Abstract
In this issue of Science Translational Medicine, Guo et al. discuss the intriguing possibility that intracellular tumor antigen–specific monoclonal antibodies (mAbs) inhibit tumor growth and metastasis and prolong survival of tumor-bearing mice. Here, I discuss the implications of using intracellular targets in mAb-based immunotherapy as well as the possible underlying mechanisms of action.
Because of their specificity and their homogeneous and reproducible characteristics, monoclonal antibodies (mAbs) have greatly facilitated the identification of clinically relevant molecules that are highly expressed by tumor cells [tumor antigens (TAs)] (1). mAbs also have been developed and implemented as effective immunotherapeutic agents for the treatment of malignant diseases (2, 3). mAbs that are currently being used in the clinic to treat various cancers target easily accessible TAs that are expressed on the outer membranes of selected tumor cells. In this issue of Science Translational Medicine, Guo et al. (4) present suggestive evidence that intracellular TAs with higher expression in cancer cells than in normal cells may also be useful targets for mAb-based immunotherapy.
KEEPING TABS ON MABS
Oncologists’ confidence in TA-specific mAbs as therapeutic agents has been boosted by convincing data from clinical trials that show improvements in outcome in an increasing number of cancer patients (5). As a result, several TA-specific mAbs have become part of the standard armamentarium used for the treatment of certain solid tumors and hematological malignancies. Examples include CD20-specific rituximab, human epidermal growth factor receptor 2 (HER2)–specific trastuzumab, and epidermal growth factor receptor (EGFR)–specific cetuximab used to treat lymphoma, some breast cancers, and head and neck cancers and colorectal carcinoma, respectively.
The TAs used as targets of mAb-based immunotherapy share several characteristics. First, these TAs are expressed in higher amounts on malignant cells relative to normal cells. Nevertheless, the targeting of normal tissues by TA-directed therapeutic mAbs does not cause side effects in the majority of the patients, even though these mAbs may induce regression of malignant lesions.
Second, TAs used as therapeutic targets mediate the antitumor effects of their corresponding mAbs through multiple mechanisms. For example, upon association with their corresponding mAb, TAs not only may inhibit signaling pathways, but also may trigger immune effector mechanisms such as complement- and cell-dependent cytotoxicity of target tumor cells. Furthermore, a growing body of in vitro, preclinical (animal model), and clinical data suggest that mAbs may induce or enhance a TA-specific T cell–mediated immune response by increasing dendritic cell (DC) uptake of TAs and subsequent presentation of TA-derived peptides to cognate T cells (5, 6). The time required for clinical responses to manifest after the administration of TA-specific mAbs argues in favor of an important role for T cell–mediated immunity in TA-specific mAb-based immunotherapy (5).
Third, the antitumor activity of the TA-specific mAbs is enhanced by combination with standard cytotoxic chemotherapeutic agents and radiotherapy (7) but is hampered by the development of tumor cell variants that use multiple mechanisms to escape from recognition and destruction by TA-specific mAbs (5, 6).
Last, the epitopes recognized by the current clinically active TA-specific mAbs are expressed on the membrane of tumor cells and, thus, are readily available for reactivity with mAbs. This cellular distribution of TA epitopes targeted by therapeutic mAbs has been a key requirement in the selection of TAs to be targeted by mAb-based immunotherapy. Now, Guo et al. (4) challenge this requirement. The surprising new data raise a number of questions that must be addressed in order to decipher the molecular mechanisms underlying the antitumor activity of intracellular TA–specific mAbs and to fully appreciate their clinical potential.
BURIED TREASURES
Using several animal model systems, Guo et al. provide new evidence to corroborate their earlier observation (8) that targeting intra-cellular oncoproteins with mAbs can inhibit the growth of human tumors grafted in immunocompromised nude mice (which lack T cells). In the new work, the authors sought to increase the clinical relevance of their model systems by using (i) immunocompetent mice grafted with syngeneic tumors and (ii) MMTV-PymT transgenic mice that develop spontaneous breast cancer.
In order to investigate the role of intra-cellular TAs in the therapeutic efficacy of TA-specific mAb-based immunotherapy, Guo et al. (4) engrafted into immunocompetent mice syngeneic melanoma cells that either (i) did or did not express the endogenous intracellular antigen PRL-3, a cancer-associated phosphatase or (ii) overexpressed intracellular enhanced green fluorescent protein (EGFP), a general reporter protein. Administration of intracellular TA–specific mAbs to syngeneic tumor–bearing mice caused a significant reduction in the sizes of their corresponding TA (PRL-3 or EGFP)–expressing tumors and in tumor metastases, as well as prolonged survival relative to mice that did not express the targeted TAs. Similar results were obtained in the MMTV-PymT transgenic mice that spontaneously develop mammary tumors; targeting with a mAb specific for the polyomavirus middle T oncoprotein reduced the progression and metastasis of tumors that expressed the intracellular targeted TA and prolonged median survival of the mice.
Taken together, these results show that intracellular TA–specific mAbs can have strong antitumor activity and that targeted intracellular TA expression is an absolute requirement for the antitumor activity of the mAbs tested. Furthermore, the antitumor activity observed with the EGFP-specific mAb indicates that, at least in this case, the targeted intracellular TA did not need to have a function in tumor cell biology for its mAb to be an effective tumor therapeutic.
In a potentially clinically relevant result, Guo et al. (4) showed that immunization of C57BL/6 mice with the intracellular TA PRL-3 or EGFP induced humoral immunity to the immunizing TA—that is, production of PRL-3– or EGFP-specific antibodies by the host. This immune response inhibited the metastatic spread to lungs, adrenal glands, and ovaries of melanoma cells that expressed the targeted intracellular TAs. This effect was influenced by the intracellular TA expression level, as metastatic spread to the lungs of melanoma cells that produced low amounts of PRL-3 was not inhibited by PRL-3–specific humoral immunity. In addition, the immune response induced in MMTV-PymT transgenic female mice by immunizations with the middle T antigen caused an approximately 10-fold reduction in the weight of mammary gland tumors and prolonged the survival of immunized mice by about 20%.
The interpretation of this series of experiments would have benefited from the use of control mice that had been immunized with an unrelated TA instead of unimmunized mice. This approach would have allowed one to assess, in both mouse models, the role of TA-specific immunity and of nonspecific activation of the immune system in vaccinated mice. Although the noted differences between immunized and control mice were statistically significant, antitumor effects were not detected in at least 20% of the TA-immunized mice. It would be of interest to know whether the lack of antitumor effects in a fraction of the immunized mice reflects defects in the immune response elicited or escape mechanisms used by tumor cells to avoid recognition by the host immune system. Such information would facilitate the design of targeted strategies to enhance the therapeutic efficacy of cancer vaccination with intracellular TAs.
An additional question raised by the new results that remains to be answered is whether the antitumor effects observed in the immunized animals were mediated only by induced humoral immunity or also involved T cell immunity. Last, the authors did not perform experiments to compare, in a systematic way, the therapeutic efficacy of cancer vaccination versus that of passive administration of TA-specific mAbs. Nevertheless, the results presented suggest that the latter modality is more effective in reducing metastases and incidence of breast cancer. It remains to be determined whether these differences reflect (i) the characteristics of the intracellular TA–specific endogenous and exogenous antibodies in terms of fine specificity, association constant, or immunoglobulin class and subclass or (ii) interference of cellular immunity elicited by the vaccination.
Even in the mAb-treated animals, fewer than 20% of the treated mice were completely “cured”; the majority succumbed to the disease despite therapy. Whether these results reflect the lack of optimization of the therapeutic schedule in terms of mAb amount and number and frequency of mAb administrations, or the escape of tumor cell variants from the antitumor activity of the mAbs tested, must be determined. If disease progression or recurrence reflects the growth of tumor cells that either are not recognized by the mAbs or are not susceptible to their anti-tumor activity, then it will be crucial to characterize the molecular basis of these escape mechanisms. This information may suggest the rational design of targeted therapies to counteract the identified escape mechanisms used by some tumor cells. Such combination therapies might include the administration of cytokines such as interferons, which have been shown to enhance the expression of some membrane-bound TAs (9), and cytotoxic chemotherapeutic agents that have been shown to enhance the antitumor activity of mAbs that recognize membrane-bound TAs (7). An additional question that should be addressed is whether cancer-initiating cells are susceptible to the antitumor activity of intracellular TA–specific mAbs, given the suggested role of these cells in metastatic spread and disease recurrence and their chemo- and radio-resistance (10).
SPARING NORMAL CELLS
The unexpected therapeutic effficacy of the intracellular TA–specific mAb-based immunotherapy described by Guo et al. (4) might define new molecular mechanisms of immunotherapy and markedly increase the number of TAs suitable for use as mAb-directed therapeutic targets. In addition, the higher immunogenicity of intracellular TAs as compared with membrane-bound TAs will facilitate the use of cancer vaccines for the treatment of malignant disease. As pointed out by Guo et al., cancer vaccination is substantially less expensive than exogenous mAb administration. Furthermore, vaccination does not cause the large fluctuations in serum antibody concentrations observed in patients treated with exogenous therapeutic mAbs and induces immunological memory.
With respect to the potential clinical relevance of the therapeutic results presented, it is noteworthy that like their membrane-bound TA-specific mAb counterparts, intracellular TA–specific therapeutic mAbs carried out their antitumor activities without causing adverse effects in normal tissues of treated mice even though they express the targeted TAs. However, no information is provided about the underlying mechanism (or mechanisms) behind these results. Can they be explained by the difference in expression levels of the targeted TAs in malignant and normal cells? If so, the optimization of intracellular TA–specific, mAb-based immunotherapy may benefit from quantitative information about the minimum amount of the mAb-targeted intracellular TA required in malignant cells to observe antitumor activity and the maximum amount of the TA that can be present in normal cells and not cause side effects. This information will greatly facilitate the selection of target intracellular TAs and the stratification and monitoring of patients to be treated with this type of immunotherapy.
The challenge will be to develop methods that can precisely measure TA expression in malignant tumors and normal tissues in an accurate and reproducible way. Furthermore, this methodology must be suited to clinical settings, in which noninvasive assays are preferred. Alternative, but not exclusive, mechanisms to explain the differential sensitivity of malignant and normal cells to intracellular TA–specific mAbs include functional differences between malignant and normal cells in the mechanisms that underlie TA interactions with their corresponding mAbs.
FINE-TUNING THERAPEUTIC ANTIBODIES
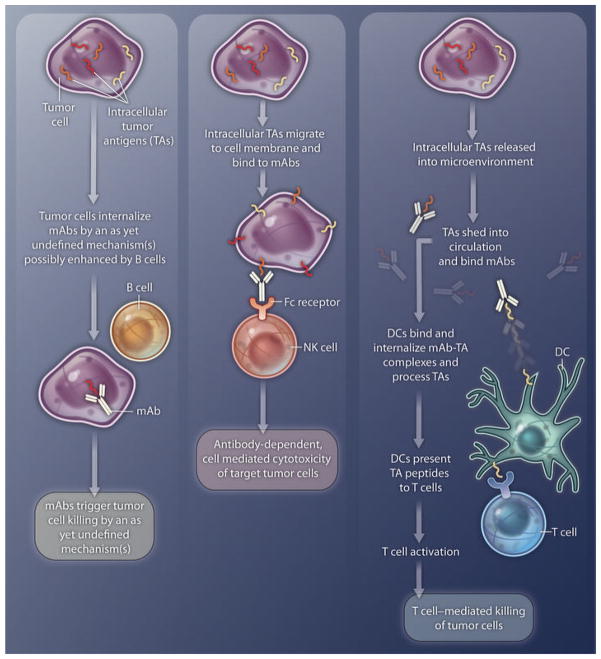
Two key questions that have been addressed only to a limited extent by Guo et al. (4) are related to the molecular basis of the interactions of mAbs with the corresponding intracellular TAs and the role of immune cells in the antitumor activity of intracellular TA–specific mAbs (Fig. 1). Among the potential mechanisms listed but not investigated by the authors is transport of the intracellular TAs to the cell membrane, where TAs react with mAbs. This mode of action is supported by data that show that other intracellular molecules, such as calreticulin, are expressed on the cell membrane when cells are exposed to chemotherapeutic agents or ionizing radiation (11). Direct experiments are required to test this potential mechanism and to define variables that modulate the transport of intracellular TAs. This information may contribute to our understanding of why the antitumor activity of intracellular TA–specific mAbs was not described earlier and may help to optimize immunotherapeutic strategies with these types of mAbs.
Fig. 1. Inside mAb.
Shown are three possible mechanisms for the antitumor activity of intracellular TA–specific mAbs. TAs might: (i) interact with mAbs that have been internalized (through an unknown mechanism that may be enhanced by B cells) by tumor cells, which are then destroyed by yet-to-be-defined mechanisms; (ii) migrate to the cell membrane and bind to mAbs, and the TA-mAb complex is recognized by Fc receptors on NK cells, which then carry out antibody-dependent tumor-cell killing; or (iii) be shed into the microenvironment or circulation and form complexes with circulating mAbs. The complex might then be taken up by DCs, which process the TAs and present the resulting TA peptides to cognate T cells. Activated T cells then mediate tumor-cell killing.
The authors provide convincing evidence that the antitumor activity of intracellular TA–specific mAbs depends on participation by B cells. In two mouse strains genetically manipulated to be devoid of mature B cells, intracellular TA–specific mAbs could not inhibit tumor growth or metastases. The authors also showed that B cells appear to enhance antibody internalization in cultured cancer cells. This dependence of the antitumor activity on B cells is not unique to intracellular TA–specific mAbs; as pointed out by the authors, similar results were observed with the death receptor DR5–specific mAb MD5-1 in a mouse breast and colon adenocarcinoma model (12).
Two other possible mechanisms for the antitumor activity of mAbs that recognize intracellular TAs are described in Fig. 1. Intracellular TAs might move to the cell membrane and bind to mAbs, and the TA-mAb complex can then be recognized by Fc receptors on natural killer (NK) cells, which carry out antibody-dependent tumor-cell killing (middle panel). Or, the TAs might be shed into the microenvironment or circulation and form complexes with circulating mAbs. The complexes might then be taken up by DCs, which process the TAs and present the resulting TA peptides to cognate T cells. Activated T cells may then mediate tumor-cell killing (right panel).
Guo et al. (4) state that T cells do not play a role in the antitumor activity of intracellular TA–specific mAbs because this effect is observed in nude mice, which lack T cells. However, whether this conclusion is applicable to immunocompetent mice remains to be tested, especially because intracellular TA–specific endogenous antibodies elicited by vaccination were able to control tumor growth and metastasis.
Acknowledgments
Funding: S.F. is supported by Public Health Service grants R01 CA113369 and R01 CA138188 awarded by the U.S. National Cancer Institute.
REFERENCES AND NOTES
- 1.Campoli M, Ferrone S. In: Cancer: Principles & Practice of Oncology. DeVita V, Hellman S, Rosenberg S, editors. Lippincott Williams and Wilkins; New York: 2009. pp. 1–18. [Google Scholar]
- 2.Weiner LM, Dhodapkar MV, Ferrone S. Monoclonal antibodies for cancer immunotherapy. Lancet. 2009;373:1033–1040. doi: 10.1016/S0140-6736(09)60251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiner LM, Surana R, Wang S. Monoclonal antibodies: Versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010;10:317–327. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo K, Li J, Tang JP, Tan CPB, Hong CW, Al-Aidaroos AQO, Varghese L, Huang C, Zeng Q. Targeting intracellular oncoproteins with antibody therapy or vaccination. Sci Transl Med. 2011;3:99ra85. doi: 10.1126/scitranslmed.3002296. [DOI] [PubMed] [Google Scholar]
- 5.Ferris RL, Jaffee EM, Ferrone S. Tumor antigen-targeted, monoclonal antibody-based immunotherapy: Clinical response, cellular immunity, and immunoescape. J Clin Oncol. 2010;28:4390–4399. doi: 10.1200/JCO.2009.27.6360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campoli M, Ferris R, Ferrone S, Wang X. Immunotherapy of malignant disease with tumor antigen-specific monoclonal antibodies. Clin Cancer Res. 2010;16:11–20. doi: 10.1158/1078-0432.CCR-09-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Machiels JP, Schmitz S. Molecular-targeted therapy of head and neck squamous cell carcinoma: Beyond cetuximab-based therapy. Curr Opin Oncol. 2011;23:241–248. doi: 10.1097/CCO.0b013e328344f581. [DOI] [PubMed] [Google Scholar]
- 8.Guo K, Tang JP, Tan CP, Wang H, Zeng Q. Monoclonal antibodies target intracellular PRL phosphatases to inhibit cancer metastases in mice. Cancer Biol Ther. 2008;7:750–757. doi: 10.4161/cbt.7.5.5764. [DOI] [PubMed] [Google Scholar]
- 9.Hance KW, Rogers CJ, Zaharoff DA, Canter D, Schlom J, Greiner JW. The antitumor and immunoadjuvant effects of IFN-alpha in combination with recombinant poxvirus vaccines. Clin Cancer Res. 2009;15:2387–2396. doi: 10.1158/1078-0432.CCR-08-1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Visvader JE. Cells of origin in cancer. Nature. 2011;469:314–322. doi: 10.1038/nature09781. [DOI] [PubMed] [Google Scholar]
- 11.Zitvogel L, Kepp O, Senovilla L, Menger L, Chaput N, Kroemer G. Immunogenic tumor cell death for optimal anticancer therapy: The calreticulin exposure pathway. Clin Cancer Res. 2010;16:3100–3104. doi: 10.1158/1078-0432.CCR-09-2891. [DOI] [PubMed] [Google Scholar]
- 12.Haynes NM, Hawkins ED, Li M, McLaughlin NM, Hämmerling GJ, Schwendener R, Winoto A, Wensky A, Yagita H, Takeda K, Kershaw MH, Darcy PK, Smyth MJ. CD11c+ dendritic cells and B cells contribute to the tumoricidal activity of anti-DR5 antibody therapy in established tumors. J Immunol. 2010;185:532–541. doi: 10.4049/jimmunol.0903624. [DOI] [PubMed] [Google Scholar]