Abstract
The oncoprotein MDM2 negatively regulates the activity and stability of the p53 tumor suppressor, and is an important molecular target for anticancer therapy. Aided by mirror image phage display and native chemical ligation, we have previously discovered several proteolysis-resistant duodecimal D-peptide antagonists of MDM2, termed DPMI-α, β, γ. The prototypic D-peptide inhibitor DPMI-α binds (25-109)MDM2 at an affinity of 220 nM, and kills tumor cells in vitro and inhibits tumor growth in vivo by reactivating the p53 pathway. Herein, we report the design of a super-active D-peptide antagonist of MDM2, termed DPMI-δ, of which the binding affinity for (25-109)MDM2 has been improved over DPMI-α by three orders of magnitude (Kd = 220 pM). X-ray crystallographic studies validate DPMI-δ as an exceedingly potent inhibitor of the p53-MDM2 interaction, promising to be a highly attractive lead drug candidate for anticancer therapeutic development.
Keywords: p53, MDM2, MDMX, D-peptides, antitumor agents, drug design, mirror image phage display, native chemical ligation
Functional inhibition of the p53 tumor suppressor protein by its negative regulators MDM2 and MDMX, whose genes MDM2 and MDMX are often amplified and/or over-expressed in many tumors harboring wild type TP53, directly contributes to tumor development and progression.1 MDM2 is an E3 ubiquitin ligase that specifically targets p53 for proteosomal degradation2 – a process potentiated by MDM2 hetero-oligomerization with its homolog MDMX.3 Both MDM2 and MDMX can also antagonize p53 transcription activity by sequestering p53 transactivation domain via their N-terminal p53-binding domains.4 Disrupting the p53-MDM2/MDMX inhibitory complex to rescue wild type p53 function has been validated as a viable therapeutic strategy for cancer treatment.5 Different structural classes of MDM2/MDMX antagonists exist as potential anticancer drug candidates, including low molecular weight compounds,6 small peptides and peptidomimetics,7 and miniature proteins,8 among others. Using mirror image phage display coupled with native chemical ligation,9 we have previously discovered several 12-mer D-peptide antagonists of MDM2, termed DPMI-α, β, γ, that are resistant to proteolytic degradation.10 The prototypic D-peptide inhibitor DPMI-α binds (25-109)MDM2 at an affinity of 220 nM, and kills tumor cells in vitro and inhibits tumor growth in vivo by reactivating the p53 pathway. An ultrahigh affinity (Kd = 220 pM), protease-resistant D-peptide is designed to antagonize MDM2 by specifically targeting its p53-binding cavity, promising to be a highly attractive lead drug candidate for anti-cancer therapeutic development.
We have previously shown that DPMI-α (TNWYANLEKLLR) adopts a left-handed α-helical conformation, burying several bulky hydrophobic side chains (highlighted in bold typeface) into the p53-binding cavity of 25-109MDM2 (Figure 1A). Among those, Trp3 and Leu7 are the two most critical residues of DPMI-α, contributing a combined free energy of 7.6 kcal/mol to 25-109MDM2 binding – an equivalent Kd value of 10−6 M.10a Sequence analysis of 18 phage-selected binding clones indicated that while Trp3 was totally conserved, Leu7 was not, as both Phe and Trp residues were also found at position 7. In fact, mutational analysis identified Phe7 as the best residue, registering a 3.5-fold stronger binding to MDM2 than Leu7. These findings largely led to the design of DPMI-β (TAWYANFEKLLR), which contains the N2A/L7F double mutation and binds (25-109)MDM2 with a Kd value 35 nM.10a Of note, a separate mirror image phage screening under more stringent conditions identified DPMI-γ (DWWPLAFEALLR), which contains a Phe residue at position 7 and binds (25-109)MDM2 at an affinity of 53 nM.10b
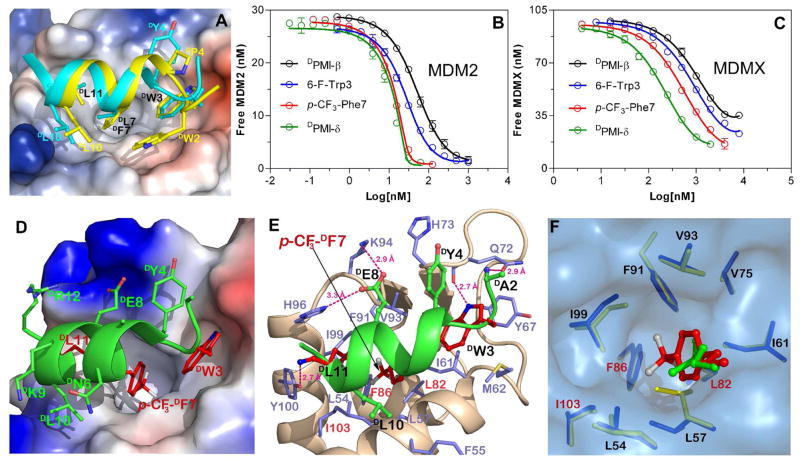
Figure 1.
(A) MDM2-binding modes of DPMI-α and DPMI-γ peptides. The structures of DPMI-α-(25-109)MDM2 (PDB:3LNJ) and DPMI-γ-(25-109)MDM2 (PDB:3IWY) are superimposed based on MDM2 molecules with DPMI-α (cyan) and DPMI-γ (yellow) displayed on the molecular surface of MDM2 complexed with DPMI-γ. The electrostatic potential displayed on MDM2 surface is colored red for negative, blue for positive, and white for apolar. The D-peptides are depicted in a Cα ribbon diagram where only the side chains of the residues involved in MDM2 binding are shown as ball-and-sticks. Interactions of 30 nM (25-109)MDM2 (B) or 100 nM (24-108)MDMX (C) with a two-fold dilution series of DPMI-β, p-CF3-Phe7- DPMI-β, 6-F-Trp3-DPMI-β and DPMI-δ as quantified by SPR-based competitive binding assays. The Kd values obtained from three independent measurements are tabulated in Table 1. (D) p-CF3-Phe7- DPMI-β bound in the hydrophobic pocket of MDM2. The D-peptide is shown as ribbon and its side chains are shown as ball-and-sticks. The three most critical residues for MDM2 binding, DTrp3, p-CF3-DPhe7 and DLeu11, are colored in red as in panel E. (E) The p-CF3-Phe7-DPMI-β-(25-109)MDM2 complex interface. Contact residues of MDM2 and p-CF3-Phe7-DPMI-β are shown as sticks and ball-and-sticks, respectively, and hydrogen bonds as red dashes. The p-CF3-Phe7-DPMI-β peptide is anchored in the p53-binding cavity of MDM2 primarily through multiple hydrophobic interactions involving DTrp3, p-CF3-DPhe7 and DLeu11 and the side chains of DTyr4 and DLeu10. In addition, five inter-molecular H-bonds are formed, including DAla2 N-Glu72 Oε1, DTrp3 Nε1-Gln72 O, DGlu8 Oε1-Lys94 Nζ, DGlu8 Oε2-His96 Nδ1 Nε2, and DLeu11 O-Ty100 Oη. (F) Comparison of the binding pockets of p-CF3-DPhe7 and DLeu7. The structures of p-CF3-Phe7-DPMI-β-(25-109)MDM2 (red/blue) and DPMI-α-(25-109)MDM2 (green/yellow, PDB:3LNJ) are superimposed based on MDM2 molecules. The residues lining the p53-binding pocket are depicted as sticks over the molecular surface of MDM2 complexed with p-CF3-Phe7- DPMI-β-(25-109)MDM2. Leu82, Phe86 and Ile103 of MDM2 make contacts exclusively with p-CF3-DPhe7, which is buried 3.8 Å deeper within the p53-binding pocket than DLeu7. The side chains of Leu57 and Ile99 of MDM2 shift (from yellow to blue) to accommodate the trifluoromethyl group of p-CF3-DPhe7 in an enlarged binding pocket.
Structural analysis of DPMI-α-(25-109)MDM2 and DPMI-γ-(25-109)MDM2 suggested that the aromatic side chain of a Phe7 residue in DPMI’s would not fully occupy its cognate binding site on MDM2. Therefore, we hypothesized that modifications to Phe7 side chain to improve its size and/or hydrophobicity would enhance MDM2 binding by these D-peptide ligands. To test this hypothesis, we used DPMI-β as our model peptide, and first evaluated the positional effect of chlorination of the phenyl ring of Phe7 of DPMI-β on MDM2 binding. A fluorescence polarization (FP)-based competition assay was developed to quantify the ability of three Cl-Phe7- DPMI-β peptides (chlorination at positions 2,3 and 4), along with 4-Br-Phe7- DPMI-β, to compete for MDM2 binding with N-acetyl-(15-29)p53 to which carboxyfluorescein (FAM) was conjugated via its Lys24 side chain. The following order of binding activity was obtained on the basis of IC50 values: 4-Cl-Phe ≈ 4-Br-Phe > Phe > 2-Cl-Phe ≫ 3-Cl-Phe (Figure S1 and Table S1). Clearly, chlorination or bromination at the para position of Phe7 enhanced DPMI-β binding to MDM2, while chlorination at the meta and ortho positions weakened it.
In light of these initial findings, we concentrated on the para position of Phe7 and synthesized five additional p-X-Phe7-DPMI-β peptides, where X = F, I, CH3, CF3, and CN. To improve FP assay sensitivity and dynamic range, a more potent, FAM-labeled p-Br-Phe7- DPMI-β peptide was used under otherwise identical experimental conditions. As shown in Figure S2 and Table S2, the following order of MDM2-binding activity ensued for p-X- Phe7-DPMI-β: CF3 > I > Br > Cl > CH3 > F > CN > H (Phe). The trifluoromethyl substitution at the para position of Phe7 emerged as the best modification to enhance DPMI-β binding to MDM2. For accurate quantification, we performed a previously established, surface plasmon resonance (SPR)-based competitive binding assay8b,11 for (25-109)MDM2 interacting with DPMI-β and p-CF3- Phe7-DPMI-β. As shown in Figure 1B and Table 1, whereas DPMI-β bound MDM2 at an affinity of 37.8 nM, in good agreement with the published value of 34.5 nM,10a p-CF3-Phe7-DPMI-β bound MDM2 with a Kd value of 450 pM – a dramatic increase in binding affinity by 80-fold.
Table 1.
Dissociation equilibrium constants (Kd, nM) of DPMI-β, 6-F-Trp3-DPMI-β, P-CF3-Phe7-DPMI-β, and DPMI-δ for synthetic (25-109)MDM2 and (24-108)MDMX.[a]
| DPMI-β | 6-F-Trp3-DPMI-β | p-CF3-Phe7-DPMI-β | DPMI-δ | |
|---|---|---|---|---|
| MDM2 | 37.8±0.9 | 14.0±1.0 | 0.45±0.41 | 0.22±0.21 |
| MDMX | 1440±41 | 1040±59 | 569±25 | 200±10 |
Each Kd value (mean ± S.D.) was obtained from three independent measurements
To better understand the structural basis of the enhanced binding of the trifluoromethylated peptide to MDM2, we determined the crystal structure of (25-109)MDM2 in complex with p-CF3-Phe7-DPMI-β at 1.8 Å resolution (Table S3, Figure S3-S4). As displayed in Figure 1D, the left-handed helix of p-CF3-Phe7-DPMI-β anchors deep inside the hydrophobic p53-binding cleft of MDM2 and establishes multiple hydrophobic interactions within the pocket primarily through the bulky side chains of DTrp2, p-CF3-DPhe7 and DLeu11 as well as the side chains of DTyr4 and DLeu10. Overall, p-CF3-Phe7-DPMI-β binding to MDM2 closely resembles its parental peptide DPMI-α as previously reported (Figure S5-S6). However, p-trifluoromethylation of DPhe7 induces new interactions within the pocket with Leu82, Phe86 and Ile103 of MDM2 (Figure 1E), and significantly enlarges the total buried surface area (BSA) of the D-peptide in the complex (from 561 Å2 to 640 Å2). In addition, one more H-bond is formed between DAla2 N of p-CF3-Phe7-DPMI-β and Glu72 Oε1 of MDM2. To accommodate the large side chain of p-CF3-DPhe7 two residues of MDM2 (Leu57 and Ile99) reorient in the p53-binding pocket (Figure 1F).
Importantly, structural analysis of the p-CF3-Phe7-DPMI-β-(25-109)MDM2 complex revealed that Trp3 would also be permissible to fluorination at multiple positions of its side chain. We replaced Trp3 in DPMI-β with 6-F-Trp, and the resultant D-peptide 6-F-Trp3-DPMI-β bound to (25-109)MDM2 with a Kd value of 14 nM as determined by the SPR-based competitive binding assay (Figure 1B and Table 1), representing a 2.5-fold enhancement in binding affinity relative to DPMI-β. When 6-F-Trp3 was incorporated into p-CF3-Phe7- DPMI-β, the resultant double mutant 6-F-Trp3/p-CF3-Phe7-DPMI-β, termed DPMI-δ, bound (25-109)MDM2 at an affinity of 220 pM, suggesting that the energetic effects of Trp3 and Phe7 modifications were additive. These results were confirmed by an independent assay based on FP techniques (Figure S2 and Table S2). It is worth noting that the N-terminal peptide (residues 1-24) of MDM2 is known to form a partially structured “lid” in the apo protein, occluding ligand binding to MDM2 in a ligand size-dependent manner.12 The “lid” has been shown to reduce the binding affinity for MDM2 of 12-mer L-peptide ligands by five fold.12c It may be anticipated that the Kd value of DPMI-δ reported here for (25-109)MDM2 would be higher than that for full-length MDM2.
D-peptide ligands, unlike their L-peptide counterparts, display a much greater disparity between MDM2 and MDMX binding, with a strong preference for MDM2 over MDMX.10,11 We quantified the interactions of (24-108)MDMX with DPMI-β, p-CF3-Phe7-DPMI-β, 6-F-Trp3-DPMI-β and DPMI-δ using SPR techniques, and the data are shown in Figure 1C and Table 1. Unexpectedly, p-trifluoromethylation of Phe7 enhanced DPMI-β binding to MDMX by only 2.5-fold, while fluorination of Trp3 slightly improved it. As a result, DPMI-δ bound to (24-108)MDMX with a Kd value of 200 nM - three orders of magnitude weaker than its binding to MDM2. These SPR results are in accord with FP measurements (Figure S7 and Table S4). Obviously, understanding the structural basis of the strong preference of D-peptide ligands for binding to MDM2 over MDMX will provide important insights into designing specific antagonists to target either protein.
Fluorocarbons are known to be substantially more hydrophobic than corresponding hydrocarbons.13 In fact, fluorinated aliphatic amino acids have been commonly used in protein de novo design to improve protein stability while having little impact on protein structure.14 It has been suggested that fluorination of alkanes enhances hydrophobicity due to an increased molecular size, thus a greater free-energy penalty for hydration.15 The high electronegativity of fluorine also enables the strongly polar C-F bond to engage in inductive interactions with surrounding polar groups and to alter hydration dynamics at fluorinated molecular surfaces.16 We have demonstrated that although p-trifluoromethylation of Phe7 gave rise to the greatest improvement, iodination, bromination or even chlorination at the para position of the phenyl ring was similarly effective in improving DPMI-β binding to MDM2. Of note, replacement of a critical Trp residue by 6-Cl-Trp has been shown to dramatically enhance the binding affinity of several peptide and peptidomimetic antagonists for MDM2 due to enhanced van der Waals interactions and polarization effects between the 6-Cl-Trp side chain and its interacting partners of MDM2.17 Given that the p53-binding cavity of MDM2/MDMX is hydrophobic in nature, halogenation (and fluorination in particular) will likely become a powerful tool for the design of exceedingly potent activators of p53 for therapeutic use.18
Different structural classes of drug candidates such as small peptides with unsurpassed affinity and specificity are urgently needed to combat cancer and infectious disease. L-peptides have been traditionally considered to be “undruggable” due primarily to their strong susceptibility to proteolytic degradation in vivo and inability to efficiently traverse the cell membrane. Drug discovery based on the scaffold of protease-resistant D-peptides,19 when coupled with advanced drug delivery technologies, offers a viable and robust solution to the problems both academia and industry are facing today. Our work on the design of ultrahigh affinity D-peptide antagonists of MDM2/MDMX to activate the p53 tumor suppressor may spearhead the development of new classes of anticancer therapeutics.
Supplementary Material
Acknowledgments
This work was supported in part by the National Institutes of Health Grants AI072732 and AI087423 and the Overseas Scholars Collaborative Research Grant 81128015 by the National Natural Science Foundation of China (to W.L.), and by the Science and Technology Commission of Shanghai Municipality Grant 11430707900 and the National Basic Research Program of China (973 Program) Grant 2010CB934000 (to W-Y.L.). L.Z. was supported by Xi’an Jiaotong University School of Medicine as a Guanghua Scholar, and X.C. by the China Scholarship Council. Portions of this research were carried out the University of Mary-land X-ray Crystallography Shared Service and at the Stanford Synchrotron Radiation Lightsource, a Directorate of SLAC National Accelerator Laboratory and an Office of Science User Facility operated for the U.S. Department of Energy Office of Science by Stanford University. The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program (P41RR001209), and the National Institute of General Medical Sciences.
Footnotes
ASSOCIATED CONTENT
Experimental procedures including synthesis of peptides and proteins, surface plasmon resonance (SPR)-based competitive binding assay, fluorescence polarization assay, crystallization of the p-CF3-Phe7- DPMI-β-(25-109)MDM2 complex, data collection, structure solution, and refinement as well as Tables S1-S4 and Figures S1-S7. The coordinates and structure factors have been deposited in the PDB with accession code 3TPX. This information is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]; (b) Levine AJ, Oren M. The first 30 years of p53: growing ever more complex. Nat Rev Cancer. 2009;9:749–758. doi: 10.1038/nrc2723. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Toledo F, Wahl GM. Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat Rev Cancer. 2006;6:909–923. doi: 10.1038/nrc2012. [DOI] [PubMed] [Google Scholar]; (d) Marine J, Dyer M. MDMX: from bench to bedside. J Cell Sci. 2007;120:371–378. doi: 10.1242/jcs.03362. [DOI] [PubMed] [Google Scholar]
- 2.(a) Honda R, Tanaka H, Yasuda H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 1997;420:25–27. doi: 10.1016/s0014-5793(97)01480-4. [DOI] [PubMed] [Google Scholar]; (b) Kubbutat M, Jones S, Vousden K. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]; (c) Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 3.(a) Wade M, Wang YV, Wahl GM. The p53 orchestra: Mdm2 and Mdmx set the tone. Trends Cell Biol. 2010;20:299–309. doi: 10.1016/j.tcb.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kruse JP, Gu W. Modes of p53 Regulation. Cell. 2009;137:609–622. doi: 10.1016/j.cell.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Huang L, Yan Z, Liao X, Li Y, Yang J, Wang ZG, Zuo Y, Kawai H, Shadfan M, Ganapathy S, et al. The p53 inhibitors MDM2/MDMX complex is required for control of p53 activity in vivo. Proc Natl Acad Sci USA. 2011;108:12001–12006. doi: 10.1073/pnas.1102309108. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Vousden K, Prives C. Blinded by the Light: The Growing Complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]; (e) Pant V, Xiong S, Iwakuma T, Quintás-Cardama A, Lozano G. Heterodimerization of Mdm2 and Mdm4 is critical for regulating p53 activity during embryogenesis but dispensable for p53 and Mdm2 stability. Proc Natl Acad Sci USA. 2011;108:11995–12000. doi: 10.1073/pnas.1102241108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Oliner JD, Pietenpol JA, Thiagalingam S, Gyuris J, Kinzler KW, Vogelstein B. Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature. 1993;362:857–860. doi: 10.1038/362857a0. [DOI] [PubMed] [Google Scholar]; (b) Momand J, Zambetti GP, Olson DC, George D, Levine AJ. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]; (c) Shvarts A, Steegenga WT, Riteco N, van Laar T, Dekker P, Bazuine M, van Ham RCA, van der Houven van Oordt W, Hateboer G, van der Eb AJ, et al. MDMX: a novel p53-binding protein with some functional properties of MDM2. EMBO J. 1996;15:5349–5357. [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Brown CJ, Lain S, Verma CS, Fersht AR, Lane DP. Awakening guardian angels: drugging the p53 pathway. Nat Rev Cancer. 2009;9:862–873. doi: 10.1038/nrc2763. [DOI] [PubMed] [Google Scholar]; (b) Shangary S, Wang S. Small-molecule inhibitors of the MDM2-p53 protein-protein interaction to reactivate p53 function: a novel approach for cancer therapy. Annu Rev Pharmacol Toxicol. 2009;49:223–241. doi: 10.1146/annurev.pharmtox.48.113006.094723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]; (b) Shangary S, Qin D, McEachern D, Liu M, Miller RS, Qiu S, Nikolovska-Coleska Z, Ding K, Wang G, Chen J, et al. Temporal activation of p53 by a specific MDM2 inhibitor is selectively toxic to tumors and leads to complete tumor growth inhibition. Proc Natl Acad Sci USA. 2008;105:3933–3938. doi: 10.1073/pnas.0708917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Chène P. Inhibiting the p53-MDM2 interaction: an important target for cancer therapy. Nat Rev Cancer. 2003;3:102–109. doi: 10.1038/nrc991. [DOI] [PubMed] [Google Scholar]; (b) Robinson JA. β-Hairpin Peptidomimetics: Design, Structures and Biological Activities. Acc Chem Res. 2008;41:1278–1288. doi: 10.1021/ar700259k. [DOI] [PubMed] [Google Scholar]; (c) Zhan C, Lu W. Peptide activators of the p53 tumor suppressor. Curr Pharm Des. 2011;17:603–609. doi: 10.2174/138161211795222577. [DOI] [PubMed] [Google Scholar]; (d) Bernal F, Wade M, Godes M, Davis TN, Whitehead DG, Kung AL, Wahl GM, Walensky LD. A Stapled p53 Helix Overcomes HDMX-Mediated Suppression of p53. Cancer Cell. 2010;18:411. doi: 10.1016/j.ccr.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Murray JK, Gellman SH. Targeting protein-protein interactions: lessons from p53/MDM2. Biopolymers. 2007;88:657–686. doi: 10.1002/bip.20741. [DOI] [PubMed] [Google Scholar]; (f) Fasan R, Dias RLA, Moehle K, Zerbe O, Vrijbloed JW, Obrecht D, Robinson JA. Using a beta-hairpin to mimic an alpha-helix: cyclic peptidomimetic inhibitors of the p53-HDM2 protein-protein interaction. Angew Chem Int Ed. 2004;43:2109–2112. doi: 10.1002/anie.200353242. [DOI] [PubMed] [Google Scholar]
- 8.(a) Li C, Liu M, Monbo J, Zou G, Li C, Yuan W, Zella D, Lu WY, Lu W. Turning a scorpion toxin into an antitumor mini-protein. J Am Chem Soc. 2008;130:13546–13548. doi: 10.1021/ja8042036. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Li C, Pazgier M, Liu M, Lu WY, Lu W. Apamin as a template for structure-based rational design of potent peptide activators of p53. Angew Chem Int Ed. 2009;48:8712–8715. doi: 10.1002/anie.200904550. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kritzer JA, Zutshi R, Cheah M, Ran FA, Webman R, Wongjirad TM, Schepartz A. Miniature Protein Inhibitors of the p53-hDM2 Interaction. ChemBioChem. 2006;7:29–31. doi: 10.1002/cbic.200500324. [DOI] [PubMed] [Google Scholar]; (d) Hu B, Gilkes DM, Chen J. Efficient p53 activation and apoptosis by simultaneous disruption of binding to MDM2 and MDMX. Cancer Res. 2007;67:8810–8817. doi: 10.1158/0008-5472.CAN-07-1140. [DOI] [PubMed] [Google Scholar]
- 9.(a) Dawson PE, Muir T, Clark-Lewis I, Kent SBH. Synthesis of proteins by native chemical ligation. Science. 1994;266:776–779. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]; (b) Dawson PE, Kent SBH. Synthesis of native proteins by chemical ligation. Annu Rev Biochem. 2000;69:923–960. doi: 10.1146/annurev.biochem.69.1.923. [DOI] [PubMed] [Google Scholar]; (c) Schumacher TN, Mayr LM, Minor DL, Milhollen MA, Burgess MW, Kim PS. Identification of D-peptide ligands through mirror-image phage display. Science. 1996;271:1854–1857. doi: 10.1126/science.271.5257.1854. [DOI] [PubMed] [Google Scholar]; (d) Eckert DM, Malashkevich VN, Hong LH, Carr PA, Kim PS. Inhibiting HIV-1 entry: discovery of D-peptide inhibitors that target the gp41 coiled-coil pocket. Cell. 1999;99:103–115. doi: 10.1016/s0092-8674(00)80066-5. [DOI] [PubMed] [Google Scholar]
- 10.(a) Liu M, Pazgier M, Li C, Yuan W, Li C, Lu W. A left-handed solution to peptide inhibition of the p53-MDM2 interaction. Angew Chem Int Ed. 2010;49:3649–3652. doi: 10.1002/anie.201000329. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Liu M, Li C, Pazgier M, Li C, Mao Y, Lv Y, Gu B, Wei G, Yuan W, Zhan C, et al. D-peptide inhibitors of the p53-MDM2 interaction for targeted molecular therapy of malignant neoplasms. Proc Natl Acad Sci USA. 2010;107:14321–14326. doi: 10.1073/pnas.1008930107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Pazgier M, Liu M, Zou G, Yuan W, Li C, Li C, Li J, Monbo J, Zella D, Tarasov SG, et al. Structural basis for high-affinity peptide inhibition of p53 interactions with MDM2 and MDMX. Proc Natl Acad Sci USA. 2009;106:4665–4670. doi: 10.1073/pnas.0900947106. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Li C, Pazgier M, Li C, Yuan W, Liu M, Wei G, Lu WY, Lu W. Systematic mutational analysis of peptide inhibition of the p53-MDM2/MDMX interactions. J Mol Biol. 2010;398:200–213. doi: 10.1016/j.jmb.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) McCoy MA, Gesell JJ, Senior MM, Wyss DF. Flexible lid to the p53-binding domain of human Mdm2: implications for p53 regulation. Proc Natl Acad Sci USA. 2003;100:1645–1648. doi: 10.1073/pnas.0334477100. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Showalter SA, Bruschweiler-Li L, Johnson E, Zhang F, Bruschweiler R. Quantitative lid dynamics of MDM2 reveals differential ligand binding modes of the p53-binding cleft. J Am Chem Soc. 2008;130:6472–6478. doi: 10.1021/ja800201j. [DOI] [PubMed] [Google Scholar]; (c) Zhan C, Varney K, Yuan W, Zhao L, Lu W. Interrogation of MDM2 Phosphorylation in p53 Activation Using Native Chemical Ligation: The Functional Role of Ser17 Phosphorylation in MDM2 Reexamined. J Am Chem Soc. 2012;134:6855–6864. doi: 10.1021/ja301255n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(a) Yoder NC, Yüksel D, Dafik L, Kumar K. Bioorthogonal noncovalent chemistry: Fluorous phases in chemical biology. Curr Opin Chem Biol. 2006;10:576–583. doi: 10.1016/j.cbpa.2006.10.007. [DOI] [PubMed] [Google Scholar]; (b) Biffinger JC, Kim HW, DiMagno SG. The polar hydrophobicity of fluorinated compounds. ChemBioChem. 2004;5:622–627. doi: 10.1002/cbic.200300910. [DOI] [PubMed] [Google Scholar]; (c) Müller K, Faeh C, Diederich F. Fluorine in pharmaceuticals: looking beyond intuition. Science. 2007;317:1881–1886. doi: 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]
- 14.(a) Bilgiçer B, Fichera A, Kumar K. A Coiled Coil with a Fluorous Core. J Am Chem Soc. 2001;123:4393–4399. doi: 10.1021/ja002961j. [DOI] [PubMed] [Google Scholar]; (b) Jäckel C, Salwiczek M, Koksch B. Fluorine in a Native Protein Environment—How the Spatial Demand and Polarity of Fluoroalkyl Groups Affect Protein Folding. Angew Chem Int Ed. 2006;45:4198–4203. doi: 10.1002/anie.200504387. [DOI] [PubMed] [Google Scholar]; (c) Tang Y, Tirrell DA. Biosynthesis of a highly stable coiled-coil protein containing hexafluoroleucine in an engineered bacterial host. J Am Chem Soc. 2001;123:11089–11090. doi: 10.1021/ja016652k. [DOI] [PubMed] [Google Scholar]; (d) Tang Y, Ghirlanda G, Petka W, Nakajima T, DeGrado W, Tirrell D. Fluorinated coiled-coil proteins prepared in vivo display enhanced thermal and chemical stability. Angew Chem Int Ed. 2001;113:1542–1544. doi: 10.1002/1521-3773(20010417)40:8<1494::AID-ANIE1494>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 15.Dalvi VH, Rossky PJ. Molecular origins of fluorocarbon hydrophobicity. Proc Natl Acad Sci USA. 2010;107:13603–13607. doi: 10.1073/pnas.0915169107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwon O, Yoo T, Othon C, Van Deventer J, Tirrell D, Zewail A. Hydration dynamics at fluorinated protein surfaces. Proc Natl Acad Sci USA. 2010;107:17101–17106. doi: 10.1073/pnas.1011569107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.(a) Hintersteiner M, Kimmerlin T, Garavel G, Schindler T, Bauer R, Meisner NC, Seifert JM, Uhl V, Auer M. A highly potent and cellularly active beta-peptidic inhibitor of the p53/hDM2 interaction. ChemBioChem. 2009;10:994–998. doi: 10.1002/cbic.200800803. [DOI] [PubMed] [Google Scholar]; (b) Fasan R, Dias RLA, Moehle K, Zerbe O, Obrecht D, Mittl PRE, Grütter MG, Robinson JA. Structure-activity studies in a family of beta-hairpin protein epitope mimetic inhibitors of the p53-HDM2 protein-protein interaction. ChemBioChem. 2006;7:515–526. doi: 10.1002/cbic.200500452. [DOI] [PubMed] [Google Scholar]; (c) García-Echeverría C, Chène P, Blommers MJ, Furet P. Discovery of potent antagonists of the interaction between human double minute 2 and tumor suppressor p53. J Med Chem. 2000;43:3205–3208. doi: 10.1021/jm990966p. [DOI] [PubMed] [Google Scholar]; (d) Grässlin A, Amoreira C, Baldridge KK, Robinson JA. Thermodynamic and computational studies on the binding of p53-derived peptides and peptidomimetic inhibitors to HDM2. ChemBioChem. 2009;10:1360–1368. doi: 10.1002/cbic.200900008. [DOI] [PubMed] [Google Scholar]
- 18.Huang Y, Wolf S, Koes D, Popowicz GM, Camacho CJ, Holak TA, Dömling A. Exhaustive Fluorine Scanning toward Potent p53-Mdm2 Antagonists. ChemMedChem. 2011;7:49–52. doi: 10.1002/cmdc.201100428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dooley CT, Chung NN, Wilkes BC, Schiller PW, Bidlack JM, Pasternak GW, Houghten RA. An all D-amino acid opioid peptide with central analgesic activity from a combinatorial library. Science. 1994;266:2019–2022. doi: 10.1126/science.7801131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.