Abstract
A truncated form of 24kDa FGF-2 consisting of 86 NH2-terminal amino acids (ATE+31) inhibits cell migration in vitro and tumor development and angiogenesis in vivo. Focal adhesion kinase (FAK) is phosphorylated on tyrosine and serine sites after cell stimulation by growth factors. This study examined the effect of ATE+31 on FAK phosphorylation in human glioma cells. FAK and Pyk phosphorylation were evaluated at serines known to be involved with cell migration. We demonstrated that ATE+31 at 3 × 10−11M decreases phosphorylation levels of Tyr407-FAK and Ser732-FAK in the presence of platelet-derived growth factor (PDGF), that ATE+31 in the presence of PDGF alters the distribution of FAK and other phosphotyrosine proteins in the adhesion contacts, and that ATE+31 in the presence of PDGF has no effect on the activation of Pyk2. These data suggest that the inhibition of cell migration by ATE+31 occurs via Tyr407-FAK and Ser732-FAK.
Keywords: Malignant glioma, FAK, phosphorylation, Pyk2
Introduction
Cell migration plays a vital role in a variety of biological and disease processes such as embryonic development, inflammation, wound healing, and cancer metastasis [1]. Various growth factor receptors and integrins have essential roles in the regulation of cell migration by growth factors [2, 3, 4, 5]. Platelet-derived growth factor (PDGF) initiates glioma cell migration after binding to the tyrosine kinase receptor, PDGFr. PDGF, via PDGFr and intergrin αvβ3, is known to promote cell migration in fibroblast and glioma cells via activation of adhesion kinase (FAK) and Src family of proto-oncogenes.
Focal adhesion kinase (FAK) is a non-receptor tyrosine kinase protein that serves as a major mediator of cell migration [6]. FAK activation following growth factor treatment or integrin engagement occurs concomitantly with phosphorylation of specific tyrosines, notably tyrosines 397, 407, 576, 577, 861 and 925. The initial step in FAK activation involves autophosphorylation of tyrosine 397 (Y397-FAK) followed by phosphorylation of other tyrosine and serine/threonine residues within FAK. Among the six tyrosine phosphorylation sites in FAK, Y407 has been suggested to be a key residue involved in mediating cell migration [7, 8].
Basic fibroblast growth factor (FGF-2) is produced as multiple forms of varying molecular weights, all derived from a single gene through alternative translational initiation sites [9]. One of these forms, 24kDa FGF-2, has been shown to have dual functions, stimulation of cell proliferation and inhibition of cell migration [10]. Deletion mutagenesis of the 24kDa FGF2 gene was used to engineer a protein that could inhibit the migration of a variety of tumor and non-tumor cells with no effect on cell proliferation. ATE+31 is an 8400 MW protein which inhibits the migration of a variety of normal and malignant cells as well as angiogenesis and tumor growth in vivo [11, 12].
We report that ATE+31 inhibits glioma cell migration by blocking the phosphorylation of serine732 and tyrosine407 within FAK. The decrease in phosphorylation levels at these specific two sites correlate with the change in the distribution of FAK and other additional phosphotyrosine proteins in focal adhesions, indicating that ATE+31 inhibits cell migration through the suppression of FAK phosphorylation.
Material and Methods
Cells
Human U-87 MG glioma cell line was a kind gift from Dr. Carol Kruse (Sidney Kimmel Cancer Center, La Jolla, CA). For all experiments, cells were serum starved overnight and plated onto vitronectin-coatd plates or coverslips to allowing seeding and spreading. Five hours after seeding, cells were then treated with or without ATE+31 (3 × 10−11 M) for 1 hr, followed by 10 minutes treatment with or without PDGF (5ng/ml).
Construction ATE+31 expression vector and generation of ATE+31
ATE+31 was constructed and expressed as described [11].
Transient transfection of U-87 MG cells expressing EGFP-mFAK
A plasmid DNA containing a EGFP fused to N-terminus of murine FAK was kindly provided by Dr. Li-Huei Tsai, Massachusetts Institute of Technology [7]. Approximately 8 × 105 cells were seeded onto 60mm plate the day before transfection. Five microgram of pEGFP-mFAK was used for each transfection using Superfect tranfection reagent (Qiagen). Transfection procedure was performed according to the manufacture’s protocol. Thirty-six hours post transfection, cells were serum starved overnight and plated onto vitronectin coated coverslips for immunofluorescence staining.
Migration Assay
Cell migration assays were performed using modified Boyden chambers with a 6.5-mm diameter, 8.0 µm porous polycarbonate membrane (Nalge Nunc International). Both sides of the membrane were coated with vitronectin at 1µg/ml. Three hundred microliters of serum-starved cells (5 × 105 cells/ml) were plated on the top chamber of the insert and PDGF (10 ng/ml) was placed in the lower compartment. ATE+31 was added at the indicated concentration in the upper and lower compartments. Cells were allowed to migrate for 2 h at 37° C in 5% CO2. At the end of the assay, the upper surface of the membrane was wiped with a cotton-tip applicator to remove non-migratory cells. Cells on the lower surface were fixed in 1 % paraformaldehyde, stained with 1% crystal violet and counted under a light microscope.
Immunoblot Analysis
Cell lysates (prepared with RIPA buffer) representing equal amounts of protein were subjected to electrophoresis on 10% polyacrylamide gels. After transfer to nitrocellulose membranes, the extracts were probed with antibodies to Lyn (Santa Cruz Biotechnology), phospho-FAK[Y397], phospho-FAK[p Y407], phospho-FAK[p S732] (BioSource/Invitrogen), phospho-Pyk2[pY402] and anti-phospho-Src[pY416] (Cell Signaling Technology) and total FAK and Pyk2 (Upstate Cell Signaling Solutions). Antibody complexes were detected with goat anti-mouse or rabbit IgG conjugated to horseradish peroxidase (Bio-Rad) and the ECL chemiluminescence reagent (GE Healthcare).
Immunofluorescence staining
For visualization of eGFP-mFAK and actin, U-87 MG cells were transfected with eGFP-mFAK using Superfect transfection reagent (Qiagen). Thirty-six hours post transfection, cells were serum-starved overnight and then plated on glass coverslips coated with vitronectin (5µg/ml). The cells were treated with or without ATE+31 in the presence or absence of PDGF 5 hr after plating, fixed with 3.7% paraformaldehyde at room temperature, permeabilized with 0.2% Triton X-100, and stained with phalloiden conjugated with Alexa fluoro dye 488. For visualization of phosphotyrosine and actin, cells were stained with antibody against phosphotyrosine (Upstate/Millipore). All cells were mounted in VectorShield (Vector Laboratories) for visualization under a confocal microscope (Olympus FV1000).
Results
ATE+31 inhibits U-87 MG cell migration in a concentration dependent manner
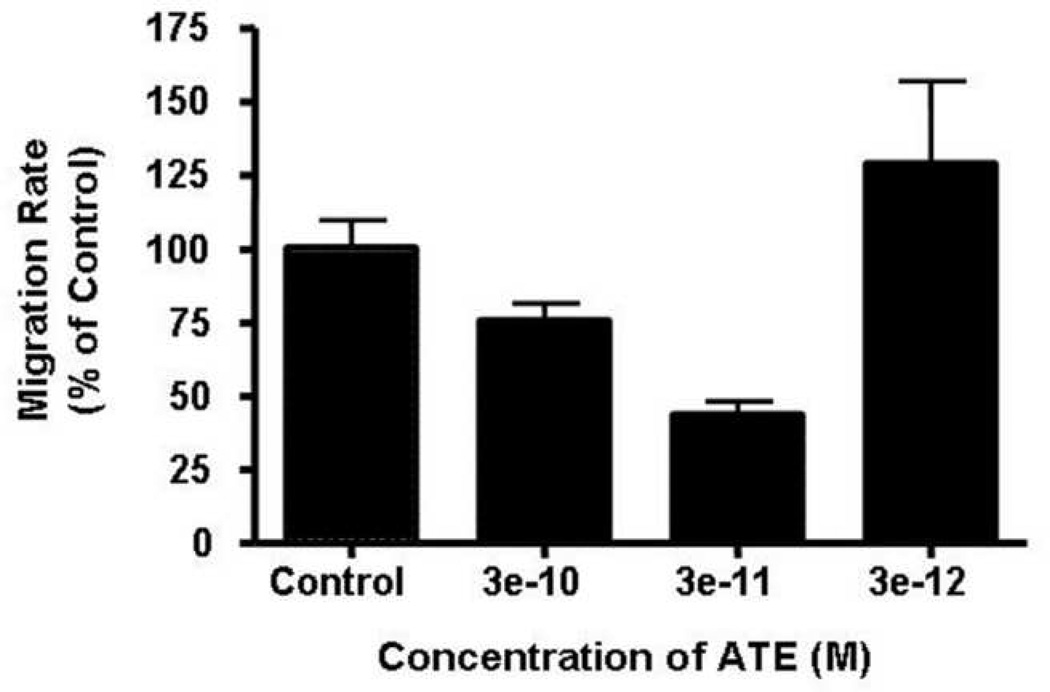
ATE+31 inhibits migration of a variety of tumor and normal cells at concentrations around 10−10M [11]. To determine the effective concentration of ATE+31 for the treatment of U-87 MG cells, the cells were exposed to 3 × 10−10 to 3 × 10−12 M ATE+31 and the rates of migration were determined in Boyden Chamber assays. ATE+31 inhibited U-87 MG cell migration in a dose dependent manner with a 70% reduction in migration rate occurring at 3 × 10−11M.
ATE+31 has no effect on phosphorylation of Y397 in FAK nor Y416 in Lyn in the presence of PDGF
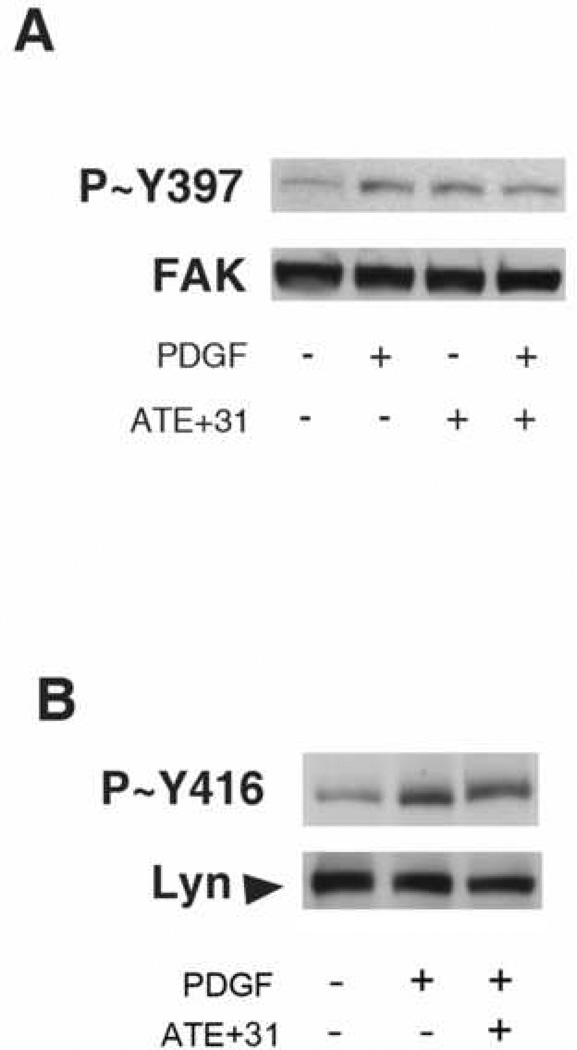
Enhanced phosphorylation of FAK results in increased signaling complex formation with other scaffolding proteins, enhanced FAK kinase activity, adhesion turnover, and motility. Tyr397 is an autophosphorylation site that becomes phosphorylated in response to treatment with growth factors, lipoproteins, and activation of integrins. The autophosphorylation of this tyrosine triggers downstream events leading to cell migration. Src recruitment to FAK also occurs after growth factor treatment of cultured cells and the FAK–Src complex is important in the regulation of growth factor–stimulated cell migration [6]. Src kinase protein becomes activated immediately after phosphorylation at Y397-FAK and subsequently phosphorylates additional Y397-FAK and other tyrosine residues in FAK. Among Src family members, including c-Src, Yes, Fyn, Lyn, Fgr and Lck, it has been shown that U-87 MG cells express high levels of Fyn and Lyn and have low levels of c-Src and Yes protein. However, PDGF stimulation activates Lyn kinase preferentially in vitronectin-adherent glioblastoma cells and increases motility in a Lyn kinase-dependent manner while having no effect of Fyn [5]. Thus, under the condition employed in this study, a possible inhibitory target of ATE+31 would be the suppression of Lyn kinase, disrupting the complete phosphorylation of Y397 as well as the other tyrosines on FAK that are phosphorylated in a Src dependent manner (Y576 and Y861). To determine whether PDGF-induced FAK/Src phosphorylation of these activation sites is altered by ATE+31 treatment, the effect of ATE+31 on the PDGF-induced phosphorylation of FAK-Y397 and Lyn-Y416 was evaluated. As shown in Figure 2A, an increase in phosphorylation of Y397 in response to PDGF was unaffected by the presence of ATE+31. The phosphorylation level of Y397 also remained unchanged in cells treated ATE+31 alone. Similar results were observed with Lyn. ATE+31 had no effect on the activation of Y416-Lyn by PDGF (Figure 2B). Further evidence that Src dependent FAK phosphorylation was not involved with the effect of ATE+31 was sought by analyzing the phosphorylation levels of Y576 -and Y861- FAK. The phosphorylation states of both of these tyrosines are elevated with PDGF treatment in a Src-dependent manner [13]. In neither case was the phosphorylation level affected by ATE+31 (data not shown), a result consistent with the failure of ATE+31 to inhibit the phosphorylation of Lyn Y416.
Figure 2. Phosphorylation of Y397-FAK and Y416-Lyn were not affected by ATE+31.
U-87 MG cells were treated with 3 × 10−11 M ATE+31 for 1 hr and then 10 ng/ml PDGF for 10min. Cell extracts were analyzed for Y-397 FAK tyrosine phosphorylation and total FAK (A) and Y416 tyrosine phosphorylation and total Lyn (B) by Western blot analysis.
ATE+31 blocks phosphorylation of Y407 and S732 in FAK
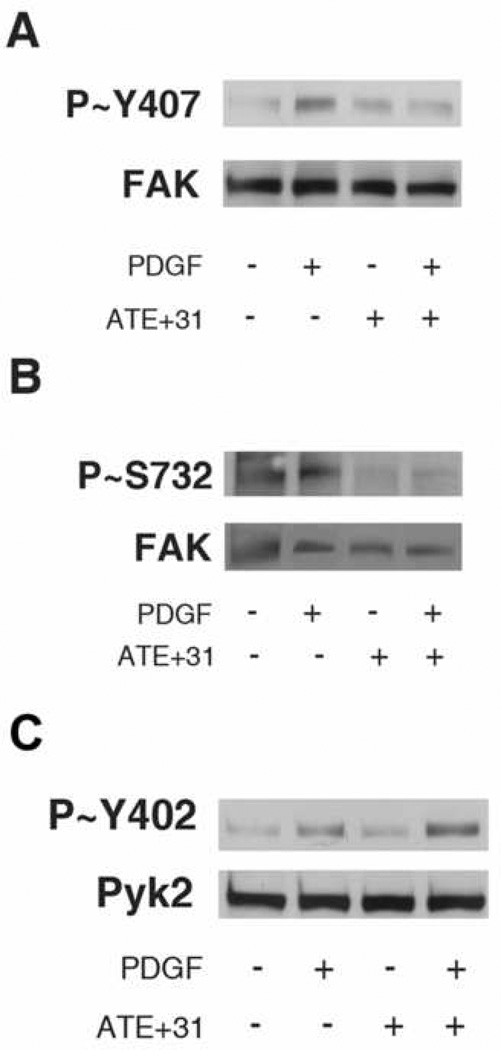
Another FAK phospho-tyrosine implicated in the control of cell migration and independent of Src kinase activation is Y407-FAK [14, 8]. Analysis of the effect of ATE+31 on PDGF activated Y407-FAK phosphorylation showed that cells treated with ATE+31 partially reduced the phosphorylation level of Y407-FAK in the presence of PDGF (Figure 3A). LeBœuf et al. [8], demonstrated that Y407 –FAK phosphorylation is downstream of S732 phosphorylation in endothelial cells and that blocking S732–FAK phosphorylation inhibits phosphorylation of Y407-FAK. To determine whether the phosphorylation states of Y407-FAK and S732-FAK may be linked in U-87 MG cells, we examined whether the decrease in PDGF-dependent phosphorylation of Y407-FAK by ATE+31 was associated with the phosphorylation level of S732 in FAK. Cells were treated as described above and the level of S732 determined by immunoblotting. The phosphorylation level of S732 was significantly reduced in ATE+31 treated PDGF-induced cells (Figure 3B).
Figure 3. Phosphorylation of Y407-FAK, S732-FAK, and Pyk2 with ATE+31 treatment.
U-87 MG cells were treated with 3 × 10−11 M ATE+31 for 1 hr and then 10 ng/ml PDGF for 10 min. Cell extracts were analyzed by Western blot analysis for Y407-FAK tyrosine phosphorylation (A), S732-FAK serine phosphorylation (B), and Y402-Pyk2 tyrosine phosphorylation (C). Total Pyk2 and total FAK are also shown.
Pyk2 activation is not affected by ATE+31 in PDGF-induced Cells
Proline-rich tyrosine kinase 2 (Pyk2) is a FAK-related protein that has high sequence homology and a conserved structural domain with FAK. Several groups have reported that Pyk2 plays a role in cell migration, and that the ratio between the two proteins may be important in regulating cell migration in some cells [15, 16, 17]. In FAK-deficient MEF cells, Pyk2 levels increase, possibly compensating for the absence of FAK [6, 18]. We have shown previously that ATE+31 does not inhibit cell migration in FAK deficient MEF cells, suggesting that ATE+31 does not have an effect on the function of Pyk2 [19].
Activation of Pyk2 is indicated by an increase in phosphorylation level of Y402-Pyk2 after stimulation by growth factor. Our data showed that stimulation with PDGF in U-87 MG cells activates Pyk2 as indicated by an increase in phosphorylation of Y402. The phosphorylation level of Y402-Pyk2 in cells treated with ATE+31 alone was similar to untreated cells. Cells treated with PDGF alone and cells treated with PDGF and ATE+31 showed an increase in the phosphorylation levels of Y402-Pyk2 (Figure 4).
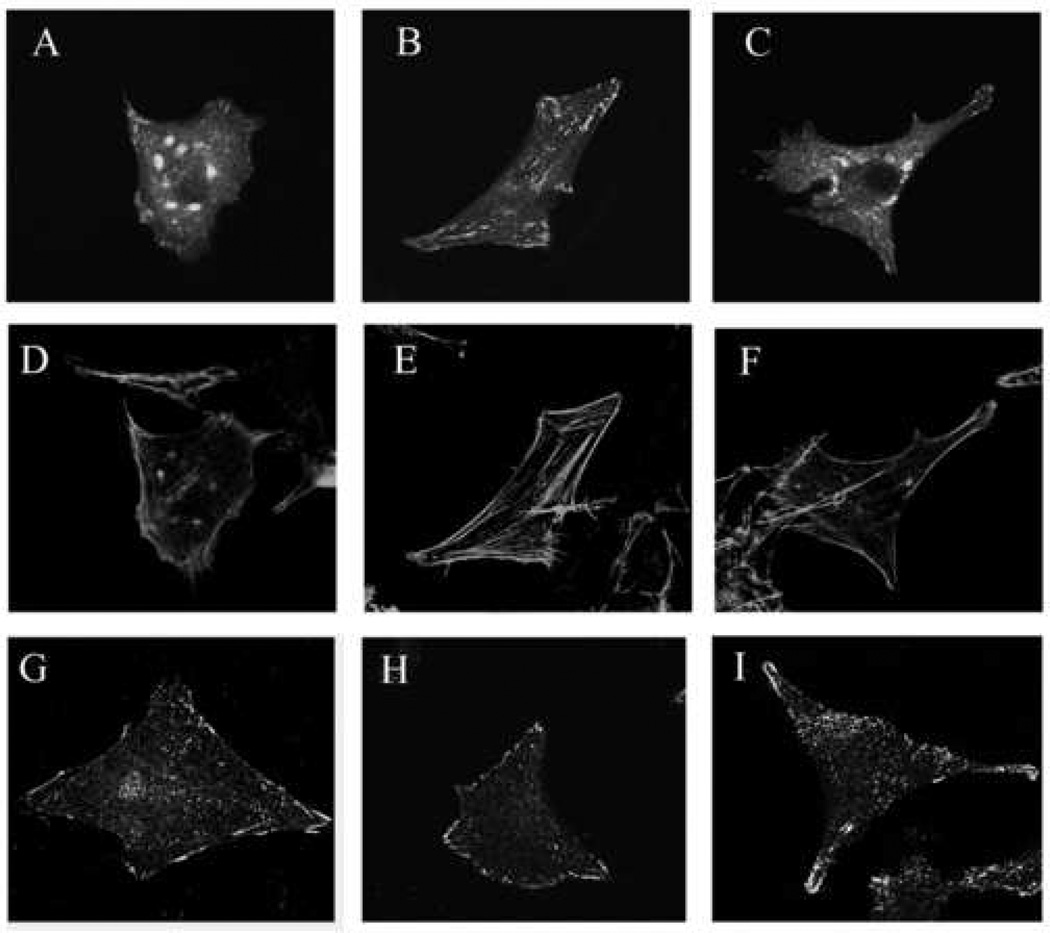
Figure 4. Effect of ATE+31 on FAK, microfilament, and focal adhesion distribution.
U-87 MG cells were seeded on coverslips coated with 5 µg/ml vitronectin for five hrs and then treated with either saline or ATE+31 for 1 hr, followed by treatment with either saline or 10 ng/ml PDGF for 10 min. Cells were fixed with 3.7% paraformaldehyde. Cells to be analyzed for the distribution of FAK were previously transfected with eGFP-FAK (Panels A–C). Actin microfilaments and adhesion plaques were visualized by staining the cells with either Alex fluoro dye 546-conjugated phalloiden (Panels D–F) or anti-phosphotyrosine antibody followed by Alexa fluorophore 488-conjugated seconday antibody (Panels G–I), respectivley. Images were captured at 100X magnification by fluorescence confocal microscopy. Panels A–C. Distribution of GFP-FAK; D–F. Distribution of Actin; G–I. Distribution of Focal adhesion plaques. A,D,G. Untreated cells; B,E, H. PDGF treatment; C, F, I. PDGF plus ATE+31.
ATE+31 alters distribution of FAK and phosphotyrosine proteins in PDGF-induced Cells
Changes in the organization of microfilaments and the distribution of focal adhesions occur in response to effecter molecules that regulate the migration rate of the cells. To determine whether ATE+31 affects focal adhesion and microfilament re-organization following PDGF treatment, we examined the distribution of FAK and actin by confocal microscopy using a fusion protein of eGFP and murine FAK. In the absence of PDGF, the eGFP-mFAK is found at the periphery of the cell and distributed throughout the cytoplasm. In both locations the fluorescence appears in short, almost punctuate structures except for a single area at the upper part of the cell. Treatment with PDGF resulted in a coalescence of FAK into thicker more fluorescently intense fibers predominately within the cell cytoplasm and at specific locations at the cell periphery. When the experiment was repeated with ATE+31 present, the distribution of FAK returned to the pattern observed with no treatment at all. (Figure 5A–C). Notably, an abundance of eGFP-mFAK observed as dense oval shaped structures was also present within the perinuclear region, an observation reported previously in primary cortical neurons transfected with FAK [7]. The eGFP-mFAK in the perinuclear region are not detected in non-transfected or transfected cells that express very low level of eGFP-mFAK. This perinuclear concentration of FAK disappeared when cells were treated with PDGF and reappeared when the PDGF was administered in the presence of ATE+31 (Figure 5C).
We also examined the formation of stress fibers in the presence and absence of ATE+31 in PDGF treated U-87 MG cells. In untreated cells, a thin layer of actin stress fibers was found at the cell periphery with little observed within the cytoplasm. With PDGF, stress fibers became more prominent along the periphery and appeared in the cytoplasm as thick strands. In the presence of ATE+31 stress fiber organization returned to the pattern observed in control cells, consistent with the reversal of FAK organization found under the same conditions. (Figure 5D–E).
Focal adhesions contain highly tyrosine phosphorylated proteins such as αvβ3 integrin, vinculin and paxillin [3, 20, 21] and can be visualized by staining permeabilized cells with anti-phosphotyrosine antibodies. In untreated cells, phosphotyrosine staining occurred predominately at the cell periphery particularly at the leading or trailing edges of the cell. (Figure 5G). After PDGF treatment, phosphotyrosine staining became more punctuate at cell periphery and extended into the cytoplasm (Figure 5H). The puntate staining of phosphotyrosine observed in the cytoplasm correlates with substantial stress fibers formed by actins (Figure 5E). When PDGF-induced cells were treated with ATE+31, the phosphotyrosine staining pattern returned to that observed in untreated cells in 60–70% of the cell population (Figure 5I). This pattern of staining also correlates with reduction of stress fiber formation in the cytoplasm (Figure 5F).
Discussion
The phosphorylation of specific tyrosine and serines modulates the function of FAK in different ways. The results presented here indicate that ATE+31 inhibits cell migration through the modulation of Y407-FAK and S732-FAK phosphorylation leading to specific functional changes in FAK. In endothelial cells, Y407 phosphorylation plays an important role in regulating cell migration. Phosphorylation of Tyr407 within FAK is required to recruit paxillin and vinculin to FAK and to ensure formation of focal adhesions [14]. Phosphorylation of S732-FAK is necessary for phosphorylation of Y407-FAK and is mediated by HSP90/ROCK-dependent pathway [8]. Our results are consistent with these data and show that a decrease in phosphorylation level of Y407-FAK is associated with a decrease in cell migration rate. These results are slightly different than those reported by Lim et al., which showed that in NIH3T3 fibroblast cells, phosphorylation of Y407-FAK inhibits autophosphorylation at Y397-FAK which reduces FAK kinase activity and cell migration [22]. It is possible that the role of Y407-FAK is cell type specific in the way it affects FAK function.
The question that arises from these results is which serine-threonine kinases are involved in phosphorylation of S732-FAK. One possible candidate is Rho-dependent kinase (ROCK). ROCK appears to be directly involved in phosphorylation of S732-FAK in endothelial cells since phosphorylation of S732-FAK becomes VEGF independent in endothelial cells expressing a constitutively active form of ROCK [8]. Phosphorylation of Y402-FAK in endothelial cells requires prior phosphorylation of S732-FAK by ROCK. Another candidate which could be involved in phosphorylation of S732-FAK is Cdk5. Cdk5 expression is found highest in postmitotic neurons of central nervous system and plays a pivotal role for proper development of the neocortex [7]. Xie et al., reported that S732-FAK phosphorylation by Cdk5 is important for microtubule organization, nuclear movement, and neuronal migration [7].
The balance between FAK and Pyk2 in cells has been implicated in regulating cell migration. The functional role of Pyk2 has been controversial. Lipinski et al., reported that Pyk2 plays an important role in promoting cell migration and that it exhibits an opposing role to that of FAK in cultured glioblastoma cells. They also demonstrated that an overexpession of Pyk2 stimulated migration, whereas overexpression of FAK inhibited cell migration [17]. Du et al., have also reported an opposing functional roles between FAK and Pyk2 in regulating organization of actin-associated cytoskeleton structures and cell rounding in Swiss 3T3 fibroblast cell [15]. Our data in Figure 4 showed that the loss of FAK function inhibited by ATE+31 does not lead to an upregulation or hyper-activation of Pyk2.
Regulation of adhesion disassembly has been linked to the tyrosine phosphorylation of FAK. We interpret the loss of FAK and phosphotyrosine staining at the periphery of the cells (Figure 5) as representing adhesion disassembly in response to growth factor treatment presumably in part due to the phosphorylation of FAK. However, after ATE+31 treatment, the loss of adhesions, indicated by the change in the distribution of FAK and phosphotyrosine proteins from cytoplasm to cell periphery, is reduced considerably. This reorganization occurs concomitantly with a decline in the level of FAK phosphorylation detected by Western blot. The appearance of eGFP-mFAK in the perinuclear region is consistent with the distribution of concentrated FAK observed in perinuclear region where centrosome is located in primary cortical neurons [7]. In cultured neurons, the phosphorylation level of S732- FAK affects the localization of FAK along a centrosome-associated microtubule fork that abuts the nucleus. FAK affects the organization of the microtubule fork and subsequently can lead to impairment of nuclear movement in vitro, and neuronal positioning defects in vivo.
In summary, phosphorylation of Y407-FAK and S732-FAK are important for FAK in modulating cell migration. Moreover, ATE+31 provides a useful tool to assess the role of adhesion signaling in mediating cell migration. It may be a potential therapeutic compound in the treatment of cancers.
Figure 1. Effects of ATE+31 on migration rates of U-87 MG cells.
The effect of ATE+31 on U-87 MG cell migration were tested in modified Boyden Chamber assay at various concentrations. ATE+31 at indicated concentration were employed and the cell migration rates in response to 10 ng/ml PDGF were measured. Results are presented as a percent of the migration rate of U-87 MG cells in the presence of PDGF alone.
Acknowledgements
This work was supported by a grant #RO1-081209 from the National Institutes of Health
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Horwitz R, Webb D. Cell Migration. Curr. Biol. 2003;13:R756–R759. doi: 10.1016/j.cub.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Rafelski SM, Theriot JA. Crawling toward a unified model of cell mobility: spatial and temporal regulation of actin dynamics. Annu. Rev Biochem. 2004;73:209–239. doi: 10.1146/annurev.biochem.73.011303.073844. [DOI] [PubMed] [Google Scholar]
- 3.Zamir E, Katz B-Z, Aota S, Yamada KM, Geiger B, Kam Z. Molecular diversity of cell-matrix adhesions. J. Cell Sci. 1999;112:1655–1669. doi: 10.1242/jcs.112.11.1655. [DOI] [PubMed] [Google Scholar]
- 4.Heldin CH, Westermark B. Mechanism of Action and In Vivo Role of Platelet-Derived Growth Factor. Physiol. Rev. 1999;79:1283–1361. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- 5.Ding Q, Stewart J, Jr, Olman MA, Klobe MR. The Pattern of Enhancement of Src Kinase Activity on Platelet-derived Growth Factor Stimulation of Glioblastoma Cells Is Affected by the Integrin Engaged. J. Biol. Chem. 2003;278:39882–39891. doi: 10.1074/jbc.M304685200. [DOI] [PubMed] [Google Scholar]
- 6.Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, Schlaepfer DD. FAK integrates growth-factor and integrin signals to promote cell migration. Nat. Cell Biol. 2000;2:249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- 7.Xie Z, Sanada K, Samuels BA, Shih H, Tsai L. Serine 732 Phosphorylation of FAK by Cdk5 Is Important for Microtubule Organization, Nuclear Movement, and Neuronal Migration. Cell. 2003;114:469–482. doi: 10.1016/s0092-8674(03)00605-6. [DOI] [PubMed] [Google Scholar]
- 8.LeBœuf F, Houle F, Sussman M, Huot J. Phosphorylation of Focal Adhesion Kinase (FAK) on Ser732 Is Induced by Rho-dependent Kinase and Is Essential for Proline-rich Tyrosine Kinase-2–mediated Phosphorylation of FAK on Tyr407 in Response to Vascular Endothelial Growth Factor. Mol Biol Cell. 2006;17:3508–3520. doi: 10.1091/mbc.E05-12-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moscatelli D, Joseph-Silverstein J, Presta M, Rifkin DB. Multiple forms of an angiogenesis factor: basic fibroblast growth factor. Biochimie. 1988;70:83–87. doi: 10.1016/0300-9084(88)90162-9. [DOI] [PubMed] [Google Scholar]
- 10.Piotrowicz RS, Maher PA, Levin EG. Dual Activities of 22–24 kDa Basic-Fibroblast Growth Factor: Inhibition of Migration and Stimulation of Proliferation. J. Cell. Physiol. 1999;178:144–153. doi: 10.1002/(SICI)1097-4652(199902)178:2<144::AID-JCP3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 11.Ding L, Doñate F, Parry GCN, Guan X, Maher P, Levin EG. Inhibition of Cell Migration and Angiogenesis by the Amino Terminal Fragment of 24kD Basic Fibroblast Growth Factor. J. Biol. Chem. 2002;277:31056–31061. doi: 10.1074/jbc.M203658200. [DOI] [PubMed] [Google Scholar]
- 12.Levin EG, Sikora L, Ding L, Rao SP, Sriramarao P. Inhibition of tumor cell migration by a truncated form of 24kDa FGF-2 leads to suppression of tumor growth and angiogenesis in vivo. Am J. Pathol. 2004;164:1183–1190. doi: 10.1016/S0002-9440(10)63206-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sundberg LJ, Galante LM, Bill HM, Mack CP, Taylor JM. An Endogenous Inhibitor of Focal Adhesion Kinase Blocks Rac1/JNK but Not Ras/ERK-dependent Signaling in Vascular Smooth Muscle Cells. J. Biol. Chem. 2003;278:29783–29791. doi: 10.1074/jbc.M303771200. [DOI] [PubMed] [Google Scholar]
- 14.LeBœuf F, Houle F, Huot J. Regulation of Vascular Endothelial Growth Factor Receptor 2-mediated Phosphorylation of Focal Adhesion Kinase by Heat Shock Protein 90 and Src Kinase Activities. J. Biol. Chem. 2004;279:39175–39185. doi: 10.1074/jbc.M405493200. [DOI] [PubMed] [Google Scholar]
- 15.Du Q-S, Ren X-R, Xie Y, Wang Q, Mei L, Xiong WC. Inhibition of PYK2-induced actin cytoskeleton reorganization, PYK2 autophosphorylation and focal adhesion targeting by FAK. J. Cell Sci. 2001;114:2977–2987. doi: 10.1242/jcs.114.16.2977. [DOI] [PubMed] [Google Scholar]
- 16.Lakkakorpi PT, Bett AJ, Lipfert L, Rodan GA, Duong LT. PYK2 Autophosphorylation, but Not Kinase Activity, Is Necessary for Adhesion-induced Association with c-Src, Osteoclast Spreading, and Bone Resorption. J. Biol. Chem. 2003;278:11502–11512. doi: 10.1074/jbc.M206579200. [DOI] [PubMed] [Google Scholar]
- 17.Lipinski CA, Tran NL, Menashi E, Rohl C, Kloss J, Bay RC, Berens ME, Loftus JC. The tyrosine kinase pyk2 promotes migration and invasion of glioma cells. Neoplasia. 2005;7:435–445. doi: 10.1593/neo.04712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim Y, Lim ST, Tomar A, Gardel M, Bernard-Trifilo JA, Chen XL, Uryu SA, Canete-Soler R, Zhai J, Lin H, Schlaepfer WW, Nalbant P, Bokoch G, Ilic D, Waterman-Storer C, Schlaepfer DD. PyK2 and FAK connections to p190Rho guanine nucleotide exchange factor regulate RhoA activity, focal adhesion formation, and cell motility. J. Cell Biol. 2008;180:187–203. doi: 10.1083/jcb.200708194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levin EG. Cancer Therapy Through Control of Cell Migration. Curr Cancer Drug Targets. 2006;7:505–518. doi: 10.2174/156800905774574048. [DOI] [PubMed] [Google Scholar]
- 20.Maher PA, Pasquale EB, Wang JY, Singer SJ. Phosphotyrosine-Containing Proteins are Concentrated in Focal Adhesions and Intercellular Junctions in Normal Cells. Proc. Natl. Acad. Sci. USA. 1985;82:6576–6580. doi: 10.1073/pnas.82.19.6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geiger B, Tokuyasu KT, Dutton AH, Singer SJ. Vinculin, an intracellular protein localized at specialized sites where microfilament bundles terminate at cell membranes. Proc, Natl. Acac. Sci. USA. 1980;77:4127–4131. doi: 10.1073/pnas.77.7.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim Y, Park H, Jeon J, Han I, Kim J, Jho EH, Oh ES. Focal Adhesion Kinase Is Negatively Regulated by Phosphorylation at Tyrosine 407. J. Biol. Chem. 2007;282:10398–10404. doi: 10.1074/jbc.M609302200. [DOI] [PubMed] [Google Scholar]