SUMMARY
Mechanosensitive cilia are vital to signaling and development across many species. In sensory hair cells, sound and movement are transduced by apical hair bundles. Each bundle is comprised of a single primary cilium (kinocilium) flanked by multiple rows of actin-filled projections (stereocilia). Extracellular tip links that interconnect stereocilia are thought to gate mechanosensitive channels. In contrast to stereocilia, kinocilia are not critical for hair-cell mechanotransduction. However, by sequentially imaging the structure of hair bundles and mechanosensitivity of individual lateral-line hair cells in vivo, we uncovered a central role for kinocilia in mechanosensation during development. Our data demonstrate that nascent hair cells require kinocilia and kinocilial links for mechanosensitivity. Although nascent hair bundles have correct planar polarity, the polarity of their responses to mechanical stimuli is initially reversed. Later in development, a switch to correctly polarized mechanosensitivity coincides with the formation of tip links and the onset of tip link-dependent mechanotransduction.
INTRODUCTION
Hair cells are the specialized mechanosensory receptors of the auditory and vestibular systems that convert head movements and sound waves into electrical signals (reviewed in Schwander et al., 2010). Projecting from the apical surface of each hair cell is a stiff hair bundle, consisting of rows of interconnected stereocilia tethered to a single kinocilium. Stereocilia are densely packed with actin filaments and form parallel rows that increase progressively in height, creating a staircase-like structure; adjacent to the tallest row of stereocilia is the kinocilium, a true cilium with a 9 + 2 arrangement of microtubules. Cadherin 23 (Cdh23) and Protocadherin 15 (Pcdh15) form extracellular tip links that connect adjacent rows of stereocilia, as well as kinocilial links that tether the tallest row of stereocilia to the kinocilium (Ahmed et al., 2006; Goodyear and Richardson, 2003; Siemens et al., 2004; Söllner et al., 2004). This morphological polarity, a stereocilia-based staircase leading to the kinocilium, is believed to restrict functional polarity, or hair-cell mechanosensitivity, to a single direction of bundle deflection (Shotwell et al., 1981). Deflection of the hair bundle towards the kinocilium is thought to tension tip links and open mechanosensitive channels, depolarizing the hair cell (Pickles et al., 1984). Conversely, deflection away from the kinocilium is thought to relieve tip-link tension and close mechanosensitive channels.
During hair-cell development, the kinocilium is the first structure to appear on the apical cell surface (Denman-Johnson and Forge, 1999; Tilney et al., 1992). Subsequently, rows of stereocilia emerge progressively and elongate adjacent to the kinocilium. Stereocilial elongation is accompanied by the addition of mechanotransduction components, including tip links. Hair bundles at this stage —comprising a full complement of stereocilia correctly polarized in a staircase formation, with detectable tip links — mark the onset of functionally polarized mechanotransduction currents (Géléoc and Holt, 2003; Lelli et al., 2009; Si et al., 2003). In auditory hair cells of mammals and birds, kinocilia regress soon after the onset of mechanotransduction, whereas vestibular hair cells maintain kinocilia throughout maturity (Denman-Johnson and Forge, 1999; Kikuchi and Hilding, 1966; Tanaka and Smith, 1978).
Although kinocilia are retained in mature vestibular hair cells, their exact role remains unclear. Previously it has been hypothesized that the kinocilium may have a mechanosensitive function, but currently there is no evidence to support this idea (Engström et al., 1962; Hillman, 1969; Shin et al., 2005). Rather, removal of the kinocilium from mature bullfrog vestibular hair cells does not affect mechanotransduction currents, suggesting that the kinocilium plays no role in mechanosensation (Hudspeth and Jacobs, 1979). This lack of function is in contrast to mammalian ciliated mechanosensory cells, and to invertebrate and flagellate mechanoreceptors, where cilia act as direct sensors of extracellular mechanical signals (Fujiu et al., 2011; Kahn-Kirby and Bargmann, 2006; McGrath et al., 2003; Nauli et al., 2003; Walker et al., 2000).
As all hair cells initially have kinocilia, we hypothesized that kinocilia may have a mechanosensitive function during development. To study kinocilial function during development, we applied in vivo imaging of hair-cell activity and structure in conjunction with scanning electron microscopy (SEM). Surprisingly, we find that hair cells are mechanosensitive much earlier than previously reported — before FM 1-43 label and tip links are detectable. In these nascent hair cells, however, functional polarity is reversed relative to hair-bundle morphological polarity. Furthermore, we show that hair cells require a kinocilium and kinocilial links for these nascent responses, providing substantial evidence that kinocilia have mechanosensitive function in hair cells.
RESULTS
Direct comparison of hair-bundle development and function by confocal and electron microscopy
To examine the function of kinocilia during development, we utilized the zebrafish posterior lateral-line organ, which is an auxiliary sensory organ present in fish and amphibians, specialized for local water-flow detection. The lateral line consists of superficial clusters of hair cells called neuromasts, arranged along the length of the animal (Figures 1A-B, 3A and 3D). Hair cells of the lateral-line organ are born in pairs, and sister hair cells adopt opposing polarities along the anterior-posterior (A-P) axis of the fish (López-Schier et al., 2004; Seiler et al., 2005) (Figure 1A). The morphological and planar polarity of a given hair bundle is determined by the asymmetric localization of the actin-rich stereocilia relative to the kinocilium (Figures 1A, 1A’, S1A and S1A’).
Figure 1. Direct comparison of hair-bundle development using transgenic β-actin-GFP, FM 1-43 and SEM.
(A) Top down view of neuromast hair bundles labeled with Tg(myo6b:β-actin-GFP). Three stages of immature hair-bundle development can be observed: early, intermediate and late. The unlabeled region on either the caudal or rostral side of each bundle is the insertion point of the kinocilium.
(A’) Arrows indicate planar polarity and functional polarity of hair bundles in (A). Dashed arrows indicate predicted planar polarity.
(B) SEM image of a neuromast at 3 dpf; asterisk denotes the many tall kinocilia. (C) SEM image of a mature hair bundle.
(D, E and F) Confocal images of hair bundles expressing β-actin-GFP. White dashed arrows indicate hair bundles of respective stage examined.
(D’, E’ and F’) White dashed lines outline cell bodies after FM 1-43 label of hair bundle examined in (D, E and F).
(D”, E” and F”) SEM images of hair bundles in (D, E and F).
All images were taken at 3 dpf. k = kinocilium, white arrowheads denote kinocilial links, solid white arrows denote tip links. Anterior is left, dorsal is up. Scale bars: 2.5 μm (A, B, D, E and F), 500 nm (C), 5 μm (D’, E’ and F’), 1 μm (D”, E” and F”). See also Figure S1.
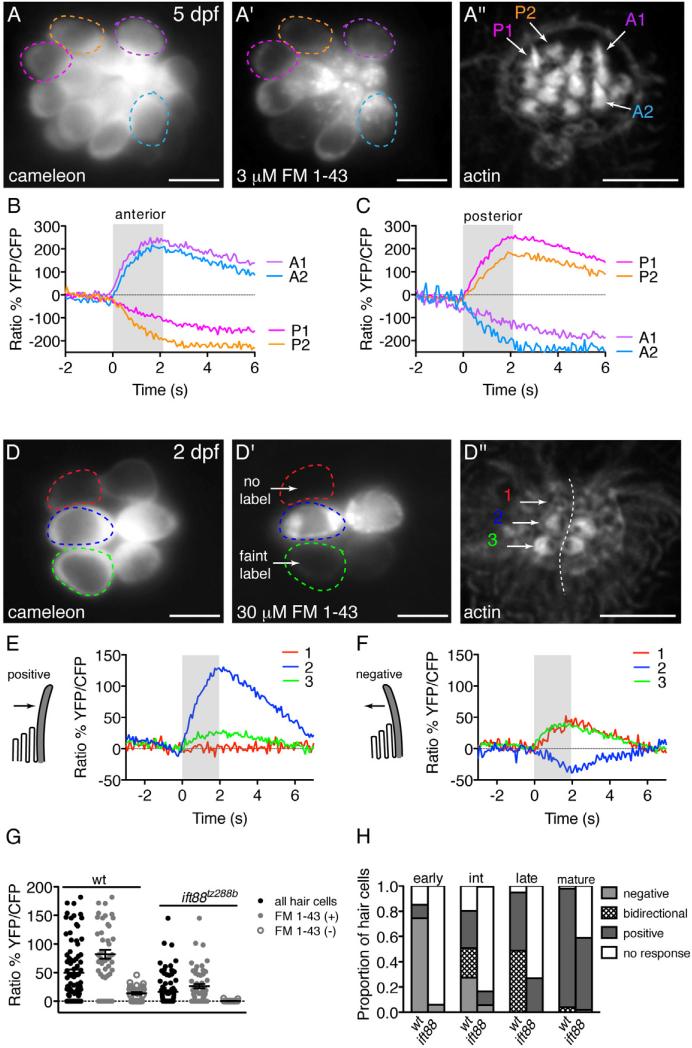
Figure 3. Functional polarity of mature and immature zebrafish hair cells.
(A) A 5 dpf neuromast expressing cameleon. Circles represent cells analyzed and are color coded and correspond to data in (A’-C).
(A’ and A”) FM 1-43 and phalloidin stain of the neuromast in (A). Two anterior-polarized hair bundles (A1 and A2) and two posterior-polarized hair bundles (P1 and P2) are indicated.
(B and C) Response of four individual hair cells highlighted in (A) to the same anterior (B) and posterior (C) stimulus.
(D, D’ and D”) Same layout as (A-A”) except analysis is at 2 dpf. Hair cell stages are as follows: cell 1: early, cell 2: mature, cell 3: intermediate. (D) A neuromast expressing cameleon. (D’) FM 1-43 strongly labeled a pair of mature cells (cell 2 and sister), faintly labeled a pair of intermediate cells (cell 3 and sister), yet did not label a pair of early hair cells (cell 1 and sister). (D”) Phalloidin label of the neuromast in (D-D’).
(E and F) Mechanically-evoked calcium responses corresponding to cells 1-3. Negative and positive deflections are indicated with schematics. Kinocilium is denoted as the tall, gray structure. Cell 1 responded to only negative deflections. Cell 2 responded to positive deflections. Cell 3 responded to both negative and positive deflections.
(G) Hair-cell responses (black circles) from wildtype (n = 13 neuromasts) and ift88tz288b (n = 21 neuromasts) at 2 dpf. This same data was broken down into FM 1-43 labeled hair cells (gray circles) and FM 1-43 unlabeled hair cells (open gray circles).
(H) Proportion of negative, bi-directional and positive responses at 2 dpf (n = 188 wildtype and 72 ift88tz288b cells).
Errors bars are s.em. Scale bars: 5 μm. For stimulus controls, see also Figure S2.
For our study, we first developed a basic framework for characterizing the various stages of development in terms of morphology and function. To accomplish this in vivo, we generated transgenic fish expressing β-actin-GFP under control of a hair-cell specific myo6b promoter and used confocal microscopy to image actin-GFP in hair bundles (Figures 1A-1F). These animals were then assessed for mechanosensitivity with FM 1-43, a vital dye that labels hair cells with functional mechanotransduction channels (Gale et al., 2001) (Figures 1D’-1F’). Finally, ultrastructural features of the same hair bundles were examined using SEM (Figures 1D”-1F”).
The results of in vivo imaging followed sequentially by SEM revealed multiple stages of hair-bundle development within each neuromast. We therefore categorized stages of hair-bundle development. Hair bundles were defined as mature or immature, and immature hair bundles were further sub-classified as early, intermediate or late (Figures 1A, Figures 1D-1F”). Actin localization showed that early hair bundles had a thin and uniform circumferential actin ring at the hair-cell surface. High-resolution SEM analysis revealed that these early bundles had 1 or 2 rows of stereocilia at a height roughly equal to the kinocilium (Figures 1D and 1D”, Table 1). Interestingly, early sister hair cells had correct planar polarity relative to each other and to the A-P axis of the fish (Figure 1D”, Figure S1C). Any minor misalignments in early bundle were comparable to those observed in mature bundles (Figure S1C). At the intermediate stage, actin localization reflected an established morphological polarity and SEM showed that on average, three rows of stereocilia were present. In these intermediate bundles, the height of stereocilia was double that of early bundles, and the kinocilium was twice the height of the tallest stereocilia (Figures 1E and 1E”, Table 1). Late stage hair bundles showed a further increase in height, had 3 to 4 rows of stereocilia, and a kinocilium which was at least five times the height of the tallest stereocilia (Figures 1F and 1F”, Table 1). Only late stage hair bundles consistently labeled with FM 1-43 and had detectable tip links in SEM images (Figures 1F’ and 1F”). This is in agreement with previous studies correlating FM 1-43 label with the presence of tip links and the onset of mechanosensitivity in mouse and chick hair cells (Géléoc and Holt, 2003; Si et al., 2003). Finally, mature hair bundles had between 4 to 5 rows of stereocilia, a bundle height of approximately 1.5 μm, and a kinocilial height of 14 μm (Figure 1C, Table 1). This coordinated approach provided a fundamental reference for the development of hair-bundle morphology and the onset of mechanosensitive function that we applied to subsequent analyses.
Table 1.
Hair-bundle morphological development
| early | intermediate | late | mature | |
|---|---|---|---|---|
| kinocilium height (μm) | 0.33 ± 0.03 | 1.07 ± 0.09 | 5.20 ± 0.54 | 14.42 ± 0.50 |
| bundle height (mm) | 0.26 ± 0.01 | 0.49 ± 0.04 | 0.77 ± 0.04 | 1.42 ± 0.06 |
| rows of stereocilia | 1.6 ± 0.1 | 3.0 ± 0.1 | 3.6 ± 0.1 | 4.4 ± 0.1 |
| early | intermediate | late | mature | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| genotype | wt | ift88 | wt | ift88 | wt | ift88 | wt | ift88 | ||
| negative | 74% | 6% | 27% | - | 6% | - | - | - | - | - |
| bi-directional | - | - | 2% | 22% | - | - | 49% | - | 4% | - |
| positive | 11% | - | 4% | 25% | - | 11% | 46% | 27% | 94% | 71% |
| no response | 15% | 94% | 12% | 8% | 83% | - | 5% | 73% | 2% | 29% |
| number of hair cells | 47 | 18 | 51 | 18 | 39 | 15 | 51 | 21 | ||
n = 25
(±) represents standard error of the mean
Polarized responses in developing hair cells
(-) denotes no observed response
FM 1-43 negative cells
FM 1-43 positive cells
Loss of kinocilia does not disrupt hair-bundle development
Upon establishing methods to track and categorize the stages of hair-bundle development, we then examined the role of kinocilia in the onset of mechanosensitivity. For this analysis, we characterized ovl/ift88 mutants that lack normal kinocilia (Tsujikawa and Malicki, 2004). Using SEM and immunohistochemistry we confirmed that in ift88tz288b mutants kinocilia were severely stunted (mature kinocilia length: 14.42 μm in wildtype, n = 25; 0.29 μm in ift88tz288b, n = 11; example: Figures 2A-2D). We occasionally observed slightly elongated (> 5 μm) kinocilia in younger larvae (2 days post fertilization (dpf)) but never kinocilia that were mature in length (data not shown). This is consistent with previous reports using a maternal-zygotic ift88 mutant that suggest in younger larvae there is residual ift88 maternal RNA in ift88tz288b mutants (Huang and Schier, 2009). Importantly, SEM images of ift88tz288b hair bundles showed that while kinocilia were severely stunted in both immature and mature hair bundles, hair-bundle development was not affected (Figures 2A and 2B). In contrast to a study of auditory hair cells in mice, loss of Ift88 and kinocilia did not result in hair-bundle planar polarity defects (Figures 2C and 2D) (Jones et al., 2008). Normal planar polarity indicates that either the kinocilium is not required to establish the coordinated organization of zebrafish hair bundles, or that in ift88tz288b hair bundles, although severely stunted, kinocilia are still able to fulfill this role.
Figure 2. ovl/ift88 mutant hair cells lack kinocilia yet develop normally.
(A and B) SEM image of an ift88tz288b immature (A) and mature (B) hair bundle. (C and D) Tubulin and actin stain of bundles at 5 dpf in wildtype (C) and ift88tz288b (D).
(E) The average number of hair cells per neuromast at 2 dpf in wildtype and ift88tz288b neuromasts (n > 13 neuromasts).
(F) Proportion of FM 1-43 labeled hair cells in ift88tz288b and wildtype neuromasts at 2 dpf (n > 13 neuromasts).
(G) The average FM 1-43 intensity per neuromast at 2 dpf (n > 13 neuromasts). An unpaired Student's t-test was used to compare number of hair cells per neuromast in (E and G). A Chi squared Fisher exact test was used to compare proportion of FM 1-43 labeled hair cells in (F). k = kinocilium. Scale bars: 500 nm (A and B), 5 μm (C and D). Error bars are s.e.m.
In addition to unaltered hair-bundle development, we observed that at 2 dpf, a stage when each neuromast contains hair cells at multiple developmental stages — early, intermediate, late and mature —ift88tz288b mutant neuromasts had a similar number of hair cells compared to wildtype neuromasts (Figures 2E). Furthermore, we found that in ift88tz288b mutant hair cells, FM 1-43 label progressed on a similar scale in developing hair cells as in wildtype larvae (Figures 2F and 2G), suggesting that a functional, developmental comparison was valid.
The onset of mechanosensitivity precedes FM 1-43 label and requires kinocilia
Normal labeling of mutant hair cells with FM 1-43 suggested that mechanotransduction was unaffected in ift88tz288b larvae. In many species, labeling with FM 1-43 is an accepted method to assess hair-cell activity, however, using a second method for assessing physiological responses in parallel is more comprehensive. Previous studies of hair-cell mechanosensitivity in zebrafish have relied on measurements of extracellular microphonic potentials (Trapani and Nicolson, 2011). Such recordings represent the sum response of all hair cells in a sensory patch. Because each neuromast contains hair cells at different stages (Figures 1), microphonic potentials are not suitable for determining the onset of mechanotransduction. We therefore developed a method to measure hair-cell activity at the resolution of single cells. For these experiments we generated transgenic fish expressing cameleon D3cpv (Palmer et al., 2006), a genetically encoded FRET (fluorescence resonance energy transfer)-based calcium indicator, in hair cells (Figures 3A and 3D).
Using intact transgenic larvae expressing cameleon, we first characterized the responses of wildtype hair cells to confirm that FM 1-43 labeling correlated with mechanosensitivity. For this analysis, we examined neuromasts at 5 dpf and 2 dpf, to compare populations of mature and immature hair cells respectively (Figures 3A-3F). When stimulated along the A-P axis using a fluid-jet, hair cells displayed robust mechanically-evoked calcium responses. Subsequently, when we labeled cells with FM 1-43, we confirmed that the majority of cells at 5 dpf and 2 dpf that labeled with FM 1-43 were mechanosensitive, as defined by a rise in intracellular calcium levels (5 dpf: 94% n = 128 cells; 2 dpf: 84% n = 44 cells).
When we extended our analysis to the immature, FM 1-43 unlabeled cells at 2 dpf, we made a striking observation. In contrast to previous studies, we reproducibly detected mechanosensitive calcium responses in cells that did not label with FM 1-43 (Figures 3D-3F, cell 1; Figure 3G: 67% n = 39 cells). It should be noted that the responses of FM 1-43 unlabeled cells were significantly smaller than responses of FM 1-43 labeled cells (Figure 3G). Although smaller, mechanosensitive responses in FM 1-43 unlabeled cells were reliably detected over a wide range of fluid-jet pressures, using numerous stimulus paradigms and also using a piezo-driven glass fiber to deflect hair bundles (Figures S2E, S2H, and S2I). Previous studies have shown that hair cells with incompletely assembled, improperly tensioned, or closed channels could be labeled by deflecting hair bundles in the presence of FM 1-43 (Lelli et al., 2009). However, we found that neither application of additional FM 1-43, nor sustained deflection in the presence of FM 1-43 could label immature FM 1-43 unlabeled cells that were mechanosensitive (Figures S4A-S4A’” and S4B-S4B’”). These observations suggest that despite their lack of FM 1-43 label, immature responses are robust and immature cells possess functional mechanosensitive channels.
Because the kinocilium is the first apical structures to appear in developing hair cells, one obvious question is whether the kinocilium is required for mechanosensitive responses in immature cells that do not label with FM 1-43. When we examined mechanically-evoked calcium responses in ift88tz288b mutants using our transgenic line expressing cameleon we found that similar to wildtype cells, in ift88tz288b mutants, the majority of FM 1-43 labeled cells were mechanosensitive (Figure 3G: 71% n = 55 cells). Of the ift88tz288b cells that labeled with FM 1-43, the magnitude of the responses was significantly reduced (Figure 3G). However, in contrast to wildtype larvae, we observed that ift88tz288b cells without FM 1-43 label were rarely mechanosensitive (Figure 3G: 6% n = 35 cells). Relative to wildtype, the reduction in response size and loss of a subset of immature responses suggests that either full-length kinocilia are required for transmitting the forces of mechanical stimuli to the bundle or that kinocilia are intrinsically involved in mechanosensitivity during development.
Morphological polarity does not accurately predict functional polarity in immature hair cells
To parse out the exact role of the kinocilia in the acquisition of mechanosensitivity, we characterized developing hair-cell responses in more detail. At 2 dpf, hair cells are positioned in a highly stereotyped fashion within each neuromast, along a line perpendicular to the axis of mechanosensitivity (Figure 3D”). Sister cells are paired across this line, and are fated to have opposing polarities (Figure 3D”) (López-Schier et al., 2004). In this arrangement, when mature, all cells initially present on the left side of the neuromast will respond to posterior stimuli, and those on the right side will respond to anterior stimuli.
In line with this polarized arrangement, in mature cells at 2 dpf, deflection in the positive direction (towards the kinocilium) was accompanied by a rise in intracellular calcium levels (Figure 3E, cell 2), whereas deflection in the negative direction (away from the kinocilium) resulted in a decrease in intracellular calcium, (Figure 3F, cell 2). When stained with phalloidin, the observed functional polarity of each mature cell at 2 dpf correlated with the morphological planar polarity of its hair bundle (Figures 3D-3F, cell 2). Similar results were obtained at 5 dpf when the majority of neuromast hair cells are mature (Figures 3A-3C).
In contrast to mature cells, we observed that the functional polarity of FM 1-43 unlabeled cells was often completely reversed — FM 1-43 unlabeled, immature cells frequently showed an increase in intracellular calcium when stimulated in the negative direction (70% derived from Table 1 early and intermediate FM 1-43 unlabeled cells; Figure 3F, cell 1). Further, in FM 1-43 labeled cells that were still immature, we observed a large subset that responded bi-directionally (increase in intracellular calcium to both negative and positive deflections) (45% derived from Table 1 intermediate and late FM 1-43 labeled cells; Figures 3B and 3C cell 3). Inappropriate functional polarity was surprising since our SEM analysis of hair-bundle ultrastructure revealed that developing bundles had correct planar polarity, which is thought to be predictive of correct functional polarity (Figures 1 and S1C).
Because correct hair-bundle polarity did not correlate with functional polarity in developing cells, it was unclear whether functional polarity was restricted to the A-P axis as in mature cells. To test A-P axis restriction, we examined responses to stimuli oriented at 45° or 90° relative to the A-P axis (Figures S1D-S1G). We observed that in all cells, off-axis responses were substantially reduced (Figure S1D). Residual off-axis responses are likely due to the fact that many hair bundles, even mature ones, are not perfectly aligned along the A-P axis (Figures S1A-S1C). Importantly, the reduction in off-axis response was independent of developmental stage and FM 1-43 label indicating that even the youngest developing hair cells are directionally restricted along the A-P axis (Figures S1D-S1G).
We also performed several controls to determine if the atypical functional polarity in immature hair cells was robust and reproducible. We found that similar results were observed for a range of fluid-jet intensities and using a piezo-driven glass fiber to deflect hair bundles (Figures S2F and S2G). These results indicate that immature functional polarity is robust in response to stimulation with either glass fiber or fluid jet, and the signal generated correlates with the strength of the stimulus.
Functional polarity reverses during hair-bundle development; reversed responses require kinocilia
After confirming the reproducibility of immature hair-cell responses, we next examined whether (1) the patterns of immature functional polarity we observed in wildtype larvae correlated with specific stages of hair-bundle development and (2) if kinocilia were required for atypical functional polarity at specific stages of development.
To test for a correlation between developmental stage and type of response, we first analyzed the functional polarity of individual developing hair cells at 2 dpf, and then determined the developmental stage of each cell based on kinocilial length, FM 1-43 label, and localization of actin-rich bundles (Figures 3D-3D”). Using this approach, we identified a complete reversal of functional polarity during hair-bundle development (Figure 3H, Table 1). Immature early cells predominantly responded to negative deflections, and did not label with FM 1-43 (Figure 3H; Table 1: 75% negative, 11% positive, 15% no response, n = 47). The functional polarity of intermediate cells was more heterogeneous (Figure 3H; Table 1: 27% negative, 24% bi-directional, 29% positive, 20% no response, n = 51), and nearly half of the cells labeled faintly with FM 1-43. Intermediate cells that responded to positive or bi-directional deflections labeled more consistently with FM 1-43 (Table 1). Finally, we observed that all immature cells with late hair bundles were labeled strongly by FM 1-43 and responded to only positive or bi-directional deflections (Figure 3H; Table 1: 49% bi-directional, 46% positive, 5% no response, n = 39). In morphologically mature hair bundles analyzed at 2 dpf, functional polarity was almost exclusively restricted to the positive direction (Figure 3H; Table 1: 4% bi-directional, 94% positive, 2% no response, n = 51). Collectively, these data indicate that developmental changes in hair bundle morphology strongly correlate with changes in functional polarity.
Upon establishing a developmental trend in functional polarity, we next sought to confirm that the loss of mechanosensitivity in FM 1-43 unlabeled ift88tz288b cells was due to a loss at a specific developmental stage rather than affecting all stages of development. On the whole, we found that compared to wildtype larvae, in ift88tz288b mutants, the percentage of mechanosensitive cells at 2 dpf was decreased (Table 1: wild type 89% n = 188, ift88 tz288b 32% n = 72). In wildtype larvae, half of these mechanosensitive cells labeled with FM 1-43 and responded exclusively to positive deflections (Table 1: wild type 47% n = 167). In ift88tz288b mutants, the vast majority of mechanosensitive cells labeled with FM 1-43 and responded exclusively to positive deflections (Table 1: ift88tz288b 91% n = 23). These results are consistent with a loss of mechanosensitivity restricted to hair cells that did not label with FM 1-43. Further, in ift88tz288b mutants, we observed a near absence of mechanosensitive cells at early and intermediate stages of development (Figure 3H; Table 1: wild type 83% n = 98, ift88 tz288b 11% n = 36). At these stages, responses to negative deflections were largely abolished in ift88tz288b mutants (Figure 3H; Table 1: wildtype 50%, ift88tz288b 6%). In addition, at intermediate and late stages we observed no bi-directional responses (Figure 3H; Table 1: wildtype 34% n = 90, ift88tz288b 0% n = 33). The occasional responses to negative deflections observed in ift88tz288b mutants are likely due to residual ift88 maternal RNA and the sporadic presence of kinocilia at 2 dpf in these animals (Huang and Schier, 2009). Based on these results, we conclude that kinocilia are not simply acting to couple forces to stereocilia but rather are specifically required for the reversed functional polarity seen in early and intermediate FM 1-43 unlabeled cells and at later stages for the reversed response component of bi-directional responses. Moreover, these data confirm that kinocilia are required for mechanosensitivity during the initial stages of hair-cell development.
Timescale of functional polarity reversal
Though it was clear that kinocilia were required for the reversed functional polarity in immature hair cells, the time scale underlying these functional changes during development was unresolved. To understand the temporal basis of these functional changes, we took advantage of the rapid development of zebrafish hair bundles (bundles develop from early to mature in 14-16 hours) and conducted long-term in vivo imaging by making hourly measurements of functional polarity in developing cells for 5 hrs. These experiments identified two transitions in functional polarity during hair cell development (Figure 4A).
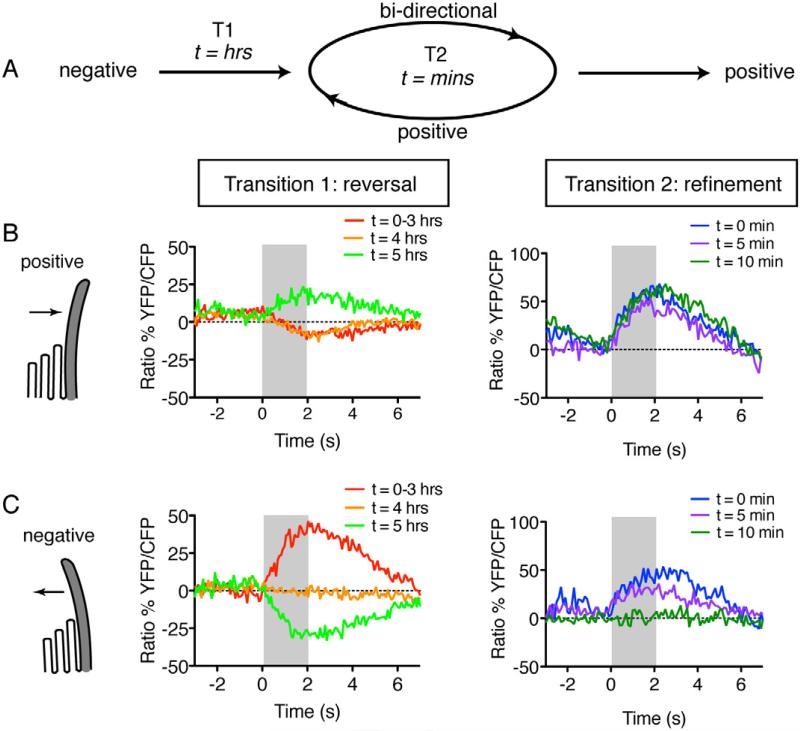
Figure 4. Development of mature functional polarity: first reversal, then refinement.
(A) Overall time scale and functional polarity associated with each transition. Early immature cells first go through Transition 1: reversal. During this period, immature cells reverse their functional polarity from the negative to positive direction. Later immature cells enter Transition 2: refinement. At this stage functional polarity is in rapid flux between bi-directional and positive deflections. Once mature, cells respond to only positive deflections.
(B) Deflection in the positive direction for cells in Transition 1 (T1), or Transition 2 (T2), respectively.
(C) Deflection in the negative direction for the same cells as in (B).
The first transition we observed was in immature early cells. Most of these cells remained responsive only to deflections in the negative direction for the entire 5 hr time period (16/19). However, 3/19 showed a marked functional polarity reversal, transitioning from the negative to the positive direction (Transition 1: Figures 4B and 4C). This transition was relatively slow — it occurred over several hours, and once it occurred, these cells did not revert back to exclusively reversed functional polarity. During this time window we observed no gross changes in cell movements that could account for a reversal in functional polarity. The functional reversal we observe is distinct from previous studies that find sister lateral-line hair cells often rotate after cytokinesis (Wibowo et al., 2011). Post-cytokinetic rotation occurs much earlier during development of lateral line hair-cells, prior to when hair cells insert into the apical epithelium and form hair bundles (data not shown).
The second type of transition we observed was in bi-directionally responding cells. While only 6/77 cells responded bi-directionally at the initiation of imaging, 8 additional cells responded bi-directionally at least once over the subsequent 5 hr imaging period. Further, in less than 1 hr, bi-directionally responding cells could change their functional polarity and respond to positive deflections, or vice versa, a phenomenon we observed in 12/14 bi-directionally responding cells. By increasing our image acquisition rate, we found that 5 minutes was sufficient for a cell to convert from bi-directional to positive functional polarity, or vice versa (Transition 2: Figures 4B and 4C), indicating that at this later developmental stage, cells oscillate rapidly between bi-directional and positive uni-directional functional polarity.
Structural correlates of kinocilial-based mechanosensation
Although we observed a developmental progression in the acquisition of functional polarity, it was unclear how immature, kinocilial-based mechanosensation was functionally distinct from traditional stereocilial-based mechanotransduction. To investigate the structural correlates of kinocilial-based mechanosensation, we first recorded mechanically-evoked calcium responses in developing hair cells and then used SEM to examine hair bundle ultrastructure of these same cells (Figure 5).
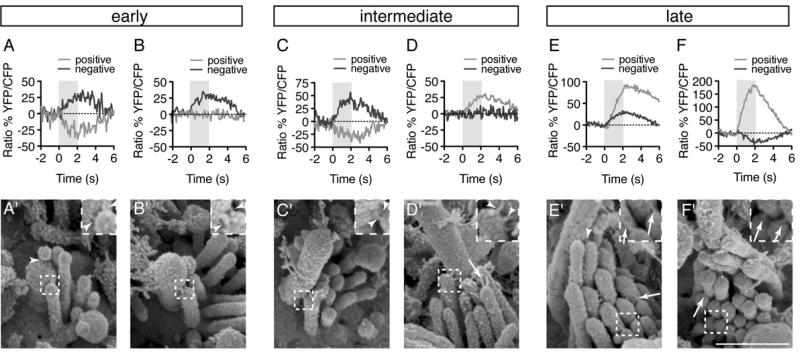
Figure 5. Correlative calcium imaging and SEM reveals a change in link architecture during development.
(A-F) Calcium responses were measured in response to positive and negative hair bundles deflections from cells at each stage of bundle development.
(A’-F’) SEM images of the hair bundles from hair cells represented in (A-F)
All images were taken at 3 dpf, arrowheads indicate kinocilial links, arrows indicate tip links, scale bar is 500 nm. Inset is 2X magnification. For additional link examples see also Figure S3.
For these sequential imaging experiments, we examined 6 early, 15 intermediate and 9 late hair bundles. All cells with early bundles responded to negative deflections (Figures 5A-5B’). SEM imaging demonstrated that half of the early cells had one complete row of stereocilia and half had bundles with two nearly complete rows (example of a single complete row: Figure 5A’; example of 2 rows: Figure 5B’; n = 3 with 1 row, n = 3 with 2 rows). In contrast to early cells, cells which responded bi-directionally (example: Figures 5E and 5E’; n = 3 intermediate, 2 late), or only to positive deflections (example: Figures 5D, 5D’, 5F, and 5F’; n = 4 intermediate, 7 late), had bundles with detectable tip links. These data demonstrate a correlation between tip link formation and the acquisition of mature functional polarity.
Our sequential calcium imaging and SEM experiments revealed a structural feature that was common to all stages of development: kinocilial links. On average we observed two kinocilial links radiating from the kinocilia to the tip of each stereocilium (Figures 5A’-5D’ and S4). Linkages were horizontally oriented or slightly oblique with either an upward or downward angle (Figures 5 and S4). This link architecture is quite different than the arrangement observed in the mouse, chick and bullfrog, where dozens of lateral kinocilial links form a dense network connecting the kinocilium to adjacent stereocilia (Goodyear et al., 2005; Hillman, 1969; Tsuprun et al., 2004).
Because the frequency and location of kinocilial links in zebrafish hair cells appeared more similar to tip links, we reasoned that kinocilial links, via deflection of the kinocilia, might function to gate mechanosensitive channels in immature bundles lacking tip links. Consistent with this hypothesis, ift88tz288b hair bundles, which lack mature kinocilia and atypical immature responses, also lack kinocilial links (Figures 2A and 2B). The absence of atypical responses in ift88tz288b hair cells and the similarities between zebrafish tip and kinocilial links suggest that kinocilia utilize kinocilial links for their mechanosensitive function.
Kinocilial-based mechanosensitivity is pharmacologically similar to mature, tip link-based transduction and requires kinocilial link components Cdh23 and Pcdh15
To further substantiate a role for kinocilial links in kinocilial-based mechanosensation, we examined zebrafish with null mutations in Cdh23 or Pcdh15, components of tip and kinocilial links. We found that similar to mature hair cells at 5 dpf (Figure S4E), Cdh23 and Pcdh15 were both required for mechanosensitive responses at 2 dpf in hair cells at all stages (Figure 6A: cdh23 n = 81, pcdh15 n = 86). In addition, we observed that transient treatment of wildtype hair bundles with BAPTA, which breaks tip and kinocilial links (Assad et al., 1991; Goodyear and Richardson, 2003) also eliminated all responses (Figure 6A). Along with our SEM observations, these results indicate that kinocilial links are part of the gating component required for kinocilial-based mechanosensation in developing hair cells.
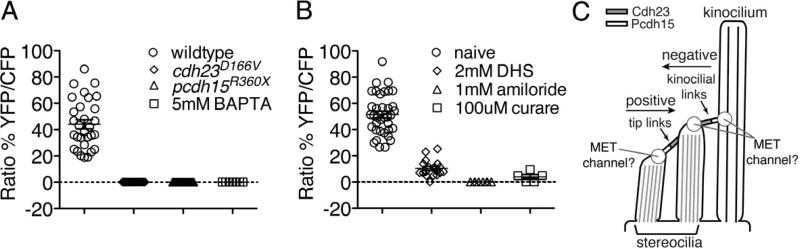
Figure 6. Nascent hair cells require similar linkage components and channel as mature cells.
(A) All mutant cdh23 and pcdh15 cells at 2 dpf did not respond to stimulation. Treatment of wildtype hair cells with BAPTA also eliminated all responses (P < 0.001, n > 7 neuromasts).
(B) The mechanotransduction channel blockers amiloride, curare and DHS significantly blocked responses at 2 dpf (P < 0.001, n > 5 neuromasts).
(C) Diagram of link asymmetry in hair bundles, and the role of tip links versus kinocilial links in mediating mechanosensitivity in the positive and negative direction, respectively.
Circles indicate potential location for mechanosensitive channels that mediate these responses. An unpaired and paired Student's t-tests were used in (A) and (B) respectively. Error bars are s.e.m. See also Figure S4.
Because Cdh23 and Pcdh15 mediate the atypical responses in early cells, we hypothesized that other components of the transduction machinery may be required for reversed functional polarity. To determine if immature, kinocilial-based responses were mediated by a channel similar to that in mature hair cells, we treated wildtype hair cells with the known mechanotransduction channel blockers dihydrostreptomycin (DHS), amiloride, and curare (Farris et al., 2004). Similar to mature cells at 5 dpf (Figure S4D), each of these channel blockers significantly reduced the response magnitude in all hair cells at 2 dpf (Figure 6B: naïve 51.5%, DHS 10.3%, amiloride 0%, curare 3.9%). While the DHS block was less complete at 2 dpf than at 5 dpf, the degree of inhibition at 2 dpf was independent of FM 1-43 labeling (Figure S4C), suggesting that partial DHS insensitivity may be a general feature of immature hair cells, as previously proposed (Santos et al., 2006). These results suggest that in comparison to tip links, kinocilial-based mechanosensation may utilize a pharmacologically similar mechanosensitive channel.
DISCUSSION
In this study, we investigated whether kinocilia have a mechanosensitive role during hair-cell development by using a combination of non-invasive imaging techniques and ultrastructural analysis. Sequential analysis of function and structure in individual hair cells enabled us to demonstrate the dependence of immature mechanosensitive responses on the kinocilium and its associated linkages.
The use of single-cell in vivo calcium imaging revealed several unexpected findings. First, mechanosensitivity in nascent hair cells initiates much earlier than anticipated — many immature hair cells are mechanosensitive, yet do not label with FM 1-43, a vital dye that permeates mechanotransduction channels. Second, the functional polarity of many FM 1-43 unlabeled hair cells is reversed with respect to the morphological planar polarity of the hair bundle. Third, mechanosensitive responses are detectable before tip links are readily observed and well before hair bundles are fully developed. In these hair cells, the presence of a kinocilium and its associated kinocilial links are required to confer mechanosensitivity. By contrast, mature functional polarity requires tip links. These results suggest that a switch from kinocilial links to tip links underlies changes in functional polarity during development of hair cells.
Molecular requirements for reversed functional polarity of immature hair cells
In immature hair cells, a kinocilium and kinocilial links tethered to an adjacent stereocilium appear to be sufficient for mediating mechanosensitivity. As both tip links and kinocilial links are composed of Cdh23 and Pcdh15, the specific requirement for kinocilial links is evident from experiments with ift88tz288b mutants. In these mutants, kinocilia are severely stunted and do not form kinocilial links, but bundles of stereocilia with tip links still form and adopt a staircase arrangement similar to wildtype hair bundles (Figure 2B). Yet, in ift88tz288b mutant hair cells, there is a striking loss of reversed and bi-directional functional polarity. In contrast, responses to positive deflections are preserved. These results, in tandem with a requirement for Cdh23 and Pcdh15, suggest that early reversed functional polarity, including the reversed component of all bi-directional responses, is mediated by kinocilia and kinocilial links.
While our experiments show that Pcdh15 and Cdh23 are required for mechanosensitive responses throughout development, it is not apparent how two linkages with the same molecular composition can mediate opposite functional polarities. One major difference between tip links and kinocilial links is in the asymmetric distribution of the cadherins along the plane of functional polarity. For tip links, there is evidence that Pcdh15 is at the lower end, whereas Cdh23 is at the upper end of each linkage (Kazmierczak et al., 2007). This asymmetric distribution is reversed in kinocilial links relative to tip links, with Pcdh15 attached to the kinocilium and Cdh23 attached to adjacent stereocilia (Figure 6C) (Goodyear et al., 2010; Lelli et al., 2010). This reversed asymmetry is intriguing considering the reversed functional polarity of kinocilial-based responses in immature hair cells. In addition to asymmetry of the linkage components themselves, high-resolution calcium imaging in rat auditory hair cells suggests that mechanotransduction channels are also asymmetrically localized: they are present only at the lower end of each tip link (Beurg et al., 2009). This result is in contrast to an earlier study in bullfrog vestibular hair cells that reported calcium entry into all stereocilia, suggesting that channels may be located at both ends of each link (Denk et al., 1995). Although link asymmetry is an attractive hypothesis to explain immature reversed functional polarity, the location of the mechanotransduction channel may vary according to hair-cell type or species. Whether signaling occurs within the kinocilium or the tallest stereocilium in developing zebrafish hair cells and how this form of mechanosensitivity is functionally reversed awaits further investigation.
Apart from rat auditory hair cells, the location of the mechanotransduction channel is not known in many vertebrate hair cells. However, pharmacological properties of the channel appear to be conserved across species (Farris et al., 2004; Marcotti et al., 2005; Rüsch et al., 1994; Trapani and Nicolson, 2011). Our data show that similar to mature responses, all immature responses are completely blocked by amiloride and curare, but in contrast to mature responses, display partial DHS sensitivity. In addition, the channels of immature cells are FM 1-43 impermeant — suggesting that at least some properties of the mechanotransduction channel differ in kinocilial-based responses. In mature hair cells, sufficient tension in the tip link-mechanotransduction channel complex maintains a high open probability of the channels (Gale et al., 2001), allowing the permeant-channel blockers DHS and FM 1-43 to enter and block channels. Because FM 1-43 does not label immature cells and DHS block is incomplete, it is possible that mechanosensitive channels in these cells may not be open at rest. However, this possibility seems unlikely since sustained deflections in the presence of FM 1-43 failed to produce dye labeling (Figures S4B-S4B’”). In addition, while many early immature cells showed an increase in intracellular calcium levels upon negative deflection, they also showed a corresponding decrease in calcium concentration upon positive deflection, consistent with the idea of active channel closure (Figures 5A and 5C). A more likely explanation is that the channels in immature cells are open at rest, but possess a unique channel or subunit composition that prohibits FM 1-43 entry and hampers DHS permeability.
Structural and molecular changes underlying the developmental progression of functional polarity
Short and long-term imaging of calcium responses in single hair cells revealed that initially, nascent cells are responsive to only negative deflections for several hours before switching to mature or bi-directional functional polarity. The transitional changes in functional polarity are likely to involve both structural and molecular mechanisms. The relatively long interval proceeding the initial switch from reversed functional polarity presumably reflects the time required for large-scale structural maturation of hair bundles, including lengthening of the kinocilium, and the addition of multiple rows of stereocilia and associated tip links (Figures 5A’, 5B’ and 5C’ versus Figures 5D’, 5E’ and 5F’). It is also possible that changes in the angle of kinocilial links during development could account for the responses to negative deflections. For example, in early cells, stereocilia are sometimes taller than the kinocilia, resulting in a downward angle of the link towards the kinocilium (Figure 5A’). This link angle might be optimally oriented for response to negative stimuli. However, we also observed early and intermediate bundles with kinocilia longer than stereocilia; in these cases, the kinocilial links were oriented horizontally or at a slight upward angle and these cells responded robustly to negative deflections (Figures 5B’ and 5C’). Therefore, there is no apparent correlation between functional polarity and the angle of kinocilial links, and it is unlikely that the angle of this linkage accounts for the developmental changes in functional polarity.
After the initial maturation of the hair bundle and the appearance of tip links, the functional polarity of single cells transitions into a second developmental state in which functional polarity oscillates rapidly within minutes between bi-directional and mature functional polarity. Such fast oscillation is surprising and might be explained by continuous variations in tip-link angle produced during bundle maturation. Variation in the angle of tip links would presumably influence linkage tensioning and ultimately channel activity. However, we did not observe bi-directional responses in ift88tz288b mutants in which tip links are unaffected, and therefore this scenario is unlikely. A more consistent explanation is that responses to positive deflections are strictly tip link-based, whereas responses to negative deflections within the bi-directional response are kinocilial-based. In this situation, rapid fluctuations in functional polarity may be due to a transient loss or gain of kinocilial-based responses. Here, a molecular-based mechanism operating on the same time scale as the fluctuations could account for the rapid changes, such as selective downregulation or post-translational modification of kinocilial-based mechanotransduction components. While the exact mechanisms underlying functional polarity reversal are not known at this point, we propose that this process requires a concomitant switch from kinocilial-based to stereocilial-based mechanosensation.
Significance of kinocilial-based mechanotransduction
Similar to other vestibular hair cells, kinocilial links are maintained in mature zebrafish hair cells (Figure S3). However, it is uncertain whether kinocilial-based responses are lost upon maturity or whether they are simply masked by responses generated from tip links. It also seems unlikely that this phenomenon is restricted to zebrafish, as both bi-directional and reversed functional polarity have been reported in immature outer hair cells of rat cochlear explants (Waguespack et al., 2007). The presence of kinocilial links in both hair-cell types, at least at immature stages, suggests that a developmental progression in the acquisition of functional polarity, mediated in part by the kinocilium, may be a common theme in hair-cell development.
A role for the kinocilium in mechanosensitivity is consistent with many other documented examples of ciliary mechanosensation, such as in kidney epithelial cells and invertebrate mechanoreceptor cells (Kahn-Kirby and Bargmann, 2006; Nauli et al., 2003; Walker et al., 2000). Indeed, it has been hypothesized that the kinocilium is a key structural element underlying the evolution of mechanosensation, as has been studied in single-celled organisms such as the choanoflagellates (Fritzsch et al., 2007).
While the functional significance of kinocilial-based responses in early developing hair cells is not yet clear, one early function of kinocilial responses may be in promoting hair cell survival. In zebrafish vestibular hair cells, loss of the kinocilium ultimately results in cell death (Tsujikawa and Malicki, 2004). Interestingly, in mouse auditory cells, specific elimination of kinocilial links also results in degeneration of hair cells (Webb et al., 2011). This degeneration is surprising since mature auditory hair cells do not maintain kinocilia after development. It is therefore possible that calcium signals mediated by kinocilial-based mechanotransduction in immature cells may directly contribute to cell survival or initiate critical differentiation cues, two known important functions of primary cilia signaling in other contexts (Li et al., 2011; Yoshimura et al., 2011).
EXPERIMENTAL PROCEDURES
Zebrafish husbandry
Zebrafish (Danio rerio) were grown at 28°C using standard methods. Larvae were raised in E3 embryo media (5 mM NaCl, 0.17 KCl, 0.33 mM CaCl2 and 0.33 mM MgSO4). Zebrafish mutant alleles used in this study include: cdh23D166V (tj264a allele), pcdh15R306X (th263b allele), ovl/ift88tz288b and have been described previously (Seiler et al., 2005; Söllner et al., 2004; Tsujikawa and Malicki, 2004). Experiments were performed using the following A-P oriented lateral-line neuromasts: first otic neuromast, first dorsal and first four ventral neuromasts of the posterior line.
Vector construction and transgenic lines
Plasmid construction was based on the Tol2/Gateway zebrafish kit (Kwan et al., 2007). The myo6b enhancer (Obholzer et al., 2008) was cloned into the 5’ entry vector (p5E, 228); β-actin (#BC063950) and cameleon D3cpv (a kind gift from Roger Tsien, UC-San Diego) (Palmer et al., 2006) were cloned into the middle entry vector (pME, 237). From the tol2 kit, p3E vector containing GFP (386), or polyA (302) were recombined with our engineered plasmids to create the following constructs: myo6b:D3cpv-pA and myo6b:β-actin-GFP. To generate transgenic fish, plasmid DNA and tol2 transposase mRNA was injected into zebrafish embryos as previously described (Kwan et al., 2007).
Immunofluorescence and confocal imaging
A mouse anti-acetylated α-tubulin antibody was used (clone 6-11B-1, Sigma) to stain kinocilia and Alexa Fluor 568 conjugated phalloidin (Invitrogen) was used to stain hair bundles. Immunohistochemistry was performed on whole-mount larvae following standard immunostaining procedures (see Supplementary Experimental Procedures).
Live and fixed samples were imaged on an upright Zeiss LSM 700 laser-scanning confocal microscope with a 63X 0.95 NA Achroplan or 63X 1.4 NA Plan-Apochromat objective lens respectively. Excitation wavelengths of 488 nm (GFP or YFP), 555 nm (Alexa568 conjugated phalloidin or FM 1-43), or 639 nm (Alexa 647 conjugated secondary) were used with a scan rate of 7 s per pixel with Kalman averaging, 3.0 - 3.5 optical zoom, and 0.5 μm z-sections.
Calcium imaging
To prepare larvae for calcium imaging, individual larvae were first anesthetized with 0.03% 3-amino benzoic acid ethylester (MESAB, Western Chemical Inc.), and pinned onto a Sylgard-filled recording chamber. To suppress movement, larvae were injected with alpha-bungarotoxin (125 μM) into the heart. Larvae were then rinsed with extracellular solution (in mM: 140 NaCl, 2 KCl, 2 CaCl2, 1 MgCl2 and 10 HEPES, pH 7.3, osm 310) without MESAB and allowed to recover.
Stimulation of neuromast hair cells has been previously described (also see Supplementary Experimental Procedures) (Trapani and Nicolson, 2011). Optical recordings were performed on a Zeiss Axioexaminer upright compound microscope equipped with a Dual View beam splitter (Optical Insights) a shutter (Sutter Instruments), and an Orca ER CCD camera (Hamamatsu). Fluorescence images were acquired using MetaMorph (Molecular Devices). Acquisitions were taken at 10-20 Hz, with 4 × 4 binning, using a 63X 1.0 NA Plan-Apochromat Zeiss water-immersion objective. Filter/dichroic pairs were as follows: excitation, 420/40; excitation dichroic, 455; CFP emission, 480/30; emission dichroic, 505; YFP emission, 535/30 (Chroma). For information regarding spectral bleed through, photobleaching and image analysis see Supplementary Experimental Procedures.
Vital labeling and pharmacology
To visualize mature hair cells, larvae were immersed in a 30 μM (2 dpf) or 3 μM (5 dpf) solution of FM 1-43, (Invitrogen) in E3 for 20 s, and immediately washed 4 times in E3. Larvae were imaged by confocal microscopy as described above or by widefield microscopy with the following filter set: excitation 535/50 565LP and emission 620/60, (Chroma). For quantification of FM 1-43 intensity measurements, the intensity of each neuromast was normalized by the number of FM 1-43 labeled hair cells.
The mechanotransduction blockers dihydrostreptomycin (DHS), amiloride, and tubo-curarine (curare) (Sigma) were prepared in extracellular solution. For calcium imaging-experiments, the water-jet micropipette solution was also exchanged with extracellular solution containing drug. To cleave tip links, larvae were incubated in 5 mM 1,2-Bis(2-Aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA, Invitrogen) in extracellular solution for 10 min and washed thoroughly prior to calcium imaging.
Electron microscopy
All SEM images were taken of larvae at 3 dpf. This stage is early enough to observe many developing hair bundles, but late enough to adequately preserve larvae. For our sequential analysis individual larvae were anesthetized with 0.03% MESAB and pinned onto a Sylgard-filled chamber. β-actin-GFP and FM 1-43 confocal imaging or calcium measurements were performed as described above. After imaging, the cupula surrounding neuromasts was removed by incubating larvae in a high dose of MESAB (0.12%) for 25 s. Specimens were then fixed in 2.5% glutaraldehyde, 2 mM CaCl2 in 0.1 M cacodylate buffer (Electron Microscopy Sciences) for 1.5 hrs at room temperature. Samples were washed and post-fixed in 80 mM cacodylate buffer containing 50 mM osmium tetroxide (Electron Microscopy Sciences) and 4 mM CaCl2 for 10 min on ice. Embryos were washed twice with water and then dehydrated in steps from 50% to 100% ethanol at room temperature. Larvae were then critical point dried in CO2 and mounted on carbon covered aluminum stubs. The head and tail tip of each larvae were painted with silver to minimize charging. Lastly samples were sputter coated with gold-palladium. A FEI Sirion XL30 scanning electron microscope was used to acquire images, with a beam strength of 2 kV.
Statistical analysis
A nonparametric Mann-Whitney test (unpaired) or a Wilcoxon matched-pairs signed rank test (paired) was used to compare differences between populations of hair-cell calcium responses. A t-test (paired or unpaired) was used to compare average neuromast calcium responses. A Chi-squared test was used to compare proportion of hair cells responding or proportion labeling with FM 1-43.
Supplementary Material
HIGHLIGHTS.
Kinocilia have a central role in mechanosensation in hair cell during development
Functional polarity is reversed in developing, kinocilial-based mechanotransduction
Functional polarity reverses during hair-cell development
Reversal is coincident with a switch from kinocilial to stereocilial transduction
ACKNOWLEDGMENTS
We thank the Zebrafish International Resource Center for providing ift88 mutants and Chi-bin Chien at the University of Utah for developing and distributing the zebrafish Tol2 gateway system. We thank Greg Baty at Portland State University's Center for Electron Microscopy and Nanofabrication for assistance with image acquisition, and Kateri Spinelli (OHSU) and Gregory Frolenkov at the University of Kentucky for advice on SEM sample preparation. A. Silk, L. Sheets and J. Trapani provided useful comments on the manuscript. This research was supported in part by NIH grant R01 DC006880 and HHMI funding to T.N and K.S.K., and an NIH training grant T32 DK 7680-17 to K.S.K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahmed ZM, Goodyear R, Riazuddin S, Lagziel A, Legan PK, Behra M, Burgess SM, Lilley KS, Wilcox ER, Riazuddin S, Griffith AJ, Frolenkov GI, Belyantseva IA, Richardson GP, Friedman TB. The tip-link antigen, a protein associated with the transduction complex of sensory hair cells, is protocadherin-15. J Neurosci. 2006;26:7022–34. doi: 10.1523/JNEUROSCI.1163-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assad JA, Shepherd GM, Corey DP. Tip-link integrity and mechanical transduction in vertebrate hair cells. Neuron. 1991;7:985–94. doi: 10.1016/0896-6273(91)90343-x. [DOI] [PubMed] [Google Scholar]
- Beurg M, Fettiplace R, Nam J-H, Ricci AJ. Localization of inner hair cell mechanotransducer channels using high-speed calcium imaging. Nat Neurosci. 2009;12:553–8. doi: 10.1038/nn.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey DP, Hudspeth AJ. Analysis of the microphonic potential of the bullfrog's sacculus. J Neurosci. 1983;3:942–61. doi: 10.1523/JNEUROSCI.03-05-00942.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk W, Holt JR, Shepherd GM, Corey DP. Calcium imaging of single stereocilia in hair cells: localization of transduction channels at both ends of tip links. Neuron. 1995;15:1311–21. doi: 10.1016/0896-6273(95)90010-1. [DOI] [PubMed] [Google Scholar]
- Denman-Johnson K, Forge A. Establishment of hair bundle polarity and orientation in the developing vestibular system of the mouse. J Neurocytol. 1999;28:821–35. doi: 10.1023/a:1007061819934. [DOI] [PubMed] [Google Scholar]
- Engström H, Ades HW, Hawkins JE. Structure and Functions of the Sensory Hairs of the Inner Ear. J. Acoust. Soc. Am. 1962;34:1356–63. [Google Scholar]
- Farris HE, LeBlanc CL, Goswami J, Ricci AJ. Probing the pore of the auditory hair cell mechanotransducer channel in turtle. J Physiol. 2004;558:769–92. doi: 10.1113/jphysiol.2004.061267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Beisel KW, Pauley S, Soukup G. Molecular evolution of the vertebrate mechanosensory cell and ear. Int J Dev Biol. 2007;51:663–78. doi: 10.1387/ijdb.072367bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiu K, Nakayama Y, Iida H, Sokabe M, Yoshimura K. Mechanoreception in motile flagella of Chlamydomonas. Nat Cell Biol , . 2011 doi: 10.1038/ncb2214. [DOI] [PubMed] [Google Scholar]
- Gale JE, Marcotti W, Kennedy HJ, Kros CJ, Richardson GP. FM1-43 dye behaves as a permeant blocker of the hair-cell mechanotransducer channel. J Neurosci. 2001;21:7013–25. doi: 10.1523/JNEUROSCI.21-18-07013.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear RJ, Forge A, Legan PK, Richardson GP. Asymmetric distribution of cadherin 23 and protocadherin 15 in the kinocilial links of avian sensory hair cells. J Comp Neurol. 2010;518:4288–97. doi: 10.1002/cne.22456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear RJ, Marcotti W, Kros CJ, Richardson GP. Development and properties of stereociliary link types in hair cells of the mouse cochlea. J Comp Neurol. 2005;485:75–85. doi: 10.1002/cne.20513. [DOI] [PubMed] [Google Scholar]
- Goodyear RJ, Richardson GP. A novel antigen sensitive to calcium chelation that is associated with the tip links and kinocilial links of sensory hair bundles. J Neurosci. 2003;23:4878–87. doi: 10.1523/JNEUROSCI.23-12-04878.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Géléoc GSG, Holt JR. Developmental acquisition of sensory transduction in hair cells of the mouse inner ear. Nat Neurosci. 2003;6:1019–20. doi: 10.1038/nn1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman DE. New ultrastructural findings regarding a vestibular ciliary apparatus and its possible functional significance. Brain Res. 1969;13:407–12. doi: 10.1016/0006-8993(69)90301-1. [DOI] [PubMed] [Google Scholar]
- Huang P, Schier AF. Dampened Hedgehog signaling but normal Wnt signaling in zebrafish without cilia. Development. 2009;136:3089–98. doi: 10.1242/dev.041343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth AJ, Jacobs R. Stereocilia mediate transduction in vertebrate hair cells (auditory system/cilium/vestibular system). Proc Natl Acad Sci U S A. 1979;76:1506–9. doi: 10.1073/pnas.76.3.1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C, Roper VC, Foucher I, Qian D, Banizs B, Petit C, Yoder BK, Chen P. Ciliary proteins link basal body polarization to planar cell polarity regulation. Nat Genet. 2008;40:69–77. doi: 10.1038/ng.2007.54. [DOI] [PubMed] [Google Scholar]
- Kahn-Kirby AH, Bargmann CI. TRP channels in C. elegans. Annu Rev Physiol. 2006;68:719–36. doi: 10.1146/annurev.physiol.68.040204.100715. [DOI] [PubMed] [Google Scholar]
- Kazmierczak P, Sakaguchi H, Tokita J, Wilson-Kubalek EM, Milligan RA, Müller U, Kachar B. Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature. 2007;449:87–91. doi: 10.1038/nature06091. [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Hilding DA. The development of the stria vascularis in the mouse. Acta Otolaryngol. 1966;62:277–91. doi: 10.3109/00016486609119573. [DOI] [PubMed] [Google Scholar]
- Kwan KM, Fujimoto E, Grabher C, Mangum BD, Hardy ME, Campbell DS, Parant JM, Yost HJ, Kanki JP, Chien C-B. The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev Dyn. 2007;236:3088–99. doi: 10.1002/dvdy.21343. [DOI] [PubMed] [Google Scholar]
- Lelli A, Asai Y, Forge A, Holt JR, Géléoc GSG. Tonotopic gradient in the developmental acquisition of sensory transduction in outer hair cells of the mouse cochlea. J Neurophysiol. 2009;101:2961–73. doi: 10.1152/jn.00136.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelli A, Kazmierczak P, Kawashima Y, Müller U, Holt JR. Development and regeneration of sensory transduction in auditory hair cells requires functional interaction between cadherin-23 and protocadherin-15. J Neurosci. 2010;30:11259–69. doi: 10.1523/JNEUROSCI.1949-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Saito M, Chuang J-Z, Tseng Y-Y, Dedesma C, Tomizawa K, Kaitsuka T, Sung C-H. Ciliary transition zone activation of phosphorylated Tctex-1 controls ciliary resorption, S-phase entry and fate of neural progenitors. Nat Cell Biol. 2011;13:402–11. doi: 10.1038/ncb2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Schier H, Starr CJ, Kappler JA, Kollmar R, Hudspeth AJ. Directional cell migration establishes the axes of planar polarity in the posterior lateral-line organ of the zebrafish. Dev Cell. 2004;7:401–12. doi: 10.1016/j.devcel.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Marcotti W, van Netten SM, Kros CJ. The aminoglycoside antibiotic dihydrostreptomycin rapidly enters mouse outer hair cells through the mechano-electrical transducer channels. J Physiol. 2005;567:505–21. doi: 10.1113/jphysiol.2005.085951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Somlo S, Makova S, Tian X, Brueckner M. Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell. 2003;114:61–73. doi: 10.1016/s0092-8674(03)00511-7. [DOI] [PubMed] [Google Scholar]
- Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AEH, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–37. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- Obholzer N, Wolfson S, Trapani JG, Mo W, Nechiporuk A, Busch-Nentwich E, Seiler C, Sidi S, Söllner C, Duncan RN, Boehland A, Nicolson T. Vesicular glutamate transporter 3 is required for synaptic transmission in zebrafish hair cells. J Neurosci. 2008;28:2110–8. doi: 10.1523/JNEUROSCI.5230-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AE, Giacomello M, Kortemme T, Hires SA, Lev-Ram V, Baker D, Tsien RY. Ca2+ indicators based on computationally redesigned calmodulin-peptide pairs. Chem Biol. 2006;13:521–30. doi: 10.1016/j.chembiol.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Pickles JO, Comis SD, Osborne MP. Cross-links between stereocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hear Res. 1984;15:103–12. doi: 10.1016/0378-5955(84)90041-8. [DOI] [PubMed] [Google Scholar]
- Rüsch A, Kros CJ, Richardson GP. Block by amiloride and its derivatives of mechano-electrical transduction in outer hair cells of mouse cochlear cultures. J Physiol. 1994;474:75–86. doi: 10.1113/jphysiol.1994.sp020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos F, MacDonald G, Rubel EW, Raible DW. Lateral line hair cell maturation is a determinant of aminoglycoside susceptibility in zebrafish (Danio rerio). Hear Res. 2006;213:25–33. doi: 10.1016/j.heares.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Schwander M, Kachar B, Müller U. Review series: The cell biology of hearing. J Cell Biol. 2010;190:9–20. doi: 10.1083/jcb.201001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler C, Finger-Baier KC, Rinner O, Makhankov YV, Schwarz H, Neuhauss SCF, Nicolson T. Duplicated genes with split functions: independent roles of protocadherin15 orthologues in zebrafish hearing and vision. Development. 2005;132:615–23. doi: 10.1242/dev.01591. [DOI] [PubMed] [Google Scholar]
- Shin J-B, Adams D, Paukert M, Siba M, Sidi S, Levin M, Gillespie PG, Gründer S. Xenopus TRPN1 (NOMPC) localizes to microtubule-based cilia in epithelial cells, including inner-ear hair cells. Proc Natl Acad Sci U S A. 2005;102:12572–7. doi: 10.1073/pnas.0502403102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shotwell SL, Jacobs R, Hudspeth AJ. Directional sensitivity of individual vertebrate hair cells to controlled deflection of their hair bundles. Ann N Y Acad Sci. 1981;374:1–10. doi: 10.1111/j.1749-6632.1981.tb30854.x. [DOI] [PubMed] [Google Scholar]
- Si F, Brodie H, Gillespie PG, Vazquez AE, Yamoah EN. Developmental assembly of transduction apparatus in chick basilar papilla. J Neurosci. 2003;23:10815–26. doi: 10.1523/JNEUROSCI.23-34-10815.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemens J, Lillo C, Dumont RA, Reynolds A, Williams DS, Gillespie PG, Müller U. Cadherin 23 is a component of the tip link in hair-cell stereocilia. Nature. 2004;428:950–5. doi: 10.1038/nature02483. [DOI] [PubMed] [Google Scholar]
- Söllner C, Rauch G-J, Siemens J, Geisler R, Schuster SC, Müller U, Nicolson T, Tübingen 2000 Screen Consortium Mutations in cadherin 23 affect tip links in zebrafish sensory hair cells. Nature. 2004;428:955–9. doi: 10.1038/nature02484. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Smith CA. Structure of the chicken's inner ear: SEM and TEM study. Am J Anat. 1978;153:251–71. doi: 10.1002/aja.1001530206. [DOI] [PubMed] [Google Scholar]
- Tanimoto M, Ota Y, Inoue M, Oda Y. Origin of inner ear hair cells: morphological and functional differentiation from ciliary cells into hair cells in zebrafish inner ear. J Neurosci. 2011;31:3784–94. doi: 10.1523/JNEUROSCI.5554-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney LG, Tilney MS, DeRosier DJ. Actin filaments, stereocilia, and hair cells: how cells count and measure. Annu Rev Cell Biol. 1992;8:257–74. doi: 10.1146/annurev.cb.08.110192.001353. [DOI] [PubMed] [Google Scholar]
- Trapani JG, Nicolson T. Mechanism of spontaneous activity in afferent neurons of the zebrafish lateral-line organ. J Neurosci. 2011;31:1614–23. doi: 10.1523/JNEUROSCI.3369-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujikawa M, Malicki J. Intraflagellar transport genes are essential for differentiation and survival of vertebrate sensory neurons. Neuron. 2004;42:703–16. doi: 10.1016/s0896-6273(04)00268-5. [DOI] [PubMed] [Google Scholar]
- Tsuprun V, Goodyear RJ, Richardson GP. The structure of tip links and kinocilial links in avian sensory hair bundles. Biophys J. 2004;87:4106–12. doi: 10.1529/biophysj.104.049031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waguespack J, Salles FT, Kachar B, Ricci AJ. Stepwise morphological and functional maturation of mechanotransduction in rat outer hair cells. J Neurosci. 2007;27:13890–902. doi: 10.1523/JNEUROSCI.2159-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RG, Willingham AT, Zuker CS. A Drosophila mechanosensory transduction channel. Science. 2000;287:2229–34. doi: 10.1126/science.287.5461.2229. [DOI] [PubMed] [Google Scholar]
- Wibowo I, Pinto-Teixeira F, Satou C, Higashijima S, López-Schier H. Compartmentalized Notch signaling sustains epithelial mirror symmetry. Development. 2011;138:1143–52. doi: 10.1242/dev.060566. [DOI] [PubMed] [Google Scholar]
- Webb SW, Grillet N, Andrade LR, Xiong W, Swarthout L, Della Santina CC, Kachar B, Müller U. Regulation of PCDH15 function in mechanosensory hair cells by alternative splicing of the cytoplasmic domain. Development. 2011;138:1607–17. doi: 10.1242/dev.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura K, Kawate T, Takeda S. Signaling through the primary cilium affects glial cell survival under a stressed environment. Glia. 2011;59:333–44. doi: 10.1002/glia.21105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.