Abstract
BACKGROUND
The majority of the prostatic cancers are adenocarcinomas characterized by glandular formation and the expression of luminal differentiation markers androgen receptor (AR) and prostate-specific antigen (PSA). Most adenocarcinomas are indolent and androgen-dependent. Hormonal therapy that inhibits AR signaling produces symptomatic relief in patients with advanced and metastatic adenocarcinomas. Prostatic small cell neuroendocrine carcinoma (SCNC) is a variant form of prostate cancer (PC). In contrast to adenocarcinoma, the tumor cells of SCNC do not form glands and are negative for AR and PSA. SCNC is extremely aggressive and does not respond to hormonal therapy. The purpose of this study was to compare the important and relevant features of two most commonly used PC cell lines, LNCaP and PC3, with prostatic adenocarcinoma and SCNC.
METHODS
Xenograft tumors of LNCaP and PC3 were prepared and compared with human prostatic adenocarcinoma and SCNC for the expression of key signaling molecules by immunohistochemistry and Western blot analysis.
RESULTS
LNCaP cells express AR and PSA and their growth is inhibited by androgen withdrawal, similar to human prostatic adenocarcinoma. PC3 cells do not express AR and PSA and their proliferation is independent of androgen, similar to SCNC. Adenocarcinoma cells and LNCaP cells are negative for neuroendocrine markers and stem cell-associated marker CD44 while SCNC and PC3 cells are positive. LNCaP cells have identical cytokeratin profiles to adenocarcinoma while PC3 cells have cytokeratin profiles similar to SCNC.
CONCLUSION
LNCaP cells share common features with adenocarcinoma while PC3 cells are characteristic of SCNC.
Keywords: prostate cancer, small cell carcinoma, adenocarcinoma, PC3, LNCaP
INTRODUCTION
Prostate cancer (PC) is the most common malignancy in men and the second leading cause of cancer-related deaths [1]. Normal prostate epithelium contains luminal epithelial cells, basal cells and a small component of neuroendocrine (NE) cells that are scattered throughout the prostate [2–5]. The majority of the PCs are classified as adenocarcinomas characterized by an absence of basal cells and uncontrolled proliferation of malignant tumor cells with features of luminal differentiation including glandular formation and the expression of androgen receptor (AR) and prostate-specific antigen (PSA). Interestingly, every single case of prostatic adenocarcinoma also contains a small population (usually ~1%) of NE tumor cells [2–5]. The NE cells in adenocarcinoma share many important features with those in the benign prostate. For example, in contrast to the non-NE luminal-type tumor cells, the NE cells in benign prostate and adenocarcinoma do not express AR and PSA [6,7].
A minority of the prostatic epithelial malignancies are variant forms including ductal type adenocarcinoma, mucinous (colloid) carcinoma, signet ring cell carcinoma, and small cell (neuroendocrine) carcinoma (SCNC) [8]. Prostatic SCNCs are considered indistinguishable from pulmonary and other extra-pulmonary SCNCs with a solid, sheet-like growth pattern, usually with areas of tumor necrosis. Tumor cells are small, with fine chromatin pattern, scant cytoplasm, and nuclear molding. Mitotic figures and crush artifact are frequent findings [3,9,10]. SCNCs of the prostate are rare tumors and account for no more than 1% of all carcinomas of the prostate. Although they may arise de novo, such tumors are often seen as recurrent tumors in patients who have a history of conventional prostatic adenocarcinomas and received hormonal therapy [11,12]. SCNC may be present either as a pure form or as a component of mixed tumors which also contain conventional adenocarcinoma. Similar to the NE cells in benign prostate and prostatic adenocarcinoma, the tumor cells in SCNC lack the expression of AR and PSA [9,10,13], which explains the clinical observation that such tumors, unlike adenocarcinomas, do not respond to hormonal therapy that stops androgen production and inhibits AR function [14,15]. In contrast to the majority of prostatic adenocarcinomas that pursue an indolent clinical course, SCNC is highly aggressive, usually presenting with locally advanced disease or distant metastasis, and the patients usually die within months of the diagnosis [16,17]. Therefore, SCNC is a different tumor than prostatic adenocarcinoma and the two entities should be clearly distinguished.
Because PC is a highly prevalent disease, it has been the focus of significant research activities for many years. Numerous articles have been published studying PC using various models including established cell lines derived from metastatic human PCs, xenograft models, and genetically engineered mouse models of PC. Among these models, cell lines have had the longest history and been most widely used in publications. Two of the most commonly used cell lines are LNCaP [18,19] and PC3 cells [20], derived from lymph node and bone metastases, respectively. It has been well established through numerous studies that LNCaP cells express AR and PSA, are androgen-dependent with relatively indolent biologic behavior similar to the vast majority of the PCs encountered clinically [19,21]. PC3 cells, on the other hand, do not express AR and PSA and are androgen-independent [20–21]. They show highly aggressive behavior which is unlike most clinical cases of PCs. For many years, these two cell lines have been used by researchers to represent different spectrums of PC with LNCaP as the indolent form and PC3 as the aggressive form of PC. They have also been used to represent androgen-dependent and castration-resistant tumors, respectively. Here we present evidence showing that unlike LNCaP cells which are typical of conventional adenocarcinoma of the prostate, PC3 cells have features that are characteristic of prostatic SCNCs.
MATERIALS AND METHODS
Materials
PC-3 and LNCaP cells were obtained from the American Type culture Collection (ATCC); fetal bovine serum (FBS), RPMI medium 1640, Dulbecco’s modified Eagle’s medium (DMEM), sodium pyruvate, L-glutamine, penicillin, and streptomycin were purchased from Hyclone; charcoal/dextran-treated FBS medium was purchased from Cellgro; monoclonal anti-neuron-specific enolase (NSE) antibody was from DAKO (Carpinteria, CA); monoclonal antichromogranin A (CgA) was from Neomarkers (Fremont, CA); polyclonal anti-PSA antibody, polyclonal anti-AR antibody, and monoclonal anti-β-actin antibody were from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal anti-CD44 antibody was from eBiosciences (San Diego, CA). Monoclonal anti-P53 antibody was from EMD (San Diego, CA). Polyclonal anti-CK5 and monoclonal anti-CK8 antibodies were from Covance (Emeryville, CA).
Cell Culture
LNCaP cells were maintained in RPMI 1640 supplemented with 10% FBS and penicillin–streptomycin, whereas PC-3 cells were maintained in the DMEM containing 10% FBS, penicillin–streptomycin, L-glutamine, pyruvate sodium. Cells were grown at 37°C with 5% CO2.
Growth Curve
Equal numbers of cells were seeded into 96-well plates and maintained with the normal medium supplemented with regular FBS or charcoal-stripped FBS (androgen-deprived). Growth curves were obtained by the Promega CellTiter (Madison) assay according to manufacturer’s instructions on days 1, 3, 5, and 7. The experiments were performed in quadruplicates.
Xenograft Tumors and Human Tumors
LNCaP and PC3 cells were used to generate xenograft tumors in male severe combined immunodeficient (SCID) mice as described [22]. When the cells reached ~80% confluency, they were washed with cold PBS and trypsinized. The cells were washed once in media and resuspended to a concentration of 1 × 106 cells/50 μl (PC3) or 2 × 106 cells/50 μl and mixed with an equal volume of cultrex (R&D Systems). The mixture was then injected into the flanks of immunodeficient mice for tumor development. The tumors were harvested, fixed in formalin, and embedded in paraffin. Paraffin sections were prepared for immunohistochemical studies.
For human adenocarcinoma, we used a tissue microarray containing tissue cores from 80 cases as published previously [23]. Regular sections from five cases of the small cell carcinoma cases were used as reported in a previous publication [24].
Immunohistochemistry
The detailed method has been reported previously [23]. Briefly, paraffin sections of 4-μm thickness were deparafinized with xylene and rehydrated through graded ethanol. Endogenous peroxidase activity was blocked with 3% hydrogen perozide in methanol for 10 min. The primary antibodies were used as follows: anti-AR: 1:100; anti-CD44, 1:200; anti-CgA, 1:1,000; anti-P53: 1:200; anti-PSA (Dako/A0562): 1:5,000; anti-NSE: 1:1,000; anti-CK5, 1:1,000; and anti-CK8, 1:1,000. The signal was detected with Dako Envision System Labelled Polymer HRP anti-mouse (cat 4001) or anti-rabbit (cat# 4003) for 30 min and developed with diaminobenzidine (DAB) for 10 min. Sections were counterstained with hematoxylin.
Western Blotting
Cultured cells were washed three time with cold phosphate-buffered saline (PBS) and lysed with lysis buffer (20 mM KCl, 150 mM NaCl, 1% NP-40, 1% Triton X-100, 50 mM NaF, 50 mM Tris, 1 mM DTT, 1 mM EGTA, 1× protease inhibitor, and 10% glycerol) for 15 min on the ice. The cells were homogenized and centrifuged for 30 min at 4°C. The protein concentration in the supernatant was determined with the Bio-Rad protein assay kit. Equal amounts of proteins were separated on 8% SDS–polyacrylamide gels and transferred to polyvinylidene fluoride (PVDF) membrane. The membrane was blocked with non-fat dry milk for 1 hr, hybridized with primary antibody in non-fat dry milk overnight, washed with PBS for 30 min, incubated with secondary antibody for 1 hr, washed with PBS 1 hr, and detected with an ECL kit (Bio-Rad).
Quantitative RT-PCR
Total RNA was purified from cells with Fermentas Gene JET™ RNA purification Kit according to the manufacture’s protocol. RNA was reverse transcribed by transcriptor reverse transcriptase (Fermentas RT-PCR Kit). The following specific forward and reverse primers were used: for NSE, 5′-GAGACAAACAG CGTTACTTAG-3′ and 5′-AGCTGCCCCTGCCTTAC-3′; for CgA, 5′-GCGGTGGAAGAGCCATCAT-3′ and 5′-TCTGTGGCTTCACCACTTTTCTC-3′; for CD44, 5′-AAGGTGGAGCAAACACAACC-3′ and 5′-TCCAC TTGGCTTTCTGTCCT-3′; for GAPDH, 5′-CATGGGT GTGAACCATGAGA-3′ and 5′-CAGTGATGGCATGGACTGTG-3′.
Real-time PCR was performed with SA Biosciences RT2 Real-time™ SYBR Kit. Total reaction volume was 15 μl and a cycle comprised of 95°C for 8 min, 95°C for 30 sec; for a total of 43 cycles followed by 95°C for 15 sec, 60°C for 1 min.
RESULTS
Expression of Luminal Differentiation Markers AR and PSA in LNCaP and PC3 Cells
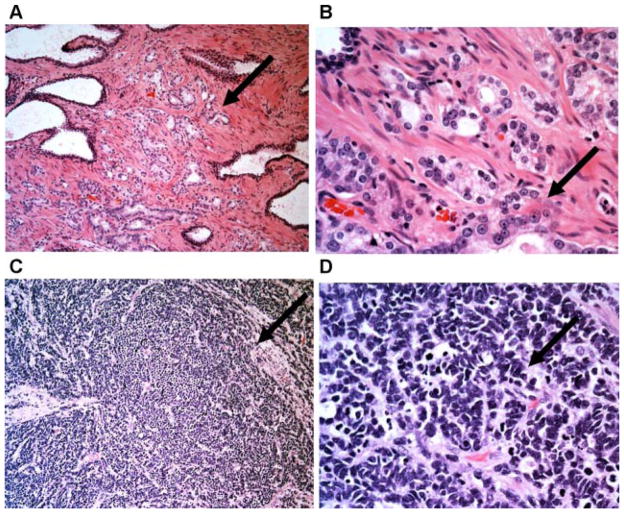
The majority of clinically encountered adenocarcinomas of the prostate are acinar type adenocarcinomas which recapitulates normal acinar structures characterized by glandular formation. The tumor cells often have prominent nucleoli (Fig. 1A,B). In contrast, prostatic SCNCs show high-grade NE features including a diffuse, solid growth pattern, and a high mitotic rate. The tumor cells have a fine, homogeneous nuclear chromatin pattern, without prominent nucleoli or glandular formation (Fig. 1C,D).
Fig. 1.
Distinct histologic features of prostatic adenocarcinoma and SCNC. The upper panel (A,B) are low and high power pictures of prostatic adenocarcinoma with cancerous glands recapitulating the morphologic features of normal prostatic glands/ducts, and the tumor cells forming glandular structures. Note that many tumor cells show prominent nucleoli. The lower panel (C,D) are low and high power pictures of prostatic SCNC with high-grade neuroendocrine morphology including diffuse, solid growth pattern, high N/C ratio, fine nuclear chromatin pattern, and frequent mitotic figures. Note that there is no glandular formation and no prominent nucleoli (H&E100× (A,C) and 400× (B,D)). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
An important feature of prostatic adenocarcinoma is that the tumor cells express markers characteristic of prostatic luminal cells such as AR and PSA. Many of the available PC cells lines, such as LNCaP, LAPC4, 22RV1, and VCaP, do show these features [21]. However, it is well known that PC3 cells are negative for such luminal differentiation markers [21], a feature that is shared by prostatic SCNC [9,10,13].
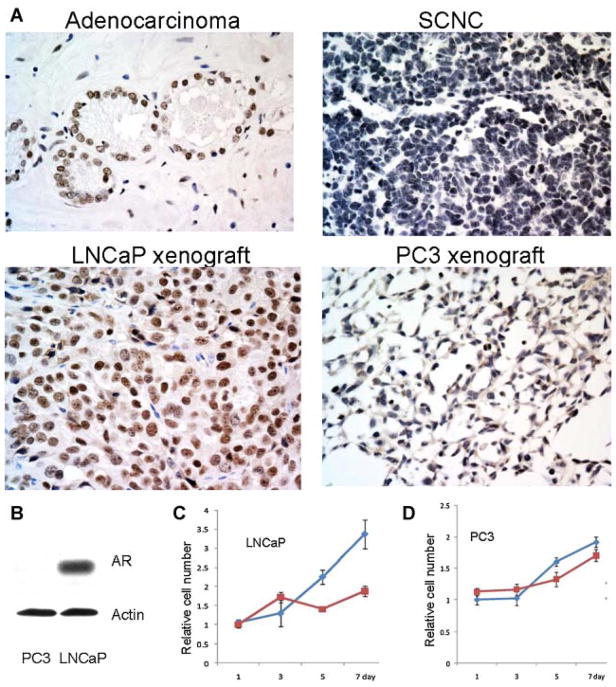
Immunohistochemical studies confirmed the previous reports and showed that while the cells of LNCaP xenograft tumors expressed AR similar to adenocarcinoma, the PC3 xenograft tumor cells did not express AR, similar to small cell carcinoma (Fig. 2A). Consistent with the above findings, Western blot assay showed that AR was expressed in LNCaP cells but not PC3 cells (Fig. 2B).
Fig. 2.
Androgen receptor is expressed in prostatic adenocarcinoma and LNCaP cells but not in prostatic SCNC and PC3 cells. A: Immunohistochemical study with an anti-AR antibody shows positive staining in adenocarcinoma but not SCNC. The LNCaP xenograft tumor cells are positive for AR but the PC3 xenograft tumors are negative (IHC, 400× magnification). B: Western blotting shows that LNCaP cells express AR while PC3 cells do not. C,D: Growth assays show that withdrawal of androgen inhibits the growth of LNCaP cells but not PC3 cells. Blue line: media with FBS; red line: media with charcoal-stripped FBS. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Decades of clinical observations have shown that prostatic adenocarcinoma responds to androgen deprivation therapy while the same therapy is not effective in prostatic SCNC [13], consistent with immunohistochemical finding showing that the former expresses AR while the latter is negative for AR. Growth assays showed that both types of cells proliferated well in media supplemented with normal FBS which contains steroid hormones including androgen. When cultured in media supplemented with charcoal-stripped FBS which is devoid of androgen, the proliferation of the AR positive LNCaP cells was significantly inhibited while the AR negative PC3 cells proliferated normally (Fig. 2C), supporting the notion that LNCaP cells are similar to adenocarcinoma cells and PC3 cells are similar to tumor cells of SCNC.
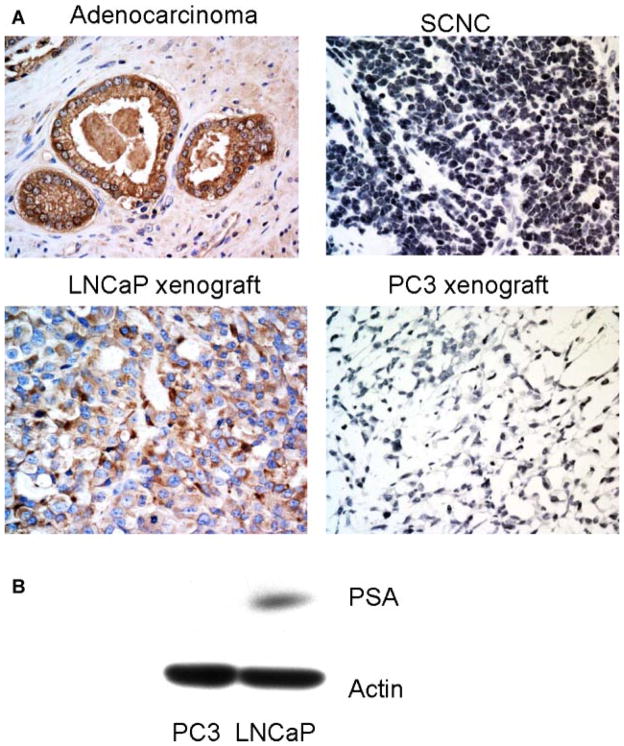
PSA expression is a hallmark of prostatic luminal cells and its expression is strictly controlled by AR activity. Immunohistochemical study shows that similar to cells of adenocarcinoma, LNCaP cells were positive for PSA while both SCNC and PC3 cells were negative for PSA (Fig. 3A), a finding that was confirmed by Western blot assay (Fig. 3B).
Fig. 3.
PSA is expressed in prostatic adenocarcinoma and LNCaP cells but not in prostatic SCNC and PC3 cells. A: Immunohistochemical study with an anti-PSA antibody shows positive staining in prostatic adenocarcinoma but not SCNC. The LNCaP xenograft tumor cells are positive for PSA but the PC3 xenograft tumors are negative (IHC, 400×). B: Western blot analysis shows that LNCaP cells express PSA but the PC3 cells do not. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Expression of Neuroendocrine Markers in LNCaP and PC3 Cells
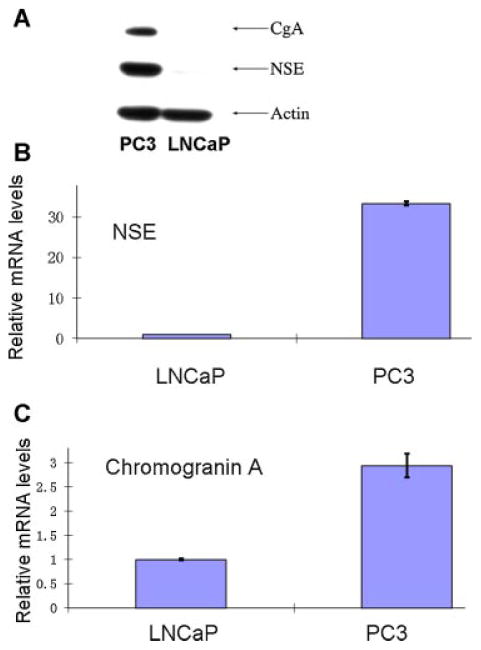
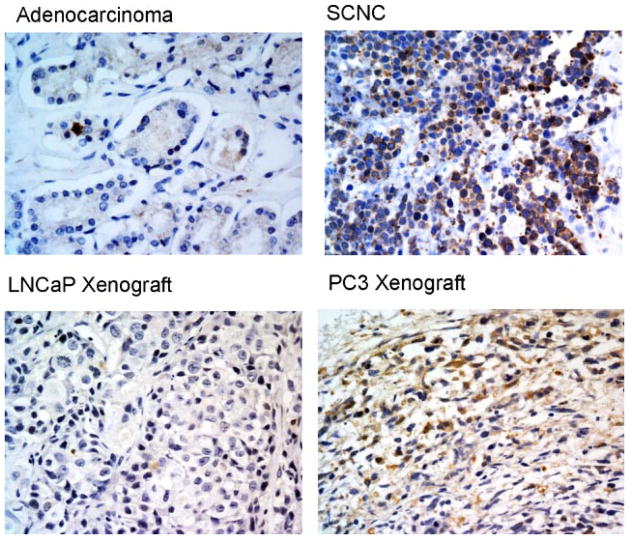
CgA and NSE are two of the markers commonly used to identify benign and neoplastic NE cells including those in the prostate [5]. RT-PCR and Western blotting studies showed that LNCaP cells, similar to the bulk, non-NE tumor cells of prostatic adenocarcinoma, were negative for the NE markers. In contrast, PC3 cells expressed high levels of these two NE markers (Fig. 4), consistent with previously published results [21,25–28]. Immunohistochemical studies showed that human prostatic adenocarcinoma cells were negative for NE markers NSE except the scattered NE cells, while SCNC showed extensive NE marker positivity (Fig. 5). Similarly, the LNCaP xenograft tumor cells were negative for NSE while PC3 xenograft tumor cells were positive (Fig. 5).
Fig. 4.
PC3 cells express NE cell markers but LNCaP cells do not. A: Western blot assay shows that PC3 cells, not LNCaP cells, express NE markers chromogranin A and NSE. B,C: show the results of qPCR studies showing higher mRNA levels for CgA and NSE in PC3 cells than in LNCaP cells. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Fig. 5.
Neuroendocrine marker NSE is expressed in prostatic SCNC and PC3 cells but not in prostatic adenocarcinoma and LNCaP cells. Immunohistochemical study with an anti-NSE antibody shows positive staining in prostatic SCNC but not in adenocarcinoma. The LNCaP xenograft tumor cells are negative for NSE but the PC3 xenograft tumors are positive (IHC, 400×). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Expression of CD44 and Cytokeratins in LNCaP and PC3 Cells
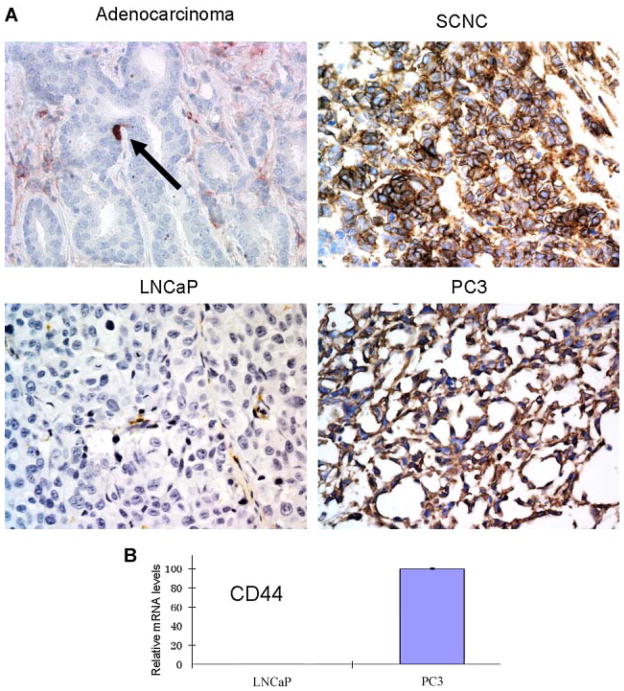
CD44 is a putative cancer stem cell-associated marker in various human tumors [29]. Using a variety of cell lines and xenograft models of PC, the Tang’s group showed that CD44 expression is associated with a small populations of tumor cells with increased tumorigenicity [30]. We have shown that in human prostatic adenocarcinoma, CD44 is selectively expressed in the scattered NE tumor cells but not in the bulk, luminal type tumor cells [28]. We have also shown that CD44 is commonly expressed in NE tumor cells of prostatic SCNC [24]. Immunohistochemical studies showed that LNCaP xenograft tumor cells were negative for CD44, similar to the bulk tumor cells of prostatic adenocarcinoma, while PC3 xenograft tumor cells expressed CD44 strongly and diffusely, similar to tumor cells of SCNC (Fig. 6A). Quantitative RT-PCR study in cultured cells confirmed the above findings (Fig. 6B).
Fig. 6.
CD44 is expressed in prostatic SCNC and PC3 cells but not in the luminal type tumor cells of prostatic adenocarcinoma and LNCaP cells. A: Immunohistochemical study shows positive staining in prostatic SCNC but not in adenocarcinoma (with the exception of rare NE tumor cells, arrow). The LNCaP xenograft tumor cells are negative for CD44 but the PC3 xenograft tumors are positive (IHC, 400×). B: qPCR study shows very low levels of CD44 mRNA in LNCaP cells but high levels in PC3 cells. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
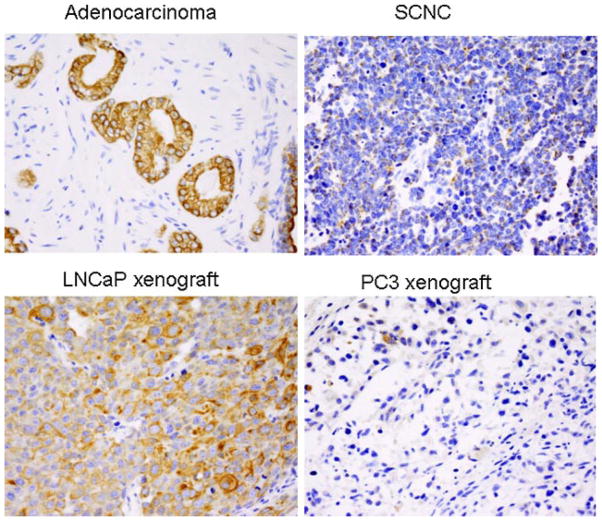
Benign prostate glands contain basal and luminal cells and the two types of cells have distinct cytokeratin profiles. CK5 and CK14 are considered basal cytokeratins while CK8 and CK18 are luminal cytokeratins [31,32]. Prostatic adenocarcinomas express luminal cytokeratins but not basal cytokeratins [31,32]. It has been reported that while the tumor cells of prostatic adenocarcinoma show a diffuse and strong staining pattern for CK8, those of prostatic SCNC show focal and dot-like staining pattern [33]. We compared the cytokeratin profiles of LNCaP and PC3 cells with those of adenocarcinoma and SCNC and showed that both cells were negative for basal cell cytokeratin CK5 as expected (data not shown). IHC staining for luminal cytokeratin CK8 showed strong and diffuse staining in LNCaP cells similar to adenocarcinoma cells, while PC3 cells showed focal positive staining similar to SCNC cells, further demonstrating that PC3 cells have features characteristic of prostatic SCNC (Fig. 7).
Fig. 7.
CK8 is strongly and diffusely expressed in prostatic adenocarcinoma and LNCaP cells but only focally and weakly in SCNC and PC3 cells. Immunohistochemical study with an anti-CK8 antibody shows positive staining in prostatic adenocarcinoma cells but a dot-like staining pattern in tumor cells of SCNC. The LNCaP xenograft tumor cells are diffusely positive for CK18 but the PC3 xenograft diffuse tumors are only focally positive (IHC, 400×). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
Various experimental models have proven extremely useful in studying many important aspects of PC such as etiology, molecular pathways of signal transduction, imaging, prevention, and therapy. Cell lines were the dominant experimental models for many years and are still the main tools used in many of today’s publications even after animal models have become widely available in recent years. Cell lines provide many unique advantages, such as being easy to grow with commonly available reagents and equipment, the ability to grow large amount of materials for many experiments in a short period of time, and the resulting material being pure populations of cells suitable for molecular biology and biochemical studies. LNCaP and PC3 have been the most commonly used PC cell lines but they possess significantly different characteristics, the most important being that LNCaP cells express luminal differentiation markers AR and PSA while PC3 cells do not. Consequently, LNCaP cells are androgen-dependent, and androgen withdrawal inhibits their growth, while PC3 cells are androgen-independent and proliferate normally in androgen-deprived media. In addition, xenograft tumors of LNCaP are slow growing and less invasive, while PC3 xenograft tumors proliferate rapidly and are more invasive. In in vitro assays, PC3 cells possess higher migratory capability than LNCaP cells. As a result, some researchers consider LNCaP and PC3 cells to represent less aggressive and more aggressive forms of prostatic adenocarcinoma, respectively. In some publications, the two cell lines have also been used to represent androgen-dependent and castration-resistant PCs, respectively. Therefore, certain molecular differences between the two cell lines have been considered to be responsible for the aggressiveness or the progression of prostatic adenocarcinoma.
The above notion is inconsistent with histologic observations in human tumors. Gleason grading has been one of the most useful histologic parameter in predicting the biologic behavior and clinical outcomes of prostatic adenocarcinoma. Prostatic adenocarcinoma, which comprises over 90% of all PCs, nearly always expresses AR and PSA irrespective of Gleason grade, so do the vast majority of castration-resistant PCs [34–38]. The recently reported efficacy of MDV3100 and Abiraterone in treating castration-resistant PC further supports the notion that in the late stage of PC, AR is still expressed, being functional and critical for the survival of the tumor cells [39]. Therefore, having a cell line that is negative for AR and PSA to represent an aggressive type or a castration-resistant form of prostate adenocarcinoma is problematic and likely misleading.
On the other hand, rare cases of PCs are variant forms including prostatic SCNC which shows NE phenotype. Prostatic SCNC is extremely aggressive and usually causes deaths in months. It is different from prostatic adenocarcinoma in that the tumor cells do not express luminal differentiation markers AR, PSA and are not responsive to hormonal therapy which provides short-term benefits in nearly all patients with advanced and metastatic prostatic adenocarcinoma. Therefore, SCNC is an entirely different disease than prostatic adenocarcinoma and it is important to distinguish the two entities clinically and pathologically for treatment and prognosis purposes [13].
Here we have provided convincing evidence demonstrating that LNCaP cells have features of prostatic adenocarcinoma including the expression of luminal differentiation markers AR and PSA, while PC3 cells have features typical of prostatic SCNC such as being negative for AR and PSA but positive for NE markers. Consistent with these observations, androgen withdrawal induces growth inhibition of LNCaP cells but not PC3 cells. It must be noted that androgen withdrawal can induce NE differentiation in LNCaP cells [40], and may also do so in human adenocarcinoma [41,42], while the NE phenotype and other features similar to SCNC observed in PC3 cells are present under normal culture conditions without any external stimuli [21,25–28].
The patterns of expression of CD44, a putative cell surface marker for normal and cancer stem cells including PC stem cells, are also consistent with our conclusion. Tang’s group has shown that expression of CD44 identifies PC cells with increased tumor initiation potential [30]. Both his group and ours have observed that while LNCaP cells do not express CD44, nearly 100% of PC3 cells express this marker [28,30]. We have also demonstrated that CD44 is not expressed in human prostatic adenocarcinoma (with the exception of the rare NE tumor cells) [28] but it is strongly and diffusely expressed in human prostatic SCNC [24], further supporting our conclusion.
The cell of origin for prostatic adenocarcinoma remains controversial. Traditionally luminal cells are considered to be the likely origin because the precursor of prostatic adenocarcinoma (high-grade prostatic intraepithelial neoplasia, or HGPIN) is characterized by cytologic atypia in luminal cells histologically. This theory is supported by a recent study in an animal model [43]. However, other studies have shown that basal cells are the likely cells of origin for adenocarcinoma [44–46]. Nevertheless, the bulk tumor cells of adenocarcinoma share features of luminal cells such as the expression of AR, PSA, and luminal cytokeratins, as do LNCaP cells. The cell of origin for prostatic SCNC is even less clear. The tumor cells of prostatic SCNC do not express basal cell markers or luminal cell markers AR and PSA. In addition, anti-CK8 staining shows focal, dot-like staining pattern, a featured characteristic of small cell carcinoma [13]. PC3 cells share all the characteristics of prostatic SCNC.
Results similar to what we have presented in this manuscript have been reported from time to time in various publications and represent well-established facts widely known to PC researchers. In this manuscript, we have for the first time put all of them together in a rational manner and arrived at the important conclusion that PC3 is likely a cell line of prostatic SCNC. At the time of manuscript preparation, a Pubmed search using “prostate AND PC3 or PC-3” returned more than 5,000 articles all related to PC. It can be assumed that in all of those studies, PC3 cells were considered to represent an aggressive form or advanced (castration-independent) stage of prostate adenocarcinoma. Our observations and conclusions have significant implications as molecular mechanisms and therapeutic efficacies observed with PC3 cells are likely applicable to prostatic SCNC but not to the adenocarcinoma.
Despite all the recent progresses, LNCaP and PC3 cells, with features characteristic of adenocarcinoma (AR+, PSA+, androgen-dependent, diffuse positivity for CK8) and SCNC (NE markers+, castration-resistant, CD44+, focal positivity for CK8), respectively, will continue to help us understand the molecular mechanisms of the two different tumors and aid in our effort in developing novel therapies. The main objective of our manuscript is to point out to investigators that PC3 cells should not be considered a cell line representing prostatic adenocarcinoma, a notion that has been held ever since this cell line was established more than 30 years ago.
CONCLUSION
We conclude that while LNCaP cells have features of prostatic adenocarcinoma, PC3, a cell line often considered to represent a more aggressive form or castration-resistant PC, actually shares important features with prostatic SCNC, a conclusion that has important implications to the field of PC research.
Acknowledgments
Grant sponsor: American Cancer Society; Grant sponsor: DOD Prostate Cancer Research Program; Grant sponsor: UCLA Prostate Cancer SPORE (PI: Reiter); Grant sponsor: Prostate Cancer Research Foundation (PI: Witte); Grant sponsor: Prostate Cancer Research Foundation (PI: Rettig).
J.H. is supported by grants from American Cancer Society, DOD Prostate Cancer Research Program, UCLA Prostate Cancer SPORE (PI: Reiter), a Challenge Award from the Prostate Cancer Research Foundation (PI: Witte), a Creativity Award from the Prostate Cancer Research Foundation (PI: Rettig). We thank Dr. Rob Reiter for providing the xenograft tumors.
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- 1.Cooperberg MR, Park S, Carroll PR. Prostate cancer 2004: Insights from national disease registries. Oncology (Williston Park) 2004;18:1239–1247. discussion 48–50, 56–58. [PubMed] [Google Scholar]
- 2.Sun Y, Niu J, Huang J. Neuroendocrine differentiation in prostate cancer. Am J Transl Res. 2009;1:148–162. [PMC free article] [PubMed] [Google Scholar]
- 3.Huang J, Wu C, di Sant’Agnese PA, Yao JL, Cheng L, Na Y. Function and molecular mechanisms of neuroendocrine cells in prostate cancer. Anal Quant Cytol Histol. 2007;29:128–138. [PubMed] [Google Scholar]
- 4.Yuan TC, Veeramani S, Lin MF. Neuroendocrine-like prostate cancer cells: Neuroendocrine transdifferentiation of prostate adenocarcinoma cells. Endocr Relat Cancer. 2007;14:531–547. doi: 10.1677/ERC-07-0061. [DOI] [PubMed] [Google Scholar]
- 5.Vashchenko N, Abrahamsson PA. Neuroendocrine differentiation in prostate cancer: Implications for new treatment modalities. Eur Urol. 2005;47:147–155. doi: 10.1016/j.eururo.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Bonkhoff H. Neuroendocrine differentiation in human prostate cancer. Morphogenesis, proliferation and androgen receptor status. Ann Oncol. 2001;12 (Suppl 2):S141–S144. doi: 10.1093/annonc/12.suppl_2.s141. [DOI] [PubMed] [Google Scholar]
- 7.Huang J, Yao JL, di Sant’agnese PA, Yang Q, Bourne PA, Na Y. Immunohistochemical characterization of neuroendocrine cells in prostate cancer. Prostate. 2006;66:1399–1406. doi: 10.1002/pros.20434. [DOI] [PubMed] [Google Scholar]
- 8.Grignon DJ. Unusual subtypes of prostate cancer. Mod Pathol. 2004;17:316–327. doi: 10.1038/modpathol.3800052. [DOI] [PubMed] [Google Scholar]
- 9.Yao JL, Madeb R, Bourne P, Lei J, Yang X, Tickoo S, Liu Z, Tan D, Cheng L, Hatem F, Huang J, Anthony di Sant’Agnese P. Small cell carcinoma of the prostate: An immunohistochemical study. Am J Surg Pathol. 2006;30:705–712. doi: 10.1097/00000478-200606000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Wang W, Epstein JI. Small cell carcinoma of the prostate: A morphologic and immunohistochemical study of 95 cases. Am J Surg Pathol. 2008;32:65–71. doi: 10.1097/PAS.0b013e318058a96b. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka M, Suzuki Y, Takaoka K, Suzuki N, Murakami S, Matsuzaki O, Shimazaki J. Progression of prostate cancer to neuroendocrine cell tumor. Int J Urol. 2001;8:431–436. doi: 10.1046/j.1442-2042.2001.00347.x. discussion 7. [DOI] [PubMed] [Google Scholar]
- 12.Miyoshi Y, Uemura H, Kitami K, Satomi Y, Kubota Y, Hosaka M. Neuroendocrine differentiated small cell carcinoma presenting as recurrent prostate cancer after androgen deprivation therapy. BJU Int. 2001;88:982–983. doi: 10.1046/j.1464-4096.2001.00936.x. [DOI] [PubMed] [Google Scholar]
- 13.Wagner D, Huang J. Small cell carcinoma of the prostate. eMedicine from WebMD. 2009 http://emedicine.medscape.com/article/1611899-overview.
- 14.Amato RJ, Logothetis CJ, Hallinan R, Ro JY, Sella A, Dexeus FH. Chemotherapy for small cell carcinoma of prostatic origin. J Urol. 1992;147:935–937. doi: 10.1016/s0022-5347(17)37427-x. [DOI] [PubMed] [Google Scholar]
- 15.Papandreou CN, Daliani DD, Thall PF, Tu SM, Wang X, Reyes A, Troncoso P, Logothetis CJ. Results of a phase II study with doxorubicin, etoposide, and cisplatin in patients with fully characterized small-cell carcinoma of the prostate. J Clin Oncol. 2002;20:3072–3080. doi: 10.1200/JCO.2002.12.065. [DOI] [PubMed] [Google Scholar]
- 16.Tetu B, Ro JY, Ayala AG, Johnson DE, Logothetis CJ, Ordonez NG. Small cell carcinoma of the prostate. Part I. A clinicopathologic study of 20 cases. Cancer. 1987;59:1803–1809. doi: 10.1002/1097-0142(19870515)59:10<1803::aid-cncr2820591019>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 17.Oesterling JE, Hauzeur CG, Farrow GM. Small cell anaplastic carcinoma of the prostate: A clinical, pathological and immunohistological study of 27 patients. J Urol. 1992;147:804–807. doi: 10.1016/s0022-5347(17)37390-1. [DOI] [PubMed] [Google Scholar]
- 18.Horoszewicz JS, Leong SS, Chu TM, Wajsman ZL, Friedman M, Papsidero L, Kim U, Chai LS, Kakati S, Arya SK, Sandberg AA. The LNCaP cell line—A new model for studies on human prostatic carcinoma. Prog Clin Biol Res. 1980;37:115–132. [PubMed] [Google Scholar]
- 19.Horoszewicz JS, Leong SS, Kawinski E, Karr JP, Rosenthal H, Chu TM, Mirand EA, Murphy GP. LNCaP model of human prostatic carcinoma. Cancer Res. 1983;43:1809–1818. [PubMed] [Google Scholar]
- 20.Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW. Establishment and characterization of a human prostatic carcinoma cell line (PC-3) Invest Urol. 1979;17:16–23. [PubMed] [Google Scholar]
- 21.van Bokhoven A, Varella-Garcia M, Korch C, Johannes WU, Smith EE, Miller HL, Nordeen SK, Miller GJ, Lucia MS. Molecular characterization of human prostate carcinoma cell lines. Prostate. 2003;57:205–225. doi: 10.1002/pros.10290. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka H, Kono E, Tran CP, Miyazaki H, Yamashiro J, Shimomura T, Fazli L, Wada R, Huang J, Vessella RL, An J, Horvath S, Gleave M, Rettig MB, Wainberg ZA, Reiter RE. Monoclonal antibody targeting of N-cadherin inhibits prostate cancer growth, metastasis and castration resistance. Nat Med. 2010;16:1414–1420. doi: 10.1038/nm.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang J, Yao JL, Zhang L, Bourne PA, Quinn AM, di Sant’Agnese PA, Reeder JE. Differential expression of interleukin-8 and its receptors in the neuroendocrine and non-neuroendocrine compartments of prostate cancer. Am J Pathol. 2005;166:1807–1815. doi: 10.1016/S0002-9440(10)62490-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon RA, di Sant’Agnese PA, Huang LS, Xu H, Yao JL, Yang Q, Liang S, Liu J, Yu R, Cheng L, Oh WK, Palapattu GS, Wei J, Huang J. CD44 expression is a feature of prostatic small cell carcinoma and distinguishes it from its mimickers. Hum Pathol. 2009;40:252–258. doi: 10.1016/j.humpath.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 25.Zhang C, Soori M, Miles FL, Sikes RA, Carson DD, Chung LW, Farach-Carson MC. Paracrine factors produced by bone marrow stromal cells induce apoptosis and neuroendocrine differentiation in prostate cancer cells. Prostate. 2011;71:157–167. doi: 10.1002/pros.21231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marchiani S, Tamburrino L, Nesi G, Paglierani M, Gelmini S, Orlando C, Maggi M, Forti G, Baldi E. Androgen-responsive and -unresponsive prostate cancer cell lines respond differently to stimuli inducing neuroendocrine differentiation. Int J Androl. 2010;33:784–793. doi: 10.1111/j.1365-2605.2009.01030.x. [DOI] [PubMed] [Google Scholar]
- 27.Uysal-Onganer P, Kawano Y, Caro M, Walker MM, Diez S, Darrington RS, Waxman J, Kypta RM. Wnt-11 promotes neuroendocrine-like differentiation, survival and migration of prostate cancer cells. Mol Cancer. 2010;9:55. doi: 10.1186/1476-4598-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palapattu GS, Wu C, Silvers CR, Martin HB, Williams K, Salamone L, Bushnell T, Huang LS, Yang Q, Huang J. Selective expression of CD44, a putative prostate cancer stem cell marker, in neuroendocrine tumor cells of human prostate cancer. Prostate. 2009;69:787–798. doi: 10.1002/pros.20928. [DOI] [PubMed] [Google Scholar]
- 29.Marhaba R, Klingbeil P, Nuebel T, Nazarenko I, Buechler MW, Zoeller M. CD44 and EpCAM: Cancer-initiating cell markers. Curr Mol Med. 2008;8:784–804. doi: 10.2174/156652408786733667. [DOI] [PubMed] [Google Scholar]
- 30.Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S, Reilly JG, Chandra D, Zhou J, Claypool K, Coghlan L, Tang DG. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25:1696–1708. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- 31.Yang Y, Hao J, Liu X, Dalkin B, Nagle RB. Differential expression of cytokeratin mRNA and protein in normal prostate, prostatic intraepithelial neoplasia, and invasive carcinoma. Am J Pathol. 1997;150:693–704. [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Hayward S, Cao M, Thayer K, Cunha G. Cell differentiation lineage in the prostate. Differentiation. 2001;68:270–279. doi: 10.1046/j.1432-0436.2001.680414.x. [DOI] [PubMed] [Google Scholar]
- 33.Petraki C, Vaslamatzis M, Petraki K, Revelos K, Alevizopoulos N, Papanastasiou P, Gregorakis A. Prostate cancer with small-cell morphology: An immunophenotypic subdivision. Scand J Urol Nephrol. 2005;39:455–463. doi: 10.1080/00365590500199855. [DOI] [PubMed] [Google Scholar]
- 34.Holzbeierlein J, Lal P, LaTulippe E, Smith A, Satagopan J, Zhang L, Ryan C, Smith S, Scher H, Scardino P, Reuter V, Gerald WL. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004;164:217–227. doi: 10.1016/S0002-9440(10)63112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buchanan G, Irvine RA, Coetzee GA, Tilley WD. Contribution of the androgen receptor to prostate cancer predisposition and progression. Cancer Metastasis Rev. 2001;20:207–223. doi: 10.1023/a:1015531326689. [DOI] [PubMed] [Google Scholar]
- 36.Sadi MV, Barrack ER. Image analysis of androgen receptor immunostaining in metastatic prostate cancer. Heterogeneity as a predictor of response to hormonal therapy. Cancer. 1993;71:2574–2580. doi: 10.1002/1097-0142(19930415)71:8<2574::aid-cncr2820710823>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 37.Pertschuk LP, Schaeffer H, Feldman JG, Macchia RJ, Kim YD, Eisenberg K, Braithwaite LV, Axiotis CA, Prins G, Green GL. Immunostaining for prostate cancer androgen receptor in paraffin identifies a subset of men with a poor prognosis. Lab Invest. 1995;73:302–305. [PubMed] [Google Scholar]
- 38.Culig Z, Hobisch A, Hittmair A, Peterziel H, Cato AC, Bartsch G, Klocker H. Expression, structure, and function of androgen receptor in advanced prostatic carcinoma. Prostate. 1998;35:63–70. doi: 10.1002/(sici)1097-0045(19980401)35:1<63::aid-pros9>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 39.Sharifi N. New agents and strategies for the hormonal treatment of castration-resistant prostate cancer. Expert Opin Invest Drugs. 2010;19:837–846. doi: 10.1517/13543784.2010.494178. [DOI] [PubMed] [Google Scholar]
- 40.Burchardt T, Burchardt M, Chen MW, Cao Y, de la Taille A, Shabsigh A, Hayek O, Dorai T, Buttyan R. Transdifferentiation of prostate cancer cells to a neuroendocrine cell phenotype in vitro and in vivo. J Urol. 1999;162:1800–1805. [PubMed] [Google Scholar]
- 41.Ahlegren G, Pedersen K, Lundberg S, Aus G, Hugosson J, Abrahamsson P. Neuroendocrine differentiation is not prognostic of failure after radical prostatectomy but correlates with tumor volume. Urology. 2000;56:1011–1015. doi: 10.1016/s0090-4295(00)00838-4. [DOI] [PubMed] [Google Scholar]
- 42.Jiborn T, Bjartell A, Abrahamsson PA. Neuroendocrine differentiation in prostatic carcinoma during hormonal treatment. Urology. 1998;51:585–589. doi: 10.1016/s0090-4295(97)00684-5. [DOI] [PubMed] [Google Scholar]
- 43.Wang X, Kruithofde Julio M, Economides KD, Walker D, Yu H, Halili MV, Hu YP, Price SM, Abate-Shen C, Shen MM. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature. 2009;461:495–500. doi: 10.1038/nature08361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mulholland DJ, Xin L, Morim A, Lawson D, Witte O, Wu H. Lin-Sca-1+CD49fhigh stem/progenitors are tumor-initiating cells in the Pten-null prostate cancer model. Cancer Res. 2009;69:8555–8562. doi: 10.1158/0008-5472.CAN-08-4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lawson DA, Zong Y, Memarzadeh S, Xin L, Huang J, Witte ON. Basal epithelial stem cells are efficient targets for prostate cancer initiation. Proc Natl Acad Sci USA. 2010;107:2610–2615. doi: 10.1073/pnas.0913873107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldstein AS, Huang J, Guo C, Garraway IP, Witte ON. Identification of a cell of origin for human prostate cancer. Science. 2010;329:568–571. doi: 10.1126/science.1189992. [DOI] [PMC free article] [PubMed] [Google Scholar]