Abstract
Rationale
Both cardiomyocyte-restricted proteasome functional enhancement and pharmacological proteasome inhibition (PSMI) were shown to attenuate myocardial ischemia/reperfusion (I/R) injury. The role of cardiac proteasome dysfunction during I/R and the perspective to diminish I/R injury by manipulating proteasome function remain unclear.
Objectives
We sought to determine proteasome adequacy in I/R hearts, create a mouse model of cardiomyocyte-restricted PSMI (CR-PSMI), and test CR-PSMI impact on I/R injury.
Methods and Results
Myocardial I/R were modeled by ligation (30min) and subsequent release of the left anterior descending artery in mice overexpressing GFPdgn, a validated surrogate proteasome substrate. At 24h of reperfusion, myocardial proteasome activities were significantly lower while total ubiquitin conjugates and GFPdgn protein levels were markedly higher in all regions of the I/R hearts than the sham controls, indicative of proteasome functional insufficiency. CR-PSMI in intact mice was achieved by transgenic (tg) overexpression of a peptidase-disabled mouse β5 subunit (T60A-β5) driven by an attenuated mouse mhc6 promoter. Overexpressed T60A-β5 can replace endogenous β5 and inhibits proteasome chymotrypsin-like activities in the heart. Mice with moderate CR-PSMI showed no abnormalities at the baseline but displayed markedly more pronounced structural and functional damage during I/R, compared with non-tg littermates. The exacerbation of I/R injury by moderate CR-PSMI was associated with significant increases in the protein level of PTEN and protein kinase Cδ (PKCδ), decreased Akt activation, and reduced PKCε.
Conclusions
Myocardial I/R causes proteasome functional insufficiency in cardiomyocytes and moderate CR-PSMI augments PTEN and PKCδ, suppresses Akt and PKCε, increases cardiomyocyte apoptosis, and aggravates I/R injury in mice.
Keywords: proteasome inhibition, myocardial reperfusion injury, transgenic mouse, Akt, PKCδ
INTRODUCTION
The ubiquitin-proteasome system (UPS) mediates the degradation of most proteins in the cell. The UPS is pivotal to protein quality control (PQC) because it is responsible for degrading all abnormal proteins, including terminally misfolded proteins. Through targeted degradation of normal proteins that are no longer needed (e.g., activated kinases and their regulators), the UPS also regulates virtually all cellular processes.1, 2 PQC and the regulatory protein degradation are essential to maintaining normal cell function and cell survival; hence, cardiac UPS dysfunction can cause not only cardiac malfunction but also increased cardiomyocyte death in the heart.3–5 In general, UPS-mediated proteolysis involves ubiquitination of target proteins and the degradation of the ubiquitinated proteins by the proteasome. The most studied proteasome is the 26S proteasome which consists of a 20S proteasome flanked by the 19S proteasome at one or both ends. Proteasomal proteolysis takes place in the interior chamber of the 20S. The 20S is composed of an axial stack of four rings: two anti-parallel inner β rings flanked by two outer α rings. Each α or β ring is formed by 7 subunits, known as α1 through α7 and β1 through β7. Three peptidase activities: chymotrypsin-like, trypsin-like, caspase-like have been identified in eukaryotic proteasomes, residing respectively in β5, β2, and β1 subunits. Most proteasome inhibitors, including the clinically used bortezomib, target the β5 subunit, inhibit its catalytic activity, and thereby inhibit the 20S proteasome.2
Based largely on the in vitro proteasome peptidase activity assays, alterations of proteasome function were associated with a variety of cardiac pathological conditions,6, 7 e.g., load-dependent cardiac disorders,8–10 hypertrophic or dilated cardiomyopathy,10–14 and ischemic heart disease.14–20 Apparently, the altered proteasome activities in a pathological condition can be a pathogenic factor contributing to progression of the disease, a compensatory response, or simply an epiphenomenon; hence, it is important to test these possibilities in a given disease, for a better understanding of the role of UPS dysfunction in pathogenesis. To do so, we must answer at least two important questions: (1) is the altered proteasome function sufficient to maintain proteostasis in the cell, and (2) what is its impact on the progression of the disease?
To help examine questions like the first one, we previously developed a stable transgenic (tg) mouse model expressing a modified green fluorescence protein (GFPdgn) that is a verified surrogate substrate of the UPS.21 We used the GFPdgn tg mice to unveil proteasome functional insufficiency (PFI), despite a marked increase of proteasome peptidase activities, in the heart of mouse models of cardiac proteinopathy.22, 23 However, proteasome functional sufficiency has not been determined in many other cardiac pathological conditions, e.g., myocardial ischemia/reperfusion (I/R) injury, although most reports show decreased proteasome peptidase activities in I/R hearts.16, 20, 24 Thus, in the first part of the present study, we inquired PFI occurrence in mouse hearts with acute regional myocardial I/R in vivo.
To answer the second question, we must manipulate proteasome function specifically in cardiomyocytes without affecting other cell types of the heart and other organs/systems. However, an animal model of cardiomyocyte-restricted proteasomal inhibition (CR-PSMI) has not been described although we have recently reported a mouse model of cardiomyocyte-restricted proteasome functional enhancement.25, 26 We used the latter model to demonstrate that proteasome functional enhancement in cardiomyocytes protects against acute myocardial I/R injury,26 contradicting to several reports which demonstrated via pharmacologic means a protective effect of ubiquitous proteasome inhibition (PSMI) against myocardial I/R injury.18, 27–30 To address this apparent discrepancy, we have established here the first tg mouse model of CR-PSMI and used it to test the impact of CR-PSMI on acute I/R injury in intact mice.
In the present study, we demonstrated that regional myocardial I/R induces PFI in cardiomyocytes of the heart. Second, we discovered that overexpression of a protease-disabled missense (T60A) mutant precursor of β5 subunit in cardiomyocytes can effectively replace endogenous β5, and thereby inhibit proteasome chymotrypsin-like activity; thus, we established a stable tg mouse model of CR-PSMI. Finally, we demonstrated that moderate CR-PSMI is well tolerated by mice at the baseline condition but it augments the pro-apoptotic kinase protein kinase Cδ (PKCδ) and the phosphatase and tensin homolog (PTEN) signaling, suppresses PKCε and the activation of pro-survival kinase Akt, aggravates cardiomyocyte apoptosis, and exacerbates myocardial I/R injury.
METHODS
A detailed Methods section can be found in the online-only Data Supplement.
Transgenic mice
All mice used in this study are in the FVB/N inbred background. The creation and characterization of the tg mice with expression of a modified green fluorescence protein (GFPdgn) have been previously described.21 GFPdgn was engineered via carboxyl fusion of degron CL1 to an enhanced green fluorescence protein (GFP) and is a proven substrate for the UPS.21, 31
To establish a genetic model of CR-PSMI, we created stable tg mouse lines that overexpress a Myc-tagged missense mutation (T60A) of the murine precursor of β5 subunit of the 20S proteasome (hereafter known as T60A-β5) under the control of an attenuated murine mhc6 promoter. The latter consists of a full-length mhc6 promoter in which 3 GATA sites and 2 TREs (thyroid response elements) were ablated but other cis-acting regions important for cardiac-specific expression were left intact.32
LV pressure–volume relationship analysis
To assess left ventricle (LV) pressure–volume (P-V) relationship, mice were anesthetized with isoflurane (2%) in medical grade oxygen, then intubated and mechanically ventilated. The LV was catheterized via the right carotid artery with a 1.2-F mouse P-V catheter (Scisense, London, Ontario). The instrumented animal was stabilized for 10min and then data were recorded with a sampling rate of 1,500 Hz with Ponemah software (Data Sciences International, Valley View, OH) during steady-state conditions. For subsequent analysis of P-V loops Ponemah software was used. The raw conductance volumes were corrected for parallel conductance by the hypertonic saline bolus.
In vivo myocardial I/R and assessment of infarct size
Regional myocardial I/R was modeled in mice by surgical ligation of the left anterior descending coronary artery (LAD) for 30min followed by releasing of the ligation for 24h. Determined by pilot studies, this protocol yields an average area at risk (AAR) of 40% and an average infarct size of 55% in young wild type FVB/N mice (Online Figure I). Infarct size was determined as previously reported.26
Terminal deoxynucleotidyl transferase end-labeling (TUNEL) assay
The heart was excised 24h after reperfusion. Two tissue samples corresponding respectively to the AAR and the remote area (RA) of the LV free wall were collected, fixed in 4% paraformaldehyde in PBS for 24h at 4°C, and further processed for TUNEL assays as previously described.4
Statistical Analysis
All continuous variables are presented as mean±SD. Differences between two groups were evaluated for statistical significance using two-tailed Student’s t-test. When difference among 3 or more groups was evaluated, one-way analysis of variance (ANOVA) or when appropriate, 2-way ANOVA, followed by the Holm-Sidak test for pair-wise comparisons were performed. The P value <0.05 were considered statistically significant.
RESULTS
PFI in the heart of mice with acute focal myocardial I/R
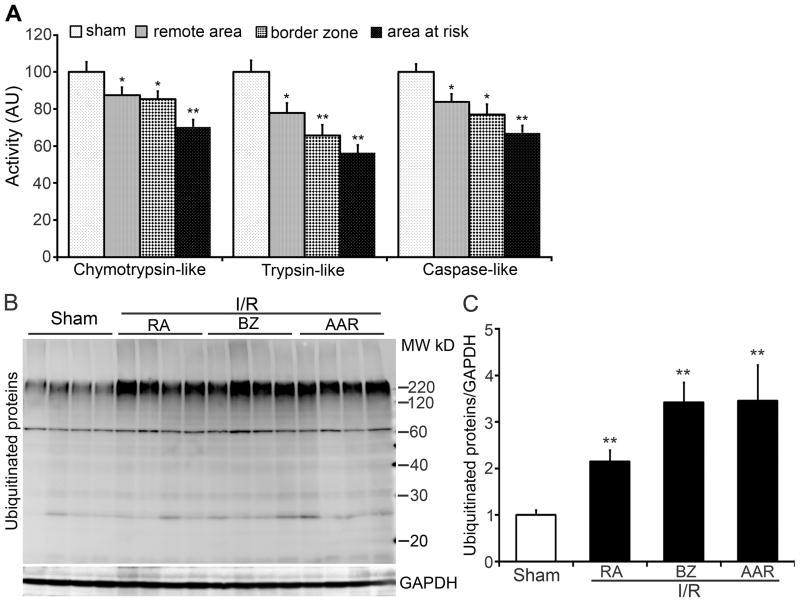
To determine the impact of acute myocardial I/R on cardiac proteasome function, GFPdgn tg mice were subject to surgical LAD ligation for 30 min followed by release of the ligation to allow reperfusion. At 24h after reperfusion, myocardial samples were collected respectively from AAR (the area undergone ischemia during coronary ligation), the remote area (RA), and the border zone (BZ, a 1-mm-wide zone between AAR and RA) of the LV free wall for assessing proteasome peptidase activities and the steady state ubiquitinated proteins. For the sham surgery control group, the tissue of the entire LV free wall was collected. Crude protein extracts were used for measuring 20S proteasome peptidase activities. All three peptidase activities were significantly decreased in all the three zones of I/R hearts, compared with the sham controls (Figure 1A). The total ubiquitinated proteins, especially the high molecular weight species, were significantly increased in all zones of I/R hearts, compared with the sham controls (Figure 1B, 1C).
Figure 1. Myocardial ischemia/reperfusion (I/R) impairs proteasome peptidase activities in mouse hearts.
GFPdgn tg mice were subject to myocardial I/R created by ligation (30min) and subsequent release of the ligation (24h) of the anterior descending artery. Crude protein extracts from myocardial tissues of the left ventricle (LV) of the sham surgery group or of the LV area at risk (AAR), border zone (BZ), and remote area (RA) of the I/R group were used for proteasomal peptidase activity assays (A) and western blot analysis for ubiquitinated proteins (B, C). n=6 mice/group; *p<0.05, **p<0.01 vs. sham.
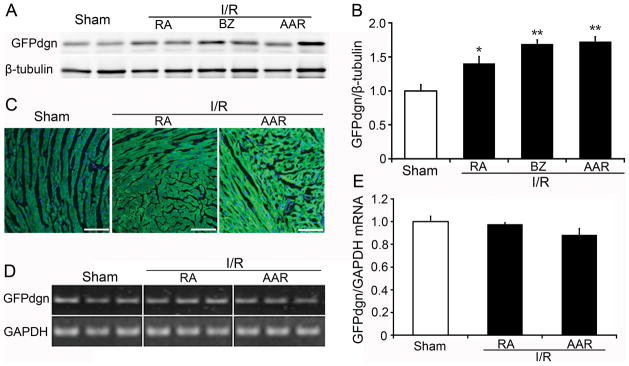
To test if the decreased proteasome peptidase activities affect on UPS proteolytic function, we measured cardiac expression of GFPdgn, a surrogate protein substrate for the UPS. A significant increase in GFPdgn protein levels in all three zones of I/R hearts was observed quantitatively using western blot analyses (Figure 2A, 2B) and qualitatively via confocal microscopy (Figure 2C). RT-PCR analyses demonstrated that the steady state GFPdgn mRNA levels were not increased (Figure 2D, 2E). In the absence of increases in synthesis, the increased GFPdgn protein levels indicate that the removal of abnormal proteins by the UPS is impaired. This impairment is presumably caused by PFI because 20S proteasome peptidase activities were significantly decreased in the I/R hearts.
Figure 2. Increase of GFPdgn protein levels in GFPdgn tg mouse hearts after in vivo focal I/R.
A and B, Western blot analyses show increases in the myocardial GFPdgn protein level in the indicated zones (RA, BZ, and AAR) of the LV free wall after I/R. Representative images (A) and densitometry data (B) are presented. N=4 mice per group; *p<0.05, **p<0.01 vs. sham. C, Representative images of myocardial GFPdgn direct green fluorescence in the indicated zones of the LV. Nuclei were stained blue with DAPI. Scale bar=100μm. D and E, RT-PCR assays show that I/R did not increase GFPdgn mRNA levels in the area at risk and the remote area. N=6 mice/group.
Establishment of a mouse model of moderate CR-PSMI
To date, no animal model of CR-PSMI has been reported but such a model would benefit remarkably investigation into the pathophysiological significance of PFI in cardiomyocytes of intact animals. Hence, we sought to create one.
As mentioned earlier, proteasome peptidase activities reside in β1, β2, andβ5 subunits of the 20S. The N-terminal threonine of the three matured β-subunits performs the nucleophilic attack for peptide bond hydrolysis.33 The catalytically active N-terminal threonine residue resides in the interior face of the 20S chamber.34 The C termini of β subunits are on the outer surface of proteasomes so that carboxyl fusion of an “epitope tags” to a β subunit would not affect the configuration and presumably activity of the proteasome.35, 36 In both yeast and cultured mammalian cells, the activity of β5 subunit appears to be the most important component for proteasome activities. For proteasome assembly and maturation the hierarchy of importance among the three proteolytic subunits is β5>β2>β1.36–38 Thus, we decided to create a catalytically inactive mutant β5 subunit through mutating its N-terminal catalytic threonine to alanine. All 3 peptidase subunits are synthesized in the cell as precursors. The N-terminus is post-translationally processed by autocatalytic activities during proteasome assembly and maturation, which exposes the N-terminal threonine. Various mutation studies further confirmed the importance of Thr76 inβ5 precursor of yeast (also known as pre2). Replacing Thr76 with Ala (T76A) in pre2 decreased its chymotrypsin-like activity markedly but had little effect on trypsin-like and caspase-like activities.36, 37 This indicates that T76A-pre2 can incorporate into the 20S proteasome, functioning as a dominant negative mutant.
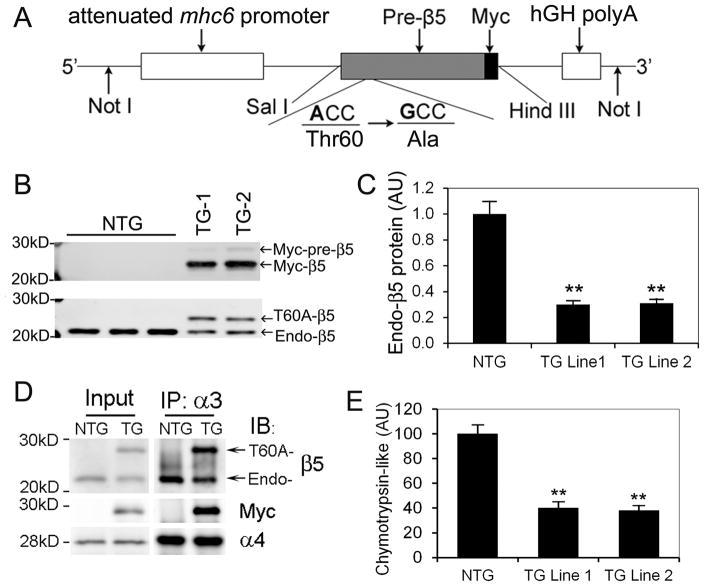
Alignment of β5 sequences across species shows that T60 of mouse β5 (Psmb5) corresponds to T76 of yeast pre2. Thus, we chose to create a T60A-β5 from mouse wild type (WT-) β5 precursor cDNA. We differentiated T60A-β5 from endogenous β5, with addition of a myc tag to the carboxyl terminus of T60A-β5. Our pilot studies confirmed that overexpression of T60A-β5 in mammalian cell lines and neonatal rat ventricular myocytes led to expression-level-dependent decreases in the chymotrypsin-like activity and inhibition of proteasomal proteolytic function (data not shown). Hence, we generated stable tg mouse lines that overexpress myc-tagged T60A-β5 precursor under the control of an attenuated mouse Mhc6 promoter (Figure 3A). Basic characterization of these mice revealed that overexpressed T60A-β5 precursor was successfully converted to mature T60A-β5, decreased endogenous β5 (Figure 3B, C), incorporated into the 20S proteasome as revealed by co-immunoprecipitation with the endogenous β5 andα4 subunits of the 20S (Figure 3D), and decreased the chymotrypsin-like activity in the heart by approximately 60% and 65% in tg line 1 and line 2, respectively (Figure 3E). Immunofluorescence confocal microscopy revealed that myc-T60A-β5 proteins are enriched in the nucleus and at the Z-line levels in the cytoplasm (Online Figure II), demonstrating the distribution pattern of endogenous 20S proteasomes observed in the cardiomyocytes.39 No abnormality in cardiac function, morphology, or growth was detected during the first 6 months of life of these tg mouse lines (data not shown). Consistent with the moderate nature of CR-PSMI in these tg lines, myocardial total ubiquitinated proteins in T60A-β5 tg mice at the baseline do not differ from Ntg littermates (Online Figure III). Notably, as described above, both tg lines exhibit the same phenotype. The experiments described hereafter used tg line 1.
Figure 3. Baseline characterization of a tg mouse model of moderate cardiomyocyte-restricted proteasome inhibition (CR-PSMI).
Tg mouse lines with a moderate expression of Myc-tagged T60A-β5 precursor under the control of an attenuated mhc6 promoter were created. A, Schematic illustration of the transgenic (TG) construct used for fertilized egg microinjections to create the T60A-β5 Tg mouse founders. B, Sample images of western blot analyses for Myc (upper panel) and the β5 subunit (lower panel) of the 20S proteasome in ventricular myocardium of mice from 2 independent tg lines (Line 1, TG-1; Line 2, TG-2) at 8 weeks. C, A summary of myocardial endogenous β5 (Endo-β5) protein expression in T60A-β5 TG and NTG littermate mice at 8 weeks. D, Representative images of immunoblot analyses (IB) of the indicated proteins in immunoprecipitated (IP) 20S proteasomes from the ventricular myocardium of Myc-T60A-β5 TG and NTG littermate mice at the baseline condition. Antibodies againstα3 subunit of the 20S were used for IP 20S proteasomes from crude protein extracts from ventricular myocardium. E, Expression of T60A-β5 suppressed the chymotrypsin-like activity of 20S proteasomes in the heart. **p<0.01 vs. Ntg; n=4 mice/group.
Moderate CR-PSMI exacerbates cardiac dysfunction in I/R mice
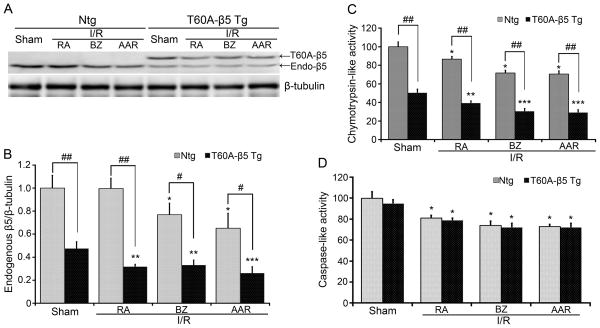
To determine the effect of CR-PSMI on I/R injury, T60A-β5 tg and non-tg (Ntg) littermate mice were subject to the ischemia (30min) and reperfusion (24hrs) protocol as described above. To verify T60A-β5 expression and its impact on proteasome function in the AAR, BZ and RA of I/R hearts, we assessed the expression of endogenous β5 and tg T60A-β5 proteins and the changes of the activities of the β5- dependent and independent peptidases (Figure 4). Compared with Ntg sham, endogenous β5 protein levels were significantly decreased in the AAR and BZ but not RA of the Ntg I/R hearts. Compared with the tg sham, both endogenous β5 and tg T60A-β5 proteins were expressed at a relatively lower level in all three zones of I/R hearts (Figure 4A, 4B). As a result, β5-dependent chymotrypsin-like activities were markedly lower in all three zones of I/R hearts in the T60A-β5 tg mice than the Ntg mice (Figure 4C). Proteasome caspase-like activities, which are β5-independent, showed no significant difference between the tg and the Ntg groups (Figure 4D).
Figure 4. Effects of T60A-β5 tg expression in cardiomyocytes on endogenous β5 expression and proteasomal peptidase activities of I/R and sham hearts.
T60A-β5 tg and Ntg littermate mice were subject to myocardial I/R as described in Figure 1. Crude protein extracts from the indicated zones of the LV were used for western blot analyses of endogenous β5 and tg T60A-β5 (A, B) or for proteasomal chymotrypsin-like (C) and caspase-like (D) activity assays. A and B, Representative images (A) and a summary of densitometric data (B) of the western blot analyses for the endogenous β5 expressions. n=4. C and D, Changes in the indicated proteasomal peptidase activities. n=6. *p< 0.05, **p<0.01, ***p<0.005 vs. Ntg sham; #p< 0.05, ##p<0.01.
In a separate cohort, LV function was measured at the terminal experiment using P-V relationship analysis. As summarized in Table 1 and illustrated by Online Figure IV, no statistically significant difference in any of the parameters examined was observed between the Ntg sham and the Tg sham groups. Compared with the Ntg sham, the Ntg I/R group demonstrated marked impairment of systolic and diastolic function. The systolic function impairment was reflected by significant decreases in LV end-systolic pressure (Pes), the maximal rate of pressure increasing (dP/dtmax), ejection fraction (EF), stroke work (SW), and the preload recruited stroke work (PRSW) and by significant increases in end-systolic volume (Ves). The impairment of diastolic function was indicated by significant increases in end-diastolic pressure (Ped) and Tau and by decreases in the maximal rate of pressure decreasing (dP/dtmin). As a result, both stoke volume (SV) and cardiac output per minute (CO) were significantly smaller in the Ntg I/R group, compared with the Ntg sham group. Importantly, the I/R induced changes in all parameters except Tau were significantly exacerbated in the T60A-β5 Tg I/R group. These findings demonstrate that CR-PSMI aggravates cardiac malfunction induced by regional myocardial I/R.
TABLE 1.
In vivo LV function based on P-V loop measurements in Ntg and T60A-β5 Tg mice treated with and without I/R (mean±SD)
| Parameters | Ntg
|
Tg
|
||
|---|---|---|---|---|
| Sham (n=6) | I/R (n=7) | Sham (n=11) | I/R (n=12) | |
| HR, beats/min | 532 ± 35 | 560 ± 80 | 502 ± 67 | 517 ± 63 |
| Pes, mmHg | 120 ± 15.2 | 109 ± 8.2* | 114 ± 10.4 | 99 ± 13.3††‡ |
| Ves, μL | 8.0 ± 4.3 | 15.5 ± 5.6** | 10.2 ± 6.7 | 23.6 ± 9.5††‡‡ |
| Ped, mmHg | 3.5 ± 0.4 | 5.3 ± 1.1** | 3.9 ± 0.8 | 7.7 ± 3.3††‡ |
| Ved, μL | 36.3 ± 5.3 | 40.0 ± 3.2 | 37.5 ± 7.2 | 45.2 ± 8.5††‡ |
| SV, μL | 28.1 ± 3.1 | 24.5 ± 4.1* | 27.5 ± 6.4 | 21.6 ± 4.0††‡ |
| SW, mmHgXμL | 3413 ± 645 | 2667 ± 380** | 3156 ± 851 | 2142 ± 484††‡‡ |
| CO, μl/min | 15045 ± 1465 | 13512 ± 1591* | 13898 ± 4193 | 11175 ± 2610†‡‡ |
| EF, % | 78.8 ± 11.4 | 61.7 ± 11.5** | 74.2 ± 14 | 49.6 ± 13.6††‡‡ |
| dP/dtmax, mmHg/s | 11329 ± 2877 | 9429 ± 1075* | 11890 ± 2625 | 7257 ± 1643††‡‡ |
| dP/dtmin, mmHg/s | 10238 ± 1974 | 7490 ± 1135** | 9376 ± 1352 | 5931 ± 931††‡‡ |
| Tau, ms | 9.0 ± 1.0 | 13.3 ± 5.0* | 9.8 ± 1.7 | 13.8 ± 3.9†† |
| PRSW, mmHg | 105 ± 5.6 | 66 ± 16.0** | 97 ± 11 | 42 ± 20††‡‡ |
HR, heart rate; Pes, end-systolic pressure; Ves, end-systolic volume; Ped, end-diastolic pressure; Ved, end-diastolic volume; SV, stroke volume; SW, stroke work; CO, cardiac output; %EF, ejection fraction; dP/dtmax, the maximal rate of pressure increasing; dP/dtmin, the maximal rate of pressure decreasing; Tau, relaxation time constant calculated by Glantz method; PRSW, preload recruited stroke work (slope of stroke work- Ved relationship).
P< 0.05,
P< 0.01 vs. Ntg sham;
P 0.05,
P<0.01 vs. Tg sham;
P<0.05,
P<0.01 vs. Ntg I/R.
Moderate CR-PSMI increases infarct size and cardiomyocyte apoptosis in I/R hearts
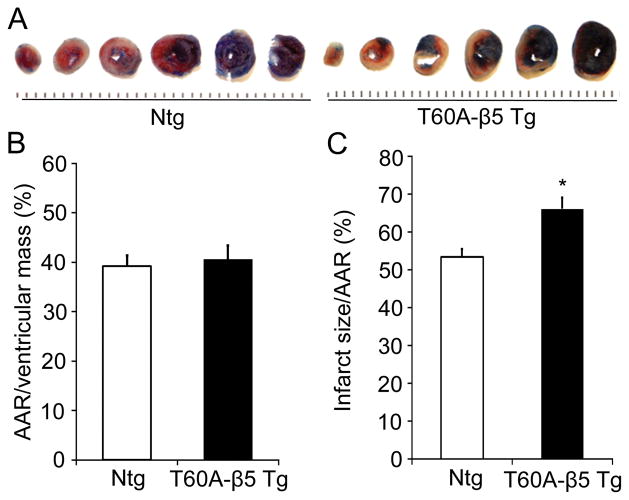
Ischemia and infarct size determination showed that the LAD ligation induced ischemic area or AAR was not significantly different between the Ntg I/R and the Tg I/R groups but at 24 hours after reperfusion, the infarct size of the Tg I/R group was significantly greater than that of the Ntg I/R group (Figure 5). These results indicate further that moderate CR-PSMI exacerbates I/R injury.
Figure 5. Moderate CR-PSMI significantly increases the infarct size in I/R mice.
T60A-β5 Tg and Ntg littermates at 10~12 weeks of age were subject to LAD ligation and release as described in Figure 1. Infarct size was determined at 24h after reperfusion. A, Representative comparison of infarct size between a Tg mouse heart and a littermate Ntg mouse heart. B and C, Morphometric analysis of area at risk (B) and infarct size (C) in T60A-β5 Tg and Ntg mouse hearts. n=6, *p<0.05 vs. Ntg.
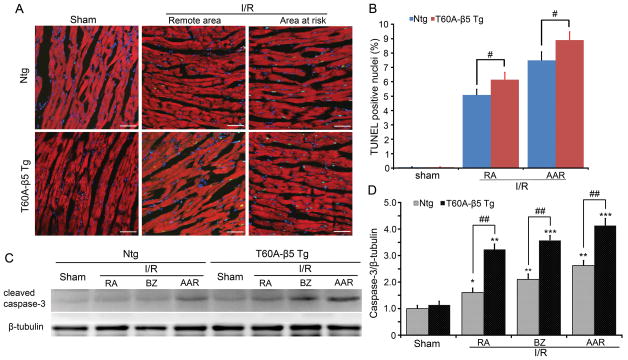
To explore the potential mechanism underlying the detrimental effect of moderate CR-PSMI on I/R hearts, we first examined the prevalence of cardiomyocyte apoptosis via the TUNEL assay and the detection of activated/cleaved caspase 3 in various areas of the LV. Essentially no TUNEL positive cardiomyocytes were detected in Ntg or Tg sham control hearts but I/R induced an increase in TUNEL positive cardiomyocytes in the AAR and the remote areas of both Ntg and Tg hearts. The increases were more pronounced in the T60A-β5 Tg I/R hearts than Ntg I/R hearts (Figure 6A, 6B). Western blot analysis revealed that the level of cleaved caspase3 was significantly increased in the AAR, BZ, and RA of the I/R hearts of Ntg and Tg groups. The increase was more pronounced in the T60A-β5 Tg hearts than Ntg hearts (Figure 6C, 6D).
Figure 6. Evaluation of cardiomyocyte apoptosis in Ntg and T60A-β5 Tg mice undergone myocardial I/R.
A, Representative images of TUNEL staining (green) in the sham and the indicated zones of the LV of I/R hearts. Nuclei were stained blue with DAPI. Cardiomyocytes are identified by the green fluorescence resulting from Alexa-568-phalloidin staining. Scale bar=50μm. B, The number of TUNEL-positive nuclei is expressed as a percentage of total nuclei detected by DAPI staining. n=6 mice/group, *P<0.05 vs. Ntg. C and D, Western blot analysis myocardial levels of the cleaved (i.e., activated) caspase-3 in all zones of the T60A-β5 Ntg and Tg mouse hearts. N=4; *p< 0.05, **p<0.01, ***p<0.005 vs. Ntg sham; #p< 0.05, ##p<0.01.
Moderate CR-PSMI augments PKCδ and PTEN and suppresses PKCε and Akt activation
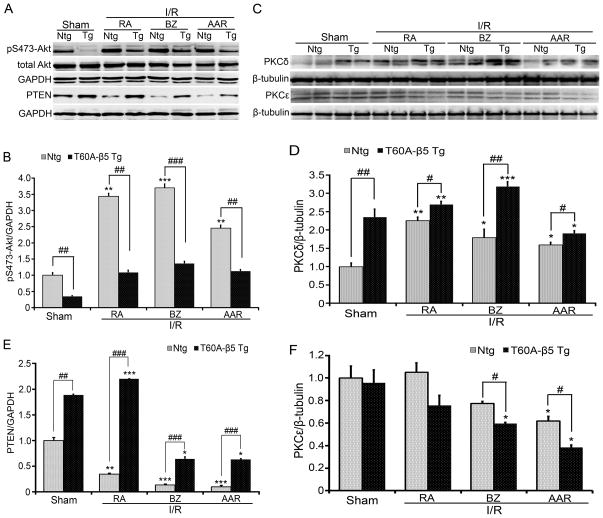
Akt activation and PKCε protect against myocardial I/R injury while increased expression of PTEN and PKCδ does the opposite at least in part through suppressing Akt.40, 41 These key signaling molecules are degraded by the UPS. Hence, in a separate cohort, we probed Akt activation through measuring Ser473-phospophrylated Akt (pS473-Akt) protein levels and measured the steady state protein levels of PTEN, Akt, PKCδ, and PKCε in LV regions that were differentially affected by LAD ligation/release. Compared with the Ntg sham group, PTEN protein levels were significantly higher and pS473-Akt levels were markedly lower in the T60A-β5 Tg sham group. I/R triggered marked decreases in PTEN in all three regions of the Ntg hearts but the decrease was completely reversed in RA and significantly attenuated in BZ and AAR by CR-PSMI (Figure 7A, 7C). I/R triggered a marked increase in pS473-Akt in all the three regions of the Ntg hearts but this increase was completely blocked by CR-PSMI (Figure 7A, 7B). PKCδ was significantly higher but PKCε remained unchanged in the T60A-β5 Tg sham group, compared with the Ntg sham group. I/R triggered an upregulation of PKCδ in all the three zones of the Ntg hearts and this upregulation was significantly enhanced by moderate CR-PSMI (Figure 7D, 7E). I/R did not alter PKCε levels in the RA but tended to down-regulate PKCε in the BZ and significantly down-regulated PKCε in the AAR of Ntg hearts. The downregulation of PKCε in the BZ and AAR was aggravated by CR-PSMI (Figure 7D, 7F).
Figure 7. Effects of CR-PSMI on Akt activation and PKCδ protein expression in sham and I/R hearts.
A ~ C, Representative images (A) and a summary of densitometry data of western blot analyses for total Akt and S473-phosphorylated Akt (p-S473-Akt, B) and PTEN (C) in sham hearts and in the indicated zones of I/R hearts. GAPDH was probed for loading control. D ~ F, Representative images (D) and a summary of densitometry data of western blot analyses for PKCδ (E) and PKCε (F) in sham hearts and in the indicated zones of I/R hearts. β-Tubulin was probed for loading control. n=4 mice/group; *p< 0.05, **p<0.01, ***p<0.005 vs. Ntg sham; #p< 0.05, ##p<0.01, ###p<0.005.
DISCUSSION
Myocardial I/R injury is an important pathological process occurring both in the natural course and during the intervention (e.g., revascularization) of ischemic heart disease, in addition to heart transplantation.42 Although significant progress has been made toward understanding I/R injury the molecular mechanisms underlying I/R are incompletely understood and finding effective clinical intervention to reduce the injury remains an important challenge.42 Several previous studies have suggested a role of altered cardiac proteasome function in I/R injury;20, 24, 26, 28, 30 however, these reports often contradict one another, regarding the adequacy of the altered proteasome function in maintaining cardiac proteostasis during I/R and the feasibility to inhibit proteasome function for ameliorating I/R injury.16, 29, 30 Taking advantage of a previously validated UPS reporter mouse, we demonstrated here for the first time that PFI occurs in mouse hearts undergoing regional myocardial I/R. Moreover, we established the first animal model of CR-PSMI and used this model to illustrate that a moderate inhibition of proteasome function in cardiomyocytes can be tolerated in mice at the baseline condition but it remarkably exacerbates I/R injury potentially via increasing PTEN and PKCδ protein levels, suppressing PKCε and Akt signaling, and increasing cardiomyocyte death.
PFI in cardiomyocytes of I/R hearts
In previous reports, proteasome functional status in I/R hearts was assessed primarily by measuring proteasome peptidase activities, the expression of some proteasome subunits, and the abundance of ubiquitin conjugates. Virtually all reports showed increases in ubiquitin conjugates. Most reports showed decreased 20S proteasome activities in I/R hearts. We confirmed these findings in the GFPdgn tg mouse LV with regional I/R. The decreases of proteasome peptidase activities observed in I/R myocardium are likely attributable to both decreased proteasome abundance, as evidenced by the decreases of β5 in both the BZ and AAR of Ntg I/R hearts, and posttranslational modifications such as oxidation of proteasome subunits as previously reported.17, 20 Notably, the degree (10%~40%) of decreases in the 20S proteasome peptidase activities observed in the RA, BZ, and AAR of I/R hearts alone should not be sufficient to impair the degradation of native proteins if the terminally damaged/misfolded proteins, which need proteasomes for their removal, are not increased. This is because previous reports have shown that it takes much more severe inhibition of the 20S proteasome (~75%) to accumulate surrogate proteasome substrates.21, 43 During I/R the production of terminally misfolded/damaged proteins (e.g., oxidized proteins) is inevitably increased; hence, the global increase of ubiquitinated proteins in I/R hearts is consistent with PFI but does not necessarily demonstrate PFI. This is because global increases of ubiquitinated proteins in the cell can also be caused by many other factors, such as increased production of ubiquitinated proteins (i.e., increased ubiquitination), decreased deubiquitination, increased atypical ubiquitination that yields ubiquitinated proteins not degradable by the proteasome, and impaired macroautophagy.2 Therefore, we probed the sufficiency of UPS function using GFPdgn, a validated surrogate substrate for the UPS. To our surprise, besides in cardiomyocytes of the AAR and the BZ, GFPdgn was also accumulated in cardiomyocytes of the RA where blood perfusion was not manipulated. These findings indicate that PFI occurs in the cardiomyocytes of I/R hearts.
Moderate CR-PSMI is well tolerated at the baseline but is detrimental during I/R
Multiple pharmacological agents are capable of inhibiting proteasome function and a pharmacological approach can have a few advantages. It is relatively cost- and time- efficient, and easier to achieve different degrees of inhibition. However, it is extremely difficult, if not impossible, to achieve homogeneous CR-PSMI in intact animals without affecting proteasomal function in other tissues and organs using pharmacological inhibitors, regardless to the specificity issues of proteasome inhibitors. Therefore, a genetic approach to specifically inhibit the 20S proteasome fulfills the purpose of this study better.
In the present study, we created the first tg mouse model of CR-PSMI via cardiac overexpression of a dominant negative mutant β5 subunit. Our baseline characterization on cardiac growth/development, the fetal gene program, and heart function of these mice (data not shown) has revealed that moderate CR-PSMI does not cause discernible abnormal phenotype in mice during the first 6 months of life (the longest time observed). However, CR-PSMI exacerbates myocardial I/R induced cardiac functional and structural impairment. At the function side, our P-V relationship analysis showed that at 24hr reperfusion following 30min ischemia, as expected, Ntg mice displayed significant decreases in both LV contractility and relaxation. The I/R induced LV functional impairment, especially the systolic malfunction, was more pronounced in T60A-β5 tg mice. Similarly, the same I/R procedure caused a greater infarct size in T60A-β5 tg mice than Ntg littermates. Consistent with these findings, chemotherapy using proteasome inhibitor bortezomib is generally well tolerated by multiple myeloma patients without preexisting cardiac conditions, whereas the therapy was associated with the development of heart failure and other cardiac dysfunction in elderly patients or patients with preexisting cardiac conditions.44, 45 The present findings are also in agreement with our recent report that cardiomyocyte-restricted proteasome functional enhancement via PA28α overexpression protects against I/R injury in mice.26 Taken together, through both gain-of-function (our previous study) and loss-of-function (the present study) approaches, we have now demonstrated that PFI in cardiomyocytes contributes to myocardial I/R injury. This conclusion is indirectly supported by several recent studies which suggest preserving cardiac proteasome function protects against I/R injury.16, 19
Notably, the above conclusion appears to tangentially dispute several previous reports which show a protective effect of proteasome inhibitors on I/R hearts in vivo or ex vivo.27, 28, 30 This is because PSMI in the present study is cardiomyocyte-restricted while the previously reported in vivo PSMI was achieved by systemic administration of pharmacological inhibitors and was ubiquitous to the body and/or to all tissue/cell types of the heart. Inflammation and the innate immune responses play a major role in I/R injury.42 Indeed, there is evidence that the protective effect of pharmacological PSMI observed in previous studies is attributable to its anti-inflammatory effects,28, 30 such as inhibition of the NFκB pathway and leukocyte infiltration.27, 30 Apparently, non-cardiomyocyte compartments of the heart (e.g., vasculature) and other systems (e.g., leukocytes and the immune system) play an important role in I/R associated inflammatory responses. In aggregate, it is suggested that the benefit of ubiquitous PSMI during myocardial I/R is derived primarily from the inhibition of proteasomes in non-cardiomyocytes. Hence, it is reasonable to speculate that measures to selectively inhibit the proteasome in non-cardiomyocyte compartments would be more effective in protecting against myocardial I/R injury than ubiquitous PSMI.
Potential mechanisms underlying the detrimental effect of CR-PSMI
Oxidative stress is markedly increased during reperfusion,42 resulting in a burst of production of oxidized proteins, including oxidative modifications of proteasome subunits and thereby inhibiting proteasome function.20, 24 The removal of oxidized proteins is 20S proteasome dependent.46–49 Therefore, it is not surprising that PFI occurs in the cardiomyocytes of I/R hearts. Hence, as supported by the findings that I/R triggered increases of total ubiquitinated proteins were remarkably aggravated by CR-PSMI (Online Figure V), additional CR-PSMI further impairs the degradation of oxidized/misfolded proteins in cardiomyocytes, exacerbating cardiomyocyte dysfunction and even causing cell death. Indeed, both TUNEL assays and detection of activated form of caspase 3 revealed that CR-PSMI significantly increased the prevalence of apoptotic cardiomyocytes in I/R hearts.
Beyond PQC, UPS-mediated protein degradation also regulates cell signaling pathways. PTEN is degraded by the UPS and it negatively regulates Akt. The Akt-mediated pro-survival signaling is activated and plays a protective role during myocardial I/R.40 The effect of PSMI on PTEN and Akt activation in the cardiomyocytes of I/R hearts has rarely been reported.16 Here we found that myocardial PTEN was significantly increased, and consistently, Akt activation was suppressed by moderate CR-PSMI in mice under the basal condition (Online Figure VI). Moreover, Akt activation during I/R, as indicated by the increase of Ser473-phosphorylated Akt, was abolished by moderate CR-PSMI. This is perhaps mediated by increased expression of PTEN and PKCδ asboth PTEN and PKCδ can inhibit Akt.42 These results suggest that suppressing Akt activation may have contributed to CR-PSMI induced increases in cardiomyocyte apoptosis in I/R hearts. Ischemic preconditioning (IPC) can powerfully protect against subsequent I/R injury. Two recent reports suggest that preservation of proteasome function contributes to the IPC protection.16, 20 By activating on the mitochondrial pathway of cell death, PKCδ is a well-known mediator of I/R injury,50 whereas PKCε protects against I/R injury.41 It was shown that IPC enhanced PKCδ degradation by the UPS and pharmacologically induced PSMI resulted in mitochondrial accumulation of PKCδ and a loss of IPC protective effects.16 In the present study, we observed that genetically induced CR-PSMI increased the abundance of PKCδ and decreased PKCε in I/R hearts, suggesting that CR-PSMI induced exacerbation of I/R injury might also be related to upregulation of PKCδ and downregulation of PKCε in the heart.
In summary, the present study demonstrates that PFI occurs in cardiomyocytes during myocardial I/R, that moderate CR-PSMI exacerbates cardiac dysfunction and cardiomyocyte death during regional myocardial I/R, and that the detrimental effects of CR-PSMI during myocardial I/R are associated with suppressing the activation of pro-survival kinase Akt and PKCε while enhancing pro-apoptotic pathways mediated by PKCδ.
Supplementary Material
Novelty and Significance.
What Is Known?
Myocardial proteasome peptidase activities were altered by acute ischemia/reperfusion (I/R).
Both genetically achieved cardiomyocyte-restricted proteasome functional enhancement and pharmacologically induced ubiquitous proteasome inhibition (PSMI) paradoxically protect against I/R injury.
What New Information Does This Article Contribute?
The establishment of the first animal model of cardiomyocyte-restricted PSMI (CR-PSMI), revealing that moderate CR-PSMI is well tolerated by mice.
CR-PSMI aggravates acute I/R injury.
Elevated oxidative stress during I/R inevitably increases oxidized proteins. Oxidized proteins are degraded by the 20S proteasome in a ubiquitin- dependent or independent manner. Unfortunately, the 20S can be modified and functionally impaired by oxidative stress. Hence, PFI likely occurs in the cardiomyocytes during I/R but this has not been directly demonstrated. Although enhancing proteasome function can protect against I/R injury, proteasome inhibitors have also been shown to reduce myocardial I/R injury. To address this apparent paradox, it is important to assess the impact of CR-PMSI on myocardial I/R injury. Here we report that a peptidase-inactivated mutant β5 subunit of the 20S, when expressed in cardiomyocytes, can replace endogenous β5 and effectively inhibits proteasome function. These studies establish the first animal model of CR-PSMI and show that moderate CR-PSMI suppresses the activation of a key survival kinase Akt potentially via increasing PTEN. This augments the pro-apoptotic kinase PKCδ, promotes cardiomyocyte apoptosis, and thereby aggravates myocardial I/R injury in mice. These findings indicate that in cardiomyocytes PFI plays an important pathogenic role in acute I/R injury, suggesting that the previously observed protective effects of proteasome inhibitors could be related to their activity in the non-cardiomyocyte compartment. Thus, proteasome inhibitors may be more effective in reducing I/R injury if their effects on cardiomyocytes can be minimized.
Acknowledgments
We thank Ms. Andrea Jahn for outstanding technical assistance in maintaining mouse colonies and genotype determination, Mr. Suleman Said for technical support on tissue sample preparation for immunostaining, and Dr. Stephen Armstrong for assistance in manuscript preparation. Dr. X. Wang is a recipient of the Established Investigator Award of the American Heart Association.
SOURCES OF FOUNDING
This work was in part supported by NIH grants R01HL072166, R01HL085629, and R01HL068936, and American Heart Association grants 0740025N (to X. W.), and 0620032Z (to H.Z.).
Non-standard Abbreviations
- AAR
area at risk
- BZ
border zone: the zone between ischemic and non-ischemic area
- CR-PSMI
cardiomyocyte-restricted proteasome inhibition
- GFPdgn
enhanced green fluorescence protein with carboxyl fusion of degron CL1
- I/R
ischemia-reperfusion
- Ntg
non-transgenic
- PFI
proteasome functional insufficiency
- PKC
protein kinase C
- PQC
protein quality control
- PSMI
proteasome inhibition
- PTEN
phosphatase and tensin homolog
- RA
the remote area, i.e., the blood flow of this area is not directly reduced
- T60A-β5
a mouse mutant β5 subunit with its Threonine60 mutated to Alanine
- TG, Tg, tg,
transgenic
- UPS
ubiquitin-proteasome system
Footnotes
DISCLOSURE
None.
References
- 1.Wang X, Robbins J. Heart failure and protein quality control. Circ Res. 2006;99:1315–1328. doi: 10.1161/01.RES.0000252342.61447.a2. [DOI] [PubMed] [Google Scholar]
- 2.Wang X, Li J, Zheng H, Su H, Powell SR. Proteasome functional insufficiency in cardiac pathogenesis. Am J Physiol Heart Circ Physiol. 2011;301:H2207–2219. doi: 10.1152/ajpheart.00714.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Usui S, Maejima Y, Pain J, Hong C, Cho J, Park JY, Zablocki D, Tian B, Glass DJ, Sadoshima J. Endogenous muscle atrophy f-box mediates pressure overload-induced cardiac hypertrophy through regulation of nuclear factor-kappab. Circ Res. 2011;109:161–171. doi: 10.1161/CIRCRESAHA.110.238717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su H, Li J, Menon S, Liu J, Kumarapeli AR, Wei N, Wang X. Perturbation of cullin deneddylation via conditional csn8 ablation impairs the ubiquitin-proteasome system and causes cardiomyocyte necrosis and dilated cardiomyopathy in mice. Circ Res. 2011;108:40–50. doi: 10.1161/CIRCRESAHA.110.230607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su H, Li F, Ranek MJ, Wei N, Wang X. Cop9 signalosome regulates autophagosome maturation. Circulation. 2011;124:2117–2128. doi: 10.1161/CIRCULATIONAHA.111.048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drews O, Tsukamoto O, Liem D, Streicher J, Wang Y, Ping P. Differential regulation of proteasome function in isoproterenol-induced cardiac hypertrophy. Circ Res. 2010;107:1094–1101. doi: 10.1161/CIRCRESAHA.110.222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willis MS, Townley-Tilson WH, Kang EY, Homeister JW, Patterson C. Sent to destroy: The ubiquitin proteasome system regulates cell signaling and protein quality control in cardiovascular development and disease. Circ Res. 2010;106:463–478. doi: 10.1161/CIRCRESAHA.109.208801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsukamoto O, Minamino T, Kitakaze M. Functional alterations of cardiac proteasomes under physiological and pathological conditions. Cardiovasc Res. 2010;85:339–346. doi: 10.1093/cvr/cvp282. [DOI] [PubMed] [Google Scholar]
- 9.Depre C, Wang Q, Yan L, Hedhli N, Peter P, Chen L, Hong C, Hittinger L, Ghaleh B, Sadoshima J, Vatner DE, Vatner SF, Madura K. Activation of the cardiac proteasome during pressure overload promotes ventricular hypertrophy. Circulation. 2006;114:1821–1828. doi: 10.1161/CIRCULATIONAHA.106.637827. [DOI] [PubMed] [Google Scholar]
- 10.Kassiotis C, Ballal K, Wellnitz K, Vela D, Gong M, Salazar R, Frazier OH, Taegtmeyer H. Markers of autophagy are downregulated in failing human heart after mechanical unloading. Circulation. 2009;120:S191–197. doi: 10.1161/CIRCULATIONAHA.108.842252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Otsuka K, Terasaki F, Shimomura H, Tsukada B, Horii T, Isomura T, Suma H, Shibayama Y, Kitaura Y. Enhanced expression of the ubiquitin-proteasome system in the myocardium from patients with dilated cardiomyopathy referred for left ventriculoplasty: An immunohistochemical study with special reference to oxidative stress. Heart Vessels. 2010;25:474–484. doi: 10.1007/s00380-010-0006-3. [DOI] [PubMed] [Google Scholar]
- 12.Predmore JM, Wang P, Davis F, Bartolone S, Westfall MV, Dyke DB, Pagani F, Powell SR, Day SM. Ubiquitin proteasome dysfunction in human hypertrophic and dilated cardiomyopathies. Circulation. 2010;121:997–1004. doi: 10.1161/CIRCULATIONAHA.109.904557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birks EJ, Latif N, Enesa K, Folkvang T, Luong le A, Sarathchandra P, Khan M, Ovaa H, Terracciano CM, Barton PJ, Yacoub MH, Evans PC. Elevated p53 expression is associated with dysregulation of the ubiquitin-proteasome system in dilated cardiomyopathy. Cardiovasc Res. 2008;79:472–480. doi: 10.1093/cvr/cvn083. [DOI] [PubMed] [Google Scholar]
- 14.Weekes J, Morrison K, Mullen A, Wait R, Barton P, Dunn MJ. Hyperubiquitination of proteins in dilated cardiomyopathy. Proteomics. 2003;3:208–216. doi: 10.1002/pmic.200390029. [DOI] [PubMed] [Google Scholar]
- 15.Geng Q, Romero J, Saini V, Baker TA, Picken MM, Gamelli RL, Majetschak M. A subset of 26s proteasomes is activated at critically low atp concentrations and contributes to myocardial injury during cold ischemia. Biochem Biophys Res Commun. 2009;390:1136–1141. doi: 10.1016/j.bbrc.2009.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Churchill EN, Ferreira JC, Brum PC, Szweda LI, Mochly-Rosen D. Ischaemic preconditioning improves proteasomal activity and increases the degradation of deltapkc during reperfusion. Cardiovasc Res. 2010;85:385–394. doi: 10.1093/cvr/cvp334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Powell SR, Divald A. The ubiquitin-proteasome system in myocardial ischaemia and preconditioning. Cardiovasc Res. 2010;85:303–311. doi: 10.1093/cvr/cvp321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baker TA, Geng Q, Romero J, Picken MM, Gamelli RL, Majetschak M. Prolongation of myocardial viability by proteasome inhibition during hypothermic organ preservation. Biochem Biophys Res Commun. 2010;401:548–553. doi: 10.1016/j.bbrc.2010.09.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takagi H, Hsu CP, Kajimoto K, Shao D, Yang Y, Maejima Y, Zhai P, Yehia G, Yamada C, Zablocki D, Sadoshima J. Activation of pkn mediates survival of cardiac myocytes in the heart during ischemia/reperfusion. Circ Res. 2010;107:642–649. doi: 10.1161/CIRCRESAHA.110.217554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Divald A, Kivity S, Wang P, Hochhauser E, Roberts B, Teichberg S, Gomes AV, Powell SR. Myocardial ischemic preconditioning preserves postischemic function of the 26s proteasome through diminished oxidative damage to 19s regulatory particle subunits. Circ Res. 2010;106:1829–1838. doi: 10.1161/CIRCRESAHA.110.219485. [DOI] [PubMed] [Google Scholar]
- 21.Kumarapeli AR, Horak KM, Glasford JW, Li J, Chen Q, Liu J, Zheng H, Wang X. A novel transgenic mouse model reveals deregulation of the ubiquitin-proteasome system in the heart by doxorubicin. FASEB J. 2005;19:2051–2053. doi: 10.1096/fj.05-3973fje. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Chen Q, Huang W, Horak KM, Zheng H, Mestril R, Wang X. Impairment of the ubiquitin-proteasome system in desminopathy mouse hearts. FASEB J. 2006;20:362–364. doi: 10.1096/fj.05-4869fje. [DOI] [PubMed] [Google Scholar]
- 23.Chen Q, Liu JB, Horak KM, Zheng H, Kumarapeli AR, Li J, Li F, Gerdes AM, Wawrousek EF, Wang X. Intrasarcoplasmic amyloidosis impairs proteolytic function of proteasomes in cardiomyocytes by compromising substrate uptake. Circ Res. 2005;97:1018–1026. doi: 10.1161/01.RES.0000189262.92896.0b. [DOI] [PubMed] [Google Scholar]
- 24.Bulteau AL, Lundberg KC, Humphries KM, Sadek HA, Szweda PA, Friguet B, Szweda LI. Oxidative modification and inactivation of the proteasome during coronary occlusion/reperfusion. J Biol Chem. 2001;276:30057–30063. doi: 10.1074/jbc.M100142200. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Powell SR, Wang X. Enhancement of proteasome function by pa28α overexpression protects against oxidative stress. FASEB J. 2011;25:883–893. doi: 10.1096/fj.10-160895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Horak KM, Su H, Sanbe A, Robbins J, Wang X. Enhancement of proteasomal function protects against cardiac proteinopathy and ischemia/reperfusion injury in mice. J Clin Invest. 2011;121:3689–3700. doi: 10.1172/JCI45709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bao J, Sato K, Li M, Gao Y, Abid R, Aird W, Simons M, Post MJ. Pr-39 and pr-11 peptides inhibit ischemia-reperfusion injury by blocking proteasome-mediated i kappa b alpha degradation. Am J Physiol Heart Circ Physiol. 2001;281:H2612–2618. doi: 10.1152/ajpheart.2001.281.6.H2612. [DOI] [PubMed] [Google Scholar]
- 28.Yu X, Patterson E, Kem DC. Targeting proteasomes for cardioprotection. Curr Opin Pharmacol. 2009;9:167–172. doi: 10.1016/j.coph.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 29.Pye J, Ardeshirpour F, McCain A, Bellinger DA, Merricks E, Adams J, Elliott PJ, Pien C, Fischer TH, Baldwin AS, Jr, Nichols TC. Proteasome inhibition ablates activation of nf-kappa b in myocardial reperfusion and reduces reperfusion injury. Am J Physiol Heart Circ Physiol. 2003;284:H919–926. doi: 10.1152/ajpheart.00851.2002. [DOI] [PubMed] [Google Scholar]
- 30.Campbell B, Adams J, Shin YK, Lefer AM. Cardioprotective effects of a novel proteasome inhibitor following ischemia and reperfusion in the isolated perfused rat heart. J Mol Cell Cardiol. 1999;31:467–476. doi: 10.1006/jmcc.1998.0880. [DOI] [PubMed] [Google Scholar]
- 31.Bence NF, Sampat RM, Kopito RR. Impairment of the ubiquitin-proteasome system by protein aggregation. Science. 2001;292:1552–1555. doi: 10.1126/science.292.5521.1552. [DOI] [PubMed] [Google Scholar]
- 32.Sanbe A, Gulick J, Hanks MC, Liang Q, Osinska H, Robbins J. Reengineering inducible cardiac-specific transgenesis with an attenuated myosin heavy chain promoter. Circ Res. 2003;92:609–616. doi: 10.1161/01.RES.0000065442.64694.9F. [DOI] [PubMed] [Google Scholar]
- 33.Schmidtke G, Kraft R, Kostka S, Henklein P, Frommel C, Lowe J, Huber R, Kloetzel PM, Schmidt M. Analysis of mammalian 20s proteasome biogenesis: The maturation of beta-subunits is an ordered two-step mechanism involving autocatalysis. EMBO J. 1996;15:6887–6898. [PMC free article] [PubMed] [Google Scholar]
- 34.Groll M, Ditzel L, Lowe J, Stock D, Bochtler M, Bartunik HD, Huber R. Structure of 20s proteasome from yeast at 2.4 a resolution. Nature. 1997;386:463–471. doi: 10.1038/386463a0. [DOI] [PubMed] [Google Scholar]
- 35.Arendt CS, Hochstrasser M. Identification of the yeast 20s proteasome catalytic centers and subunit interactions required for active-site formation. Proc Natl Acad Sci U S A. 1997;94:7156–7161. doi: 10.1073/pnas.94.14.7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jager S, Groll M, Huber R, Wolf DH, Heinemeyer W. Proteasome beta-type subunits: Unequal roles of propeptides in core particle maturation and a hierarchy of active site function. J Mol Biol. 1999;291:997–1013. doi: 10.1006/jmbi.1999.2995. [DOI] [PubMed] [Google Scholar]
- 37.Chen P, Hochstrasser M. Autocatalytic subunit processing couples active site formation in the 20s proteasome to completion of assembly. Cell. 1996;86:961–972. doi: 10.1016/s0092-8674(00)80171-3. [DOI] [PubMed] [Google Scholar]
- 38.Heinemeyer W, Fischer M, Krimmer T, Stachon U, Wolf DH. The active sites of the eukaryotic 20 s proteasome and their involvement in subunit precursor processing. J Biol Chem. 1997;272:25200–25209. doi: 10.1074/jbc.272.40.25200. [DOI] [PubMed] [Google Scholar]
- 39.Gomes AV, Zong C, Edmondson RD, Li X, Stefani E, Zhang J, Jones RC, Thyparambil S, Wang GW, Qiao X, Bardag-Gorce F, Ping P. Mapping the murine cardiac 26s proteasome complexes. Circ Res. 2006;99:362–371. doi: 10.1161/01.RES.0000237386.98506.f7. [DOI] [PubMed] [Google Scholar]
- 40.Downey JM, Davis AM, Cohen MV. Signaling pathways in ischemic preconditioning. Heart Fail Rev. 2007;12:181–188. doi: 10.1007/s10741-007-9025-2. [DOI] [PubMed] [Google Scholar]
- 41.Ping P, Song C, Zhang J, Guo Y, Cao X, Li RC, Wu W, Vondriska TM, Pass JM, Tang XL, Pierce WM, Bolli R. Formation of protein kinase c(epsilon)-lck signaling modules confers cardioprotection. J Clin Invest. 2002;109:499–507. doi: 10.1172/JCI13200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nat Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lindsten K, Menendez-Benito V, Masucci MG, Dantuma NP. A transgenic mouse model of the ubiquitin/proteasome system. Nat Biotechnol. 2003;21:897–902. doi: 10.1038/nbt851. [DOI] [PubMed] [Google Scholar]
- 44.Richardson PG, Mitsiades C, Hideshima T, Anderson KC. Bortezomib: Proteasome inhibition as an effective anticancer therapy. Annu Rev Med. 2006;57:33–47. doi: 10.1146/annurev.med.57.042905.122625. [DOI] [PubMed] [Google Scholar]
- 45.Enrico O, Gabriele B, Nadia C, Sara G, Daniele V, Giulia C, Antonio S, Mario P. Unexpected cardiotoxicity in haematological bortezomib treated patients. Br J Haematol. 2007;138:396–397. doi: 10.1111/j.1365-2141.2007.06659.x. [DOI] [PubMed] [Google Scholar]
- 46.Pickering AM, Linder RA, Zhang H, Forman HJ, Davies KJ. Nrf2-dependent induction of proteasome and pa28alphabeta regulator are required for adaptation to oxidative stress. J Biol Chem. 2012;287:10021–10031. doi: 10.1074/jbc.M111.277145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grune T, Merker K, Sandig G, Davies KJ. Selective degradation of oxidatively modified protein substrates by the proteasome. Biochem Biophys Res Commun. 2003;305:709–718. doi: 10.1016/s0006-291x(03)00809-x. [DOI] [PubMed] [Google Scholar]
- 48.Shringarpure R, Grune T, Mehlhase J, Davies KJ. Ubiquitin conjugation is not required for the degradation of oxidized proteins by proteasome. J Biol Chem. 2003;278:311–318. doi: 10.1074/jbc.M206279200. [DOI] [PubMed] [Google Scholar]
- 49.Davies KJ. Degradation of oxidized proteins by the 20s proteasome. Biochimie. 2001;83:301–310. doi: 10.1016/s0300-9084(01)01250-0. [DOI] [PubMed] [Google Scholar]
- 50.Churchill EN, Mochly-Rosen D. The roles of pkcdelta and epsilon isoenzymes in the regulation of myocardial ischaemia/reperfusion injury. Biochem Soc Trans. 2007;35:1040–1042. doi: 10.1042/BST0351040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.