Abstract
Background
Neglected tropical diseases impact over 1 billion of the world's poorest populations and require special attention. However, within the NTDs recognized by the World Health Organization, some are also dually categorized as emerging and re-emerging infectious diseases requiring more detailed examination on potential global health risks.
Methods
We reviewed the 17 NTDs classified by the WHO to determine if those NTDs were also categorized by the US Centers for Disease Control and Prevention as emerging and re-emerging infectious diseases (‘‘EReNTDs’’). We then identified common characteristics and risks associated with EReNTDs.
Results
Identified EReNTDs of dengue, rabies, Chagas Disease, and cysticercosis disproportionately impact resource-poor settings with poor social determinants of health, spread through globalization, are impacted by vector control, lack available treatments, and threaten global health security. This traditionally neglected subset of diseases requires urgent attention and unique incentive structures to encourage investment in innovation and coordination.
Discussion
Multi-sectorial efforts and targeted public–private partnerships would spur needed R&D for effective and accessible EReNTD treatments, improvement of social determinants of health, crucial low-income country development, and health system strengthening efforts. Utilization of One Health principles is essential for enhancing knowledge to efficaciously address public health aspects of these EReNTDs globally.
Keywords: neglected tropical diseases, emerging and re-emerging infectious diseases, global health, innovation, One Health, public–private partnerships
Neglected tropical diseases (NTDs) reflect a country's socio-economic progress (1). The World Health Organization (WHO) estimates 1 billion people from 149 endemic countries and territories are affected by 17 NTDs (1). Impoverished environments exacerbate disease severity, including poor water sanitation, inadequate housing, rapid urbanization and poor disease vector control, and often lack access to needed healthcare and effective case detection/management (1).
Long-term disability from disfigurement, pregnancy complications, impaired childhood development and growth, and reduced productivity from NTDs fuel an endless ‘poverty trap’ (1–3). In addition, NTD's have a disproportionate impact on these already marginalized communities, compared to developed countries (1).
Although NTDs contribute significantly to poor global health outcomes, and economic stagnation, (1–3) their relatively lower mortality compared with diseases such as HIV/AIDS or malaria, makes them ‘hidden,’ resulting in inadequate funding and diminished attention in global health priority setting (1) and as innovation targets (2–4). This dearth of diagnostic, treatment, and public health interventions often leads to unaffordable, ineffective, inconvenient, unsafe, or simply unavailable treatments in these already impoverished settings (4, 5). In fact, only ∼1% of developed drugs are targeted for tropical diseases, with an even smaller fraction devoted to NTDs (6).
Though improvements have been made, many NTDs remain severely unaddressed (3, 4). International organizations, industry, and academia, as well as public–private partnerships (PPPs) have begun to focus more on NTD drug discovery (7). Yet these efforts alone have been insufficient.
Importantly, these diseases are dynamic and expanding. Exacerbating ‘standard’ NTD global health threats are those NTDs also classified as ‘emerging or re-emerging infectious diseases’ (EReNTDs), i.e. NTDs ‘whose incidence in humans has increased within the past two decades or threatens to increase in the near future’8. EReNTDs present a unique global health challenge since they not only manifest in underserved populations, but are also largely ignored in drug development and may not be included within current disease prevention or control programs (1, 9). Indeed, despite their impact, they may not even be classified as public health emergencies of international concern under International Health Regulations. These diseases, largely neglected in global health, yet impacting millions, are spreading to non-endemic regions.
Yet EReNTDs deserve the world's urgent attention. Not only do they significantly impact resource-poor communities, they have potential to spread to regional and global populations. The rapid pace of globalization through interconnected travel and trade and environmental and demographic factors such as climate change, population growth, international migration, and rapid urbanization all contribute to this potential (1, 10) . EReNTDs are a threat to global health and should be specifically addressed through comprehensive, targeted efforts (1, 10).
Emerging and re-emerging infectious diseases
We define EReNTDs as those WHO NTDs meeting US Centers for Disease Control and Prevention (CDC) criteria for emerging or re-emerging infectious disease (1, 11). Using these parameters, EReNTDs include Dengue, Chagas disease, rabies, and cysticercosis (see Table 1). Other diseases such as African typanosomiasis may also be considered ‘re-emerging’ based on past epidemiological data but have not been specifically categorized as such (1–3, 12).
Table 1.
WHO neglected tropical diseases (those in red also classified as emerging and re-emerging infectious diseases by US Centers for Disease Control and Prevention)
| Buruli ulcer | Chagas disease (American typanosomiasis) |
| Cysticercosis | Dengue/severe dengue |
| Dracunculiasis (guinea-worm disease) | Echinococcosis |
| Fascioliasis | Human African typanosomiasis |
| Leishmaniasis | Lepropsy |
| Lymphatic filariasis | Onchocerciasis |
| Rabies | Schistosomiasis |
| Soil transmitted helminthiasis | Trachoma |
| Yaws |
Dengue represents a tremendous public health challenge, estimated to place at risk 2.5 billion people and estimates indicating more than 50 million infections globally on an annual basis (13). It is endemic in all WHO regions except Europe, is the most dangerous and widespread flavivirus globally, and a leading cause of childhood hospitalization and mortality (1–3, 9, 14, 15). WHO reports Dengue outbreaks are increasing and spreading in geographic scope, and has deemed it an international public health priority. Yet underreporting may severely underestimate its actual severity and spread (1–4).
Dengue fever is clearly an EReNTD. Dengue has emerged in countries with no previous experience, and has re-emerged in those that have not had a case in >20 years (4, 5, 15). Factors that lead to increased local outbreaks include rapid urbanization, lack of vector control, basic infrastructure failures (e.g. waste disposal), and lack of hygienic household water storage (6, 16, 17). This concatenation of factors has created vulnerabilities for societies with limited clinical or disease surveillance experience of EReNTDs.
However, unlike traditional notions of NTDs, Dengue is not confined solely to resource-poor endemic settings and is increasingly associated with international travel (3, 4, 16), especially in vector-friendly habitats such as heavily urbanized areas (7, 16). Outbreaks in Hawaii and other non-endemic areas highlights the growing risk of disease transmission of this global EReNTD (8, 9).
The primary public health intervention for Dengue is environmental management using insecticides for vector control and emphasis on early case detection (1, 9). Though these environmental-driven interventions may be effective, lack of a prophylaxis as a primary method to prevent or treat such a wide-spread disease continues to present challenges. Efforts are underway to develop Dengue vaccine candidates, though no licensed antivirals or vaccines are available to treat or prevent the disease (18).
Dengue is also considered a growing threat due to a rise in mortality associated with increasing virulence of the disease potentially caused by viral evolution. This may result from reinfection of cases from one of four different Dengue serotypes, with initial infection only providing partial immunity/protection, leading to changes in the disease (9, 15). These viral shifts may exacerbate spread of disease due to increased virulence and should be addressed with development of effective prevention controls.
Chagas disease, too, is spreading beyond its endemic presence in Latin America and is estimated to impact some 10 million people worldwide (1). The spread of the disease has resulted from increased travel and trade, including to non-endemic areas such as Europe, USA, Canada, Australia, and Japan. Roughly 14 million persons have migrated from countries where Chagas disease is endemic to non-endemic areas (19). This spread is also facilitated by other factors including immigration, urbanization, vertical transmission from mother-to-child, and potentially tainted donor blood or organ tissue (1, 19). Though Chagas disease spread can be addressed using adequate surveillance, early diagnosis, and effective vector control, existing drug treatments require prolonged treatment regimes, have potentially severe adverse effects, and have not been proven effective for the chronic phase of Chagas or administration in adult patients (20). Due to these challenges, efforts toward development of new, cost–effective antiprotozoal drugs, such as a potential Trypanosoma cruzi vaccine are underway to improve disease control (3, 20). Again, like Dengue, efforts have fallen short: preventive chemotherapy and rapid-impact packages, containing a combination of four to six drugs delivered acutely to interrupt disease transmission of different parasites and certain NTDs, may not effectively address Chagas disease (such as during the chronic phase of the disease) (3). In addition, even these drugs are expensive, highly toxic, long-term, and often difficult to administer; may have patient compliance and follow-up failures; do not have pediatric formulations; and can lead to drug resistance (1, 2, 7, 14). There is also currently no vaccine for Chagas disease (1, 7).
Approximately 55,000 deaths and more than 15 million people are estimated to be affected by rabies annually (1, 7, 21). Fortunately, it is treatable with post-exposure prophylaxis, but if left untreated can lead to eventual paralysis, coma, and death (1). Though rabies is widely distributed globally, the highest risk occurs in Africa and southeast Asia, with an estimated 270,000 lives saved from post-exposure prophylaxis in these regions (1). But lack of awareness regarding preventive measures and failure to seek care are key challenges to effectively controlling rabies (1).
Rabies has emerged or re-emerged in diverse countries including South Korea, Indonesia, Bhutan, and South Africa, and transmission expansion to additional animal species raises concern about global disease strategy focusing on vector control through targeted immunizations in animal reservoirs (1, 22–24). Indeed, observations of new virus types of rabies infection in previously uncommon reservoirs including bats, transmission of rabies from bats to humans in Latin America, potential rabies exposure to travelers in Bali from monkeys, transmission of rabies from foxes to dogs following wide-spread vaccination campaigns, and potential threat of zoonotic disease spread due to the international animal trade have emerged (23, 25, 26). However, the source of approximately 99% of deaths associated with rabies continues to originate from dogs, highlighting the need for coordinated action and directed efforts toward vaccination interventions and education in this high-risk animal population (21).
Human cysticercosis’, estimated prevalence is >50 million people, and it is an increasingly common parasitic disease globally, including in previously non-endemic regions (27). Cysticerci infection is endemic to many countries in Latin America and southeast Asia, India, Haiti, and China, but is most recognized as a public health crisis in sub-Saharan Africa (1). It is particularly devastating for subsistence farmers in developing countries. It can wreak economic havoc on agricultural/food systems dependent on pig-production since infected pig carcasses are condemned (1). As an EReNTD, cysticercosis has emerged in sub-Saharan Africa due to increasing popularity of pork consumption and may be imported into non-endemic regions such as USA via migration of agricultural workers and international travel (28, 29).
Little reliable information is available for the disease epidemiology of this EReNTD (1). Public health strategies include preventive chemotherapy for treatment of helminthic infections and vector control through porcine mass vaccinations (1). Although there have been efforts to inoculate pigs to impact disease incidence and spread, costs and the challenges of high risk areas have limited the success of these efforts (30). Currently no human vaccine is available, however, health education regarding food safety precautions refraining from consumption of unclean foods, washing of fruits and vegetables, and adequate cooking of pork products may serve as an effective preventative measure (27, 31).
Common characteristics
Emerging and re-emerging neglected tropical diseases share many characteristics and common risks. EReNTDs are widespread and impact millions. They disproportionately burden resource-poor communities, but have also recently spread to higher income settings through globalized trade and travel. They have negative economic impact on local communities, are heavily influenced by poor social determinants of health, can be fatal, are heavily dependent upon effective vector control to prevent spread, and often lack sufficient management options (i.e. cost–effective prevention and treatment). None have an approved vaccine, with the exception of targeted pre-exposure immunization in rabies. Though varying somewhat by specific EReNTD, current treatment options, public health interventions, health promotion and education efforts, and available diagnostic tools are largely insufficient to combat EReNTDs across the growing number of countries where they are now being observed.
This set of diseases will likely continue to expand, both in incidence and geographic scope due to the fact they are emerging or re-emerging infectious diseases and are largely ‘neglected’ lacking sufficient disease control and robust treatment options. Additionally, the frenetic advance of globalization is likely to continue to provide opportunities for EReNTD spread. Although not ‘officially’ classified by the CDC as emerging or re-emerging, other NTDs may also be undergoing epidemiological or geographical shifts due to genetic mutation or change in host/vector distribution, potentially leading to greater non-endemic region spread.
Public–private engagement
Multi-sector engagement through PPPs and product development partnerships are increasingly being recognized as a strategy to address NTDs (4, 7, 32, 33). These integrated partnerships engage stakeholders from industry, academia, private foundations, and other non-profit entities in shared innovation efforts (4, 32).
For example, successful global partnerships for NTD programs include Merck's partnership for onchocerciasis, Pfizer's partnership with the International Trachoma Initiative, and the GlaxoSmithKline, WHO, and Merck consortium providing medicines for lymphatic filariasis (3). Other NTD partnerships include those forged by WHO's Special Programme for Research and Training in Tropical Diseases (TDR), leading to the creation of over 10 products for tropical diseases; the Drugs for Neglected Diseases Initiative, a partnership supported by the Gates Foundation to address African trypanosomiasis; and the Grand Challenges in Global Health initiative, focusing on innovation efforts benefiting the developing world (4, 14, 32). In addition, in 2012, 13 pharmaceutical companies, states, the Bill and Melinda Gates Foundation, the World Bank and other organizations announced a new PPP to eliminate or control 10 NTDs by the end of the decade (34).
Public–private partnerships that specifically engage in drug discovery targeted for development of NTD treatments may also be supported by funding and research and development (R&D) activity in ‘innovative developing countries’ such as Brazil, China, and India (4, 35, 36). However, overall R&D investment funding is still largely skewed toward the ‘big 3’ infectious diseases of HIV, tuberculosis, and malaria, with neglected disease funding driven primarily by public and philanthropic donors, not private industry (37). In addition, PPPs that focus on drug development exclusively may not recognize the unique and diverse set of challenges posed by EReNTDs that disproportionately impact communities in resource-poor settings where lack of access to medicines and other social determinants of health may impede effectiveness of drug-based intervention.
Enhanced PPP: a one health package
In order to address current limitations of existing PPPs and the need to combat globally expanding EReNTDs, existing PPP networks should be expanded beyond traditional NTD drug discovery efforts. Though drug R&D is a crucial component in any solution to the problem of EReNTDs, drug innovation alone is insufficient, particularly in poor communities. Instead, efforts should encompass activities beyond drug development in a comprehensive ‘One Health’ package involving multi-disciplinary participation, multi-sectorial engagement, and governance by multi-national actors across the entire spectrum of EReNTD needs: prevention, surveillance, vector control, economic and social development, and treatment.
Multi-disciplinary PPPs
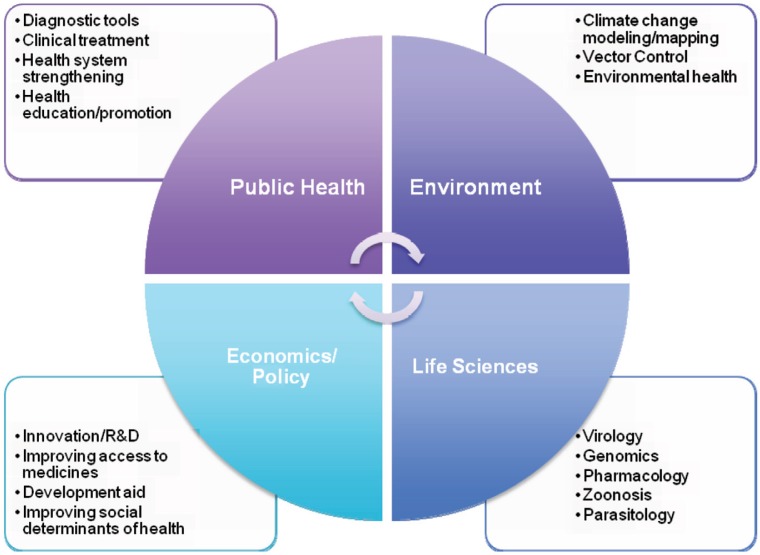
Addressing EReNTDs requires attention to virology, parasitology, zoonoses, genomics, pharmacology, veterinary sciences, public health, geography, environmental factors, social determinant of health, and economic development indicators (1, 8, 9). Interdisciplinary innovation by broad-based teams should be a PPP theme (see Fig. 1). Beyond areas of human (veterinary medicine), public health and epidemiology, experts in animal health, veterinarian sciences, microbial research, environmental health, as well as economics, trade and travel, political science, policy, and law should be considered stakeholders in addressing and adopting strategic management of EReNTDs.
Fig. 1.
Multi-disciplinary public-private partnerships for emerging and re-emerging neglected tropical diseases.
The ‘One Health Initiative’ which aims to promote engagement between human, animal, agricultural, and environmental sectors, to improve health for humans and animals may serve as a viable model (38). By recognizing the interconnectedness of various sectors in global health management, One Health advocates for integrative health risk management using knowledge sharing, education, and effective governance among these system participants to provide a comprehensive, strategic approach to future health challenges (39, 40). Building on such a base, PPPs addressing EReNTDs should integrate this broad array of stakeholders to ensure adequate development and deployment of coordinated interventions for sustained pubic health promotion.
Efforts should target greater health innovation in resource-poor settings by leveraging broader networks of partnerships integrating technology solutions (33). This should include not only industry and the public sector, but also civil society groups, non-profit drug development organizations, financing institutions (such as non-profit venture-capital firms or venture capital firms that focus on life sciences in developing countries, e.g. Bioventures) and ground-based NGOs to encompass a broad range of needed expertise and experience (7, 41).
At a minimum, basic staples of strategic EReNTD planning should include assessments of:
public health promotion/education;
vector control and disease surveillance;
diagnostic tools;
cost–effective clinical treatment;
local health system needs for intervention delivery;
logistics capacity (e.g. storage and delivery of drugs/vaccines); and
policy efforts to improve conditions of the poor and medication access.
Once this foundation is assessed, strategically pooling resources and experiences from a greater swath of projects and identifying and integrating various technology and delivery solutions for EReNTDs should then occur. In addition, EReNTD planning should expressly include development of a strategic research framework to support One Health-based policymaking across multiple sectors, such as the integrated decision-making process advocated by the National League of Cities (42, 43). Further, explicit focus on using open source software development for drug discovery, vaccine stabilization for greater transport efficiency, electronic/mobile text-based health promotion and health workforce training, and improved water purification and utilization of household water treatment technologies should be explored as possible multi-disciplinary solutions (44–46).
As an example, EReNTDs such as Dengue could benefit from this comprehensive approach. PPPs could determine key risk factors associated with Dengue from a multi-sector perspective, and identify a combination of cost–effective measures to tackle them. This includes public sector participation in developing better disease surveillance systems through identified Dengue vectors, using serotypes to monitor disease transmission globally, and performing environmental impact scans and studies to identify local and global at-risk populations for focused efforts. The collection of this data and investment in this surveillance infrastructure can then support collaborative private sector efforts to develop more targeted solutions and technology specifically addressing identified risk factors pertinent to each region/community. This could include more targeted drug/vaccine development and vector control tailored for certain environments/populations.
Once vector identification, transmission, impacts, and other risks are identified by PPPs, the public sector can provide targeted water and waste sanitation programs with integrated urban planning to improve Dengue vector control efforts. Public health agencies can then engage in education campaigns through community-based and wireless technologies (if supported) for prevention efforts and detection. This can be coupled with integration of targeted development aid from other NGOs and non-public health actors to improve basic social determinants of health. Private sector actors can support these efforts by engaging in drug/vaccine and other technology development with sensitivity to equitable access by using open source access or patent pools models in intellectual property management, such as use of the World Intellectual Property Organisation's Research system, a new open innovation platform. By co-developing these coordinated EReNTD PPP strategies, a package of targeted interventions can be developed to meet local needs, address all facets of EReNTD transmission and control, and engage as many relevant stakeholders as needed to fight the disease.
Governance: using WHO TDR
Public–private partnerships engaging a wide-ranging membership of diverse actors requires effective governance to ensure collaboration, distribution of responsibilities, and coordination of roles to adequately address EReNTDs (47). This need for global health governance among a plethora of highly fragmented and diverse actors represents a potential opportunity for multi-national organizations such as WHO to reassert their increasingly eroding role in global health.
Success of WHO TDR in partnering and mobilizing industry may be a viable model for EReNTD-targeted PPPs (14, 32). Established in 1974, TDR is the culmination of WHO, World Bank, and the United Nations Development Program (UNDP) efforts against NTDs. It has led public–private collaboration and provided needed funding, quality control, drug and discovery tools and networks, and technical expertise for development and clinical trial implementation. Further, it has assisted in procuring raw materials and formulating products for public sector use (4, 14, 32).
For EReNTDs, TDR can act as conduit for engagement with targeted PPPs. Beyond continuing to provide technical assistance; it could also act as a central repository and funding agency for EReNTD projects. More crucially, it could coordinate and match potential resources and partners to develop EReNTD disease-specific projects, and provide ongoing technical assistance as needed. In addition, to address multi-sectorial issues associated with EReNTDs, TDR could also act as the central coordinator and advocate for a research framework for One Health policymaking for NTDs (42), collaborating with international specialized agencies including the World Bank (for development projects and health strengthening programs), UN Environment Program (for environmental assessments and interventions), UNDP (for poverty alleviation), World Organisation for Animal Health/United Nations Food and Agriculture Organization (for animal health and veterinarian sciences) and UN Children's Fund (for specific maternal and child health interventions). TDR could also leverage its ability to provide WHO pre-qualification for health interventions and related technologies enabling prior approval to attract greater private and public sector participation (35).
Conclusion
Neglected tropical diseases are a global scourge affecting millions of the globe's poorest. Exacerbating these risks are EReNTDs now spreading rapidly to non-endemic regions. Enhanced public–private coordinated efforts addressing both clinical and social factors must be engaged to address this growing global health concern.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
References
- 1.WHO. Working to overcome the global impact of neglected tropical diseases: first WHO report on neglected tropical diseases [Internet] 2010. Available from: http://whqlibdoc.who.int/publications/2010/9789241564090_eng.pdf [cited 19 October 2011]
- 2.Hotez PJ, Pecoul B. ‘Manifesto’ for advancing the control and elimination of neglected tropical diseases. PLoS Negl Trop Dis. 2010;4:e718. doi: 10.1371/journal.pntd.0000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotez PJ, Molyneux DH, Fenwick A, Kumaresan J, Sachs SE, Sachs JD, et al. Control of neglected tropical diseases. N Engl J Med. 2007;357:1018–27. doi: 10.1056/NEJMra064142. [DOI] [PubMed] [Google Scholar]
- 4.Croft SL. Public-private partnership: from there to here. Trans R Soc Trop Med Hyg. 2005;99:9–14. doi: 10.1016/j.trstmh.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Trouiller P, Torreele E, Olliaro P, White N, Foster S, Wirth D, et al. Drugs for neglected diseases: a failure of the market and a public health failure? Trop Med Int Health. 2001;6:945–51. doi: 10.1046/j.1365-3156.2001.00803.x. [DOI] [PubMed] [Google Scholar]
- 6.Maurer SM, Rai A, Sali A. Finding cures for tropical diseases: is open source an answer? PLoS Med. 2004;1:e56. doi: 10.1371/journal.pmed.0010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nwaka S, Ramirez B, Brun R, Maes L, Douglas F, Ridley R. Advancing drug innovation for neglected diseases – criteria for lead progression. PLoS Negl Trop Dis. 2009;3:e440. doi: 10.1371/journal.pntd.0000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Snowden FM. Emerging and reemerging diseases: a historical perspective. Immunol Rev. 2008;225:9–26. doi: 10.1111/j.1600-065X.2008.00677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morens DM, Folkers GK, Fauci AS. The challenge of emerging and re-emerging infectious diseases. Nature. 2004;430:242–9. doi: 10.1038/nature02759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lashley FR. Emerging infectious diseases: vulnerabilities, contributing factors and approaches. Expert Rev Anti Infect Ther. 2004;2:299–316. doi: 10.1586/14787210.2.2.299. [DOI] [PubMed] [Google Scholar]
- 11.National Center for Infectious Diseases. Emerging infectious diseases: disease information: NCID: CDC [Internet] 2005. Available from: http://www.cdc.gov/ncidod/diseases/eid/disease_sites.htm [cited 22 October 2011]
- 12.Smith DH, Pepin J, Stich AH. Human African trypanosomiasis: an emerging public health crisis. Br Med Bull. 1998;54:341–55. doi: 10.1093/oxfordjournals.bmb.a011692. [DOI] [PubMed] [Google Scholar]
- 13.Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, et al. Dengue: a continuing global threat. Nat Rev Micro. 2010;8:S7–16. doi: 10.1038/nrmicro2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mrazek MF, Mossialos E. Stimulating pharmaceutical research and development for neglected diseases. Health Policy. 2003;64:75–88. doi: 10.1016/s0168-8510(02)00138-0. [DOI] [PubMed] [Google Scholar]
- 15.Guha-Sapir D. Dengue fever: new paradigms for a changing epidemiology. Emerg Themes Epidemiol. 2005;2:1. doi: 10.1186/1742-7622-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilder-Smith A, Gubler DJ. Geographic expansion of dengue: the impact of international travel. Med Clin North Am. 2008;92:1377–90. doi: 10.1016/j.mcna.2008.07.002. x. [DOI] [PubMed] [Google Scholar]
- 17.Gubler DJ, Clark GC. Dengue/dengue hemorrhagic fever: the emergence of a global health problem. Emerging Infectious Diseases. Centers for Disease Control. 1995;1:55. doi: 10.3201/eid0102.952004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmitz J, Roehrig J, Barrett A, Hombach J. Next generation dengue vaccines: a review of candidates in preclinical development. Vaccine. 2011;29:7276–84. doi: 10.1016/j.vaccine.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 19.Jackson Y, Myers C, Diana A, Marti HP, Wolff H, Chappuis F, et al. Congenital transmission of Chagas disease in Latin American immigrants in Switzerland. Emerg Infect Dis. 2009;15:601–3. doi: 10.3201/eid1504.080438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quijano-Hernandez I, Dumonteil E. Advances and challenges towards a vaccine against Chagas disease. Vaccines. 2011;7:1184–91. doi: 10.4161/hv.7.11.17016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO. Rabies [Internet] WHO. 2011. Available from: http://www.who.int/mediacentre/factsheets/fs099/en/ [cited 21 June 2012]
- 22.Tenzin, Sharma B, Dhand NK, Timsina N, Ward MP. Reemergence of rabies in Chhukha district, Bhutan, 2008. Emerg Infect Dis. 2010;16:1925–30. doi: 10.3201/eid1612.100958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gautret P, Lim PL, Shaw M, Leder K. Rabies post-exposure prophylaxis in travellers returning from Bali, Indonesia, November 2008 to March 2010. Clin Microbiol Infect. 2011;17:445–7. doi: 10.1111/j.1469-0691.2010.03271.x. [DOI] [PubMed] [Google Scholar]
- 24.Kim CH, Lee CG, Yoon HC, Nam HM, Park CK, Lee JC, et al. Rabies, an emerging disease in Korea. J Vet Med Series B. 2006;53:111–5. doi: 10.1111/j.1439-0450.2006.00928.x. [DOI] [PubMed] [Google Scholar]
- 25.Marano N, Arguin PM, Pappaioanou M. Impact of globalization and animal trade on infectious disease ecology. Emerg Infect Dis. 2007;13:1807–9. doi: 10.3201/eid1312.071276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider MC, Romijn PC, Uieda W, Tamayo H, da Silva DF, Belotto A, et al. Rabies transmitted by vampire bats to humans: an emerging zoonotic disease in Latin America? Rev Panam Salud Publica. 2009;25:260–9. doi: 10.1590/s1020-49892009000300010. [DOI] [PubMed] [Google Scholar]
- 27.Kraft R. Cysticercosis: an emerging parasitic disease. Am Fam Physician. 2007;76:91–6. [PubMed] [Google Scholar]
- 28.Wallin MT, Kurtzke JF. Neurocysticercosis in the United States: review of an important emerging infection. Neurology. 2004;63:1559–64. doi: 10.1212/01.wnl.0000142979.98182.ff. [DOI] [PubMed] [Google Scholar]
- 29.Hotez PJ. Neglected Infections of Poverty in the United States of America. PLoS Negl Trop Dis. 2008;2:e256. doi: 10.1371/journal.pntd.0000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sciutto E, Fragoso G, de Aluja AS, Hernández M, Rosas G, Larralde C. Vaccines against cysticercosis. Curr Top Med Chem. 2008;8:415–23. doi: 10.2174/156802608783790839. [DOI] [PubMed] [Google Scholar]
- 31.Cysticercosis [Internet] PubMed Health. 2012. Available from: http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0001651/ [cited 21 June 2012]
- 32.Nwaka S, Hudson A. Innovative lead discovery strategies for tropical diseases. Nat Rev Drug Discov. 2006;5:941–55. doi: 10.1038/nrd2144. [DOI] [PubMed] [Google Scholar]
- 33.Gardner CA, Acharya T, Yach D. Technological and social innovation: a unifying new paradigm for global health. Health Affairs. 2007;26:1052–61. doi: 10.1377/hlthaff.26.4.1052. [DOI] [PubMed] [Google Scholar]
- 34.World Bank. Private and public partners unite to combat 10 neglected tropical diseases by 2020 [Internet] 2012. Available from: http://web.worldbank.org/WBSITE/EXTERNAL/NEWS/0,contentMDK:23100187∼pagePK:64257043∼piPK:437376∼theSitePK:4607,00.html [cited February 2 2012]
- 35.Morel CM. Health innovation networks to help developing countries address neglected diseases. Science. 2005;309:401–4. doi: 10.1126/science.1115538. [DOI] [PubMed] [Google Scholar]
- 36.Maurer SA. Choosing the right incentive strategy for research and development in neglected diseases. Bull World Health Organ. 2006;84:376–81. doi: 10.2471/blt.06.029835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moran M, Guzman J, Ropars A-L, McDonald A, Jameson N, Omune B, et al. Neglected disease research and development: how much are we really spending? PLoS Med. 2009;6:e1000030. doi: 10.1371/journal.pmed.1000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pappaioanou M, Spencer H. ‘One Health’ initiative and ASPH. Public Health Rep. 2008;123:261. [PMC free article] [PubMed] [Google Scholar]
- 39.One Health Initiative [Internet] Available from: http://www.onehealthinitiative.com/ [cited 21 June 2012]
- 40.Kaplan B, Kahn LH, Monath TP, Woodall J. ‘ONE HEALTH’ and parasitology. Parasit Vectors. 2009;2:36. doi: 10.1186/1756-3305-2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masum H, Singer PA. ‘Venture capital on a shoestring: bioventures’ pioneering life sciences fund in South Africa. BMC Int Health Hum Rights. 2010;10(Suppl 1):S8. doi: 10.1186/1472-698X-10-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coker R, Rushton J, Mounier-Jack S, Karimuribo E, Lutumba P, Kambarage D, et al. Towards a conceptual framework to support one-health research for policy on emerging zoonoses. Lancet Infect Dis. 2011;11:326–31. doi: 10.1016/S1473-3099(10)70312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.National League of Cities [Internet] 2011. Available from: http://www.onehealthinitiative.com/publications/NLC%20One%20Health%20resolution%20November%202011.pdf [cited 21 June 2012]
- 44.Kristensen D, Chen D, Cummings R. Vaccine stabilization: research, commercialization, and potential impact. Vaccine. 2011;29:7122–4. doi: 10.1016/j.vaccine.2011.05.070. [DOI] [PubMed] [Google Scholar]
- 45.Shannon MA, Bohn PW, Elimelech M, Georgiadis JG, Mariñas BJ, Mayes AM. Science and technology for water purification in the coming decades. Nature. 2008;452:301–10. doi: 10.1038/nature06599. [DOI] [PubMed] [Google Scholar]
- 46.Zurovac D, Sudoi RK, Akhwale WS, Ndiritu M, Hamer DH, Rowe AK, et al. The effect of mobile phone text-message reminders on Kenyan health workers’ adherence to malaria treatment guidelines: a cluster randomised trial. Lancet. 2011;378:795–803. doi: 10.1016/S0140-6736(11)60783-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishtar S. Public – private ‘partnerships’ in health – a global call to action. Health Res Policy Syst. 2004;2:5. doi: 10.1186/1478-4505-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]