Abstract
Objective
The importance of an anti-angiogenic state as a mechanism of disease in preeclampsia is now recognized. Assays for the determination of concentrations of soluble vascular endothelial growth factor receptor (sVEGFR)-1, sVEGFR-2, placental growth factor (PlGF) and soluble endoglin (sEng) have been developed for research and clinical laboratories. A key question is whether these factors should be measured in plasma or serum. The purpose of this study was to determine if there are differences in the concentrations of these analytes between plasma and serum in normal pregnancy and in preeclampsia.
Methods
Samples of maternal blood were obtained by venipuncture and collected in EDTA (lavender top) and serum collection tubes (red top). A standard laboratory procedure was implemented for the centrifugation, aliquoting and storage of samples. Plasma and serum from 70 women with normal pregnancies and 34 patients with preeclampsia, were assayed for sVEGFR-1, sVEGFR-2, PlGF and sEng by ELISA. Non-parametric paired tests were used for analyses.
Results
A significant difference between plasma and serum concentration was observed for sVEGFR-1 and sVEGFR-2 in normal pregnancy, and for sVEGFR-1, sVEGFR-2, PlGF and sEng in women with preeclampsia.
Conclusion
The concentrations of sVEGFR-1, sVEGFR-2, PlGF and sEng when measured in maternal plasma and in serum are different. Therefore, the matrix used for the assay (serum versus plasma) needs to be considered when selecting thresholds for predictive studies and interpreting the growing body of literature on this subject.
Keywords: preeclampsia, anti-angiogenic state, sFlt-1, sVEGFR-1, sVEGFR-2, PlGF, placental growth factor, sEng, soluble Endoglin, vascular endothelial growth factor, pregnancy
INTRODUCTION
An imbalance between angiogenic and anti-angiogenic factors has been proposed to play a central role in the pathogenesis of preeclampsia. Indeed, patients with preeclampsia have lower plasma/serum concentrations of angiogenic factors such as vascular endothelial growth factor (VEGF)[1–4] and placental growth factor (PlGF) [2,4–20] and higher concentrations of anti-angiogenic factors including soluble VEGF receptor-1 (sVEGFR-1), also referred to as sFlt-1,[4,10,12–14,16,18,20–31] and soluble endoglin (sEng).[17,18,31–37] Recently, sVEGFR-2 has also been implicated in the pathophysiology of preeclampsia.[38–40] Since these differences can be observed before the clinical diagnosis of the disease,[6–11,15,26,35,41–47] it has been proposed that the measurement of plasma/serum concentrations of angiogenic and anti-angiogenic factors in the first and/or second trimester of pregnancy,[7,11,14,16–18,48–53] alone or in combination with the results of Doppler velocimetry of the uterine arteries,[20,29,37,54–60] may serve as an assessment tool to identify women at risk to develop preeclampsia.
Among men and non-pregnant women, the concentrations of VEGF and sVEGFR-1 have been reported to be significantly higher in serum than in plasma.[61–67] However, it is unknown if there is a difference in the concentrations of these analytes when measured in maternal serum or plasma during pregnancy. This information is important to avoid inaccurate risk assessment. The objective of this study was to determine if there are differences in the concentrations of sVEGFR-1, sVEGFR-2, PlGF and sEng in the serum and plasma, both in women with a normal pregnancy and in patients with preeclampsia.
METHODS
Study design
This cross-sectional study included 34 patients with preeclampsia and 70 women with a normal pregnancy, identified in our clinical database and bank of biological samples. Women with multiple pregnancies and/or those with fetuses with chromosomal or congenital anomalies were excluded. All patients were enrolled at the Sotero del Rio Hospital, Santiago, Chile and provided written informed consent prior to the collection of blood samples. The utilization of samples for research purposes was approved by the Institutional Review Boards of the Sotero del Rio Hospital, Santiago, Chile (an affiliate of the Pontificia Catholic University of Santiago, Chile), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS. Many of these samples have been previously employed to study the biology of inflammation, hemostasis, angiogenesis regulation, and growth factor concentrations.
Definitions
Patients were considered to have a normal pregnancy if they did not have any medical, obstetrical, or surgical complications, and if they delivered a term (≥37 weeks), singleton neonate of appropriate birth weight for gestational age [68] without complications. Preeclampsia was diagnosed in the presence of hypertension (systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg) and proteinuria (≥300 mg in a 24-hour urine collection, two dipstick measurement of 1+ or one dipstick measurement ≥2+) according to ACOG [69] and the National High Blood Pressure Education Program.[70] Small-for-gestational age (SGA) neonate was defined as a birthweight <10th percentile for the gestational age at birth according to the Chilean birth weight distribution of a Hispanic population.[68]
Sample collection and immunoassays
Maternal blood samples were obtained by venipuncture and collected in serum collection tubes (red top) and EDTA containing tubes (lavender top). The samples were centrifuged shortly after collection, and stored at −70°C until assay. The serum and plasma concentrations of sVEGFR-1, sVEGFR-2, PlGF, and sEng were determined by sensitive and specific immunoassays obtained from R&D Systems (Minneapolis, MN). All four immunoassays utilized the quantitative sandwich enzyme immunoassay technique. The concentrations of sVEGFR-1, sVEGFR-2, PlGF, and sEng were determined by interpolation from the standard curve. The inter- and intra-assay coefficients of variation obtained in our laboratory were: sVEGFR-1: 1.4% and 3.9%, respectively; sVEGFR-2: 2% and 4%, respectively; PlGF: 6% and 4.8%, respectively; and sEng: 2.3% and 4.6% respectively. The sensitivity of the assays was: sVEGFR-1: 16.97 pg/ml; sVEGFR-2: 19.07 pg/ml; PlGF: 9.52 pg/ml; and sEng: 0.08 ng/ml. The laboratory personnel performing the assays were blinded to the clinical information of each subject.
Statistical analysis
The normality of the data was tested using the Kolmogorov-Smirnov test. Since the concentrations of sVEGFR-1, sVEGFR-2, PlGF and sEng were not normally distributed, non-parametric tests were used for analyses. Paired-Wilcoxon ranks tests were used for comparison of the concentrations of sVEGFR-1, sVEGFR-2, PlGF and sEng in plasma and serum samples from the same patients. The median percentage of difference between paired plasma and serum concentrations for each analyte was calculated (plasma was considered as the reference sample). The statistical package used was SPSS v.15.0 (SPSS Inc., Chicago, IL, USA). A p-value of <0.05 was considered significant.
RESULTS
Table I displays the demographic and clinical characteristics of the study population. In the normal pregnancy group, the median serum concentrations of sVEGFR-1 and sVEGFR-2 were significantly higher than those of plasma, while those of PlGF and sEng did not change significantly (Table II). The median percentage of difference of the maternal serum over plasma concentrations for sVEGFR-1, sVEGFR-2, PlGF and sEng were 14.2%, 6.5%, 4%, and −1.1%, respectively. Serum concentrations of sVEGFR-1 and sVEGFR-2 were significantly higher than those of plasma through all trimesters (Table III). No significant differences were observed in the median percentage of difference of the maternal serum over plasma concentrations of any analyte among trimesters.
Table I.
Demographic and clinical characteristics of the study population
| Normal pregnancy (n= 70) | Preeclampsia (n=34) | |
|---|---|---|
| Maternal age (years) | 25.5 (15–39) | 22.5 (18–38) |
|
| ||
| Pre-pregnancy BMI (Kg/m2) | 23.5 (17.1–37.4) | 25.7 (18.6–36.3) |
|
| ||
| Nulliparity (%) | 25.7 (18/70) | 64.7 (22/34) |
|
| ||
| Systolic blood pressure (mmHg) | 120 (110–137) | 160 (140–220) |
|
| ||
| Diastolic blood pressure (mmHg) | 80 (65–88) | 100 (90–150) |
|
| ||
| Gestational age at delivery (weeks) | 39.4 (37–41.4) | 36.4 (28–41) |
|
| ||
| Birth weight (g) | 3,400 (2,770–4,090) | 2,400 (830–4,580) |
|
| ||
| Gestational age at maternal plasma/serum collection (weeks) | 28.9 (6.1–40) | 36.1 (27.6–40.9) |
| Early-onset preeclampsia (%) | - | 38.2 (13/34) |
| SGA (%) | - | 26.5 (9/34) |
The results are expressed as percentage (proportion) or median (range)
BMI: body mass index.
Early-onset preeclampsia: preeclampsia diagnosed <34 weeks of gestation
SGA: small for gestational age
Table II.
Comparison of the maternal plasma and serum concentrations of sVEGFR-1, sVEGFR-2, PlGF and sEng in patients with a normal pregnancy
| Analyte | Plasma (n=70) | Serum (n=70) | p* |
|---|---|---|---|
| sVEGFR-1 (pg/mL) | 2,081.8 (264.8–19,210.9) | 2,288.6 (277.7–15,861.8) | <0.001 |
| sVEGFR-2 (pg/mL) | 9,161.7 (6,201.1–13,817.8) | 10,231.4 (6,379.4–16,168) | <0.001 |
| PlGF (pg/mL) | 189.2 (6.8–1,896.3) | 197.5 (6.8–1,841.8) | 0.1 |
| sEng (ng/mL) | 8.6 (4.5–38.4) | 8.4 (4.7–44.9) | 0.7 |
The results are expressed as median (range)
Wilcoxon ranks test
Table III.
Comparison of the maternal plasma and serum concentrations of sVEGFR-1, sVEGFR-2, PlGF and sEng in patients with a normal pregnancy in the first, second and third trimester
| Analyte | Plasma (n=70) | Serum (n=70) | p* |
|---|---|---|---|
| First trimester | n=10 | n=10 | |
| sVEGFR-1 (pg/mL) | 1,403.7 (264.8–3,259.1) | 1,699.6 (277.7–3,568.9) | 0.005 |
| sVEGFR-2 (pg/mL) | 10,178.8 (6,916.3–11,100.4) | 10,708.6 (7,183.5–11,804.7) | 0.04 |
| PlGF (pg/mL) | 27 (6.8–58.9) | 29.2 (6.8–50.5) | 0.5 |
| sEng (ng/mL) | 7.3 (6.4–12) | 7.3 (5.8–11.3) | 0.1 |
| Second trimester | n=22 | n=22 | |
| sVEGFR-1 (pg/mL) | 1,542.9 (386.0–4,133.6) | 1,698.7 (512.2–4,686.2) | <0.001 |
| sVEGFR-2 (pg/mL) | 8,970.9 (6,673.4–13,787.5) | 10,192.9 (6,469.5–13,573.9) | <0.001 |
| PlGF (pg/mL) | 145.3 (26.1–1,896.3) | 157.3 (27.3–1,841.8) | 0.5 |
| sEng (ng/mL) | 6.5 (4.5–38.4) | 6.6 (4.7–43.6) | 0.6 |
| Third trimester | n=38 | n=38 | |
| sVEGFR-1 (pg/mL) | 2,793.3 (827.1–19,210.9) | 3,083.3 (885–15,861.8) | <0.001 |
| sVEGFR-2 (pg/mL) | 8,996.4 (6,201.1–13,817.8) | 9,947.8 (3,379.4–16,168) | 0.003 |
| PlGF (pg/mL) | 304.4 (39.9–1,606.3) | 292.1 (41.8–1,540) | 0.2 |
| sEng (ng/mL) | 10 (5.6–34.9) | 10.1 (5.6–44.9) | 1.0 |
The results are expressed as median (range)
Wilcoxon ranks test
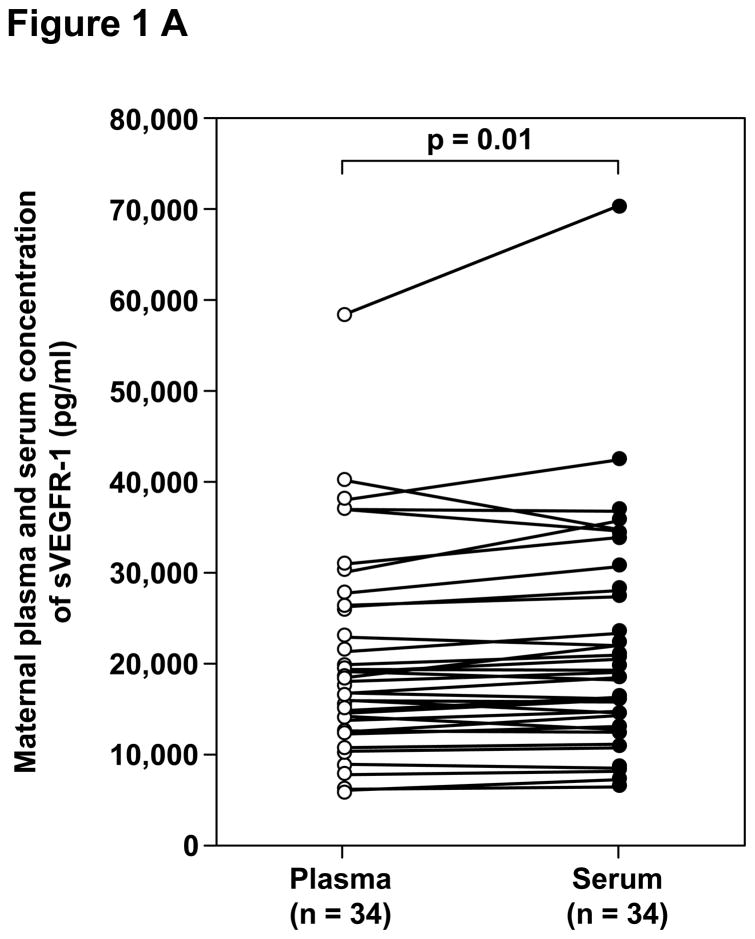
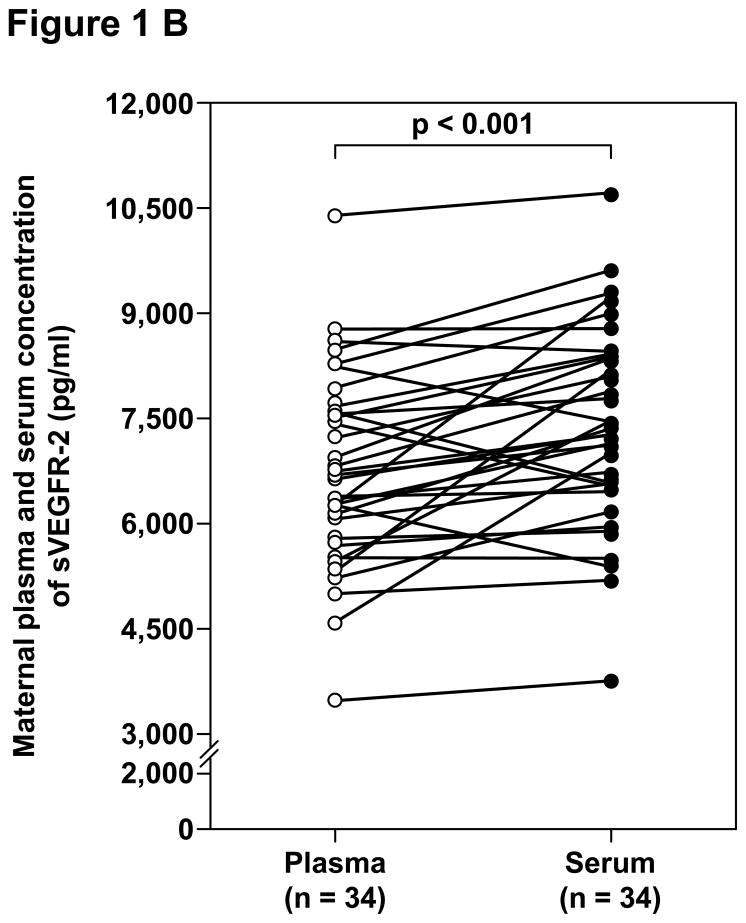
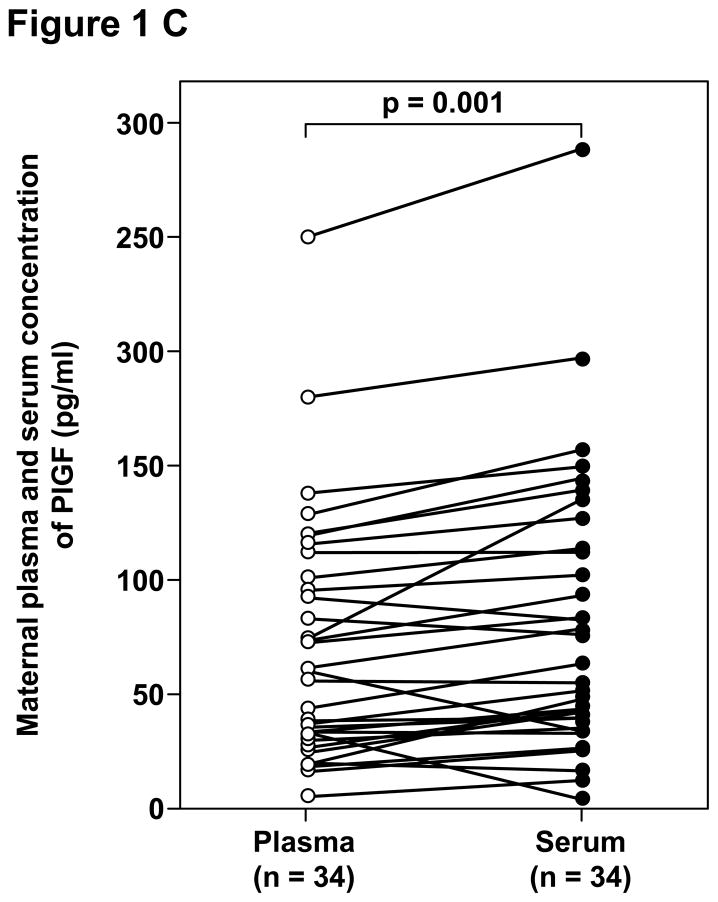
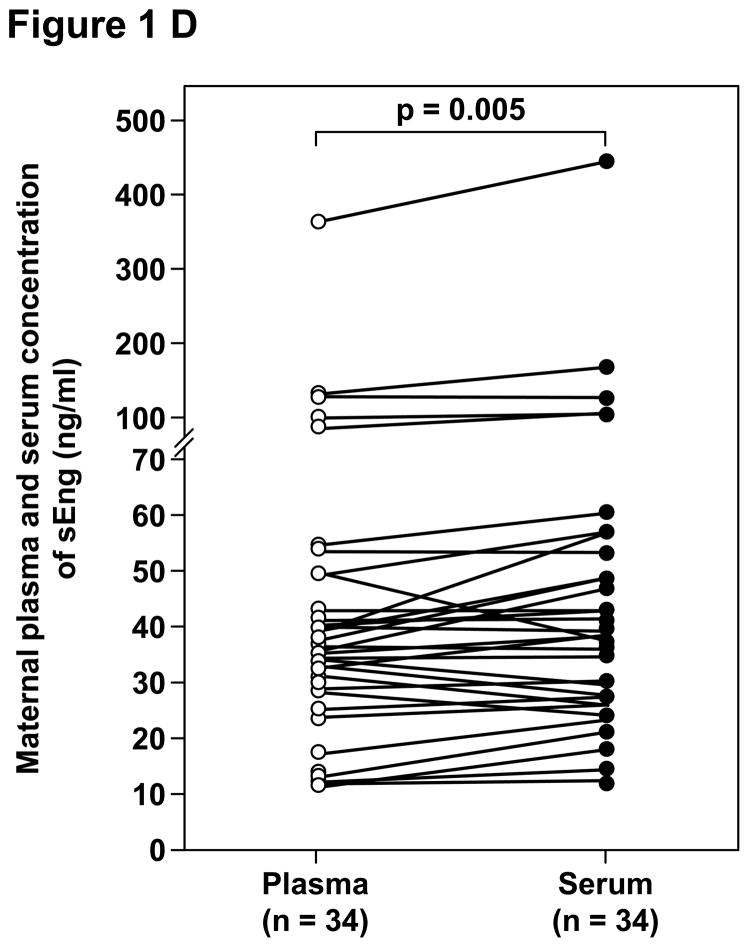
Among patients with preeclampsia, the median concentrations of sVEGFR-1, sVEGFR-2, PlGF and sEng were significantly higher in serum than in plasma (Table IV and Figure 1). The median percentages of difference of the maternal serum over plasma concentrations were 6.5%, 8.3%, 14.6% and 9.2%, respectively.
Table IV.
Comparison of the maternal plasma and serum concentrations of sVEGFR-1, sVEGFR-2, PlGF and sEng in patients with preeclampsia
| Analyte | Plasma (n=34) | Serum (n=34) | p* |
|---|---|---|---|
| sVEGFR-1 (pg/mL) | 17,747.7 (5,557.8 – 58,140.6) | 18,284.5 (5,955.4 – 70,199.1) | 0.01 |
| sVEGFR-2 (pg/mL) | 6,668 (3,487.8 – 10,399.1) | 7,298 (3,746.4 – 10,725.4) | <0.001 |
| PlGF (pg/mL) | 60.5 (6.8 – 250.5) | 61.4 (6.8 – 289.9) | 0.001 |
| sEng (ng/mL) | 35.9 (11.2 – 353.2) | 39.1 (12 – 437.5) | 0.005 |
The results are expressed as median (range)
Wilcoxon ranks test
Figure 1.
Paired comparisons of the maternal plasma and serum concentrations of: A. sVEGFR-1, B. sVEGFR-2, C. PlGF, and D. sEng in patients with preeclampsia.
DISCUSSION
Principal findings of the study
The concentration of angiogenic and anti-angiogenic factors in plasma and serum of pregnant women is different. In normal pregnancy, the concentrations of sVEGFR-1 and sVEGFR-2, but not those of PlGF or sEng, were significantly higher in serum than in plasma. In contrast, among patients with preeclampsia, a significant difference was observed for sVEGFR-1, sVEGFR-2, PlGF and sEng.
Angiogenic and anti-angiogenic factors in plasma and in serum
Despite the accumulating body of evidence about the changes in the maternal concentrations of circulating angiogenic and anti-angiogenic factors in preeclampsia, the comparability between plasma and serum values has received little attention. In contrast, this has been more extensively addressed in non-pregnant patients for VEGF, a molecule which has been studied for a long time for its role in pathological vascularization, such as inflammatory diseases and malignancies. The concentration of VEGF in the serum is significantly higher than in the plasma of patients with cancer[61,65–67] and also in healthy individuals.[61–67] This difference is mainly attributed to the release of VEGF by platelets during the clotting process in serum preparation,[61–65,67] and it varies according to the technique of plasma preparation (e.g. EDTA plasma, citrate plasma, CTAD plasma, platelet poor plasma, platelet rich plasma) [65] as well as the duration of clotting and temperature.[63] Because the procedures for serum preparation and temperature are not standardized among laboratories, it has been suggested that the use of plasma should be preferred[62,67] when studying VEGF as a marker of tumor angiogenesis. Of note, the concentration of free VEGF in maternal plasma and serum is close to the limit of detection in both normal pregnancy and preeclampsia, and changes in its concentration are difficult to detect [10]. For this reason, VEGF has not been proposed as a biomarker for risk assessment in preeclampsia, and it was not included in this study.
In agreement with our findings, an increase in sVEGFR-1 concentration in serum compared to plasma has previously been demonstrated in healthy males and non-pregnant female volunteers [71] and in neonatal cord blood obtained from the umbilical vein at cesarean section.[28] Similarly, the concentration of sEng in umbilical vein blood has been previously found to be significantly higher in serum than in plasma.[36]
Differences between plasma and serum concentration of angiogenic and anti-angiogenic factors in preeclampsia
To our knowledge, this is the first study comparing the concentration of angiogenic and anti-angiogenic factors in plasma and serum in normal pregnancy and in pregnancies complicated with preeclampsia. Our findings indicate that the concentrations of sVEGFR-1, sVEGFR-2, PlGF and sEng in patients with preeclampsia, and those of sVEGFR-1 and sVEGFR-2 in normal pregnant women, vary significantly between plasma and serum and that values measured in serum are higher than those from plasma. Such difference should be considered when comparing the results of studies which have used different biological samples, but it becomes even more meaningful if the measurement of these angiogenic and anti-angiogenic factors is introduced in clinical practice as a screening or diagnostic test. In that case, standardization of the method is crucial because thresholds established in serum may not have the same predictive value in plasma and vice versa.
The cause of the observed differences is not known. It can be speculated that, similar to what has been observed for VEGF in non-pregnant individuals, the clotting process can influence the production or release of angiogenic and anti-angiogenic factors by several cell types so that each factor measured in plasma and serum is derived by different cellular sources. Indeed, sVEGFR-1 is known to be produced by endothelial cells, monocytes-macrophages, and neutrophils[71,72] as well as the placenta.[73,74] Endothelial cells are probably the main source of circulating sVEGFR-2,[75] but the contribution of other cells, such as circulating endothelial progenitor cells[75,76] and megakaryocytes,[75] is also possible. sEng is released by vascular endothelial cells,[77,78] macrophages,[79] immature erythroid cells[80] and syncytiotrophoblast.[78] Finally, while PlGF was first isolated in the placenta,[81,82] it is also expressed by other tissues such as activated endothelial cells,[83] inflammatory cells,[83] erytroblasts,[83] neurons,[84] and keratinocytes during wound healing.[85]
In conclusion, the concentration of sVEGFR-1 and sVEGFR-2 in normal pregnancy is significantly different when measured in plasma and in serum. Interestingly, this difference is significant for sVEGFR-1, sVEGFR-2, PlGF and sEng in patients with preeclampsia. Therefore, the matrix used for each angiogenic and anti-angiogenic factor must be taken into account when reviewing the growing body of evidence that links these factors with the pathophysiology of preeclampsia, as well as in the clinical implementation of the determination of these analytes for the risk assessment for preeclampsia and other obstetrical syndromes.
Acknowledgments
This research was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Reference List
- 1.Lyall F, Greer IA, Boswell F, Fleming R. Suppression of serum vascular endothelial growth factor immunoreactivity in normal pregnancy and in pre-eclampsia. Br J Obstet Gynaecol. 1997;104:223–228. doi: 10.1111/j.1471-0528.1997.tb11050.x. [DOI] [PubMed] [Google Scholar]
- 2.Reuvekamp A, Velsing-Aarts FV, Poulina IE, Capello JJ, Duits AJ. Selective deficit of angiogenic growth factors characterises pregnancies complicated by pre-eclampsia. Br J Obstet Gynaecol. 1999;106:1019–1022. doi: 10.1111/j.1471-0528.1999.tb08107.x. [DOI] [PubMed] [Google Scholar]
- 3.Livingston JC, Chin R, Haddad B, McKinney ET, Ahokas R, Sibai BM. Reductions of vascular endothelial growth factor and placental growth factor concentrations in severe preeclampsia. Am J Obstet Gynecol. 2000;183:1554–1557. doi: 10.1067/mob.2000.108022. [DOI] [PubMed] [Google Scholar]
- 4.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torry DS, Wang HS, Wang TH, Caudle MR, Torry RJ. Preeclampsia is associated with reduced serum levels of placenta growth factor. Am J Obstet Gynecol. 1998;179:1539–1544. doi: 10.1016/s0002-9378(98)70021-3. [DOI] [PubMed] [Google Scholar]
- 6.Tidwell SC, Ho HN, Chiu WH, Torry RJ, Torry DS. Low maternal serum levels of placenta growth factor as an antecedent of clinical preeclampsia. Am J Obstet Gynecol. 2001;184:1267–1272. doi: 10.1067/mob.2001.113129. [DOI] [PubMed] [Google Scholar]
- 7.Tjoa ML, van Vugt JM, Mulders MA, Schutgens RB, Oudejans CB, van Wijk IJ. Plasma placenta growth factor levels in midtrimester pregnancies. Obstet Gynecol. 2001;98:600–607. doi: 10.1016/s0029-7844(01)01497-1. [DOI] [PubMed] [Google Scholar]
- 8.Taylor RN, Grimwood J, Taylor RS, McMaster MT, Fisher SJ, North RA. Longitudinal serum concentrations of placental growth factor: evidence for abnormal placental angiogenesis in pathologic pregnancies. Am J Obstet Gynecol. 2003;188:177–182. doi: 10.1067/mob.2003.111. [DOI] [PubMed] [Google Scholar]
- 9.Krauss T, Pauer HU, Augustin HG. Prospective analysis of placenta growth factor (PlGF) concentrations in the plasma of women with normal pregnancy and pregnancies complicated by preeclampsia. Hypertens Pregnancy. 2004;23:101–111. doi: 10.1081/PRG-120028286. [DOI] [PubMed] [Google Scholar]
- 10.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 11.Thadhani R, Mutter WP, Wolf M, Levine RJ, Taylor RN, Sukhatme VP, Ecker J, Karumanchi SA. First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J Clin Endocrinol Metab. 2004;89:770–775. doi: 10.1210/jc.2003-031244. [DOI] [PubMed] [Google Scholar]
- 12.Masuyama H, Suwaki N, Nakatsukasa H, Masumoto A, Tateishi Y, Hiramatrsu Y. Circulating angiogenic factors in preeclampsia, gestational proteinuria, and preeclampsia superimposed on chronic glomerulonephritis. Am J Obstet Gynecol. 2006;194:551–556. doi: 10.1016/j.ajog.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 13.Robinson CJ, Johnson DD, Chang EY, Armstrong DM, Wang W. Evaluation of placenta growth factor and soluble Fms-like tyrosine kinase 1 receptor levels in mild and severe preeclampsia. Am J Obstet Gynecol. 2006;195:255–259. doi: 10.1016/j.ajog.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 14.Moore Simas TA, Crawford SL, Solitro MJ, Frost SC, Meyer BA, Maynard SE. Angiogenic factors for the prediction of preeclampsia in high-risk women. Am J Obstet Gynecol. 2007;197:244–248. doi: 10.1016/j.ajog.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 15.Unal ER, Robinson CJ, Johnson DD, Chang EY. Second-trimester angiogenic factors as biomarkers for future-onset preeclampsia. Am J Obstet Gynecol. 2007;197:211–214. doi: 10.1016/j.ajog.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 16.Vatten LJ, Eskild A, Nilsen TI, Jeansson S, Jenum PA, Staff AC. Changes in circulating level of angiogenic factors from the first to second trimester as predictors of preeclampsia. Am J Obstet Gynecol. 2007;196:239–6. doi: 10.1016/j.ajog.2006.10.909. [DOI] [PubMed] [Google Scholar]
- 17.Lim JH, Kim SY, Park SY, Yang JH, Kim MY, Ryu HM. Effective prediction of preeclampsia by a combined ratio of angiogenesis-related factors. Obstet Gynecol. 2008;111:1403–1409. doi: 10.1097/AOG.0b013e3181719b7a. [DOI] [PubMed] [Google Scholar]
- 18.De Vivo A, Baviera G, Giordano D, Todarello G, Corrado F, D’anna R. Endoglin, PlGF and sFlt-1 as markers for predicting pre-eclampsia. Acta Obstet Gynecol Scand. 2008;87:837–842. doi: 10.1080/00016340802253759. [DOI] [PubMed] [Google Scholar]
- 19.Teixeira PG, Cabral AC, Andrade SP, Reis ZS, da Cruz LP, Pereira JB, Martins BO, Rezende CA. Placental growth factor (PlGF) is a surrogate marker in preeclamptic hypertension. Hypertens Pregnancy. 2008;27:65–73. doi: 10.1080/10641950701825937. [DOI] [PubMed] [Google Scholar]
- 20.Diab AE, El-Behery MM, Ebrahiem MA, Shehata AE. Angiogenic factors for the prediction of pre-eclampsia in women with abnormal midtrimester uterine artery Doppler velocimetry. Int J Gynaecol Obstet. 2008;102:146–151. doi: 10.1016/j.ijgo.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 21.Koga K, Osuga Y, Yoshino O, Hirota Y, Ruimeng X, Hirata T, Takeda S, Yano T, Tsutsumi O, Taketani Y. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab. 2003;88:2348–2351. doi: 10.1210/jc.2002-021942. [DOI] [PubMed] [Google Scholar]
- 22.Tsatsaris V, Goffin F, Munaut C, Brichant JF, Pignon MR, Noel A, Schaaps JP, Cabrol D, Frankenne F, Foidart JM. Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: pathophysiological consequences. J Clin Endocrinol Metab. 2003;88:5555–5563. doi: 10.1210/jc.2003-030528. [DOI] [PubMed] [Google Scholar]
- 23.Chaiworapongsa T, Romero R, Espinoza J, Bujold E, Mee KY, Goncalves LF, Gomez R, Edwin S. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am J Obstet Gynecol. 2004;190:1541–1547. doi: 10.1016/j.ajog.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 24.McKeeman GC, Ardill JE, Caldwell CM, Hunter AJ, McClure N. Soluble vascular endothelial growth factor receptor-1 (sFlt-1) is increased throughout gestation in patients who have preeclampsia develop. Am J Obstet Gynecol. 2004;191:1240–1246. doi: 10.1016/j.ajog.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Bujold E, Romero R, Chaiworapongsa T, Kim YM, Kim GJ, Kim MR, Espinoza J, Goncalves LF, Edwin S, Mazor M. Evidence supporting that the excess of the sVEGFR-1 concentration in maternal plasma in preeclampsia has a uterine origin. J Matern Fetal Neonatal Med. 2005;18:9–16. doi: 10.1080/14767050500202493. [DOI] [PubMed] [Google Scholar]
- 26.Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Kim MR, Espinoza J, Bujold E, Goncalves L, Gomez R, Edwin S, et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med. 2005;17:3–18. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- 27.Shibata E, Rajakumar A, Powers RW, Larkin RW, Gilmour C, Bodnar LM, Crombleholme WR, Ness RB, Roberts JM, Hubel CA. Soluble fms-like tyrosine kinase 1 is increased in preeclampsia but not in normotensive pregnancies with small-for-gestational-age neonates: relationship to circulating placental growth factor. J Clin Endocrinol Metab. 2005;90:4895–4903. doi: 10.1210/jc.2004-1955. [DOI] [PubMed] [Google Scholar]
- 28.Staff AC, Braekke K, Harsem NK, Lyberg T, Holthe MR. Circulating concentrations of sFlt1 (soluble fms-like tyrosine kinase 1) in fetal and maternal serum during pre-eclampsia. Eur J Obstet Gynecol Reprod Biol. 2005;122:33–39. doi: 10.1016/j.ejogrb.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 29.Crispi F, Dominguez C, Llurba E, Martin-Gallan P, Cabero L, Gratacos E. Placental angiogenic growth factors and uterine artery Doppler findings for characterization of different subsets in preeclampsia and in isolated intrauterine growth restriction. Am J Obstet Gynecol. 2006;195:201–207. doi: 10.1016/j.ajog.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 30.Wathen KA, Tuutti E, Stenman UH, Alfthan H, Halmesmaki E, Finne P, Ylikorkala O, Vuorela P. Maternal serum-soluble vascular endothelial growth factor receptor-1 in early pregnancy ending in preeclampsia or intrauterine growth retardation. J Clin Endocrinol Metab. 2006;91:180–184. doi: 10.1210/jc.2005-1076. [DOI] [PubMed] [Google Scholar]
- 31.Salahuddin S, Lee Y, Vadnais M, Sachs BP, Karumanchi SA, Lim KH. Diagnostic utility of soluble fms-like tyrosine kinase 1 and soluble endoglin in hypertensive diseases of pregnancy. Am J Obstet Gynecol. 2007;197:28–6. doi: 10.1016/j.ajog.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 32.Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 33.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 34.Rana S, Karumanchi SA, Levine RJ, Venkatesha S, Rauh-Hain JA, Tamez H, Thadhani R. Sequential changes in antiangiogenic factors in early pregnancy and risk of developing preeclampsia. Hypertension. 2007;50:137–142. doi: 10.1161/HYPERTENSIONAHA.107.087700. [DOI] [PubMed] [Google Scholar]
- 35.Robinson CJ, Johnson DD. Soluble endoglin as a second-trimester marker for preeclampsia. Am J Obstet Gynecol. 2007;197:174–175. doi: 10.1016/j.ajog.2007.03.058. [DOI] [PubMed] [Google Scholar]
- 36.Staff AC, Braekke K, Johnsen GM, Karumanchi SA, Harsem NK. Circulating concentrations of soluble endoglin (CD105) in fetal and maternal serum and in amniotic fluid in preeclampsia. Am J Obstet Gynecol. 2007;197:176. doi: 10.1016/j.ajog.2007.03.036. [DOI] [PubMed] [Google Scholar]
- 37.Stepan H, Geipel A, Schwarz F, Kramer T, Wessel N, Faber R. Circulatory soluble endoglin and its predictive value for preeclampsia in second-trimester pregnancies with abnormal uterine perfusion. Am J Obstet Gynecol. 2008;198:175–176. doi: 10.1016/j.ajog.2007.08.052. [DOI] [PubMed] [Google Scholar]
- 38.Schlembach D, Beinder E. Angiogenic factors in preeclampsia. J Soc Gynecol Investig. 2003;10:316A. [Google Scholar]
- 39.Kim SY, Park SY, Kim JW, Kim YM, Yang JH, Kim MY, Ahn H, Shin JS, Kim JO, Ryu HM. Circulating endothelial progenitor cells, plasma VEGF, VEGFR-1 and VEGFR-2 in preeclampsia. Am J Obstet Gynecol. 2005;193:S74. [Google Scholar]
- 40.Chaiworapongsa T, Romero R, Gotsch F, Espinoza J, Nien JK, Goncalves L, Edwin S, Kim YM, Erez O, Kusanovic JP, et al. Low maternal concentrations of soluble vascular endothelial growth factor receptor-2 in preeclampsia and small for gestational age. J Matern Fetal Neonatal Med. 2008;21:41–52. doi: 10.1080/14767050701831397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Livingston JC, Haddad B, Gorski LA, Neblett P, Ahokas RA, Ramsey R, Sibai BM. Placenta growth factor is not an early marker for the development of severe preeclampsia. Am J Obstet Gynecol. 2001;184:1218–1220. doi: 10.1067/mob.2001.113877. [DOI] [PubMed] [Google Scholar]
- 42.Su YN, Lee CN, Cheng WF, Shau WY, Chow SN, Hsieh FJ. Decreased maternal serum placenta growth factor in early second trimester and preeclampsia. Obstet Gynecol. 2001;97:898–904. doi: 10.1016/s0029-7844(01)01341-2. [DOI] [PubMed] [Google Scholar]
- 43.Chappell LC, Seed PT, Briley A, Kelly FJ, Hunt BJ, Charnock-Jones DS, Mallet AI, Poston L. A longitudinal study of biochemical variables in women at risk of preeclampsia. Am J Obstet Gynecol. 2002;187:127–136. doi: 10.1067/mob.2002.122969. [DOI] [PubMed] [Google Scholar]
- 44.Park CW, Park JS, Shim SS, Jun JK, Yoon BH, Romero R. An elevated maternal plasma, but not amniotic fluid, soluble fms-like tyrosine kinase-1 (sFlt-1) at the time of mid-trimester genetic amniocentesis is a risk factor for preeclampsia. Am J Obstet Gynecol. 2005;193:984–989. doi: 10.1016/j.ajog.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 45.Ohkuchi A, Hirashima C, Matsubara S, Suzuki H, Takahashi K, Arai F, Watanabe T, Kario K, Suzuki M. Alterations in placental growth factor levels before and after the onset of preeclampsia are more pronounced in women with early onset severe preeclampsia. Hypertens Res. 2007;30:151–159. doi: 10.1291/hypres.30.151. [DOI] [PubMed] [Google Scholar]
- 46.Erez O, Romero R, Espinoza J, Fu W, Todem D, Kusanovic JP, Gotsch F, Edwin S, Nien JK, Chaiworapongsa T, et al. The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J Matern Fetal Neonatal Med. 2008;21:279–287. doi: 10.1080/14767050802034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, Kusanovic JP, Gotsh F, Erez O, Mazaki-Tovi S, et al. A Longitudinal Study of Angiogenic (Placental Growth Factor) and Anti Angiogenic (soluble Endoglin and soluble VEGF Receptor-1) Factors in Normal Pregnancy and Patients Destined to develop Preeclampsia and Deliver a Small-For-Gestational-Age Neonate. J Matern Fetal Neonatal Med. 2008;21:9–23. doi: 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Polliotti BM, Fry AG, Saller DN, Mooney RA, Cox C, Miller RK. Second-trimester maternal serum placental growth factor and vascular endothelial growth factor for predicting severe, early-onset preeclampsia. Obstet Gynecol. 2003;101:1266–1274. doi: 10.1016/s0029-7844(03)00338-7. [DOI] [PubMed] [Google Scholar]
- 49.Hertig A, Berkane N, Lefevre G, Toumi K, Marti HP, Capeau J, Uzan S, Rondeau E. Maternal serum sFlt1 concentration is an early and reliable predictive marker of preeclampsia. Clin Chem. 2004;50:1702–1703. doi: 10.1373/clinchem.2004.036715. [DOI] [PubMed] [Google Scholar]
- 50.Akolekar R, Zaragoza E, Poon LC, Pepes S, Nicolaides KH. Maternal serum placental growth factor at 11 + 0 to 13 + 6 weeks of gestation in the prediction of pre-eclampsia. Ultrasound Obstet Gynecol. 2008;32:732–739. doi: 10.1002/uog.6244. [DOI] [PubMed] [Google Scholar]
- 51.Baumann MU, Bersinger NA, Mohaupt MG, Raio L, Gerber S, Surbek DV. First-trimester serum levels of soluble endoglin and soluble fms-like tyrosine kinase-1 as first-trimester markers for late-onset preeclampsia. Am J Obstet Gynecol. 2008;199:266. doi: 10.1016/j.ajog.2008.06.069. [DOI] [PubMed] [Google Scholar]
- 52.Than NG, Romero R, Hillermann R, Cozzi V, Nie G, Huppertz B. Prediction of preeclampsia - a workshop report. Placenta. 2008;29(Suppl A):S83–5. doi: 10.1016/j.placenta.2007.10.008. Epub@2007 Dec 3.:S83–S85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kusanovic JP, Romero R, Chaiworapongsa T, Erez O, Mittal P, Vaisbuch E, Mazaki-Tovi S, Gotsch F, Edwin S, Gomez R, et al. A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in teh identification of patients destined to develop preeclampsia. J Matern Fetal Neonatal Med. 2009 doi: 10.3109/14767050902994754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Madazli R, Kuseyrioglu B, Uzun H, Uludag S, Ocak V. Prediction of preeclampsia with maternal mid-trimester placental growth factor, activin A, fibronectin and uterine artery Doppler velocimetry. Int J Gynaecol Obstet. 2005;89:251–257. doi: 10.1016/j.ijgo.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 55.Parra M, Rodrigo R, Barja P, Bosco C, Fernandez V, Munoz H, Soto-Chacon E. Screening test for preeclampsia through assessment of uteroplacental blood flow and biochemical markers of oxidative stress and endothelial dysfunction. Am J Obstet Gynecol. 2005;193:1486–1491. doi: 10.1016/j.ajog.2005.02.109. [DOI] [PubMed] [Google Scholar]
- 56.Yu CK, Smith GC, Papageorghiou AT, Cacho AM, Nicolaides KH. An integrated model for the prediction of preeclampsia using maternal factors and uterine artery Doppler velocimetry in unselected low-risk women. Am J Obstet Gynecol. 2005;193:429–436. doi: 10.1016/j.ajog.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 57.Espinoza J, Romero R, Nien JK, Gomez R, Kusanovic JP, Goncalves LF, Medina L, Edwin S, Hassan S, Carstens M, et al. Identification of patients at risk for early onset and/or severe preeclampsia with the use of uterine artery Doppler velocimetry and placental growth factor. Am J Obstet Gynecol. 2007;196:326–13. doi: 10.1016/j.ajog.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stepan H, Unversucht A, Wessel N, Faber R. Predictive value of maternal angiogenic factors in second trimester pregnancies with abnormal uterine perfusion. Hypertension. 2007;49:818–824. doi: 10.1161/01.HYP.0000258404.21552.a3. [DOI] [PubMed] [Google Scholar]
- 59.Crispi F, Llurba E, Dominguez C, Martin-Gallan P, Cabero L, Gratacos E. Predictive value of angiogenic factors and uterine artery Doppler for early-versus late-onset pre-eclampsia and intrauterine growth restriction. Ultrasound Obstet Gynecol. 2008;31:303–309. doi: 10.1002/uog.5184. [DOI] [PubMed] [Google Scholar]
- 60.Poon LC, Kametas NA, Maiz N, Akolekar R, Nicolaides KH. First-trimester prediction of hypertensive disorders in pregnancy. Hypertension. 2009;53:812–818. doi: 10.1161/HYPERTENSIONAHA.108.127977. [DOI] [PubMed] [Google Scholar]
- 61.Verheul HM, Hoekman K, Luykx-de BS, Eekman CA, Folman CC, Broxterman HJ, Pinedo HM. Platelet: transporter of vascular endothelial growth factor. Clin Cancer Res. 1997;3:2187–2190. [PubMed] [Google Scholar]
- 62.Banks RE, Forbes MA, Kinsey SE, Stanley A, Ingham E, Walters C, Selby PJ. Release of the angiogenic cytokine vascular endothelial growth factor (VEGF) from platelets: significance for VEGF measurements and cancer biology. Br J Cancer. 1998;77:956–964. doi: 10.1038/bjc.1998.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Webb NJ, Bottomley MJ, Watson CJ, Brenchley PE. Vascular endothelial growth factor (VEGF) is released from platelets during blood clotting: implications for measurement of circulating VEGF levels in clinical disease. Clin Sci (Lond) 1998;94:395–404. doi: 10.1042/cs0940395. [DOI] [PubMed] [Google Scholar]
- 64.Nielsen HJ, Werther K, Mynster T, Brunner N. Soluble vascular endothelial growth factor in various blood transfusion components. Transfusion. 1999;39:1078–1083. doi: 10.1046/j.1537-2995.1999.39101078.x. [DOI] [PubMed] [Google Scholar]
- 65.Wynendaele W, Derua R, Hoylaerts MF, Pawinski A, Waelkens E, de Bruijn EA, Paridaens R, Merlevede W, van Oosterom AT. Vascular endothelial growth factor measured in platelet poor plasma allows optimal separation between cancer patients and volunteers: a key to study an angiogenic marker in vivo? Ann. Oncol. 1999;10:965–971. doi: 10.1023/a:1008377921886. [DOI] [PubMed] [Google Scholar]
- 66.Adams J, Carder PJ, Downey S, Forbes MA, MacLennan K, Allgar V, Kaufman S, Hallam S, Bicknell R, Walker JJ, et al. Vascular endothelial growth factor (VEGF) in breast cancer: comparison of plasma, serum, and tissue VEGF and microvessel density and effects of tamoxifen. Cancer Res. 2000;60:2898–2905. [PubMed] [Google Scholar]
- 67.Jelkmann W. Pitfalls in the measurement of circulating vascular endothelial growth factor. Clin Chem. 2001;47:617–623. [PubMed] [Google Scholar]
- 68.Gonzalez RP, Gomez RM, Castro RS, Nien JK, Merino PO, Etchegaray AB, Carstens MR, Medina LH, Viviani PG, Rojas IT. A national birth weight distribution curve according to gestational age in chile from 1993 to 2000. Rev Med Chil. 2004;132:1155–1165. doi: 10.4067/s0034-98872004001000001. [DOI] [PubMed] [Google Scholar]
- 69.ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet. 2002;77:67–75. [PubMed] [Google Scholar]
- 70.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 71.Barleon B, Reusch P, Totzke F, Herzog C, Keck C, Martiny-Baron G, Marme D. Soluble VEGFR-1 secreted by endothelial cells and monocytes is present in human serum and plasma from healthy donors. Angiogenesis. 2001;4:143–154. doi: 10.1023/a:1012245307884. [DOI] [PubMed] [Google Scholar]
- 72.Rajakumar A, Michael HM, Rajakumar PA, Shibata E, Hubel CA, Karumanchi SA, Thadhani R, Wolf M, Harger G, Markovic N. Extra-placental expression of vascular endothelial growth factor receptor-1, (Flt-1) and soluble Flt-1 (sFlt-1), by peripheral blood mononuclear cells (PBMCs) in normotensive and preeclamptic pregnant women. Placenta. 2005;26:563–573. doi: 10.1016/j.placenta.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 73.Clark DE, Smith SK, He Y, Day KA, Licence DR, Corps AN, Lammoglia R, Charnock-Jones DS. A vascular endothelial growth factor antagonist is produced by the human placenta and released into the maternal circulation. Biol Reprod. 1998;59:1540–1548. doi: 10.1095/biolreprod59.6.1540. [DOI] [PubMed] [Google Scholar]
- 74.He Y, Smith SK, Day KA, Clark DE, Licence DR, Charnock-Jones DS. Alternative splicing of vascular endothelial growth factor (VEGF)-R1 (FLT-1) pre-mRNA is important for the regulation of VEGF activity. Mol Endocrinol. 1999;13:537–545. doi: 10.1210/mend.13.4.0265. [DOI] [PubMed] [Google Scholar]
- 75.Ebos JM, Bocci G, Man S, Thorpe PE, Hicklin DJ, Zhou D, Jia X, Kerbel RS. A naturally occurring soluble form of vascular endothelial growth factor receptor 2 detected in mouse and human plasma. Mol Cancer Res. 2004;2:315–326. [PubMed] [Google Scholar]
- 76.Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 77.Cheifetz S, Bellon T, Cales C, Vera S, Bernabeu C, Massague J, Letarte M. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J Biol Chem. 1992;267:19027–19030. [PubMed] [Google Scholar]
- 78.Gougos A, St JS, Greaves A, O’Connell PJ, d’Apice AJ, Buhring HJ, Bernabeu C, van Mourik JA, Letarte M. Identification of distinct epitopes of endoglin, an RGD-containing glycoprotein of endothelial cells, leukemic cells, and syncytiotrophoblasts. Int Immunol. 1992;4:83–92. doi: 10.1093/intimm/4.1.83. [DOI] [PubMed] [Google Scholar]
- 79.Lastres P, Bellon T, Cabanas C, Sanchez-Madrid F, Acevedo A, Gougos A, Letarte M, Bernabeu C. Regulated expression on human macrophages of endoglin, an Arg-Gly-Asp-containing surface antigen. Eur J Immunol. 1992;22:393–397. doi: 10.1002/eji.1830220216. [DOI] [PubMed] [Google Scholar]
- 80.Buhring HJ, Muller CA, Letarte M, Gougos A, Saalmuller A, van Agthoven AJ, Busch FW. Endoglin is expressed on a subpopulation of immature erythroid cells of normal human bone marrow. Leukemia. 1991;5:841–847. [PubMed] [Google Scholar]
- 81.Maglione D, Guerriero V, Viglietto G, li-Bovi P, Persico MG. Isolation of a human placenta cDNA coding for a protein related to the vascular permeability factor. Proc Natl Acad Sci US A. 1991;88:9267–9271. doi: 10.1073/pnas.88.20.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khaliq A, Li XF, Shams M, Sisi P, Acevedo CA, Whittle MJ, Weich H, Ahmed A. Localisation of placenta growth factor (PIGF) in human term placenta. Growth Factors. 1996;13:243–50. doi: 10.3109/08977199609003225. [DOI] [PubMed] [Google Scholar]
- 83.Tordjman R, Delaire S, Plouet J, Ting S, Gaulard P, Fichelson S, Romeo PH, Lemarchandel V. Erythroblasts are a source of angiogenic factors. Blood. 2001;97:1968–1974. doi: 10.1182/blood.v97.7.1968. [DOI] [PubMed] [Google Scholar]
- 84.Autiero M, Luttun A, Tjwa M, Carmeliet P. Placental growth factor and its receptor, vascular endothelial growth factor receptor-1: novel targets for stimulation of ischemic tissue revascularization and inhibition of angiogenic and inflammatory disorders. J Thromb Haemost. 2003;1:1356–1370. doi: 10.1046/j.1538-7836.2003.00263.x. [DOI] [PubMed] [Google Scholar]
- 85.Failla CM, Odorisio T, Cianfarani F, Schietroma C, Puddu P, Zambruno G. Placenta growth factor is induced in human keratinocytes during wound healing. J Invest Dermatol. 2000;115:388–395. doi: 10.1046/j.1523-1747.2000.00085.x. [DOI] [PubMed] [Google Scholar]