE 3.
Blockade of the PD-1/PD-L1 pathway significantly increases frequency of cytokine-producing NY-ESO-1-specific CD8+ T cells. A, Representative flow cytometry analysis from one melanoma patient (MP3) showing percentages of IFN-γ- and TNF-α-producing A2/NY-ESO-1157–165 tet+CD8+ T cells among total CD8+ T cells and (B and C), pooled data from melanoma patients (n = 6) showing the variation in the number of IFN-γ- and TNF-α-producing NY-ESO-1 tet+ cells for 105 CD8+ T cells (left and center panels), and the percentages of NY-ESO-1 tet+CD8+ T cells that produce IFN-γ and TNF-α among total NY-ESO-1 tet+CD8+ T cells (right panels). PBMCs were incubated for 6 days with (After IVS with peptide) or without (After IVS without peptide) peptide NY-ESO-1157–165 and Abs against PD-L1 (aPD-L1), PD-L2, PD-1 (aPD-1), or an isotype control (IgG) before evaluating intracellular cytokine production of NY-ESO-1 tet+CD8+ T cells in response to cognate peptide. D and E, Fold change of the number of IFN-γ- and TNF-α-producing NY-ESO-1, EBV, and MART-1 tet+CD8+ T cells after 6-day IVS with cognate peptide and Abs against PD-L1, PD-L2, PD-1, or an isotype control Ab. The ratio of the number of cytokine-producing tet+CD8+ T cells from melanoma patients (n = 6) in the presence of indicated Ab treatment and isotype control Ab is shown. The p values were calculated using the Wilcoxon signed rank test.
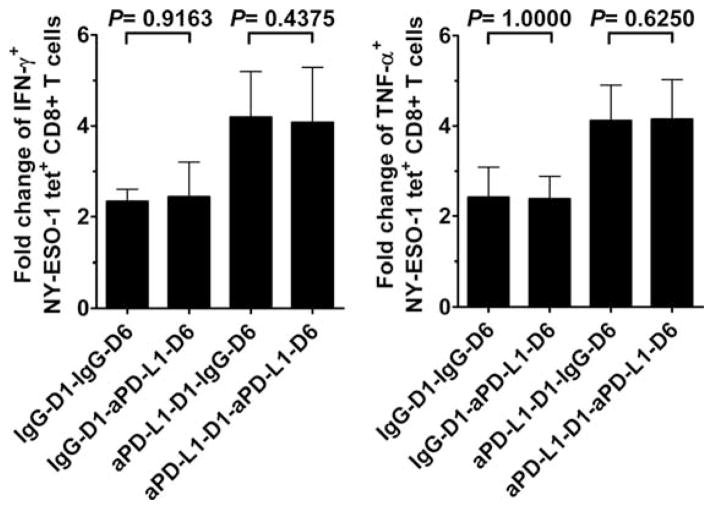
FIGURE 4.
Effect of PD-1/PD-L1 pathway blockade performed after IVS on the frequency of cytokine-producing NY-ESO-1-specific CD8+ T cells. Summary data from melanoma patients (n = 6) showing the mean fold change and SD in the number of IFN-γ-producing (IFN-γ+) and TNF-α-producing (TNF-α+) NY-ESO-1 tet+CD8+ T cells after 6-day stimulation with peptide NY-ESO-1157–165 in the presence of anti-PD-L1 or isotype control Abs added at day 1 (D1) and/or at day 6 (D6) before restimulation with cognate peptide and intracellular cytokine staining of NY-ESO-1 tet+CD8+ T cells. The ratio of cytokine-producing NY-ESO-1 tet+CD8+ T cells per 105 total CD8+ T cells measured after 6-day IVS with and without cognate peptide is displayed. The p values were calculated using the Wilcoxon signed rank test.
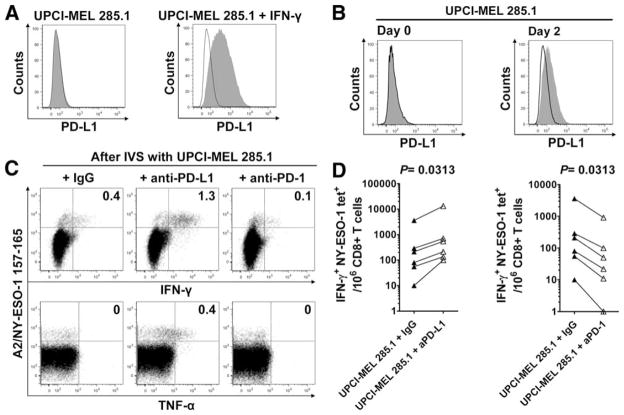
In agreement with previous studies (26), we observed that naturally occurring NY-ESO-1-specific CD8+ T cells produce IFN-γ, known to further promote PD-L1 expression by melanoma cells (9, 27), thus potentially increasing PD-1/PD-L1 interactions in the tumor microenvironment. Accordingly, we observed that HLA-A2+, NY-ESO-1+ UPCI-MEL 285.1 melanoma cells up-regulated PD-L1 expression after 48 h incubation in the presence of either IFN-γ (500 IU/ml; Fig. 5A) or HLA-A2-restricted IFN-γ-producing NY-ESO-1-specific CD8+ T cells (Fig. 5B). We next investigated the role of PD-1/PD-L1 pathway on cytokine production by NY-ESO-1-specific CD8+ T cells upon stimulation with NY-ESO-1+ melanoma cells. CD8+ T cells isolated from six melanoma patients (MP3, MP4, MP6, MP7, MP8, and MP9) among the nine who exhibited the highest levels of circulating NY-ESO-1-specific CD8+ T cells (Table I) were incubated in vitro for 6 days with the HLA-A2-matched NY-ESO-1+ melanoma cell line UPCI-MEL 285.1 with Abs against PD-L1, PD-1, or an isotype control. Following incubation with UPCI-MEL 285.1 cells, we measured the percentage of NY-ESO-1-specific CD8+ T cells that produced cytokines upon restimulation with the same melanoma cell line (Fig. 5, C and D). As compared with the incubation with melanoma cells and isotype control, the numbers of NY-ESO-1-specific CD8+ T cells that produced IFN-γ and/or TNF-α increased after IVS with melanoma cells and anti-PD-L1 Ab and decreased after IVS with melanoma cells and anti-PD-1 Ab in all six patients. Therefore, blockade of the PD-1/PD-L1 pathway in T cell/melanoma cell coculture appears to promote the expansion of cytokine-producing NY-ESO-1-specific CD8+ T cells, consistent with our data obtained using PBMCs.
FIGURE 5.
PD-1/PD-L1 pathway blockade increases the number of cytokine-producing NY-ESO-1-specific CD8+ T cells upon stimulation with NY-ESO-1-expressing tumor cells. A, Up-regulation of PD-L1 expression by the HLA-A2+NY-ESO-1+ UPCI-MEL 285.1 melanoma cells upon treatment with IFN-γ. Melanoma cells were cultured without (left) or with (right) IFN-γ (500 IU/ml) for 48 h, stained with anti-PD-L1 Abs (gray) or an isotype control Ab (black line), and analyzed by flow cytometry. B, Expression of PD-L1 by the HLA-A2+NY-ESO-1+ UPCI-MEL 285.1 melanoma cells before (Day 0) and after (Day 2) incubation with CD8+ T cells purified from PBMCs of one HLA-A2+ melanoma patient (MP3) with spontaneous NY-ESO-1-specific CD8+ T cells. Melanoma cells were stained with anti-PD-L1 Abs (gray) or with an isotype control Ab (black line) and analyzed by flow cytometry. C, Representative flow cytometry analysis from one melanoma patient (MP3) showing percentages of IFN-γ- and TNF-α-producing A2/NY-ESO-1157–165 tet+ cells among total CD8+ T cells, and (D) pooled data from melanoma patients (n = 6) showing the variation in the number of IFN-γ-producing NY-ESO-1 tet+ cells for 106 CD8+ T cells in the presence of UPCI-MEL 285.1 cells after 6-day IVS with UPCI-MEL 285.1 cells and anti-PD-L1 (aPD-L1), anti-PD-1 (aPD-1), or isotype control Abs (IgG). The p values were calculated using the Wilcoxon signed rank test.
PD-1/PD-L1 pathway blockade significantly increases the frequency of proliferating and total NY-ESO-1-specific CD8+ T cells
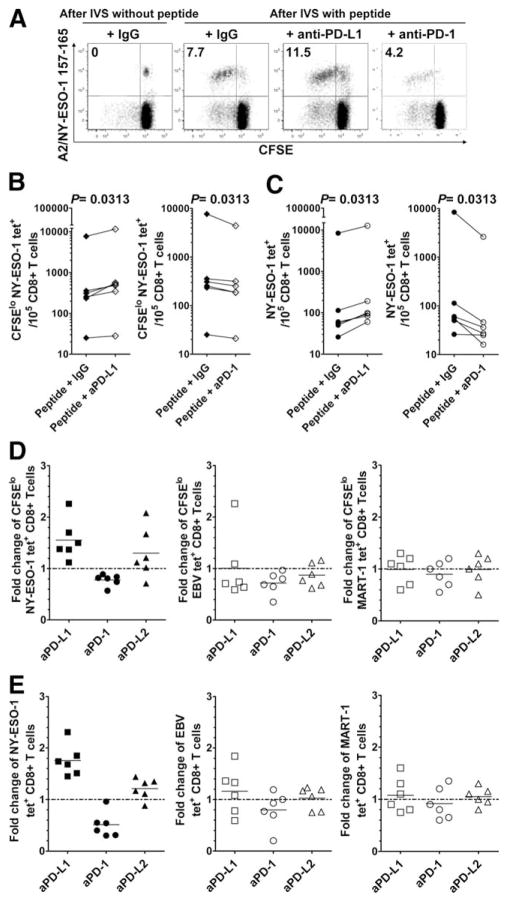
To evaluate whether PD-1/PD-L1 pathway blockade increases the capacity of NY-ESO-1-specific CD8+ T cells to proliferate in response to the cognate Ag in a multiday assay, CFSE-labeled PBMCs from six melanoma patients were stimulated for 6 days with either NY-ESO-1, EBV, or MART-1 peptides in the presence of Abs against PD-1, PD-L1, PD-L2, or an isotype control. As a control, PBMCs were incubated for 6 days without cognate peptide. On day 6, the frequency of proliferating (CFSElow) and total NY-ESO-1, EBV, and MART-1 tet+CD8+ T cells was assessed by flow cytometry. The addition of anti-PD-L1 Ab, but not anti-PD-L2 (data not shown), augmented the number of CFSElow and total NY-ESO-1 tet+CD8+ T cells (p = 0.0313) (Fig. 6A–C), resulting in a 1.5-fold and a 1.8-fold change in the number of CFSElow and total NY-ESO-1 tet+CD8+ T cells, respectively (Fig. 6, D and E, left panels). Alternatively, stimulation of PD-1 by the agonist anti-PD-1 Ab resulted in decreased numbers of both CFSElow and total NY-ESO-1 tet+CD8+ T cells (p = 0.0313; 0.8-fold and 0.5-fold change, respectively). Consistent with their lower levels of PD-1 expression, blockade of the PD-1 pathway did not significantly increase the number of CFSElow and total EBV and MART-1 tet+CD8+ T cells (Fig. 6, D and E, center and right panels).
FIGURE 6.
PD-1/PD-L1 pathway blockade significantly increases the frequency of proliferating and total NY-ESO-1-specific CD8+ T cells. A, Representative flow cytometry analysis from one melanoma patient (MP3) showing percentages of CFSElow NY-ESO-1 tet+CD8+ T cells among total CD8+ T cells, and (B and C) pooled data from melanoma patients (n = 6) showing the variation in the number of CFSElow and total NY-ESO-1 tet+ cells for 105 CD8+ T cells. CFSE-labeled PBMCs were incubated for 6 days with (After IVS with peptide) or without (After IVS without peptide) peptide NY-ESO-1157–165 and Abs against PD-L1 (aPD-L1), PD-L2, PD-1 (aPD-1), or an isotype control (IgG). D and E, Fold change of the number of CFSElow (D) and total (E) NY-ESO-1, EBV, and MART-1 tet+CD8+ T cells after 6-day IVS with cognate peptide and Abs against PD-L1, PD-L2, PD-1, or an isotype control (n = 6). The ratio of the number of CFSElow and total NY-ESO-1, EBV, and MART-1 tet+CD8+ T cells in the presence of indicated Ab treatment and isotype control Ab is shown. The p values were calculated using the Wilcoxon signed rank test.
Discussion
Although there is ample evidence that patients with cancers can develop immune responses directed against Ags expressed by their own tumors, the correlation between tumor Ag-specific immune responses and positive clinical outcome remains elusive in humans (28). In particular, spontaneous immune responses to NY-ESO-1, a strictly tumor-specific T cell target, are detectable only in patients with advanced NY-ESO-1-expressing cancer (4, 29, 30). Therefore, understanding the failure of spontaneous NY-ESO-1-specific T cell responses to promote tumor regression may help us in the design of novel therapeutic interventions aimed at overcoming the tumor evasion of host immune responses. Here, we show for the first time that tumor-induced NY-ESO-1-specific CD8+ T cells that are detectable ex vivo in patients with advanced NY-ESO-1-expressing mela-noma (i.e., stage IV melanoma) up-regulate PD-1 expression in contrast to CD8+ T cells directed against other tumor (MART-1) or viral (Flu, EBV) Ags. In agreement with previous observations of HIV-specific CD8+ T cells (21, 31), the up-regulation of PD-1 expression by spontaneous NY-ESO-1-specific CD8+ T cells appears to occur along with T cell activation, which suggests high levels of tumor Ag-induced T cell activation.
Two studies have previously reported the role of PD-1/PD-L1 blockade on human tumor-specific T cells in vitro. Blank et al. have retrovirally transduced PBMCs isolated from one HLA-A2+ healthy donor with high-affinity, CD8-independent TCRs isolated from HLA-A0201 transgenic mice (9). These T cells have been genetically engineered to become HLA-A2-restricted p53-specific tumor-reactive T cells and were expanded in vitro with anti-CD3/CD28 Abs before testing their effector functions in the presence or absence of anti-PD-L1 Ab in short stimulation assays (4–16 h). In contrast to our data on NY-ESO-1-specific CD8+ T cells isolated ex vivo from PBMCs of melanoma patients and which spontaneously up-regulate PD-1 expression, in this experimental setting (i.e., short stimulation assays) the authors observed an increase of cytokine-producing CD8+ T cells after PD-1/PD-L1 blockade. Wong et al. have reported the effects of a fully humanized PD-1-abrogating Ab on the in vitro expansion and function of vaccine-induced MART-1 and gp100-specific CD8+ T cells (32). In this study, melanoma patients were immunized with analog peptides (MART-1 27L and gp100 209M). The authors tested the impact of the PD-1/PD-L1 blockade, using purified immature dendritic cells previously prepared in vitro for peptide stimulation with the analog peptide. These experimental conditions may possibly have contributed to increase the activation status of analog peptide-specific CD8+ T cells and therefore PD-1 up-regulation by these cells in vitro. Notably, and in contrast to the anti-PD-1 Ab used by Wong et al. (32), our choice of the anti-PD-1 Ab (AF 1086; R&D Systems) was based on a previous study by Petrovas et al. (20) showing that this Ab acts in an agonist fashion. In this study, using plate-bound anti-PD-1, the authors observed that PD-1+ CD8+ T cells were the most sensitive to apoptosis and were the cells that augmented apoptosis to the greatest extent upon PD-1 ligation.
MART-1-specific CD8+ T cells isolated from PBMCs of the same melanoma patients with stage IV melanoma and recognizing the MART-1 Ag, commonly expressed by the large majority of the melanoma cells, expressed lower levels of PD-1 than did NY-ESO-1-specific CD8+ T cells, albeit at a significantly higher level than MART-1-specific CD8+ T cells isolated from healthy donors. Therefore, although high tumor load appears to play a critical role in the up-regulation of PD-1 expression by tumor Ag-specific CD8+ T cells, it may not be the sole factor in determining the levels of PD-1 up-regulation by CD8+ T cells. Several hypotheses may be raised to explain these findings. The first one is that MART-1 CD8+ T cells are stimulated by a potential source of Ag that could be either the MART-1 Ag, which is expressed in skin melanocytes and pigmented cells in the eye, and/or cross-reactive peptides derived from viral, bacterial, or human proteins (33). In this scenario, a significant number of circulating MART-1-specific CD8+ T cells in healthy donors and in melanoma patients may represent low-affinity T cells with low or no tumor reactivity and therefore may not be activated in melanoma patients. Alternatively, the absence of PD-1 up-regulation by MART-1-specific CD8+ T cells may be due to the significant fraction of the MART-1-specific CD8+ T cells in PBMCs of melanoma patients and healthy donors that are phenotypically and functionally naive (34, 35). The existence of such cells may explain our observations of low levels of MART-1-specific CD8+ T cells that are frequently detectable in PBMCs of healthy donors and that do not upegulate PD-1 expression. In melanoma patients, the coexistence of nontumor-reactive naive and tumor-reactive effector memory MART-1-specific CD8+ T cells may explain the higher PD-1 levels observed on MART-1 CD8+ T cells (34, 35).
In healthy donors, EBV-specific CD8+ T cells expressed higher PD-1 levels than in melanoma patients, which may possibly reflect higher levels of EBV Ag chronically stimulating lytic cycle Ag-specific CD8+ T cells (36). CD8+ T cell responses against lytic EBV epitopes (like the EBV BMLF1 epitope used in our study) and latent EBV epitopes appear to be immunodominant in younger donors with acute infections and older donors who are long-term carriers, respectively (37). Therefore, the melanoma patients included in our study with a mean age of 63 years may possibly represent a long-term carrier population with lower load of lytic Ag than in younger healthy donors. A larger study including a larger number of healthy donors and melanoma patients with different disease stages from different age groups will need to be performed to better investigate this issue.
NY-ESO-1-specific CD8+ T cells isolated from PBMCs of patients with stage IV melanoma produced IFN-γ in their large majority, TNF-α for a minority of cells, and no IL-2, exhibiting the same cytokine profile as MART-1-specific CD8+ T cells, which express low levels of PD-1, whereas EBV-specific CD8+ T cells, which also express low levels of PD-1, produced significantly higher levels of TNF-α and IL-2, albeit similar amounts of IFN-γ. Notably, PD-1/PD-L1 blockade had no direct effect on the capacity of PD-1+ NY-ESO-1-specific CD8+ T cells to produce cytokines in ex vivo assays in the context of a short stimulation with cognate peptide. Therefore, and in agreement with previous studies of PD-1-expressing HIV-specific CD8+ T cells (20, 31), our findings show that PD-1 up-regulation has no direct effect on T cell effector function (i.e., cytokine secretion).
One critical finding in our study is the impact of the PD-1/PD-L1 pathway manipulation in combination with prolonged Ag stimulation upon the numbers of cytokine-producing, proliferating, and total PD-1+ NY-ESO-1-specific CD8+ T cells. The numbers of IFN-γ- and TNF-α-producing, proliferating, and total PD-1+ NY-ESO-1-specific CD8+ T cells increased significantly after prolonged stimulation with cognate peptide and anti-PD-L1 Ab. In contrast, they decreased significantly after prolonged stimulation with cognate peptide and agonist anti-PD-1 Ab. Collectively, and together with our observations that PD-1/PD-L1 blockade did not significantly increase the percentages of NY-ESO-1 tet+CD8+ T cells that produced cytokines among total NY-ESO-1 tet+CD8+ T cells either in short-term or multiday cultures, our findings suggest that PD-1 acts as a regulator of NY-ESO-1-specific CD8+ T cell expansion in the context of chronic Ag exposure and has no major impact on their functionality on a cell-per-cell basis. In this regard, our data are in line with one previous study demonstrating that the primary mechanism by which PD-1 affects CD8+ T cell proliferation is through the regulation of cell survival (20).
Of note, the effects of PD-1/PD-L1 pathway manipulation were often modest and varied from patient to patient, supporting the hypothesis that other factors besides PD-1 up-regulation play an important role in regulating the ability of Ag-specific T cells to proliferate (20). In addition to PD-1 expression, other factors have been proposed as key regulators of T cell exhaustion such as duration and level of Ag exposure and availability of CD4+ T cell help (17, 38), suggesting that PD-1/PD-L1 blockade may better work in patients with reduced tumor burden (postsurgery) in combination with vaccine interventions stimulating NY-ESO-1-spe-cific CD8+ and CD4+ T cells.
Interestingly, we have shown that manipulation of the PD-1/PD-L1 pathway regulates the expansion of functional NY-ESO-1-specific CD8+ T cells in the context of a chronic stimulation with PD-L1+ NY-ESO-1+ melanoma cells, expressing normally processed and presented epitopes, making our findings more relevant to what occurs at the tumor sites.
Collectively, our data demonstrate that in contrast to EBV-, Flu-, or Melan-A/MART-1-specific CD8+ T cells, NY-ESO-1-specific CD8+ T cells up-regulate PD-1 expression. The data also show that in the context of a prolonged Ag presentation by PD-L1+ APCs, the blockade of the PD-1/PD-L1 pathway can partially restore NY-ESO-1-specific CD8+ T cell numbers and functions. Therefore, the findings support the use of PD-1/PD-L1 blockade in the context of combinatorial immunotherapeutic interventions targeting the multiple tumor-induced immunosuppressive signals. Such trials will define whether the increased number of tumor-reactive NY-ESO-1-specific CD8+ T cells in patients may further promote broader antitumor T cell responses through epitope spreading (39, 40), increasing the likelihood of objective clinical responses. Whether the disruption of the PD-1/PD-L1 pathway may also result in serious autoimmune effects in vivo will need to be carefully monitored.
Acknowledgments
We thank Dr. Rafi Ahmed (Emory University, Atlanta, GA) for helpful discussions. We also thank Lisa Spano for editorial assistance.
Footnotes
This work was supported by National Institutes of Health/National Cancer Institute Grants CA90360 and CA112198 (to H.M.Z.) and a Cancer Research Institute grant (to H.M.Z.).
Abbreviations used in this paper: CGA, cancer-germline Ag; ECD, energy-coupled dye; Flu, influenza; IVS, in vitro stimulation; MFI, mean fluorescence intensity; PD-1, programmed death 1 receptor; PD-L1, programmed death ligand 1; PD-L2, programmed death ligand 2; tet, tetramer.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- 2.Haas GG, Jr, D’Cruz OJ, De Bault LE. Distribution of human leukocyte antigen-ABC and -D/DR antigens in the unfixed human testis. Am J Reprod Immunol Microbiol. 1988;18:47–51. doi: 10.1111/j.1600-0897.1988.tb00234.x. [DOI] [PubMed] [Google Scholar]
- 3.Jager E, Nagata Y, Gnjatic S, Wada H, Stockert E, Karbach J, Dunbar PR, Lee SY, Jungbluth A, Jager D, et al. Monitoring CD8 T cell responses to NY-ESO-1: correlation of humoral and cellular immune responses. Proc Natl Acad Sci USA. 2000;97:4760– 4765. doi: 10.1073/pnas.97.9.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stockert E, Jager E, Chen YT, Scanlan MJ, Gout I, Karbach J, Arand M, Knuth A, Old LJ. A survey of the humoral immune response of cancer patients to a panel of human tumor antigens [see comments] J Exp Med. 1998;187:1349–1354. doi: 10.1084/jem.187.8.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jager E, Stockert E, Zidianakis Z, Chen YT, Karbach J, Jager D, Arand M, Ritter G, Old LJ, Knuth A. Humoral immune responses of cancer patients against “Cancer-Testis” antigen NY-ESO-1: correlation with clinical events. Int J Cancer. 1999;84:506–510. doi: 10.1002/(sici)1097-0215(19991022)84:5<506::aid-ijc10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 6.Gajewski TF. Identifying and overcoming immune resistance mechanisms in the melanoma tumor microenvironment. Clin Cancer Res. 2006;12:2326s–2330s. doi: 10.1158/1078-0432.CCR-05-2517. [DOI] [PubMed] [Google Scholar]
- 7.Blank C, Brown I, Peterson AC, Spiotto M, Iwai Y, Honjo T, Gajewski TF. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004;64:1140–1145. doi: 10.1158/0008-5472.can-03-3259. [DOI] [PubMed] [Google Scholar]
- 8.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blank C, Kuball J, Voelkl S, Wiendl H, Becker B, Walter B, Majdic O, Gajewski TF, Theobald M, Andreesen R, Mackensen A. Blockade of PD-L1 (B7–H1) augments human tumor-specific T cell responses in vitro. Int J Cancer. 2006;119:317–327. doi: 10.1002/ijc.21775. [DOI] [PubMed] [Google Scholar]
- 10.Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 11.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okazaki T, Iwai Y, Honjo T. New regulatory co-receptors: inducible co-stimulator and PD-1. Curr Opin Immunol. 2002;14:779–782. doi: 10.1016/s0952-7915(02)00398-9. [DOI] [PubMed] [Google Scholar]
- 13.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 14.Dong H, Zhu G, Tamada K, Chen L. B7–H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 15.Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, Shalabi A, Shin T, Pardoll DM, Tsuchiya H. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193:839– 846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 17.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682– 687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 19.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 20.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, Koup RA. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 22.Peng G, Li S, Wu W, Tan X, Chen Y, Chen Z. PD-1 upregulation is associated with HBV-specific T cell dysfunction in chronic hepatitis B patients. Mol Immunol. 2008;45:963–970. doi: 10.1016/j.molimm.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 23.Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol. 2007;81:9249–9258. doi: 10.1128/JVI.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, Ferrari C. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keir ME, Francisco LM, Sharpe AH. PD-1 and its ligands in T-cell immunity. Curr Opin Immunol. 2007;19:309–314. doi: 10.1016/j.coi.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 26.Valmori D, Dutoit V, Lienard D, Rimoldi D, Pittet MJ, Champagne P, Ellefsen K, Sahin U, Speiser D, Lejeune F, et al. Naturally occurring human lymphocyte antigen-A2 restricted CD8+ T-cell response to the cancer testis antigen NY-ESO-1 in melanoma patients. Cancer Res. 2000;60:4499– 4506. [PubMed] [Google Scholar]
- 27.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Tumor-associated B7–H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793– 800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 28.Rosenberg SA, Sherry RM, Morton KE, Scharfman WJ, Yang JC, Topalian SL, Royal RE, Kammula U, Restifo NP, Hughes MS, et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol. 2005;175:6169– 6176. doi: 10.4049/jimmunol.175.9.6169. [DOI] [PubMed] [Google Scholar]
- 29.Chen YT, Gure AO, Tsang S, Stockert E, Jager E, Knuth A, Old LJ. Identification of multiple cancer/testis antigens by allogeneic antibody screening of a melanoma cell line library. Proc Natl Acad Sci USA. 1998;95:6919– 6923. doi: 10.1073/pnas.95.12.6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jager E, Chen YT, Drijfhout JW, Karbach J, Ringhoffer M, Jager D, Arand M, Wada H, Noguchi Y, Stockert E, et al. Simultaneous humoral and cellular immune response against cancer-testis antigen NY-ESO-1: definition of human histocompatibility leukocyte antigen (HLA)-A2-binding peptide epitopes. J Exp Med. 1998;187:265–270. doi: 10.1084/jem.187.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sauce D, Almeida JR, Larsen M, Haro L, Autran B, Freeman GJ, Appay V. PD-1 expression on human CD8 T cells depends on both state of differentiation and activation status. AIDS. 2007;21:2005–2013. doi: 10.1097/QAD.0b013e3282eee548. [DOI] [PubMed] [Google Scholar]
- 32.Wong RM, Scotland RR, Lau RL, Wang C, Korman AJ, Kast WM, Weber JS. Programmed death-1 blockade enhances expansion and functional capacity of human melanoma antigen-specific CTLs. Int Immunol. 2007;19:1223–1234. doi: 10.1093/intimm/dxm091. [DOI] [PubMed] [Google Scholar]
- 33.Loftus DJ, Castelli C, Clay TM, Squarcina P, Marincola FM, Nishimura MI, Parmiani G, Appella E, Rivoltini L. Identification of epitope mimics recognized by CTL reactive to the melanoma/melanocyte-derived peptide MART-127–35. J Exp Med. 1996;184:647– 657. doi: 10.1084/jem.184.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pittet MJ, Zippelius A, Valmori D, Speiser DE, Cerottini JC, Romero P. Melan-A/MART-1-specific CD8 T cells: from thymus to tumor. Trends Immunol. 2002;23:325–328. doi: 10.1016/s1471-4906(02)02244-5. [DOI] [PubMed] [Google Scholar]
- 35.Zippelius A, Pittet MJ, Batard P, Rufer N, de Smedt M, Guillaume P, Ellefsen K, Valmori D, Lienard D, Plum J, et al. Thymic selection generates a large T cell pool recognizing a self-peptide in humans. J Exp Med. 2002;195:485– 494. doi: 10.1084/jem.20011658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laichalk LL, Thorley-Lawson DA. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein-Barr virus in vivo. J Virol. 2005;79:1296–1307. doi: 10.1128/JVI.79.2.1296-1307.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vescovini R, Telera A, Fagnoni FF, Biasini C, Medici MC, Valcavi P, di Pede P, Lucchini G, Zanlari L, Passeri G, et al. Different contribution of EBV and CMV infections in very long-term carriers to age-related alterations of CD8+ T cells. Exp Gerontol. 2004;39:1233–1243. doi: 10.1016/j.exger.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 38.Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J Exp Med. 2006;203:2223–2227. doi: 10.1084/jem.20061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vanderlugt CL, Miller SD. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat Rev. 2002;2:85–95. doi: 10.1038/nri724. [DOI] [PubMed] [Google Scholar]
- 40.Markiewicz MA, Fallarino F, Ashikari A, Gajewski TF. Epitope spreading upon P815 tumor rejection triggered by vaccination with the single class I MHC-restricted peptide P1A. Int Immunol. 2001;13:625– 632. doi: 10.1093/intimm/13.5.625. [DOI] [PubMed] [Google Scholar]