Abstract
Several studies have shown that a higher lymphocyte count 3–4 weeks after allogeneic stem cell transplantation (SCT) is associated with better transplant outcome. However the factors determining early lymphocyte recovery are not defined. To further explore the relationship between lymphocyte recovery and outcome we analyzed lymphocyte counts and other engraftment parameters in 157 patients with leukemia (48 acute myeloid leukemia, 80 chronic myeloid leukemia, and 29 acute lymphoblastic leukemia [ALL]) receiving T-cell depleted myeloablative stem cell transplantation (SCT) from an HLA-identical sibling. In multivariate analysis the day 30 absolute lymphocyte count (LC30) above the median of 450/µl was associated with improved survival (71±5 vs. 38±6%, p<0.0001), less relapse (21±5 vs. 44±7%, p=0.009), less non-relapse mortality (9±3 vs. 36±6%, p<0.0001) and less acute GVHD (34±5 vs. 51±6%, p=0.025). The beneficial effect of a higher LC30 influenced outcome in patients with both standard and high risk disease but did not affect survival and relapse in ALL. We found that a higher LC30 correlated with higher lymphocyte counts at all time points between 30–90 days post SCT and also with more rapid neutrophil and platelet engraftment. These results indicate that LC30 is a surrogate for robust engraftment and identifies an "at risk" population of patients after T-cell-depleted SCT.
Keywords: AML, ALL, CML, day 30 lymphocyte count, allogeneic stem cell transplantation
Introduction
Several studies have shown that early recovery of the lymphocyte count after allogeneic stem cell transplantation (SCT) is associated with better survival, reduced relapse and lower transplant related mortality1–5. A recent analysis found that a low absolute lymphocyte count (=100/µl) was also associated with poor outcome following acute graft versus host disease (aGVHD) 6. We previously reported improved transplant outcome for chronic myelogenous leukemia (CML) in patients with high day 30 lymphocyte counts (LC30) after T-cell-depleted myeloablative stem cell transplantation from an HLA-identical sibling 7. Natural killer (NK) cells are the first lymphocytes to recover after peripheral blood stem cell transplantation and play an important role in the innate host defenses. Niederwieser et al showed in the early post-transplant period, the majority of peripheral blood lymphocytes were NK cells 8, which can mediate cytotoxicity without prior sensitization and may be responsible for early graft-versus-leukemia (GVL) effects 9; 10. We have earlier shown that NK cells are the dominant population in the LC30 and that LC30 and NK30 were highly correlated. Furthermore, higher levels of NK30 were associated with improved transplant outcome in CML after SCT 7.
These findings suggested that the LC30 may serve as a surrogate for NK cell recovery. However, the simplicity and reproducibility of LC30 measurement between transplant centers encouraged us to investigate lymphocyte recovery further in a large diverse population of transplant recipients to further explore the predictive value of LC30 and to better elucidate the relationship of the LC30 with other measurements of engraftment at various times post-transplant.
Here we show, in a cohort of 157 patients undergoing T cell depleted SCT, that LC30 was correlated with standard engraftment parameters and was a powerful predictor of transplant outcome in myeloid malignancies but not in acute lymphoblastic leukemia.
PATIENTS AND METHODS
Study group
One-hundred-sixty consecutive patients with leukemias received a T cell depleted SCT from an HLA identical sibling in NHLBI institutional review board approved protocols. The study populations included 80 CML patients previously reported 7. Written informed consent was obtained according to principles outlined in the Declaration of Helsinki. Of these patients, 157 survived until day 30 and formed the study cohort.
Conditioning regimens and transplant approach
Transplant approach, details of cyclosporine (CSA) dosing schedule, post-transplant T-cell add back, monitoring of minimal residual disease, chimerism, infection prophylaxis and CMV monitoring were described previously 7; 11.Three transplant regimens were used: (1) 52 patients received 13.6 Gy total body irradiation (TBI), cyclophosphamide (Cy) 120mg/kg, standard dose (SD) CSA (target levels 200–400 µg/l ; (2) 41 received TBI/Cy and low dose (LD) CSA (target levels 100–200 µg/l) ; (3) 64 received 12.0 Gy TBI/Cy/fludarabine (Flu) 125mg/m2 and LD CSA. All patients received a T cell depleted transplant (dose range 0.2 - 2.0×105 CD3+ cells/kg) with delayed donor-lymphocyte infusions 1–3 months after transplantation. The stem cell source was G-CSF mobilized peripheral blood for all patients, except 28 in the first cohort who received bone marrow transplants.
Day 30 lymphocyte monitoring (LC30)
Of 160 patients transplanted 157 [48 acute myeloblastic leukemia (AML) or myelodysplastic syndrome which had progressed to AML, 80 chronic myelogenous leukemia (CML), and 29 acute lymphoblastic leukemia (ALL)] survived to day +30 post transplant for LC30 evaluation from a routine blood count (in all cases obtained before any post-transplant DLI). The three patients excluded from analysis died from CMV pneumonitis day 15, acute respiratory distress syndrome day 23, and intracranial hemorrhage day 28 post-transplantation. Their absolute lymphocyte count on day of death was 140, 289 and 80/µl respectively.
Definitions
Patients with ALL and AML, in first complete remission and patients with CML in chronic phase (CP) were considered to have standard risk (SR) disease. All other patients, including those who underwent transplantation in CR2 or greater, those with primary refractory or relapsed disease, and those with secondary AML were defined as having high risk (HR) disease. Overall survival (OS) was calculated from the interval between the date of transplantation and death, or last follow-up visit. Relapsed disease for AML and ALL was definedby morphologic or cytogenetic evidence, either in peripheralblood or in bone marrow. Relapsed disease for CML was defined by hematological or cytogenetic or molecular evidence of recurrence. Non-relapse mortality (NRM) was defined as the time from transplantation until death from infectious cause, graft failure, GVHD, or any other cause unrelated to disease. Estimates for relapse, GVHD incidence and NRM were made using cumulative incidence estimates. Engraftment was defined as absolute neutrophil count of >500/µl, and unsupported platelet count of >20,000 for 3 consecutive days or detection of donor DNA by PCR-short tandem repeat (STR).
Statistical methods
Summary statistics, such as proportions, means, standard deviations, 95% confidence intervals, medians and ranges, were used to describe the patient characteristics, pretransplant variables and post-transplant outcomes. Standard techniques in survival analysis, including Kaplan-Meier estimates and the Cox Proportional Hazard Models, were used to estimate the time-to-event distributions of OS, DFS/current LFS, relapse, GVHD and TRM. Statistical associations between pre-transplant variables were investigated using correlation analysis, including Pearson’s Correlation Coefficients and Spearman’s Rank Correlation Coefficients, and multiple regression analysis. Statistical tests based on t-tests, chi-squared tests and F-tests were used to evaluate the statistical significance of covariates in multiple regression models or the Cox Proportional Hazard Models. Variables included in univariate analysis were: age (continuous, < vs. = median), gender, donor-patient sex match (female to male vs. others), disease risk (HR vs. SR), type of transplant (BMT vs PBSCT), CD34 dose (continuous, < vs. = median), CD3 dose (0.2 vs. >0.2×105/kg CD3+ cells, LC30 (continuous, < vs. = median), and CSA dose (SD vs. LD). Analysis was performed with LC30 taken as a continuous variable, < vs. = median for whole cohort and each disease category with cohort median and disease specific median value of LC30. Multivariate analysis was performed using the Cox models. Data analysis was performed using SPSS 14 for Windows (SPSS Inc., Chicago, IL) software.
Results
Patients
Cohort distribution and patient characteristics are shown in Table 1 and 2 respectively.
Table 1.
Cohort descriptions
| Cohort† | n | Stem cell source |
TBI (Gy) | Flu (125mg/m2) |
Cy mg/kg |
|---|---|---|---|---|---|
| 1 | 28 | BM | 1360 | - | 120 |
| 2 | 65 | PB | 1360 | - | 120 |
| 3 | 64 | PB | 1200 | 125 | 120 |
TBI-Total body irradiation, Flu-Fludarabine, Cy-Cyclophosphamide, BM-bone marrow, PB-G-CSF mobilized peripheral blood
Non-significant difference between cohorts in terms of age (< vs.= median), gender, disease distribution, disease risk
Table 2.
Patient characteristics
| Variables (n=157) | Details |
|---|---|
| Age | Median 34 years (range 10–56) |
| Gender | Male 89 (57%); Female 68 (43%) |
| Disease distribution | AML 48 (31%); ALL 29 (19%); CML 80 (50%) |
| Disease risk | HR 80 (51%); SR 77 (49%) |
| AML- HR 27, SR 21; ALL- HR 26, SR 3; CML- HR 27, SR 53 |
|
| CD34 cell dose (x106/kg)† | Median 5 (range 0.78–15.9) |
| CSA dose* | LD 105 (67%); SD 52 (33%) |
| CD3+ cell dose/kg with graft | 0.2x105 64 (41%); >0.2x105 93 (59%) |
| Follow-up surviving patients | Median 52 (range 12–146) |
| 100 day TRM | N=14 (9%) |
| LC30** | Median 448/µl (range 0–3295) |
AML-acute myeloid leukemia, ALL-acute lymphoblastic leukemia, CML-chronic, myeloid leukemia, HR-high risk and SR-standard risk for relapse after SCT, TRM-transplant related mortality
CD34 cell dose and LC30 were significantly correlated (correlation coefficient 0.578, p < 0.0001)
SD-standard dose (200 – 400 mcg/L), LD-low dose (100 – 200 mcg/L)
HR and SR groups were equally balanced between LC30 > vs. < 450/µl (40 vs. 37 for SR and 38 vs. 42 for HR disease, p=0.346).
Validation of the LC30 as a representative measurement of early lymphocyte recovery
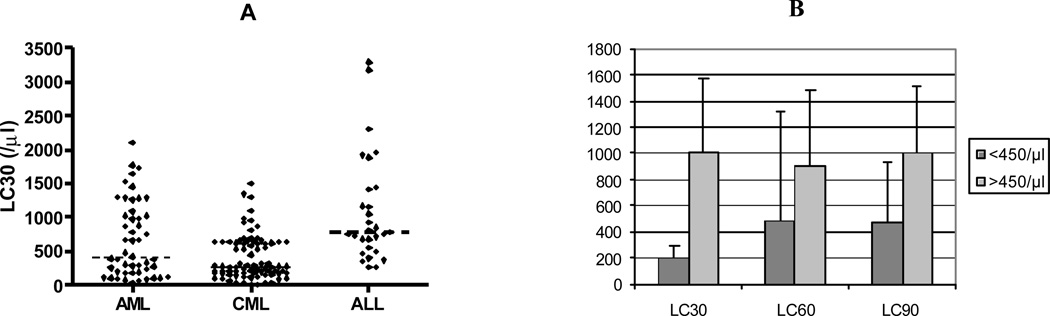
Median LC30 of cohort was 448/µl (<450 vs. >450 taken in a cut-off model for analysis), range 0–3295 (AML 403/µl, range 31 – 2105; CML 287/µl, range 0–1498 and ALL 778/ µl, range 265–3295) (Figure 1A). Figure 1B shows the absolute lymphocyte count on day 30 (LC30), 60 (LC60) and 90 (LC90) [above and below median] post-transplant among patients who survive at least 90 days after SCT. Patients with LC30 above median had higher lymphocyte counts on day 14 and retained correspondingly higher lymphocyte count on day 60 and 90 while patients with = median LC30 had lower counts on day 14 and slower recovery on day 60 and 90 than the high LC30 subset. Since day 14 lymphocyte counts were very low, we chose the LC30 as the earliest reliable measurement for subsequent analyses. One hundred three patients received DLI between day 30–45 and 22 day 60. DLI had no significant impact on LC60 and LC90 recovery compared to LC30 (p=0.38 for LC60 and 0.24 for LC90).
Figure 1.
A. LC30 distribution in AML, ALL and CML; B. comparison LC30, day 60 lymphocyte (LC60) and day 90 lymphocyte (LC90) counts post-transplantation between group with LC30 < and > 450/µl.
Transplant outcome and LC30
Engraftment
Two patients (1.3 %, one with CML and the other ALL) failed to engraft. Both patients were rescued successfully after receiving a second graft. Their transplant outcome in relation to LC30/NK30 was calculated from the second graft.
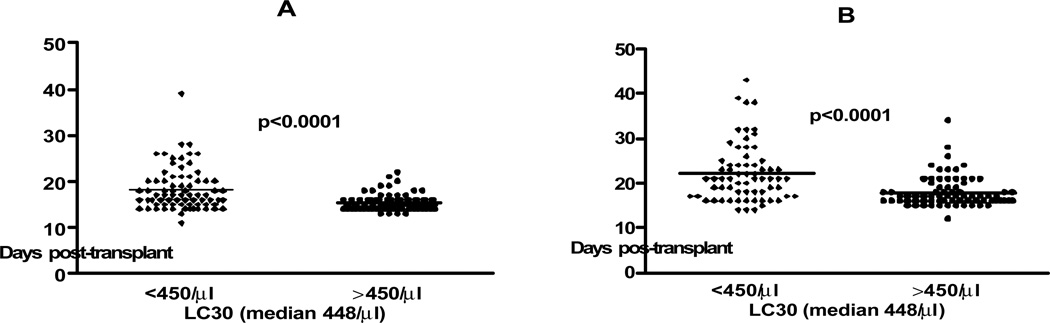
Median time to neutrophil and platelet engraftment was 16 days (range 11–39) and 18 (range 12–43). Patients with LC30 >450/µl had faster neutrophil engraftment (15.5 ± 0.2 vs. 18 ± 0.5 days for LC30 <450/µl; p<0.0001) and faster platelet engraftment (18 ± 0.4 vs. 22 ± 0.8 days for LC30 <450/µl; p<0.0001) (Figure 2). Single tandem repeat (STR) chimerism was available in 55 patients. Median time to full donor lymphoid chimerism was 60 days (range 14–798). There was no correlation between LC30 (either as a continuous or a categorical variable) and time to full donor T cell chimerism (p=0.445).
Figure 2.
Time to A. neutrophil and B. platelet engraftment in patients with LC30 < vs >450/µl
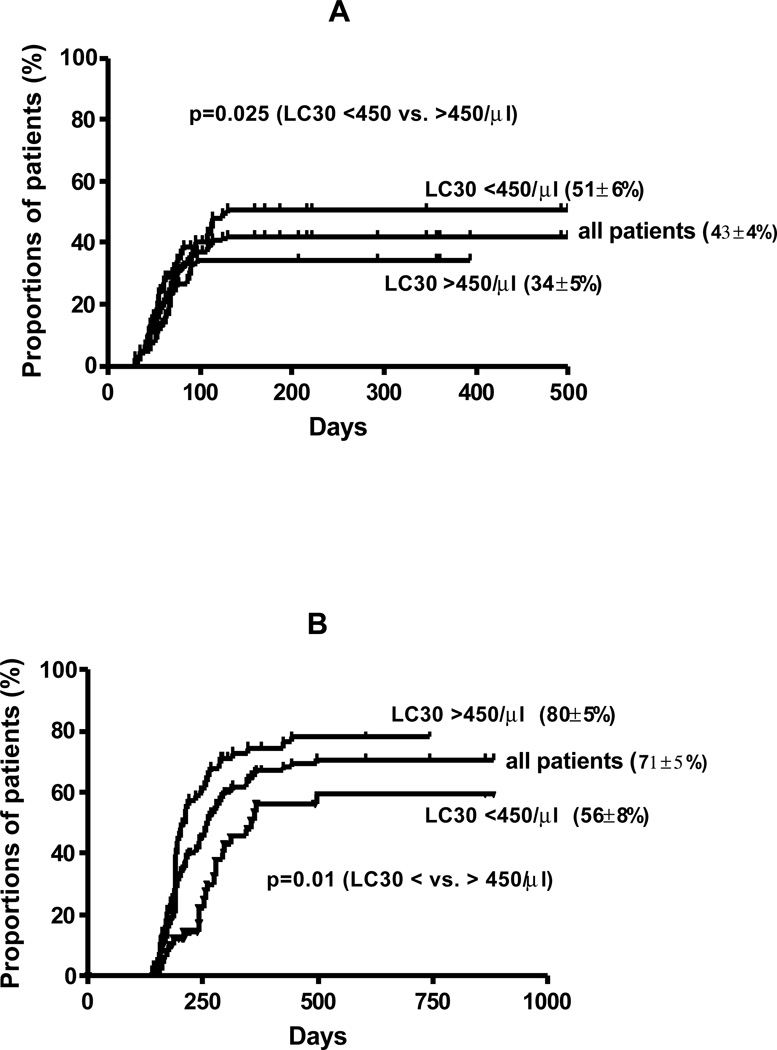
Acute GVHD
Sixty three patients developed acute GVHD grade (II-IV) with a cumulative incidence of 43 ± 4 % (Figure 3A). On univariate analysis, risk of acute GVHD was associated with HR disease (57 vs. 26% for SR disease; p<0.001), and LC30 <450/µl (p=0.025) (Figure 3A). There was no effect of stem cell source (BMT vs. PBSCT), disease type, age, gender, CSA dose, CD34+ and CD3+ cell dose on incidence of acute GVHD. Multivariate analysis showed that independent factors associated with more aGVHD were HR disease (RR 3.0, 95% CI 1.7–5.0; p<0.0001) and LC30 <450/µl (RR 1.8, 95% CI 1.1–2.9; p=0.027).
Figure 3.
Cumulative incidence of A. acute GVHD and B. chronic GVHD and incidence in patients with LC30 above and below 450/µl
Chronic GVHD
Eighty one of 129 evaluable patients surviving more than 100 days developed chronic GVHD [limited 58 (45%); extensive 23 (18%)] with a cumulative incidence of 71 ± 5 % (Figure 3B). On univariate analysis, SR disease (71 vs. 53% for HR disease; p=0.021), and LC30 >450/µl (p=0.01) were associated with increased risk of chronic GVHD (Figure 3B). There was no effect of type of SCT (BMT vs. PBSCT), disease type, age, gender, CSA dose, CD34+ and CD3+ cell dose on incidence of chronic GVHD. Multivariate analysis showed LC30 >450/µl (RR 0.55, 95% CI 0.34–0.87; p=0.01) was independently associated with an increased incidence of chronic GVHD.
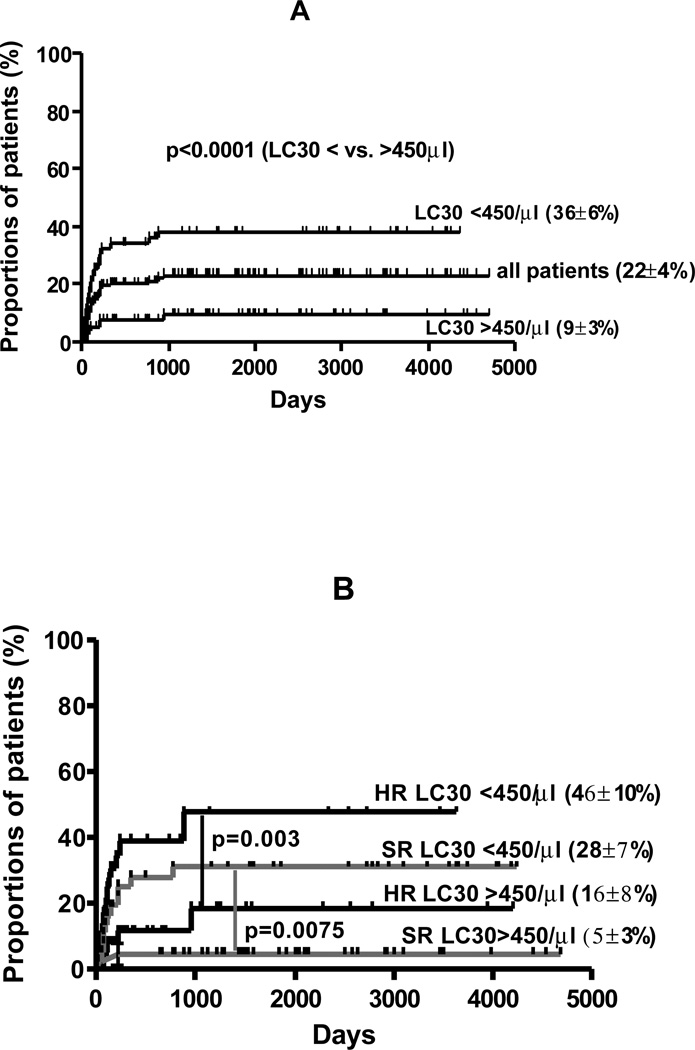
NRM
Thirty-one of 157 (21%) patients died from non-relapse causes, with a cumulative incidence of NRM of 22 ± 4 % (Figure 4A). Causes of death were: bacterial/fungal infection (3); viral infection (8); GVHD-related (8); acute respiratory distress syndrome/ idiopathic pneumonitis (8); late graft failure (1); transfusion reaction (1); intracranial bleeding (1); motor vehicle accident (1). Factors associated with a significant decreased risk of NRM were LC30 >450/µl (p<0.0001), (Figure 4A); CD 34+ cell dose = median (10 vs. 30% for CD34+ cell dose < median; p=0.002) and absence of grade II-IV acute GVHD (13 vs. 27%, p=0.02). In multivariate analysis, acute GVHD (RR 2.3; 95% CI 1.1–4.9; p=0.024) and LC30 <450/µl (RR 3.6; 95% CI 1.2–10.6; p=0.022) were independently associated with an increased risk of NRM, (Figure 4B).
Figure 4.
A. cumulative incidence of non-relapse mortality; B. LC30, disease risk (HR- high risk; SR- standard risk) and NRM
Relapse
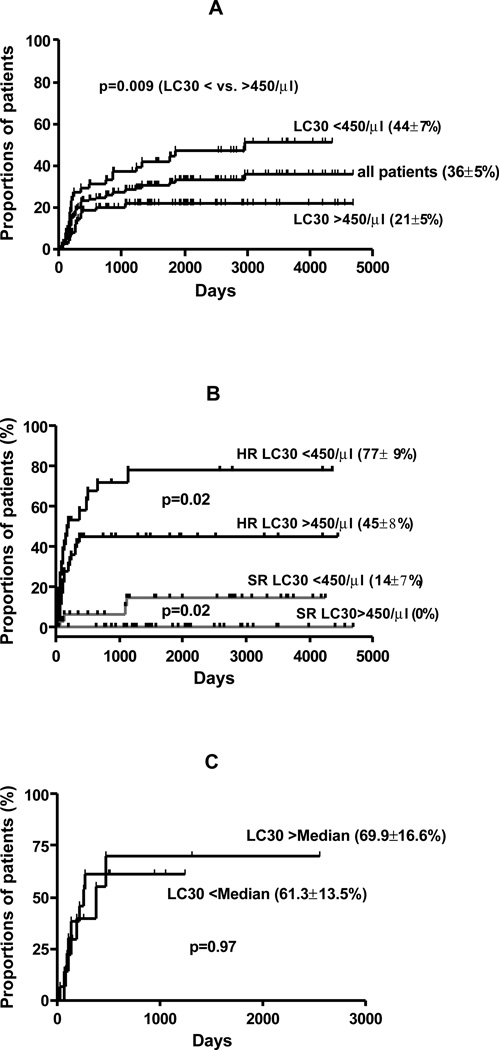
Forty three patients relapsed with a cumulative incidence of 36 ± 5% (Figure 5A). Median time to relapse was 3.6 months (range 1–37). On univariate analysis, increased risk of relapse was associated with PBSCT (majority CML patients) compared to BMT (31 vs 8%; p=0.009), patients with ALL (52% vs. 40% for AML and 11% for CML; p<0.0001), HR disease (49 vs. 5% for SR disease; p<0.0001); absence of chronic GVHD (44 vs. 17% for patients with chronic GVHD; p=0.001) and LC30 <450/µl (p=0.009), (Figure 5A). There was no effect of age, gender, CSA dose, or CD34+ and CD3+ cell dose on incidence of relapse. Multivariate analysis showed HR disease (RR 15.6; 95% CI 5.4–45.1; p<0.001), LC30 <450/µl (RR 3.0; 95% CI 1.5–6.0; p=0.002) and absence of chronic GVHD (RR 0.32; 95% CI 0.16–0.65; p=0.001) to be independently associated with an increased risk of relapse. Cumulative incidence of relapse in relation to disease risk status and LC30 is shown in Figure 5B. Patients with SR disease who achieved an LC30 of = 450/µl had no relapse. In contrast patients with HR disease who failed to achieve LC30 of >450/µl, experienced a relapse rate of 77 ± 9%. Interestingly the impact of LC30 was only found in AML/MDS and CML, but not in ALL (whether analyzed either by cohort median or the disease specific median value of LC30 [Figure 5C]).
Figure 5.
A. cumulative incidence of relapse; B. LC30, disease risk (HR- high risk; SR- standard risk) and relapse; C. LC30 and relapse in acute lymphoblastic leukemia (ALL)
Survival
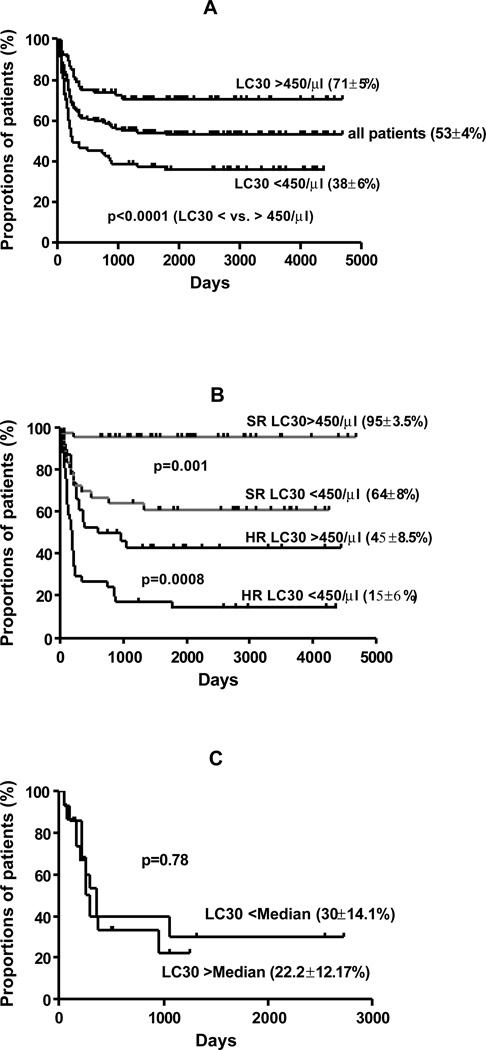
Currently 87 of 157 patients are alive and 83 are disease free (actuarial OS is shown in Figure 6A). In univariate analysis, factors associated with improved survival were SR risk disease (81 vs. 31% for HR disease; p<0.001), higher LC30 > 450/µl (p<0.001), (Figure 1A), CD34+ cell dose above median of 5.0×106/kg (67 vs 44%; p=0.002), absence of aGVHD (grade II-IV) (p=0.03) and development of chronic GVHD (79 vs 48%; p<0.0001). There was no impact of type of SCT, age, gender, CD3+ cell and CSA dose on survival.
Figure 6.
A. Overall survival; B. LC30, disease risk (HR- high risk; SR- standard risk) and survival; C. LC30 and survival in acute lymphoblastic leukemia (ALL)
Multivariate Cox regression analysis showed SR disease (RR 7.1, 95% CI 3.2–15.5; p<0.0001), LC30 >450/µl (RR 2.7, 95% CI 1.03–5.1; p=0.047) and development of chronic GVHD (RR 0.44, 95% CI 0.23–0.83; p=0.01) were independently associated with improved survival.
Interestingly, patients with HR disease and those patients who failed to achieve an LC30 of at least 450/µl had a very poor outcomes with an actuarial survival of only 15 ± 6%. However the patients with SR disease and LC30 of >450/µl had an actuarial survival of 95 ± 3.5% (Figure 6B). In disease-specific analysis, there was no impact of LC30 on survival in ALL compared to AML and CML (analyzed with both cohort median and disease specific median value of LC30 [Figure 6C]).
Discussion
Our results highlight a powerful predictive effect of the day 30 post-transplant lymphocyte count (LC30) on transplant outcome. When treated as a dichotomous variable (absolute lymphocyte count above or below the median of 448/µl), the LC30 emerged as a unique, and powerful factor for predicting survival, NRM, acute GVHD and relapse. The only other factor significantly affecting outcomes in multivariate analysis were the well-established variables known to affect transplant outcome (disease status, and development of acute or chronic GVHD). The differences in actuarial outcome were not only significant between high and low LC30 sets but also associated with, notably a RR of 3.0 for lower relapse with higher LC30. Beneficial effects of LC30 were not seen in ALL despite higher LC30 numbers in our study. These results should be interpreted with caution because of the small sample size of the ALL group (n=29) with a preponderance of HR disease (n=26).
These data confirm other observations indicating the importance of lymphocyte recovery as a prognostic factor for outcome after allogeneic stem cell transplantation 1–4; 12. The measurement of lymphocyte count has practical significance because it identifies a group of patients (notably HR patients with poor lymphocyte recovery) who might be suitable candidates for early interventions or aggressive approaches to improve transplant outcome. Other studies also show early lymphocyte recovey to be correlated with better outcome in non-T-cell depleted transplants 1; 3; 12, particularly in myeloid leukemias. Early lymphocyte recovery has also been shown to identify a patient subgroup at risk for increased NRM (from infections and acute GVHD), and graft failure 4; 6; 7.
In this analysis we sought to validate the widely used choice of day 21–30 for measuring lymphocyte count for predictive purposes post transplant. We showed that LC30 as a dichotomous value above or below the median reliably identified patient groups with either a relatively high or a relatively low lymphocyte count between day 14 to 90 post transplant. Thus the measurement of LC30 reliably characterized lymphocyte recovery over the entire first three months post-transplant. Furthermore, we found that patients with higher than median LC30 were those who had a more rapid platelet and neutrophil recovery suggesting that LC30 measurement is a surrogate for speed of donor engraftment (Figure 2).
Lymphocytes recovering in the first month post transplant consist of two major populations: NK cells and CD3+ T cells. NK cells recover early after allogeneic SCT, generally about 3–4 days earlier than granulocyte and monocytes 8; 13–15 but early recovery of NK cell function does not occur in patients recovering from chemotherapy without stem cell support 16. Our earlier analyses in a small CML patient cohort from this dataset indicated that NK cell and total lymphocyte count on day 30 were strongly correlated 17. Our data point to the transplanted CD34 cell as the origin of recovering NK cells, since all transplants were selected for CD34 cells and depleted of lymphocytes using a cocktail of anti CD2, CD6 and CD7 antibodies. This cocktail would largely eliminate mature CD2+ NK cells from the graft. Furthermore, we found that CD34 dose and LC30 was positively correlated. There is some data relating NK cell recovery to transplant outcome: Scholl et al 18 showed that patients with better NK cell recovery had less severe acute GVHD. In our previous report patients with CML had better leukemia control early post-transplant if they achieved higher than median higher NK cell counts 7.
A role for donor CD3+ T cells in early lymphocyte recovery and subsequent clinical outcome appeared to be a less likely explanation for our findings since donor T cell percentage varied between 0–100% on day 14 or day 30 and chimerism did not correlate with high or low LC30. In these T cell depleted SCT; T cell engraftment is characteristically slow and variable. Interestingly, in an earlier analysis we did not find any benefit on outcome for early complete donor lymphoid chimerism in a subset of 132 patients in this series 11.
The observation that a GVL effect of LC30 was not found in ALL patients and the finding that better NK recovery on D30 was predictive for less aGVHD bears a striking resemblance to the findings of the Perugia transplant group in haploidentical T cell depleted transplants who related NK KIR incompatibility (as a surrogate for donor-vsrecipient NK alloreactivity) with a survival benefit, less acute GVHD and a beneficial effect on relapse in myeloid but not lymphoid malignancies 19. The relationship between NK alloreactivity and NK recovery post-transplant is unclear but the similarity of the relationship between NK characteristics and outcome in the Perugia series and our own HLA identical T cell depleted transplants suggest that KIR expression might in some way affects the pace of NK recovery.
In conclusion, LC30, appears to be a surrogate for robust engraftment of all hematopoietic lineages, including NK cells. Since the lymphocyte count is reliably and widely measured in most transplant centers, and can tbe retrieved from retrospective transplant data, analyses of large patient groups transplanted with different regimens to determine the general predictive value of LC30 measurement should be possible. Such studies might define the types of diseases and transplant approaches impacted most by the pattern of lymphocyte recovery and lead to development of patient-specific strategies to improve outcome in those who fail to achieve satisfactory lymphocyte counts in the first month post transplant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
B.N.S.- conceived and designed the study, collected patient data, provided clinical care, performed statistical analysis and wrote the report; A.M.- collected patient data, provided clinical care; S.M.; K.R.; A. Y.; L.W.; J.S.; A.S.; ; R.C.- advised on study design and commented on the manuscript; R.K.- performed chimerism study, advised on study design and commented on the manuscript; A.J.B.- supervised the study, conceived and designed the study, provided clinical care and wrote the report.
Reference List
- 1.Powles R, Singhal S, Treleaven J, et al. Identification of patients who may benefit from prophylactic immunotherapy after bone marrow transplantation for acute myeloid leukemia on the basis of lymphocyte recovery early after transplantation. Blood. 1998;91:3481–3486. [PubMed] [Google Scholar]
- 2.Kim DH, Kim JG, Sohn SK, et al. Clinical impact of early absolute lymphocyte count after allogeneic stem cell transplantation. Br. J. Haematol. 2004;125:217–224. doi: 10.1111/j.1365-2141.2004.04891.x. [DOI] [PubMed] [Google Scholar]
- 3.Kumar S, Chen MG, Gastineau DA, et al. Effect of slow lymphocyte recovery and type of graft-versus-host disease prophylaxis on relapse after allogeneic bone marrow transplantation for acute myelogenous leukemia. Bone Marrow Transplant. 2001;28:951–956. doi: 10.1038/sj.bmt.1703262. [DOI] [PubMed] [Google Scholar]
- 4.Chakrabarti S, Brown J, Guttridge M, et al. Early lymphocyte recovery is an important determinant of outcome following allogeneic transplantation with CD34+ selected graft and limited T-cell addback. Bone Marrow Transplant. 2003;32:23–30. doi: 10.1038/sj.bmt.1704082. [DOI] [PubMed] [Google Scholar]
- 5.Einsele H, Ehninger G, Steidle M, et al. Lymphocytopenia as an unfavorable prognostic factor in patients with cytomegalovirus infection after bone marrow transplantation. Blood. 1993;82:1672–1678. [PubMed] [Google Scholar]
- 6.Lee KH, Choi SJ, Lee JH, et al. Prognostic factors identifiable at the time of onset of acute graft-versus-host disease after allogeneic hematopoietic cell transplantation. Haematologica. 2005;90:939–948. [PubMed] [Google Scholar]
- 7.Savani BN, Rezvani K, Mielke S, et al. Factors associated with early molecular remission after T cell-depleted allogeneic stem cell transplantation for chronic myelogenous leukemia. Blood. 2006;107:1688–1695. doi: 10.1182/blood-2005-05-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niederwieser D, Gastl G, Rumpold H, et al. Rapid reappearance of large granular lymphocytes (LGL) with concomitant reconstitution of natural killer (NK) activity after human bone marrow transplantation (BMT) Br. J. Haematol. 1987;65:301–305. doi: 10.1111/j.1365-2141.1987.tb06857.x. [DOI] [PubMed] [Google Scholar]
- 9.Jiang YZ, Barrett AJ, Goldman JM, Mavroudis DA. Association of natural killer cell immune recovery with a graft-versus-leukemia effect independent of graftversus- host disease following allogeneic bone marrow transplantation. Ann. Hematol. 1997;74:1–6. doi: 10.1007/s002770050246. [DOI] [PubMed] [Google Scholar]
- 10.Sconocchia G, del PD, Barrett AJ. Non-classical antileukemia activity of early recovering NK cells after induction chemotherapy and HLA-identical stem cell transplantation in myeloid leukemias. Leukemia. 2006;20:1632–1633. doi: 10.1038/sj.leu.2404300. [DOI] [PubMed] [Google Scholar]
- 11.Montero A, Savani BN, Shenoy A, et al. T cell depleted peripheral blood stem cell allotransplantation with T cell add back for patients with hematological malignancies: effect of chronic GVHD on outcome. Biol. Blood Marrow Transplant. 2006;12:1318–1325. doi: 10.1016/j.bbmt.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 12.Saliba RM, Komanduri KV, Giralt S, de Souza J, Patab PA, Oran B, Rondon G, Couriel D, Champlin R, de Lima M. Correlates and outcome of absolute lymphocyte count (ALC) on day 30 post allogeneic stem cell transplantation (SCT) for treatment of AML. Biol. Blood Marrow Transplant. 2007;13(2 (suppl 2)):98. [Google Scholar]
- 13.Talmadge JE, Reed E, Ino K, et al. Rapid immunologic reconstitution following transplantation with mobilized peripheral blood stem cells as compared to bone marrow. Bone Marrow Transplant. 1997;19:161–172. doi: 10.1038/sj.bmt.1700626. [DOI] [PubMed] [Google Scholar]
- 14.Roberts MM, To LB, Gillis D, et al. Immune reconstitution following peripheral blood stem cell transplantation, autologous bone marrow transplantation and allogeneic bone marrow transplantation. Bone Marrow Transplant. 1993;12:469–475. [PubMed] [Google Scholar]
- 15.Anderson KC, Ritz J, Takvorian T, et al. Hematologic engraftment and immune reconstitution posttransplantation with anti-B1 purged autologous bone marrow. Blood. 1987;69:597–604. [PubMed] [Google Scholar]
- 16.Reittie JE, Gottlieb D, Heslop HE, et al. Endogenously generated activated killer cells circulate after autologous and allogeneic marrow transplantation but not after chemotherapy. Blood. 1989;73:1351–1358. [PubMed] [Google Scholar]
- 17.Savani BN, Mielke S, Rezvani K, et al. Total Lymphocyte and Natural Killer (NK) Cell Count Day 30 Post-Transplant Strongly Predict Transplant Outcome after T Cell Depleted Allogeneic Stem Cell Transplantation. ASH Annual Meeting Abstracts. 2006;108:2993. [Google Scholar]
- 18.Scholl S, Mugge LO, Issa MC, et al. Impact of early NK cell recovery on development of GvHD and CMV reactivation in dose-reduced regimen prior to allogeneic PBSCT. Bone Marrow Transplant. 2005;35:183–190. doi: 10.1038/sj.bmt.1704752. [DOI] [PubMed] [Google Scholar]
- 19.Ruggeri L, Mancusi A, Burchielli E, et al. Natural killer cell alloreactivity in allogeneic hematopoietic transplantation. Curr. Opin. Oncol. 2007;19:142–147. doi: 10.1097/CCO.0b013e3280148a1a. [DOI] [PubMed] [Google Scholar]