Abstract
The discovery and de-discovery of the xenotropic murine leukemia virus-related virus (XMRV) has been a tumultuous roller-coaster ride for scientists and patients. The initial associations of XMRV with chronic fatigue syndrome and prostate cancer, while providing much hope and optimism, have now been discredited and/or retracted following overwhelming evidence that 1) numerous patient cohorts from around the world are XMRV-negative, 2) the initial reports of XMRV-positive patients were due to contamination with mouse DNAs, XMRV plasmid DNA, or virus from the 22Rv1 cell line and 3) XMRV is a laboratory-derived virus generated in the mid 1990’s through recombination during passage of a prostate tumor xenograft in immuno-compromised mice. While these developments are disappointing to scientists and patients, they provide a valuable road map of potential pitfalls to the would-be microbe hunters.
Introduction
Xenotropic murine leukemia virus-related virus (XMRV), a new gammaretrovirus, was reported to be associated with human prostate cancer (PC) in 2006 and chronic fatigue syndrome (CFS) in 2009. This discovery was followed by a dediscovery phase in which the evidence supporting a link between XMRV and human disease was refuted. Here, we will briefly summarize the evidence for and against a role for XMRV in human disease.
Evidence for XMRV infection in humans
In 2006, Urisman et al. identified XMRV from patients with a rare familial PC using Virochip microarray and PCR analysis [1]. The identified viral sequences were closely related to xenotropic murine leukemia viruses, hence the name XMRV. This finding, if verified, would have identified a new potential human pathogen that had crossed species from mice to humans. Further support for the viral link was that forty percent (8/20) of the PC patients that were homozygous for the R462Q mutation in the RNase L gene, an interferon-responsive endoribonuclease involved in innate antiviral activity, were XMRV-positive [2]. Even though patients carrying the RNase L mutation might be more susceptible to viral infection, no known virus had yet been identified in PC patients. In September 2009, Schlaberg et al. reported that 27% of their PC patients were XMRV-positive by PCR and immunohistochemistry [3]; however, they did not find any association with the RNase L mutation. These results not only confirmed the detection of XMRV in human PC, but also, alarmingly, suggested that XMRV might be present in a large percentage of all PC patients, not just rare PCs in families that may have deficiencies in their antiviral responses.
In October 2009, excitement further brewed after Lombardi et al. published in Science that 67% of CFS patients, as well as 3.7% of healthy controls, were positive for XMRV [4]. The authors claimed detection of anti-XMRV antibodies in patients, detection of XMRV DNA by PCR, and isolation of infectious virus from patients’ CD4+ T cells. The Schlaberg et al. and Lombardi et al. papers seemed to provide two independent confirmations of the Urisman et al. study. Furthermore, the reports sounded the alarm that XMRV, a new human virus with unknown pathogenic potential, was present in a significant proportion of the apparently healthy population (3.7%) and might be associated with other human diseases of unknown etiology.
In September 2010, Lo et al. reported that they could detect fragments of polytropic MLV-related sequences, but not XMRV, in 32/37 CFS patients and 3/44 healthy donors by PCR [5], suggesting that other gammaretroviruses could also be present in CFS patients. Even though Lo et al. failed to detect XMRV in CFS patients, their report was viewed as a confirmation of the Lombardi et al. study.
At the time, these four papers together provided support for the idea that XMRV was linked to PC and CFS, and that a new retrovirus could potentially be passed between individuals through the blood supply (like HIV-1) or other unknown routes of transmission since healthy patient controls had also been found positive for XMRV.
Evidence against XMRV infection in humans
Absence of XMRV in multiple patient cohorts and normal blood donors
An eruption of papers followed as researchers began to investigate different prostate and CFS patient cohorts for XMRV. Although a few reports cited frequent or rare associations between XMRV and PC or CFS [6–10], far more papers (>30) failed to find any association at all [9–43]. XMRV was also found to not be associated with immunocompromised HIV- or HCV-infected patients [44–52] or linked to breast cancer, lupus, lymphomas, autism, multiple sclerosis, or human vaccines [53–62]. The failure to detect XMRV in PC and CFS patients was first reported from Germany [40], UK [39, 43], and the Netherlands [38]. These observations led some to propose that XMRV was geographically restricted to the United States. However, other investigators also failed to detect XMRV in PC or CFS patient cohorts in the United States, making this explanation unlikely [23, 31, 33, 37]. Furthermore, XMRV was not found in 1000 U.S. blood donors tested by Abbott Diagnostics [20] and 17,249 blood donors tested by the American Red Cross from six different U.S. regions [63]. The explanations for the discrepancies between the XMRV-positive and XMRV-negative studies rested on increasingly-farfetched arguments such as the definitions used for selection of CFS patients in specific cohorts, the status of the CFS disease, the PCR buffers, primers, or magnesium concentrations.
XMRV inhibition by human restriction factors
All vertebrates possess intracellular defense mechanisms that restrict the replication of invading pathogens. If XMRV were a true human pathogen it would need to circumvent numerous host antiviral restriction mechanisms to establish a spreading viral infection. APOBEC3 proteins, TRIM5α, and BST2/tetherin are recently characterized host restriction factors that potently inhibit HIV-1 and other retroviruses. The human APOBEC3G (A3G) and APOBEC3F (A3F) block HIV-1 replication (in the absence of the HIV-1 Vif protein) by inducing G-to-A hypermutation, and inhibiting reverse transcription and integration [64]. The HIV-1 Vif protein interacts with A3G and A3F to target them for proteosomal degradation, and hence escape the restriction by A3G and A3F. MLV does not encode a Vif-like protein and its sensitivity to inhibition by A3G and A3F led us investigate how XMRV could efficiently replicate in human PBMCs, which express A3G and A3F, as reported by Lombardi et al.. In March 2010, Groom et al. [65] and Paprotka et al. [66] showed that, in single-replication-cycle experimental systems, XMRV replication was indeed potently inhibited by human APOBEC3 proteins. It was also shown that XMRV replication was inhibited by BST2/tetherin, but not human TRIM5α [65]. The effects of APOBEC3 proteins on XMRV replication were also confirmed by other laboratories [67, 68].
We subsequently determined that while XMRV could infect activated peripheral blood cells from normal human donors, it could not establish a spreading infection and increase virus production exponentially as observed in a A3G/A3F-negative human T cell line [69] (Figure 1). The ability of primate host restriction mechanisms to limit XMRV replication has been observed in independent studies in which pigtail macaques were experimentally infected with >1010 RNA copy equivalents of XMRV, a huge dose of infectious particles (>105), compared to similar experiments with HIV-1 in which infectious particle doses of 50 – 100 are sufficient to productively infect the animals [70]. Despite this high infectious dose, virus replication peaked at low levels of around 2000 copies/ml and became undetectable 4 weeks after infection. Sequencing of proviral DNAs revealed extensive G-to-A hypermutation, indicating APOBEC3-mediated restriction. Similar results were independently obtained by infection of rhesus macaques with XMRV [71]. These studies indicate that if infection of humans occurred with XMRV, its replication would most likely be severely limited by APOBEC3 and other host restriction factors.
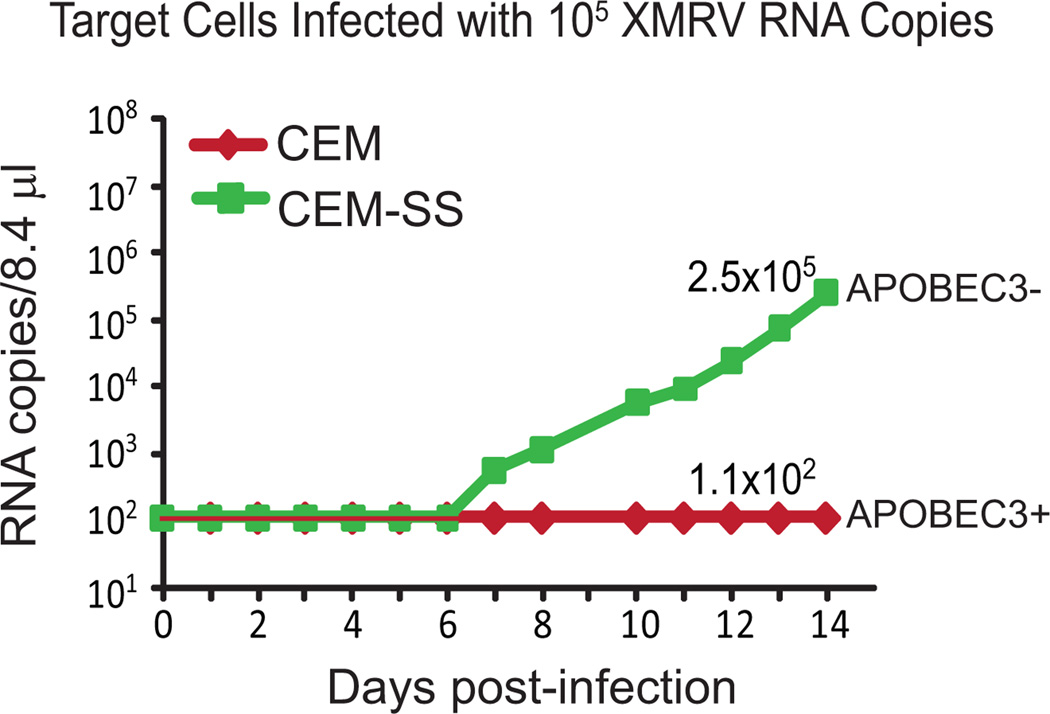
Figure 1. Replication kinetics of XMRV in APOBEC3 positive and negative cells.
CEM (APOBEC3G/3F+) and CEM-SS (APOBEC3G/3F−) were infected with 1 × 105 RNA copies of XMRV from cell supernatants derived from the 22Rv1 cell line. Robust replication was seen in the CEM-SS cells, but no increase in XMRV RNA copies was observed up to 14 days post-infection in the CEM cells, indicating that there was little or no XMRV replication and spread in cells containing APOBEC3G or APOBEC3F.
XMRV is a laboratory-derived recombinant between two endogenous murine retroviruses
In 2009, the Miller group showed that a human prostate cancer-derived cell line, 22Rv1, produced large amounts of XMRV (~2 × 1010 RNA copies/ml) [72]. Based on the Urisman et al. and Schlaberg et al. reports, it was assumed that the prostate tumor used to generate 22Rv1 was infected with XMRV. The XMRV produced from the 22Rv1 cell line was nearly identical to all patient-associated XMRV sequences, which is unexpected for a retrovirus replicating at high rates in humans, because error-prone replication is expected to generate extensive genetic diversity. Based on the lack of genetic diversity between 22Rv1-derived and patient-derived XMRVs [73, 74], similarities between XMRV and murine XMVs, and previous reports of infection of human xenografts with murine or other host-derived retroviruses, we hypothesized that XMRV might have been generated during xenograft passage of the patient tumor (CWR22) that gave rise to 22Rv1 cells [75]. CWR22 xenografts had been passaged in nude mice, most likely outbred strains NU/NU from Charles River Laboratories or Hsd nude from Harlan Laboratories. Early xenografts from passage 3, 7, and 8 (several months per passage) were obtained along with late xenograft samples, including a passage that was used to derive the 22Rv1 cell line. PCR analysis using XMRV-specific primers showed that the early xenografts did not harbor XMRV; however, the late xenografts and the subsequent 22Rv1 cell line were positive for XMRV. These results suggested that the original patient tumor did not contain XMRV, and XMRV was not required to maintain and propagate the PC cells. Likewise, if replication-competent XMRV were present in the original CWR22 xenograft, it would have had ample time to spread to all of the cells during the several years of passage in mice.
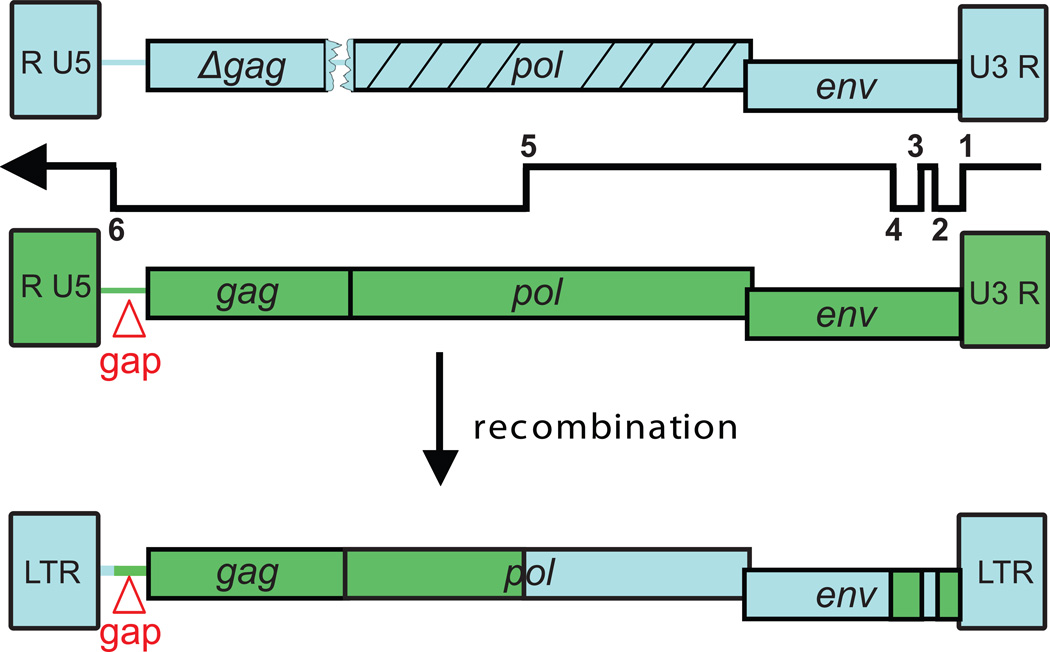
Even though the xenograft samples were XMRV-negative, we detected extremely low levels of XMRV envelope sequences (<1–3 copies/100 cells; 1–3%) [75]. Subsequent studies showed that the XMRV envelope signal was coming from contaminating mouse DNA (1 -3%) associated with the xenograft samples. It is nearly impossible to remove all infiltrating mouse tissue from an excised human tumor xenograft, and in this instance it worked to our advantage. PCR and sequencing analysis of the xenografts identified sequences for two unknown mouse endogenous proviruses, PreXMRV-1 and PreXMRV-2 (Figure 2). PreXMRV-1 mapped to mouse chromosome 3 and contained LTRs and a >3000-nt stretch in pol and env that was 99.9% identical to XMRV. PreXMRV-1 also contained a 16-nt deletion in gag and a frameshift in pol that would render it replication incompetent. PreXMRV-2 mapped to mouse chromosome 12 and contained >3000-nt stretch in gag and pol that was 99.9% identical to XMRV. All reading frames were open suggesting that PreXMRV-2 could be replication competent. Aligning the two proviruses showed that these two proviruses fit together like a puzzle (Figure 2); only 6 template switches between the two viral genomes were required to generate a recombinant that differed from XMRV by only 4-nt changes, only one of which led to an amino acid change.
Figure 2. XMRV recombination junctions.
XMRV was generated by six template switches during reverse transcription between PreXMRV-1 (blue) and PreXMRV-2 (green). Red triangle, 24 bp gap in the gag leader sequence. LTR, long terminal repeats.
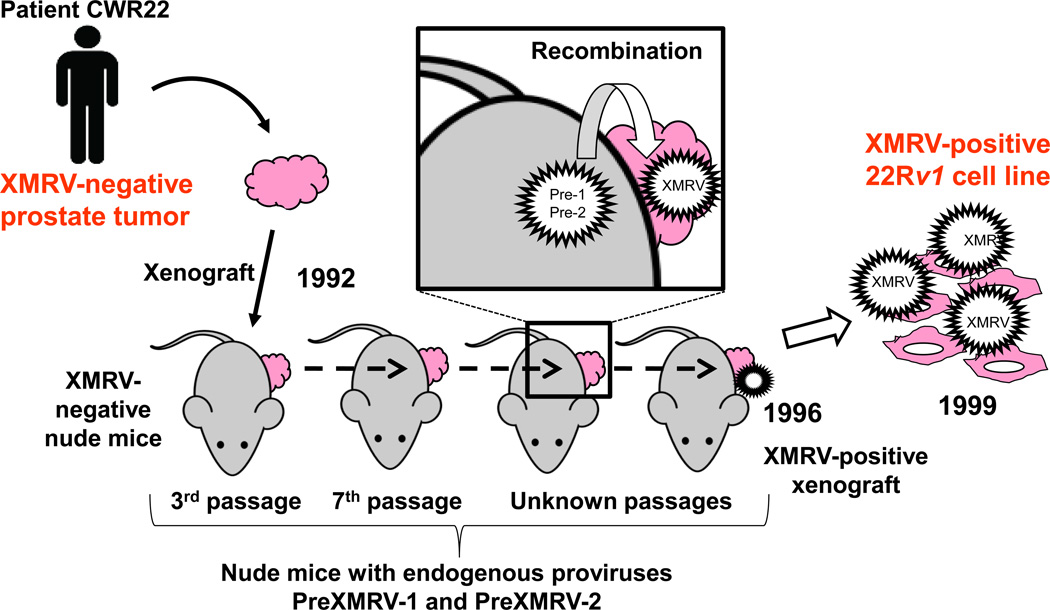
These data supported the hypothesis that these two viruses were copackaged during xenografting and underwent recombination to generate XMRV, which subsequently spread throughout the xenograft (Figure 3). This event occurred around 1993–1996, since the late xenograft samples after 1996 were all positive for XMRV but the early xenograft samples before 1993 were not. This timeline then proposes that any patient diagnosed with CFS or PC before 1993 could not have been infected with XMRV; thus, any archived patient samples from before 1993 should test XMRV negative.
Figure 3. Origin of XMRV.
Timeline showing the transplantation of the original patient CWR22 prostate tumor into nude mice in 1992. Both the prostate tumor and the nude mice are XMRV-negative. The nude mice contain two endogenous proviruses, PreXMRV-1 (Pre-1) and PreXMRV-2 (Pre-2), which copackage and undergo recombination to generate XMRV (box inset). XMRV spreads throughout the human prostate tissue, from which the 22Rv1 cell line is derived, and hence is XMRV-positive.
Further studies showed that nude mouse strains NU/NU and Hsd contained endogenous proviral copies of PreXMRV-1 and/or PreXMRV-2 by chromosomal site-specific PCR [76]. Exhaustive scanning for the distribution of XMRV, PreXMRV-1 or PreXMRV-2 in 94 laboratory or wild-derived strains showed that 1) no strain contained XMRV, 2) PreXMRV-1 was found predominantly in Asian mice, 3) PreXMRV-2 was found predominantly in European mice, and 4) only 3 laboratory strains (NU/NU, Hsd nude and C57BR/cd) were positive for both PreXMRV-1 and PreXMRV-2 [76]. Together, these data indicated that it was very unlikely that the two XMRV ancestors would have been present in the same mouse, which would be a prerequisite for generating a recombinant in the wild. It was also very unlikely that the recombinant could have occurred prior to the 1990s and itself circulating as an endogenous provirus.
While our studies inferred that the original prostate tumor CWR22 was XMRV-negative, the original tumor sample was not available for us to study. Recently the Silverman lab obtained archived paraffin-embedded tissue blocks from the prostate tumor CWR22 and demonstrated it to be negative for XMRV [77]. In another study, Yang et al. also showed that archived early xenograft samples from CWR22 in their laboratory were XMRV-negative [78]. These independent studies provide further evidence that XMRV was generated during propagation of the CWR22 xenograft in nude mice.
The probability of generating XMRV independently is vanishingly small
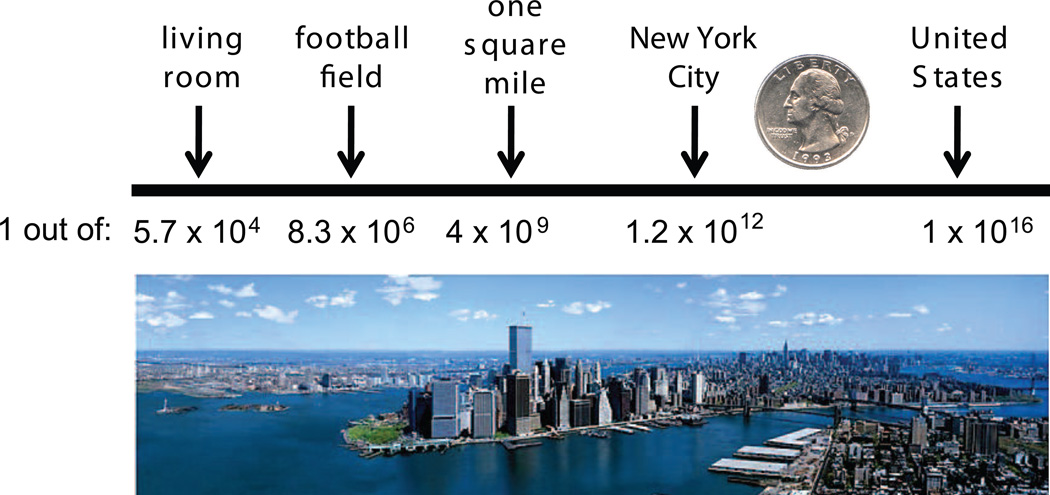
The chance that PreXMRV-1 and PreXMRV-2 could have recombined and independently generated a virus identical in sequence to XMRV is vanishing small. Surveys of the Hsd nude population show that 53% of the mice contain both proviruses [76] and they are not normally expressed (Delviks-Frankenberry, et al., unpublished). Xenotropic viruses generated by recombination between PreXMRV-1 and PreXMRV-2 would need host human tissue xenografts to infect and propagate, since the Xpr1 receptor on Hsd nude mouse cells (Xpr1n) does not support XMRV entry [76, 79]. Given that it took several years of passage before XMRV arose, the necessary events must be very rare. Finally, the most compelling argument against generation of XMRV independently is that even if the two viral RNAs would copackage to form a heterozygus virion, the chance that the exact same recombinant (XMRV) would be generated is on the order of 1.3 × 10−12 [75]. To illustrate this low probability, we compared the size of a quarter (about one square inch) with different areas (living room, football field, etc.), and determined that New York City has approximately 1.2 × 1012 inches. Thus, the probability of generating XMRV independently is about the same as a quarter dropped from a helicopter landing on another quarter on the ground in New York City (Figure 4). Current experiments examining the recombination potential between PreXRMV-1 and PreXMRV-2 in tissue culture show that, as expected, these two proviruses can recombine and form replication competent viruses; however, none of these viruses were generated using the same template switches that generated XMRV (Delviks-Frankenberry, et al., unpublished). Together, these results imply that all XMRV sequences identified in patient samples originated from the 22Rv1 cell line (or the CWR22 xenografts) and therefore arose through laboratory contamination.
Figure 4. Probability of independently generating XMRV.
Different areas are shown in square inches. The probability of generating XMRV, a virus identical in sequence, by recombination between PreXMRV-1 and PreXMRV-2 would be about the same as of dropping a quarter from a helicopter and have it fall on top of another quarter in New York City.
Independent studies directly refute evidence supporting XMRV and X-MLV infection in humans
Mouse DNA contamination is frequent
There is also strong evidence supporting the conclusion that detection of MLV-related sequences by PCR in patient samples is not due to infection. In December 2010, several papers showed that contamination with mouse DNA [74, 80–87] is a common occurrence not only in human DNA or tissue samples, but also common laboratory reagents such as PCR buffers and RT-PCR enzymes. Mouse DNA contamination was the most likely explanation for the presence of polytropic MLV-like sequences found in CFS patient samples in the Lo et al. study [24] and perhaps some reports of XMRV in human samples, since the PCR primers were not designed to exclude endogenous MLV sequences. Indeed, all reported XMRV-specific primers are expected to detect either PreXMRV-1 or PreXMRV-2. Another source of contamination is cloned XMRV plasmid DNA. The Silverman lab showed that the CFS patient samples reported to be positive for XMRV by Lombardi et al. were unambiguously contaminated with the VP62 XMRV plasmid, leading to a partial retraction of the paper by the authors [88]. Yet another potential source of contamination is the XMRV virus produced from the 22Rv1 cell line, which was widely used in prostate cancer labs for at least 10 years before it was shown to produce large amounts of infectious XMRV [72]. Cross contamination of cell lines through normal tissue culture practices in laboratories where the 22Rv1 cell line was used has been reported [89].
No chromosomal integration sites are confirmed for XMRV in patients
One of the strongest arguments in favor of XMRV infection in humans was a report of 14 chromosomal integration sites demonstrating linkage of XMRV proviral and human genomic sequences from prostate tumors [90, 91]. The only way such combinations of sequence could arise is following infection of a human cell by XMRV. These findings were challenged by a report from the Towers lab, which showed that two of the 14 integration sites that were presumably recovered from human prostate tumors were previously identified by the same lab as integration sites in an experimentally infected human cell line [92]. Since retroviral integration sites are selected randomly (or nearly randomly) by the virus, the results indicated that PCR contamination was almost certainly responsible for at least two of the integration sites, and strongly implied that all of the presumed chromosomal integration sites were the result of contamination. The authors of the original study later conceded that the prostate tumor XMRV integration sites could not be confirmed [93].
Previously identified XMRV-positive patients are XMRV-negative in independent studies
To date, more than 50 papers have been published citing no association between XMRV and CFS, PC, or other diseases. Knox et al. substantially advanced the view that XMRV is not involved in human disease by analyzing 43 CFS patients that had been previously determined to be XMRV-positive in the original Lombardi et al. paper. Their results showed that all of these patients were negative for XMRV DNA/RNA, infectious virus, or XMRV-specific antibodies [29]. Likewise, Shin et al. also tested 14 patients from the original Lombardi et al. paper and showed that these patients were XMRV-negative [41]. Furthermore, testing of blinded samples from 15 healthy controls and 15 patients previously reported to be XMRV/MLV-positive (including 5 samples from the Lo et al. study that were positive for MLV-like sequences) by 9 independent laboratories failed to detect XMRV or MLV in patient samples [24]. Additionally, while the other seven labs could reliably distinguish between the XMRV positive and negative controls, the Whittemore Peterson Institute and the Ruscetti lab could not reliably detect XMRV in the positive controls, and found some positives among the negative controls. These results diminished any confidence in the assays and results reported in the Lombardi et al. and Lo et al. studies and any evidence that XMRV or a related virus was infecting CFS patients.
Kearney and colleagues, including Dr. Ruscetti, a coauthor of Lombardi et al., provided further evidence that plasma samples and cell lines processed in the Ruscetti lab were contaminated with mouse genomic and mitochondrial DNA as well as XMRV [94]. The XMRV sequences present in these samples were not genetically different from XMRV produced in the 22Rv1 cell line. A human cell line (293T) that was maintained in the Ruscetti laboratory was shown to be contaminated with XMRV, suggesting a possible source for the contamination that lead to the isolation of infectious virus from patients in the Lombardi et al. study.
Treatment of CFS patient PBMCs with 5-azacytidine was omitted from Lombardi et al. paper
In September 2011, Abbie Smith, a graduate student and virology blogger, (http://scienceblogs.com/erv/) revealed that Dr. Judy Mikovits, the corresponding author of the Lombardi et al. study, presented a figure at a meeting that turned out to be identical to one in the original paper, but with different patient numbers and experimental conditions (John Cohen, ScienceInsider, http://news.sciencemag.org/scienceinsider/2011/10/xmrv-researcher-fired.html). This revelation led the authors to concede that the patient-derived PBMCs in Lombardi et al. had been treated with 5-azacytidine, an agent used to demethylate DNA and induce transcription from latent genes and proviruses, but did not include this treatment in the paper because “it was not germane.” The omission of such critical information from the paper cast further doubt on the validity of the entire study.
Retraction of the Lombardi et al. and Lo et al. studies
On December 22, 2011, Science Editor Bruce Alberts fully retracted Lombardi et al. because 1) the authors had failed to reliably detect XMRV or other MLVs in CFS patients, 2) evidence of poor quality control, 3) the fact that the authors had already partially retracted several figures, and 4) for admittedly omitting important information from the legend of Figure 2C. On January 3, 2012, Lo et al. retracted their paper for several reasons, including the fact that they were unable to detect antiviral antibodies in patients, isolate virus by culture, or demonstrate integration of XMRV in the human genome. As Lo et al. stated, they retracted the conclusions of their paper because “the association of murine gammaretroviruses with CFS has not withstood the test of time or of independent verification”. The Urisman et al. paper, containing the original report of XMRV, as well as the Schlaberg et al. paper, claiming association of XMRV with PC, stands unretracted.
Conclusions
Although, as of this writing, one large study of the XMRV-CFS association remains to be reported, the results to date lead to only one conclusion. What lessons can future virologists glean from the XMRV experience? First, rule out DNA contamination, which is an increasingly frequent occurrence as techniques become ever more sensitive. Second, any claims of a novel virus in humans must be substantiated with fully sequencing the entire viral genome, not just PCR fragments, and providing evidence that the virus is replication competent. Third, experimental samples and controls should be collected and processed at the same time in parallel, since differential treatment of samples and controls could lead to contamination and false association. Finally, contamination of cell lines with viruses is probably far more prevalent than previously suspected; akin to mycoplasma testing, methods and protocols should be established to facilitate routine checking of cell lines for viral contamination.
HIGHLIGHTS.
XMRV is a laboratory-derived virus
Mouse DNA contamination is abundant
XMRV is not linked to CFS or prostate cancer
No evidence of XMRV infection in humans
Acknowledgements
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. JMC was supporte by research grant R37 CA089441 from the National Cancer Institute and was a Research Professor of the American Cancer Society with support from the FM Kirby Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1. Urisman A, Molinaro RJ, Fischer N, Plummer SJ, Casey G, Klein EA, Malathi K, Magi-Galluzzi C, Tubbs RR, Ganem D, et al. Identification of a novel Gammaretrovirus in prostate tumors of patients homozygous for R462Q RNASEL variant. PLoS Pathog. 2006;2:e25. doi: 10.1371/journal.ppat.0020025. * First paper to claim an association between XMRV and prostate cancer.
- 2.Silverman RH. A scientific journey through the 2-5A/RNase L system. Cytokine Growth Factor Rev. 2007;18:381–388. doi: 10.1016/j.cytogfr.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schlaberg R, Choe DJ, Brown KR, Thaker HM, Singh IR. XMRV is present in malignant prostatic epithelium and is associated with prostate cancer, especially high-grade tumors. Proc Natl Acad Sci U S A. 2009;106:16351–16356. doi: 10.1073/pnas.0906922106. * Second paper to claim an association between XMRV and prostate cancer.
- 4. Lombardi VC, Ruscetti FW, Das Gupta J, Pfost MA, Hagen KS, Peterson DL, Ruscetti SK, Bagni RK, Petrow-Sadowski C, Gold B, et al. Detection of an infectious retrovirus, XMRV, in blood cells of patients with chronic fatigue syndrome. Science. 2009;326:585–589. doi: 10.1126/science.1179052. * First paper to claim an association between XMRV and chronic fatigue syndrome.
- 5. Lo SC, Pripuzova N, Li B, Komaroff AL, Hung GC, Wang R, Alter HJ. Detection of MLV-related virus gene sequences in blood of patients with chronic fatigue syndrome and healthy blood donors. Proc Natl Acad Sci U S A. 2010;107:15874–15879. doi: 10.1073/pnas.1006901107. * MLV-like sequences are claimed to be associated with chronic fatigue syndrome.
- 6.Arnold RS, Makarova NV, Osunkoya AO, Suppiah S, Scott TA, Johnson NA, Bhosle SM, Liotta D, Hunter E, Marshall FF, et al. XMRV infection in patients with prostate cancer: novel serologic assay and correlation with PCR and FISH. Urology. 2010;75:755–761. doi: 10.1016/j.urology.2010.01.038. [DOI] [PubMed] [Google Scholar]
- 7.Fischer N, Hellwinkel O, Schulz C, Chun FK, Huland H, Aepfelbacher M, Schlomm T. Prevalence of human gammaretrovirus XMRV in sporadic prostate cancer. J Clin Virol. 2008;43:277–283. doi: 10.1016/j.jcv.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 8.Danielson BP, Ayala GE, Kimata JT. Detection of xenotropic murine leukemia virus-related virus in normal and tumor tissue of patients from the southern United States with prostate cancer is dependent on specific polymerase chain reaction conditions. J Infect Dis. 2010;202:1470–1477. doi: 10.1086/656146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verhaegh GW, de Jong AS, Smit FP, Jannink SA, Melchers WJ, Schalken JA. Prevalence of human xenotropic murine leukemia virus-related gammaretrovirus (XMRV) in Dutch prostate cancer patients. Prostate. 2010;71:415–420. doi: 10.1002/pros.21255. [DOI] [PubMed] [Google Scholar]
- 10.Switzer WM, Jia H, Zheng H, Tang S, Heneine W. No association of xenotropic murine leukemia virus-related viruses with prostate cancer. PLoS One. 2011;6:e19065. doi: 10.1371/journal.pone.0019065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendoza R, Silverman RH, Klein EA, Miller AD. No Biological Evidence of XMRV in Blood or Prostatic Fluid from Prostate Cancer Patients. PLoS One. 2012;7:e36073. doi: 10.1371/journal.pone.0036073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groom HC, Warren AY, Neal DE, Bishop KN. No evidence for infection of UK prostate cancer patients with XMRV, BK virus, Trichomonas vaginalis or human papilloma viruses. PLoS One. 2012;7:e34221. doi: 10.1371/journal.pone.0034221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blomberg J, Sheikholvaezin A, Elfaitouri A, Blomberg F, Sjosten A, Mattson Ulfstedt J, Pipkorn R, Kallander C, Ohrmalm C, Sperber G. Phylogeny-directed search for murine leukemia virus-like retroviruses in vertebrate genomes and in patients suffering from myalgic encephalomyelitis/chronic fatigue syndrome and prostate cancer. Adv Virol. 2011;2011:341294. doi: 10.1155/2011/341294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oakes B, Qiu X, Levine S, Hackett J, Jr, Huber BT. Failure to Detect XMRVSpecific Antibodies in the Plasma of CFS Patients Using Highly Sensitive Chemiluminescence Immunoassays. Adv Virol. 2011;2011:854540. doi: 10.1155/2011/854540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kearney MF, Lee K, Bagni RK, Wiegand A, Spindler J, Maldarelli F, Pinto PA, Linehan WM, Vocke CD, Delviks-Frankenberry KA, et al. Nucleic Acid, Antibody, and Virus Culture Methods to Detect Xenotropic MLV-Related Virus in Human Blood Samples. Adv Virol. 2011;2011:272193. doi: 10.1155/2011/272193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arredondo M, Hackett J, Jr, Bethencourt FR, Trevino A, Escudero D, Collado A, Qiu X, Swanson P, Soriano V, Mendoza CD. Prevalence of Xenotropic Murine Leukemia Virus-Related Virus Infection in Different Risk Populations in Spain. AIDS Res Hum Retroviruses. 2012 doi: 10.1089/AID.2011.0149. [DOI] [PubMed] [Google Scholar]
- 17.Mo F, Wyatt AW, Wu C, Lapuk AV, Marra MA, Gleave ME, Volik SV, Collins CC. Next-generation sequencing of prostate tumors provides independent evidence of xenotropic murine leukemia virus-related gammaretrovirus contamination. J Clin Microbiol. 2012;50:536–537. doi: 10.1128/JCM.06170-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steffen I, Tyrrell DL, Stein E, Montalvo L, Lee TH, Zhou Y, Lu K, Switzer WM, Tang S, Jia H, et al. No evidence for XMRV nucleic acids, infectious virus or anti-XMRV antibodies in Canadian patients with chronic fatigue syndrome. PLoS One. 2011;6:e27870. doi: 10.1371/journal.pone.0027870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang S, Zhao J, Haleyur Giri Setty MK, Devadas K, Gaddam D, Viswanath R, Wood O, Zhang P, Hewlett IK. Absence of detectable XMRV and other MLV-related viruses in healthy blood donors in the United States. PLoS One. 2011;6:e27391. doi: 10.1371/journal.pone.0027391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiu X, Swanson P, Tang N, Leckie GW, Devare SG, Schochetman G, Hackett J., Jr Seroprevalence of xenotropic murine leukemia virus-related virus in normal and retrovirus-infected blood donors. Transfusion. 2012;52:307–316. doi: 10.1111/j.1537-2995.2011.03395.x. [DOI] [PubMed] [Google Scholar]
- 21.Stieler K, Schindler S, Schlomm T, Hohn O, Bannert N, Simon R, Minner S, Schindler M, Fischer N. No detection of XMRV in blood samples and tissue sections from prostate cancer patients in Northern Europe. PLoS One. 2011;6:e25592. doi: 10.1371/journal.pone.0025592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luczkowiak J, Martinez-Prats L, Sierra O, Fiorante S, Rubio R, Pulido F, Otero JR, Delgado R. Lack of the detection of XMRV or polytropic MLV-related sequences in blood cells from HIV-1-infected patients in Spain. J Acquir Immune Defic Syndr. 2012;59:101–104. doi: 10.1097/QAI.0b013e318238b596. [DOI] [PubMed] [Google Scholar]
- 23.Ali MA, Dale JK, Kozak CA, Goldbach-Mansky R, Miller FW, Straus SE, Cohen JI. Xenotropic murine leukemia virus-related virus is not associated with chronic fatigue syndrome in patients from different areas of the us in the 1990s. Virol J. 2011;8:450. doi: 10.1186/1743-422X-8-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Simmons G, Glynn SA, Komaroff AL, Mikovits JA, Tobler LH, Hackett J, Jr, Tang N, Switzer WM, Heneine W, Hewlett IK, et al. Failure to confirm XMRV/MLVs in the blood of patients with chronic fatigue syndrome: a multi-laboratory study. Science. 2011;334:814–817. doi: 10.1126/science.1213841. ** A nine-independent lab study confirming a lack of XMRV in patients previously.
- 25.Cool M, Bouchard N, Masse G, Laganiere B, Dumont A, Hanna Z, Phaneuf D, Morisset R, Jolicoeur P. No detectable XMRV in subjects with chronic fatigue syndrome from Quebec. Virology. 2011;420:66–72. doi: 10.1016/j.virol.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 26.Akgul B, Pfister D, Knuchel R, Heidenreich A, Wieland U, Pfister H. No evidence for a role of xenotropic murine leukaemia virus-related virus and BK virus in prostate cancer of German patients. Med Microbiol Immunol. 2012;201:245–248. doi: 10.1007/s00430-011-0215-0. [DOI] [PubMed] [Google Scholar]
- 27.Mi Z, Lu Y, Zhang S, An X, Wang X, Chen B, Wang Q, Tong Y. Absence of xenotropic murine leukemia virus-related virus in blood donors in China. Transfusion. 2012;52:326–331. doi: 10.1111/j.1537-2995.2011.03267.x. [DOI] [PubMed] [Google Scholar]
- 28.Jerome KR, Diem K, Huang ML, Selke S, Corey L, Buchwald D. Xenotropic murine leukemia virus-related virus in monozygotic twins discordant for chronic fatigue syndrome. Diagn Microbiol Infect Dis. 2011;71:66–71. doi: 10.1016/j.diagmicrobio.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Knox K, Carrigan D, Simmons G, Teque F, Zhou Y, Hackett J, Jr, Qiu X, Luk KC, Schochetman G, Knox A, et al. No evidence of murine-like gammaretroviruses in CFS patients previously identified as XMRV-infected. Science. 2011;333:94–97. doi: 10.1126/science.1204963. ** Patients in the original Lombardi et al. paper were retested and found to be XMRV-negative.
- 30.Schutzer SE, Rounds MA, Natelson BH, Ecker DJ, Eshoo MW. Analysis of cerebrospinal fluid from chronic fatigue syndrome patients for multiple human ubiquitous viruses and xenotropic murine leukemia-related virus. Ann Neurol. 2011;69:735–738. doi: 10.1002/ana.22389. [DOI] [PubMed] [Google Scholar]
- 31.Sakuma T, Hue S, Squillace KA, Tonne JM, Blackburn PR, Ohmine S, Thatava T, Towers GJ, Ikeda Y. No evidence of XMRV in prostate cancer cohorts in the Midwestern United States. Retrovirology. 2011;8:23. doi: 10.1186/1742-4690-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furuta RA, Miyazawa T, Sugiyama T, Kuratsune H, Ikeda Y, Sato E, Misawa N, Nakatomi Y, Sakuma R, Yasui K, et al. No association of xenotropic murine leukemia virus-related virus with prostate cancer or chronic fatigue syndrome in Japan. Retrovirology. 2011;8:20. doi: 10.1186/1742-4690-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Satterfield BC, Garcia RA, Jia H, Tang S, Zheng H, Switzer WM. Serologic and PCR testing of persons with chronic fatigue syndrome in the United States shows no association with xenotropic or polytropic murine leukemia virusrelated viruses. Retrovirology. 2011;8:12. doi: 10.1186/1742-4690-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sabunciyan S, Mandelberg N, Rabkin CS, Yolken R, Viscidi R. No difference in antibody titers against xenotropic MLV related virus in prostate cancer cases and cancer-free controls. Mol Cell Probes. 2011;25:134–136. doi: 10.1016/j.mcp.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hohn O, Strohschein K, Brandt AU, Seeher S, Klein S, Kurth R, Paul F, Meisel C, Scheibenbogen C, Bannert N. No evidence for XMRV in German CFS and MS patients with fatigue despite the ability of the virus to infect human blood cells in vitro. PLoS One. 2010;5:e15632. doi: 10.1371/journal.pone.0015632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong P, Li J, Li Y. Failure to detect Xenotropic murine leukaemia virus-related virus in Chinese patients with chronic fatigue syndrome. Virol J. 2010;7:224. doi: 10.1186/1743-422X-7-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Switzer WM, Jia H, Hohn O, Zheng H, Tang S, Shankar A, Bannert N, Simmons G, Hendry RM, Falkenberg VR, et al. Absence of evidence of xenotropic murine leukemia virus-related virus infection in persons with chronic fatigue syndrome and healthy controls in the United States. Retrovirology. 2010;7:57. doi: 10.1186/1742-4690-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Kuppeveld FJ, de Jong AS, Lanke KH, Verhaegh GW, Melchers WJ, Swanink CM, Bleijenberg G, Netea MG, Galama JM, van der Meer JW. Prevalence of xenotropic murine leukaemia virus-related virus in patients with chronic fatigue syndrome in the Netherlands: retrospective analysis of samples from an established cohort. BMJ. 2010;340:c1018. doi: 10.1136/bmj.c1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erlwein O, Kaye S, McClure MO, Weber J, Wills G, Collier D, Wessely S, Cleare A. Failure to detect the novel retrovirus XMRV in chronic fatigue syndrome. PLoS One. 2010;5:e8519. doi: 10.1371/journal.pone.0008519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hohn O, Krause H, Barbarotto P, Niederstadt L, Beimforde N, Denner J, Miller K, Kurth R, Bannert N. Lack of evidence for xenotropic murine leukemia virusrelated virus(XMRV) in German prostate cancer patients. Retrovirology. 2009;6:92. doi: 10.1186/1742-4690-6-92. ** First paper to report absence of XMRV in a large patient cohort.
- 41. Shin CH, Bateman L, Schlaberg R, Bunker AM, Leonard CJ, Hughen RW, Light AR, Light KC, Singh IR. Absence of XMRV retrovirus and other murine leukemia virus-related viruses in patients with chronic fatigue syndrome. J Virol. 2011;85:7195–7202. doi: 10.1128/JVI.00693-11. ** Patients from the Lombardi et al. paper were found to be negative for XMRV.
- 42. Aloia AL, Sfanos KS, Isaacs WB, Zheng Q, Maldarelli F, De Marzo AM, Rein A. XMRV: a new virus in prostate cancer? Cancer Res. 2010;70:10028–10033. doi: 10.1158/0008-5472.CAN-10-2837. * A total of 800 U.S. prostate cancer patients test negative for XMRV.
- 43.Groom HC, Boucherit VC, Makinson K, Randal E, Baptista S, Hagan S, Gow JW, Mattes FM, Breuer J, Kerr JR, et al. Absence of xenotropic murine leukaemia virus-related virus in UK patients with chronic fatigue syndrome. Retrovirology. 2010;7:10. doi: 10.1186/1742-4690-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gingaras C, Danielson BP, Vigil KJ, Vey E, Arduino RC, Kimata JT. Absence of XMRV in peripheral blood mononuclear cells of ARV-treatment naive HIV-1 infected and HIV-1/HCV coinfected individuals and blood donors. PLoS One. 2012;7:e31398. doi: 10.1371/journal.pone.0031398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Delviks-Frankenberry KA, Chaipan C, Bagni R, Wyvill K, Yarchoan R, Pathak VK. Lack of Detection of Xenotropic Murine Leukemia Virus-Related Virus in HIV-1 Lymphoma Patients. Adv Virol. 2011;2011:797820. doi: 10.1155/2011/797820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spindler J, Hackett J, Jr, Qiu X, Wiegand A, Boltz VF, Swanson P, Bream JH, Jacobson LP, Li X, Rinaldo CR, et al. Prevalence of XMRV nucleic acid and antibody in HIV-1-Infected men and in men at risk for HIV-1 Infection. Adv Virol. 2011;2011:268214. doi: 10.1155/2011/268214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maggi F, Focosi D, Lanini L, Sbranti S, Mazzetti P, Macera L, Davini S, De Donno M, Mariotti ML, Antonelli G, et al. Xenotropic murine leukaemia virusrelated virus is not found in peripheral blood cells from treatment-naive human immunodeficiency virus-positive patients. Clin Microbiol Infect. 2012;18:184–188. doi: 10.1111/j.1469-0691.2011.03580.x. [DOI] [PubMed] [Google Scholar]
- 48.Gray ER, Garson JA, Breuer J, Edwards S, Kellam P, Pillay D, Towers GJ. No evidence of XMRV or related retroviruses in a London HIV-1-positive patient cohort. PLoS One. 2011;6:e18096. doi: 10.1371/journal.pone.0018096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang S, Zhao J, Viswanath R, Nyambi PN, Redd AD, Dastyar A, Spacek LA, Quinn TC, Wang X, Wood O, et al. Absence of detectable xenotropic murine leukemia virus-related virus in plasma or peripheral blood mononuclear cells of human immunodeficiency virus Type 1-infected blood donors or individuals in Africa. Transfusion. 2010;51:463–468. doi: 10.1111/j.1537-2995.2010.02932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cornelissen M, Zorgdrager F, Blom P, Jurriaans S, Repping S, van Leeuwen E, Bakker M, Berkhout B, van der Kuyl AC. Lack of detection of XMRV in seminal plasma from HIV-1 infected men in The Netherlands. PLoS One. 2010;5:e12040. doi: 10.1371/journal.pone.0012040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kunstman KJ, Bhattacharya T, Flaherty J, Phair JP, Wolinsky SM. Absence of xenotropic murine leukemia virus-related virus in blood cells of men at risk for and infected with HIV. AIDS. 2010;24:1784–1785. doi: 10.1097/qad.0b013e32833b76fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Korn K, Reil H, Ensser A, Knoll A. No evidence of XMRV infection in immunocompromised patients and HIV-positive individuals from Germany. Infection. 2012;40:181–184. doi: 10.1007/s15010-012-0249-2. [DOI] [PubMed] [Google Scholar]
- 53.Khan G, Philip PS, Naase M, Al Zarouni KM. No evidence for the involvement of XMRV or MCV in the pathogenesis of breast cancer. Br J Cancer. 2012;106:1166–1170. doi: 10.1038/bjc.2012.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robinson MJ, Tuke PW, Erlwein O, Tettmar KI, Kaye S, Naresh KN, Patel A, Walker MM, Kimura T, Gopalakrishnan G, et al. No Evidence of XMRV or MuLV Sequences in Prostate Cancer, Diffuse Large B-Cell Lymphoma, or the UK Blood Donor Population. Adv Virol. 2011;2011:782353. doi: 10.1155/2011/782353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Switzer WM, Zheng H, Simmons G, Zhou Y, Tang S, Shankar A, Kapusinszky B, Delwart EL, Heneine W. No evidence of murine leukemia virus-related viruses in live attenuated human vaccines. PLoS One. 2011;6:e29223. doi: 10.1371/journal.pone.0029223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Waugh EM, Jarrett RF, Shield L, Montgomery D, Dean RT, Mitchell A, Greaves MF, Gallagher A. The retrovirus XMRV is not directly involved in the pathogenesis of common types of lymphoid malignancy. Cancer Epidemiol Biomarkers Prev. 2011;20:2232–2236. doi: 10.1158/1055-9965.EPI-11-0561. [DOI] [PubMed] [Google Scholar]
- 57.Balada E, Castro-Marrero J, Felip L, Vilardell-Tarres M, Ordi-Ros J. Xenotropic murine leukemia virus-related virus (XMRV) in patients with systemic lupus erythematosus. J Clin Immunol. 2011;31:584–587. doi: 10.1007/s10875-011-9535-5. [DOI] [PubMed] [Google Scholar]
- 58.Lintas C, Guidi F, Manzi B, Mancini A, Curatolo P, Persico AM. Lack of infection with XMRV or other MLV-related viruses in blood, post-mortem brains and paternal gametes of autistic individuals. PLoS One. 2011;6:e16609. doi: 10.1371/journal.pone.0016609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Satterfield BC, Garcia RA, Gurrieri F, Schwartz CE. PCR and serology find no association between xenotropic murine leukemia virus-related virus (XMRV) and autism. Mol Autism. 2010;1:14. doi: 10.1186/2040-2392-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barnes E, Flanagan P, Brown A, Robinson N, Brown H, McClure M, Oxenius A, Collier J, Weber J, Gunthard HF, et al. Failure to detect xenotropic murine leukemia virus-related virus in blood of individuals at high risk of bloodborne viral infections. J Infect Dis. 2010;202:1482–1485. doi: 10.1086/657167. [DOI] [PubMed] [Google Scholar]
- 61.Maric R, Pedersen FS, Kjeldbjerg A, Moeller-Larsen A, Bahrami S, Brudek T, Petersen T, Christensen T. Absence of xenotropic murine leukaemia virusrelated virus in Danish patients with multiple sclerosis. J Clin Virol. 2010;49:227–228. doi: 10.1016/j.jcv.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 62.Jeziorski E, Foulongne V, Ludwig C, Louhaem D, Chiocchia G, Segondy M, Rodiere M, Sitbon M, Courgnaud V. No evidence for XMRV association in pediatric idiopathic diseases in France. Retrovirology. 2010;7:63. doi: 10.1186/1742-4690-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dodd RY, Hackett J, Jr, Linnen JM, Dorsey K, Wu Y, Zou S, Qiu X, Swanson P, Schochetman G, Gao K, et al. Xenotropic murine leukemia virus-related virus does not pose a risk to blood recipient safety. Transfusion. 2012;52:298–306. doi: 10.1111/j.1537-2995.2011.03450.x. * Greater then 17,000 blood donors tested and found negative for XMRV.
- 64.Smith JL, Bu W, Burdick RC, Pathak VK. Multiple ways of targeting APOBEC3- virion infectivity factor interactions for anti-HIV-1 drug development. Trends Pharmacol Sci. 2009;30:638–646. doi: 10.1016/j.tips.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Groom HC, Yap MW, Galao RP, Neil SJ, Bishop KN. Susceptibility of xenotropic murine leukemia virus-related virus (XMRV) to retroviral restriction factors. Proc Natl Acad Sci U S A. 2010;107:5166–5171. doi: 10.1073/pnas.0913650107. * APOBEC3 proteins and BST2/tetherin, but not TRIM5α, block XMRV replication.
- 66. Paprotka T, Venkatachari NJ, Chaipan C, Burdick R, Delviks-Frankenberry KA, Hu WS, Pathak VK. Inhibition of xenotropic murine leukemia virus-related virus by APOBEC3 proteins and antiviral drugs. J Virol. 2010;84:5719–5729. doi: 10.1128/JVI.00134-10. * Host proteins human APOBEC3G, human APOBEC3F and murine A3 inhibit XMRV replication.
- 67.Bogerd HP, Zhang F, Bieniasz PD, Cullen BR. Human APOBEC3 proteins can inhibit xenotropic murine leukemia virus-related virus infectivity. Virology. 2011;410:234–239. doi: 10.1016/j.virol.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stieler K, Fischer N. Apobec 3G efficiently reduces infectivity of the human exogenous gammaretrovirus XMRV. PLoS One. 2010;5:e11738. doi: 10.1371/journal.pone.0011738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chaipan C, Dilley KA, Paprotka T, Delviks-Frankenberry KA, Venkatachari NJ, Hu WS, Pathak VK. Severe restriction of xenotropic murine leukemia virusrelated virus replication and spread in cultured human peripheral blood mononuclear cells. J Virol. 2011;85:4888–4897. doi: 10.1128/JVI.00046-11. * XMRV is unable to produce a spreading infection in PBMCs in tissue culture.
- 70. Del Prete GQ, Kearney MF, Spindler J, Wiegand A, Chertova E, Roser JD, Estes JD, Hao XP, Trubey CM, Lara A, et al. Restricted replication of xenotropic murine leukemia virus-related virus in pigtailed macaques. J Virol. 2011;86:3152–3166. doi: 10.1128/JVI.06886-11. * Pigtail macaques infected with XMRV clear the virus and XMRV genomes are hypermutated.
- 71.Zhang A, Bogerd H, Villinger F, Das Gupta J, Dong B, Klein EA, Hackett J, Jr, Schochetman G, Cullen BR, Silverman RH. In vivo hypermutation of xenotropic murine leukemia virus-related virus DNA in peripheral blood mononuclear cells of rhesus macaque by APOBEC3 proteins. Virology. 2011;421:28–33. doi: 10.1016/j.virol.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Knouf EC, Metzger MJ, Mitchell PS, Arroyo JD, Chevillet JR, Tewari M, Miller AD. Multiple integrated copies and high-level production of the human retrovirus XMRV (xenotropic murine leukemia virus-related virus) from 22Rv1 prostate carcinoma cells. J Virol. 2009;83:7353–7356. doi: 10.1128/JVI.00546-09. * 22Rv1cells produce XMRV.
- 73.Katzourakis A, Hue S, Kellam P, Towers GJ. Phylogenetic analysis of murine leukemia virus sequences from longitudinally sampled chronic fatigue syndrome patients suggests PCR contamination rather than viral evolution. J Virol. 2011;85:10909–10913. doi: 10.1128/JVI.00827-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hue S, Gray ER, Gall A, Katzourakis A, Tan CP, Houldcroft CJ, McLaren S, Pillay D, Futreal A, Garson JA, et al. Disease-associated XMRV sequences are consistent with laboratory contamination. Retrovirology. 2010;7:111. doi: 10.1186/1742-4690-7-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Paprotka T, Delviks-Frankenberry KA, Cingoz O, Martinez A, Kung HJ, Tepper CG, Hu WS, Fivash MJ, Jr, Coffin JM, Pathak VK. Recombinant origin of the retrovirus XMRV. Science. 2011;333:97–101. doi: 10.1126/science.1205292. ** XMRV was shown to be a recombinant of endogenous proviruses PreXMRV-1 and PreXMRV-2.
- 76. Cingoz O, Paprotka T, Delviks-Frankenberry KA, Wildt S, Hu WS, Pathak VK, Coffin JM. Characterization, mapping, and distribution of the two XMRV parental proviruses. J Virol. 2012;86:328–338. doi: 10.1128/JVI.06022-11. ** In depth srceen of wild and laboratory mice showing distribution of PreXMRV-1, PreXMRV-2 and lack of XMRV
- 77.Das Gupta J, Luk KC, Tang N, Gaughan C, Klein EA, Kandel ES, Hackett J, Jr, Silverman RH. Absence of XMRV and Closely Related Viruses in Primary Prostate Cancer Tissues Used to Derive the XMRV-Infected Cell Line 22Rv1. PLoS One. 2012;7:e36072. doi: 10.1371/journal.pone.0036072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang J, Battacharya P, Singhal R, Kandel ES. Xenotropic murine leukemia virusrelated virus (XMRV) in prostate cancer cells likely represents a laboratory artifact. Oncotarget. 2011;2:358–362. doi: 10.18632/oncotarget.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yan Y, Liu Q, Kozak CA. Six host range variants of the xenotropic/polytropic gammaretroviruses define determinants for entry in the XPR1 cell surface receptor. Retrovirology. 2009;6:87. doi: 10.1186/1742-4690-6-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Smith RA. Contamination of clinical specimens with MLV-encoding nucleic acids: implications for XMRV and other candidate human retroviruses. Retrovirology. 2010;7:112. doi: 10.1186/1742-4690-7-112. * Showed mouse contamination in clinical samples.
- 81.Zheng H, Jia H, Shankar A, Heneine W, Switzer WM. Detection of murine leukemia virus or mouse DNA in commercial RT-PCR reagents and human DNAs. PLoS One. 2011;6:e29050. doi: 10.1371/journal.pone.0029050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wolff D, Gerritzen A. Presence of murine leukemia virus (MLV)-related virus gene sequences in a commercial RT-PCR reagent. Clin Lab. 2011;57:631–634. [PubMed] [Google Scholar]
- 83.Erlwein O, Robinson MJ, Dustan S, Weber J, Kaye S, McClure MO. DNA extraction columns contaminated with murine sequences. PLoS One. 2011;6:e23484. doi: 10.1371/journal.pone.0023484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tuke PW, Tettmar KI, Tamuri A, Stoye JP, Tedder RS. PCR master mixes harbour murine DNA sequences. Caveat emptor! PLoS One. 2011;6:e19953. doi: 10.1371/journal.pone.0019953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sato E, Furuta RA, Miyazawa T. An endogenous murine leukemia viral genome contaminant in a commercial RT-PCR kit is amplified using standard primers for XMRV. Retrovirology. 2010;7:110. doi: 10.1186/1742-4690-7-110. * Showed mouse DNA contamination in commercial RT-PCR kits.
- 86. Oakes B, Tai AK, Cingoz O, Henefield MH, Levine S, Coffin JM, Huber BT. Contamination of human DNA samples with mouse DNA can lead to false detection of XMRV-like sequences. Retrovirology. 2010;7:109. doi: 10.1186/1742-4690-7-109. * Showed frequent contaminaqtion of human DNA samples with mouse DNA
- 87. Robinson MJ, Erlwein OW, Kaye S, Weber J, Cingoz O, Patel A, Walker MM, Kim WJ, Uiprasertkul M, Coffin JM, et al. Mouse DNA contamination in human tissue tested for XMRV. Retrovirology. 2010;7:108. doi: 10.1186/1742-4690-7-108. * Showed frequent contamination of human tissues with mouse DNA.
- 88.Silverman RH, Das Gupta J, Lombardi VC, Ruscetti FW, Pfost MA, Hagen KS, Peterson DL, Ruscetti SK, Bagni RK, Petrow-Sadowski C, et al. Partial Retraction. Science. 2011 doi: 10.1126/science.1212182. [DOI] [PubMed] [Google Scholar]
- 89.Sfanos KS, Aloia AL, Hicks JL, Esopi DM, Steranka JP, Shao W, Sanchez-Martinez S, Yegnasubramanian S, Burns KH, Rein A, et al. Identification of replication competent murine gammaretroviruses in commonly used prostate cancer cell lines. PLoS One. 2011;6:e20874. doi: 10.1371/journal.pone.0020874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dong B, Kim S, Hong S, Das Gupta J, Malathi K, Klein EA, Ganem D, Derisi JL, Chow SA, Silverman RH. An infectious retrovirus susceptible to an IFN antiviral pathway from human prostate tumors. Proc Natl Acad Sci U S A. 2007;104:1655–1660. doi: 10.1073/pnas.0610291104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim S, Kim N, Dong B, Boren D, Lee SA, Das Gupta J, Gaughan C, Klein EA, Lee C, Silverman RH, et al. Integration site preference of xenotropic murine leukemia virus-related virus, a new human retrovirus associated with prostate cancer. J Virol. 2008;82:9964–9977. doi: 10.1128/JVI.01299-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Garson JA, Kellam P, Towers GJ. Analysis of XMRV integration sites from human prostate cancer tissues suggests PCR contamination rather than genuine human infection. Retrovirology. 2011;8:13. doi: 10.1186/1742-4690-8-13. * Reported XMRV integation sites in human tumors were a result of contamination.
- 93.Rusmevichientong A, Das Gupta J, Elias PS, Silverman RH, Chow SA. Analysis of single-nucleotide polymorphisms in patient-derived retrovirus integration sites reveals contamination from cell lines acutely infected by xenotropic murine leukemia virus-related virus. J Virol. 2011;85:12830–12834. doi: 10.1128/JVI.05624-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kearney MF, Spindler J, Wiegand A, Shao W, Anderson EM, Maldarelli F, Ruscetti FW, Mellors JW, Hughes SH, Le Grice SF, et al. Multiple sources of contamination in samples from patients reported to have XMRV infection. PLoS One. 2012;7:e30889. doi: 10.1371/journal.pone.0030889. ** Plasma samples processed through the Ruscetti laboratory are found contaminated with XMRVs and/or MLVs.