Abstract
Introduction
Seven arenaviruses cause viral hemorrhagic fever in humans: the Old World arenaviruses Lassa and Lujo, and the New World Clade B arenaviruses Machupo (MACV), Junín (JUNV), Guanarito (GTOV), Sabiá (SABV), and Chapare (CHPV). All of these viruses are Risk Group 4 biosafety pathogens. MACV causes human disease outbreak with high case-fatality rates. To date, at least 1,200 cases with ≈200 fatalities have been recorded 1, 2.
Areas covered
This review summarizes available systems and technologies for the identification of antivirals against MACV, animal models for in vivo evaluation of novel inhibitors, present treatment of arenaviral diseases, overview of efficacious small molecules and other therapeutics reported to date, and strategies to identify novel inhibitors for anti-arenaviral therapy.
Expert opinion
New high-throughput approaches to quantitate infection rates of areaviruses, as well as viruses modified to carry reporter genes, will accelerate compound screens and drug discovery efforts. RNAi, gene expression profiling and proteomics studies will identify host targets for therapeutic intervention. New discoveries in the cell entry mechanism of MACV and other arenaviruses as well as extensive structural studies of arenaviral L and NP could facilitate the rational design of antivirals effective against all pathogenic New World arenaviruses.
Keywords: antivirals, arenavirus, Machupo, therapeutics, viral hemorrhagic fever
1. Introduction
Enveloped RNA viruses from four different families can infect humans and cause severe clinical syndromes termed “viral hemorrhagic fever” (VHF): Arenaviridae, Bunyaviridae, Filoviridae, and Flaviviridae 3, 4. The family Arenaviridae includes the largest number of VHF-causing agents, with case-fatality rates as high as 30%. Lassa virus (LASV) is an Old World arenavirus that causes Lassa fever in West Africa. More than 300,000 LASV infections are reported in endemic areas per year with several thousand deaths5. Machupo (MACV), Guanarito (GTOV), Junín (JUNV) and Sabiá (SABV) viruses are New World arenaviruses that cause Bolivian, “Venezuelan,” Argentinian and “Brazilian” hemorrhagic fever, respectively 6. Among these, JUNV is the most important pathogen causing annual outbreaks in a progressively expanding region in north central Argentina, with almost 5 million individuals at risk of infection7. LASV, MACV, GTOV, JUNV,and SABV have been classified as National Institute of Allergy and Infectious Diseases Category A Priority Pathogens 8, Select Agents 9, and Risk Group 4 biosafety pathogens in part because of their lethality and the significant risk of their misuse 10, 11. Recently, two new arenaviruses, Chapare virus (CHPV) and Lujo virus have been isolated from severe cases of undiagnosed hemorrhagic fevers in Bolivia and southern Africa (Zambia), respectively 12, 13, illustrating that new pathogenic arenaviruses may emerge every few years.
The family Arenaviridae includes a single genus, Arenavirus, currently comprised of 24 recognized species 14–20. Based on antigenic properties and sequence phylogeny, arenaviruses have been divided into two distinct groups: The Old World arenaviruses (Lassa–lymphocytic choriomeningitis serocomplex) include viruses indigenous to Africa, the ubiquitous lymphocytic choriomeningitis virus (LCMV) and Lujo virus. The New World arenaviruses (Tacaribe serocomplex) include viruses indigenous to the Americas 14, 21–23. MACV is a member of group B New World arenaviruses 21, 24, which also contains the human pathogenic viruses GTOV, JUNV, SABV, and CHPV and the non-pathogenic Tacaribe virus (TCRV), Amapari virus (AMAV), and Cupixi virus 18, 21, 25.
Rodents of the superfamily Muroidea are the natural hosts of most arenaviruses, and the geographical distribution of each arenavirus is determined by the range of its corresponding host. New World arenaviruses are found in rodents of the family Cricetidae, subfamily Sigmodontinae, in specialized ecological niches in South and North America 14, 26. Field studies strongly support the concept of only a single major reservoir host for each virus 26. The big laucha (Calomys callosus) is the principal host for MACV; the drylands laucha (Calomys musculinus), for JUNV; and the short-tailed zygodont (Zygodontomys brevicauda) for GTOV 27–30. The phylogenetic diversity of arenaviruses is possibly the result of long-term co-evolution of the viruses and their corresponding hosts 31, 32. Humans become infected through contact with infected rodents or inhalation of aerosolized virus from contaminated rodent blood, excreta or secreta 11.
1.1 Clinical significance
Bolivian hemorrhagic fever (BHF) was first recognized in 1959 on the island of Orobayaya. 470 cases were reported in the years up to 1962 in the Beni region of northeastern Bolivia. The disease was described as a new hemorrhagic fever only later, in 1964, by MacKenzie and coworkers 33. Machupo virus, named after a river close to the outbreak area, was isolated in 1963 from the spleen of a fatal human case studied in the town of San Joaquín and identified as the etiological agent of BHF 34. The virus is responsible for a series of localized BHF outbreaks occurring from 1962 to 1964 involving more than 1,000 patients, of which 180 died. Similarly to Argentinean hemorrhagic fever (AHF), caused by JUNV, the outbreak frequency peaked during the annual harvest (April–July). The case-fatality rate of BHF is approximately 5–30%. After 20 years of no reported cases, mainly as a result of rodent control measures 35, an outbreak involving 19 people was reported in 1994. Eight more cases were described in 1999, 18 cases in 2000, 20 suspected cases in 2007 and 200 in 20082.
1.2 The virion and the viral lifecycle
Arenaviruses are pleomorphic enveloped viruses ranging from 50 to 300 nm in diameter (reviewed in 11, 15, 36, 37). The arenavirus genome consists of two single-stranded RNA molecules, designated L (large) and S (small). Each segment encodes two different proteins in two non-overlapping reading frames of opposite polarities. The two open reading frames on each genomic segment are separated by an intergenic noncoding region (IGR) with the potential to form one or more energetically stable stem-loop (hairpin) structures 38, 39. The L segment (≈7200 nt) encodes the viral RNA-dependent RNA polymerase (L) and a small zinc-binding matrix protein (Z). The S segment (≈3500 nt) encodes the nucleoprotein (NP), which associates with viral RNA in the form of bead-like structures to form nucleocapsids, and the envelope glycoprotein precursor (GPC), which is proteolytically processed into two mature envelope proteins GP1 and GP2 and a stable signal peptide40–43. Extracted virion RNA is not infectious and therefore arenaviruses are considered by some as negative-sense RNA viruses despite the presence of the ambisense coding strategy.
Virus entry
Arenavirus cell entry is mediated by the GP spike complex on the virion surface. This complex is a trimer of heterdimers comprised of the integral membrane protein GP2, which is noncovalently attached to the highly glycosylated peripheral protein GP144–48. The complex also includes the signal peptide of the arenavirus GPC42, 49, 50, which is associated non-covalently with the cytoplasmic domain of GP251. In the case of MACV, as well as other pathogenic New World arenaviruses, cell entry occurs after GP1 binds the cellular receptor transferrin receptor 1 (TfR1)52–55. Following TfR1 binding, the virus is most likely endocytosed through clathrin-coated vesicles56, 57 into intracellular endosomal compartments56, 58–62. This internalization process has been reported to be dependent on the cellular clathrin coat-associated protein Eps15 and dynamin63 and on an intact actin network64. Activation of the PI3K/AKT signaling pathway was also shown65.
GP2-dependent low pH-induced fusion of the virions with cellular membranes occurs in late endosomes. The current hypothesis suggests that the putative signal peptide-GP2 interface stabilizes the prefusion state of the GP complex at neutral pH, and triggers the conformational changes leading to membrane fusion at acidic pH66, 67. Both myristoylation of the signal peptide and a positively charged amino acid residue, lysine residue 33, are specifically required for its function as fusion modulator50, 66. Exposure to low pH destabilizes the high-energy prefusion state of the GP spike complex resulting in irreversible conformational changes, in which the GP1 subunit is released from the GP spike68, 69. The hydrophobic fusion peptide at the N-terminus of GP270, 71 becomes exposed and inserts into the target membrane. This results in a series of conformational rearrangements in the GP2 subunit leading to the postfusion six-α-helix core structure. Subsequently, the viral envelope and the target membrane are pulled together and give rise to the fusion pore72. Viral nucleocpsids are then delivered into the cell cytoplasm, where arenavirus transcription and replication exclusively occurs 37.
Virus transcription and protein expression
Arenavirus RNA synthesis is initiated after delivery of the two encapsidated S and L segments, each associated with the RNA-dependent RNA polymerase, L, into the cytosol. L initiates from the 3′ end of the genomic template and produces subgenomic, genome-complementary (antigenomic), NP and L mRNAs terminating at non-specific sites at the IGR. These mRNAs are capped and not polyadenylated73–75. The 5′ ends of viral mRNAs contain, in addition to a cap structure, several extra random nontemplated bases, resembling the mRNAs of orthomyxoviruses and bunyaviruses73, 76, 77. Therefore, it has been inferred that the arenaviral L protein uses a transcription-initiation mechanism involving an oligonucleotide-cap primer of host origin. NP, L, and the viral RNA form the transcriptionally active unit, the ribonucleoprotein (RNP) complex78–80. Transcription of GPC and Z mRNAs occurs only after one round of arenavirus replication, in which S and L antigenomes are synthesized. Consequently, NP mRNA and NP protein accumulate earlier than GPC mRNA and the GP1 and GP2 glycoproteins. Furthermore, NP is much more abundant in cells than GP1 and GP26, 37.
GP is translated as a single precursor protein (GPC) into the lumen of the endoplasmatic reticulum, where the signal peptide is cleaved off 81. GPC then undergoes extensive N-linked glycosylation47, 48, and is thought to oligomerize prior to being proteolytically processed by the subtilase SKI-1/S1P to generate the two mature glycoproteins GP1 and GP240–43, 82. Proteolytic maturation of GPC as well as its trafficking from the ER to the cell surface is dependent on the signal peptide51, 66, 81, 83. The signal peptide associates with and masks endogenous dibasic ER-retention/retrieval signals present in the cytoplasmic tail of GP2, and thereby ensures that only the fully assembled GP spike is transported to the plasma membrane51.
Virus replication
During replication, L reads through the IGR transcription-termination signal and generates uncapped antigenomic and genomic RNAs 84 that contain a single nontemplated G on the 5’ end76, 77. A model for this extra nontemplated G contends that a dinucleotide primer (pppGpC) used in replication initiation first base-pairs with positions 2 and 3 of the template RNA and then produces a single G overhang when the nascent RNA slips two bases “backward” on the template85.
Virus assembly and budding
The newly synthesized full-length antigenomic and genomic RNA species are encapsidated by NP to generate RNP complexes for further mRNA transcription and for production of virus progeny77. It is thought that arenaviral Z plays an important role in regulating the dynamics of arenaviral infection by a direct feedback loop where increased viral gene expression and higher levels of Z act to suppress RNA synthesis and balance the infection process80, 86–88. Z inhibits RNA synthesis by locking the L polymerase in a catalytically inactive promoter-bound state89, 90. It is plausible that the resulting Z–L–RNA complex may serve as a critical intermediate to ensure genomic RNA is packaged along with L poised to reinitiate viral RNA synthesis in the newly infected cell90. The integrity of the RING domain within Z is essential for Z-L interaction and therefore for Z inhibitory activity87.
Arenaviruses bud from the plasma membrane91, 92 and Z is the main driving force for assembly and egress93–95. Z is strongly membrane-associated and accumulates near the inner surface of the plasma membrane where budding takes place95. Myristoylation of Z is critical for its membrane association, as well as for its binding to the viral GP complex, specifically with the signal peptide component96–98. Interaction of NP and Z protein was also reported, suggesting that Z might recruit NP, complexed in the virus RNP core, to the patches in the cellular membranes enriched in envelope GP spikes where virus assembly and budding takes place99, 100.
Consistent with its features as a bona fide budding protein, Z of MACV and all other pathogenic New World arenaviruses contains the canonical proline-rich late (L) domain PSAP motif. L domains are highly conserved and have been shown to mediate interaction with host cell proteins required for virus budding. For example, the cellular multivesicular body pathway proteins Vps4A, Vps4B, and Tsg101 are involved in LASV budding101 and Z was shown to recruit Tsg101 to the plasma membrane94.
Virus-host interactions
LCMV and LASV Z interacts with several cellular proteins, such as the oncoprotein promyelocytic leukemia protein (PML)102, the ribosomal protein P0103, the eukaryotic translation initiation factor eIF4E104 and the proline-rich homeodomain protein105. PML is relocated to the cytoplasm following infection with the arenavirus LCMV. In the cytoplasm, the RING domains of PML and Z repress translation by directly inhibiting translation initiation factor eIF4E106.
Blocking the interferon (IFN) response
At least two arenavirus proteins, NP and Z, inhibit the host’s antiviral IFN response. For instance, MACV and JUNV NP inhibits IFNβ and IRF3-dependent promoter activation, as well as nuclear translocation of IRF3107, a function that was first demonstrated for the Old World arenaviruses LCMV108 and later also for Latinos virus, LASV, Pichindé virus, and Whitewater Arroyo virus107. Mapping of NP residues that are critical for this anti-IFN activity identified a highly conserved DIEG motif among all arenavirus NPs109. Z from MACV, JUNV, GTOV, and SABV binds RIG-I, resulting in IFNβ response down regulation110.
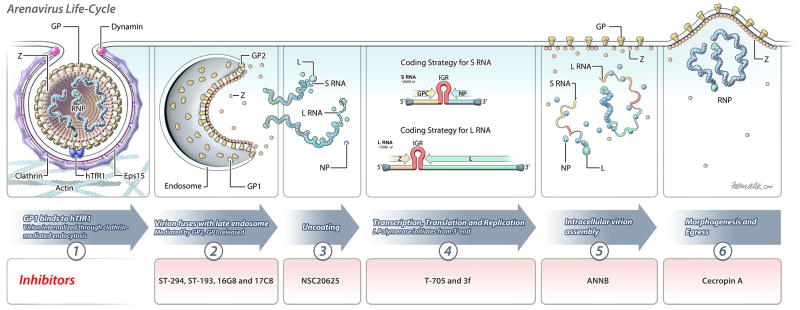
The arenavirus lifecycle is depicted in Figure 1.
Figure 1.
Schematic depiction of the arenavirus (MACV) lifecycle. Stages, which are thought to be targeted by the identified inhibitors, are indicated.
1.3 Animal models
Critical evaluation of candidate drugs identified in vitro is dependent on the availability of suitable animal models. “Suitable” means that, ideally, a given animal infected with an agent faithfully represents the disease caused by the virus in a human. Unfortunately, animal model development for Risk Group 4 agents, such as MACV, is complicated by the lack of human clinical data on the one hand, and the difficulties of performing animal experimentation within BSL-4 containment on the other hand. Animal model development for New World hemorrhagic fever arenaviruses is lagging behind that of other, more common, diseases because of these reasons. To date, only few animals have been evaluated for their susceptibility to MACV, and even fewer have been described on a basic level in terms of gross and histologic pathology.
Nonhuman primates
Although rhesus monkeys (Macaca mulatta), crab-eating macaques (Macaca fascicularis), grivets (Chlorocebus aethiops), and Geoffroy’s tamarins (Saguinus geoffroyi) can be lethally infected with MACV, pathologic descriptions have only been published for rhesus monkeys and grivets111–113. Rhesus monkeys uniformly die after subcutaneous injection. The clinical presentation of the infected animals is representative of what has been observed among humans: the disease begins after an incubation period of 1 to 2 weeks with fever, weakness, dehydration, cutaneous hyperesthesia, and anorexia. Vomiting, mild diarrhea, conjunctivitis with periorbital edema, flushing over the head and upper torso, skin petechiae, tremors and hemorrhages from the gums, vagina, and gastrointestinal tract are typical clinical signs. Death occurs due to multiorgan failure 7–12 days after disease onset2, 114. Likewise, the pathology of rhesus monkeys closely mirrors what has been recorded for very few autopsies that have been performed among deceased patients: lymphadenopathy; splenomegaly; hepatomegaly; meningeal edema; and hydropericardium. Hepatic necroses are the most characteristic sign of infection. However, infected rhesus monkeys also develop epithelial necroses, necrotizing enteritis, and necroses of the adrenal cortices, as well lymphoid depletion, signs that have not been observed in humans112. Grivets usually also die after subcutaneous infection with MACV. However, the clinical signs of infected animals diverge more from the human disease course than those of rhesus monkeys: hepatic necrosis, necrotizing enteritis, acute suppurative bronchopneumonia, and hemorrhages into the skin, lungs, intestine, liver, and lymph nodes were reported, as well as necroses in liver, intestine, skin, oral cavities, and adrenal cortices. Additionally, acute thrombosis was noted.
Nonhuman primates are often considered the most relevant models for human disease because of their evolutionary proximity to humans. It is therefore thought that nonhuman primates may most closely mimic human host responses, although this is far from proven. The logistical aspects of nonhuman primate research in maximum containment, and the costs associated with these studies therefore make small animal models attractive for at least initial drug efficacy studies.
Small animal models
As in the case of nonhuman primates, the literature on MACV only scantly reports on small animal model candidates. Early studies showed that MACV is lethal in mature strain 13 guinea pigs (Cavia porcellus), suckling laboratory mice, and suckling hamsters113. Unfortunately, the exact clinical presentation, and even more important, the pathology of infected guinea pigs and laboratory mice has not been reported in detail and it therefore remains unclear how useful guinea pigs could be in drug efficacy studies.
Suckling golden hamsters usually die only after intracerebral infection with MACV that has previously been adapted to hamsters. Deceased animals do not have gross pathologic abnormalities, but splenic lymphoid depletion, focal lymphoid necrosis, and hepatic fatty changes, which are a hallmark of infection on the microscopic level115. However, the lack of specific reagents for hamsters, and the fact that these animals only become sick after intracerebral infection raises doubts about the suitability of these animals for drug studies.
Recently developed alternatives to hamsters are adult signal transducer and activator of transcription 1 (STAT-1) knockout mice, which are defective in type I, II, and III IFN signaling. In these animals, MACV appeared to have an early tropism for the spleen and kidney (day 3) and spread by day 5 to all other organs sampled. Viral titers remained high until the time of death (day 7). The most significant histopathological findings were mild to moderate hepatic necroses, moderate to marked lymphoid depletion in lymph nodes, spleen and thymus, and pancreatitis. Importantly, ribavirin protected these mice against MACV disease, thereby supporting the potential usefulness of these animals for drug efficacy studies 116.
1.4 Current treatment and vaccines
Currently, virus-specific treatments approved for use against BHF are not available and treatment therefore consists primarily of supportive care (antivirals against arenaviruses have been recently reviewed in 117).
Vaccines
Despite the bioterrorism and public health risks associated with BHF, to date there are no FDA-licensed vaccines. The only vaccines for the prevention of human arenavirus disease are limited to a single, safe, efficacious, live-attenuated vaccine, designated Candid 1 (Candidate no. 1) for the prevention of JUNV infection118–121. Candid 1, which is classified as an Investigational New Drug (IND) in the United States, was derived from the wild-type JUNV strain XJ13 through serial passage both in vivo and in vitro122. A recent study suggests that the major determinant of attenuation in mice is located in the transmembrane domain of the G2 glycoprotein (F427I mutation) 123. Candid 1 has been evaluated in large-scale controlled trials among at-risk populations of agricultural workers in Argentina, where it showed a protective efficacy greater or equal to 84%. Vaccination of more than 150,000 high-risk individuals in the endemic areas has led to a consistent reduction in AHF cases with no serious side effects observed among vaccinees 7, 121, 124. The vaccine also cross-protects experimental animals against MACV challenge125.
Passive antibody therapy
Studies to assess the effect of immune plasma on the course and outcome of BHF in animal models suggest that passive antibody therapy might be useful for the treatment of BHF126. In the case of the related JUNV/AHF, transfusion of immune convalescent plasma with defined doses of JUNV-neutralizing antibodies is the present therapeutic intervention and treatment method. Convalescent serum is administered within the first 8 days of disease to provide an adequate dose of neutralizing antibodies. Lethality is then reduced to less than 1%, but about 10% of treated patients develop a transient cerebellar-cranial nerve syndrome 3 to 6 weeks later119, 127–129.
It is important to note that many considerations argue for the need for alternative treatments. First, plasma therapy is not as efficient in preventing JUNV/AHF when initiated after 8 days of illness; second, a late neurological syndrome is observed in 10% of plasma-treated patients128; third, maintaining adequate stocks of plasma is difficult due to the limited number of BHF cases and the absence of a program for convalescent serum collection; fourth, there is the risk of transfusion-borne diseases130.
Antivirals
Current antiviral therapy is limited to an off- label use of the nonimmunosuppressive guanosine analogue, ribavirin (1-β-D-ribofuranosyl-1-H-1,2,4-triazole-3-carboxamide), an IMP dehydrogenase inhibitor which has only partial efficacy against some arenaviruses and is associated with significant toxicity in humans7, 119, 131–141. Ribavirin can lead to adverse side effects such as thrombocytosis, severe anemia, and, at least in animal models, birth defects137, 142. Recent studies suggest that the antiviral activity of ribavirin on arenaviruses is not mediated by depletion of the intracellular GTP pool, but might be exerted, at least partially, by lethal mutagenesis143, 144.
2. Body
2.1 Systems for MACV drug discovery
2.1.1 Infectious virus
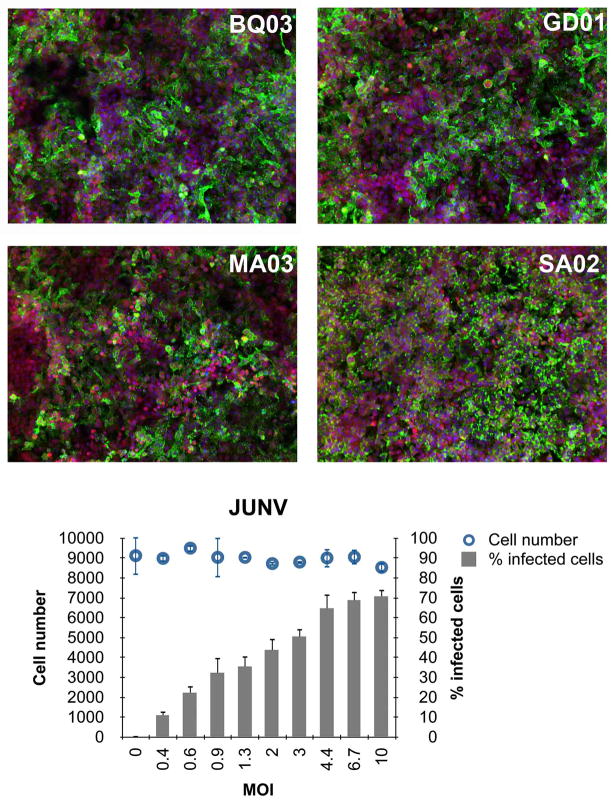
Drug screens for compounds against pathogenic arenaviruses are highly challenging as the infections have to be performed in a Biosafety Level 4 (BSL4) environment, with all of the procedures for handling these infectious agents usually being conducted within biological safety cabinets. Traditional methods to assess infectious arenavirus replication, such as plaque or real-time PCR assays, are low-throughput and therefore inadequate for large-scale primary drug screening. Recently, we have developed a high throughput/high-content image-based primary screening assay for infectious JUNV (Figure 2). The infection assay, performed in a 96-well format, is set up for higher throughput than the traditional methods currently used for therapeutic testing. This image-based screen allows for cellular quantitation of the viral infection based on fluorescence intensity in whole cells (percentage of cells that stain positive for viral antigen) and could also identify compounds that exhibit cellular toxicity. During image processing, a wide variety of cellular measurements, including shapes, textures, intensities, and localizations, can be captured, and these characteristics are then used to create unique representations, or “phenotypic signatures,” of each cell. Such quantitative measurement will help discard false positives, as well as identify small-molecule “hits” for lead optimization. Similar approaches can be applied for developing screening assays for other pathogenic arenaviruses—after taking into consideration the biology (cell types, viral pathogenicity, cell morphology, and antigen localization) and issues related to image acquisition and analysis.
Figure 2.
HeLa cells in 96-well plates were infected with JUNV, Romero strain, at the indicated MOIs for 1 h. Two days later cells were fixed in formalin for 72 h, washed, permeabilized, and blocked with 3%BSA/PBS. Cells were stained with anti-JUNV GPBQ03 or GD01 antibody, or with anti-JUNV NP MA03 or SA02 antibody (1:1,000 dilution) followed by Alexa-488 goat anti mouse IgG (shown in green). Hoechst and HCS CellMask Deep Red were used for nuclear and cytoplasmic staining, respectively (shown in blue and red). Images were analyzed within the Opera (PerkinElmer) environment using standard Acapella scripts. The intensity and subcellular localization of viral infection was determined by Alexa-488 fluorescence. The algorithm was used to identify objects such as nuclei based on Hoechst dye and cytoplasm based on CellMask Deep Red stain. For each condition, images from 6 fields/well (≈9000 cells) were acquired with the script calculating the percent positive cells and the mean fluorescence intensities in the cytoplasmic, membrane, or nuclear region. Top panel shows representative images for each of the antibodies. Bottom panel shows infection rates and cell number for each MOI used. Averages of triplicate wells are shown.
Other systems that might be adapted to high-throughput chemical screening are infectious viruses carrying reporter genes, such as eGFP or luciferase. Such a system has been created for Ebola virus145; however, generating a cDNA clone from which the recombinant virus could be rescued is a prerequisite (such systems are referred to as reverse genetics). To date, reverse genetics systems have been developed for the pathogenic arenaviruses, LCMV146, 147, JUNV148, 149 and LASV150 as well as Pichindé virus151. With the exception of LCMV152, these systems have yet to be advanced to include a reporter protein.
2.1.2 Surrogate systems
Pathogenic arenaviruses are BSL-4 pathogens, and therefore experimental work with infectious viruses is restricted to high security and maximum containment laboratories. To study or screen for antivirals that inhibit specific arenaviral lifecycle events under standard BSL-2 conditions, several virus surrogate systems have been developed.
A surrogate system to study arenavirus cell entry consists of retroviral vectors (most commonly Moloney murine leukemia virion-like particles) that carry the arenaviral GP spike complex in their envelope and that encode a reporter gene, such as eGFP or luciferase, to facilitate quantification of transduction efficiencies. This system is feasible because enveloped viruses can incorporate heterologous viral GPs into their lipid membranes during budding153 and because arenavirus cell attachment and entry are mediated exclusively by the envelope GP spike complex. Thus, these pseudotyped virion-like particles acquire the receptor specificity of the arenavirion from which the heterologous GP spike is derived154, 155. To date, such pseudotypes systems have been described for MACV, JUNV, GTOV, LASV and other, non-pathogenic, arenaviruses 53, 54, 156, 157 and allow rapid and reliable screening of small molecule libraries in an high-throughput format.
Replicon and mini-genome systems are alternative surrogate systems that enable study of arenavirus transcription and replication under BSL-2 conditions (recently reviewed in 158). A modified MACV replicon system has recently been created by Kranzusch et al. 159. The system is based on two plasmids: one that encodes the MACV L gene under the control of the T7 promoter and another that contains a modified MACV S segment, in which the GPC gene is replaced by an eGFP gene to enable a quantifiable readout. A similar LASV replicon system with a luciferase reporter has also been established 78. Transmission-incompetent infectious JUNV replicon particles also have been reported. Here, the antigenomic S segment contains a perfect replacement of GPC by either GFP or Gaussia luciferase (GLuc). To generate these particles, cells are transfected with three plasmids containing the L segment, the modified S segment, and the GPC gene160. Mini-genome systems have also been generated for LCMV161, Pichindé virus151, and TCRV80.
Finally, a surrogate system to rapidly and quantitatively measure arenavirus Z-mediated budding has also been established. This system is based on a chimeric LASV Z protein fused at its C-terminus to GLuc. The budding activity of this chimeric protein can be determined by measuring levels of GLuc activity in tissue culture supernatants of Z-GLuc-transfected cells. This cell-based BSL2 system is also amenable to high-throughput screens162.
2.2 Technologies for MACV drug discovery
2.2.1 Small molecule/compound screening
High-throughput screening of molecules for their antiviral effects has been increasingly used by both public laboratories and private companies to identify novel drugs against arenaviruses. These efforts have to date identified six chemically distinct classes of small molecule compounds that specifically inhibit GP-mediated membrane fusion with differing selectivities against New World and/or Old World arenaviruses 79, 163–165.
The first attempt to identify inhibitors of arenavirus infection using a high-throughput screening assay employed a virus-induced cytopathic effect-based assay with the non-pathogenic New World arenavirus TCRV. Approximately 400,000 small molecule compounds were screened and one highly active and specific small molecule inhibitor, ST-294, was found to inhibit TCRV in vitro, as well as other New World pathogenic viruses such as MACV, JUNV and GTOV, at concentrations in the nanomolar range. This molecule also demonstrated favorable pharmacodynamic properties (metabolically stable and orally bioavailable), as well as in vivo activity against TCRV in a newborn mouse model 163. Mechanism-of-action studies suggest that this compound targets GP2 and is a viral entry inhibitor 163.
Another screen for LASV entry inhibitors used a chemically diverse, random library of about 400,000 small molecule compounds against lentivirus-based pseudotypes incorporating LASV GP. The initial hit rate of the primary screen (>75% inhibition at 5 μM) was about 1.2%. However, 90 to 95% of these initial hits were found to be non-specific for LASV GP and/or cytotoxic in subsequent counter-screens (specificity assays using pseudotypes with unrelated GPs and inhibition of a panel of RNA and DNA viruses, cytotoxicity assays, confirmation assays against authentic LASV, and validation of antiviral activity with resynthesized compound). A benzimidazole derivative, ST-37, was identified, with a potent antiviral activity. Subsequent SAR and lead optimization yielded a modified compound, ST-193, with a potent in vitro activity against arenaviral entry mediated by diverse arenavirus envelope proteins. This antiviral is effective against pseudotypes of MACV, JUNV, GTOV, and LASV with an IC50 of 0.2–12 nM164.
Finally, to identify inhibitors of arenavirus infection using arenavirus GP as a target, Lee et al performed positive screening of more than 80,000 synthetic small molecules against LASV pseudotypes. This screen yielded initially ≈1% hits. Subsequent counter-screening for specificity using pseudotypes of the unrelated vesicular stomatitis Indiana virus yielded 32 single compounds and 53 compound mixtures that specifically blocked LASV GP-mediated infection. Two lead compounds, 16G8 and 17C8, were found to have high activity against MACV, JUNV, GTOV and other New World arenaviruses, as well as against LASV. These compounds act at the level of GP-mediated membrane fusion with IC50s ≈200–350 nm79. Despite different chemical structures, evidence suggests that all these diverse inhibitors act through the pH-sensitive interface of the signal peptide and GP2 subunits in the GP spike complex and prevent virus entry by stabilizing the prefusion spike complex against pH-induced activation in the endosome163–165.
Other types of inhibitors that target viral RNA synthesis have also been reported. T-705 (6-fluoro-3-hydroxy-2-pyrazinecarboxamide), a compound with broad antiviral activity against RNA viruses, including influenza A virus166, flaviviruses167, 168, bunyaviruses, and several nonpathogenic arenaviruses169–171 was found to be active in vitro against MACV, JUNV, and GTOV. T-705 most likely acts as a purine nucleoside analog specifically targeting L172. Studies employing the Pichindé virus hamster model of acute arenaviral disease showed that T-705 could protect against viral infection with efficacy in late stage infection169.
Another compound, 10-allyl-6-chloro-4-methoxy-9(10H)-acridone, designated 3f, was found to inhibit the multiplication of JUNV and other arenaviruses in an in vitro screen of N-substituted acridone derivatives. Acridone-based substances have been recently reported as potent inosine monophosphate dehydrogenase inhibitors173. Indeed, 3f strongly inhibited JUNV RNA synthesis in part by reducing the cellular GTP pool174, 175.
In the search for Z-targeted agents, antiretroviral zinc-finger active compounds with diverse chemical structures, including azoic compounds, hydrazide derivatives, disulfide-based reagents and others were screened in vitro against JUNV and other arenaviruses. The most active inhibitors, named NSC20625, 3–7, and 2–71, demonstrated a broad range of action against arenaviruses, including JUNV and other nonpathogenic arenaviruses. The aromatic disulfide NSC20625 is a very potent virucidal agent that destroys virion infectivity, generating particles that enter the host cell but are unable to complete the viral replication cycle176, 177. Follow-up studies showed that inactivated JUNV and LCMV particles retain the biological functions of the virion GP in virus binding and uptake, but are blocked at the uncoating of viral nucleocapsid from endosomes178. The treatment of purified JUNV with NSC20625 eliminated infectivity apparently through irreversible modifications in the matrix Z protein as detected by alterations in its electrophoretic migration profile and changes in its subcellular localization. In the case of LCMV, the compound was found to induce unfolding and oligomerization of Z to high-molecular-mass aggregates without affecting cellular RING-containing proteins such as PML179. However, NSC20625 did suppress LCMV Z-mediated PML nuclear bodies disruption, most likely by disrupting the Z-PML interaction180.
The screening against JUNV and other, nonpathogenic, arenaviruses of an extended series of aromatic and aliphatic disulfides, thiuram disulfides and thiosulfones, resulted in the identification of even more effective virucidal and antiviral agents.
Two new compounds, the aromatic carboxamide-derivative NSC4492 and the amine-derivative NSC71033, showed an improved efficacy to inactivate JUNV (submicromolar concentrations) in comparison to other disulfide-based compounds. The thiuram disulfide NSC14560 has an antiviral effect at low micromolar concentrations. The compound acts at a stage following viral entry most likely by interacting with the zinc-binding motifs present in the arenavirus Z protein181.
The action of five azo-based compounds against JUNV was evaluated in vitro. The compound 2-azo-(1'-(2'-nitroso)naphthyl)-benzoate (ANNB) was the most effective arenavirus inhibitor with EC50 values in the micromolar range and without inactivating properties. ANNB most likely acts at the level of intracellular virion assembly. By contrast, azodicarbonamide (ADA) is very effective in inactivating JUNV182.
Antiviral activity of antimicrobial cationic peptides, such as cecropin A and melittin, against JUNV was also shown. Cecropin A acts at the level of viral morphogenesis and egress from infected cells183 . Azoles obtained from carbohydrates were also found to inhibit JUNV replication184, 185.
2.2.2 siRNA screening
Genome-wide interference (RNAi) libraries are now available that allow the knockdown of all proteins known to be encoded by the human genome. These screens allow the dissection of virus–host interactions and have the potential to identify human host factors that are crucial for replication of pathogenic arenaviruses. Consequently, they provide great opportunities for the identification of drug targets. Targeting host cell determinants that are temporarily dispensable for the host but crucial for virus replication could also prevent viral escape. Although RNAi library screens have been used extensively to study important viruses, including HIV-1, flaviviruses and influenza A virus186–188, no similar large-scale studies have been reported to date with pathogenic arenaviruses.
3. Conclusion
In recent years, great strides have been made in the discovery and in vitro evaluation of compounds that are efficacious against various New World hemorrhagic fever arenavirus at nanomolar concentrations. Unfortunately, as has become clear through this review, these compounds cannot be further evaluated and characterized because of the lack of adequate animal models. It is of utmost importance to further study MACV infection in animals to establish an actual model of BHF that not only is characterized down to molecular markers, but that also fulfills the stringent requirements of the FDA for compound licensing brought forward in the “Animal Rule”. Of course, the development of such animal models should be guided by the “3R” (refinement, reduction, replacement) principle to minimize animal suffering and only be undertaken once sufficient human clinical data are available for comparisons. Furthermore, it will be necessary to re-evaluate the common notion that nonhuman primates are the best possible models for BHF. Rodent models hold great promise for drug evaluation, and compartmentalization, i.e. the evaluation of specific pathologic events in different types of animals rather than a single one, ought to be considered. Clearly, much more detailed studies need to be performed before it can be concluded which type of animal is most suitable to address a given problem in arenavirus research. This holds true especially for antiviral efficacy studies, as there might be species-to-species variation in regard to the ability of the antiviral to bind to its host target or the way how the antiviral is metabolized by the host.
4. Expert opinion
Drug discovery studies using MACV and other pathogenic New World arenaviruses have been limited to date mainly because work with these infectious agents is confined to maximum containment facilities. Furthermore, traditional methods of infectious virus quantification, such as plaque assays and qRT-PCR are time-consuming, tedious, and not amenable to high-throughput screening for drug discovery. Development of surrogate systems, consisting of only partial viral components, facilitated work under less stringent BSL-2 conditions and the establishment of more target specific and more high-throughput compound screening capabilities. Recent independent small molecules high-throughput screens have identified novel promising antivirals using these surrogate systems. To advance and accelerate identification of arenavirus candidate inhibitors, future efforts should focus on developing high-throughput methodologies for quantification of arenavirus infection rates, similar to the one we have developed and optimized for JUNV (Figure 2). Another important tool for areanvirus drug discovery would be the generation of a reverse genetics system for MACV, as well as the construction of recombinant pathogenic New World arenaviruses carrying reporter genes for more simplified and rapid assay implementation. Most antivirals identified and characterized to date against MACV infection target the early entry stage (pH-dependent fusion), viral RNA synthesis, or the arenaviral Z protein. Although these compounds were found to be effective in vitro, their efficacy remains to be proven in vivo in an appropriate BHF animal models or animal models of other pathogenic arenaviruses (such as AHF). Recently, two new rodent animal models have been characterized for MACV and JUNV infections116, 189. As these models and the guinea pig BHF and VHF models are more adequate models of New World pathogenic arenaviral human disease than rodent models of non-pathogenic arenaviruses, they should be used more extensively for initial in vivo evaluation of potential antiviral therapies.
Finally, arenaviruses encode for only four proteins and because of that rely heavily on the host cell machinery for productive infections. Identification of antivirals against host factors, which are crucial for arenaviral multiplication, will necessitate better understanding of arenavirus-host cell interaction. Future comprehensive RNAi screens, gene expression profiling and proteomics studies will largely advance our understanding of key host factors and regulatory networks that are important for pathogen survival. These in turn will likely lead to the identification of novel targets for therapeutics intervention.
Recent excellent reviews have already addressed targeting arenaviral fusion, GPC protein processing, and viral budding for therapeutic intervention117, 158, 190. Therefore, we will focus our discussion on targeting arenavirus entry, transcription and replication, as well as targeting NP’s function as an IFN antagonist.
Targeting arenaviral entry
Arenavirus cell entry is the first step of infection and a crucial determinant for cellular tropism, host range, and virus pathogenesis. As such, it represents a very attractive target for antiviral discovery to suppress the beginning of viral infection. It is clear that there is a close competition between virus spread and the patient’s antiviral immune response. While the rapid viral dissemination critically depends on efficient attachment of the virus to host cells and subsequent entry, drugs targeting these steps will give the host’s immune system an advantage by providing a wider window of opportunity for the generation of an efficient antiviral immune response. Recent discoveries in the field of cellular entry of MACV and other pathogenic New World arenaviruses make targeting this step even more attractive and promising.
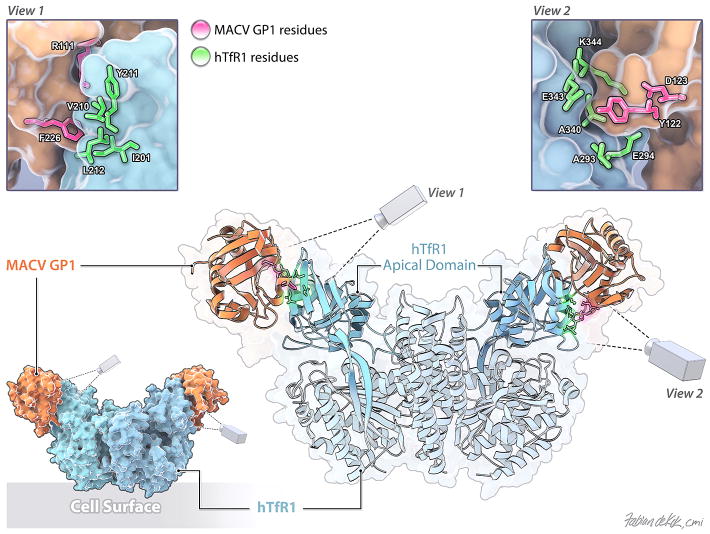
The human cellular receptor for all pathogenic New World arenaviruses (MACV, GTOV, JUNV, CHPV, and SABV) is TfR153, 55. In addition, the closely related non-pathogenic arenaviruses AMAV and TCRV can also use TfR1 orthologs of their principal reservoir hosts to infect nonhuman cells52. Interestingly, however, both AMAV and TCRV can enter human cells in a human TfR1-independent manner52, 191. This reveals a complex pattern of receptor utilization for New World arenaviruses, suggesting a possible relationship between receptor engagement and disease potential, i.e. it is possible that the ability of an arenavirus to bind to human TfR1 is a major factor that determines whether the virus is pathogenic for humans. Recent functional and structural studies identified the MACV GP1-hTfR1 binding site (Figure 3). First, the GP1-binding site on hTfR1 has been mapped to the apical domain of TfR1, and in particular, to a region surrounding the prominent tyrosine residue 21154. Indeed, the presence of this residue predicts whether one or more of these arenaviruses can utilize a particular TfR1 ortholog54. Second, the co-crystal structure of MACV GP1 bound to TfR1192 suggested GP1 residues important for this association and provided structural insight of GP1 residues that contact TfR1. Third, functional mutagenesis studies pinpointed MACV GP1 residues critical for hTfR1 association and viral entry193. Several of these GP1 residues are conserved among other pathogenic New World arenaviruses, indicating a common basis of receptor interaction. These studies, therefore, provide a solid platform for the future design and development of small molecule inhibitors and antivirals specifically targeting viral glycoprotein-host receptor interaction and subsequent cell entry.
Figure 3.
Structure of the MACV GP1-hTfR1 complex (as described in192) with the cell surface orientated to the bottom (PDB ID number: 3KAS). The TfR1 apical domain is colored cyan. MACV GP1 is colored in apricot. Two enlarged views of the TfR1:MACV GP1 contact sites are shown. MACV residues important for TfR1 binding are labeled and colored in magenta. TfR1 residues are labeled and colored in bright green.
TfR1 is an essential protein involved in iron uptake. It binds iron-bound transferrin and transports it to an acidic cellular compartment in which iron is released and subsequently transported across the vesicle membrane into the cytoplasm194, 195. Because of this important function of TfR1, targeting its interaction with pathogenic arenaviruses might result in significant cytotoxicity. However, the above-described studies clearly demonstrated that all New World hemorrhagic fever arenaviruses bind a common region of the apical domain of TfR154, 55, which does not overlap with the binding site of transferrin or another TfR1-binding factor, hereditary hemochromatosis protein, HFE196–198. Furthermore, any cytotoxicity risks associated with targeting the GP1-hTfR1 interaction are further mitigated by the short duration of treatment required in acute, fast-progressing arenavirus hemorrhagic fevers. Thus, compound screens and drug discovery approaches targeting the virus-receptor interactions may facilitate the identification and design of safe, effective, and general therapeutics that could inhibit the initial step of the infection process of all pathogenic New World arenaviruses.
One obvious approach to inhibit arenaviral cellular binding and entry is the use of humanized antibodies against TfR1, which inhibit the arenaviral GP1 association; similar to the ones previously described54, 55. As these antibodies bind the apical domain of TfR1, they would most likely not interfere with cellular iron uptake or other cellular functions of hTfR1. Administration of such antibodies, similar to immune serum administered to AHF patients, might not completely prevent infection. However, it might limit replication and slow the spread of the virus in an infected patient, and therefore slow the disease progression, allowing for the development of neutralizing antibodies, which are thought to be central for recovery199. Smaller soluble forms of TfR1 (for example, in the form of an apical domain) might be beneficial in a similar manner. TfR1 expression is regulated through iron-responsive elements at the 3’ untranslated regions of the its mRNA200, 201. When cellular iron concentrations are low, cell-surface expression of TfR1 is increased. High levels of surface TfR1 could promote more vigorous replication of the incoming pathogenic arenavirus. Therefore, it is plausible that iron supplementation, commonly used to treat anemia, could slow arenaviral replication by downregulating TfR1 expression. Such an approach, however, may have to bypass tight control of iron intake in the duodenum. Finally, small molecules and drug screen assays using either purified soluble TfR1 together with purified arenaviral GP1 in an ELISA-like platform or the MoMLV pseudotype system might be good strategies for identifying novel antiviral candidates for the treatment of arenaviral hemorrhagic fevers. We suggest that animal and clinical testing of these possible therapeutic approaches, first in MACV and JUNV rodent models and then in rhesus monkeys, is straightforward and warranted.
Targeting transcription and replication
Antivirals targeting areanaviral transcription and replication may function by a number of mechanisms. First, small molecules targeted at the viral RNA could alter its structure and consequently interfere with RNA-protein interactions and the formation of RNA-protein complexes. For example, in the case of HIV-1, aminoglycosides have been shown to selectively disrupt the Rev-RRE202 and Tat-TAR203 interactions. Similar type of antivirals might be also applicable for blocking the interaction between the MACV L polymerase and its binding site at positions 2–5 of the conserved 3’ viral promoter159. Second, antivirals targeting the viral polymerase could interfere with its crucial enzymatic functions in viral transcription and replication. Recently, the crystal structure of the N-terminal 196 residues of the L polymerase (NL1) of LCMV revealed a type II endonuclease a/b architecture similar to the N-terminal end of the influenza A virus PA protein. Functional studies demonstrated that NL1 has indeed RNA endonuclease activity; it is able to bind and cleave RNA and exhibits sequence-specificity with a preference for uracil-containing substrates. Mutagenesis and mini-genome-based assays revealed a correlation between endonuclease activity and selective production of mRNA, suggesting that this activity might be involved in the cap-snatching mechanism employed by arenaviruses. These studies also further defined the arenavirus endonuclease active site motif as E-X38-P-D-X(11,13)-E-X12-K-X3-D-X2-K. This active site of LCMV NL1 includes four acidic residues (E51, D89, E102 and D119), as well as the two important lysine residues K115 and K122 neighboring D119. Interestingly, P88, D89 and E102 are evolutionary-conserved within arenaviruses204. From an antiviral design point-of-view, this endonuclease domain is conserved and active across the members of the virus families Arenaviridae, Bunyaviridae and Orthomyxoviridae, and thus represents an attractive target for therapeutic intervention. Furthermore, any endonuclease inhibitors that have been reported for influenza A virus or would be identified in the future may be applicable to arenaviruses and bunyaviruses205.
Finally, recent studies describing the structure of LASV NP in the presence and absence of RNA have provided several new and potentially vulnerable targets on NP for the development of antivirals. RNA-free LASV NP forms trimers in solution with the N- and C- terminal domains oriented in a head-to-tail fashion, forming a ring-shaped structure with a three-fold symmetry206–208. In the absence of RNA, the N-terminal domain of LASV NP folds into a novel structure with a deep nucleotide-binding cavity that has been proposed to bind the cellular m7GTP cap structure for viral RNA transcription206. However, functional studies using the LASV mini-genome replicon assay demonstrated that exchange of conserved side chains in the cavity of the N terminus of NP in most cases resulted in a global defect in virus RNA synthesis rather than a specific defect in mRNA synthesis207. Furthermore, a recent crystal structure of the LASV NP–RNA complex indicated that this site is likely not a binding site for cap, but rather is a binding site for the viral genome208. This latter structure revealed that ssRNA binds in a deep, basic, crevice that channels between the head and body regions of NP’s N-terminal domain. The importance of the observed RNA-binding site to NP function has been demonstrated in mutagenesis studies using the LASV mini-genome system. Furthermore, similar functional assays suggest that the interaction between the N- and C-terminal domains of LASV NP (possibly because of a secondary RNA-binding site between the two NP domains), as well as NP–NP association (possibly to allow processive movement of the polymerase L) is important for viral replication and transcription207, 208.
These structural studies suggest a new model: RNA binding by NP is controlled through a gating mechanism of conformational changes within the N-terminal domain. The trimeric form of NP constitutes and stabilizes a closed conformation unable to bind RNA. In order for RNA to bind, NP must undergo structural rearrangements that may ultimately result in reorganization of the oligomer. These features make NP an excellent target for small molecules that prevent NP binding of RNA. Each NP residue that contacts the RNA and which is functionally important for viral transcription and replication is 100% conserved among arenaviruses. Particularly attractive targets for inhibitors of arenavirus replication are the packing interactions between the conserved residues Y308 and R300 (in LASV) and the purine at position 3 of the RNA. Finally, antivirals that target the NP–NP interface, the interface between the N- and C-terminal domains of NP, or that stabilize the RNP to prevent conformational changes induced by the polymerase L might also be effective in inhibiting arenavirus replication.
Targeting arenaviral NP and its suppression of the innate IFN response
The arenaviral nucleoprotein NP has been implicated in suppression of the host innate immune system, but the mechanism by which this occurs has remained elusive. The crystal structure of the immunosuppressive C-terminal half of LASV NP illustrated that its 3D fold closely mimics that of the DEDDh family of exonucleases. Biochemical experiments demonstrate that NP indeed has a bona fide exonuclease activity, with strict specificity for double-stranded RNA substrates in a 3’ to 5’ direction. The active sites of DEDDh exonucleases form a negatively charged cavity, which contains the strictly conserved catalytic residues Asp-Glu-Asp-Asp, and a histidine in close apposition to the DEDD core. In LASV NP, the putative exonuclease catalytic residues D389, E391, D466, D533, and H528 are located in a negatively charged cavity and mutations of these residues abrogate exonuclease activity, IRF3 translocation into the nucleus, as well as Sendai virus-induced IFN-beta activation206, 209. These data suggest that that proper exonuclease function of arenaviral NP is critical for innate immune suppression in vitro. Furthermore, as the residues in the DEDDh active site are completely conserved among all arenavirus NP proteins, this exonuclease activity might be a shared feature of arenavirus NPs, and therefore a valid target for therapeutic intervention. Indeed, mutations of LCMV NP residues previously identified as essential for arenaviral anti-IFN activity109 are now shown to lie within the exonuclease active site or in its vicinity.
The high-resolution crystal structure determinations of arenavirus NP thus reveal several new and potentially vulnerable targets on NP for the development of antivirals and provide a structural template necessary for the design and analysis of such antivirals.
Acknowledgments
The content of this publication does not necessarily reflect the views or policies of the US Department of Defense, the US Department of the Army, the US Department of Health and Human Services or of the institutions and companies affiliated with the authors.
Footnotes
Declaration of interest
SR Radoshitzky and S Bavari’s research is supported by the Joint Science and Technology Office Transformational Medical Technologies (proposal #TMTI0048_09_RDE_T). F de Kok-Mercado performed this work as an employee of Battelle Memorial Institute under its prime contract with NIAID (Contract No. HHSN272200200016I). JH Kuhn performed this work as an employee of Tunnell Consulting, Inc., a subcontractor to Battelle Memorial Institute under its prime contract with NIAID (Contract No. HHSN272200200016I).
References
- 1.Peters CJ, Kuehne RW, Mercado RR, et al. Hemorrhagic fever in Cochabamba, Bolivia, 1971. Am J Epidemiol. 1974 Jun;99(6):425–33. doi: 10.1093/oxfordjournals.aje.a121631. [DOI] [PubMed] [Google Scholar]
- 2.Aguilar PV, Camargo W, Vargas J, et al. Reemergence of Bolivian hemorrhagic fever, 2007–2008. Emerging infectious diseases. 2009 Sep;15(9):1526–8. doi: 10.3201/eid1509.090017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray M. Pathogenesis of viral hemorrhagic fever. Curr Opin Immunol. 2005 Aug;17(4):399–403. doi: 10.1016/j.coi.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Geisbert TW, Jahrling PB. Exotic emerging viral diseases: progress and challenges. Nat Med. 2004 Dec;10(12 Suppl):S110–21. doi: 10.1038/nm1142. [DOI] [PubMed] [Google Scholar]
- 5.McCormick JB, Fisher-Hoch SP. Lassa fever. Curr Top Microbiol Immunol. 2002;262:75–109. doi: 10.1007/978-3-642-56029-3_4. [DOI] [PubMed] [Google Scholar]
- 6.Buchmeier MJ, de La Torre JC, Peters CJ. Arenaviridae:the viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. 5. Philadelphia: Lippincott Williams & Wilkins; 2006. pp. 1791–827. [Google Scholar]
- 7.Enria DA, Briggiler AM, Sanchez Z. Treatment of Argentine hemorrhagic fever. Antiviral Res. 2008 Apr;78(1):132–9. doi: 10.1016/j.antiviral.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.NIAID Category Priority Pathogens. 2012 http://www.niaid.nih.gov/topics/biodefenserelated/biodefense/pages/cata.aspx.
- 9.National Select Agent Registry. 2012 http://www.selectagents.gov/
- 10*.Borio L, Inglesby T, Peters CJ, et al. Hemorrhagic fever viruses as biological weapons: medical and public health management. J Am Med Assoc. 2002 May 8;287(18):2391–405. doi: 10.1001/jama.287.18.2391. This article describes the rationale why the development of antivirals against exotic agents such as New World arenaviruses is deemed critical. [DOI] [PubMed] [Google Scholar]
- 11.Charrel RN, de Lamballerie X. Arenaviruses other than Lassa virus. Antiviral Res. 2003 Jan;57(1–2):89–100. doi: 10.1016/s0166-3542(02)00202-4. [DOI] [PubMed] [Google Scholar]
- 12**.Briese T, Paweska JT, McMullan LK, et al. Genetic detection and characterization of Lujo virus, a new hemorrhagic fever-associated arenavirus from southern Africa. PLoS Path. 2009 May;5(5):e1000455. doi: 10.1371/journal.ppat.1000455. This article describes a novel arenavirus that possibly challenges the long-held notion of only two distinct (Old World and New World) arenavirus groups. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delgado S, Erickson BR, Agudo R, et al. Chapare Virus, a Newly Discovered Arenavirus Isolated from a Fatal Hemorrhagic Fever Case in Bolivia. PLoS Path. 2008 Apr;4(4):e1000047. doi: 10.1371/journal.ppat.1000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clegg JC. Molecular phylogeny of the arenaviruses. Curr Top Microbiol Immunol. 2002;262:1–24. doi: 10.1007/978-3-642-56029-3_1. [DOI] [PubMed] [Google Scholar]
- 15.Jay MT, Glaser C, Fulhorst CF. The arenaviruses. J Am Vet Med Assoc. 2005 Sep 15;227(6):904–15. doi: 10.2460/javma.2005.227.904. [DOI] [PubMed] [Google Scholar]
- 16.Lecompte E, Ter Meulen J, Emonet S, et al. Genetic identification of Kodoko virus, a novel arenavirus of the African pigmy mouse (Mus Nannomys minutoides) in West Africa. Virology. 2007 Jul 20;364(1):178–83. doi: 10.1016/j.virol.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Oldstone MB. Arenaviruses. I. The epidemiology molecular and cell biology of arenaviruses. Introduction. Curr Top Microbiol Immunol. 2002;262:V–XII. [PubMed] [Google Scholar]
- 18.Charrel RN, Lemasson JJ, Garbutt M, et al. New insights into the evolutionary relationships between arenaviruses provided by comparative analysis of small and large segment sequences. Virology. 2003 Dec 20;317(2):191–6. doi: 10.1016/j.virol.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Erickson BR, Delgado S, Aguda R, et al. A newly discovered arenavirus associated with a fatal hemorrhagic fever case in Bolivia. Abstracts of the XIIIth International Conference on Negative Strand Viruses; 2006 June 17–22; Salamanca, Spain. 2006. p. 165. [Google Scholar]
- 20.Salvato MS, Clegg JCS, Buchmeier MJ, et al. Family Arenaviridae. In: King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ, editors. Virus Taxonomy - Ninth Report of the International Committee on Taxonomy of Viruses. London, United Kingdom: Elsevier/Academic Press; 2011. pp. 715–23. [Google Scholar]
- 21.Bowen MD, Peters CJ, Nichol ST. The phylogeny of New World (Tacaribe complex) arenaviruses. Virology. 1996 May 1;219(1):285–90. doi: 10.1006/viro.1996.0248. [DOI] [PubMed] [Google Scholar]
- 22.Rowe WP, Pugh WE, Webb PA, et al. Serological relationship of the Tacaribe complex of viruses to lymphocytic choriomeningitis virus. J VirolJ Virol. 1970 Mar;5(3):289–92. doi: 10.1128/jvi.5.3.289-292.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cajimat MN, Fulhorst CF. Phylogeny of the Venezuelan arenaviruses. Virus Res. 2004 Jun 15;102(2):199–206. doi: 10.1016/j.virusres.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez JP, Sanchez A, Rico-Hesse R. Molecular phylogeny of Guanarito virus, an emerging arenavirus affecting humans. Am J Trop Med Hyg. 1995 Jul;53(1):1–6. [PubMed] [Google Scholar]
- 25.Charrel RN, Feldmann H, Fulhorst, et al. Phylogeny of New World arenaviruses based on the complete coding sequences of the small genomic segment identified an evolutionary lineage produced by intrasegmental recombination. Biochem Biophys Res Commun. 2002 Sep 6;296(5):1118–24. doi: 10.1016/s0006-291x(02)02053-3. [DOI] [PubMed] [Google Scholar]
- 26.Salazar-Bravo J, Ruedas LA, Yates TL. Mammalian reservoirs of arenaviruses. Curr Top Microbiol Immunol. 2002;262:25–63. doi: 10.1007/978-3-642-56029-3_2. [DOI] [PubMed] [Google Scholar]
- 27**.Johnson KM, Mackenzie RB, Webb PA, et al. Chronic infection of rodents by Machupo virus. Science. 1965 Dec 17;150(703):1618–9. doi: 10.1126/science.150.3703.1618. This important manuscripts establishes the mechanism by which Machupo virus is maintained in nature. [DOI] [PubMed] [Google Scholar]
- 28.Johnson KM, Kuns ML, Mackenzie RB, et al. Isolation of Machupo virus from wild rodent Calomys callosus. Am J Trop Med Hyg. 1966 Jan;15(1):103–6. doi: 10.4269/ajtmh.1966.15.103. [DOI] [PubMed] [Google Scholar]
- 29.Fulhorst CF, Bowen MD, Salas RA, et al. Natural rodent host associations of Guanarito and pirital viruses (Family Arenaviridae) in central Venezuela. Am J Top Med Hyg. 1999 Aug;61(2):325–30. doi: 10.4269/ajtmh.1999.61.325. [DOI] [PubMed] [Google Scholar]
- 30.Mills JN, Ellis BA, McKee KT, Jr, et al. Junin virus activity in rodents from endemic and nonendemic loci in central Argentina. Am J Trop Med Hyg. 1991 Jun;44(6):589–97. doi: 10.4269/ajtmh.1991.44.589. [DOI] [PubMed] [Google Scholar]
- 31.Hugot JP, Gonzalez JP, Denys C. Evolution of the Old World Arenaviridae and their rodent hosts: generalized host-transfer or association by descent? Infect Genet Evol. 2001 Jul;1(1):13–20. doi: 10.1016/s1567-1348(01)00003-x. [DOI] [PubMed] [Google Scholar]
- 32.Bowen MD, Peters CJ, Nichol ST. Phylogenetic analysis of the Arenaviridae: patterns of virus evolution and evidence for cospeciation between arenaviruses and their rodent hosts. Mol Phylogenet Evol. 1997 Dec;8(3):301–16. doi: 10.1006/mpev.1997.0436. [DOI] [PubMed] [Google Scholar]
- 33**.Mackenzie RB, Beye HK, Valverde L, et al. Epidemic hemorrhagic fever in Bolivia. I. A preliminary report of the epidemiologic and clinical findings in a new epidemic area in South America. Am J Trop Med Hyg. 1964 Jul;13:620–5. One of several important papers that describes the initial discovery of Machupo virus and Bolivian hemorrhagic fever. [PubMed] [Google Scholar]
- 34.Johnson KM, Wiebenga NH, Mackenzie RB, et al. Virus Isolations from Human Cases of Hemorrhagic Fever in Bolivia. Proc Soc Exp Biol Med. 1965 Jan;118:113–8. doi: 10.3181/00379727-118-29772. [DOI] [PubMed] [Google Scholar]
- 35**.Kuns ML. Epidemiology of Machupo virus infection. II. Ecological and control studies of hemorrhagic fever. Am J Trop Med Hyg. 1965 Sep;14(5):813–6. doi: 10.4269/ajtmh.1965.14.813. One of several important papers that describes the initial discovery of Machupo virus and Bolivian hemorrhagic fever. [DOI] [PubMed] [Google Scholar]
- 36.Buchmeier MJ. Arenaviruses: protein structure and function. Curr Top Microbiol Immunol. 2002;262:159–73. doi: 10.1007/978-3-642-56029-3_7. [DOI] [PubMed] [Google Scholar]
- 37.Meyer BJ, de la Torre JC, Southern PJ. Arenaviruses: genomic RNAs, transcription, and replication. Curr Top Microbiol Immunol. 2002;262:139–57. doi: 10.1007/978-3-642-56029-3_6. [DOI] [PubMed] [Google Scholar]
- 38.Auperin DD, Galinski M, Bishop DH. The sequences of the N protein gene and intergenic region of the S RNA of pichinde arenavirus. Virology. 1984 Apr 15;134(1):208–19. doi: 10.1016/0042-6822(84)90286-1. [DOI] [PubMed] [Google Scholar]
- 39.Wilson SM, Clegg JC. Sequence analysis of the S RNA of the African arenavirus Mopeia: an unusual secondary structure feature in the intergenic region. Virology. 1991 Feb;180(2):543–52. doi: 10.1016/0042-6822(91)90068-m. [DOI] [PubMed] [Google Scholar]
- 40.Buchmeier MJ, Southern PJ, Parekh BS, et al. Site-specific antibodies define a cleavage site conserved among arenavirus GP-C glycoproteins. J Virol. 1987 Apr;61(4):982–5. doi: 10.1128/jvi.61.4.982-985.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kunz S, Edelmann KH, de la Torre JC, et al. Mechanisms for lymphocytic choriomeningitis virus glycoprotein cleavage, transport, and incorporation into virions. Virology. 2003 Sep 15;314(1):168–78. doi: 10.1016/s0042-6822(03)00421-5. [DOI] [PubMed] [Google Scholar]
- 42**.Lenz O, ter Meulen J, Klenk HD, et al. The Lassa virus glycoprotein precursor GP-C is proteolytically processed by subtilase SKI-1/S1P. Proc Natl Acad Sci U S A. 2001 Oct 23;98(22):12701–5. doi: 10.1073/pnas.221447598. This paper identifies the protease necessary for arenaviral GPC processing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beyer WR, Popplau D, Garten W, et al. Endoproteolytic processing of the lymphocytic choriomeningitis virus glycoprotein by the subtilase SKI-1/S1P. J Virol. 2003 Mar;77(5):2866–72. doi: 10.1128/JVI.77.5.2866-2872.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daelli MG, Coto CE. Inhibition of the production of infectious particles in cells infected with Junin virus in the presence of tunicamycin. Rev Arg Microbiol. 1982;14(3):171–6. [PubMed] [Google Scholar]
- 45.Eichler R, Lenz O, Garten W, et al. The role of single N-glycans in proteolytic processing and cell surface transport of the Lassa virus glycoprotein GP-C. Virol J. 2006;3:41. doi: 10.1186/1743-422X-3-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gangemi JD, Rosato RR, Connell EV, et al. Structural polypeptides of Machupo virus. J Gen Virol. 1978 Oct;41(1):183–8. doi: 10.1099/0022-1317-41-1-183. [DOI] [PubMed] [Google Scholar]
- 47.Padula PJ, de Martinez Segovia ZM. Replication of Junin virus in the presence of tunicamycin. Intervirology. 1984;22(4):227–31. doi: 10.1159/000149555. [DOI] [PubMed] [Google Scholar]
- 48.Wright KE, Spiro RC, Burns JW, et al. Post-translational processing of the glycoproteins of lymphocytic choriomeningitis virus. Virology. 1990 Jul;177(1):175–83. doi: 10.1016/0042-6822(90)90471-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Froeschke M, Basler M, Groettrup M, et al. Long-lived signal peptide of lymphocytic choriomeningitis virus glycoprotein pGP-C. J Biol Chem. 2003 Oct 24;278(43):41914–20. doi: 10.1074/jbc.M302343200. [DOI] [PubMed] [Google Scholar]
- 50*.York J, Romanowski V, Lu M, et al. The signal peptide of the Junin arenavirus envelope glycoprotein is myristoylated and forms an essential subunit of the mature G1-G2 complex. J Virol. 2004 Oct;78(19):10783–92. doi: 10.1128/JVI.78.19.10783-10792.2004. This manuscript is the first of many studies that elaborates on the important role of the arenaviral GPC signal peptide in GPC maturation, trafficking, and fusion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Agnihothram SS, York J, Nunberg JH. Role of the stable signal peptide and cytoplasmic domain of G2 in regulating intracellular transport of the Junin virus envelope glycoprotein complex. J Virol. 2006 Jun;80(11):5189–98. doi: 10.1128/JVI.00208-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52*.Abraham J, Kwong JA, Albarino CG, et al. Host-Species Transferrin Receptor 1 Orthologs Are Cellular Receptors for Nonpathogenic New World Clade B Arenaviruses. PLoS Path. 2009 Apr;5(4) doi: 10.1371/journal.ppat.1000358. This manuscript establishes that tranferrin receptor 1 from hosts of nonpathogenic New World arenaviruses is a receptor for these viruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53**.Radoshitzky SR, Abraham J, Spiropoulou CF, et al. Transferrin receptor 1 is a cellular receptor for New World haemorrhagic fever arenaviruses. Nature. 2007 Mar 1;446(7131):92–6. doi: 10.1038/nature05539. This manuscript identifies human transferrin receptor 1 as a cellular receptor for Machupo virus and other New World hemorrhagic fever arenaviruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54*.Radoshitzky SR, Kuhn JH, Spiropoulou CF, et al. Receptor determinants of zoonotic transmission of New World hemorrhagic fever arenaviruses. Proc Natl Acad Sci U S A. 2008 Feb 19;105(7):2664–9. doi: 10.1073/pnas.0709254105. This manuscript chrachterizes the site on human tranferrin receptor 1 to which arenaviral GP1 binds as well as commonalities between human tranferrin receptor 1 and tranferrin receptor 1 from hosts of hemorrhagic New World arenaviruses that possibly facilitate zoonotic transmission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Helguera G, Jemielity S, Abraham J, et al. An antibody recognizing the apical domain of human transferrin receptor 1 efficiently inhibits the entry of all New World hemorrhagic fever arenaviruses. J Virol. Jan 25; doi: 10.1128/JVI.06397-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martinez MG, Cordo SM, Candurra NA. Characterization of Junin arenavirus cell entry. J Gen Virol. 2007 Jun;88(Pt 6):1776–84. doi: 10.1099/vir.0.82808-0. [DOI] [PubMed] [Google Scholar]
- 57.Vela EM, Zhang L, Colpitts TM, et al. Arenavirus entry occurs through a cholesterol-dependent, non-caveolar, clathrin-mediated endocytic mechanism. Virology. 2007 Aug 13; doi: 10.1016/j.virol.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Castilla V, Mersich SE, Candurra NA, et al. The entry of Junin virus into Vero cells. Arch Virol. 1994;136(3–4):363–74. doi: 10.1007/BF01321064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Glushakova SE, Lukashevich IS. Early events in arenavirus replication are sensitive to lysosomotropic compounds. Arch Virol. 1989;104(1–2):157–61. doi: 10.1007/BF01313817. [DOI] [PubMed] [Google Scholar]
- 60.Glushakova SE, Iakuba AI, Vasiuchkov, et al. Lysosomotropic agents inhibit the penetration of arenaviruses into a culture of BHK-21 and Vero cells. Vopr Virusol. 1990;35(2):146–50. [PubMed] [Google Scholar]
- 61.Kunz S, Borrow P, Oldstone MB. Receptor structure, binding, and cell entry of arenaviruses. Curr Top Microbiol Immunol. 2002;262:111–37. doi: 10.1007/978-3-642-56029-3_5. [DOI] [PubMed] [Google Scholar]
- 62.Oldenburg J, Reignier T, Flanagan ML, et al. Differences in tropism and pH dependence for glycoproteins from the Clade B1 arenaviruses: Implications for receptor usage and pathogenicity. Virology. 2007 Jul 20;364(1):132–9. doi: 10.1016/j.virol.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martinez MG, Forlenza MB, Candurra NA. Involvement of cellular proteins in Junin arenavirus entry. Biotechnol J. 2009;4(6):866–70. doi: 10.1002/biot.200800357. [DOI] [PubMed] [Google Scholar]
- 64.Martinez MG, Cordo SM, Candurra NA. Involvement of cytoskeleton in Junin virus entry. Virus Res. 2008 Dec;138(1–2):17–25. doi: 10.1016/j.virusres.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 65.Linero FN, Scolaro LA. Participation of the phosphatidylinositol 3-kinase/Akt pathway in Junin virus replication in vitro. Virus Res. 2009 Oct;145(1):166–70. doi: 10.1016/j.virusres.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.York J, Nunberg JH. Role of the stable signal peptide of Junin arenavirus envelope glycoprotein in pH-dependent membrane fusion. J Virol. 2006 Aug;80(15):7775–80. doi: 10.1128/JVI.00642-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.York J, Nunberg JH. Intersubunit interactions modulate pH-induced activation of membrane fusion by the Junin virus envelope glycoprotein GPC. J Virol. 2009 May;83(9):4121–6. doi: 10.1128/JVI.02410-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Di Simone C, Buchmeier MJ. Kinetics and pH dependence of acid-induced structural changes in the lymphocytic choriomeningitis virus glycoprotein complex. Virology. 1995 May 10;209(1):3–9. doi: 10.1006/viro.1995.1225. [DOI] [PubMed] [Google Scholar]
- 69.Di Simone C, Zandonatti MA, Buchmeier MJ. Acidic pH triggers LCMV membrane fusion activity and conformational change in the glycoprotein spike. Virology. 1994 Feb;198(2):455–65. doi: 10.1006/viro.1994.1057. [DOI] [PubMed] [Google Scholar]
- 70.Glushakova SE, Lukashevich IS, Baratova LA. Prediction of arenavirus fusion peptides on the basis of computer analysis of envelope protein sequences. FEBS Lett. 1990 Aug 20;269(1):145–7. doi: 10.1016/0014-5793(90)81140-j. [DOI] [PubMed] [Google Scholar]
- 71.Glushakova SE, Omelyanenko VG, Lukashevitch IS, et al. The fusion of artificial lipid membranes induced by the synthetic arenavirus 'fusion peptide'. Biochim Biophys Acta. 1992 Oct 5;1110(2):202–8. doi: 10.1016/0005-2736(92)90360-x. [DOI] [PubMed] [Google Scholar]
- 72.Eschli B, Quirin K, Wepf A, et al. Identification of an N-terminal trimeric coiled-coil core within arenavirus glycoprotein 2 permits assignment to class I viral fusion proteins. J Virol. 2006 Jun;80(12):5897–907. doi: 10.1128/JVI.00008-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Meyer BJ, Southern PJ. Concurrent sequence analysis of 5' and 3' RNA termini by intramolecular circularization reveals 5' nontemplated bases and 3' terminal heterogeneity for lymphocytic choriomeningitis virus mRNAs. J Virol. 1993 May;67(5):2621–7. doi: 10.1128/jvi.67.5.2621-2627.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Singh MK, Fuller-Pace FV, Buchmeier MJ, et al. Analysis of the genomic L RNA segment from lymphocytic choriomeningitis virus. Virology. 1987 Dec;161(2):448–56. doi: 10.1016/0042-6822(87)90138-3. [DOI] [PubMed] [Google Scholar]
- 75.Southern PJ, Singh MK, Riviere Y, et al. Molecular characterization of the genomic S RNA segment from lymphocytic choriomeningitis virus. Virology. 1987 Mar;157(1):145–55. doi: 10.1016/0042-6822(87)90323-0. [DOI] [PubMed] [Google Scholar]
- 76.Garcin D, Kolakofsky D. A novel mechanism for the initiation of Tacaribe arenavirus genome replication. J Virol. 1990 Dec;64(12):6196–203. doi: 10.1128/jvi.64.12.6196-6203.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Raju R, Raju L, Hacker D, et al. Nontemplated bases at the 5' ends of Tacaribe virus mRNAs. Virology. 1990 Jan;174(1):53–9. doi: 10.1016/0042-6822(90)90053-t. [DOI] [PubMed] [Google Scholar]
- 78.Hass M, Golnitz U, Muller S, et al. Replicon system for Lassa virus. J Virol. 2004 Dec;78(24):13793–803. doi: 10.1128/JVI.78.24.13793-13803.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79**.Lee AM, Rojek JM, Spiropoulou CF, et al. Unique small molecule entry inhibitors of hemorrhagic fever arenaviruses. J Biol Chem. 2008 Jul 4;283(27):18734–42. doi: 10.1074/jbc.M802089200. One of several important papers that describe high-throughput screnning against New World arenaviruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lopez N, Jacamo R, Franze-Fernandez MT. Transcription and RNA replication of tacaribe virus genome and antigenome analogs require N and L proteins: Z protein is an inhibitor of these processes. J Virol. 2001 Dec;75(24):12241–51. doi: 10.1128/JVI.75.24.12241-12251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81**.Eichler R, Lenz O, Strecker T, et al. Identification of Lassa virus glycoprotein signal peptide as a trans-acting maturation factor. EMBO Rep. 2003 Nov;4(11):1084–8. doi: 10.1038/sj.embor.7400002. This manuscript describes the unique and important function of the arenaviral GPC signal peptide in GPC maturation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82*.Rojek JM, Lee AM, Nguyen N, et al. Site 1 protease is required for proteolytic processing of the glycoproteins of the South American hemorrhagic fever viruses Junin, Machupo, and Guanarito. J Virol. 2008 Jun;82(12):6045–51. doi: 10.1128/JVI.02392-07. This paper expands the findings of reference 42 to New World hemorrhagic fever arenaviruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Eichler R, Lenz O, Strecker T, et al. Signal peptide of Lassa virus glycoprotein GP-C exhibits an unusual length. FEBS Lett. 2003 Mar 13;538(1–3):203–6. doi: 10.1016/s0014-5793(03)00160-1. [DOI] [PubMed] [Google Scholar]
- 84.Leung WC, Ghosh HP, Rawls WE. Strandedness of Pichinde virus RNA. J Virol. 1977 Apr;22(1):235–7. doi: 10.1128/jvi.22.1.235-237.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Garcin D, Kolakofsky D. Tacaribe arenavirus RNA synthesis in vitro is primer dependent and suggests an unusual model for the initiation of genome replication. J Virol. 1992 Mar;66(3):1370–6. doi: 10.1128/jvi.66.3.1370-1376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cornu TI, de la Torre JC. RING finger Z protein of lymphocytic choriomeningitis virus (LCMV) inhibits transcription and RNA replication of an LCMV S-segment minigenome. J Virol. 2001 Oct;75(19):9415–26. doi: 10.1128/JVI.75.19.9415-9426.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cornu TI, de la Torre JC. Characterization of the arenavirus RING finger Z protein regions required for Z-mediated inhibition of viral RNA synthesis. J Virol. 2002 Jul;76(13):6678–88. doi: 10.1128/JVI.76.13.6678-6688.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cornu TI, Feldmann H, de la Torre JC. Cells expressing the RING finger Z protein are resistant to arenavirus infection. J Virol. 2004 Mar;78(6):2979–83. doi: 10.1128/JVI.78.6.2979-2983.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jacamo R, Lopez N, Wilda M, et al. Tacaribe virus Z protein interacts with the L polymerase protein to inhibit viral RNA synthesis. J Virol. 2003 Oct;77(19):10383–93. doi: 10.1128/JVI.77.19.10383-10393.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kranzusch PJ, Whelan SP. Arenavirus Z protein controls viral RNA synthesis by locking a polymerase-promoter complex. Proc Natl Acad Sci U S A. Nov 21; doi: 10.1073/pnas.1112742108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dalton AJ, Rowe WP, Smith GH, et al. Morphological and cytochemical studies on lymphocytic choriomeningitis virus. J Virol. 1968 Dec;2(12):1465–78. doi: 10.1128/jvi.2.12.1465-1478.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tesh RB, Jahrling PB, Salas R, et al. Description of Guanarito virus (Arenaviridae: Arenavirus), the etiologic agent of Venezuelan hemorrhagic fever. Am J Trop Med Hyg. 1994 Apr;50(4):452–9. doi: 10.4269/ajtmh.1994.50.452. [DOI] [PubMed] [Google Scholar]
- 93.Eichler R, Strecker T, Kolesnikova L, et al. Characterization of the Lassa virus matrix protein Z: electron microscopic study of virus-like particles and interaction with the nucleoprotein (NP) Virus Res. 2004 Mar 15;100(2):249–55. doi: 10.1016/j.virusres.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 94**.Perez M, Craven RC, de la Torre JC. The small RING finger protein Z drives arenavirus budding: implications for antiviral strategies. Proc Natl Acad Sci U S A. 2003 Oct 28;100(22):12978–83. doi: 10.1073/pnas.2133782100. This paper is the first to demonstrate the role of arenaviral Z protein as a budding factor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Strecker T, Eichler R, Meulen J, et al. Lassa virus Z protein is a matrix protein and sufficient for the release of virus-like particles [corrected] J Virol. 2003 Oct;77(19):10700–5. doi: 10.1128/JVI.77.19.10700-10705.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Capul AA, Perez M, Burke E, et al. Arenavirus Z-glycoprotein association requires Z myristoylation but not functional RING or late domains. J Virol. 2007 Sep;81(17):9451–60. doi: 10.1128/JVI.00499-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Perez M, Greenwald DL, de la Torre JC. Myristoylation of the RING finger Z protein is essential for arenavirus budding. J Virol. 2004 Oct;78(20):11443–8. doi: 10.1128/JVI.78.20.11443-11448.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Strecker T, Maisa A, Daffis S, et al. The role of myristoylation in the membrane association of the Lassa virus matrix protein Z. Virology journal. 2006;3:93. doi: 10.1186/1743-422X-3-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Salvato MS. Molecular biology of the prototype arenavirus, lymphocytic choriomeningitis virus. In: Salvato MS, editor. The Arenaviridae. New York: Plenum Press; 1993. pp. 133–56. [Google Scholar]
- 100.Salvato MS, Schweighofer KJ, Burns J, et al. Biochemical and immunological evidence that the 11 kDa zinc-binding protein of lymphocytic choriomeningitis virus is a structural component of the virus. Virus research. 1992 Mar;22(3):185–98. doi: 10.1016/0168-1702(92)90050-j. [DOI] [PubMed] [Google Scholar]
- 101.Urata S, Noda T, Kawaoka Y, et al. Cellular factors required for Lassa virus budding. J Virol. 2006 Apr;80(8):4191–5. doi: 10.1128/JVI.80.8.4191-4195.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Borden KL, Campbell Dwyer EJ, et al. An arenavirus RING (zinc-binding) protein binds the oncoprotein promyelocyte leukemia protein (PML) and relocates PML nuclear bodies to the cytoplasm. J Virol. 1998 Jan;72(1):758–66. doi: 10.1128/jvi.72.1.758-766.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Borden KL, Campbelldwyer EJ, Carlile GW, et al. Two RING finger proteins, the oncoprotein PML and the arenavirus Z protein, colocalize with the nuclear fraction of the ribosomal P proteins. J Virol. 1998 May;72(5):3819–26. doi: 10.1128/jvi.72.5.3819-3826.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Campbell Dwyer EJ, Lai H, et al. The lymphocytic choriomeningitis virus RING protein Z associates with eukaryotic initiation factor 4E and selectively represses translation in a RING-dependent manner. J Virol. 2000 Apr;74(7):3293–300. doi: 10.1128/jvi.74.7.3293-3300.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Djavani M, Topisirovic I, Zapata JC, et al. The proline-rich homeodomain (PRH/HEX) protein is down-regulated in liver during infection with lymphocytic choriomeningitis virus. J Virol. 2005 Feb;79(4):2461–73. doi: 10.1128/JVI.79.4.2461-2473.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kentsis A, Dwyer EC, Perez JM, et al. The RING domains of the promyelocytic leukemia protein PML and the arenaviral protein Z repress translation by directly inhibiting translation initiation factor eIF4E. J Mol Biol. 2001 Sep 28;312(4):609–23. doi: 10.1006/jmbi.2001.5003. [DOI] [PubMed] [Google Scholar]
- 107*.Martinez-Sobrido L, Giannakas P, Cubitt B, et al. Differential inhibition of type I interferon induction by arenavirus nucleoproteins. J Virol. 2007 Nov;81(22):12696–703. doi: 10.1128/JVI.00882-07. This paper expands the important finding from reference 108 to other arenaviruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108**.Martinez-Sobrido L, Zuniga EI, Rosario D, et al. Inhibition of the type I interferon response by the nucleoprotein of the prototypic arenavirus lymphocytic choriomeningitis virus. J Virol. 2006 Sep;80(18):9192–9. doi: 10.1128/JVI.00555-06. This paper identifies Old World arenavirus NP to be an interferon antagonist. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Martinez-Sobrido L, Emonet S, Giannakas P, et al. Identification of amino acid residues critical for the anti-interferon activity of the nucleoprotein of the prototypic arenavirus lymphocytic choriomeningitis virus. J Virol. 2009 Nov;83(21):11330–40. doi: 10.1128/JVI.00763-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110**.Fan L, Briese T, Lipkin WI. Z proteins of New World arenaviruses bind RIG-I and interfere with type I interferon induction. J Virol. Feb;84(4):1785–91. doi: 10.1128/JVI.01362-09. This paper identifies Old World arenavirus Z to be an interferon antagonist. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Eddy GA, Scott SK, Wagner FS, et al. Pathogenesis of Machupo virus infection in primates. Bull World Health Organ. 1975;52(4–6):517–21. [PMC free article] [PubMed] [Google Scholar]
- 112.Terrell TG, Stookey JL, Eddy GA, et al. Pathology of Bolivian hemorrhagic fever in the rhesus monkey. Am J Pathol. 1973 Nov;73(2):477–94. [PMC free article] [PubMed] [Google Scholar]
- 113.Webb PA, Justines G, Johnson KM. Infection of wild and laboratory animals with Machupo and Latino viruses. Bull World Health Organ. 1975;52(4–6):493–9. [PMC free article] [PubMed] [Google Scholar]
- 114.Stinebaugh BJ, Schloeder FX, Johnson KM, et al. Bolivian hemorrhagic fever. A report of four cases. Am J Med. 1966 Feb;40(2):217–30. doi: 10.1016/0002-9343(66)90103-3. [DOI] [PubMed] [Google Scholar]
- 115.Terrell TG, Stookey JL, Spertzel RO, et al. Comparative histopathology of two strains of Bolivian hemorrhagic fever virus infections in suckling hamsters. Am J Trop Med Hyg. 1973 Nov;22(6):814–8. doi: 10.4269/ajtmh.1973.22.814. [DOI] [PubMed] [Google Scholar]
- 116**.Bradfute SB, Stuthman KS, Shurtleff AC, et al. A STAT-1 knockout mouse model for Machupo virus pathogenesis. Virol J. 2011;8:300. doi: 10.1186/1743-422X-8-300. This paper establishes the first mouse model for Machupo virus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Charrel RN, Coutard B, Baronti C, et al. Arenaviruses and hantaviruses: from epidemiology and genomics to antivirals. Antiviral Res. May;90(2):102–14. doi: 10.1016/j.antiviral.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 118.Barrera Oro JG, McKee KT., Jr Toward a vaccine against Argentine hemorrhagic fever. Bull Pan Am Hlth Organ. 1991;25(2):118–26. [PubMed] [Google Scholar]
- 119.Enria DA, Maiztegui JI. Antiviral treatment of Argentine hemorrhagic fever. Antiviral Res. 1994 Jan;23(1):23–31. doi: 10.1016/0166-3542(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 120**.Maiztegui J, Feinsod F, Briggiler A, et al. Inoculation of the 1st Argentinean volunteers with attenuated candid-1 strain Junin virus. Medicina. 1987;47(6):565. First manuscript describing the evaluation of the Candid 1 live-attenuated JUNV vaccine in humans. [Google Scholar]
- 121.Maiztegui JI, McKee KT, Jr, Barrera Oro JG, et al. Protective efficacy of a live attenuated vaccine against Argentine hemorrhagic fever. AHF Study Group. J Infect Dis. 1998 Feb;177(2):277–83. doi: 10.1086/514211. [DOI] [PubMed] [Google Scholar]
- 122.Goni SE, Iserte JA, Ambrosio AM, et al. Genomic features of attenuated Junin virus vaccine strain candidate. Virus Genes. 2006 Feb;32(1):37–41. doi: 10.1007/s11262-005-5843-2. [DOI] [PubMed] [Google Scholar]
- 123**.Albarino CG, Bird BH, Chakrabarti AK, et al. The major determinant of attenuation in mice of the Candid1 vaccine for Argentine hemorrhagic fever is located in the G2 glycoprotein transmembrane domain. J Virol. 2011 Oct;85(19):10404–8. doi: 10.1128/JVI.00856-11. This study identifies the basis for attenuation of the JUNV vaccine strain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Enria DA, Ambrosio AM, Briggiler AM, et al. Candid#1 vaccine against Argentine hemorrhagic fever produced in Argentina. Immunogenicity and safety. Medicina. 2010;70(3):215–22. [PubMed] [Google Scholar]
- 125.Barrera-Oro JG, Lupton HW. Cross-protection against Machupo virus with Candid # 1 live-attenuated Junin virus vaccine. I. The postvaccination prechallenge immune response. Second International Conference on the Impact of Viral Diseases on the Development of Latin American Countries and the Caribbean Region; Buenos Aires, Argentina. 1988. [Google Scholar]
- 126.Eddy GA, Wagner FS, Scott SK, et al. Protection of monkeys against Machupo virus by the passive administration of Bolivian haemorrhagic fever immunoglobulin (human origin) Bull World Health Organ. 1975;52(4–6):723–7. [PMC free article] [PubMed] [Google Scholar]
- 127.Enria DA, Briggiler AM, Fernandez NJ, et al. Importance of dose of neutralising antibodies in treatment of Argentine haemorrhagic fever with immune plasma. Lancet. 1984 Aug 4;2(8397):255–6. doi: 10.1016/s0140-6736(84)90299-x. [DOI] [PubMed] [Google Scholar]
- 128.Enria DA, de Damilano AJ, Briggiler AM, et al. Late neurologic syndrome in patients with Argentinian hemorrhagic fever treated with immune plasma. Medicina. 1985;45(6):615–20. [PubMed] [Google Scholar]
- 129.Maiztegui JI, Fernandez NJ, de Damilano AJ. Efficacy of immune plasma in treatment of Argentine haemorrhagic fever and association between treatment and a late neurological syndrome. Lancet. 1979 Dec 8;2(8154):1216–7. doi: 10.1016/s0140-6736(79)92335-3. [DOI] [PubMed] [Google Scholar]
- 130.García CC, Sepúlveda CS, Damonte EB. Novel therapeutic targets for arenavirus hemorrhagic fevers. Future Virol. 2011;6(1):27–44. [Google Scholar]
- 131.Barry M, Russi M, Armstrong L, et al. Brief report: treatment of a laboratory-acquired Sabia virus infection. New England J Med. 1995 Aug 3;333(5):294–6. doi: 10.1056/NEJM199508033330505. [DOI] [PubMed] [Google Scholar]
- 132.Huggins JW. Prospects for treatment of viral hemorrhagic fevers with ribavirin, a broad- spectrum antiviral drug. Rev Infect Dis. 1989 May-Jun;11(Suppl 4):S750–61. doi: 10.1093/clinids/11.supplement_4.s750. [DOI] [PubMed] [Google Scholar]
- 133*.Kenyon RH, Canonico PG, Green DE, et al. Effect of ribavirin and tributylribavirin on argentine hemorrhagic fever (Junin virus) in guinea pigs. Antimicrob Agents Chemother. 1986 Mar;29(3):521–3. doi: 10.1128/aac.29.3.521. One of many studies that establishes ribavirin as a potential treatment for arenavirual infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Khan SH, Goba A, Chu M, et al. New opportunities for field research on the pathogenesis and treatment of Lassa fever. Antiviral Res. 2008 Apr;78(1):103–15. doi: 10.1016/j.antiviral.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 135*.Kilgore PE, Ksiazek TG, Rollin PE, et al. Treatment of Bolivian hemorrhagic fever with intravenous ribavirin. Clin Infect Dis. 1997 Apr;24(4):718–22. doi: 10.1093/clind/24.4.718. One of many studies that establishes ribavirin as a potential treatment for arenavirual infections. [DOI] [PubMed] [Google Scholar]
- 136*.McCormick JB, King IJ, Webb PA, et al. Lassa fever. Effective therapy with ribavirin. New England J Med. 1986 Jan 2;314(1):20–6. doi: 10.1056/NEJM198601023140104. One of many studies that establishes ribavirin as a potential treatment for arenavirual infections. [DOI] [PubMed] [Google Scholar]
- 137.McKee KT, Jr, Huggins JW, Trahan CJ, et al. Ribavirin prophylaxis and therapy for experimental argentine hemorrhagic fever. Antimicrob Agents Chemother. 1988 Sep;32(9):1304–9. doi: 10.1128/aac.32.9.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Petkevich AS, Sabynin VM, Lukashevich IS, et al. Effect of ribovirin (virazole) on arenavirus reproduction in cell cultures. Vopr Virusol. 1981 Mar-Apr;(2):244–5. [PubMed] [Google Scholar]
- 139*.Rodriguez M, McCormick JB, Weissenbacher MC. Antiviral effect of ribavirin on Junin virus replication in vitro. Rev Arg Microbiol. 1986;18(2):69–74. One of many studies that establishes ribavirin as a potential treatment for arenavirual infections. [PubMed] [Google Scholar]
- 140.Snell NJ. Ribavirin--current status of a broad spectrum antiviral agent. Expert Opin Pharmacother. 2001 Aug;2(8):1317–24. doi: 10.1517/14656566.2.8.1317. [DOI] [PubMed] [Google Scholar]
- 141.Weissenbacher MC, Calello MA, Merani MS, et al. Therapeutic effect of the antiviral agent ribavirin in Junin virus infection of primates. J Med Virol. 1986 Nov;20(3):261–7. doi: 10.1002/jmv.1890200308. [DOI] [PubMed] [Google Scholar]
- 142.Enria DA, Briggiler AM, Levis S, et al. Tolerance and antiviral effect of ribavirin in patients with Argentine hemorrhagic fever. Antiviral Res. 1987 Jul;7(6):353–9. doi: 10.1016/0166-3542(87)90017-9. [DOI] [PubMed] [Google Scholar]
- 143.Moreno H, Gallego I, Sevilla N, et al. Ribavirin can be mutagenic for arenaviruses. J Virol. Jul;85(14):7246–55. doi: 10.1128/JVI.00614-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Olschlager S, Neyts J, Gunther S. Depletion of GTP pool is not the predominant mechanism by which ribavirin exerts its antiviral effect on Lassa virus. Antiviral Res. Aug;91(2):89–93. doi: 10.1016/j.antiviral.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 145.Towner JS, Paragas J, Dover JE, et al. Generation of eGFP expressing recombinant Zaire ebolavirus for analysis of early pathogenesis events and high-throughput antiviral drug screening. Virology. 2005 Feb 5;332(1):20–7. doi: 10.1016/j.virol.2004.10.048. [DOI] [PubMed] [Google Scholar]
- 146**.Flatz L, Bergthaler A, de la Torre JC, et al. Recovery of an arenavirus entirely from RNA polymerase I/II-driven cDNA. Proc Natl Acad Sci U S A. 2006 Mar 21;103(12):4663–8. doi: 10.1073/pnas.0600652103. One of several papers that establish reverse genetics for non-hemorrhagic fever arenaviruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147**.Sanchez AB, de la Torre JC. Rescue of the prototypic Arenavirus LCMV entirely from plasmid. Virology. 2006 Jul 5;350(2):370–80. doi: 10.1016/j.virol.2006.01.012. One of several papers that establish reverse genetics for non-hemorrhagic fever arenaviruses. [DOI] [PubMed] [Google Scholar]
- 148*.Albarino CG, Bergeron E, Erickson BR, et al. Efficient reverse genetics generation of infectious junin viruses differing in glycoprotein processing. J Virol. 2009 Jun;83(11):5606–14. doi: 10.1128/JVI.00276-09. This is one of two papers that establish reverse genetics for New World hemorrhagic fever arenaviruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149*.Emonet SF, Seregin AV, Yun NE, et al. Rescue from cloned cDNAs and in vivo characterization of recombinant pathogenic Romero and live-attenuated Candid #1 strains of Junin virus, the causative agent of Argentine hemorrhagic fever disease. J Virol. Feb;85(4):1473–83. doi: 10.1128/JVI.02102-10. This is one of two papers that establish reverse genetics for New World hemorrhagic fever arenaviruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Albarino CG, Bird BH, Chakrabarti AK, et al. Efficient rescue of recombinant Lassa virus reveals the influence of S segment noncoding regions on virus replication and virulence. J Virol. 2011 Apr;85(8):4020–4. doi: 10.1128/JVI.02556-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lan S, McLay Schelde L, Wang J, et al. Development of infectious clones for virulent and avirulent pichinde viruses: a model virus to study arenavirus-induced hemorrhagic fevers. J Virol. 2009 Jul;83(13):6357–62. doi: 10.1128/JVI.00019-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Popkin DL, Teijaro JR, Lee AM, et al. Expanded potential for recombinant trisegmented lymphocytic choriomeningitis viruses: protein production, antibody production, and in vivo assessment of biological function of genes of interest. J Virol. Aug;85(15):7928–32. doi: 10.1128/JVI.00486-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Suomalainen M, Garoff H. Incorporation of homologous and heterologous proteins into the envelope of Moloney murine leukemia virus. J Virol. 1994 Aug;68(8):4879–89. doi: 10.1128/jvi.68.8.4879-4889.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wool-Lewis RJ, Bates P. Characterization of Ebola virus entry by using pseudotyped viruses: identification of receptor-deficient cell lines. J Virol. 1998 Apr;72(4):3155–60. doi: 10.1128/jvi.72.4.3155-3160.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Burns JC, Friedmann T, Driever W, et al. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8033–7. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Reignier T, Oldenburg J, Noble B, et al. Receptor use by pathogenic arenaviruses. Virology. 2006 Sep 15;353(1):111–20. doi: 10.1016/j.virol.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 157.Rojek JM, Spiropoulou CF, Kunz S. Characterization of the cellular receptors for the South American hemorrhagic fever viruses Junin, Guanarito, and Machupo. Virology. 2006 Jun 5;349(2):476–91. doi: 10.1016/j.virol.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 158.Emonet SE, Urata S, de la Torre JC. Arenavirus reverse genetics: new approaches for the investigation of arenavirus biology and development of antiviral strategies. Virology. Mar 15;411(2):416–25. doi: 10.1016/j.virol.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159*.Kranzusch PJ, Schenk AD, Rahmeh AA, et al. Assembly of a functional Machupo virus polymerase complex. Proc Natl Acad Sci U S A. 2010 Nov 16;107(46):20069–74. doi: 10.1073/pnas.1007152107. This paper characterizes the promoter requirement of the Machupo virus L polymerase and provides electron-microscopic structural information on the viral polymerase. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Albarino CG, Bird BH, Chakrabarti AK, et al. Reverse genetics generation of chimeric infectious Junin/Lassa virus is dependent on interaction of homologous glycoprotein stable signal peptide and G2 cytoplasmic domains. J Virol. 2011 Jan;85(1):112–22. doi: 10.1128/JVI.01837-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lee KJ, Novella IS, Teng MN, et al. NP and L proteins of lymphocytic choriomeningitis virus (LCMV) are sufficient for efficient transcription and replication of LCMV genomic RNA analogs. J Virol. 2000 Apr;74(8):3470–7. doi: 10.1128/jvi.74.8.3470-3477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Capul AA, de la Torre JC. A cell-based luciferase assay amenable to high-throughput screening of inhibitors of arenavirus budding. Virology. 2008 Dec 5;382(1):107–14. doi: 10.1016/j.virol.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163**.Bolken TC, Laquerre S, Zhang Y, et al. Identification and characterization of potent small molecule inhibitor of hemorrhagic fever New World arenaviruses. Antiviral Res. 2006 Feb;69(2):86–97. doi: 10.1016/j.antiviral.2005.10.008. This paper describes the first high-throughput screnning against New World arenaviruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164**.Larson RA, Dai D, Hosack VT, et al. Identification of a broad-spectrum arenavirus entry inhibitor. J Virol. 2008 Nov;82(21):10768–75. doi: 10.1128/JVI.00941-08. One of several papers that describe high-throughput screnning against New World arenaviruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.York J, Dai D, Amberg SM, et al. pH-induced activation of arenavirus membrane fusion is antagonized by small-molecule inhibitors. J Virol. 2008 Nov;82(21):10932–9. doi: 10.1128/JVI.01140-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kiso M, Takahashi K, Sakai-Tagawa Y, et al. T-705 (favipiravir) activity against lethal H5N1 influenza A viruses. Proc Natl Acad Sci U S A. Jan 12;107(2):882–7. doi: 10.1073/pnas.0909603107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Julander JG, Shafer K, Smee DF, et al. Activity of T-705 in a hamster model of yellow fever virus infection in comparison with that of a chemically related compound, T-1106. Antimicrob Agents Chemother. 2009 Jan;53(1):202–9. doi: 10.1128/AAC.01074-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Morrey JD, Taro BS, Siddharthan V, et al. Efficacy of orally administered T-705 pyrazine analog on lethal West Nile virus infection in rodents. Antiviral Res. 2008 Dec;80(3):377–9. doi: 10.1016/j.antiviral.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Gowen BB, Smee DF, Wong MH, et al. Treatment of late stage disease in a model of arenaviral hemorrhagic fever: T-705 efficacy and reduced toxicity suggests an alternative to ribavirin. PLoS One. 2008;3(11):e3725. doi: 10.1371/journal.pone.0003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gowen BB, Wong MH, Jung KH, et al. In vitro and in vivo activities of T-705 against arenavirus and bunyavirus infections. Antimicrob Agents Chemother. 2007 Sep;51(9):3168–76. doi: 10.1128/AAC.00356-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gowen BB, Wong MH, Jung KH, et al. Efficacy of favipiravir (T-705) and T-1106 pyrazine derivatives in phlebovirus disease models. Antiviral Res. May;86(2):121–7. doi: 10.1016/j.antiviral.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mendenhall M, Russell A, Juelich T, et al. T-705 (favipiravir) inhibition of arenavirus replication in cell culture. Antimicrob Agents Chemother. 2011 Feb;55(2):782–7. doi: 10.1128/AAC.01219-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Watterson SH, Chen P, Zhao Y, et al. Acridone-based inhibitors of inosine 5'-monophosphate dehydrogenase: discovery and SAR leading to the identification of N-(2-(6-(4-ethylpiperazin-1-yl)pyridin-3-yl)propan-2-yl)-2- fluoro-9-oxo-9,10-dihydroacridine-3-carboxamide (BMS-566419) J Med Chem. 2007 Jul 26;50(15):3730–42. doi: 10.1021/jm070299x. [DOI] [PubMed] [Google Scholar]
- 174.Sepulveda CS, Fascio ML, Mazzucco, et al. Synthesis and evaluation of N-substituted acridones as antiviral agents against haemorrhagic fever viruses. Antivir Chem Chemother. 2008;19(1):41–7. doi: 10.1177/095632020801900106. [DOI] [PubMed] [Google Scholar]
- 175.Sepulveda CS, Garcia CC, Fascio ML, et al. Inhibition of Junin virus RNA synthesis by an antiviral acridone derivative. Antiviral Res. Jan;93(1):16–22. doi: 10.1016/j.antiviral.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 176.Garcia CC, Candurra NA, Damonte EB. Antiviral and virucidal activities against arenaviruses of zinc-finger active compounds. Antivir Chem Chemother. 2000 May;11(3):231–7. doi: 10.1177/095632020001100306. [DOI] [PubMed] [Google Scholar]
- 177.Garcia CC, Candurra NA, Damonte EB. Mode of inactivation of arenaviruses by disulfide-based compounds. Antiviral Res. 2002 Sep;55(3):437–46. doi: 10.1016/s0166-3542(02)00076-1. [DOI] [PubMed] [Google Scholar]
- 178.Garcia CC, Ellenberg PC, Artuso MC, et al. Characterization of Junin virus particles inactivated by a zinc finger-reactive compound. Virus Res. 2009 Jul;143(1):106–13. doi: 10.1016/j.virusres.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 179.Garcia CC, Djavani M, Topisirovic I, et al. Arenavirus Z protein as an antiviral target: virus inactivation and protein ollgomerization by zinc finger-reactive compounds. J Gen Virol. 2006 May;87:1217–28. doi: 10.1099/vir.0.81667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Garcia CC, Topisirovic I, Djavani M, et al. An antiviral disulfide compound blocks interaction between arenavirus Z protein and cellular promyelocytic leukemia protein. Biochem Biophys Res Commun. Mar 19;393(4):625–30. doi: 10.1016/j.bbrc.2010.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sepulveda CS, Garcia CC, Damonte EB. Inhibition of arenavirus infection by thiuram and aromatic disulfides. Antiviral Res. Sep;87(3):329–37. doi: 10.1016/j.antiviral.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 182.Garcia CC, Candurra NA, Damonte EB. Differential inhibitory action of two azoic compounds against arenaviruses. Int J Antimicrob Agents. 2003 Apr;21(4):319–24. doi: 10.1016/s0924-8579(02)00390-4. [DOI] [PubMed] [Google Scholar]
- 183.Albiol Matanic VC, Castilla V. Antiviral activity of antimicrobial cationic peptides against Junin virus and herpes simplex virus. Int J Antimicrob Agents. 2004 Apr;23(4):382–9. doi: 10.1016/j.ijantimicag.2003.07.022. [DOI] [PubMed] [Google Scholar]
- 184.Barradas JS, Errea MI, D'Accorso NB, et al. Synthesis and antiviral activity of azoles obtained from carbohydrates. Carbohydr Res. 2008 Sep 22;343(14):2468–74. doi: 10.1016/j.carres.2008.06.028. [DOI] [PubMed] [Google Scholar]
- 185.Barradas JS, Errea MI, D'Accorso NB, et al. Imidazo[2,1-b]thiazole carbohydrate derivatives: Synthesis and antiviral activity against Junin virus, agent of Argentine hemorrhagic fever. Eur J Med Chem. Jan;46(1):259–64. doi: 10.1016/j.ejmech.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 186.Karlas A, Machuy N, Shin Y, et al. Genome-wide RNAi screen identifies human host factors crucial for influenza virus replication. Nature. Feb 11;463(7282):818–22. doi: 10.1038/nature08760. [DOI] [PubMed] [Google Scholar]
- 187.Zhou H, Xu M, Huang Q, Gates AT, et al. Genome-scale RNAi screen for host factors required for HIV replication. Cell Host Microbe. 2008 Nov 13;4(5):495–504. doi: 10.1016/j.chom.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 188.Li Q, Brass AL, Ng A, et al. A genome-wide genetic screen for host factors required for hepatitis C virus propagation. Proc Natl Acad Sci U S A. 2009 Sep 22;106(38):16410–5. doi: 10.1073/pnas.0907439106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189**.Kolokoltsova OA, Yun NE, Poussard AL, et al. Mice lacking alpha/beta and gamma interferon receptors are susceptible to junin virus infection. J Virol. 2010 Dec;84(24):13063–7. doi: 10.1128/JVI.01389-10. This study establishes a mouse model for JUNV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lee AM, Pasquato A, Kunz S. Novel approaches in anti-arenaviral drug development. Virology. Mar 15;411(2):163–9. doi: 10.1016/j.virol.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Flanagan ML, Oldenburg J, Reignier T, et al. New world clade B arenaviruses can use transferrin receptor 1 (TfR1)-dependent and -independent entry pathways, and glycoproteins from human pathogenic strains are associated with the use of TfR1. J Virol. 2008 Jan;82(2):938–48. doi: 10.1128/JVI.01397-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192**.Abraham J, Corbett KD, Farzan M, et al. Structural basis for receptor recognition by New World hemorrhagic fever arenaviruses. Nat Struct Mol Biol. Apr;17(4):438–44. doi: 10.1038/nsmb.1772. This paper describes the crystal structure of MACV GP1 bound to its receptor TfR1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Radoshitzky SR, Longobardi LE, Kuhn JH, et al. Machupo virus glycoprotein determinants for human transferrin receptor 1 binding and cell entry. PLoS One. 6(7):e21398. doi: 10.1371/journal.pone.0021398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Andrews NC, Fleming MD, Levy JE. Molecular insights into mechanisms of iron transport. Curr Opin Hematol. 1999 Mar;6(2):61–4. doi: 10.1097/00062752-199903000-00001. [DOI] [PubMed] [Google Scholar]
- 195.Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004 Apr 30;117(3):285–97. doi: 10.1016/s0092-8674(04)00343-5. [DOI] [PubMed] [Google Scholar]
- 196.Cheng Y, Zak O, Aisen P, Harrison SC, et al. Structure of the human transferrin receptor- transferrin complex. Cell. 2004 Feb 20;116(4):565–76. doi: 10.1016/s0092-8674(04)00130-8. [DOI] [PubMed] [Google Scholar]
- 197.Daniels TR, Delgado T, Rodriguez JA, et al. The transferrin receptor part I: Biology and targeting with cytotoxic antibodies for the treatment of cancer. Clin Immunol. 2006 Aug 9; doi: 10.1016/j.clim.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 198**.Abraham J, Corbett KD, Farzan M, et al. Structural basis for receptor recognition by New World hemorrhagic fever arenaviruses. Nat Struct Mol Biol. 2010 Apr;17(4):438–44. doi: 10.1038/nsmb.1772. This paper describes the crystal structure of MACV GP1 bound to its receptor TfR1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Peters CJ, Jahrling PB, Liu CT, et al. Experimental studies of arenaviral hemorrhagic fevers. Curr Top Microbiol Immunol. 1987;134:5–68. doi: 10.1007/978-3-642-71726-0_2. [DOI] [PubMed] [Google Scholar]
- 200.Fleming RE, Britton RS. Iron Imports. VI. HFE and regulation of intestinal iron absorption. Am J Physiol Gastrointest Liver Physiol. 2006 Apr;290(4):G590–4. doi: 10.1152/ajpgi.00486.2005. [DOI] [PubMed] [Google Scholar]
- 201.Pantopoulos K. Iron metabolism and the IRE/IRP regulatory system: an update. Ann N Y Acad Sci. 2004 Mar;1012:1–13. doi: 10.1196/annals.1306.001. [DOI] [PubMed] [Google Scholar]
- 202.Wang Y, Hamasaki K, Rando RR. Specificity of aminoglycoside binding to RNA constructs derived from the 16S rRNA decoding region and the HIV-RRE activator region. Biochemistry. 1997 Jan 28;36(4):768–79. doi: 10.1021/bi962095g. [DOI] [PubMed] [Google Scholar]
- 203.Wang S, Huber PW, Cui M, et al. Binding of neomycin to the TAR element of HIV-1 RNA induces dissociation of Tat protein by an allosteric mechanism. Biochemistry. 1998 Apr 21;37(16):5549–57. doi: 10.1021/bi972808a. [DOI] [PubMed] [Google Scholar]
- 204**.Morin B, Coutard B, Lelke M, et al. The N-terminal domain of the arenavirus L protein is an RNA endonuclease essential in mRNA transcription. PLoS Path. 2010 Sep 16;6(9):e1001038. doi: 10.1371/journal.ppat.1001038. First description of an endouclease domain in an arenavirus polymerase. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205*.Reguera J, Weber F, Cusack S. Bunyaviridae RNA polymerases (L-protein) have an N-terminal, influenza-like endonuclease domain, essential for viral cap-dependent transcription. PLoS Path. 6(9):e1001101. doi: 10.1371/journal.ppat.1001101. Description of a similar endouclease domain in bunyavirus polymerase, suggesting that antiviral developed for other viral families could be effective against arenaviruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206**.Qi X, Lan S, Wang W, et al. Cap binding and immune evasion revealed by Lassa nucleoprotein structure. Nature. Dec 9;468(7325):779–83. doi: 10.1038/nature09605. Structural information on an arenavirus nucleoprotein reveals potentially pan-arenavirus targets for antivirals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207**.Brunotte L, Kerber R, Shang W, et al. Structure of the Lassa virus nucleoprotein revealed by X-ray crystallography, small-angle X-ray scattering, and electron microscopy. J Biol Chem. Nov 4;286(44):38748–56. doi: 10.1074/jbc.M111.278838. Structural information on an arenavirus nucleoprotein reveals potentially pan-arenavirus targets for antivirals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208**.Hastie KM, Liu T, Li S, et al. Crystal structure of the Lassa virus nucleoprotein-RNA complex reveals a gating mechanism for RNA binding. Proc Natl Acad Sci U S A. Nov 29;108(48):19365–70. doi: 10.1073/pnas.1108515108. Structural information on an arenavirus nucleoprotein reveals potentially pan-arenavirus targets for antivirals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209**.Hastie KM, Kimberlin CR, Zandonatti MA, et al. Structure of the Lassa virus nucleoprotein reveals a dsRNA-specific 3' to 5' exonuclease activity essential for immune suppression. Proc Natl Acad Sci U S A. Feb 8;108(6):2396–401. doi: 10.1073/pnas.1016404108. Structural information on an arenavirus nucleoprotein reveals potentially pan-arenavirus targets for antivirals. [DOI] [PMC free article] [PubMed] [Google Scholar]