Abstract
The view on enterococci has over the years shifted from harmless commensals to opportunistic but important pathogens mainly causing nosocomial infections. One important part of this development is the emergence of vancomycin resistance enterococci (VRE). The term VRE includes several combinations of bacterial species and resistance genes of which the most clinically important is Enterococcus faecium with vanA type vancomycin resistance. This variant is also the most common VRE among farm animals. The reason for VRE being present among farm animals is selection by extensive use of the vancomycin analog avoparcin for growth promotion. Once the use of avoparcin was discontinued, the prevalence of VRE among farm animals decreased. However, VRE are still present among farm animals and by spread via food products they could potentially have a negative impact on public health. This review is based on the PhD thesis Vancomycin Resistant Enterococci in Swedish Broilers – Emergence, Epidemiology and Elimination and makes a short summary of VRE in humans and food producing animals. The specific situation regarding VRE in Swedish broiler production is also mentioned.
Keywords: VRE, epidemiology, vancomycin, vanA, Enterococcus faecium, gene transfer
Vancomycin resistant enterococci
The term vancomycin resistant enterococci (VRE) includes several combinations of bacterial species and resistance genes. Some of which are highly important as pathogens and reservoirs of antimicrobial resistance genes, others where the resistance is intrinsic and a part of that species characteristics. To bring order in the complexity, each part of the term VRE is dealt with and elucidated separately.
Vancomycin
Vancomycin is a glycopeptide antimicrobial produced by the soil bacteria Streptomyces orientalis (1). It was developed and introduced in the 1950s (2). Glycopeptides interfere with the cell wall production resulting in a destabilized cell wall and lysis of the bacteria (2). When the bacterial cell wall is synthesized, polysaccharide-pentapeptide complexes are linked together via a transpeptidation reaction in which the end amino acid of the pentapeptide is removed (2). Glycopeptides interfere with this process by binding tightly to the D-Alanyl-D-Alanin (D-Ala-D-Ala) end of the pentapeptide and hiding it from the transpeptidase that is to catalyse the cross-linking in the peptidoglycan synthesis (2).
Vancomycin is active against most Gram positive bacteria whereas the majority of Gram negatives are resistant (3, 4). It is considered a drug of ‘last resort’ and has been classified as critically important for human medicine for treatment of patients with severe infections with multi-drug resistant Enterococcus spp. and meticillin resistant Staphylococcus aureus (MRSA) as the main indications (5). Vancomycin is also used for intestinal infections, especially pseudomembranous colitis caused by Clostridium difficile where the poor absorption of vancomycin when administered orally is advantageous (6).
Enterococci
Enterococci are intestinal bacteria colonizing humans and other mammals as well as birds, reptiles and insects (7–9). They are Gram positive, facultative anaerobes, catalase negative and non sporeforming cocci occurring either as single bacteria, in pairs or in short chains (10, 11). They can sustain various adverse conditions and can survive for several months in the environment (12, 13).
Until 1984 enterococci were considered a part of the genus Streptococcus, even though they were first described and tentatively named enterococci (entérocoque) in 1899 (11, 14). Today, 40 different species of enterococci have been described (15). The species most frequent in the intestines of humans are Enterococcus faecalis, and to a lesser extent E. faecium whereas the most common species in various farm animals are E. faecium together with E. cecorum, E. faecalis, and to some extent E. hirae (3, 16, 17).
Even though the first description in 1899 referred to enterococci as potential pathogens they were for a long time regarded as harmless intestinal bacteria without clinical importance (10, 14). Nowadays however, enterococci are recognised as important opportunistic pathogens, especially causing nosocomial infections such as urinary tract infections, wound infections and endocarditis (10). The clinically most important species in human medicine are E. faecalis and E. faecium (18). Of these, E. faecalis is the most pathogenic species but E. faecium is of increasing importance as it is generally more frequently resistant to antimicrobials (3).
Enterococcal resistance to vancomycin
Until today, nine different variants of vancomycin resistance in enterococci have been described (vanA, B, C, D, E, G, L, M and N; Table 1) (19–22). Among those, the three most common variants are the vanA, B and C types with E. faecium carrying the vanA genotype as the most common combination (10, 18). An additional variant (vanF) has also been described but thus far only in Paenibacillus popilliae (23). Since the vanF variant has a high similarity in amino acid sequences to the vanA variant, P. popilliae has been suggested as a possible origin for vancomycin resistance in enterococci (23). Other plausible sources are various glycopeptide producing organisms, even if genetic differences make an older common source more likely (24).
Table 1.
Characteristics of different types of vancomycin resistance described among Enterococcus spp
| Range of MIC (mg/L) | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Sort | Modified target | Vancomycin | Teicoplanin | Expression | Location | Transferable | |
| vanA | Acquired | D-Ala-D-Lac | 64–1000 | 16–512 | Inducible | Chromosome or plasmid | Yes |
| vanB | Acquired | D-Ala-D-Lac | 4–1000 | 0.5–1 | Inducible | Chromosome or plasmid | Yes |
| vanC | Intrinsic | D-Ala-D-Ser | 2–32 | 0.5–1 | Constitutive or inducible | Chromosome | No |
| vanD | Acquired | D-Ala-D-Lac | 64–128 | 4–64 | Constitutive or inducible | Chromosome | No |
| vanE | Acquired | D-Ala-D-Ser | (6) 8–32 | 0.5 | Inducible | Chromosome | No |
| vanG | Acquired | D-Ala-D-Ser | 16 | 0.5 | Inducible | Chromosome | Yes |
| vanL | Acquired | D-Ala-D-Ser | 8 | <8 | Inducible | Chromosome | No |
| vanM | Unknown | D-Ala-D-Lac | >128 | 64 to >256 | Inducible | Unknown | Yes |
| vanN | Acquired | D-Ala-D-Ser | 16 | 0.5 | Constitutive | Plasmid | Yes |
Common to all variants of vancomycin resistance in enterococci is the ability to cause a change in the structure of the pentapeptide incorporated in the three dimensional web of peptidoglycans composing the bacterial cell wall: from the original D-Ala-D-Ala to either D-Ala-D-Lactate (D-Ala-D-Lac) or D-Ala-D-Serine (D-Ala-D-Ser) (20). This shift results in a reduced affinity for vancomycin by 1000 and seven times respectively (10).
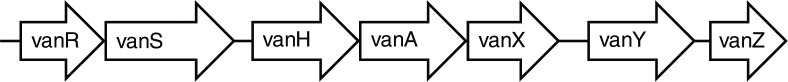
In all different variants of vancomycin resistance are several genes involved in the alteration of the cell wall structure which results in the resistance. The number and organisation of these genes are somewhat similar among the different variants. For the vanA variant, the genes are organized as in Fig. 1. vanS is a sensor gene which in the presence of a glycopeptide phosphorylate, and thus activate the regulator gene vanR (20). After activation of the gene complex, vanH mediates production of lactate from pyruvate which vanA uses to synthesize the alternative D-Ala-D-Lac end of the pentapetide (3). It is essential for resistance that production of the normal D-Ala-D-Ala end of the pentapetide does not continue. This is resolved by the vanX and vanY genes where vanX hydrolyzes and thereby interrupts the production of the pentapeptides, and vanY cleaves the pentapeptides that might still be produced (3, 25). In the absence of a glycopeptide, vanS initiates dephosphorylation of vanR resulting in deactivation of the gene (20).The function of the vanZ gene is not understood (20).
Fig. 1.
Organization of the genes involved in the vanA variant of vancomycin resistance in enterococci. Adapted from Courvalin, 2006 (20).
Clinical impact of VRE
The first cases of infection with vancomycin resistant entrerococci were seen in 1986 (26, 27). Since then, VRE have spread around the world and are now one of the most important causes of nosocomial infections, even if asymptomatic intestinal colonization is much more common than morbidity (24). In most parts of the world, VRE of the vanA variant are the most prevalent among human cases (18). But in some countries (e.g. Australia and Sweden) the majority of human cases are of the vanB type (28–31).
When infections with VRE do occur, the consequences are often worse compared to infections with vancomycin susceptible enterococci. Associations have been seen between infections with VRE and increases in therapy failure, length of hospital stay as well as mortality (24, 32). However, studies where no significant difference is seen also exist (33).
VRE in farm animals
Feeding animals low doses of antimicrobials may in certain conditions increase their productivity, for example by improving feed conversion and decreasing morbidity and mortality caused by clinical and subclinical infections (34). The growth promoting effect of antimicrobials was first discovered when broiler chickens were fed the fermentation leftovers after production of antimicrobials (35, 36). Notable is that all use of growth promoters in Sweden was forbidden in 1986 by the Feedingstuffs Act (SFS 1985:295).
The glycopeptide avoparcin was first introduced for growth promotion in 1975 (37). At that time it was used extensively in most parts of Europe and the rest of the world with the notable exception of Canada and USA where avoparcin never has been approved for animals (38). Avoparcin was mainly used for broilers and pigs but to some extent also for turkeys, veal calves and other animals (37, 39, 40). The extent of avoparcin use is demonstrated by data from Denmark where 24 kg of vancomycin was used in human medicine in 1994, and in the same year more than 24,000 kg of avoparcin was used for growth promotion (39). A similar example is Australia, where from 1992 to 1996 less than 600 kg of vancomycin but over 62,000 kg of avoparcin was imported (41).
As avoparcin confers cross-resistance to vancomycin the (mis)use of avoparcin selected for VRE (42). Hence, VRE, i.e. E. faecium carrying the vanA genotype was common in the intestinal flora of farm animals in Europe during the 1990s (43, 44). By contrast, since avoparcin has never been approved in Canada and USA, VRE had until 2008 never been isolated from farm animals in USA (45).
When the connection between avoparcin and VRE in farm animals was confirmed, the use of avoparcin was discontinued as a precautionary measure to avoid further spread of VRE to the community and into hospital settings (46). Apart from Sweden, the use of avoparcin in Europe ceased first in Denmark, Finland and Norway (47). Later it ceased in Germany and finally in the whole of the European Union as a consequence of the Commission Directive 97/6/EC (46). Avoparcin has later been banned or phased out also in other parts of the world (37, 48, 49). Once the use of avoparcin had been discontinued, the prevalence of VRE in farm animals rapidly declined (50–52).
The decreased occurrence of VRE in animals after the use of avoparcin was discontinued reinforces the theory that if the selective pressure is removed, the antimicrobial resistance will disappear (53, 54). However, when using selective media (i.e. media with vancomycin) VRE could and can still be readily detected in samples from farm animals (55–61). Furthermore, a recent study modelling persistence of VRE indicates that it will be present among farm animals for a long period of time which is also in agreement with today's view on the timeframe of reversal of antimicrobial resistance (54, 62).
Different theories about why VRE persist among farm animals have been presented. In Denmark, the use of the macrolide tylosin in pigs was suggested to co-select for vancomycin resistance among enterococci since the genes encoding the two resistances were located on the same plasmid (63). A similar but weaker correlation with co-selection by copper resistance has also been suggested (64). Another explanation that has been suggested is that plasmid addiction systems located on the same plasmid as the vanA gene would force the bacteria to retain the resistance (65).
The Swedish paradox
In Sweden, avoparcin was only used for a short period of time from the end of the 1970s until 1984 and the yearly usage by the end of that period was between 7,000 and 9,000 kg (66). Furthermore, all use of growth promoters in Sweden was forbidden in 1986 by the Feedingstuffs Act (SFS 1985:295).
In accordance with the low selective pressure for vancomycin resistance by avoparcin use that enterococci in Swedish farm animals had been exposed to, VRE were not isolated in samples from Swedish broilers or pigs in the middle of the 1990s (67, 68). However, in a study conducted 1998 to 2000 the first VRE from Swedish farm animals were isolated (69). In the following years, the occurrence of VRE increased and in samples analysed within the Swedish Veterinary Antimicrobial Resistance Monitoring programme (SVARM), the proportion of broilers colonized with VRE increased from less than 1% in 2000 to over 40% in 2005 (70). In addition, this increase was caused by the spread of one clone of E. faecium with the vanA gene (70). It is however important to keep in mind that the increase is observed only when samples are cultured on selective media, i.e. on agar containing vancomycin, indicating that the proportion of enterococci that are vancomycin resistant is low (70).
The reason(s) for the increased occurrence of VRE in Swedish broiler production is unknown. For example, plasmid addiction systems do not seem to be common among VRE from Swedish broilers and hence are probably not an important factor in the epidemiology (71). However, it has been shown that both the level of VRE contamination and the proportion of broilers colonized with VRE differ among farms (72). This was taken as an indication that if the factor(s) causing these differences were identified, it might be possible to reduce the occurrence of VRE among Swedish broilers. Unpublished results also indicate that altered disinfection routines might be a way forward to achieve this.
VRE as a foodborne zoonosis
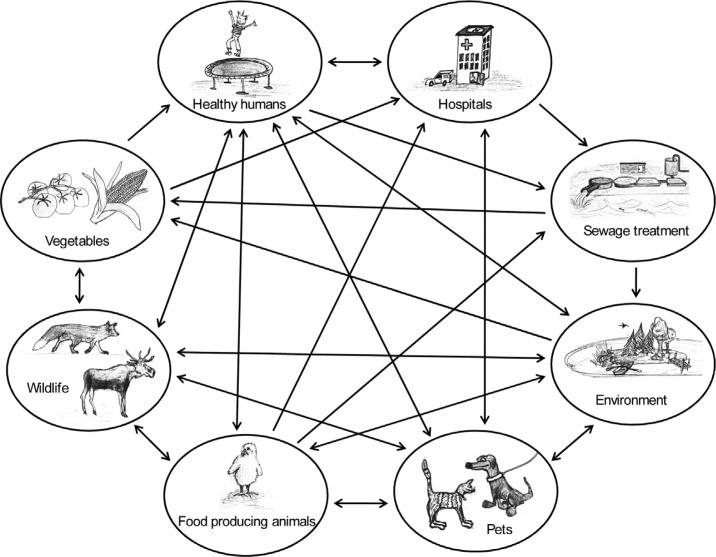
The World Health Organisation defines a zoonosis as ‘any disease or infection that is naturally transmissible from vertebrate animals to humans and vice-versa’ (73). The transmission from animals to humans can be either direct or indirect (Fig. 2). One route for indirect transfer is contaminated animal or vegetable food products (74, 75). The scenario regarding spread of zoonotic agents via the food chain, i.e. foodborne zoonoses, is that organisms which are pathogenic to man and originating from animals contaminate human food products. Meat products could for example be contaminated by faecal material at the slaughterhouses whereas vegetables may be contaminated in the field by manure or sewage water used for fertilization and irrigation.
Fig. 2.
Various routs by which zoonotic bacteria can spread between animals and humans. The same routes apply also for resistance genes. Illustrations by K. Dahl.
Normally, it is the agent per se that is zoonotic (e.g. Norovirus, Salmonella and Campylobacter spp.). However, regarding antimicrobial resistant bacteria it is not only pathogens that could have a zoonotic potential, but also the genes encoding antimicrobial resistance in commensals such as Escherichia coli and Enterococcus spp. which may transfer to more pathogenic organisms (75, 76). Here, the scenario is that once in the human intestine, the bacteria might colonize and persist or their presence may only be transient (77, 78). Even if the animal derived bacteria colonize the human intestine only for a short time, this can be sufficient for resistance genes to be transferred to other strains better adapted to colonize humans (79). These human adapted strains can then persist for long periods and also spread to other people. The zoonotic potential of certain antimicrobial resistance genes means any resistant bacteria present in farm animals may be considered a reservoir for resistance that can spread affecting both veterinary and human medicine (80).
Regarding VRE, both spread of the resistant bacteria and for some of the variants also spread of the resistance genes via horizontal transfer could occur and has been subject to extensive reviews (37, 81, 82). Both ways are possible for the vanA variant and is among other things of importance for the zoonotic potential.
Similar strains of VRE have been isolated from farm animals and humans (83). Furthermore, when the use of avoparcin was discontinued in Europe, not only did the occurrence of VRE among farm animals decrease but there was also a subsequent decrease in the occurrence of VRE in food of animal origin and in the prevalence of human colonization with VRE (51, 84). However, hospital isolates of E. faecium generally cluster in subgroups which are separate from those found in animals (85, 86). Taken together, even though strains adapted to animals can cause infections in humans, these events are of limited importance for public health.
Transfer of the vanA gene is often mediated by a transposon, a mobile genetic element that can be incorporated either in the bacterial chromosome or on plasmids (20, 87). More specifically the genes are located in and transferred by the transposon Tn1546 or closely related genetic elements (20). One example of gene transfer between animal and human adapted enterococci is when Jensen showed that VRE from pigs and broilers have specific variants of the Tn1546 transposon and that VRE from healthy humans can have any of the two variants (88). This was taken as an indication that the dissemination rout was probably from pigs and poultry to humans. Furthermore, the possibility for in vivo transfer of vancomycin resistance from VRE of animal origin to enterococci of human origin in the intestines of humans has been described (79). Transfer in mice of the vanA gene to hospital adapted enterococci has also been demonstrated (89). Taken together, this indicates that the most important way by which the presence of VRE among farm animals impact on public health is via transfer of resistance genes to strains already adapted to humans and/or hospitals.
To what extent the presence of VRE among farm animals has actually affected the situation in public health will probably never be determined. However, it is clear that both occasional infections with animal associated strains and gene transfer from such strains can happen. Furthermore, one can only speculate on what happened when the first hospital adapted VRE emerged. Such thoughts have been tested in a mathematical model by Smith et al. (90). It was concluded that agricultural use of antimicrobials can lead to the spread of antimicrobial resistance from animals to humans, either by spread of resistant strains or by gene transfer between animal adapted and human adapted strains. Furthermore, Smith et al. state that these events will have the largest impact on public health if they occur when that particular resistance is still rare among human adapted strains. So, if the first hospital adapted VRE emerged by horizontal gene transfer of the resistance genes into a vancomycin susceptible hospital adapted enterococci – where did then the resistance genes come from? And if they came from an animal associated VRE, is not then all hospital adapted VRE animal associated strictly speaking?
Acknowledgements
This review is based on my PhD thesis Vancomycin Resistant Enterococci in Swedish Broilers – Emergence, Epidemiology and Elimination fulfilled at the Department of Clinical Sciences, Swedish University of Agricultural Sciences (SLU), Uppsala, Sweden. Supervisors for the work with the thesis were Dr. Ivar Vågsholm, Dr. Björn Bengtsson, Dr. Stina Englund, Dr. Claes Fellström, Dr. Anders Franklin and Dr. Christina Greko.
Conflict of interest and funding
I declare that there is no conflict of interest. The main financial support for the work with the PhD thesis was kindly provided by the Swedish Farmers’ Foundation for Agricultural Research and SVARMpat. Other sources of financial support were Med-Vet-Net, Carl-Fredrik von Horns trust fund by the Royal Swedish Academy of Agriculture and Forestry (KSLA), SVA vaccine fund, Albert Hjärre fund, Ivar and Elsa Sandbergs fund and SLU fund for internationalisation of postgraduate studies (FUR).
References
- 1.McCormick MH, McGuire JM, Pittenger GE, Pittenger RC, Stark WM. Vancomycin, a new antibiotic. I. Chemical and biologic properties. Antibiot Annu. 1955;3:606–11. [PubMed] [Google Scholar]
- 2.Reynolds PE. Structure, biochemistry and mechanism of action of glycopeptide antibiotics. Eur J Clin Microbiol Infect Dis. 1989;8:943–50. doi: 10.1007/BF01967563. [DOI] [PubMed] [Google Scholar]
- 3.French GL. Enterococci and vancomycin resistance. Clin Infect Dis. 1998;27:S75–83. doi: 10.1086/514910. [DOI] [PubMed] [Google Scholar]
- 4.Walsh C. Antibiotics: actions, origins, resistance. Washington, DC: ASM Press; 2003. [Google Scholar]
- 5.WHO. WHO list of critically important antimicrobials for human medicine. Geneva: World Health Organisation; 2009. [Google Scholar]
- 6.Kirst HA, Thompson DG, Nicas TI. Historical yearly usage of vancomycin. Antimicrobial agents and chemotherapy. 1998;42:1303–4. doi: 10.1128/aac.42.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin JD, Mundt JO. Enterococci in insects. Appl Microbiol. 1972;24:575–80. doi: 10.1128/am.24.4.575-580.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mundt JO. Occurrence of enterococci in animals in a wild environment. Appl Microbiol. 1963;11:136–40. doi: 10.1128/am.11.2.136-140.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sherman JM. The Streptococci. Bacteriol Rev. 1937;1:3–97. doi: 10.1128/br.1.1.3-97.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher K, Phillips C. The ecology, epidemiology and virulence of Enterococcus. Microbiology. 2009;155(Pt 6):1749–57. doi: 10.1099/mic.0.026385-0. [DOI] [PubMed] [Google Scholar]
- 11.Schleifer KH, Kilpper-Bälz R. Transfer of Streptococcus faecalis and Streptococcus faecium to the genus Enterococcus nom.rev. as Enterococcus faecalis comb. nov. and Enterococcus faecium comb. nov. Int J Syst Bacteriol. 1984;34:31–4. [Google Scholar]
- 12.Barnes EM. Differential and selective media for the faecal streptococci. J Sci Food Agric. 1959;10:656–62. [Google Scholar]
- 13.Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis. 2006;6:130. doi: 10.1186/1471-2334-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thiercelin M. Sur un diplocoque saprophyte de l'intestin susceptible de devenir pathogene. Comptes rendus des seances de la Societe de biologie et de ses filiales. 1899;51:269–71. [Google Scholar]
- 15. List of Prokaryotic names with Standing in Nomenclature – Genus Enterococcus Available from http://www.bacterio.cict.fr/e/enterococcus.html [cited 28 December 2011]
- 16.Klein G. Taxonomy, ecology and antibiotic resistance of enterococci from food and the gastro-intestinal tract. Int J Food Microbiol. 2003;88:123–31. doi: 10.1016/s0168-1605(03)00175-2. [DOI] [PubMed] [Google Scholar]
- 17.Devriese LA, Hommez J, Wijfels R, Haesebrouck F. Composition of the enterococcal and streptococcal intestinal flora of poultry. J Appl Bacteriol. 1991;71:46–50. [PubMed] [Google Scholar]
- 18.Werner G, Coque TM, Hammerum AM, Hope R, Hryniewicz W, Johnson A, et al. Emergence and spread of vancomycin resistance among enterococci in Europe. Euro Surveill. 2008;13(47) [PubMed] [Google Scholar]
- 19.Xu X, Lin D, Yan G, Ye X, Wu S, Guo Y, et al. vanM, a new glycopeptide resistance gene cluster found in Enterococcus faecium. Antimicrob Agents Chemother. 2010;54:4643–7. doi: 10.1128/AAC.01710-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Courvalin P. Vancomycin resistance in gram-positive cocci. Clin Infect Dis. 2006;42:S25–34. doi: 10.1086/491711. [DOI] [PubMed] [Google Scholar]
- 21.Boyd DA, Willey BM, Fawcett D, Gillani N, Mulvey MR. Molecular characterization of Enterococcus faecalis N06-0364 with low-level vancomycin resistance harboring a novel D-Ala-D-Ser gene cluster, vanL. Antimicrob Agents Chemother. 2008;52:2667–72. doi: 10.1128/AAC.01516-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lebreton F, Depardieu F, Bourdon N, Fines-Guyon M, Berger P, Camiade S, et al. D-Ala-d-Ser VanN-type transferable vancomycin resistance in Enterococcus faecium. Antimicrob Agents Chemother. 2011;55:4606–12. doi: 10.1128/AAC.00714-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel R, Piper K, Cockerill FR, III, Steckelberg JM, Yousten AA. The biopesticide Paenibacillus popilliae has a vancomycin resistance gene cluster homologous to the enterococcal VanA vancomycin resistance gene cluster. Antimicrob Agents Chemother. 2000;44:705–9. doi: 10.1128/aac.44.3.705-709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel R. Clinical impact of vancomycin-resistant enterococci. The Journal of antimicrobial chemotherapy. 2003;51(iii):13–21. doi: 10.1093/jac/dkg272. [DOI] [PubMed] [Google Scholar]
- 25.Arthur M, Reynolds PE, Depardieu F, Evers S, Dutka-Malen S, Quintiliani R, Jr, et al. Mechanisms of glycopeptide resistance in enterococci. J Infect. 1996;32:11–6. doi: 10.1016/s0163-4453(96)80003-x. [DOI] [PubMed] [Google Scholar]
- 26.Leclercq R, Derlot E, Duval J, Courvalin P. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N Engl J Med. 1988;319:157–61. doi: 10.1056/NEJM198807213190307. [DOI] [PubMed] [Google Scholar]
- 27.Uttley AH, Collins CH, Naidoo J, George RC. Vancomycin-resistant enterococci. Lancet. 1988;1:57–8. doi: 10.1016/s0140-6736(88)91037-9. [DOI] [PubMed] [Google Scholar]
- 28.Johnson PD, Ballard SA, Grabsch EA, Stinear TP, Seemann T, Young HL, et al. A sustained hospital outbreak of vancomycin-resistant Enterococcus faecium bacteremia due to emergence of vanB E. faecium sequence type 203. J Infect Dis. 2010;202:1278–86. doi: 10.1086/656319. [DOI] [PubMed] [Google Scholar]
- 29.Bell JM, Paton JC, Turnidge J. Emergence of vancomycin-resistant enterococci in Australia: phenotypic and genotypic characteristics of isolates. J Clin Microbiol. 1998;36:2187–90. doi: 10.1128/jcm.36.8.2187-2190.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soderblom T, Aspevall O, Erntell M, Hedin G, Heimer D, Hokeberg I, et al. Alarming spread of vancomycin resistant enterococci in Sweden since 2007. Euro Surveill. 2010;15(29) doi: 10.2807/ese.15.29.19620-en. [DOI] [PubMed] [Google Scholar]
- 31.SWEDRES. SWEDRES 2009. In: Dohnhammar U, Olson-Liljequist B, editors. Solna, Sweden: Swedish Strategic Programme for the Rational Use of Antimicrobial Agents & Swedish Institute for Infectious Disease Control; 2010. pp. 33–35. [Google Scholar]
- 32.DiazGranados CA, Zimmer SM, Klein M, Jernigan JA. Comparison of mortality associated with vancomycin-resistant and vancomycin-susceptible enterococcal bloodstream infections: a meta-analysis. Clin Infect Dis. 2005;41:327–33. doi: 10.1086/430909. [DOI] [PubMed] [Google Scholar]
- 33.Garbutt JM, Ventrapragada M, Littenberg B, Mundy LM. Association between resistance to vancomycin and death in cases of Enterococcus faecium bacteremia. Clin Infect Dis. 2000;30:466–72. doi: 10.1086/313694. [DOI] [PubMed] [Google Scholar]
- 34.Butaye P, Devriese LA, Haesebrouck F. Antimicrobial growth promoters used in animal feed: effects of less well known antibiotics on gram-positive bacteria. Clin Microbiol Rev. 2003;16:175–88. doi: 10.1128/CMR.16.2.175-188.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stokstad EL, Jukes TH. Further observations on the ‘animal protein factor’. Proc Soc Exp Biol Med. 1950;78:523–28. [Google Scholar]
- 36.Stokstad EL, Jukes TH, Pierce J, Page AC, Franklin AL. The multiple nature of the animal protein factor. J Biol Chem. 1949;180:647–54. [PubMed] [Google Scholar]
- 37.Hammerum AM, Lester CH, Heuer OE. Antimicrobial-resistant enterococci in animals and meat: a human health hazard? Foodborne Pathogens Dis. 2010;7:1137–46. doi: 10.1089/fpd.2010.0552. [DOI] [PubMed] [Google Scholar]
- 38.McDonald LC, Kuehnert MJ, Tenover FC, Jarvis WR. Vancomycin-resistant enterococci outside the health-care setting: prevalence, sources, and public health implications. Emerg Infect Dis. 1997;3:311–7. doi: 10.3201/eid0303.970307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wegener HC. Historical yearly usage of glycopeptides for animals and humans: the American-European paradox revisited. Antimicrob Agents Chemother. 1998;42:3049. doi: 10.1128/aac.42.11.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bates J. Epidemiology of vancomycin-resistant enterococci in the community and the relevance of farm animals to human infection. J Hosp Infect. 1997;37:89–101. doi: 10.1016/s0195-6701(97)90179-1. [DOI] [PubMed] [Google Scholar]
- 41.Witte W. Medical consequences of antibiotic use in agriculture. Science (New York, NY) 1998;279:996–7. doi: 10.1126/science.279.5353.996. [DOI] [PubMed] [Google Scholar]
- 42.Bager F, Madsen M, Christensen J, Aarestrup FM. Avoparcin used as a growth promoter is associated with the occurrence of vancomycin-resistant Enterococcus faecium on Danish poultry and pig farms. Prev Vet Med. 1997;31:95–112. doi: 10.1016/s0167-5877(96)01119-1. [DOI] [PubMed] [Google Scholar]
- 43.Aarestrup FM. Occurrence of glycopeptide resistance among Enterococcus faecium isolates from conventional and ecological poultry farms. Microb Drug Resist (Larchmont, NY) 1995;1:255–7. doi: 10.1089/mdr.1995.1.255. [DOI] [PubMed] [Google Scholar]
- 44.Klare I, Heier H, Claus H, Reissbrodt R, Witte W. vanA-mediated high-level glycopeptide resistance in Enterococcus faecium from animal husbandry. FEMS Microbiol lett. 1995;125:165–71. doi: 10.1111/j.1574-6968.1995.tb07353.x. [DOI] [PubMed] [Google Scholar]
- 45.Donabedian SM, Perri MB, Abdujamilova N, Gordoncillo MJ, Naqvi A, Reyes KC, et al. Characterization of vancomycin-resistant Enterococcus faecium isolated from swine in three Michigan counties. J Clin Microbiol. 2010;48:4156–60. doi: 10.1128/JCM.02346-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anonymous. Commission directive 97/6/EC. Off J Eur Commun. 1997:11–3. L35 5.2.97. [Google Scholar]
- 47.Aarestrup FM, Kruse H, Tast E, Hammerum AM, Jensen LB. Associations between the use of antimicrobial agents for growth promotion and the occurrence of resistance among Enterococcus faecium from broilers and pigs in Denmark, Finland, and Norway. Microb Drug Resist (Larchmont, NY) 2000;6:63–70. doi: 10.1089/mdr.2000.6.63. [DOI] [PubMed] [Google Scholar]
- 48.Yoshimura H, Ishimaru M, Endoh YS, Suginaka M, Yamatani S. Isolation of glycopeptide-resistant enterococci from chicken in Japan. Antimicrob Agents Chemother. 1998;42:3333. doi: 10.1128/aac.42.12.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lauderdale TL, Shiau YR, Wang HY, Lai JF, Huang IW, Chen PC, et al. Effect of banning vancomycin analogue avoparcin on vancomycin-resistant enterococci in chicken farms in Taiwan. Environ Microbiol. 2007;9:819–823. doi: 10.1111/j.1462-2920.2006.01189.x. [DOI] [PubMed] [Google Scholar]
- 50.Aarestrup FM, Seyfarth AM, Emborg HD, Pedersen K, Hendriksen RS, Bager F. Effect of abolishment of the use of antimicrobial agents for growth promotion on occurrence of antimicrobial resistance in fecal enterococci from food animals in Denmark. Antimicrob Agents Chemother. 2001;45:2054–9. doi: 10.1128/AAC.45.7.2054-2059.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klare I, Badstubner D, Konstabel C, Bohme G, Claus H, Witte W. Decreased incidence of VanA-type vancomycin-resistant enterococci isolated from poultry meat and from fecal samples of humans in the community after discontinuation of avoparcin usage in animal husbandry. Microb Drug Resist (Larchmont, NY) 1999;5:45–52. doi: 10.1089/mdr.1999.5.45. [DOI] [PubMed] [Google Scholar]
- 52.van den Bogaard AE, Bruinsma N, Stobberingh EE. The effect of banning avoparcin on VRE carriage in The Netherlands. J Antimicrob Chemother. 2000;46:146–8. doi: 10.1093/jac/46.1.146. [DOI] [PubMed] [Google Scholar]
- 53.Andersson DI, Levin BR. The biological cost of antibiotic resistance. Curr Opin Microbiol. 1999;2:489–93. doi: 10.1016/s1369-5274(99)00005-3. [DOI] [PubMed] [Google Scholar]
- 54.Andersson DI, Hughes D. Antibiotic resistance and its cost: is it possible to reverse resistance? Nature Rev Microbiol. 2010;8:260–71. doi: 10.1038/nrmicro2319. [DOI] [PubMed] [Google Scholar]
- 55.Borgen K, Simonsen GS, Sundsfjord A, Wasteson Y, Olsvik O, Kruse H. Continuing high prevalence of VanA-type vancomycin-resistant enterococci on Norwegian poultry farms three years after avoparcin was banned. J Appl Microbiol. 2000;89:478–85. doi: 10.1046/j.1365-2672.2000.01137.x. [DOI] [PubMed] [Google Scholar]
- 56.Heuer OE, Pedersen K, Andersen JS, Madsen M. Vancomycin-resistant enterococci (VRE) in broiler flocks 5 years after the avoparcin ban. Microb Drug Resist (Larchmont, NY) 2002;8:133–8. doi: 10.1089/107662902760190680. [DOI] [PubMed] [Google Scholar]
- 57.Garcia-Migura L, Liebana E, Jensen LB, Barnes S, Pleydell E. A longitudinal study to assess the persistence of vancomycin-resistant Enterococcus faecium (VREF) on an intensive broiler farm in the United Kingdom. FEMS Microbiol Lett. 2007;275:319–25. doi: 10.1111/j.1574-6968.2007.00911.x. [DOI] [PubMed] [Google Scholar]
- 58.Ghidan A, Dobay O, Kaszanyitzky EJ, Samu P, Amyes SG, Nagy K, et al. Vancomycin resistant enterococci (VRE) still persist in slaughtered poultry in hungary 8 years after the ban on avoparcin. Acta Microbiol Immunol Hung. 2008;55:409–17. doi: 10.1556/AMicr.55.2008.4.5. [DOI] [PubMed] [Google Scholar]
- 59.Lim SK, Kim TS, Lee HS, Nam HM, Joo YS, Koh HB. Persistence of vanA-type Enterococcus faecium in Korean livestock after ban on avoparcin. Microbial Drug Resist (Larchmont, NY) 2006;12:136–9. doi: 10.1089/mdr.2006.12.136. [DOI] [PubMed] [Google Scholar]
- 60.Novais C, Coque TM, Costa MJ, Sousa JC, Baquero F, Peixe LV. High occurrence and persistence of antibiotic-resistant enterococci in poultry food samples in Portugal. J Antimicrob Chemother. 2005;56:1139–43. doi: 10.1093/jac/dki360. [DOI] [PubMed] [Google Scholar]
- 61.Sorum M, Johnsen PJ, Aasnes B, Rosvoll T, Kruse H, Sundsfjord A, et al. Prevalence, persistence, and molecular characterization of glycopeptide-resistant enterococci in Norwegian poultry and poultry farmers 3 to 8 years after the ban on avoparcin. Appl Environ Microbiol. 2006;72:516–21. doi: 10.1128/AEM.72.1.516-521.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnsen PJ, Townsend JP, Bohn T, Simonsen GS, Sundsfjord A, Nielsen KM. Retrospective evidence for a biological cost of vancomycin resistance determinants in the absence of glycopeptide selective pressures. J Antimicrob Chemother. 2011;66(3):608–10. doi: 10.1093/jac/dkq512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aarestrup FM. Characterization of glycopeptide-resistant enterococcus faecium (GRE) from broilers and pigs in Denmark: genetic evidence that persistence of GRE in pig herds is associated with coselection by resistance to macrolides. J Clin Microbiol. 2000;38:2774–7. doi: 10.1128/jcm.38.7.2774-2777.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hasman H, Aarestrup FM. Relationship between copper, glycopeptide, and macrolide resistance among Enterococcus faecium strains isolated from pigs in Denmark between 1997 and 2003. Antimicrob Agents Chemother. 2005;49:454–6. doi: 10.1128/AAC.49.1.454-456.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Johnsen PJ, Osterhus JI, Sletvold H, Sorum M, Kruse H, Nielsen K, et al. Persistence of animal and human glycopeptide-resistant enterococci on two Norwegian poultry farms formerly exposed to avoparcin is associated with a widespread plasmid-mediated vanA element within a polyclonal Enterococcus faecium population. Appl Environ Microbiol. 2005;71(1):159–68. doi: 10.1128/AEM.71.1.159-168.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wierup M, Lowenhielm C, Wold-Troell M, Agenas I. Animal consumption of antibiotics and chemotherapeutic drugs in Sweden during 1980, 1982 and 1984. Vet Res Commun. 1987;11:397–405. doi: 10.1007/BF00380624. [DOI] [PubMed] [Google Scholar]
- 67.Anonymous: Antimicrobial Feed Additives; SOU 1997:132, Annex D. Stockholm, Sweden: Fritzes Bookshop; 1997. [Google Scholar]
- 68.Quednau M, Ahrne S, Petersson AC, Molin G. Antibiotic-resistant strains of Enterococcus isolated from Swedish and Danish retailed chicken and pork. J Appl Microbiol. 1998;84:1163–70. doi: 10.1046/j.1365-2672.1998.00463.x. [DOI] [PubMed] [Google Scholar]
- 69.Kuhn I, Iversen A, Finn M, Greko C, Burman LG, Blanch AR, et al. Occurrence and relatedness of vancomycin-resistant enterococci in animals, humans, and the environment in different European regions. Appl Environ Microbiol. 2005;71:5383–90. doi: 10.1128/AEM.71.9.5383-5390.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nilsson O, Greko C, Top J, Franklin A, Bengtsson B. Spread without known selective pressure of a vancomycin-resistant clone of Enterococcus faecium among broilers. J Antimicrob Chemother. 2009;63:868–72. doi: 10.1093/jac/dkp045. [DOI] [PubMed] [Google Scholar]
- 71.Nilsson O, Greko C, Bengtsson B, Englund S. Genetic diversity among VRE isolates from Swedish broilers with the coincidental finding of transferrable decreased susceptibility to narasin. J Appl Microbiol. 2012;112(4):716–22. doi: 10.1111/j.1365-2672.2012.05254.x. [DOI] [PubMed] [Google Scholar]
- 72.Nilsson O, Greko C, Bengtsson B. Environmental contamination by vancomycin resistant enterococci (VRE) in Swedish broiler production. Acta Vet Scand. 2009;51:49. doi: 10.1186/1751-0147-51-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.WHO. Programmes and projects, Zoonoses and veterinary public health. Available from ( http://www.who.int/zoonoses/en/) [cited 28 December 2011]
- 74.Giraffa G. Enterococci from foods. FEMS Microbiol Rev. 2002;26:163–71. doi: 10.1111/j.1574-6976.2002.tb00608.x. [DOI] [PubMed] [Google Scholar]
- 75.Schjorring S, Krogfelt KA. Assessment of bacterial antibiotic resistance transfer in the gut. Int J Microbiol. 2011;2011:312956. doi: 10.1155/2011/312956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.EFSA. Scientific Opinion of the Panel on Biological Hazards on a request from the European Food Safety Authority on foodborne antimicrobial resistance as a biological hazard. EFSA J. 2008;765:1–87. [Google Scholar]
- 77.Berchieri A. Intestinal colonization of a human subject by vancomycin-resistant Enterococcus faecium. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 1999;5:97–100. doi: 10.1111/j.1469-0691.1999.tb00110.x. [DOI] [PubMed] [Google Scholar]
- 78.Trobos M, Lester CH, Olsen JE, Frimodt-Moller N, Hammerum AM. Natural transfer of sulphonamide and ampicillin resistance between Escherichia coli residing in the human intestine. J Antimicrob Chemother. 2009;63:80–6. doi: 10.1093/jac/dkn437. [DOI] [PubMed] [Google Scholar]
- 79.Lester CH, Frimodt-Moller N, Sorensen TL, Monnet DL, Hammerum AM. In vivo transfer of the vanA resistance gene from an Enterococcus faecium isolate of animal origin to an E. faecium isolate of human origin in the intestines of human volunteers. Antimicrob Agents chemother. 2006;50:596–9. doi: 10.1128/AAC.50.2.596-599.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Witte W. Selective pressure by antibiotic use in livestock. Int J Antimicrob Agents. 2000;16:S19–24. doi: 10.1016/s0924-8579(00)00301-0. [DOI] [PubMed] [Google Scholar]
- 81.Bonten MJ, Willems R, Weinstein RA. Vancomycin-resistant enterococci: why are they here, and where do they come from? Lancet Infect Dis. 2001;1:314–25. doi: 10.1016/S1473-3099(01)00145-1. [DOI] [PubMed] [Google Scholar]
- 82.Sundsfjord A, Simonsen GS, Courvalin P. Human infections caused by glycopeptide-resistant Enterococcus spp: are they a zoonosis? Clin Microbiol Infect. 2001;7:16–33. doi: 10.1046/j.1469-0691.2001.00055.x. [DOI] [PubMed] [Google Scholar]
- 83.Freitas AR, Coque TM, Novais C, Hammerum AM, Lester CH, Zervos MJ, et al. Human and Swine Hosts Share Vancomycin-Resistant Enterococcus faecium CC17 and CC5 and Enterococcus faecalis CC2 Clonal Clusters Harboring Tn1546 on Indistinguishable Plasmids. J Clin Microbiol. 2011;49:925–31. doi: 10.1128/JCM.01750-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pantosti A, Del Grosso M, Tagliabue S, Macri A, Caprioli A. Decrease of vancomycin-resistant enterococci in poultry meat after avoparcin ban. Lancet. 1999;354:741–2. doi: 10.1016/S0140-6736(99)02395-8. [DOI] [PubMed] [Google Scholar]
- 85.Top J, Schouls LM, Bonten MJ, Willems RJ. Multiple-locus variable-number tandem repeat analysis, a novel typing scheme to study the genetic relatedness and epidemiology of Enterococcus faecium isolates. J Clin Microbiol. 2004;42:4503–11. doi: 10.1128/JCM.42.10.4503-4511.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Willems RJ, Top J, van Santen M, Robinson DA, Coque TM, Baquero F, et al. Global spread of vancomycin-resistant Enterococcus faecium from distinct nosocomial genetic complex. Emerg Infect Dis. 2005;11:821–28. doi: 10.3201/eid1106.041204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tamarin RH. Principles of Genetics. 7th ed. Singapore: McGraw-Hill Education; 2004. [Google Scholar]
- 88.Jensen LB. Differences in the occurrence of two base pair variants of Tn1546 from vancomycin-resistant enterococci from humans, pigs, and poultry. Antimicrob Agents Chemother. 1998;42:2463–4. doi: 10.1128/aac.42.9.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lester CH, Hammerum AM. Transfer of vanA from an Enterococcus faecium isolate of chicken origin to a CC17 E. @ faecium isolate in the intestine of cephalosporin-treated mice. J Antimicrob Chemother. 2010;65:1534–36. doi: 10.1093/jac/dkq170. [DOI] [PubMed] [Google Scholar]
- 90.Smith DL, Harris AD, Johnson JA, Silbergeld EK, Morris JG., Jr Animal antibiotic use has an early but important impact on the emergence of antibiotic resistance in human commensal bacteria. Proc Natl Acad Sci USA. 2002;99:6434–9. doi: 10.1073/pnas.082188899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.CLSI. Wayne, PA: Clinical and Laboratory Standards Institute; 2010. Performance Standards for Antimicrobial Susceptibility Testing; Twentieth Informational Supplement. CLSI document M100-S20. [Google Scholar]