Abstract
Major histocompatibility complex (MHC) class I chain-related gene A (MICA), a ligand for the activating immunoreceptor natural killer group 2D (NKG2D), is expressed on stressed cells such as tumor cells. Study of expression of this molecule on tumor cells and patients’ sera is useful to define patients’ stages leading to proper selection of therapy. In this study, mouse anti-MICA monoclonal antibodies (mAbs) were produced by DNA immunization using a gene gun. Screening of anti-MICA-producing mouse and hybridomas were performed by immunoblot and cell enzyme-linked immunosorbent assay (ELISA) against MICA-positive HeLa and -negative Me1386 cell lines. MAbs were characterized against MICA-positive and -negative cell lines by immunoblot, cell ELISA and flow cytometry. The mAbs were also characterized for locus and allele specificities of MICA and MHC class I chain-related gene B (MICB) as well as for their ability to stain formalin-fixed paraffin-embedded tissues by immunohistochemistry. Although all mouse immune sera were positive with MICA-positive cells by both immunoblot and cell ELISA methods, some hybridomas were positive only with one method. The mAbs had diverse specificities to detect MICA and MICB and different abilities to stain formalin-fixed paraffin-embedded tissues. Thus, DNA immunization by gene gun is an effective method to generate immune mice for the production of mAbs with a variety of specificities against native and denatured forms of MIC proteins.
Keywords: major histocompatibility complex class I chain-related gene A, major histocompatibility complex class I chain-related gene B, monoclonal antibody, NKG2D ligand
Introduction
MICA is a member of the major histocompatibility complex (MHC) class I chain-related (MIC) gene family, which is located within the MHC class I region of human chromosome 6 (1, 2). This gene family comprises two genes, MICA and MHC class I chain-related gene B (MICB), and five pseudogenes or gene fragments (MICC, MICD, MICE, MICF and MICG) (3). MICA and MICB genes encode membrane proteins of 383 amino acids with 83% identity (2, 3), which function as ligands for NKG2D receptor expressed on NK, αβ T cells and γδ T cells (4, 5). MIC proteins are mainly expressed on the surface of epithelia (6) and are upregulated on stressed cells such as tumor cells (7, 8). A number of studies has shown that MICA expression plays a role in tumor elimination (5, 7, 9, 10). On the other hand, soluble form of MIC is found in sera of cancer patients and is correlated with an impairment of NKG2D-dependent activation of immune cells in killing of cancer cells (11, 12) leading to immune evasion. Several studies of tumor geneor immunotherapy have been developed based on MICA/ NKG2D recognition or -mediated cytotoxicity (13–16).
Thus, MICA expression either on tumor cells or in patient sera could be beneficial in the selection of proper therapy for cancer patients. In addition, levels of soluble MICA in cancer patients’ sera have been suggested to be useful for staging of the disease (17). This study aimed to produce monoclonal antibodies (mAbs) to detect MICA expression especially on formalin-fixed paraffin-embedded tissues, which are the routine specimens for pathological laboratories. Thus, we used immunoblot with reducing condition for screening mouse- or hybridoma-producing antibodies. In parallel, we also used cell enzyme-linked immunosorbent assay (ELISA) for screening antibodies against a native form of MICA. Because of a very high polymorphism of MICA and its high homology to MICB, we characterized the antibodies obtained against different alleles of MICA and MICB.
Materials and methods
Cells and tissues
HeLa (human cervical cancer cell line; ATCCCCL-2), Me1386 (human melanoma cell line), L929 (mouse fibroblast cell line; ATCC CCL-1), P3-X63-Ag8.653 (mouse myeloma cell line; ATCC CRL 1580) and 293T (human renal epithelial cell line; ATCC CRL-11268) were maintained in a 5% CO2 atmosphere at 37 °C in RPMI-1640 (Gibco; Invitrogen, Carlsbad, CA), with 10% heat-activated fetal calf serum (ICN, Costa Mesa, CA). Human cholangiocarcinoma lesions were obtained from patients who underwent surgery at Srinagarind Hospital, Khon Kaen, Thailand, with informed consent (HE450525 and HE470821) and were kindly provided by Liver Fluke and Cholangiocarcinoma Research Center, Faculty of Medicine, Khon Kaen University, Thailand. Tissue samples were fixed in 20% buffered formalin, routinely processed and embedded in paraffin.
Animals
Six- to 8-week-old female BALB/c mice purchased from and maintained in the Department of Laboratory Animal Resources at Roswell Park Cancer Institute (Buffalo, NY) were used for immunization. All animals used in this study were treated in accordance with Institutional Animal Care and Use Committee guidelines, Roswell Park Cancer Institute.
Molecular cloning and expression of MICA and MICB alleles in mammalian cells
MICA*00801 in pET26 and MICA*00901 in pcDNA3.1 plasmid-containing histidine fusion tag protein were constructed (C.-C. Chang, X. Wang & S. Ferrone, Hillman Cancer Research Institute, University of Pittsburgh, Pittsburgh, PA, USA, unpublished data). MICA and MICB alleles in pcDNA3.1 expression plasmids were constructed by mutagenesis using MICA*00201 and MICB*014 as templates for MICA (S. Barusrux, S. Raroengjai, A. Jumnainsong, K. Klumkrathok & C. Leelayuwat, The Centre for Research and Development of Medical Diagnostic Laboratories, Faculty of Associated Medical Sciences, Khon Kaen University, Khon Kaen, Thailand, manuscript in preparation) and MICB (A. Jumnainsong, H. T. Reyburn, M. Vales-Gomez & C. Leelayuwat, The Centre for Research and Development of Medical Diagnostic Laboratories, Faculty of Associated Medical Sciences, Khon Kaen University, Khon Kaen, Thailand and Department of Pathology, University of Cambridge, Cambridge, UK, manuscript in preparation), respectively.
Recombinant pcDNA3.1 plasmids carrying allele-specific MICA and MICB were transfected into L929 using the ESCORT V transfection reagent (Sigma, St Louis, MI) and 293T using FuGENE® 6 (Roche Diagnostics, Indianapolis, IN), respectively, according to the manufacturers’ instructions. pEGFPN1 plasmid-expressing green fluorescent protein was used to validate the efficiency of transfection.
Recombinant MICA protein
MICA*00801 in pET26 expression vector was transformed to Escherichia coli BL21. Bacterial cells were grown at 37 °C in Luria broth culture medium supplemented with 50 μg/ml kanamycin. A 2-h induction with 0.5 mM isopropyl-b-d-thiogalactopyranoside was performed, and cells were lysed in 20 mM sodium phosphate, pH 7.5, by six cycles of freezing–thawing. The lysate was centrifuged at 10,000 × g for 15 min and the supernatant was collected. Recombinant MICA (rMICA) was separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) and stained with Coomassie blue.
Immunization schedule
MICA*00901 plasmid DNA (MICA pDNA) was used for immunization using the Helios gene gun system (Bio-Rad Laboratories, Hercules, CA). MICA pDNA was coated with gold particle as described by the manufacturer's instructions. Three days before immunization, mice were primed by intramuscular injection of 20 μg (per mouse) of mouse granulocyte–macrophage colony-stimulating factor (GM-CSF) plasmid. Subsequently, mice were boosted at 2-week intervals by the shaved abdominal skin with 4 μg (per mouse) of MICA pDNA.
Mice were bled 1 week after each booster and sera were tested for reactivity with the respective immunogen against human MICA-negative (Me1386) and MICA-positive (HeLa) cell lines (7) by cell ELISA and immunoblot. In addition, mice sera were tested against a rMICA protein by immunoblot analysis. According to the small amount of mouse sera, the sera after two immunizations were tested by immunoblot against rMICA, whereas sera after three immunizations were tested on HeLa and Me1386 cell lysates. When serum displayed a titer of at least 1:100 in immunoblot analysis or 1:1000 in ELISA, the immunized mice were boosted with rMICA protein (20 mg). Four days following the last booster, mice were killed to generate hybridomas.
Generation of mAb secreting hybridomas
Hybridization and subcloning were performed as described (18). Briefly, splenocytes were harvested from immunized mice and fused to mouse myeloma cells P3-X36-Ag8.653 at a ratio of 1:1. Twenty 96-well, flat-bottom microtiter plates (Corning Inc., Corning, NY) were seeded with 1.5–2 × 105 cells per well. Hybridomas were subcloned by limiting dilution. Isotyping of mAb was performed using IsoStrip (Roche Diagnostics) following the manufacturer's protocol.
Cell ELISA
Cells (5 × 105/ml) were incubated with a diluted mouse sera or hybridoma supernatants for 2 h at 4 °C. Following four washes with 0.5% nonfat dry milk in phosphate buffer saline (PBS), cells were stained with a 1/5000 diluted peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) Fcγ fragment for 1 h at 4 °C followed by four washes. Binding of antibody was visualized using 3,3′,5,5′-tetramethylbenzidine (TMB) (KPL, Gaithersburg, MD). Results were expressed as optical density absorbance (OD), which was determined at 450 nm on an ELISA plate reader (Thermo Labsystems, Helsinki, Finland). A positive reaction was obtained when an OD was greater than two times of mean negative OD.
Immunoblot analysis
Western blot was performed as previously described (19). In brief, cell lysates were prepared by incubating cells with lysis buffer. Lysates equivalent to 1 × 105 cells were separated in 12% SDS-PAGE under reducing conditions. Proteins were transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA), which were then incubated overnight at 4 °C with 5% (w/v) skim milk in phosphate buffer saline (PBS) and diluted mouse serum (1:300) or diluted mAb. After washing with PBS containing 0.1% (w/v) Tween-20, membranes were incubated for 45 min at room temperature with diluted (1:5000) peroxidase-conjugated goat anti-mouse IgG Fcγ fragment and washed four times with PBS containing 0.1% Tween-20, followed by one more washing with PBS. Bound antibodies were visualized using ECL system (Amersham, Piscataway, CA) or TMB (BioFX Laboratories, Owings Mills, MD).
Flow cytometry
Cells (5 × 105/ml) were incubated with 50 μl of hybridoma supernatant for 30 min on ice. Following two washes with 1% bovine serum albumin in 1 × PBS, cells were stained with 10 μl of fluorescein isothiocyanate (FITC)-labeled rabbit anti-mouse F(ab′)2 for 30 min on ice followed by two washes. Cells bound antibodies were analyzed by FACScan with the CellQuest™ software (Becton Dickinson, San Jose, CA).
For characterization of MICA and MICB allele specificity, MIC-transfected and mock-293T cells (5 × 105) were incubated with 2 μg of mAbs (WW2G8 and WW6B7) for 20 min on ice. After two washes with fluorescent activated cell sorter (FACS) buffer, cells were then incubated for 30 min on ice with 10 μl of FITC-labeled rabbit anti-mouse F(ab′)2. Stained cells were washed twice and resuspended with FACS buffer. Cells were analyzed in FACSCalibur (Becton Dickinson, San Jose, CA) with Flowjo program (Tree Star Inc., Ashland, OR).
Immunohistochemical staining
Immunoperoxidase staining of formalin-fixed paraffin-embedded HeLa or Me1386 or human cholangiocarcinoma (bile duct cancer) tissue sections were performed using EnVision+ system (DAKO, Carpinteria, CA). In brief, 4-μm-thick tissue sections were deparaffinized with xylene and rehydrated by passage through decreasing concentrations of ethanol. Antigens were retrieved by steaming in citrate buffer (pH 6.0). Endogenous peroxidase activity was inhibited by incubation for 30 min at room temperature with 3% H2O2. Tissue sections were then sequentially incubated for 10 min with Protein Block (DAKO) and overnight at 4 °C with an optimal amount of MICA mAb. After washing with PBS, tissue sections were incubated for 30 min at room temperature with EnVision1 reagent. Peroxidase activity was detected by incubating tissue sections for 5 min with 3,3′-diaminobenzidine chromogen solution (DAKO). Tissue sections were counterstained with hematoxylin 2 (Richard-Allan Scientific, Kalamazoo, MI). Mouse anti-idiotypic IgG1 MK2-23 mAb (20) was used as negative control.
Results
Mouse immune sera against rMICA protein and MICA-positive cell line
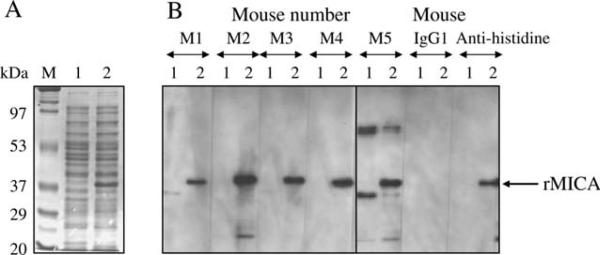
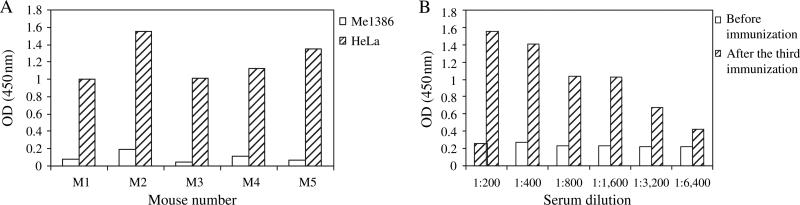
Recombinant MICA expressed in BL21 cells (Figure 1A) was used for the last boost of mice and for detection of anti-MICA immune sera by immunoblot. After the second immunization of BALB/c mice with MICA pDNA, all five mouse immune sera (1:300 dilution) showed positive band with approximately 38 kDa rMICA (Figure 1B). After the third immunization, all five immune sera showed positive band with by immunoblot a single band at approximately 62 kDa with HeLa cell lysate but not with Me1386 (data not shown). Differences in size of MICA produced by prokaryotic cells (BL21) and mammalian cells (HeLa) were because of presence or absence of glycosylation. Mouse immune sera were tested by cell ELISA at 1:200 against HeLa and Me1386 cells (Figure 2A). All five immune sera were positive with MICA-positive (HeLa) cells but not with MICA-negative (Me1386) cells. Cell ELISA was also performed by serial dilution of immune sera and gave positive results with HeLa at serum dilution of 1:6400 (Figure 2B). A mouse (M2) with immune serum giving a specific band by immunoblot and highest positive OD by cell ELISA was chosen for production of hybridomas.
Figure 1.
Mouse immune sera reactivity to recombinant major histocompatibility complex class I chain-related gene A (rMICA). MICA*00801 in pET26 plasmid was expressed in BL21. (A) Sodium dodecyl sulphate–polyacrylamide gel electrophoresis analysis of rMICA expression in BL2. (B) Immunoblot detection with mouse immune sera (after second immunization). Mouse immunoglobulin G1 and anti-histidine were used as negative and positive controls, respectively. Lane M, molecular weight marker; lane 1, mock-transformed BL21 lysate; lane 2, MICA-transformed BL21 lysate; M1–M5, mouse identification number.
Figure 2.
Mouse immune sera reactivity to major histocompatibility complex class I chain-related gene A (MICA)-positive cells by cell enzyme-linked immunosorbent assay (ELISA). Diluted mouse immune sera were tested against MICA-positive (HeLa) and -negative (Me1386) cell lines by cell ELISA. (A) Optical densities (ODs) of all five mouse immune sera (diluted at 1:200) after third immunization were shown. (B) One mouse serum (M2) was diluted by serial twofold dilution and tested for titer of anti-MICA against HeLa cells.
Generation and screening of hybridomas
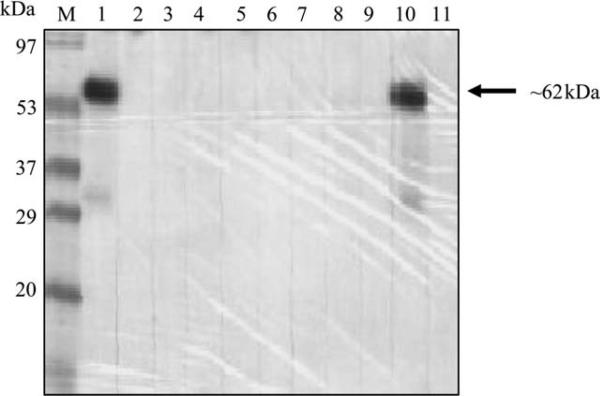
Colony growth was observed for almost all hybridomas produced. Hybridoma supernatants were pooled from 12 wells/pool. Each pool was determined for the presence of anti-MICA mAbs against HeLa cell lysate by immunoblot (Figure 3). Each well from a pool giving positive result was further tested. All hybridoma supernatants were also tested individually for anti-MICA by cell ELISA. Twenty-nine anti-MICA hybridomas were obtained, comprising 16 positive with immunoblot and cell ELISA, 5 with immuno-blot only and 8 with cell ELISA only.
Figure 3.
Immunoblot analysis of hybridoma supernatants. Screening of anti-major histocompatibility complex class I chain-related gene A (MICA)-producing hybridomas by immunoblot was performed by pool. Each lane represents the reaction of a pool of 12 hybridoma supernatants with HeLa cell (MICA positive) lysate. The MICA-specific band is approximately 62 kDa.
Generation and characterization of mAbs
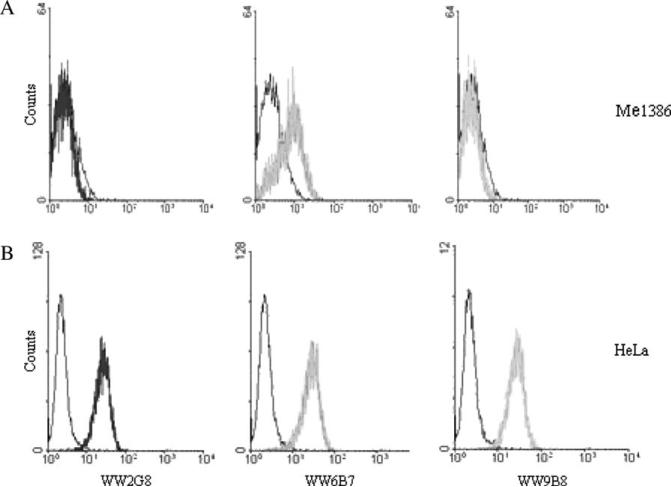
Three hybridomas, WW2G8, WW6B7 and WW9B8, which were anti-MICA positive by both immunoblot and cell ELISA, were chosen for subcloning by limiting dilution. They were all IgG1 with κ chain. Immunoblot analysis showed one band with the HeLa cell lysate of approximately 62 kDa (Figure 4). In cell ELISA, OD ratio of reaction against HeLa:Me1386 was 2.08:0.052, 2.374:0.213 and 2.538:0.102 for WW2G8, WW6B7 and WW9B8, respectively. All three mAbs were positive with HeLa cells by flow cytometry (Figure 5). Only WW6B7 showed a weak reactivity with Me1386.
Figure 4.
Characterization of major histocompatibility complex class I chain-related gene A (MICA) monoclonal antibodies against MICA-positive and -negative cell lysates by immunoblot. Supernatants of WW2G8, WW6B7 and WW9B8 were used in immunoblot analysis against HeLa (MICA positive) and Me1386 cell (MICA negative) lysates. Lane 1, Me1386 cell lysate; lane 2, HeLa cell lysate.
Figure 5.
Characterization of major histocompatibility complex class I chain-related gene A (MICA) monoclonal antibodies (mAbs) against MICA-positive and -negative cells by flow cytometry. HeLa or Me1386 cells (5 × 105) were incubated with anti-MICA mAbs and followed by fluorescein isothiocyanate-labeled rabbit anti-mouse F(ab′)2. Stained cells were analyzed in FACScan equipped with Cell-Quest™ software. Analysis of WW2G8, WW6B7 and WW9B8 against Me1386 (A) and HeLa (B) cells by flow cytometry. Isotype controls are shown in black.
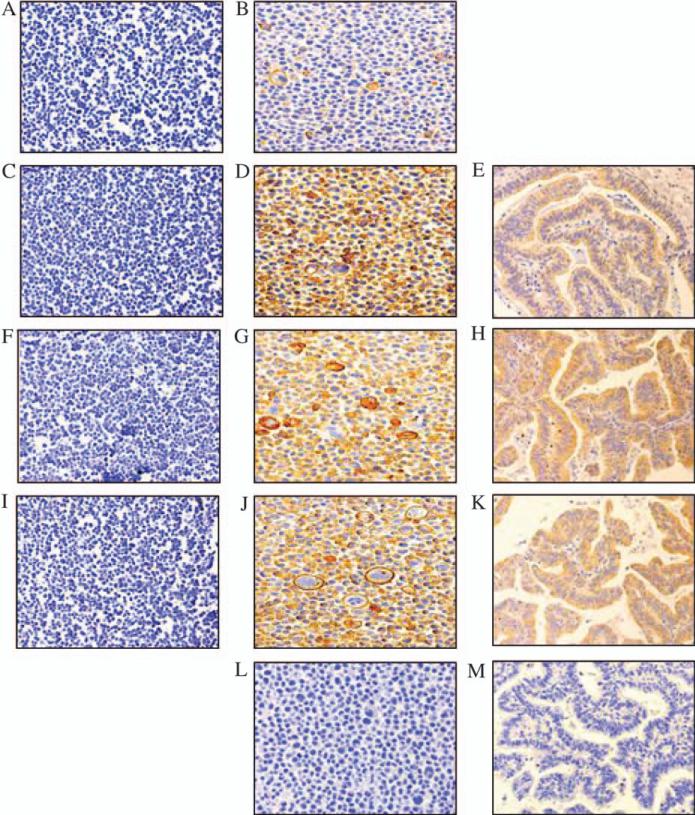
These mAbs were then used in immunohistochemical staining of formalin-fixed paraffin-embedded tissue from cholangiocarcinoma as well as HeLa and Me1386 cells. A mAb produced by peptide immunization (N’-FLRYDRQKCRAKPQGQWAEDC-C’; WJ1, H. S. Cho, X. Wang & S. Ferrone, Hillman Cancer Research Institute, University of Pittsburgh, Pittsburgh, PA, USA, unpublished data) was also included in the assay. WW6B7, WW9B8 and WJ1 were strongly positive with HeLa but not with Me1386 (Figure 6). These three mAbs could also stain formalin-fixed tissue sections. The characteristics of mAbs were summarized and shown in Table 1.
Figure 6.
Immunohistochemical staining of formalin-fixed, paraffin-embedded cell lines and cholangiocarcinoma tissue sections with anti-major histocompatibility complex class I chain-related gene A (MICA) monoclonal antibodies. Immunoperoxidase staining was performed on formalin-fixed, paraffin-embedded Me1386 cells, HeLa cells and cholangiocarcinoma tissue sections. Left (A, C, F and I), middle (B, D, G, J and L) and right column (E, H, K and M) is Me1386 cells, HeLa cells and cholangiocarcinoma tissue sections, respectively. A and B; C, D and E; F, G and H; I, J and K and L and M were stained with WW2G8, WW6B7, WW9B8, WJ1 and MK2-23, respectively. Magnification, ×20.
Table 1.
The characteristics of anti-MICA mAbs
| Characteristic: forms of MIC/methods to detect/alleles of MIC | Anti-MICAmAbs |
|||
|---|---|---|---|---|
| WW2G8 | WW6B7 | WW9B8 | WJ1 | |
| Native form of MIC | ||||
| Cell ELISA | ||||
| HeLa cells | + + + + | ++++ | ++++ | ND |
| Flow cytometry | ||||
| Me1386 cells | - | + | - | ND |
| HeLa cells | + | + | + | ND |
| MICA*00201 | + | + | ND | ND |
| 00701 | + | + | ND | ND |
| 00801 | + | + | ND | ND |
| MICB*003 | - | + | ND | ND |
| 00502 | - | + | ND | ND |
| 008 | - | + | ND | ND |
| Denatured form of MIC Immunohistochemistry | ||||
| HeLa | + | ++++ | ++++ | ++++ |
| Western blot | ||||
| MICA*00201 | ++ | ++++ | ++++ | ++++ |
| 004 | + | ++++ | ++++ | ++++ |
| 00701 | +/- | + | + | - |
| 00801 | + | +++ | ++++ | +++ |
| 00901 | - | + | - | + |
| 010 | - | + | + | + |
| 01201 | + | ++++ | +++ | ++++ |
| 017 | - | + | - | + |
| 01801 | - | ++++ | ++++ | ++++ |
| 019 | - | - | - | - |
| 045 | - | + | + | + |
| MICB*002 | - | + | - | + |
| 003 | - | - | - | - |
| 004 | - | +++ | - | +++ |
| 00502 | - | +++ | - | +++ |
| 008 | - | +++ | - | +++ |
| 013 | - | +++ | - | +++ |
MICA, major histocompatibility complex class I chain-related gene A; MICB, major histocompatibility complex class I chain-related gene B; mAbs, monoclonal antibodies; ELISA, enzyme-linked immunosorbent assay; ND, not determined.
Characterization of mAbs for MICA and MICB allele specificity
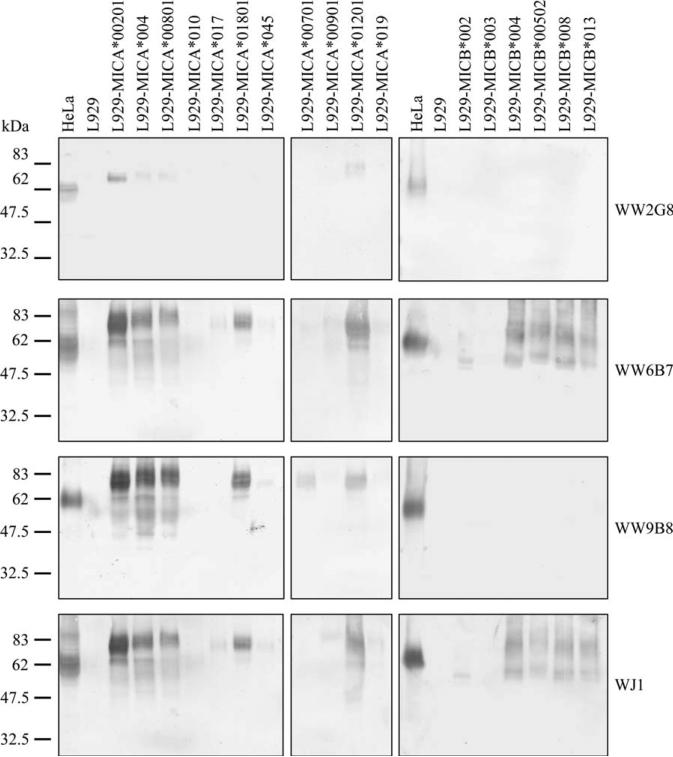
Because of the lack of MICA and MICB in mouse, L929 mouse cell line was chosen for transfection with plasmids carrying MICA and MICB genes. pEGFPN1 plasmid was used to validate the efficiency of transfection. Approximately 30% cells expressed green fluorescent protein. Each lysate from an allele of MICA- and MICB-transfected cells was analyzed by immunoblot against the three mAbs and WJ1. WW6B7 and WJ1 detected both MICA and MICB, whereas WW9B8 and WW2G8 could detect only MICA (Figure 7). Furthermore, WW2G8 could detect only some MICA alleles.
Figure 7.
Immunoblot analysis of monoclonal antibodies (mAbs) against allele-specific major histocompatibility complex class I chain-related gene A (MICA) and major histocompatibility complex class I chain-related gene B (MICB). Allele-specific MICA or MICB plasmids were transfected and expressed in L929. MIC-transfected and mock-L929 cell lysates were tested by anti-MICA mAbs by immunoblot. Each lane refers to different alleles of MICA- or MICB-transfected L929 cell lysates reacting with different mAbs shown on right. MICA*00201, *004, *00801, *010, *017, *01801, *045, *00701, *00901, *01201 and *019 and MICB*002, *003, *004, *00502, *008 and *013 were included in the analysis. HeLa and mock-L929 cell lysates were used as positive and negative controls.
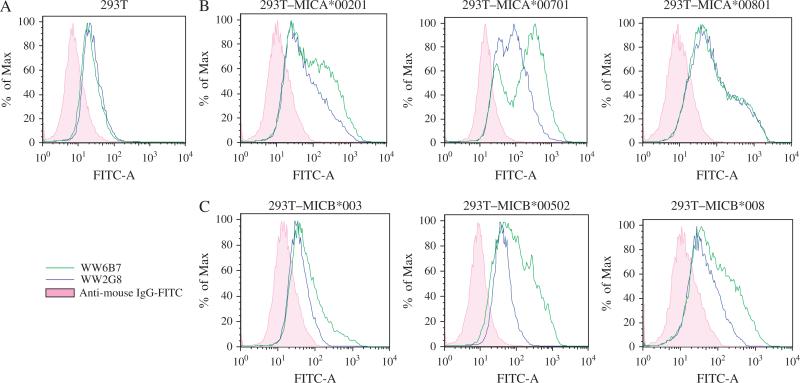
Specificities of WW2G8 and WW6B7 were also determined by flow cytometry using 293T-transfected cells. Because 293T cells also express MICA, untransfected 293T cells were also used as negative control. WW6B7 detected all alleles of MICA and MICB tested, but WW2G8 was not reactive against some of the MICB-transfected cells (Figure 8). The results were in accordance with the immunoblot analysis. Thus, WW9B8 and WJ1 were not included in the analysis.
Figure 8.
Analysis of major histocompatibility complex class I chain-related gene A (MICA) and major histocompatibility complex class I chain-related gene B (MICB) allele specificities of monoclonal antibodies (mAbs) by flow cytometry. Allele-specific MICA or MICB was transfected and expressed in 293T cells. MIC-transfected and mock-293T cells (5 × 105) were incubated with mAbs and followed by fluorescein isothiocyanate (FITC)-labeled rabbit anti-mouse F(ab′)2. Stained cells were analyzed in FACSCalibur with Flowjo program. (A) Untransfected 293T cells stained with WW2G8 and WW6B7 were used as base lines (shown in pink). (B) MICA-transfected 293T cells treated with WW2G8 and WW6B7. (C) MICB-transfected 293T cells treated with WW2G8 and WW6B7. IgG, immunoglobulin G.
Discussion
This study has shown that DNA-based immunization delivered by a gene gun is an effective method for the generation of mouse anti-MICA immune serum. The immune serum displayed a high titer by both immunoblot and cell ELISA techniques following only three immunization boosters. This could have been the results of a number of factors. First, pDNA administration by gene gun involves direct transfection of antigen-presenting cells, such as Langerhans cells (21). Second, MICA may be a highly immunogenic protein in mouse because of the lack of this protein in this animal. Third, plasmid-encoded GM-CSF used as a genetic adjuvant to DNA vaccination has been shown to be efficient in inducting both T helper (Th)1- and Th2-type responses (22–25). However, gene gun immunization (26) and prior inoculation with pGM-CSF before DNA immunization (27) give rise to the Th2-type responses, thus all the mAb isotype was IgG1. We are fortunate to be able to obtain a mAb against MICA by peptide immunization (WJ1). Peptide immunization is unpredictable and cannot guarantee for specific protein-reacting antibodies. From our experiences, only three of nine peptides could produce MIC-reactive antibodies in rabbits (28).
Pooling of hybridoma supernatants in the screening of specific antibody-producing hybridomas reduced labor time and was able to produce positive results. Although the same mouse was used, hybridomas showed different reactions using immunoblot and cell ELISA technique. Thus, both methods need to be used to screen mAb-producing hybridomas.
All three mAbs (WW2G8, WW6B7 and WW9B8) were reactive by immunoblot against 62 kDa MICA present in HeLa cell lysate, consistent with previous reports (8, 29). However, these mAbs were reactive against approximately 38 kDa rMICA protein produced in BL21. Differences in size of MICA produced by prokaryotic cells (BL21) and mammalian cells (HeLa) were apparently because of presence or absence of glycosylation. These mAbs are able to recognize MICA in a native form as positive results were also obtained by cell ELISA and flow cytometry.
WW2G8 could detect different alleles of MICA in their native forms. However, WW2G8 was less efficient in detecting MICA on HeLa cells by immunoblot, suggesting that WW2G8 does not react well with the denatured form of MICA.
On the other hand, WW6B7 may not be efficient in detecting the native protein as it was weakly positive with Me1386 cells by flow cytometry. WW6B7 was positive with both MICA- and MICB-transfected L929 lysates by immunoblot analysis. Different glycosylations between mouse and human cells affecting WW6B7 reactivity could not be excluded. Alternatively, WW6B7 might be reactive to other native proteins on Me1386 that could not be detected by immunoblot analysis and cell ELISA. Additionally, WW6B7 could detect different alleles of both MICA and MICB in their native forms.
WW6B7 and WJ1 could be used to detect both MICA and MICB expression in formalin-fixed paraffin-embedded tissues. WW6B7 has an advantage over WW9B8 as the former mAb was able to detect most of the common MICA (30–32) and MICB alleles (33, 34). It should be noted that these three mAbs were weakly reactive with MICA*010 similar to previous report (35). The negative or weak reactivity of these mAbs against MICA*00701, *00901, *010, *017, *019 and *045 as well as MICB*002 and *003 in the immunoblot analysis should also be noted, this may be a limitation of these antibodies in detecting this group of alleles. This is not surprising knowing the polymorphic nature of MICA and MICB.
In summary, mAbs with different characteristics against MIC proteins were obtained. WW2G8 and WW9B8 are specific for MICA in both native and denatured forms. WW6B7 and WJ1 are specific for both MICA and MICB in a denatured form, whereas WW2G8 has less activity to detect MICA in a denatured form. Thus, WW9B8, WW6B7 and WJ1 should be suitable for immunohistochemical staining of formalin-fixed paraffin-embedded tissue sections. These MIC mAbs could be conveniently used for tissues stored in general pathological laboratories.
Acknowledgments
We are most grateful to Prof Dr Prapon Wilairat, Department of Biochemistry, Mahidol University, Thailand, for his critical comments and suggestions to improve the presentation of this manuscript. This project was supported by a grant (RSA/5/2543) from Thailand Research Fund (TRF) and Centre for Research and Development of Medical Diagnostic Laboratories, Khon Kaen University, Thailand. CL was TRF Scholar; WW and KK were supported by Allied Health Sciences Consortium, Ministry of Education, Thailand, and TRF Royal Golden Jubilee PhD Scholarship Program, respectively.
References
- 1.Leelayuwat C, Townend DC, Degli-Esposti MA, Abraham LJ, Dawkins RL. A new polymorphic and multicopy MHC gene family related to nonmammalian class I. Immunogenetics. 1994;40:339–51. doi: 10.1007/BF01246675. [DOI] [PubMed] [Google Scholar]
- 2.Bahram S, Bresnahan M, Geraghty DE, Spies T. A second lineage of mammalian major histocompatibility complex class I genes. Proc Natl Acad Sci U S A. 1994;5:6259–63. doi: 10.1073/pnas.91.14.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bahram S. MIC genes: from genetics to biology. Adv Immunol. 2000;76:1–60. doi: 10.1016/s0065-2776(01)76018-x. [DOI] [PubMed] [Google Scholar]
- 4.Wu J, Song Y, Bakker AB, et al. An activating immunoreceptor complex formed by NKG2D and DAP10. Science. 1999;285:730–2. doi: 10.1126/science.285.5428.730. [DOI] [PubMed] [Google Scholar]
- 5.Bauer S, Groh V, Wu J, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–9. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 6.Groh V, Bahram S, Bauer S, Herman A, Beauchamp M, Spies T. Cell stress-regulated human major histocompatibility complex class I gene expressed in gastrointestinal epithelium. Proc Natl Acad Sci U S A. 1996;29:12445–50. doi: 10.1073/pnas.93.22.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pende D, Rivera P, Marcenaro S, et al. Major histocompatibility complex class I-related chain A and UL16-binding protein expression on tumor cell lines of different histotypes: analysis of tumor susceptibility to NKG2D-dependent natural killer cell cytotoxicity. Cancer Res. 2002;62:6178–86. [PubMed] [Google Scholar]
- 8.Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc Natl Acad Sci U S A. 1999;8:6879–84. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu X, Rao G, Gaffud MJ, et al. Clinicopathological significance of major histocompatibility complex class I-related chain a and B expression in thyroid cancer. J Clin Endocrinol Metab. 2006;91:2704–12. doi: 10.1210/jc.2006-0492. [DOI] [PubMed] [Google Scholar]
- 10.Busche A, Goldmann T, Naumann U, Steinle A, Brandau S. Natural killer cell-mediated rejection of experimental human lung cancer by genetic overexpression of major histocompatibility complex class I chain-related gene A. Hum Gene Ther. 2006;17:135–46. doi: 10.1089/hum.2006.17.135. [DOI] [PubMed] [Google Scholar]
- 11.Salih HR, Rammensee HG, Steinle A. Cutting edge: down-regulation of MICA on human tumors by proteolytic shedding. J Immunol. 2002;169:4098–102. doi: 10.4049/jimmunol.169.8.4098. [DOI] [PubMed] [Google Scholar]
- 12.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–8. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 13.Jinushi M, Hodi FS, Dranoff G. Therapy-induced antibodies to MHC class I chain-related protein A antagonize immune suppression and stimulate antitumor cytotoxicity. Proc Natl Acad Sci U S A. 2006;13:9190–5. doi: 10.1073/pnas.0603503103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang B, Wei H, Zheng X, Zhang J, Sun R, Tian Z. The inhibitory effects of synthetic short peptides, mimicking MICA and targeting at NKG2D receptors, on function of NK cells. Peptides. 2005;26:405–12. doi: 10.1016/j.peptides.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Germain C, Larbouret C, Cesson V, et al. MHC class I-related chain A conjugated to antitumor antibodies can sensitize tumor cells to specific lysis by natural killer cells. Clin Cancer Res. 2005;11:7516–22. doi: 10.1158/1078-0432.CCR-05-0872. [DOI] [PubMed] [Google Scholar]
- 16.Friese MA, Platten M, Lutz SZ, et al. MICA/NKG2D-mediated immunogene therapy of experimental gliomas. Cancer Res. 2003;63:8996–9006. [PubMed] [Google Scholar]
- 17.Holdenrieder S, Stieber P, Peterfi A, Nagel D, Steinle A, Salih HR. Soluble MICA in malignant diseases. Int J Cancer. 2006;118:684–7. doi: 10.1002/ijc.21382. [DOI] [PubMed] [Google Scholar]
- 18.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–7. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Campoli M, Cho HS, et al. A method to generate antigen-specific mAb capable of staining formalin-fixed, paraffin-embedded tissue sections. J Immunol Methods. 2005;299:139–51. doi: 10.1016/j.jim.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Kusama M, Kageshita T, Chen ZJ, Ferrone S. Characterization of syngeneic anti-idiotypic monoclonal antibodies to murine anti-human high molecular weight-melanoma associated antigen monoclonal antibodies. J Immunol. 1989;143:3844–52. [PubMed] [Google Scholar]
- 21.Condon C, Watkins SC, Celluzzi CM, Thompson K, Falo LD., Jr DNA-based immunization by in vivo transfection of dendritic cells. Nat Med. 1996;2:1122–8. doi: 10.1038/nm1096-1122. [DOI] [PubMed] [Google Scholar]
- 22.Moore AC, Kong WP, Chakrabarti BK, Nabel GJ. Effects of antigen and genetic adjuvants on immune responses to human immunodeficiency virus DNA vaccines in mice. J Virol. 2002;76:243–50. doi: 10.1128/JVI.76.1.243-250.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barouch DH, Santra S, Tenner-Racz K, et al. Potent CD41 T cell responses elicited by a bicistronic HIV-1 DNA vaccine expressing gp120 and GM-CSF. J Immunol. 2002;168:562–8. doi: 10.4049/jimmunol.168.2.562. [DOI] [PubMed] [Google Scholar]
- 24.Kim JJ, Simbiri KA, Sin JI, et al. Cytokine molecular adjuvants modulate immune responses induced by DNA vaccine constructs for HIV-1 and SIV. J Interferon Cytokine Res. 1999;19:77–84. doi: 10.1089/107999099314441. [DOI] [PubMed] [Google Scholar]
- 25.Iwasaki A, Stiernholm BJ, Chan AK, Berinstein NL, Barber BH. Enhanced CTL responses mediated by plasmid DNA immunogens encoding costimulatory molecules and cytokines. J Immunol. 1997;158:4591–601. [PubMed] [Google Scholar]
- 26.Feltquate DM, Heaney S, Webster RG, Robinson HL. Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization. J Immunol. 1997;158:2278–84. [PubMed] [Google Scholar]
- 27.Kusakabe K, Xin KQ, Katoh H, et al. The timing of GM-CSF expression plasmid administration influences the Th1/Th2 response induced by an HIV-1-specific DNA vaccine. J Immunol. 2000;164:3102–11. doi: 10.4049/jimmunol.164.6.3102. [DOI] [PubMed] [Google Scholar]
- 28.Leelayuwat C, Hollingsworth P, Pummer S, et al. Antibody reactivity profiles following immunization with diverse peptides of the PERB11 (MIC) family. Clin Exp Immunol. 1996;106:568–76. doi: 10.1046/j.1365-2249.1996.d01-862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zwirner NW, Fernandez-Vina MA, Stastny P. MICA, a new polymorphic HLA-related antigen, is expressed mainly by keratinocytes, endothelial cells, and monocytes. Immunogenetics. 1998;47:139–48. doi: 10.1007/s002510050339. [DOI] [PubMed] [Google Scholar]
- 30.Collins RW. Human MHC class I chain related (MIC) genes: their biological function and relevance to disease and transplantation. Eur J Immunogenet. 2004;31:105–14. doi: 10.1111/j.1365-2370.2004.00457.x. [DOI] [PubMed] [Google Scholar]
- 31.Romphruk AV, Naruse TK, Romphruk A, et al. Diversity of MICA (PERB11.1) and HLA haplotypes in Northeastern Thais. Tissue Antigens. 2001;58:83–9. doi: 10.1034/j.1399-0039.2001.580203.x. [DOI] [PubMed] [Google Scholar]
- 32.Jumnainsong A, Romphruk AV, Jearanaikoon P, et al. Association of polymorphic extracellular domains of MICA with cervical cancer in northeastern Thai population. Tissue Antigens. 2007;69:326–33. doi: 10.1111/j.1399-0039.2006.00754.x. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez S, Rodriguez-Rodero S, Martinez-Borra J, Lopez-Vazquez A, Rodrigo L, Lopez-Larrea C. MICB typing by PCR amplification with sequence specific primers. Immunogenetics. 2003;54:850–5. doi: 10.1007/s00251-002-0533-x. [DOI] [PubMed] [Google Scholar]
- 34.Jumnainsong A, Jearanaikoon P, Khahmahpahte S, et al. Associations of MICB with cervical cancer in north-eastern Thais: identification of major histocompatibility complex class I chain-related gene B motifs influencing natural killer cell activation. Clin Exp Immunol. 2008;153:205–13. doi: 10.1111/j.1365-2249.2008.03682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Z, Groh V, Strong RK, Spies T. A single amino acid substitution causes loss of expression of a MICA allele. Immunogenetics. 2000;51:246–8. doi: 10.1007/s002510050039. [DOI] [PubMed] [Google Scholar]