Abstract
Field parasitological studies consistently demonstrate the reality of polyparasitism in natural systems. However, only recently, studies from ecological and evolutionary fields have emphasised a broad spectrum of potential multiple infections-related impacts. The main goal of our review is to reunify the different approaches on the impacts of polyparasitism, not only from laboratory or human medical studies but also from field or theoretical studies. We put forward that ecological and epidemiological determinants to explain the level of polyparasitism, which regularly affects not only host body condition, survival or reproduction but also host metabolism, genetics or immune investment. Despite inherent limitations of all these studies, multiple infections should be considered more systematically in wildlife to better appreciate the importance of parasite diversity in wildlife, cumulative effects of parasitism on the ecology and evolution of their hosts.
Keywords: Wildlife, polyparasitism, multiparasitism, coinfections, parasite diversity, life-history traits
Numerous studies have investigated the impacts of single parasite species not only on host survival, host reproduction but also on various host traits (i.e. behaviour or morphological traits). However, research on infectious diseases in humans, particularly in developing countries, has emphasised the importance of multiple infections (1, 2). At the same time, ecological and evolutionary studies are still improving our knowledge on multiple parasitic infections (synonymised with concomitant infections, coinfections, polyparasitism or multiparasitism) of most vertebrate hosts in natural systems (3, 4).
Introduction
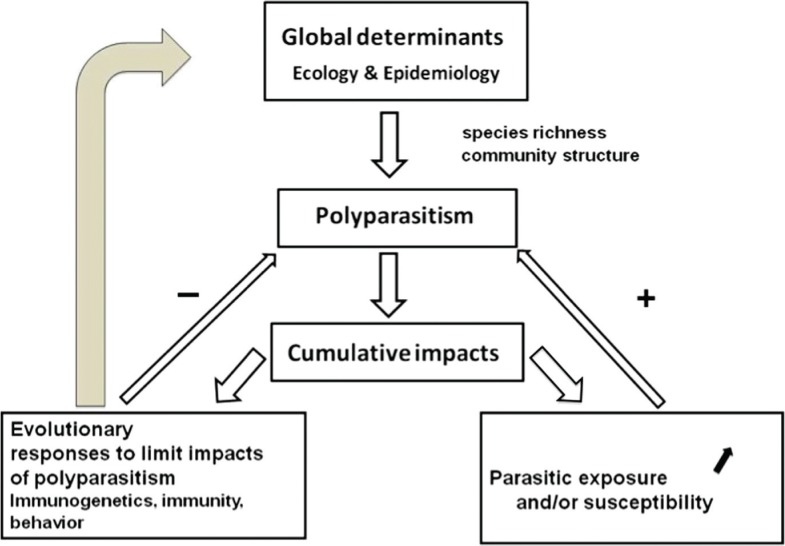
Multiple infections (simultaneous infections with multiple parasite species in an individual host), also called polyparasitism, are the rule, rather than the exception, in the wild. These simultaneous infections are however part of a more broad pattern of parasite assemblages. In fact, if parasites of different species may be encountered in the same host individual (i.e. infracommunities), infections by different and often numerous parasite species are also observed in a host population (called parasite species richness at population level) or in a host species (called parasite species richness at host species level). Moreover, infections with different parasite species are observed at all geographical scales: local (i.e. a village, a forest), regional (i.e. a country, a state) or global scales (i.e. biogeographic realm, continent), generally with parasite species increasing with the geographical scale (5). Despite being recognised for many years (1, 6), investigations on the impacts of multiple infections on individual hosts or higher parasite species richness on host populations or species are still scarce in disease ecology and in evolutionary ecology (7, 3). Our main goals with the present review are to introduce the factors that may explain higher parasite species richness (often investigated as parasite species diversity) in wild animals and to reunify observations and data from various fields (clinical, immunology, ecological parasitology, genetics, theoretical evolutionary epidemiology) to assess the reality of the impacts of multiple infections in natural individual hosts, their potential underlying mechanisms and evolutionary implications (Fig. 1).
Fig. 1.
Polyparasitism imposes cumulative impacts on their hosts with evolutionary consequences on adaptive responses to limit these impacts, which in turn may affect the determinants of multiple infections.
Determinants of polyparasitism: ecology and epidemiology matters
Parasite species richness
More than half of all living organisms are parasites, living at the expense of other free-living organisms (8). The search of the determinants of this parasite species richness has been the topic of numerous studies (7, 9, 10). The main hypotheses come from two major domains: theoretical ecology (with determinants such as latitudinal gradients, geographical range, home range) and epidemiological theory (with determinants such as population size, population density, population longevity). More recently, behavioural ecology has inspired the search of new determinants (sociality, grooming and preening behaviour). Although there is a need for a comprehensive analysis of all published studies, two major determinants seem to emerge consistently from the studies. Host geographical range, an ecologically linked factor, and host population density, an epidemiologically linked factor, appear to be the two most important drivers of parasite species richness, and hence for polyparasitism (Table 1). A host species living on a large geographical range in high population densities generally harbours a higher diversity of parasite species than a host species living in a more restricted geographical area and in low population densities.
Table 1.
Host density and geographical distribution as determinant of parasite species richness in mammal species
| Determinant | Parasite and host taxa | Correlation | References |
|---|---|---|---|
| Host density | Helminths and mammals | Positive | 103 |
| Nematodes and mammals | Positive | 104 | |
| Fleas, rodents and insectivores | Positive | 105 | |
| Helminths and primates | Positive | 9 | |
| Helminths and carnivores | Positive | 106 | |
| Macro-, microparasites and carnivores | Positive | 107 | |
| Area size (geographic distribution) | Helminths and rodents | Positive | 108 |
| Fleas and rodents | Positive | 109 | |
| Helminths and carnivores | Positive | 106 | |
| Macro-, microparasites and carnivores | Positive | 107 |
Furthermore, parasite species richness seems to be an attribute of host species such as any other host life history trait (11, 12). Parasite species richness is often similar among populations, which means that a given host species is composed of populations with roughly similar number of parasite species, although parasite species richness observed at host population level is always lower than the total parasite species counted at the global geographic range of the host species (5, 13).
Comparative studies on the determinants of parasite species richness have then attempted to explain the variability of parasite diversity among host species, host populations and even host individuals. Strong patterns have also emerged from ecological parasitology emphasising the existence of ecological rules that apply to parasite community structure (14, 15).
Polyparasitism and parasite community structure
In natural conditions, hosts are infected by multiple parasite species or by multiple genotypes of the same parasite species. Multiple infections have often been investigated through the analyses of the outcomes of competition among parasites. Competition during multiple infections includes both interspecific competition (e.g. mixed species helminth infection) and/or intraspecific competition (e.g. genetically diverse strains of microparasites). Fundamentally, competition between parasites may be direct or indirect, through competition for resources (e.g. blood) or immune system (i.e. immune-suppression or cross-immunity) (16, 17). The relative importance of the forces that determine the structure of parasite communities, resources or immunity, may then provide some consequences of multiple infections. During multiple infections with two or more parasite species, the burden of one (or more) parasite(s) might be enhanced by the other parasite(s) (synergic interactions) or, on the contrary, be suppressed (antagonist interactions) (18, 19). The recent study of Telfer and colleagues (20) has highlighted that parasitic interactions can explain more variation in infection risks than factors related to parasite exposure, host age and/or seasonality.
Lessons from empirical and theoretical studies
Laboratory and clinical studies
Several laboratory studies using animal models have focused on coinfections, but relatively few studies have investigated the impacts of such coinfections on host fitness. Clinical studies in humans have also given significant results on the impacts of coinfections.
Helminth–microparasite coinfections have been particularly well investigated. Helminths may alter the efficiency of immune system via cytokines and induce Th2-cell responses (21). As a mutual inhibition occurs between cytokine responses induced by helminths (Interleukine-4) and by microparasites (Interferon-gamma), helminths are expected to enhance microparasitic infections, which may cause coinfections to be more deleterious. This has been illustrated in helminth–tuberculosis, helminth–AIDS or helminth–malaria coinfections in humans (22, 23). In helminth–Plasmodium coinfection, the relative risk of clinical malaria is enhanced in patients infected by helminths (23). Similarly, Schistosoma mansoni infection in BALB/c mice increases the parasitemia peak of Plasmodium yoelii leading to mouse death, whereas single infection by the protozoan is non-lethal and self-resolving (24).
Virus–protozoan coinfections have been investigated in humans, notably for the malaria-AIDS model, where HIV seems to facilitate the infection by Plasmodium falciparum. The risk of contracting a new malaria infection is six times higher in adults infected with HIV than in uninfected adults (25). Both infective agents act synergistically and result in worse health conditions (26). Several other studies dealing with various combinations of protozoans and viruses in laboratory animals concluded that multiple infections are often associated with higher mortality or reduced body condition.
Protozoan–protozoan or helminth–helminth coinfections have been intensively investigated. Plasmodium yoelii infections are enhanced by Leishmania mexicana or Toxoplasma gondii in co-infected rats. Trypanosoma cruzi infections are also enhanced in mice infected with Plasmodium yoelii. If some helminth species seem to prevent infection by other heminth species, the majority of studies showed enhancement of infection (16).
Helminth coinfections may strongly affect human condition. For example, concomitant schistosome, hookworm and whipworm infections increase the severity of anaemia in children (27).
Theroretical and experimental studies
Predictions related to multiparasitism impacts on host fitness benefit from both theoretical and experimental studies investigating the expression and evolution of parasite virulence during coinfections (28–31). The main result emerging from this active field of research suggests that competitive interactions among strains select parasites that are able to exploit their host more rapidly than their parasite competitors, thereby causing increased host exploitation (30). In multiple infections, natural selection is then expected to favour higher levels of virulence. This prediction was supported by experimental studies concerning coinfections by strains/genotypes in rodent-malaria, snail-schistosome or Daphnia-microparasite models (29, 31, 32). Unfortunately, few laboratory studies have considered interspecific interactions from a virulence perspective. In multi-strain infection, we may expect cross immunity, kin selection and potential recombination between strains to occur, which should not happen in interspecific coinfection. Consequently the related outcomes could be different. Indeed, the few models dealing with interspecific interactions stressed that sub-lethal pathogens like helminths may lead to the evolution of lower virulence in multiple infections (30).
To summarise, laboratory, clinical or theoretical models regularly stressed negative impacts of multi-infection on hosts.
Mechanisms inducing deleterious effects in multiple infections
Deleterious effects may be linked to higher parasitic diversion of resources and/or cumulative damages to the hosts due to greater overall parasite loads (33). However, immunity, and particularly the cytokines’ environment, appears to be the key element in coinfection. Cytokines play a predominant role in structuring parasite community by affecting positive or negative interactions among parasites and the parasite transmission (34).
Ezeamama and colleagues (27) found that coinfections with hookworms and either schistosomes or whipworms in children were associated with higher levels of anemia than expected from single infections. These higher levels of anemia are consistent with helminth biology, due to blood feeding behaviour of hookworms and eggs extravasation through the bowel wall for schistosomes. However, the authors also stressed that a second mechanism called anemia of inflammation could operate, linked to iron deficiency. Anemia of inflammation is mediated by the production of pro-inflammatory cytokines during helminth infections (particularly IL-6), which enhances production of hepcidin, an iron regulatory peptide that inhibits iron uptake in the duodenum and iron release from macrophages (35). This example illustrates that if anemia may be related to blood consumption or loss, immune defenses are also involved. Other pathogeneses of anemia are reported in humans infected by Schistosoma japonicum or in mice infected by Trypanosoma brucei, both being related to immune-mediated hemolysis (36, 37).
Several other examples illustrated that although controlling parasite proliferation may be advantageous for the hosts, immune defenses may be detrimental by increasing the severity of disease and by reducing host fitness. All these immune-mediated diseases, termed immunopathology (38), are mainly linked to collateral effects of interleukines (i.e. enhanced production of hepcidin and iron deficiency). Strong inflammation processes may lead to severe tissue destruction or to wrong recognition of antigens including host antigens (i.e. autoimmunity). Coinfections may exacerbate immunopathology (39, 40) as was recently shown in wild mammals (41). Hence, spoliations and/or damages related to the biology of parasites and immune-mediated pathology during multiple infections may accumulate and lead to the increase of the disease severity associated with the decrease of host fitness.
However, some parasites may prevent immune pathology in case of coinfections. Helminths have been shown to protect hosts against inflammatory diseases (42). Helminth infections stimulate the dominance of T-helper 2 cells (Th2 response) and innate cell types such as mast cells and eosinophils (43). These changes in the Th2 response create an anti-inflammatory environment favourable not only for parasite survival but also surprisingly for the host. For example, infection with the trematode Fasciola hepatica suppresses immune responses to self-antigens and attenuates the clinical signs of experimental autoimmune encephalomyelitis in mice (the animal model for multiple sclerosis) (42).
Lesson from comparative and field studies on multiple infections in wildlife
An extensive review of the literature allowed us to identify the potential multiple impacts of parasite diversity and multiple infections. Our review concerned ecological and evolutionary ecological studies, which aim at understanding broad patterns at population or species level (Table 2). These studies have mainly concerned parasitic interspecific interactions and have brought interesting and sometimes intriguing results, despite being mainly correlative.
Table 2.
Studies reporting impacts of multiple infections in free-ranging vertebrates
| Level of impact | Host level | Host taxa | Response to polyparasitism |
|---|---|---|---|
| Genetics | Inter-, intraspecific | Fish | Increased variability at MHC genes (55, 58, 61, 62) |
| Birds | Optimal allele diversity at MHC level (66, 69) | ||
| Mammals | Selection and resistance to a parasite (63, 64, 69, 73–75, 110) | ||
| MHC heterozygote superiority (76) | |||
| Immunity | Inter-, intraspecific | Fish | Increased level of immune investment (87–90) |
| Birds | Increased susceptibility to other parasites (20) | ||
| Mammals | |||
| Demography | Intraspecific | Birds | Reduced survival and/or fecundity (44, 45, 48–50, 68, 93) |
| Mammals | |||
| Body condition | Intraspecific | Mammals | Deterioration of body condition (44, 47, 49) |
| Birds | |||
| Metabolism | Interspecific | Mammals | Increase in BMR (85) |
| Behaviour | Intraspecific | Mammals | Reduced escape capacity (52) |
| Sleep duration | Interspecific | Mammals | Increase in sleep duration (87) |
| Phenotype | Intraspecific | Birds | Plumage colouration (46) |
Impacts on host condition and demography
At the demographic level, multiple infections are clearly prone to reduce body condition of the host, affecting survival and reproduction in birds (44–46) and in mammals (47–50). According to the prediction for helminth–helminth coinfections, wild rabbits harbouring more than three helminth species have a worse body condition compared to rabbits only infected by one or two helminths (47), and multiple helminth infections have more deleterious impacts on body condition of the willow ptarmigan than single helminth infection (45). Jolles and colleagues (49) investigated microparasite–helminth coinfections and demonstrated that African buffaloes co-infected with Mycobacterium bovis and gastrointestinal nematodes had a reduced lifespan and a worsened body condition compared to African buffaloes infected only by bovine tuberculosis or only by digestive worms. Interestingly, nematodes facilitated tuberculosis invasion by inducing immune-suppression effects (51). Similarly, Purple martins infected by two blood parasites, Haemoproteus prognei and an unidentified filarial nematode, had consistently higher mortality rates compared to birds infected only by the filarial nematode (45).
Microparasite–microparasite coinfections seem to present a wide range of possible impacts. If multiple infections by blood protozoan parasites affect carotenoid-based colouration of blue tits’ plumage (46), other coinfections may induce dramatic mortalities, as it was established in African lions co-infected by distemper virus and hemoprotozoans (50).
Impacts may be observed at unexpected levels such as the reduced escape capacity in hares harbouring multiple parasite species (52), probably as a result of worsened physical condition.
All these results obtained in wild are in agreement with epidemiological studies that often established an increase in disease severity during coinfections. As already mentioned, several major parasitic diseases of humans (i.e. AIDS, malaria, schistosomiasis) are influenced by the presence of other infectious agents (53, 54).
Impacts on host genetic
Fundamentally, the studies on host genetics have mainly focused on MHC gene polymorphism. Investigating the maintenance of host genetic variation through coevolutionary host–pathogen interactions, a first prediction hypothesises the existence of a link between high MHC polymorphism and high diversity of parasites (55–60). In accordance with this prediction, variation at the Human Leukocyte Antigen (HLA) class I genes was found associated with local pathogen richness (57). Various studies in fish showed that individual hosts harbouring higher parasite species have higher genetic diversity at the MHC genes (55, 61, 62). However, such pattern was not observed in rodents (60, 63–65). A low diversity at MHC genes in grey slender mouse opossum was linked to high helminth parasite loads in terms of individual parasite species richness, nematode intensities and prevalence (60). The authors explained this unexpected pattern by an ‘unbalanced situation’ scenario, which might be caused by a recent loss of genetic diversity and a lack of resistance alleles (60). MHC-depleted species was supposed to suffer from higher parasite loads than MHC-diversified species. Interestingly, this prediction was supported in a tropical Asian rat (Leopoldamys sabanus) for which a more diverse MHC IIB allele repertoire was linked to reduced individual helminth species richness (65). Due to the better body condition observed in rats harbouring higher allele diversity, the authors inferred that individuals harbouring higher polymorphism at MHC level should be more resistant to multiple infections (65).
This first prediction was simultaneously challenged by the hypothesis of an optimal and intermediate number of alleles per host individual prone to limit simultaneous cumulative parasite loads during multiple infections, but also prone to limit autoimmunity (66). This optimal scenario refers to the hypothesis that individuals with strong immunity are also likely to suffer associated costs, notably energetic drain and autoimmunity (67). This second prediction was supported by Wegner and colleagues (66) who first established a link between an intermediate number of DBR alleles and a lowest cumulative parasitic intensity during multiple infections in three-spined sticklebacks in the laboratory. Importantly this relation was confirmed in field enclosures (68). The number of parasite species infecting individual bank voles was also found lowest with intermediate numbers of DRB alleles (69). The ‘optimal number of alleles’ hypothesis seems to be reinforced by field ecological studies stressing that individuals with strong immune responses may also experience deleterious effects for their survival or reproduction (41, 70).
A third prediction considers frequency-dependent selection and/or MHC heterozygote superiority, where some rare alleles or some heterozygote genotypes could lead to higher parasite resistance, at least against certain types of parasites. Association between a specific allele and disease resistance has been well documented in humans (71, 72). Parasite-driven selection and MHC polymorphism have been well supported in fish, birds and mammals (63, 73–75). For example, rabbits harbouring a particular allele showed lower infestations with hepatic coccidia, Eimeria stiedai (75). MHC heterozygote superiority against multiple parasites has found some support in the water vole (76).
Taken together, all these studies put forward complex links between MHC and parasitism but also the difficulty to make good prediction, or how to interpret results from correlative studies. Importantly, these studies argued for more field studies before definitively linking MHC diversity and parasite resistance. Finally, the links between MHC diversity and host fitness in wildlife need also more investigations (77, 78).
Impacts on host metabolism and sleep
Concerning host metabolism, there are several experimental studies that linked parasitic infestations or immune stimulation to higher basal metabolic rates (BMRs) and costs in birds and mammals (79–83). BMR scales in allometry with body mass, but the reasons why some species have higher or lower metabolic rates than predicted from their body mass still remain unclear despite several studies (84). BMR was expected to be positively linked to parasite loads. A study linked higher BMR across mammal species with higher helminth species richness (85) but another failed in the case of ectoparasitic fleas (86). Moreover, due to the intimate links between parasitism, energy and immune defenses we may also expect more broad patterns connecting these three variables or, at least, congruent results of high parasite pressures associated with high investment in immunity.
Parasite diversity was also linked to increased sleep duration in mammal taxa (87). The main explanation is a potential positive link between sleep duration and the strength of immune defences due to energy saving.
Taken together, all these results are congruent with the findings that higher helminth species richness in mammals are linked with higher white blood cell counts across species (88) but also with other studies that also linked higher immune investment in host species or populations challenged by more parasite species compared to others (89, 90) (Table 1).
Limits of the available field and comparative studies
It is remarkable that most of the predictions based on laboratory studies or on human medical studies seem to be corroborated by data from wildlife. Predictions related to stronger impacts of polyparasitism on various hosts’ traits seem highly supported. Both approaches, that is field descriptive or comparative studies, are important attempts to detect impacts of polyparasitism and may suggest or advocate some ideas or patterns that have to be formally tested. Importantly, however, most available field studies are still descriptive and correlative and cannot prove causality between multiple infections and supposed related impacts. The same criticism applies for all evolutionary comparative studies. In other words, if we want to understand underlying processes in polyparasitism we have to continue laboratory studies and switch from field correlative studies to more field experimental studies. Manipulation experiments with parasite removal using selective medication (91, 92) are of great interest to formally validate these hypotheses.
The other second limit of available field studies is that they only consider a community of few parasites, that is only helminths (44, 47, 55, 60, 68, 73) or blood protozoans (46), or focused only on two parasite species (45, 49, 50) or two parasitic clades (75) when hosts, in reality, are co-infected with various parasitic clades and considerably more parasites species. The criticism is the same for most of the comparative studies as they have concerned only helminth species richness (58, 85, 88) or blood protozoan species richness (93), ignoring all other potential important parasite taxa.
This criticism may be enhanced as most of the field studies focusing on host genetics have mainly considered parasite loads using faecal eggs counts (FEC), a technique which consists of detecting both the number of helminth morphotypes and the number of eggs (supposed to be correlated to the number of intestinal worms) in faecal samples (60, 63, 64, 69, 73, 74). Despite the great interest (and its increasing popularity) of such methodology due to its non-invasive nature for wildlife populations and the interesting information extracted from these data, there are important limits related to FEC. First, they allow detection mostly of helminths and coccidians. Ecologists may therefore underestimate helminth diversity per se due to low fecundity, low infestation levels or intermittent egg excretions in helminth parasitism. Second, egg counts do not always correlate with the number of adult worms within the intestinal tracts of the animals (94). The relationship between indirect measures and actual worm burden is then very complex, stressing that egg counts are not always a reliable method for estimating the numbers of parasites within the gastrointestinal tract and also parasite loads. These serious difficulties will probably be solved, at least to identify the full diversity of parasites with new molecular tools (such as new generation of sequencing technologies).
We have to be aware that data concerning parasite diversity available for comparative studies are still very limited. For example, an intense literature survey gathered whole helminth species richness for only 318 mammal species, a very low number, which represents <6% of all mammals (7).
Emerging diseases and polyparasitism
One goal of disease ecology is to study pathogens’ transmission and their spread over space and time (95). Recent studies have highlighted the existence and importance of heterogeneity in host characteristics in the transmission process due to higher susceptibility or infectiousness of some individuals prone to disproportionately contribute to parasite transmission, sometimes in the absence of visible disease symptoms (96). For example, «superspreaders» were implicated in the early dynamics of the severe acute respiratory syndrome epidemic in Southeast Asia, where some individuals accounted for 40 or more secondary cases (97). From the same perspective, male individuals have been shown to play a dominant role in helminth transmission in the yellow-necked mice because population-level transmission declined significantly when males, but not females, were treated to remove parasites (98).
At species level and for multi-host pathogens (that can be transmitted between several different host species) the available studies have postulated and sometimes established that some host species could amplify the disease (their presence increases transmission rates between species), whereas others may limit transmission from one species to one other (99), for a review in a biodiversity perspective). This conceptual framework could be improved by including polyparasitism as a key host species characteristic prone to affect its infectiousness. In fact, considering the strong effects of multiple infections on life history traits, behaviour, genetic structure or body condition, we may expect strong trade-offs between immune defences and all these parameters.
If some species are prone to sustain high level of immune defences to control or limit multiple parasitic species attacks (i.e. resistance) (100, 101), they may be poor amplifiers of pathogens. Conversely, host species prone to rather tolerate multiple attacks (tolerance being the ability to maintain fitness in the presence of a given pathogen load without tempting to reduce it, (100, 101) could be best candidate as amplifiers.
Identifying the extent to which immune mechanisms and/or polyparasitism may contribute to higher or lower infectiousness in some host species compared to others could undoubtedly be a promising avenue in the understanding of disease ecology of multi-host pathogens. In other words, identifying the key host species and their related immunological and/or life-history determinants represent a challenge in the open field of disease ecology and emerging diseases.
Conclusions
Integrating multiple infections is a promising avenue to infer the reality of parasitic pressures in natural populations. Even if several difficulties are encountered, there are three important tasks to resolve:
The monitoring of complete parasite communities in host individuals or populations, which can be realised thanks to new fast-improving technologies such as high-throughput sequencing approaches (103).
The re-foundation of community ecology of parasitism in the light of polyparasitism-related impacts in order to re-analyse patterns observed in so many studies, to infer new testable hypotheses based on likely processes and potentially to establish some laws.
The integration of immunity in disease ecology. New methodologies and concepts in immuno-ecology have revolutionised the way host–parasite interactions are investigated. The difficulty to disentangle immune responses facing multiparasitism in an ecological context will be only resolved by field manipulation.
Acknowledgements
This study is supported by the French ANR Biodiversity ANR 07 BDIV 012, project CERoPath, ‘Community Ecology of Rodents and their Pathogens in a changing environment’ (http://www.ceropath.org/). We thank two anonymous referees for helpful comments and suggestions.
Conflict of interest and funding
The authors declare that they have no conflict of interest.
References
- 1.Crawley MJ. The population biology of predators, parasites and diseases. London: Blackwell; 1992. [Google Scholar]
- 2.Pullan R, Brooker S. The health impact of polyparasitism in humans: are we under- estimated the burden of parasitic diseases? Parasitology. 2008;135:783–94. doi: 10.1017/S0031182008000346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rigaud T, Perrot-Minnot MJ, Brown MJF. Parasite and host assemblages: embracing the reality will improve our knowledge of parasite transmission and virulence. Proc Roy Soc Lond B. 2010;277:3693–702. doi: 10.1098/rspb.2010.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tompkins DM, Dunn AM, Smith MJ, Telfer S. Wildlife diseases: from individuals to ecosystems. J Anim Ecol. 2010;80:19–38. doi: 10.1111/j.1365-2656.2010.01742.x. [DOI] [PubMed] [Google Scholar]
- 5.Poulin R. Evolutionnary Ecology of parasites. 2nd ed. Princeton: Princeton University Press; 2007. [Google Scholar]
- 6.Petney TN, Andrews RM. Multiparasite communities in animals and humans: frequency, structure and pathogenic significance. Int J Parasitol. 1998;28:377–93. doi: 10.1016/s0020-7519(97)00189-6. [DOI] [PubMed] [Google Scholar]
- 7.Bordes F, Morand S. Parasite diversity: an overlooked metric of parasite pressures? Oikos. 2009;118:801–6. [Google Scholar]
- 8.de Meeûs T, Renaud F. Parasites within the new phylogeny of eukaryotes. Trends Par. 2002;18:247–51. doi: 10.1016/s1471-4922(02)02269-9. [DOI] [PubMed] [Google Scholar]
- 9.Nunn C, Altizer S, Jones KE, Sechrest W. Comparative tests of parasite species richness in primates. Am Nat. 2003;162:597–614. doi: 10.1086/378721. [DOI] [PubMed] [Google Scholar]
- 10.Poulin R, Morand S. Parasite biodiversity. Washington: Smithsonian Institution Press; 2004. [Google Scholar]
- 11.Krasnov BR, Korallo-Vinarskaya NP, Vinarsky M-V, Shenbrot GI, Mouillot D, Poulin R. Searching for general patterns in parasite ecology: host identity versus environmental influence on gamasid mite assemblages in small mammals. Parasitology. 2008;135:229–42. doi: 10.1017/S003118200700368X. [DOI] [PubMed] [Google Scholar]
- 12.Bordes F, Morand S. Helminth species diversity of mammals: parasite species richness is a host species attribute. Parasitology. 2008;135:1701–5. doi: 10.1017/S0031182008005040. [DOI] [PubMed] [Google Scholar]
- 13.Poulin R. Are they general laws in parasite ecology? Parasitology. 2007;134:763–76. doi: 10.1017/S0031182006002150. [DOI] [PubMed] [Google Scholar]
- 14.Guégan J-F, Morand S, Poulin R. Are there general laws in parasite community ecology? The emergence of spatial parasitology and epidemiology. In: Thomas F, Guégan J-F, Renaud F, editors. Parasitism and ecosystems. Oxford: Oxford University Press; 2004. pp. 22–42. [Google Scholar]
- 15.Morand S, Krasnov BR. Why applying ecology law to epidemiology? Trends Par. 2008;24:304–9. doi: 10.1016/j.pt.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Cox FEG. Concomitant infections, parasites and immune responses. Parasitology. 2001;122:23–38. doi: 10.1017/s003118200001698x. [DOI] [PubMed] [Google Scholar]
- 17.Graham AL. Ecological rules governing helminth-microparasite coinfection. PNAS. 2008;105:566–70. doi: 10.1073/pnas.0707221105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behnke JM, Eira C, Rogan M, Gilbert FS, Torres J, Miquel J, Lewis JW. Helminth species richness in wild wood mice, Apodemus sylvaticus, is enhanced by the presence of the intestinal nematode Heligmosomoides polygyrus. Parasitology. 2009;136:793–804. doi: 10.1017/S0031182009006039. [DOI] [PubMed] [Google Scholar]
- 19.Fenton A, Viney ME, Lello J. Detecting interspecific macroparasites interactions from ecological data: patterns and process. Ecol Let. 2010;13:606–15. doi: 10.1111/j.1461-0248.2010.01458.x. [DOI] [PubMed] [Google Scholar]
- 20.Telfer S, Lambin X, Birtles R, Beldomenico P, Burthe S, Paterson S, Begon M. Species interactions in a parasite community drive infection risk in a wildlife population. Science. 2010;330:243–46. doi: 10.1126/science.1190333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gause WC, Urban JF, Stadecker MJ. The immune responses to parasitic helminths: insights from murine models. Trends Immunol. 2003;24:269–77. doi: 10.1016/s1471-4906(03)00101-7. [DOI] [PubMed] [Google Scholar]
- 22.Benwitch Z, Kalinkovich A, Weisman Z, Borkow G, Beyers N, Beyers AD. Can eradication of helminthic infections change the face of AIDS and Tuberculosis? Immunol Today. 1999;20:485–87. doi: 10.1016/s0167-5699(99)01499-1. [DOI] [PubMed] [Google Scholar]
- 23.Druilhe P, Tall A, Sokhna C. Worms can worsen malaria: towards a new means to roll back malaria? Trends Parasitol. 2005;21:359–62. doi: 10.1016/j.pt.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Sangweme D, Shiff C, Kumar N. Plasmodium yoelii: Adverse outcome of non-lethal P. yoelii malaria during co-infection with Schistosoma mansoni in BALB/c mouse model. Exp Parasotol. 2009;122:254–59. doi: 10.1016/j.exppara.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamya MR, Gasasira AF, Yeka A, Bakyaita N, Nsobyra SL, Francis D, et al. Effects of HIV-1 infection on antimalarial treatment outcomes in Uganda: apopulation-based study. J Inf Dis. 2006;193:9–15. doi: 10.1086/498577. [DOI] [PubMed] [Google Scholar]
- 26.Otieno RO, Ouma C, Ong'echan JM, Keller CC, Were T, Waindia EN, et al. Increased severe anemia in HIV-1-exposed and HIV-1-positive infants and children during acute malaria. AIDS. 2006;20:275–80. doi: 10.1097/01.aids.0000200533.56490.b7. [DOI] [PubMed] [Google Scholar]
- 27.Ezeamama AE, Mc Garvey S, Acosta LP, Zierler S, Manalo DL, Wu HW, et al. The synergistic effects of concomitant schistosomiasis, hookworm and Trichuris infections on children's anaemia burden. PLOS Neglect Trop Dis. 2008;2:1–5. doi: 10.1371/journal.pntd.0000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mosquera J, Adler FR. Evolution of virulence: a unified framework for coinfections and superinfection. J Theor Biol. 1998;195:293–313. doi: 10.1006/jtbi.1998.0793. [DOI] [PubMed] [Google Scholar]
- 29.Taylor LH, Mackinnon MJ, Read AF. Virulence of mixed-clone and single-clone infections of the rodent malaria Plasmodium Chabaudi. Evolution. 1998;52:583–91. doi: 10.1111/j.1558-5646.1998.tb01656.x. [DOI] [PubMed] [Google Scholar]
- 30.Schjorring S, Koella JC. Sub-lethal effects of pathogens can lead to the evolution of lower virulence in multiple infections. Proc R Soc Lond B. 2003;270:189–93. doi: 10.1098/rspb.2002.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ben-Ami F, Mouton L, Ebert D. The effects of multiple infections on the expression and evolution of virulence in a Daphnia-endoparasite system. Evolution. 2008;62:1700–11. doi: 10.1111/j.1558-5646.2008.00391.x. [DOI] [PubMed] [Google Scholar]
- 32.Davies CM, Fairbrother E, Webster JP. Mixed strain schistosome infections of snails and the evolution of parasite virulence. Parasitology. 2002;124:31–8. doi: 10.1017/s0031182001008873. [DOI] [PubMed] [Google Scholar]
- 33.Holmstad PR, Jensen KH, Skorping A. Ectoparasite intensities are correlated with endoparasite infection loads in willow ptarmigan. Oikos. 2008;117:515–20. [Google Scholar]
- 34.Graham AL, Cattadori IM, Lloyd-Smith JO, Bjørnstad ON. Transmission consequences of coinfection: cytokines write large? Trends in Parasitol. 2007;23:284–91. doi: 10.1016/j.pt.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 35.Ganz T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood. 2003;102:783–88. doi: 10.1182/blood-2003-03-0672. [DOI] [PubMed] [Google Scholar]
- 36.Mahmoud AA, Woodruff AW. Mechanisms involved in the anaemia of schistosomiasis. Trans R Soc Trop Med Hyg. 1972;66:75–84. doi: 10.1016/0035-9203(72)90055-7. [DOI] [PubMed] [Google Scholar]
- 37.Babatunde OA, Clarkson AB, Shear HL. Pathogenesis of anemia in Trypanosoma brucei-infected mice. Inf Immun. 1972;36:1060–68. doi: 10.1128/iai.36.3.1060-1068.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Graham AL, Allen JE, Read A. Evolutionary causes and consequences of immunopathology. Annu Rev Ecol Evol Syst. 2005;36:373–97. [Google Scholar]
- 39.Graham AL. When T-helpers cells don't help: immunopathology during concomitant infections. Quart Rev Biol. 2002;77:409–54. doi: 10.1086/344414. [DOI] [PubMed] [Google Scholar]
- 40.Graham AL, Allen JE, Read A. Malaria-filaria coïnfection in mice makes malaria more severe unless filarial infection achieves patency. J Infect Dis. 2005;191:410–21. doi: 10.1086/426871. [DOI] [PubMed] [Google Scholar]
- 41.Graham AL, Hayward AD, Watt KA, Pilkington JG, Pemberton JM, Nussey DH. Fitness correlates of heritable variation in antibody responsiveness in a wild mammal. Science. 2010;330:662–65. doi: 10.1126/science.1194878. [DOI] [PubMed] [Google Scholar]
- 42.Walsh PK, Brady MT, Finlay CM, Boon L, Kingston H, Mills G. Infection with a helminth parasite attenuates autoimmunity through TGF--mediated suppression of Th17 and Th1 responses. J Immunol. 2009;183:1577–86. doi: 10.4049/jimmunol.0803803. [DOI] [PubMed] [Google Scholar]
- 43.Maizels RM, Balic A, Gomez-Escobar N, Nair M, Taylor MD, Allen JE. Helminth parasites-masters of regulation. Imm Rev. 2004;201:89–116. doi: 10.1111/j.0105-2896.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- 44.Holmstad PR, Hudson PJ, Skorping A. The influence of a parasite community of a host population: a longitudinal study on willow ptarmigan and their parasites. Oikos. 2005;111:377–91. [Google Scholar]
- 45.Davidar P, Morton ES. Are multiple infections more severe for purple martins than single infections? Auk. 2006;123:141–47. [Google Scholar]
- 46.del Cerro S, Merino S, Martinez de la Puente J, Lobato E, Ruiz de Castaneda R, Rivero de Aguilar J, et al. Carotenoid-based plumage colouration is associated with blood parasite richness and stress protein levels in blue tits (Cyanistes caerulus) Oecologia. 2010;162:825–35. doi: 10.1007/s00442-009-1510-y. [DOI] [PubMed] [Google Scholar]
- 47.Lello J, Boag B, Hudson PJ. The effects of single and concomitant infections on condition and fecundity of the wild rabbits (Oryctolagus cuniculus) Int J Parasitol. 2005;35:1509–15. doi: 10.1016/j.ijpara.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 48.Jolly D, Messier F. The effect of bovine tuberculosis and brucellosis on reproduction and survival of wood bison in Wood Buffalo National Park. J Anim Ecol. 2005;74:543–51. [Google Scholar]
- 49.Jolles AE, Ezenwa V, Etienne RS, Turner WC, Olff H. Interactions between macroparasites and microparasites drive infection patterns in free -ranging African buffalo. Ecology. 2008;89:2239–50. doi: 10.1890/07-0995.1. [DOI] [PubMed] [Google Scholar]
- 50.Munson L, Terio K, Kock R, Mlengeya T, Roelke ME, Dubovi E, et al. Climate extremes promote fatal co-infections during Canine distemper Epidemics in African Lions. Plos One. 2008;3:1–6. doi: 10.1371/journal.pone.0002545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ezenwa VO, Etienne RS, Luikart G, Beja-Pereira A, Jolles AE. Hidden consequences of living in a wormy world: nematode-induced immune suppression facilitates tuberculosis invasion in African Buffalo. Am Nat. 2010;176:613–24. doi: 10.1086/656496. [DOI] [PubMed] [Google Scholar]
- 52.Alzaga V, Vicente J, Villanua D, Acevedo P, Casas F, Gortazar C. Body condition and parasite intensity correlates with escape capacity in Iberian hares. Behav Ecol Soc. 2008;62:769–75. [Google Scholar]
- 53.Harms G, Feldmeier H. HIV coinfection and tropical parasitic diseases-deleterious interactions in both directions? Trop Med Int Health. 2002;7:479–88. doi: 10.1046/j.1365-3156.2002.00893.x. [DOI] [PubMed] [Google Scholar]
- 54.Sokna C, Le Hesran JY, Mbaye PA, Akiana J, Camara P, Diop M. Increase of malaria attacks among children presenting concomitant infections by Schistosoma mansoni in Senegal. Malaria J. 2004;117:597–610. doi: 10.1186/1475-2875-3-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wegner KM, Reusch TBH, Kalbe M. Multiple parasites are driving Major Histocompatibility Complex polymorphism in the wild. J Evol Biol. 2003;16:224–32. doi: 10.1046/j.1420-9101.2003.00519.x. [DOI] [PubMed] [Google Scholar]
- 56.Sommer S. The importance of immune gene variability (MHC) in evolutionary ecology and conservation. Front Zool. 2005;2:16. doi: 10.1186/1742-9994-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prugnolle F, Manica F, Charpentier M, Guégan J-F, Guernier V, Balloux F. Pathogen-driven selection and worldwide HLA class I diversity. Current Biol. 2005;15:1022–27. doi: 10.1016/j.cub.2005.04.050. [DOI] [PubMed] [Google Scholar]
- 58.Goüy de Bellocq J, Charbonnel N, Morand S. Coevolutionary relationship between helminth diversity and MHC class II polymorphism in rodents. J Evol Biol. 2008;21:1144–50. doi: 10.1111/j.1420-9101.2008.01538.x. [DOI] [PubMed] [Google Scholar]
- 59.Tollenaere C, Bryja J, Galan M, Cadet P, Deter J, Chaval Y, et al. Multiple parasites mediate balancing selection at two MHC class II genes in the fossorial water vole: insights from multivariate analyses and population genetics. J Evol Biol. 2008;21:1307–20. doi: 10.1111/j.1420-9101.2008.01563.x. [DOI] [PubMed] [Google Scholar]
- 60.Meyer-Lucht Y, Otten C, Püttker T, Pardini R, Metzger JP, Sommer S. Variety matters: adaptive genetic diversity and parasitic load in two mouse opossums from the Brazilian Atlantic coast. Conserv Genet. 2010;11:2001–13. [Google Scholar]
- 61.šimková A, Ottovà E, Morand S. MHC variability, life traits and parasite diversity of European Cyprinid fish. Evol Ecol. 2006;20:465–67. [Google Scholar]
- 62.Dionne M, Miller KM, Dodson JJ, Caron F, Bernatchez L. Clinal variation in MHC diversity with temperature: evidence for the role of host-pathogens interaction on local adaptation in Atlantic salmon. Evolution. 2007;61:2154–64. doi: 10.1111/j.1558-5646.2007.00178.x. [DOI] [PubMed] [Google Scholar]
- 63.Harf R, Sommer S. Association between major histocompatibility complex class II DRB alleles and parasite load in the hairy-footed gerbil, Gerbillurus paeba, in the southern Kalahari. Mol Ecol. 2005;14:85–91. doi: 10.1111/j.1365-294X.2004.02402.x. [DOI] [PubMed] [Google Scholar]
- 64.Meyer-Lucht Y, Sommer S. MHC diversity and the association to nematode parasitism in the yellow-necked mouse (Apodemus flavicollis) Mol Ecol. 2005;14:2233–43. doi: 10.1111/j.1365-294X.2005.02557.x. [DOI] [PubMed] [Google Scholar]
- 65.Lenz T, Wells K, Pfeiffer M, Sommer S. Diverse MHC IIB allele repertoire increases parasite resistance and body condition in the Long-tailed (Leopoldamys sabanus) BMC Evol Biol. 2009;9:269. doi: 10.1186/1471-2148-9-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wegner KM, Kalbe M, Kurtz J, Teusch TBH, Milinski M. Parasite selection for immunogenetic optimality. Science. 2003;301:1343. doi: 10.1126/science.1088293. [DOI] [PubMed] [Google Scholar]
- 67.Rolff J, Siva-Jothy MT. Invertebrate Ecological Immunity. Science. 2003;301:473–75. doi: 10.1126/science.1080623. [DOI] [PubMed] [Google Scholar]
- 68.Wegner KM, Kalbe M, Milinski M, Reusch TBH. Mortality selection during the 2003 European heat wave in three-spined sticklebacks: effects of parasites and MHC genotype. BMC Evol Biol. 2008;8:124. doi: 10.1186/1471-2148-8-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kloch A, Babik W, Bajer A, Sinski E, Radwan J. Effects of an MHC-DRB genotype and allele number on the load of gut parasites in the bank voles Myodes glareolus . Mol Ecol. 2010;19:255–65. doi: 10.1111/j.1365-294X.2009.04476.x. [DOI] [PubMed] [Google Scholar]
- 70.Stjernman M, Raberg L, Nilsson JA. Maximum host survival at intermediate parasite infection intensities. Plos One. 2008;3:1–3. doi: 10.1371/journal.pone.0002463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hill AV, Allsopp CE, Kwiatkowski D, Anstey NM, Twumasi P, Rowe PA, et al. Common West African HLA antigens are associated with protection from severe malaria. Nature. 1991;352:595–600. doi: 10.1038/352595a0. [DOI] [PubMed] [Google Scholar]
- 72.Hill AV. The genomics and genetics of human infectious diseases susceptibility. Annu Rev Genomics Hum Genet. 2001;2:373–400. doi: 10.1146/annurev.genom.2.1.373. [DOI] [PubMed] [Google Scholar]
- 73.Paterson S, Wilson K, Pemberton JM. Major histocompatibility complex variation associated with juvenile survival and parasite resistance in a large unmanaged ungulate population. PNAS USA. 1998;95:3714–19. doi: 10.1073/pnas.95.7.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schwensow N, Dausmann K, Eberle M, Fietz J, Sommer S. Functional associations of similar MHC alleles and shared parasite species in two sympatric lemurs. Inf Gen Evol. 2010;10:662–68. doi: 10.1016/j.meegid.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 75.Oppelt C, Starkloff A, Rausch P, Von Holst D, Rödel H. Major histocompatibility complex variation and age-specific endoparasite load in subadult European rabbits. Mol Ecol. 2010;19:4155–67. doi: 10.1111/j.1365-294X.2010.04766.x. [DOI] [PubMed] [Google Scholar]
- 76.Oliver MK, Telfer S, Piertney SB. Major histocompatibility complex (MHC) heterozygote superiority to natural multi-parasite infections in the water vole (Arvicola terrestris) Proc R Soc Lond B. 2009;276:1119–28. doi: 10.1098/rspb.2008.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Radwan J, Biedrzycka A, Babik W. Does reduced MHC diversity decrease viability of vertebrate populations? Biol Cons. 2009;143:537–44. doi: 10.1016/j.biocon.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smith S, Mang T, Goüy de Bellocq J, Schaschl H, Zeiotlhofer C, Hackländer K, Suchentrunk F. Homozygoty at a class II MHC locus depresses female reproductivity ability in European brown hares. Mol Ecol. 2010;19:4131–43. doi: 10.1111/j.1365-294X.2010.04765.x. [DOI] [PubMed] [Google Scholar]
- 79.Magnanou E, Fons R, Feliu C, Morand S. Physiological responses of insular wild black rat (Rattus rattus) to natural infection by the digenean trematode Fasciola hepatica. Parasitol Res. 2005;99:97–101. doi: 10.1007/s00436-005-0063-1. [DOI] [PubMed] [Google Scholar]
- 80.Khokhlova IS, Krasnov BR, Kam M, Burdelova NI, Degen AA. Energy cost of ectoparasitism: the flea Xenopsylla ramesis on the desert gerbil Gerbillus dasyurus. J Zool Lond. 2002;258:349–54. [Google Scholar]
- 81.Devevey G, Niculita-Herzel H, Biollaz F, Yvon C, Chapuisat M, Christe P. Developmental, metabolic and immunological cost of flea infestation in the common vole. Funct Ecol. 2008;22:1091–98. [Google Scholar]
- 82.Demas GE. Metabolic costs of mounting an antigen-stimulated immune response in adult and aged C57BL/6J mice. Am J Physiol. 1997;273:1631–37. doi: 10.1152/ajpregu.1997.273.5.R1631. [DOI] [PubMed] [Google Scholar]
- 83.Martin LB, Scheuerlein A, Wikelski M. Immune activity elevates energy expenditure of house sparrows: a link between direct and indirect costs? Proc R Soc Lond B. 2003;270:153–58. doi: 10.1098/rspb.2002.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.White CR, Seymour RS. Does Basal Metabolic Rate contain a useful signal? Mammalian BMR allometry and correlation with a selection of physiological, ecological and life-history variables. Physiol Bioch Zool. 2004;77:929–41. doi: 10.1086/425186. [DOI] [PubMed] [Google Scholar]
- 85.Morand S, Harvey PH. Mammalian metabolism, longevity and parasite species richness. Proc R Soc Lond B. 2000;267:1999–2003. doi: 10.1098/rspb.2000.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Korallo N, Vinarski MV, Krasnov BR, Shenbrot GI, Mouillot D, Poulin R. Are there general rules governing parasite diversity? Small mammalian hosts and gamasid mite assemblages. Div Distr. 2007;13:353–60. [Google Scholar]
- 87.Preston BT, Capellini I, McNamara P, Barton RA, Nunn CL. Parasite resistance and the adaptative significance of sleep. BMC Evol Biol. 2009;9:1–9. doi: 10.1186/1471-2148-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bordes F, Morand S. Coevolution between helminth diversity and basal immune investment in mammals: cumulative effects of polyparasitism? Parasitol Res. 2009;106:33–37. doi: 10.1007/s00436-009-1623-6. [DOI] [PubMed] [Google Scholar]
- 89.Møller AP, Merino S, Brown CR, Robertson RJ. Immune defence and host sociality: a comparative study of swallows and martins. Am Nat. 2001;152:136–45. doi: 10.1086/321308. [DOI] [PubMed] [Google Scholar]
- 90.šimková A, Lafond T, Ondrackova M, Juralda P, Ottovà E, Morand S. Parasitism, life history traits and immune defence in cyprinid fish from Central Europe. BMC Evol Biol. 2008;8:1–11. doi: 10.1186/1471-2148-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Neuhaus P. Parasite removal and its impact on litter size and body condition in Columbian ground squirrels (Spermophilus columbianus) Biol Lett. 2003;270:213–15. doi: 10.1098/rsbl.2003.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Knowles SCL, Palinauskas V, Sheldon BC. Chronic malaria infections increase family inequalities and reduce parental fitness: experimental evidence from a wild bird population. J Evol Biol. 2009;29:57–569. doi: 10.1111/j.1420-9101.2009.01920.x. [DOI] [PubMed] [Google Scholar]
- 93.Arrierero E, Møller A. Host ecology and life history traits associated with blood parasite species richness in birds. J Evol Biol. 2008;21:1504–13. doi: 10.1111/j.1420-9101.2008.01613.x. [DOI] [PubMed] [Google Scholar]
- 94.Taylor WA, Boomker J, Krecek RC, Skinner JD, Watermeyer R. Helminths in sympatric populations of mountain reedbuck (Redunca fulvorufula) and gray Rhebok (Pelea capreolus) in South Africa. J Parasitol. 2005;91:863–70. doi: 10.1645/GE-436R.1. [DOI] [PubMed] [Google Scholar]
- 95.Kilpatrick AM, Altizer S. Disease Ecology. Nature Education Knowledge. 2010;1:13. [Google Scholar]
- 96.Lloyd-Smith JO, Schreiber SJ, Kopp PE, Getz WM. Super-spreading and the effect of individual variation on disease emergence. Nature. 2005;438:355. doi: 10.1038/nature04153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li YG, Yu ITS, Xu PC, Lee JHW, Wong TW, Ooi PL, et al. Predicting super spreading events during the 2003 severe acute respiratory syndrome epidemics in Hong Kong and Singapore. Am J Epidemiol. 2004;160:719–28. doi: 10.1093/aje/kwh273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ferrari N, Cattadori IM, Nespereira J, Rizzoli A, Hudson P. The role of host sex in parasite dynamics: field experiments on the yellow- necked mouse Apodemus flavicollis . Ecol Let. 2004;7:88–94. [Google Scholar]
- 99.Keesing F, Belden LK, Daszak P, Dobson A, Harvell CD, Holt RD, et al. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature. 2010;468:647–52. doi: 10.1038/nature09575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Råberg L, Graham AL, Read AF. Decomposing health: tolerance and resistance to parasites in animals. Phil Trans Roy Soc Lond B. 2009;364:37–49. doi: 10.1098/rstb.2008.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Råberg L, Sim D, Read AF. Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science. 2007;318:812–14. doi: 10.1126/science.1148526. [DOI] [PubMed] [Google Scholar]
- 102.Nakamura S, Yang C-S, Sakon N, Ueda M, Tougan T, Yamashita A, et al. Direct metagenomic detection of viral pathogens in nasal and fecal specimens using an unbiased high-throughput sequencing approach. PLoS ONE. 2009;4:e4219. doi: 10.1371/journal.pone.0004219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Morand S, Poulin R. Density, body mass and parasite species richness of terrestrial mammals. Evol Ecol. 1998;12:717–27. [Google Scholar]
- 104.Arneberg P. Host population density and body mass as determinants of species richness in parasite communities: comparative analyses of directly transmitted nematodes of mammals. Ecography. 2002;25:88–94. [Google Scholar]
- 105.Stanko M, Miklisová D, Goüy de Bellocq J, Morand S. Mammal density and patterns of ectoparasite species richness and abundance. Oecologia. 2002;131:289–95. doi: 10.1007/s00442-002-0889-5. [DOI] [PubMed] [Google Scholar]
- 106.Torres J, Miquel J, Casanova JC, Ribas A, Feliu C, Morand S. Parasite species richness of Iberian carnivores: influences of host density and range distribution. Biodiv Cons. 2006;15:4619–32. [Google Scholar]
- 107.Lindenfors P, Nunn CL, Jones KE, Cunningham AA, Sechrest W, Gittleman JL. Parasite species richness in carnivores: effects of host body mass, latitude, geographical range and population density. Glob Ecol Biogeo. 2007;1:1–14. [Google Scholar]
- 108.Feliu C, Renaud F, Catzeflis F, Durand P, Hugot J-P, Morand S. A comparative analysis of parasite species richness of Iberian rodents. Parasitology. 1997;115:453–66. doi: 10.1017/s0031182097001479. [DOI] [PubMed] [Google Scholar]
- 109.Krasnov BR, Shenbrot GI, Khokhlova I, Degen AA. Flea species richness and parameters of host body, host geography and host ‘milieu’. J Anim Ecol. 2004;73:1121–28. [Google Scholar]
- 110.Lohm J, Grahn M, Langefors Å, Andersen Ø, Storset A, von Schantz T. Experimental evidence for major histocompatibility complex allele-specific resistance to a bacterial infection. Proc R Soc Lond B. 2002;269:2029–33. doi: 10.1098/rspb.2002.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]