Abstract
Malignant transformation of cells is often associated with changes in classical and non-classical HLA class I antigen, HLA class II antigen as well as NK cell activating ligand (NKCAL) expression. These changes are believed to play a role in the clinical course of the disease since these molecules are critical to the interactions between tumor cells and components of both innate and adaptive immune system. For some time, it has been assumed that alterations in the expression profile of HLA antigens and NKCAL on malignant cells represented loss of classical HLA class I antigen and induction of HLA class II antigen, non-classical HLA class I antigen and/or NKCAL expression. In contrast to these assumptions, experimental evidence suggests that in some cases dysplastic and malignant cells can acquire classical HLA class I antigen expression and/or lose the ability to express HLA class II antigens. In light of the latter findings as well as of the revival of the cancer immune surveillance theory, a reevaluation of the interpretation of changes in HLA antigen and NKCAL expression in malignant lesions is warranted. In this article, we first briefly describe the conventional types of changes in HLA antigen and NKCAL expression that have been identified in malignant cells to date. Second, we discuss the evidence indicating that, in at least some cell types, classical HLA class I antigen expression can be acquired and/ or the ability to express HLA class II antigens is lost. Third, we review the available evidence for the role of immune selective pressure in the generation of malignant lesions with changes in HLA antigen expression. This information contributes to our understanding of the role of the immune system in the control of tumor development and to the optimization of the design of immunotherapeutic strategies for the treatment of cancer.
Keywords: Antigen processing machinery, Cancer, Classical HLA class I antigen, Immune escape, Immune selection, HLA class II antigen, MICA, MICB, NK cell activating ligand, Non-classical HLA class I antigen, ULBP
Introduction
In humans, like in other animal species, malignant transformation of cells is often associated with changes in gene expression and in their antigenic profile. They include changes in classical and non-classical human leukocyte antigen (HLA) class I [1] and class II [2] as well as natural killer cell activating ligand (NKCAL) [3–5] expression. These changes have been convincingly documented in a number of malignant tumors by analyzing cell lines in long-term culture and surgically removed lesions [1–5]. Cell lines have provided the opportunity to identify and characterize the multiple molecular mechanisms underlying changes in HLA antigen and NKCAL expression and to analyze their functional implications. On the other hand, surgically removed lesions have provided the opportunity to prove that the changes found in cell lines are not an in vitro artifact, but reflect in vivo changes. Furthermore, they have allowed investigators to assess the clinical significance of these changes. A number of studies suggest that changes in the expression pattern of these molecules play a role in the clinical course of the disease since they have been associated, in at least some tumor types, with prognosis as well as disease-free interval and survival [1–5]. These associations are likely to reflect the critical role these molecules play in the interactions of tumor cells with components of both innate and adaptive immune system [1–5] (Fig. 1). Nevertheless, the biological and clinical significance of HLA antigen and NKCAL changes remains under debate [6]. The debate has focused on whether HLA antigen and NKCAL changes are simply the by-product of genomic instability or reflect selection of tumor cells with HLA antigen or NKCAL changes secondary to immune selective pressure. This debate also stems, at least in part, from the assumptions investigators have made over the years in terms of changes in HLA antigen and NKCAL expression in malignant lesions. In this regard, changes in classical HLA class I antigen expression in malignant lesions are assumed to represent loss [1, 2] since it has been propagated through textbooks of immunology that classical HLA class I antigens are expressed by all nucleated cells [7, 8]. On the other hand, changes in non-classical HLA class I antigen, HLA class II antigen, and NKCAL expression are assumed to represent appearance [1–5] since these antigens are believed to have a restricted distribution in normal tissues [7–9]. However, there is evidence that dysplastic and malignant cells can acquire classical HLA class I antigen expression and/or lose the ability to express HLA class II antigens. The latter observations challenge our past assumptions regarding the mechanisms underlying changes in the expression of these molecules in malignant lesions.
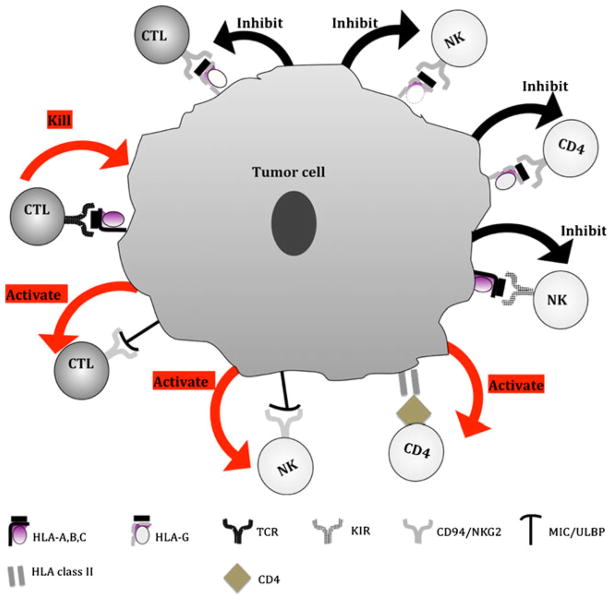
Fig. 1.
Molecular mechanisms underlying the functional properties of HLA antigen and NKCAL expressed by malignant cells. Once the classical HLA class I-β2m-peptide complex is transported to the plasma membrane, it plays a major role in the interactions between target cells and (a) activation of peptide-specific CTL through TCR; and (b) inhibition of T-cell subpopulations through inhibitory receptors KIR. In contrast to classical HLA class I antigens, the non-classical HLA class I antigens, HLA-G, inhibit CTL, CD4(+) T cells and NK cells through their interaction with the NK cells receptor CD94/NKG2. HLA class II expression by tumor cells may be potentially beneficial to TA-specific immune responses through their interaction with CD4(+) T cells, resulting in the activation of CD4(+) T-cell-mediated killing, macrophage through release of Th1 cytokines; B cells and eosinophils through release of Th2 cytokines, and CTL through release of Th1 cytokines
In light of the experimental evidence demonstrating that in some cases dysplastic and/or malignant cells can acquire classical HLA class I antigen expression and/or lose the ability to express HLA class II antigens as well as of the revival of the cancer immune surveillance theory [10], a reevaluation of the interpretation of changes in HLA antigen and NKCAL expression in malignant lesions is warranted. In this article, we review this topic since this information may contribute to our understanding of the role of the immune system in the control of tumor development and to the optimization of the design of immunotherapeutic strategies for the treatment of cancer. Moreover, this information is likely to contribute to the resolution of some of the conflicting information in the literature regarding the clinical relevance of HLA antigen and NKCAL changes in malignant disease. Specifically, we first briefly describe the types of changes in HLA antigen and NKCAL expression that have been identified in malignant cells to date. Second, we discuss the evidence indicating that, in at least some cell types, malignant transformation may actually be associated with the appearance of classical HLA class I antigens and/ or loss of the ability to express HLA class II antigens. Lastly, we summarize the evidence for the role of immune selective pressure in the generation of malignant lesions with HLA antigen defects.
Analysis of HLA antigen and NKCAL expression in malignant lesions
A conventional view
Beginning in the 1980s and continuing today, a large number of malignant lesions have been tested with classical HLA class I and HLA class II antigen-specific mAb [1, 2]. More recently, these studies have been expanded to include the analysis of the expression of non-classical HLA class I antigens such as HLA-E, -F, and -G, as well as NKCAL including the phylogenetically distant MHC class I related chain (MIC) [1–5] and the UL16-binding proteins (ULBP) [1–5]. At present, the available information regarding non-classical HLA class I antigen and NKCAL expression is still limited since the field is in an early stage. Moreover, progress in this area is hindered by the lack and/or limited availability of non-classical HLA class I antigen- and NKCAL-specific mAb, with the appropriate specificity that is suitable for IHC staining.
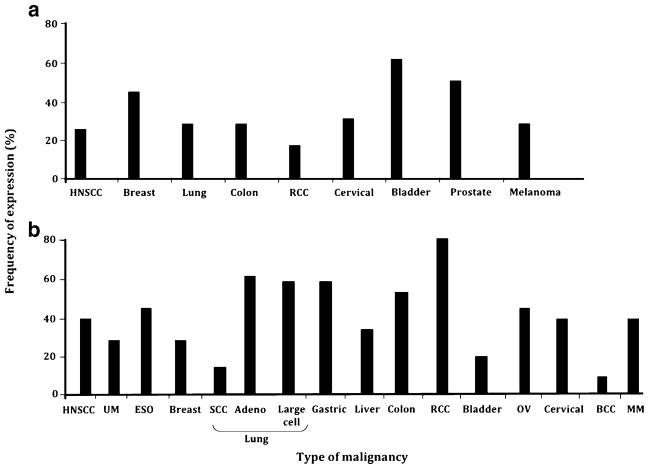
As reviewed elsewhere [1, 2], convincing evidence indicates that malignant transformation of cells is frequently associated with changes in classical HLA class I and HLA class II antigen expression (Fig. 2). These studies, which include our own, have concluded that classical HLA class I antigens are lost or downregulated, whereas HLA class II antigens are induced on malignant cells [1, 2]. The molecular mechanisms underlying changes in HLA antigen expression in malignant cells include structural gene abnormalities as well as defective regulation of HLA gene transcription and translation [1, 2]. More recently, epigenetic events associated with tumor development and progression have been found to underlie changes in HLA antigen, antigen processing machinery, co-stimulatory molecule, and tumor antigen (TA) expression in malignant cells [2]. It is noteworthy that in some tumors such as breast, colon, and cervical carcinoma, HLA class II antigen expression is not restricted to cells that have undergone malignant transformation [1, 2]. Among the non-classical HLA class I antigens, HLA-G expression has been studied the most extensively [1, 2]. Although the results in the literature conflict, there is a general agreement that malignant transformation of cells may be associated with the appearance of HLA-G, the frequency being quite different among the various types of tumors analyzed (Fig. 3) [1, 2, 11, 12]. In contrast to HLA-G, less information is available regarding HLA-E and HLA-F expression by malignant cells [1, 2, 13–16]. In surgically removed malignant lesions, HLA-E expression is expressed in glioblastoma, carcinomas of colon and ovary, lymphoma, and melanoma [1, 2, 13–16]. Only cell lines derived from EBV-transformed lymphoid cells as well as monocytic, glioblastoma, liver carcinoma, and transitional cell bladder cells have been found to express HLA-F on the cell surface [1, 2].
Fig. 2.
Frequency of classical HLA class I antigen downregulation and HLA class II antigen expression in malignant lesions of different embryological origin. Solid tumors for which more than 50 primary lesions have been analyzed for a classical and b HLA class II expression are shown. BCC cutaneous basal cell carcinoma, ESO esophageal carcinoma, HNSCC head and neck squamous cell carcinoma, MM malignant melanoma, OV ovarian carcinoma, RCC renal cell carcinoma, SCC squamous cell carcinoma, UM uveal melanoma
Fig. 3.
Frequency of non-classical HLA class I antigen, HLA-G, expression in malignant lesions of different embryological origin. Solid tumors for which more than 50 primary lesions have been analyzed for non-classical HLA class I antigen, HLA-G, expression are shown. NHL non-Hodgkin’s lymphoma, RCC renal cell carcinoma
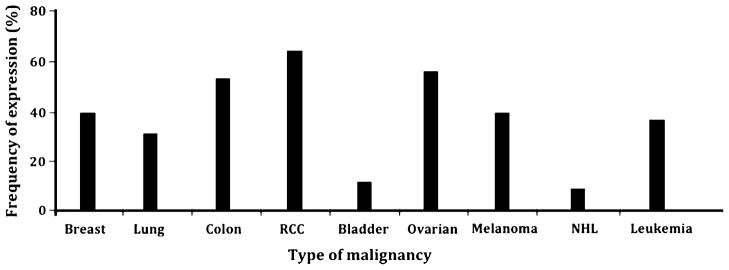
Information regarding NKCAL expression by malignant cells is derived from the analysis of only a limited number of surgically removed malignant lesions. Nevertheless, the results of these studies, which have been corroborated by those derived from the analysis of cell lines in long-term culture of different embryological origin [1–5], indicate that MICA and MICB have a much broader distribution in malignant tumors than in normal tissues, as they are expressed in solid tumors derived from the breast, lung, colon, kidney, ovary, and pancreas as well as glioblastoma, neuroblastoma, and cutaneous and uveal melanoma [1–5]. Moreover, both MICA and MICB have been found to be expressed in hematologic malignancies such as acute and chronic lymphatic leukemia, and acute and chronic myeloid leukemia [1–5]. ULBP expression has been examined only in glioma, leukemia, and melanoma [1–5]. In general, ULBP molecules are expressed less frequently than MIC molecules, and MIC and ULBP do not appear to be expressed in a coordinated fashion in the tumor cells examined. In addition, the available information suggests that the frequency of MIC and ULBP expression is independent of classical and non-classical HLA class I antigen expression.
A contemporary view
The view that HLA class I antigens are frequently lost or downregulated on malignant cells is based on the assumption that classical HLA class I antigens are expressed on all nucleated cells except for immunoprivileged tissues (e.g., brain, cornea, anterior chamber of the eye, liver, testis, fetotrophoblast, hair matrix, and proximal nail matrix) [7, 8, 17–25]. The view that malignant transformation of cells may be associated with the appearance of HLA class II antigens is based on the assumption that these antigens are constitutively expressed only by hematopoietic antigen presenting cells (APC) including B lymphocytes, dendritic cells (DC), and macrophages [7–9]. However, a number of studies in the literature which for unknown reasons are ignored by the scientific community argue against the aforementioned assumptions regarding classical HLA class I antigen as well as class II antigen expression in normal tissues. First, classical HLA class I antigens are not expressed on all nucleated cells. In addition to immunoprivileged tissues [17–25], classical HLA class I antigens are not always detected on adipocytes, chondrocytes, hepatocytes, smooth and skeletal muscle cells, epithelial cells of parathyroid, acinar pancreas, biliary duct, nested melanocytes in benign nevi, basal layer melanocytes, ureter endothelia, and sympathetic ganglia [26–39]. Moreover, in some cases, such as epithelial cells of trachea glands, bronchial glands, esophagus, and stomach, classical HLA class I antigens are expressed in the cytoplasm and not on the cell membrane [29]. Along the same lines, HLA class II antigen expression in normal tissues is not restricted to APC. In this regard, it is well known that HLA class II antigen expression can be induced on a number of cell types including, but not limited to, endothelial cells, epithelial cells, keratinocytes, fibroblasts, and mast cells as well as melanocytes upon incubation with interferon-γ (IFN-γ) [40, 41]. Furthermore, as noted above in tumors such as breast, colon, and cervical carcinoma, HLA class II antigens are expressed not only by cells that have undergone malignant transformation but also by normal breast, colon, and cervical epithelial cells [1, 2]. In addition, HLA class II antigen expression has also been observed on several normal nucleated cells of different embryological origin under basal conditions. They include the epithelium of the bronchial glands, gastrointestinal tract, urinary bladder, and thymic reticuloepithelial cells among cells of entodermic origin; epithelium of mammary gland, acinar cells of parotid, and astrocytes among cells of ectodermic origin; breast, glomerular, and peritubular renal endothelium, cervix, and endometrium among cells of the mesoderm, as well as keratinocytes and nested melanocytes in benign and atypical (dysplastic) melanocytic nevi [39, 42, 43]. Second, there are examples in the literature demonstrating that both classical HLA class I antigen and HLA class II antigen expression can be detected in sites of immune privilege under normal conditions [29, 42, 43] as well as in patients with autoimmune and inflammatory conditions such as alopecia areata, hepatitis, dermatomyositis, psoriasis, rheumatoid arthritis, and systemic lupus erythematosus [17, 29, 42–53]. Lastly, there is evidence to suggest that non-malignant, pre-malignant as well as malignant cells can acquire classical HLA class I antigen expression. In this regard, acquisition of classical HLA class I antigen expression has been observed in halo nevi [54] as well as some forms of hepatocellular [1, 2] and testicular [1, 2] carcinomas. More recently, we have performed an analysis of HLA antigen expression in a panel of surgically removed benign and atypical (dysplastic) nevi [39]. We have observed that among benign nevi, only halo nevi express HLA class I heavy chain and HLA class II β chain (Fig. 4). On the other hand, HLA class I heavy chain and HLA class II β chain were expressed in more than 70% of the atypical (dysplastic) nevi (Fig. 4). The latter are associated with an increased risk of melanoma and, at least in some cases, represent a precursor of malignant melanoma. It is noteworthy that the level of HLA class I heavy chain and of HLA class II β chain expression in atypical (dysplastic) nevi correlated with the degree of cytologic atypia and architectural disorder. The latter distinction has important clinical implications since the degree of cytologic atypia and architectural disorder are two criteria employed most often in the histologic diagnosis of atypical (dysplastic) nevi and the degree of histologic atypia in AN has been associated with melanoma risk [55, 56]. In view of the role of lymphocyte-mediated destruction of melanocytes in the development of halo nevi, it is likely that HLA antigen expression renders melanocytes more susceptible to both CD8(+) and CD4(+) T-cell recognition and killing [54, 57]. However, whether the appearance of HLA antigens on melanocytes in halo nevi as well as atypical (dysplastic) nevi reflects their induction via some form of cellular stress and/or is secondary to cytokine release by lymphocytes present within the microenvironment remains to be determined.
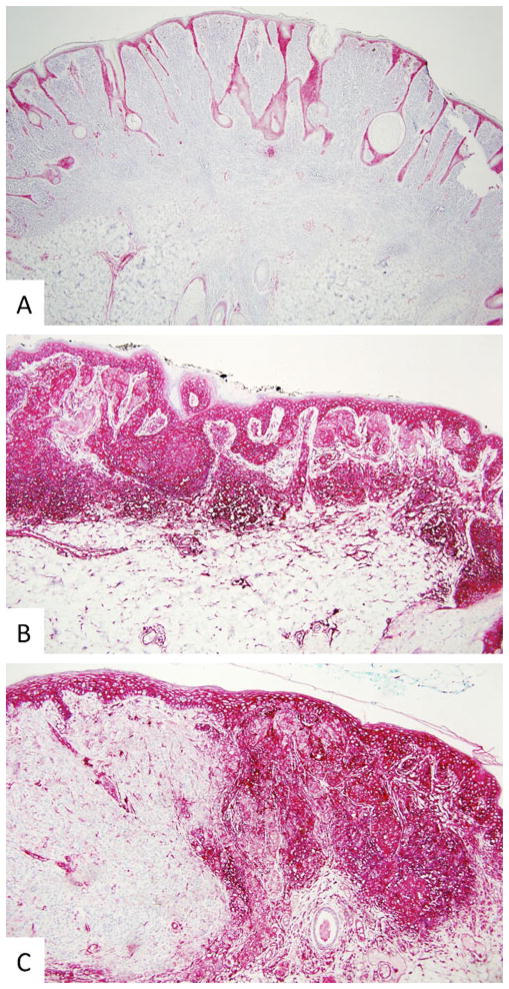
Fig. 4.
Differential HLA antigen expression in benign and atypical melanocytic nevi, in cutaneous melanoma and in surrounding normal cells. a Only normal keratinocytes, endothelial cells, and antigen-presenting cells were marked by HLA class I heavy chain-specific mAb HC-10 in the immunoperoxidase reaction in an intradermal nevus (×10). b Normal keratinocytes, endothelial cells, antigen-presenting cells, and melanocytes were marked by HLA class I heavy chain-specific mAb HC-10 in the immunoperoxidase reaction in a severely atypical nevus (×10). c Normal keratinocytes, endothelial cells, and malignant melanocytes but not intradermal nested melanocytes or vertical growth phase melanoma cells were marked by HLA class I heavy chain-specific mAb HC-10 in the immunoperoxidase reaction in a superficial spreading melanoma arising within an intradermal nevus (×10)
The data described above suggests that HLA class I antigens have a more restricted distribution in normal tissues, whereas HLA class II antigens have a broader distribution in normal tissues than originally described. Moreover, there is increasing evidence demonstrating that HLA class II antigens presenting TA-derived peptides can serve as a target for TA-specific CD4(+) T cells and be subject to immune selective pressure [58–60]. Therefore, previous assumptions regarding the HLA antigen phenotype of malignant cells may need to be reevaluated. In fact, a thorough review of the literature demonstrates that classical HLA class I antigen loss and HLA class II antigen induction do not occur in every type of malignant disease. Specifically, HLA class I antigen defects are rarely observed in tumors derived from hepatocytes, uveal melanocytes, testicular germ cells as well as hematologic malignancies [1, 2]. Moreover, colon cancer cells [61] which demonstrate microsatellite instability (MSI) as well as primary mediastinal B-cell lymphoma and classical Hodgkin lymphoma cell lines [62] lose HLA class II antigen expression due to somatic mutations affecting HLA class II antigen-regulatory genes [61, 62]. The loss of HLA class II antigen expression is associated with decreased survival in primary mediastinal B-cell lymphoma (PMBCL) patients [62]. Lastly, the inability to upregulate HLA class II antigen expression has been documented in tumors as well as cell lines derived from carcinoma of breast, stomach, colon, cervix, cutaneous epithelia, plasma cells as well as from T-cell leukemia, neuroblastoma, teratocarcinoma, choriocarcinoma, and uveal and cutaneous melanoma [1, 2].
The findings we have discussed above suggest that the manner in which investigators have depicted the HLA antigen status of malignant cells in the past may not reflect the true biology of HLA antigen expression. Whether this premise may also be applied to non-classical HLA class I antigen and NKCAL expression remains to be determined since this field is in an early stage. Nonetheless, an alternative view of the HLA antigen and NKCAL status may be that in tumors, like in cases of infection [3–5, 63–68], HLA antigen and/or NKCAL expression is upregulated and/or lost depending on the nature of the host’s immune response. In this regard, intense selective pressure acting on malignant cells is likely to evoke (via mutation and selection) a wide range of defensive strategies, including changes in HLA antigen and NKCAL expression, which enable them to survive immunological attack. In the following sections, we will review the available evidence for the role of immune selective pressure in the generation of the HLA antigen and NKCAL phenotype of tumor cells.
Role of immune selective pressure in the HLA antigen and NKCAL phenotype of malignant cells
Cancer immune surveillance
In the last decade, the cancer immune surveillance theory has been revived by countless studies in mice supporting the notion that both CD8(+) and CD4(+) T cells and NK cells are engaged in the control of tumor cell growth [4, 6, 60, 69–73]. Specifically, frequencies of spontaneously arising tumors or tumors induced by the chemical carcinogen methylcholanthrene (MCA) are higher in mice that are genetically deficient for key effector molecules of T and/or NK cells or their respective receptors, i.e., IFN-γ R1−/−[74], IFN-γ−/− [75], perforin (pfp)−/− [75–78], RAG-2−/−[79, 80], JAK2 [81, 82], ABL [79–81], STAT1−/− [74, 80], TCRJα281−/− [80, 83], TCRβ−/− [80, 84], and TCRδ−/−[80, 83]. Along similar lines, treatment of mice with MCA induces some cancers of the occult type that grow out only in the presence of concomitant immunosuppression [85]. Additional studies have provided evidence that the anti-tumor activities of NK cells may not only directly lead to tumor eradication by means of cytolysis or IFN-γ secretion but may also indirectly contribute to tumor control by inducing an efficient TA-specific immune response [86]. In humans, evidence for a role of the immune system in preventing tumor growth is derived from correlative studies in patients with solid organ and bone marrow transplants, HIV/AIDS, and primary immune deficiencies. These studies have shown that in these patients, the incidence of a variety of malignancies originating from brain, thyroid, breast, colon, liver, pancreas, kidney, prostate, cervix, bone, connective tissue as well as cutaneous squamous cell carcinoma, basal cell carcinoma, melanoma, Kaposi’s sarcoma, and hematologic malignancies is increased [87–106]. These correlative studies have been further supported by the association between infiltration of tumors with T or NK cells and positive prognosis in patients with different types of malignancies [107–112]. More recently, convincing evidence for a beneficial role of NK cells in control of human malignancies comes from the association between (1) low NK cell-like cytotoxicity of peripheral blood lymphocytes and an increased risk for cancer [108] and (2) leukemia patients receiving alloreactive NK cells in the course of allogeneic hematopoietic stem cell transplantation and an increase in disease-free relapse and survival [113, 114].
Immune selective pressure
Given the mounting evidence of cancer immune surveillance along with the observation that dysplastic and malignant cells can acquire classical HLA class I antigen and/or lose HLA class II antigen expression, it is likely that the actual status of HLA antigen as well as NKCAL expression on malignant cells reflects the complex interplay between tumor microenvironment, host’s immune system, and tumor cells. In this regard, it is assumed that tumors arise from a single normal cell by a series of cumulative genetic and epigenetic changes through a sequential evolutionary process of mutation and selection. Malignant cells within a tumor may harbor different mutations in a number of critical genes at various stages during the evolution of the tumor, providing some malignant cells with a selective advantage [115, 116]. Although genetic and epigenetic alterations drive cellular transformation, multiple signals delivered within the microenvironment through the release of intracellular components termed DAMPs (damage-associated molecular patterns) from damaged or dying malignant cells [117–120] as well as the release of soluble factors by stromal, endothelial, and immune cells are critical factors in determining the progression versus dormancy or destruction of a dysplastic or malignant cell. Therefore, this complex interplay between tumor cell heterogeneity and tumor microenvironment not only determines but also shapes the phenotype of malignant cells towards generation of mutant resistant variants [121, 122]. To this end, tumor evolution is thought to adhere to Darwinian principles by escaping both non-immune (intrinsic) and immune (extrinsic) responses against self-altered malignant cells.
The notion that the type of host immune response elicited by a tumor may determine the HLA antigen or NKCAL phenotype of a malignant cell is not novel. During the 1920s, Little and Snell [123, 124] demonstrated selection of tumor cell variants with MHC class I antigen loss in inbred mice. Moreover, metastatic tumor variants derived from transplants into normal mice regularly lose MHC class I antigen expression, while cells from similar transplants into immunocompromised (athymic nude) mice do not [125]. More recently, Harding’s group has shown that immune selective pressure targeting a H2-restricted cytotoxic T lymphocyte (CTL) epitope can isolate tumor cells lacking the targeted epitope by failing to express the MHC-anchored peptide from a tumor cell population [126]. Specifically, using matched panels of TAP1(+) and TAP1 (−) tumor cell lines generated from a parental transformed murine fibroblast line, Harding and co-workers demonstrated that both TAP1(+) and TAP1(−) cells produce tumors in athymic mice, while only TAP1(−) cells form persistent tumors in the immunocompetent autologous C57BL/6 mice [126]. Moreover, inoculation of C57BL/6 mice with mixtures of TAP1(+) and TAP1(−) cells produced tumors composed exclusively of TAP1(−) [126]. These data suggest that the selection pressure applied by MHC class I antigen-restricted, antigen-specific CTLs favors the outgrowth of cells with defective presentation of antigen-derived peptides because of TAP defects. In other words, the tumor’s MHC phenotype has been “immunoedited” in the course of the disease, resulting in the survival of tumor variants with defective presentation of antigen-derived peptides by MHC class I antigens. Along similar lines, both we [Ferrone unpublished] and others [127] have observed that adoptive transfer of antigen-specific CTL to SCID mice implanted with autologous melanoma cells leads to immunoselection of HLA class I antigen and TA loss variants. In parallel with HLA antigens, it is worth noting that there is evidence to suggest that NKCAL may also be a target of immune selection. In this regard, a higher frequency of NKCAL MICA/B loss has been observed in metastatic melanoma lesions than in primary lesions [128]. Further, experimental data in vitro have shown that MICA loss was associated with resistance to NK cell-mediated lysis of two human melanoma cell lines isolated from recurrent metastases in spite of lack of HLA class I antigen expression [129, 130].
The results obtained in the aforementioned animal studies are paralleled by those obtained in clinical settings. Jager et al. [131] have demonstrated total HLA class I antigen loss and selective HLA-A2 antigen loss in three of five and one of five, respectively, melanoma lesions which progressed in spite of the expression of the targeted TA and of the presence of a TA-specific T-cell response in patients immunized with MART-1- and tyrosinase-derived, HLA-A2-binding peptides. Similar results have been described by Khong et al. [132] who have characterized melanoma metastases that recurred after an initial dramatic clinical response in a patient immunized with gp100-, MART-1-, and tyrosinase-derived peptides. T-cell clones specific to these TA were present in the patient’s peripheral blood as well as in the isolated tumor-infiltrating lymphocytes (TILs). In one recurrent melanoma metastasis, multiple TA had been lost, but HLA class I antigen expression was retained [132]. In another recurrent metastasis, HLA class I antigens were not detectable, while TA continued to be expressed [132]. Restifo et al. [133] have described total HLA class I antigen loss in recurrent melanoma metastases in five patients who experienced an initial clinical response following T-cell-based immunotherapy. Rosenberg’s group have demonstrated loss of TA as well as HLA class I antigens in patients treated with TA-peptide- [132] and autologous cell transfer-based [134] therapies. More recently, Garrido et al. have demonstrated an increased incidence of alterations in HLA class I antigen expression in patients with recurrent bladder cancer treated with the Bacillus Calmett–Guerin (BCG) immunotherapy, whereas mitomycin treatment did not change the pattern of HLA class I antigen expression [135]. It is noteworthy that a number of molecular mechanisms have been found to underlie defects in HLA class I antigen expression [1]. Among them is loss of HLA class I antigen expression by tumor cells due to mutations in one copy of the beta-2-microglobulin (β2m) gene associated with loss of the other copy [1, 132, 136]. These findings reflect the critical role β2m plays in the expression of classical HLA class I antigens on the cell membrane [137]. Additional studies have revealed a mutational hotspot in the β2m gene that may be associated with immune selective pressure introduced by T-cell-based immunotherapy [136]. The presence of this mutational hotspot implies a relationship between the modified tumor microenvironment during immunotherapy and the type of genomic instability and/or DNA repair capacity possessed by tumor cells. The latter relationship is further supported by an elevated frequency of β2m gene CT dinucleotide deletion mutations identified in MSI (+) colon carcinoma lesions [61, 138]. The β2m gene mutations may be preferentially selected by T cell selective pressure. In this regard, the impairment of HLA class I antigen expression is a frequent even in MSI (+) colon carcinoma [140] and predominantly mediated by frameshift mutations of the β2m gene [137, 141, 142] likely reflecting immunoselective pressure. In view of these findings, the outgrowth of MSI (+) colon carcinoma cells that lack HLA class I as well as class II antigen expression is compatible with the selection of HLA class I and class II antigen-deficient tumor cells during MSI (+) colon carcinoma tumorigenesis. Although the exact mechanisms of such a selection process are not known at present, it is tempting to speculate that tumor-infiltrating CD8(+) as well as CD4(+) T cells recognize MSI (+) colon carcinoma cells that expression TA-derived peptides on the cell surface via HLA class I and class II antigens. This hypothesis is in line with the observation that MSI (+) tumors are usually infiltrated with large numbers of activated CTLs presumably recognizing neoepitopes generated by the tumor’s genomic mutator phenotype [61, 138, 139] and a recent report of a HLA-DR-restricted CD4(+) T-cell response in a MSI (+) colon carcinoma patient [143].
Conclusion
The results we have summarized demonstrate that dysplastic and malignant cells can acquire classical HLA class I antigen expression and/or lose the ability to express HLA class II antigens. Given the mounting evidence that immune selection most likely underlies the generation of immunoresistant tumor variants, our past assumptions regarding HLA antigen as well as NKCAL expressions in malignant lesions may not be accurate. It seems more likely that the status of HLA antigen as well as NKCAL expression on malignant cells reflects the complex interplay between tumor microenvironment, host’s immune system, and tumor cells. Therefore, it is our belief that more appropriately defining the HLA antigen and NKCAL phenotype of malignant cells may provide insights into the actual type of immune response generated as well as potential mechanism of immune selection in different malignancies. Previous assessments of the status of HLA antigen and NKCAL expression on tumor cells most likely do not give the “whole” picture since these studies only provide a static account of the level of expression of these molecules. From a clinical standpoint, this static picture most likely clouds our ability to determine the true clinical relevance of HLA antigen as well as NKCAL expression on tumor cells since their level is likely to change throughout the course of the disease.
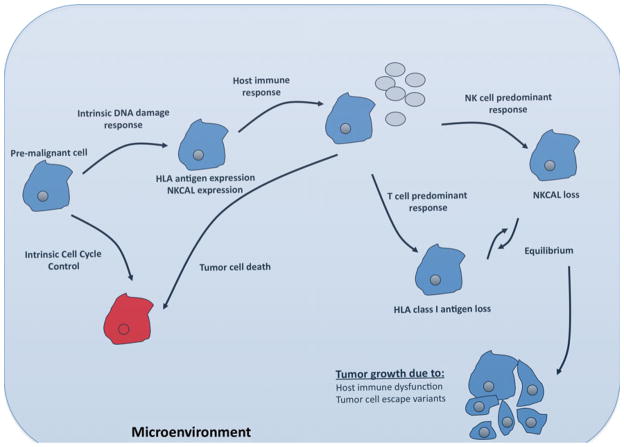
On the basis of the aforementioned experimental results and clinical data, immune selection may be viewed as conceptually equivalent to the theory of evolution proposed by Darwin more than a century ago [144, 145] (Fig. 5). During an early stage of tumor development, HLA antigen or NKCAL expression on pre-malignant or malignant cells may be increased to reflect the genetic instability of these cells and the activation of the intrinsic DNA damage response pathways [3–5]. At this stage, whether the immune system is activated most likely depends on the nature of the tumor microenvironment. In this regard, the tumor microenvironment can have potential positive or negative impacts on the ability of the host’s immune system to recognize tumor cells and to control tumor growth [146]. If a favorable tumor microenvironment exists, the net change at this stage is a shift in the “immune balance” toward activation and immune surveillance is initiated. Since HLA class I antigens play a crucial role in the control of tumor growth by CTLs in the tumor microenvironment, HLA antigen abnormalities in tumor cells may be envisioned as the result of immune selection advantageous to tumor cell survival in situations where T cells play a major role in controlling tumor growth. On the other hand, because the activity of NK cells is inhibited by MHC class I molecules [3–5], in situations where NK cells provide the major source of selective pressure, HLA antigen expression may be indeed advantageous to tumor survival. This possibility is supported by the high HLA class I antigen expression level in uveal melanoma [1, 2], carcinomas of the breast [147], lung [148] and liver [1, 2] as well as leukemia [1, 2] and lymphoma [1, 2] where NK cells are thought to control tumor progression. Nonetheless, it must be stressed that NKCAL expression is not always beneficial to immune surveillance. In this regard, non-classical HLA antigen expression by tumor cells may induce apoptosis of NK cells upon ligation of activating receptors via a Fas–FasL-dependent mechanism [1, 2, 149]. Moreover, sustained membrane-bound as well as soluble NKG2D ligand expression in vivo, either systemically or locally, actually impairs NKG2D-mediated immune control of tumor growth by downregulating the NKG2D receptor and inhibition of NK-cell-mediated killing [149]. At a later stage in tumor development, the tumor site is most likely dominated by escape variants expressing low levels of HLA antigens and medium to low levels of NKCAL on the plasma membrane, but shedding and/or secreting large amounts of soluble NKCAL or non-classical HLA class I molecules. The net influence in this period is sustained NKCAL receptor downregulation, resulting in impaired NK cell control of tumor growth, and inability of CD4(+) or CD8(+) T cells to recognize and destroy tumor cells.
Fig. 5.
Immunoselection and microevolution of tumors. Tumor evolution is thought to adhere to Darwinian principles by escaping both non-immune (intrinsic) and immune (extrinsic) responses against self-altered malignant cells. Pre-malignant cells that acquire genetic mutations may undergo apoptosis or in parallel, acquire the expression of HLA as well as NKCAL through intrinsic cell-cycle control or DNA damage response mechanisms. Those cells resistant to apoptosis may be targeted by the host’s immune system. The type of immune response elicited by each tumor is most likely dependent upon a number of factors including the etiology of the tumor, patient characteristics as well as tumor microenvironment. During this immune response, the selective pressure facilitates the outgrowth of tumor cells that have lost the molecule(s) targeted by the ongoing immune response. Equilibrium most likely develops between the tumor cell escape variants and the adapting host’s immune response. At some point, the immune response is unable to adapt to the changing tumor cell population resulting in tumor growth. It is noteworthy that a cause–effect relationship between multiple rounds of immune selection and the appearance of multiple HLA or NKCAL abnormalities has not been proved yet. Nevertheless, if correct, our view about the role of immune selection in the generation of a malignant cell phenotype implies that a tumor will grow only when it has developed enough escape mechanisms to avoid the range of immune responses a patient’s immune system is able to mount
The challenge for the tumor immunologists now is to more appropriately characterize HLA antigen and NKCAL expression on benign, dysplastic, and malignant cells in order to understand the mechanisms by which tumors become refractory to the immune system. The mechanisms underlying the ability of cells to up- or downregulate HLA antigen and/or NKCAL expression are most likely due to multiple variables. Changes in HLA antigen expression have been attributed to defects in β2m synthesis, loss of the gene(s) encoding HLA antigen heavy chain(s), mutations which inhibit HLA antigen heavy chain transcription or translation, defects in the regulatory mechanisms which control HLA antigen expression, and/or abnormalities in one or more of the antigen processing machinery components [1, 2]. More recently, epigenetic events associated with tumor development and progression have been found to underlie changes in HLA antigen, antigen processing machinery, co-stimulatory molecule, and TA expression in malignant cells [2]. In this regard, the ability of epigenetic drugs to restore the defective HLA antigen, APM component and co-stimulatory molecule expression, and the consequent increase in immune recognition of malignant cells provides us with new therapeutic tools that may improve the clinical efficacy of active-specific immuno-therapy for the treatment of malignant disease. Nonetheless, to date very little attention has been focused on the regulation of HLA antigens in malignant lesions following treatment with DNA methyltransferases (DNMT) and histone deacetylases (HDAC) inhibitors. Future studies aimed at identifying the cell-type-specific molecular mechanisms underlying the transcriptional and post-transcriptional regulation of HLA antigen and NKCAL in benign and malignant cells should be undertaken. Moreover, investigations should be directed at determining the ability of epigenetic pharmacologic agents to modulate HLA and NKCAL expression in malignant lesions. In addition, investigations need to be tailored towards characterizing the interplay between tumor microenvironment and immune effector cells. This information may suggest strategies to overcome the barriers posed by the microenvironment to an effective destruction of tumor cells mediated by immunological mechanisms. Lastly, as investigators, we must keep in mind that the status of HLA antigen and NKCAL expression on malignant cells is not static. The levels of these antigens most likely fluctuate throughout the course of a patient’s immune response. Studies should be directed towards characterizing not only the status of HLA antigen and NKCAL expression on malignant cells but also the nature of TA-specific immune responses in patients at different stages of tumor development. A better understanding of the mechanisms influencing the status of HLA antigen and NKCAL expression in malignant cells will likely translate into the optimization of the design of immuno-preventative as well as immuno-therapeutic strategies for the treatment of cancer. Moreover, the combination of immunization strategies with approaches that counteract changes in HLA antigen as well as NKCAL expression may enhance the clinical efficacy of immunotherapy.
Acknowledgments
This work was supported by an ASDS Cutting Edge Research Grant (MC) and by PHS grants PO1CA109688 (SF), RO1CA104947(SF), and RO1CA110249(SF), awarded by the National Cancer Institute.
Footnotes
This article is published as part of the Special Issue on Prognostic Impact of Anti-Cancer Immune Responses [33:5]
Contributor Information
Michael Campoli, Email: ferrones@upmc.edu, University of Pittsburgh Medical Center, Pittsburgh, PA, USA. University of Pittsburgh Cancer Institute, 5200 Centre Avenue suite 303, Pittsburgh, PA 15232, USA.
Soldano Ferrone, Department of Surgery, School of Medicine, University of Pittsburgh, Pittsburgh, PA 15232, USA. Department of Immunology, School of Medicine, University of Pittsburgh, Pittsburgh, PA 15232, USA. Department of Pathology, School of Medicine, University of Pittsburgh, Pittsburgh, PA 15232, USA. Cancer Immunology Program, School of Medicine, University of Pittsburgh, Pittsburgh, PA 15232, USA.
References
- 1.Chang CC, Campoli M, Ferrone S. Classical and nonclassical HLA class I antigen and NK cell-activating ligand changes in malignant cells: current challenges and future directions. Adv Cancer Res. 2005;93:189–234. doi: 10.1016/S0065-230X(05)93006-6. [DOI] [PubMed] [Google Scholar]
- 2.Campoli M, Ferrone S. HLA antigen changes in malignant cells: epigenetic mechanisms and biologic significance. Oncogene. 2008;27:5869–5885. doi: 10.1038/onc.2008.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nausch N, Cerwenka A. NKG2D ligands in tumor immunity. Oncogene. 2008;27:5944–5958. doi: 10.1038/onc.2008.272. [DOI] [PubMed] [Google Scholar]
- 4.Waldhauer I, Steinle A. NK cells and cancer immuno-surveillance. Oncogene. 2008;27:5932–5943. doi: 10.1038/onc.2008.267. [DOI] [PubMed] [Google Scholar]
- 5.Champsaur M, Lanier LL. Effect of NKG2D ligand expression on host immune responses. Immunol Rev. 2010;235:267–285. doi: 10.1111/j.0105-2896.2010.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol. 2003;21:807–839. doi: 10.1146/annurev.immunol.21.120601.141135. [DOI] [PubMed] [Google Scholar]
- 7.Kindt T, Osborne B, Goldsby R. Kuby immunology. 6. Freeman; San Francisco: 2006. [Google Scholar]
- 8.Murphy KM, Travers P, Walport M. Janeway’s immunobiology—immunobiology: the immune system Janeway. Garland Science; London: 2007. [Google Scholar]
- 9.Drozina G, Kohoutek J, Jabrane-Ferrat N, Peterlin BM. Expression of MHC II genes. Curr Top Microbiol Immunol. 2005;290:147–170. doi: 10.1007/3-540-26363-2_7. [DOI] [PubMed] [Google Scholar]
- 10.Bui JD, Schreiber RD. Cancer immunosurveillance, immunoediting and inflammation: independent or interdependent processes? Curr Opin Immunol. 2007;19:203–208. doi: 10.1016/j.coi.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Rouas-Freiss N, Moreau P, Menier C, LeMaoult J, Carosella ED. Expression of tolerogenic HLA-G molecules in cancer prevents antitumor responses. Semin Cancer Biol. 2007;17:413–421. doi: 10.1016/j.semcancer.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Urosevic M, Dummer R. Human leukocyte antigen-G and cancer immunoediting. Cancer Res. 2008;68:627–630. doi: 10.1158/0008-5472.CAN-07-2704. [DOI] [PubMed] [Google Scholar]
- 13.Mittelbronn M, Simon P, Loffler C, et al. Elevated HLA-E levels in human glioblastomas but not in grade I to III astrocytomas correlate with infiltrating CD8+ cells. J Neuroimmunol. 2007;189:50–58. doi: 10.1016/j.jneuroim.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Wischhusen J, Waschbisch A, Wiendl H. Immune-refractory cancers and their little helpers—an extended role for immunetolerogenic MHC molecules HLA-G and HLA-E? Semin Cancer Biol. 2007;17:459–468. doi: 10.1016/j.semcancer.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Goncalves MA, Le Discorde M, Simoes RT, et al. Classical and non-classical HLA molecules and p16(INK4a) expression in precursors lesions and invasive cervical cancer. Eur J Obstet Gynecol Reprod Biol. 2008;141:70–74. doi: 10.1016/j.ejogrb.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 16.Levy EM, Bianchini M, Von Euw EM, et al. Human leukocyte antigen-E protein is overexpressed in primary human colorectal cancer. Int J Oncol. 2008;32:633–641. [PubMed] [Google Scholar]
- 17.Ito T, Ito N, Saathoff M, et al. Immunology of the human nail apparatus: the nail matrix is a site of relative immune privilege. J Invest Dermatol. 2005;125:1139–1148. doi: 10.1111/j.0022-202X.2005.23927.x. [DOI] [PubMed] [Google Scholar]
- 18.Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix CC. CNS immune privilege: hiding in plain sight. Immunol Rev. 2006;213:48–65. doi: 10.1111/j.1600-065X.2006.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crispe IN, Giannandrea M, Klein I, John B, Sampson B, Wuensch S. Cellular and molecular mechanisms of liver tolerance. Immunol Rev. 2006;213:101–118. doi: 10.1111/j.1600-065X.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 20.Arck PC, Gilhar A, Bienenstock J, Paus R. The alchemy of immune privilege explored from a neuroimmunological perspective. Curr Opin Pharmacol. 2008;8:480–489. doi: 10.1016/j.coph.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Ito T, Meyer KC, Ito N, Paus R. Immune privilege and the skin. Curr Dir Autoimmun. 2008;10:27–52. doi: 10.1159/000131412. [DOI] [PubMed] [Google Scholar]
- 22.Mellor AL, Munn DH. Creating immune privilege: active local suppression that benefits friends, but protects foes. Nat Rev Immunol. 2008;8:74–80. doi: 10.1038/nri2233. [DOI] [PubMed] [Google Scholar]
- 23.Gilhar A. Collapse of immune privilege in alopecia areata: coincidental or substantial? J Invest Dermatol. 2010;130:2535–2537. doi: 10.1038/jid.2010.260. [DOI] [PubMed] [Google Scholar]
- 24.McKenna KC, Chen PW. Influence of immune privilege on ocular tumor development. Ocul Immunol Inflamm. 2010;18:80–90. doi: 10.3109/09273941003669950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fijak M, Bhushan S, Meinhardt A. Immunoprivileged sites: the testis. Meth Mol Biol. 2011;677:459–470. doi: 10.1007/978-1-60761-869-0_29. [DOI] [PubMed] [Google Scholar]
- 26.Ruiter DJ, Bhan AK, Harrist TJ, Sober AJ, Mihm MC., Jr Major histocompatibility antigens and mononuclear inflammatory infiltrate in benign nevomelanocytic proliferations and malignant melanoma. J Immunol. 1982;129:2808–2815. [PubMed] [Google Scholar]
- 27.Houghton AN, Thomson TM, Gross D, Oettgen HF, Old LJ. Surface antigens of melanoma and melanocytes. Specificity of induction of Ia antigens by human gamma-interferon. J Exp Med. 1984;160:255–269. doi: 10.1084/jem.160.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Natali PG, Bigotti A, Cavaliere R, Nicotra MR, Ferrone S. Phenotyping of lesions of melanocyte origin with monoclonal antibodies to melanoma-associated antigens and to HLA antigens. J Natl Cancer Inst. 1984;73:13–24. [PubMed] [Google Scholar]
- 29.Natali PG, Bigotti A, Nicotra MR, Viora M, Manfredi D, Ferrone S. Distribution of human Class I (HLA-A, B, C) histocompatibility antigens in normal and malignant tissues of nonlymphoid origin. Cancer Res. 1984;44:4679–4687. [PubMed] [Google Scholar]
- 30.Aubock J, Niederwieser D, Romani N, Fritsch P, Huber C. Human interferon-gamma induces expression of HLA-DR on keratinocytes and melanocytes. Arch Dermatol Res. 1985;277:270–275. doi: 10.1007/BF00509079. [DOI] [PubMed] [Google Scholar]
- 31.Bergman W, Willemze R, de Graaff-Reitsma C, Ruiter DJ. Analysis of major histocompatibility antigens and the mononuclear cell infiltrate in halo nevi. J Invest Dermatol. 1985;85:25–29. doi: 10.1111/1523-1747.ep12274521. [DOI] [PubMed] [Google Scholar]
- 32.Herlyn M, Guerry D, Koprowski H. Recombinant gamma-interferon induces changes in expression and shedding of antigens associated with normal human melanocytes, nevus cells, and primary and metastatic melanoma cells. J Immunol. 1985;134:4226–4230. [PubMed] [Google Scholar]
- 33.Real FX, Houghton AN, Albino AP, et al. Surface antigens of melanomas and melanocytes defined by mouse monoclonal antibodies: specificity analysis and comparison of antigen expression in cultured cells and tissues. Cancer Res. 1985;45:4401–4411. [PubMed] [Google Scholar]
- 34.Herlyn M, Rodeck U, Mancianti M, et al. Expression of melanoma-associated antigens in rapidly dividing human melanocytes in culture. Cancer Res. 1987;47:3057–3061. [PubMed] [Google Scholar]
- 35.Bergman W, Ruiter DJ, Scheffer E, van Vloten WA. Melanocytic atypia in dysplastic nevi. Immunohistochemical and cytophotometrical analysis. Cancer. 1988;61:1660–1666. doi: 10.1002/1097-0142(19880415)61:8<1660::aid-cncr2820610825>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 36.Mancianti ML, Herlyn M, Weil D, et al. Growth and phenotypic characteristics of human nevus cells in culture. J Invest Dermatol. 1988;90:134–141. doi: 10.1111/1523-1747.ep12462099. [DOI] [PubMed] [Google Scholar]
- 37.Elder DE, Rodeck U, Thurin J, et al. Antigenic profile of tumor progression stages in human melanocytic nevi and melanomas. Cancer Res. 1989;49:5091–5096. [PubMed] [Google Scholar]
- 38.David-Watine B, Israël A, Kourilsky P. The regulation and expression of MHC class I genes. Immunol Today. 1990;11:286–292. doi: 10.1016/0167-5699(90)90114-o. [DOI] [PubMed] [Google Scholar]
- 39.Campoli M, Fitzpatrick J, High W, Ferrone S. HLA antigen expression in benign melanocytic lesions: is acquisition of HLA antigen expression a biomarker of atypical (dysplastic) melanocytes? J Am Acad Dermatol. 2001 doi: 10.1016/j.jaad.2011.04.025. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsujisaki M, Igarashi M, Sakaguchi K, Eisinger M, Herlyn M, Ferrone S. Immunochemical and functional analysis of HLA class II antigens induced by recombinant immune interferon on normal epidermal melanocytes. J Immunol. 1987;138:1310–1316. [PubMed] [Google Scholar]
- 41.Marsman M, Jordens I, Griekspoor A, Neefjes J. Chaperoning antigen presentation by MHC class II molecules and their role in oncogenesis. Adv Cancer Res. 2005;93:129–158. doi: 10.1016/S0065-230X(05)93004-2. [DOI] [PubMed] [Google Scholar]
- 42.Natali PG, De Martino C, Quaranta V, et al. Expression of Ia-like antigens in normal human nonlymphoid tissues. Transplantation. 1981;31:75–78. doi: 10.1097/00007890-198101000-00017. [DOI] [PubMed] [Google Scholar]
- 43.Natali PG, Segatto O, Ferrone S, Tosi R, Corte G. Differential tissue distribution and ontogeny of DC-1 and HLA-DR antigens. Immunogenetics. 1984;19:109–116. doi: 10.1007/BF00387853. [DOI] [PubMed] [Google Scholar]
- 44.Steinhoff G. Major histocompatibility complex antigens in human liver transplants. J Hepatol. 1990;11:9–15. doi: 10.1016/0168-8278(90)90264-r. [DOI] [PubMed] [Google Scholar]
- 45.Brand DD, Kang AH, Rosloniec EF. Immunopathogenesis of collagen arthritis. Springer Semin Immunopathol. 2003;25:3–18. doi: 10.1007/s00281-003-0127-1. [DOI] [PubMed] [Google Scholar]
- 46.Kalish RS, Gilhar A. Alopecia areata: autoimmunity—the evidence is compelling. J Investig Dermatol Symp Proc. 2003;8:164–167. doi: 10.1046/j.1087-0024.2003.00802.x. [DOI] [PubMed] [Google Scholar]
- 47.Medina J, Garcia-Buey L, Moreno-Otero R. Review article: immunopathogenetic and therapeutic aspects of autoimmune hepatitis. Aliment Pharmacol Ther. 2003;17:1–16. doi: 10.1046/j.1365-2036.2003.01389.x. [DOI] [PubMed] [Google Scholar]
- 48.Mok CC, Lau CS. Pathogenesis of systemic lupus erythematosus. J Clin Pathol. 2003;56:481–490. doi: 10.1136/jcp.56.7.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chinoy H, Ollier WE, Cooper RG. Have recent immunogenetic investigations increased our understanding of disease mechanisms in the idiopathic inflammatory myopathies? Curr Opin Rheumatol. 2004;16:707–713. doi: 10.1097/01.bor.0000142339.24380.b7. [DOI] [PubMed] [Google Scholar]
- 50.Turesson C. Endothelial expression of MHC class II molecules in autoimmune disease. Curr Pharm Des. 2004;10:129–143. doi: 10.2174/1381612043453414. [DOI] [PubMed] [Google Scholar]
- 51.Goronzy JJ, Weyand CM. Rheumatoid arthritis. Immunol Rev. 2005;204:55–73. doi: 10.1111/j.0105-2896.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- 52.Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370:263–271. doi: 10.1016/S0140-6736(07)61128-3. [DOI] [PubMed] [Google Scholar]
- 53.Longhi MS, Ma Y, Mieli-Vergani G, Vergani D. Adaptive immunity in autoimmune hepatitis. Dig Dis. 2010;28:63–69. doi: 10.1159/000282066. [DOI] [PubMed] [Google Scholar]
- 54.Zeff RA, Freitag A, Grin CM, Grant-Kels JM. The immune response in halo nevi. J Am Acad Dermatol. 1997;37:620–624. doi: 10.1016/s0190-9622(97)70181-6. [DOI] [PubMed] [Google Scholar]
- 55.Shors AR, Kim S, White E, Argenyi Z, Barnhill RL, Duray P, et al. Dysplastic naevi with moderate to severe histological dysplasia: a risk factor for melanoma. Br J Dermatol. 2006;155:988–993. doi: 10.1111/j.1365-2133.2006.07466.x. [DOI] [PubMed] [Google Scholar]
- 56.Arumi-Uria M. Dysplastic nevus: the eye of the hurricane. J Cutan Pathol. 2008;35(Suppl 2):S16–S19. doi: 10.1111/j.1600-0560.2008.01142.x. [DOI] [PubMed] [Google Scholar]
- 57.Baranda L, Torres-Alvarez B, Moncada B, et al. Presence of activated lymphocytes in the peripheral blood of patients with halo nevi. J Am Acad Dermatol. 1999;41:567–572. doi: 10.1016/s0190-9622(99)80054-1. [DOI] [PubMed] [Google Scholar]
- 58.Chaux P, Vantomme V, Stroobant V, et al. Identification of MAGE-3 epitopes presented by HLA-DR molecules to CD4(+) T lymphocytes. J Exp Med. 1999;189:767–778. doi: 10.1084/jem.189.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang RF, Wang X, Rosenberg SA. Identification of a novel major histocompatibility complex class II-restricted tumor antigen resulting from a chromosomal rearrangement recognized by CD4(+) T cells. J Exp Med. 1999;189:1659–1668. doi: 10.1084/jem.189.10.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martorelli D, Muraro E, Merlo A, Turrini R, Rosato A, Dolcetti R. Role of CD4+ cytotoxic T lymphocytes in the control of viral diseases and cancer. Int Rev Immunol. 2010;29:371–402. doi: 10.3109/08830185.2010.489658. [DOI] [PubMed] [Google Scholar]
- 61.Michel S, Linnebacher M, Alcaniz J, et al. Lack of HLA class II antigen expression in microsatellite unstable colorectal carcinomas is caused by mutations in HLA class II regulatory genes. Int J Cancer. 2010;127:889–898. doi: 10.1002/ijc.25106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Steidl C, Shah SP, Woolcock BW, et al. MHC class II transactivator CIITA is a recurrent gene fusion partner in lymphoid cancers. Nature. 2011;471:377–381. doi: 10.1038/nature09754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guardiola J, Maffei A. Control of MHC class II gene expression in autoimmune, infectious, and neoplastic diseases. Crit Rev Immunol. 1993;13:247–268. [PubMed] [Google Scholar]
- 64.Girdlestone J. Transcriptional regulation of MHC class I genes. Eur J Immunogenet. 1996;23:395–413. doi: 10.1111/j.1744-313x.1996.tb00015.x. [DOI] [PubMed] [Google Scholar]
- 65.Zhou F. Molecular mechanisms of IFN-gamma to up-regulate MHC class I antigen processing and presentation. Int Rev Immunol. 2009;28:239–260. doi: 10.1080/08830180902978120. [DOI] [PubMed] [Google Scholar]
- 66.Lutteke N, Raftery MJ, Lalwani P, et al. Switch to high-level virus replication and HLA class I upregulation in differentiating megakaryocytic cells after infection with pathogenic hantavirus. Virology. 2010;405:70–80. doi: 10.1016/j.virol.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 67.Meissner TB, Li A, Biswas A, et al. NLR family member NLRC5 is a transcriptional regulator of MHC class I genes. Proc Natl Acad Sci USA. 2010;107:13794–13799. doi: 10.1073/pnas.1008684107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rayamajhi M, Humann J, Kearney S, Hill KK, Lenz LL. Antagonistic crosstalk between type I and II interferons and increased host susceptibility to bacterial infections. Virulence. 2010;1:421–425. doi: 10.4161/viru.1.5.12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pardoll DM, Topalian SL. The role of CD4+ T cell responses in antitumor immunity. Curr Opin Immunol. 1998;10:588–594. doi: 10.1016/s0952-7915(98)80228-8. [DOI] [PubMed] [Google Scholar]
- 70.Mumberg D, Monach PA, Wanderling S, et al. CD4(+) T cells eliminate MHC class II-negative cancer cells in vivo by indirect effects of IFN-gamma. Proc Natl Acad Sci USA. 1999;96:8633–8638. doi: 10.1073/pnas.96.15.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Antony PA, Piccirillo CA, Akpinarli A, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Corthay A, Skovseth DK, Lundin KU, et al. Primary antitumor immune response mediated by CD4+ T cells. Immunity. 2005;22:371–383. doi: 10.1016/j.immuni.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 73.Perez-Diez A, Joncker NT, Choi K, et al. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood. 2007;109:5346–5354. doi: 10.1182/blood-2006-10-051318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kaplan DH, Shankaran V, Dighe AS, et al. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci USA. 1998;95:7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Street SE, Cretney E, Smyth MJ. Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood. 2001;97:192–197. doi: 10.1182/blood.v97.1.192. [DOI] [PubMed] [Google Scholar]
- 76.van den Broek ME, Kagi D, Ossendorp F, et al. Decreased tumor surveillance in perforin-deficient mice. J Exp Med. 1996;184:1781–1790. doi: 10.1084/jem.184.5.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smyth MJ, Thia KY, Street SE, et al. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med. 2000;191:661–668. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smyth MJ, Thia KY, Street SE, MacGregor D, Godfrey DI, Trapani JA. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J Exp Med. 2000;192:755–760. doi: 10.1084/jem.192.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shankaran V, Ikeda H, Bruce AT, et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 80.Koebel CM, Vermi W, Swann JB, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450:903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 81.Tefferi A. JAK and MPL mutations in myeloid malignancies. Leuk Lymphoma. 2008;49:388–397. doi: 10.1080/10428190801895360. [DOI] [PubMed] [Google Scholar]
- 82.Schuetz C, Niehues T, Friedrich W, Schwarz K. Autoimmunity, autoinflammation and lymphoma in combined immunodeficiency (CID) Autoimmun Rev. 2010;9:477–482. doi: 10.1016/j.autrev.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 83.Smyth MJ, Crowe NY, Godfrey DI. NK cells and NKT cells collaborate in host protection from methylcholanthrene-induced fibrosarcoma. Int Immunol. 2001;13:459–463. doi: 10.1093/intimm/13.4.459. [DOI] [PubMed] [Google Scholar]
- 84.Girardi M, Oppenheim DE, Steele CR, et al. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294:605–609. doi: 10.1126/science.1063916. [DOI] [PubMed] [Google Scholar]
- 85.Murgia C, Pritchard JK, Kim SY, Fassati A, Weiss RA. Clonal origin and evolution of a transmissible cancer. Cell. 2006;126:477–487. doi: 10.1016/j.cell.2006.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smyth MJ, Swann J, Cretney E, Zerafa N, Yokoyama WM, Hayakawa Y. NKG2D function protects the host from tumor initiation. J Exp Med. 2005;202:583–588. doi: 10.1084/jem.20050994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kobayashi N. Malignant neoplasms in registered cases of primary immunodeficiency syndrome. Jpn J Clin Oncol. 1985;15 (Suppl 1):307–312. [PubMed] [Google Scholar]
- 88.Penn I. Tumors of the immunocompromised patient. Annu Rev Med. 1988;39:63–73. doi: 10.1146/annurev.me.39.020188.000431. [DOI] [PubMed] [Google Scholar]
- 89.Kasiske BL, Ramos EL, Gaston RS, et al. The evaluation of renal transplant candidates: clinical practice guidelines. Patient Care and Education Committee of the American Society of Transplant Physicians. J Am Soc Nephrol. 1995;6:1–34. doi: 10.1681/ASN.V611. [DOI] [PubMed] [Google Scholar]
- 90.Birkeland SA, Storm HH, Lamm LU, et al. Cancer risk after renal transplantation in the Nordic countries, 1964–1986. Int J Cancer. 1995;60:183–189. doi: 10.1002/ijc.2910600209. [DOI] [PubMed] [Google Scholar]
- 91.Penn I. Malignant melanoma in organ allograft recipients. Transplantation. 1996;61:274–278. doi: 10.1097/00007890-199601270-00019. [DOI] [PubMed] [Google Scholar]
- 92.Sheil AG, Disney AP, Mathew TH, Livingston BE, Keogh AM. Lymphoma incidence, cyclosporine, and the evolution and major impact of malignancy following organ transplantation. Transplant Proc. 1997;29:825–827. doi: 10.1016/s0041-1345(96)00151-0. [DOI] [PubMed] [Google Scholar]
- 93.Penn I. Posttransplant malignancies. Transplant Proc. 1999;31:1260–1262. doi: 10.1016/s0041-1345(98)01987-3. [DOI] [PubMed] [Google Scholar]
- 94.Nakachi K, Hayashi T, Imai K, Kusunoki Y. Perspectives on cancer immuno-epidemiology. Cancer Sci. 2004;95:921–929. doi: 10.1111/j.1349-7006.2004.tb03178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Buell JF, Gross TG, Woodle ES. Malignancy after transplantation. Transplantation. 2005;80(Suppl 2):S254–S264. doi: 10.1097/01.tp.0000186382.81130.ba. [DOI] [PubMed] [Google Scholar]
- 96.Clifford GM, Franceschi S. Cancer risk in HIV-infected persons: influence of CD4(+) count. Future Oncol. 2009;5:669–678. doi: 10.2217/fon.09.28. [DOI] [PubMed] [Google Scholar]
- 97.Kenkre VP, Stock W. Burkitt lymphoma/leukemia: improving prognosis. Clin Lymphoma Myeloma. 2009;9(Suppl 3):S231–S238. doi: 10.3816/CLM.2009.s.017. [DOI] [PubMed] [Google Scholar]
- 98.Martellotta F, Berretta M, Vaccher E, Schioppa O, Zanet E, Tirelli U. AIDS-related Kaposi’s sarcoma: state of the art and therapeutic strategies. Curr HIV Res. 2009;7:634–638. doi: 10.2174/157016209789973619. [DOI] [PubMed] [Google Scholar]
- 99.Gucalp A, Noy A. Spectrum of HIV lymphoma 2009. Curr Opin Hematol. 2009;17:362–367. doi: 10.1097/MOH.0b013e328338f6b6. [DOI] [PubMed] [Google Scholar]
- 100.DiNardo CD, Tsai DE. Treatment advances in posttransplant lymphoproliferative disease. Curr Opin Hematol. 2010;17:368–374. doi: 10.1097/MOH.0b013e328339018c. [DOI] [PubMed] [Google Scholar]
- 101.Jensen AO, Svaerke C, Farkas D, Pedersen L, Kragballe K, Sorensen HT. Skin cancer risk among solid organ recipients: a nationwide cohort study in Denmark. Acta Derm Venereol. 2010;90:474–479. doi: 10.2340/00015555-0919. [DOI] [PubMed] [Google Scholar]
- 102.Marquez C, Bair SM, Smithberger E, Cherpelis BS, Glass LF. Systemic retinoids for chemoprevention of non-melanoma skin cancer in high-risk patients. J Drugs Dermatol. 2010;9:753–758. [PubMed] [Google Scholar]
- 103.Mounier N, Spina M, Spano JP. Hodgkin lymphoma in HIV positive patients. Curr HIV Res. 2010;8:141–146. doi: 10.2174/157016210790442704. [DOI] [PubMed] [Google Scholar]
- 104.Rama I, Grinyo JM. Malignancy after renal transplantation: the role of immunosuppression. Nat Rev Nephrol. 2010;6:511–519. doi: 10.1038/nrneph.2010.102. [DOI] [PubMed] [Google Scholar]
- 105.Weinstock DM. Epstein–Barr virus, lymphoma risk and the potential role of HIV infection in IBD patients undergoing immunosuppression. Dig Dis. 2010;28:519–524. doi: 10.1159/000320411. [DOI] [PubMed] [Google Scholar]
- 106.Wieland E, Olbricht CJ, Susal C, et al. Biomarkers as a tool for management of immunosuppression in transplant patients. Ther Drug Monit. 2010;32:560–572. doi: 10.1097/FTD.0b013e3181efb3d2. [DOI] [PubMed] [Google Scholar]
- 107.Coca S, Perez-Piqueras J, Martinez D, et al. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer. 1997;79:2320–2328. doi: 10.1002/(sici)1097-0142(19970615)79:12<2320::aid-cncr5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 108.Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356:1795–1799. doi: 10.1016/S0140-6736(00)03231-1. [DOI] [PubMed] [Google Scholar]
- 109.Ishigami S, Natsugoe S, Hokita S, et al. Intranodal antitumor immunocyte infiltration in node-negative gastric cancers. Clin Cancer Res. 2000;6:2611–2617. [PubMed] [Google Scholar]
- 110.Ishigami S, Natsugoe S, Tokuda K, et al. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer. 2000;88:577–583. [PubMed] [Google Scholar]
- 111.Ishigami S, Natsugoe S, Tokuda K, et al. Clinical impact of intratumoral natural killer cell and dendritic cell infiltration in gastric cancer. Cancer Lett. 2000;159:103–108. doi: 10.1016/s0304-3835(00)00542-5. [DOI] [PubMed] [Google Scholar]
- 112.Villegas FR, Coca S, Villarrubia VG, et al. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer. 2002;35:23–28. doi: 10.1016/s0169-5002(01)00292-6. [DOI] [PubMed] [Google Scholar]
- 113.Hsu KC, Dupont B. Natural killer cell receptors: regulating innate immune responses to hematologic malignancy. Semin Hematol. 2005;42:91–103. doi: 10.1053/j.seminhematol.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 114.Ruggeri L, Mancusi A, Burchielli E, et al. NK cell alloreactivity and allogeneic hematopoietic stem cell transplantation. Blood Cells Mol Dis. 2008;40:84–90. doi: 10.1016/j.bcmd.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 115.Merlo LM, Pepper JW, Reid BJ, Maley CC. Cancer as an evolutionary and ecological process. Nat Rev Cancer. 2006;6:924–935. doi: 10.1038/nrc2013. [DOI] [PubMed] [Google Scholar]
- 116.Klein G, Imreh S, Zabarovsky ER. Why do we not all die of cancer at an early age? Adv Cancer Res. 2007;98:1–16. doi: 10.1016/S0065-230X(06)98001-4. [DOI] [PubMed] [Google Scholar]
- 117.Lotze MT, Zeh HJ, Rubartelli A, et al. The grateful dead: damage-associated molecular pattern molecules and reduction/ oxidation regulate immunity. Immunol Rev. 2007;220:60–81. doi: 10.1111/j.1600-065X.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 118.Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV, Agostinis P. Immunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation. Biochim Biophys Acta. 2010;1805:53–71. doi: 10.1016/j.bbcan.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 119.Piccinini AM, Midwood KS. DAMPening inflammation by modulating TLR signalling. Mediators Inflamm. 2010 doi: 10.1155/2010/672395. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annu Rev Immunol. 2010;28:367–388. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- 121.Malmberg KJ, Ljunggren HG. Escape from immune- and nonimmune-mediated tumor surveillance. Semin Cancer Biol. 2006;16:16–31. doi: 10.1016/j.semcancer.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 122.Ullrich E, Bonmort M, Mignot G, Kroemer G, Zitvogel L. Tumor stress, cell death and the ensuing immune response. Cell Death Differ. 2008;15:21–28. doi: 10.1038/sj.cdd.4402266. [DOI] [PubMed] [Google Scholar]
- 123.Snell GD. The genetics of transplantation. J Natl Cancer Inst. 1953;14:691–700. discussion, 1–4. [PubMed] [Google Scholar]
- 124.Klein E, Klein G, Revesz L. Permanent modification (mutation?) of a histocompatibility gene in a heterozygous tumor. J Natl Cancer Inst. 1957;19:95–114. [PubMed] [Google Scholar]
- 125.Garcia-Lora A, Martinez M, Algarra I, Gaforio JJ, Garrido F. MHC class I-deficient metastatic tumor variants immunoselected by T lymphocytes originate from the coordinated downregulation of APM components. Int J Cancer. 2003;106:521–527. doi: 10.1002/ijc.11241. [DOI] [PubMed] [Google Scholar]
- 126.Johnsen AK, Templeton DJ, Sy M, Harding CV. Deficiency of transporter for antigen presentation (TAP) in tumor cells allows evasion of immune surveillance and increases tumorigenesis. J Immunol. 1999;163:4224–4231. [PubMed] [Google Scholar]
- 127.Lozupone F, Rivoltini L, Luciani F, et al. Adoptive transfer of an anti-MART-1(27–35)-specific CD8+ T cell clone leads to immunoselection of human melanoma antigen-loss variants in SCID mice. Eur J Immunol. 2003;33:556–566. doi: 10.1002/immu.200310032. [DOI] [PubMed] [Google Scholar]
- 128.Vetter CS, Groh V, thor Straten P, Spies T, Brocker EB, Becker JC. Expression of stress-induced MHC class I related chain molecules on human melanoma. J Invest Dermatol. 2002;118:600–605. doi: 10.1046/j.1523-1747.2002.01700.x. [DOI] [PubMed] [Google Scholar]
- 129.Porgador A, Mandelboim O, Restifo NP, Strominger JL. Natural killer cell lines kill autologous beta2-microglobulin-deficient melanoma cells: implications for cancer iummunotherapy. Proc Natl Acad Sci USA. 1997;94 (24):13140–13145. doi: 10.1073/pnas.94.24.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pende D, Rivera P, Marcenaro S, et al. Major histocompatibility complex class I-related chain A and UL16-binding protein expression on tumor cell lines of different histotypes: analysis of tumor susceptibility to NKG2D-dependent natural killer cell cytotoxicity. Cancer Res. 2002;62:6178–6186. [PubMed] [Google Scholar]
- 131.Jager E, Ringhoffer M, Altmannsberger M, et al. Immunoselection in vivo: independent loss of MHC class I and melanocyte differentiation antigen expression in metastatic melanoma. Int J Cancer. 1997;71:142–147. doi: 10.1002/(sici)1097-0215(19970410)71:2<142::aid-ijc3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 132.Khong HT, Wang QJ, Rosenberg SA. Identification of multiple antigens recognized by tumor-infiltrating lymphocytes from a single patient: tumor escape by antigen loss and loss of MHC expression. J Immunother. 2004;27:184–190. doi: 10.1097/00002371-200405000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Restifo NP, Marincola FM, Kawakami Y, Taubenberger J, Yannelli JR, Rosenberg SA. Loss of functional beta 2-microglobulin in metastatic melanomas from five patients receiving immunotherapy. J Natl Cancer Inst. 1996;88:100–108. doi: 10.1093/jnci/88.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rosenberg SA, Yang JC, Robbins PF, et al. Cell transfer therapy for cancer: lessons from sequential treatments of a patient with metastatic melanoma. J Immunother. 2003;26:385–393. doi: 10.1097/00002371-200309000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Carretero R, Cabrera T, Gil H, et al. BCG immunotherapy of bladder cancer induces selection of HLA class I-deficient tumor cells. Int J Cancer. 2010 doi: 10.1002/ijc.25733. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 136.Chang CC, Campoli M, Restifo NP, Wang X, Ferrone S. Immune selection of hot-spot beta 2-microglobulin gene mutations, HLA-A2 allospecificity loss, and antigen-processing machinery component down-regulation in melanoma cells derived from recurrent metastases following immunotherapy. J Immunol. 2005;174:1462–1471. doi: 10.4049/jimmunol.174.3.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Donaldson JG, Williams DB. Intracellular assembly and trafficking of MHC class I molecules. Traffic. 2009;10:1745–1752. doi: 10.1111/j.1600-0854.2009.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kloor M, Becker C, Benner A, et al. Immunoselective pressure and human leukocyte antigen class I antigen machinery defects in microsatellite unstable colorectal cancers. Cancer Res. 2005;65:6418–6424. doi: 10.1158/0008-5472.CAN-05-0044. [DOI] [PubMed] [Google Scholar]
- 139.Ripberger E, Linnebacher M, Schwitalle Y, Gebert J, von Knebel DM. Identification of an HLA-A0201-restricted CTL epitope generated by a tumor-specific frameshift mutation in a coding microsatellite of the OGT gene. J Clin Immunol. 2003;23:415–423. doi: 10.1023/a:1025329819121. [DOI] [PubMed] [Google Scholar]
- 140.Dierssen JW, de Miranda NF, Mulder A, van Puijenbroek M, Verduyn W, Claas FH, van de Velde CJ, Jan Fleuren G, Cornelisse CJ, Corver WE, Morreau H. High-resolution analysis of HLA class I alterations in colorectal cancer. BMC Cancer. 2006;6:233. doi: 10.1186/1471-2407-6-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bicknell DC, Kaklamanis L, Hampson R, Bodmer WF, Karran P. Selection for beta 2-microglobulin mutation in mismatch repair-defective colorectal carcinomas. Curr Biol. 1996;6:1695–1697. doi: 10.1016/s0960-9822(02)70795-1. [DOI] [PubMed] [Google Scholar]
- 142.Cabrera CM, Jiménez P, Cabrera T, Esparza C, Ruiz-Cabello F, Garrido F. Total loss of MHC class I in colorectal tumors can be explained by two molecular pathways: beta2-microglobulin inactivation in MSI-positive tumors and LMP7/ TAP2 downregulation in MSI-negative tumors. Tissue Antigens. 2003;61:211–219. doi: 10.1034/j.1399-0039.2003.00020.x. [DOI] [PubMed] [Google Scholar]
- 143.Saeterdal I, Bjorheim J, Lislerud K, et al. Frameshift-mutation-derived peptides as tumor-specific antigens in inherited and spontaneous colorectal cancer. Proc Natl Acad Sci USA. 2001;98:13255–13260. doi: 10.1073/pnas.231326898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 145.Darwin C. The origin of species by means of natural selection: the preservation of favored races in the struggle for life. In: Burrow JW, editor. Natural selection. Penguin; London: 1859. p. 162. [Google Scholar]
- 146.Ferrone S, Whiteside TL. Tumor microenvironment and immune escape. Surg Oncol Clin N Am. 2007;16:755–774. doi: 10.1016/j.soc.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 147.Madjd Z, Spendlove I, Pinder SE, Ellis IO, Durrant LG. Total loss of MHC class I is an independent indicator of good prognosis in breast cancer. Int J Cancer. 2005;117:248–255. doi: 10.1002/ijc.21163. [DOI] [PubMed] [Google Scholar]
- 148.Ramnath N, Tan D, Li Q, et al. Is downregulation of MHC class I antigen expression in human non-small cell lung cancer associated with prolonged survival? Cancer Immunol Immun-other. 2006;55:891–899. doi: 10.1007/s00262-005-0085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Campoli M, Ferrone S. Tumor escape mechanisms: potential role of soluble HLA antigens and NK cells activating ligands. Tissue Antigens. 2008;72:321–334. doi: 10.1111/j.1399-0039.2008.01106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]