Abstract
Heart disease is a leading cause of death and disability in developed countries. Heart disease includes a broad range of diseases that affect the development and/or function of the cardiovascular system. Some of these diseases, such as congenital heart defects, are present at birth. Others develop over time and may be influenced by both genetic and environmental factors. Many of the known heart diseases are associated with abnormal expression of genes. Understanding the factors and mechanisms that regulate gene expression in the heart is essential for the detection, treatment and prevention of heart diseases. Polycomb Group (PcG) and Trithorax Group (TrxG) proteins are special families of chromatin factors that regulate developmental gene expression in many tissues and organs. Accumulating evidence suggests that these proteins are important regulators of development and function of the heart as well. A better understanding of their roles and functional mechanisms will translate into new opportunities for combating heart disease.
Introduction
During heart development, several cardiac progenitor pools give rise to diverse cell lineages, such as cardiomyocytes, smooth muscle cells of the blood vessels, fibroblasts that form the connective tissues, and endothelial cells of the endocardium (reviewed in Brand, 2003; Vincent and Buckingham, 2010). In order for the developing heart to take on the correct form and function, the differentiation of these cell lineages must be finely orchestrated with cardiac morphogenic events, such as looping, septation, and trabeculation. A significant part of this orchestration is at the level of transcriptional regulation, and a network of cardiac transcription factors have been shown to govern the temporal and spatial patterns of gene expression in the developing heart (reviewed in Bruneau, 2002; Clark et al., 2006; Conway et al., 2010; Cripps and Olson, 2002; Greulich et al., 2011; Kodo and Yamagishi, 2011; McCulley and Black, 2012; Nemer, 2008; Olson, 2006; Wirrig and Yutzey, 2011). Transcription factors also play important roles postnatally in maintaining the pattern of differentiated gene expression, protecting cells from apoptosis, and regulating the heart’s stress response such as hypertrophic growth (Aries et al., 2004; Balza et al., 2006; Oka et al., 2006; Oka et al., 2007; Parlakian et al., 2005; Toko et al., 2002; Wang et al., 2005; Zhang et al., 2001).
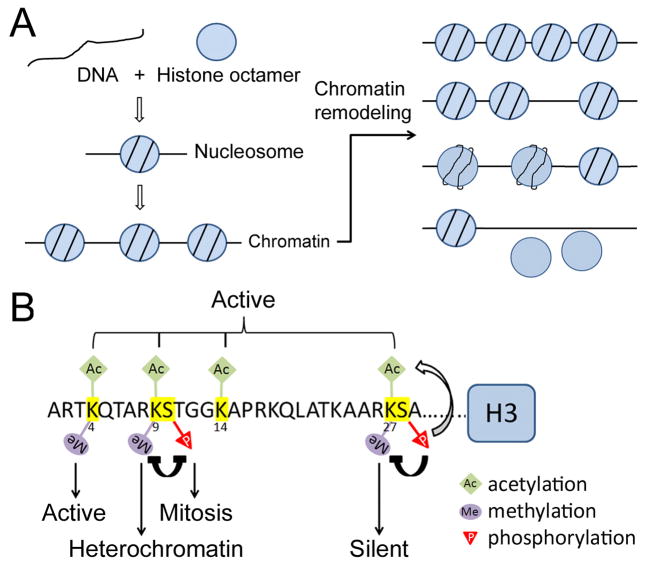
During the past two decades, the importance of epigenetic mechanisms in transcriptional regulation has been gradually recognized. Eukaryotic genomic DNA is packaged into arrays of nucleosomes, collectively known as chromatin (Fig. 1A). The chromatin in a given region can exist in different configurations that either limit or facilitate local transcription activity. Each nucleosome contains a stretch of DNA wrapped around 8 histone molecules. Each histone has an N-terminal and/or C-terminal tail that protrudes from the nucleosome and can be covalently modified by acetylation, methylation, phosphorylation and other enzymatic reactions (reviewed in Berger et al., 2002; Cheung et al., 2000; Nightingale et al., 2006) (Fig 1B). These modifications have profound effects on chromatin configuration and, therefore, transcription. Another way to change chromatin configuration is through ATP-dependent chromatin remodeling. Chromatin remodeling can change the position of nucleosome(s), nucleosome spacing, nucleosome-DNA affinity, as well as the integrity of nucleosome(s) (reviewed in Aalfs and Kingston, 2000; Fan et al., 2004; Glatt et al., 2011; Hargreaves and Crabtree, 2011) (Fig. 1A).
Figure 1. Eukaryotic genomic DNA is packaged into chromatin.
(A) Nucleosomes are the basic subunits of chromatin. Each nucleosome consists of ~146 bp of DNA wrapped approximately twice around a histone octamer (the individual monomers and histone tails are not shown). ATP-dependent chromatin remodeling can change nucleosome density, the position of nucleosome(s), nucleosome-DNA affinity, and the integrity of nucleosome(s). (B) Major modification sites on histone H3 tail. Different modifications have different effects on chromatin structure and transcriptional activity. For example, methylation of K4 and K27 is associated with transcriptionally active and silent chromatin, respectively. Modification of one residue can also promote or inhibit the modification of another residue. For example, methylation of K9 and phosphorylation of S10 inhibits each other.
The heart expresses many epigenetic factors, including both histone modifying proteins and chromatin remodelers. Several recent publications have provided excellent general reviews of epigenetic regulation of heart development and disease (Han et al., 2011; Ohtani and Dimmeler, 2011; Vallaster et al., 2012; van Weerd et al., 2011). Among epigenetic factors, Polycomb Group (PcG) and Trithorax Group (TrxG) proteins are unique in that they are able to maintain repressed and activated chromatin configurations, respectively, for extended periods of time and in the absence of the transcription factors that initiate repression or activation (reviewed in Jacobs and van Lohuizen, 2002; Pirrotta, 1995). This feature makes them particularly important in the maintenance of “cellular memory” both during lineage differentiation and in adult tissues. Studies on the development of multiple organs and tissues have corroborated such importance. Until recently, there was little information on the roles of PcG or TrxG proteins in the heart. However, emerging data strongly suggest that they are central players in the specification of cardiac lineages during heart development and in the maintenance of cellular properties in the adult heart. In this review, I will focus on these data and discuss them in conjunction with current models of PcG and TrxG functional mechanisms.
PcG and TrxG proteins: essential regulators of axial patterning and lineage development
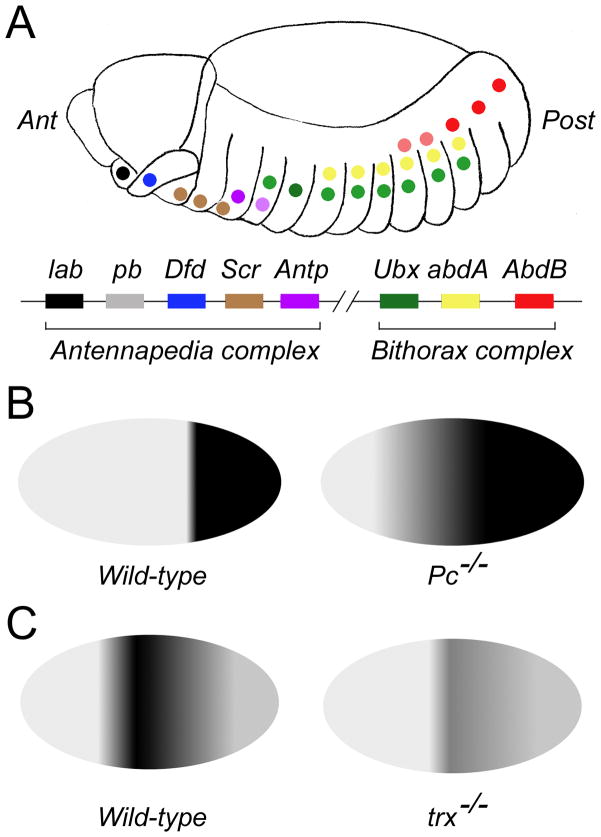
PcG proteins and their antagonists, TrxG proteins, were first identified in Drosophila as regulators of homeotic genes (Hox genes) (the early genetic studies that identified PcG and TrxG proteins were reviewed in Kennison, 1995). Hox genes are highly conserved transcriptional regulators of cell fates along the anterior-posterior axis (reviewed in Hueber and Lohmann, 2008). They are expressed in defined domains along the anterior-posterior axis and in defined temporal orders: individual Hox genes are sequentially activated in progressively posterior domains at progressively later time points (reviewed in Mallo et al., 2010). The overall result is that the boundaries of Hox expression domains are spatially staggered (Figure 2). PcG genes are required for repression of Hox genes outside of, especially anterior to, their normal expression domains (reviewed in Pirrotta, 1995; Gould, 1997). In PcG mutants, Hox genes become derepressed in anterior cells, causing those cells to take on a more posterior fate. On the other hand, TrxG genes are required for keeping Hox genes activated within their normal expression domains (reviewed in Gould, 1997). In TrxG mutants, expression of Hox genes is not properly maintained, causing posterior cells to take on a more anterior fate.
Figure 2. Regulation of Hox genes expression by PcG and TrxG genes in Drosophila.
(A) Staggered expression of Drosophila Hox genes along the anterior-posterior axis of the embryo. The Drosophila genome contains seven Hox genes arranged in two clusters: the Antennapedia complex and the Bithorax complex. The genes and their respective expression domains in the Drosophila embryo are color-coded in this diagram. Ant: anterior; Post: posterior. (B) PcG genes are required to repress Hox genes outside their normal expression domains. The diagram shows expression domain of the Hox gene AbdB in wild-type (left) vs. Pc−/− (right) embryos. AbdB is normally expressed in the posterior segments of wild-type embryos but expands anteriorly in Pc−/− embryos. (C) TrxG genes are required to maintain Hox gene expression. The diagram shows expression of the Hox gene Ubx in wild-type (left) vs. trx−/− embryos (right). Ubx expression is greatly reduced in trx−/− embryos.
In addition to their roles in axial patterning, PcG and TrxG proteins are involved in the development of many organs and cell lineages (reviewed in Surface et al., 2010; van Lohuizen 1998). Genome-wide studies show that PcG and TrxG proteins and the histone marks regulated by them are associated with thousands of chromatin loci in embryonic stem (ES) cells, and these loci are enriched for “developmental genes” (Mikkelsen et al., 2007; Ku et al., 2008; Pan et al., 2007; Zhao et al., 2007). ES cells that are deficient in PcG activity are defective in differentiation and in activating differentiation-specific genes such as lineage markers (Pasini et al., 2007; Chamberlain et al., 2008; Shen et al., 2009; Landeira et al., 2010).
Finally, mammalian PcG and TrxG proteins have been found to participate in the regulation of proliferation and tumorigenesis (reviewed in Bracken and Helin, 2009; Hess, 2004; Reisman et al., 2009). For example, the PcG protein Bmi-1 is an oncogene that can induce telomerase activity and allow cells to bypass senescence when over-expressed in mammary epithelial cells (Dimri et al., 2002). Another PcG protein, Ezh2, is over-expressed in hormone-refractory, metastatic prostate cancer. Silencing of Ezh2 by siRNA inhibits proliferation of cultured prostate cells (Varambally et al., 2002).
Functional mechanisms of PcG and TrxG proteins
PcG and TrxG proteins function at the level of chromatin, and their functional mechanisms are highly conserved. PcG proteins function in multi-protein complexes (reviewed in Muller and Verrijzer, 2009; Schuettengruber and Cavalli, 2009). Four such complexes have been identified in Drosophila: PhoRC, PRC1, PRC2, and PR-DUB. With the exception of PhoRC, corresponding complexes have also been identified in mammals. The PhoRC complex contains sequence-specific DNA binding activity and also interacts with mono- and di-methylated lysine 27 of histone H3 (H3K27) (Klymenko et al., 2006). It has been proposed that PhoRC plays the critical role of recognizing hypomethylated nucleosomes around upstream regulatory elements of PcG target genes. The PRC2 complex contains methyl-transferase activity and induces trimethylation of H3K27 (Czermin et al., 2002; Muller et al., 2002). H3K27me3 is a well-known mark for silenced chromatin and is associated with the promoters and regulatory elements of PcG target genes. The PRC1 complex binds trimethylated H3K27 and induces chromatin compaction, thereby maintaining target chromatin regions in the silenced state (Fischle et al., 2003; Francis et al., 2004) (Figure 3A). PRC1 also mono-ubiquitinates histone H2A at lysine 119, though the ubiquitinase activity of PRC1 appears to be dispensable for its silencing function (Eskeland et al., 2010). The PR-DUB complex has histone deubiquitinase activity that is specific to mono-ubiquitinated H2A (uH2A) in in vitro assays (Scheuermann et al., 2010). The exact role of H2A ubiquitination/deubiquitination in PcG-mediated repression is still unclear.
Figure 3. PcG and TrxG proteins function at the level of chromatin.
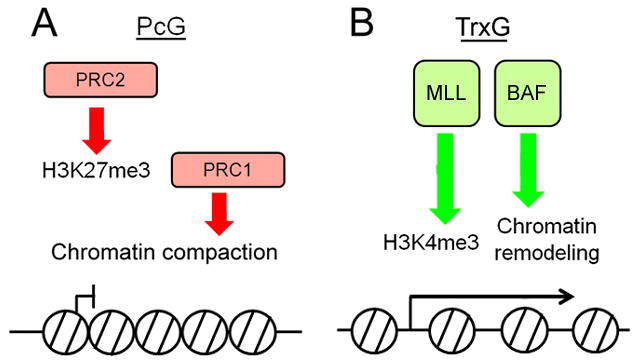
(A) Biochemical functions of the PcG complexes PRC2 and PRC1. PRC2 mediates the trimethylation of histone H3K27, a repressive histone mark. PRC1 binds H3K27me3 and compacts chromatin. (B) Biochemical functions of TrxG complexes MLL and BAF. MLL mediates trimethylation of histone H3K4. BAF has chromatin remodeling activity.
TrxG proteins also function in multi-subunit complexes (Figure 3B) (reviewed in Schuettengruber et al., 2011). Three TrxG complexes, the MLL complex, the BRM/BAF complex and a supercomplex, have been purified in mammalian cells. The MLL complex contains histone methyltransferase (HMTase) activity and trimethylates lysine 4 of histone H3 (H3K4) (Yokoyama et al., 2004). H3K4me3 is tightly associated with the promoter regions of transcriptionally active loci (Bernstein et al., 2005; Schneider et al., 2004). The BRM/BAF complex contains the SWI/SNF chromatin-remodeling ATPase Brm/Brg1 and mediates ATP-dependent nucleosome sliding (Papoulas et al., 1998; Wang et al., 1996). The supercomplex contains both HMTase activity and chromatin remodeling activity (Nakamura et al., 2002).
Several lines of evidence show that PcG and TrxG proteins antagonize the function of each other. First of all, PcG and TrxG mutations have opposite effects on axial patterning. PcG mutations cause posterior transformations, while TrxG mutations cause anterior transformations (reviewed in Kennison, 1995). Secondly, genetic experiments show that most PcG and TrxG mutations are reciprocally suppressive (Daubresse et al., 1999; Ingham, 1983; Kennison and Tamkun, 1988). Finally, the PRC1 complex blocks the chromatin remodeling ability of the BRM/BAF complex on an in vitro assembled nucleosomal template (Shao et al., 1999). Conversely, the active histone mark H3K4me3, which is generated by SET1-like and MLL complexes, inhibits histone methylation by PRC2 (Schmitges et al., 2011). Together, PcG and TrxG proteins comprise a highly conserved system that keeps the transcription state of target genes in finely controlled balance.
PcG and TrxG proteins in the maintenance of “cellular memory”
In multi-cellular organisms, different cell lineages have distinct patterns of gene expression. During differentiation, a cell’s chromatin is configured to facilitate a lineage-specific gene expression pattern. Both the chromatin configuration and the gene expression pattern it ensures are inherited by the cell’s progenies through many cell divisions, even when the developmental cues that initiate the chromatin configuration are no longer present (reviewed in Margueron and Reinberg, 2010; Simon, 1995). PcG and TrxG proteins are thought to be particularly important for the maintenance of such “cellular memory” (reviewed in Jacobs and van Lohuizen, 2002; Pirrotta, 1997).
Genetic experiments in Drosophila provided the early evidence that PcG proteins have a role in the long-term maintenance of gene silencing. For example, the expression pattern of the Hox gene Ubx is established shortly after gastrulation during Drosophila embryogenesis. In embryos mutant for the PcG gene esc, Ubx expression domain was initiated normally, but ectopic expression arose after the germ-band extension stage (Struhl and Akam, 1985). Thus, esc is dispensable for setting up the Ubx expression domain, but is needed in later stages to ensure that Ubx remains silenced in regions where it was not initially activated. On the other hand, TrxG proteins function to maintain gene activation. For example, in mouse embryos that lack the TrxG gene Mll2, the expression of Mox1 and Hoxb1, two Mll2 targets, was induced normally but later became degenerate and was eventually lost completely (Glaser et al., 2006).
How can PcG/TrxG proteins facilitate the transmission of chromatin configuration through cell divisions? While the exact mechanisms are far from well understood, some clues can be found in recent biochemical and structural studies of the PRC2 complex. During S phase, core components of PRC2 are recruited to sites of DNA replication (Hansen et al., 2008). One of the core components, EED, binds to pre-existing H3K27me3 marks through its WD40 repeats (Margueron et al., 2009). EED-H3K27me3 interaction stimulates the methyltransferase activity of PRC2. Thus, pre-existing H3K27me3 not only provides a way to recruit PRC2 complex, but also activates the complex to tri-methylate H3K27 on newly incorporated histones, providing a mechanism for efficient “self-renewal” of the H3K27me3 mark during mitosis.
Emerging roles for PcG and TrxG proteins in the regulation of heart development and function
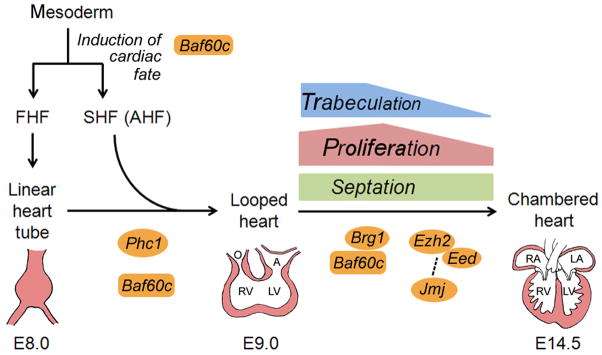
Multiple PcG and TrxG genes are normally expressed in the mouse heart. Due to the essential function of PcG/TrxG genes, constitutive knockouts of key PcG or TrxG genes often result in lethality during early embryogenesis before cardiac phenotypes can be analyzed (Bultman et al., 2000; O’Carroll et al., 2001; Voncken et al., 2003). Aided by conditional knockout models, studies in the past decade have uncovered crucial roles for two PcG complexes and one TrxG complex during cardiac development and/or in the adult heart (Fig. 4, Supplementary Table 1). The major findings of these studies are reviewed below.
Figure 4. Multiple steps of cardiac development require PcG/TrxG function.
Precardiac mesoderm give rise to cardiac progenitors in the first heart field (FHF) and second heart field (SHF, also known as anterior heart field or AHF). The TrxG protein Baf60c likely regulates the induction of cardiac fate. Cells in the FHF form the linear heart tube, which gives rise to the bulk of the left ventricle (LV) and also serves as a scaffold for subsequent heart growth. As the heart tube loops, cells in the SHF migrate to join the linear heart tube and give rise to the outflow tract (O), right ventricle (RV) and atria (A). Both Baf60c and the PcG protein Phc1 have been shown to regulate this early phase of cardiac development. The formation of the chambered heart from the looped heart involves a number of morphogenic processes such as trabeculation, proliferation and septation. Multiple PcG and TrxG proteins, including Brg1, Baf60c, Ezh2, Eed and Jmj, have been shown to regulate these processes. The dashed line between Jmj and Ezh2/Eed represents possible functional interaction. In addition to the FHF and SHF, cells from cardiac neural crest and proepicardium also contribute to the heart (not diagrammed).
Polycomb Repressor Complex 2 (PRC2) core components: Ezh2 and Eed
Ezh2 is a SET-domain histone methyltransferase and the core subunit of PRC2 (Czermin et al., 2002; Muller et al., 2002). Homozygous Ezh2 knockout embryos die before completion of gastrulation (O’Carroll et al., 2001), suggesting that Ezh2 is essential for early embryonic development. Ezh2 is highly expressed in the developing heart but down-regulated in the adult heart, while its homolog Ezh1 shows the reverse pattern (Sdek et al., 2011). Two recent studies that inactivated Ezh2 in specific cell populations in the heart showed that Ezh2 plays important roles both during heart development and in the adult heart (He et al., 2012a; Delgado-Olguin et al., 2012).
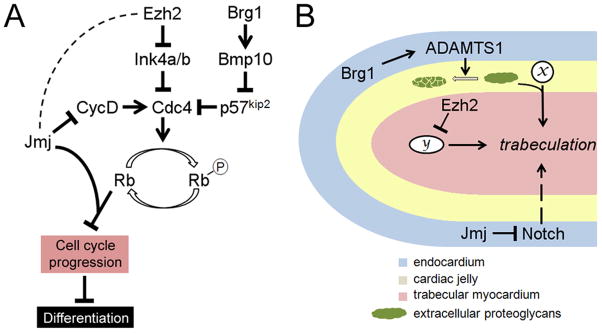
Inactivation of Ezh2 in ventricular cardiomyocytes using Nkx2.5::Cre (Ezh2NK) resulted in perinatal lethality and an array of cardiac abnormalities including hypoplasia in the compact myocardium, excessive trabeculation, septal defects and dilation in the right atrium (He et al., 2012a). Perinatal lethality and thinning of the myocardium were also observed when Eed was deleted in differentiated cardiomyocytes by TnT::Cre, which is active slightly later than Nkx2.5::Cre (He et al., 2012a). However, EedTnT embryos did not exhibit septal defects or atrial dilation. Taken together, these results suggest that PRC2 activity is required for multiple aspects of heart morphogenesis at multiple time points. What is the molecular basis for the morphological defects in Ezh2NK and EedTnT hearts? He et al. have identified more than 50 genes that are directly repressed by PRC2 in the developing heart. The list includes multiple transcription factors with known roles in various steps of heart morphogenesis, such as Isl1, Tbx2, Tbx3, Hand1, Irx5 and Six1 (Cai et al., 2003; Costantini et al., 2005; Guo et al., 2011; McFadden et al., 2005; Mesbah et al., 2008; Risebro et al., 2006; Ribeiro et al., 2007; Riley et al., 1998; Singh et al., 2011). This suggests that PRC2 is critically involved in the developmental coordination of cardiac gene expression programs. In addition, PRC2 directly represses the cyclin-dependent kinase inhibitors Ink4a/b, which may explain the hypoplasia phenotype in Ezh2NK embryos (He et al., 2012a) (Fig. 5A).
Figure 5. Roles of Ezh2, Brg1 and Jmj in cardiomyocyte proliferation and trabeulation.
(A) Pathways by which Ezh2, Brg1 and Jmj regulate fetal cardiomyocyte proliferation. Cyclins (such as cyclin D) activates cyclin-dependent kinases (such as Cdc4), which phosphorylate Rb and relieve Rb repression of a number of genes essential for cell cycle progression. Ezh2 promotes fetal cardiomyocyte proliferation by direct repression of the cyclin-dependent kinase inhibitor Ink4a/b. Brg1 also promotes fetal cardiomyocyte proliferation, and it does so by activating Bmp10, which in turn represses another cyclin-dependent kinase inhibitor p57kip2. Jmj inhibits fetal cardiomyocyte proliferation by repressing cyclin D and by acting as a co-repressor for Rb. It is unclear whether Jmj and Ezh2 functionally interact with each other in the repression of cyclin D and Ink4a/b, and if they do, whether Jmj promotes or inhibits PRC2 activity in these contexts (hence dashed line between Jmj and Ezh2). Over-proliferation of fetal cardiomyocytes may result in delayed differentiation. (B) Regulation of trabeculation by Ezh2, Brg1 and Jmj. The diagram shows a trabecula. Formation of these finger-like trabeculae is induced by signaling between the endocardium and the myocardium. Proteoglycans in the cardiac jelly modulates the trabeculation process by modulating the function of signaling molecules (x). Ezh2 expression in the myocardium is required for trabeculation, possibly by repressing an as-yet unidentified downstream effector (y). Brg1 expression in the endocardium promotes termination of trabeculation by activating the secreted proteinase ADAMTS1, which mediates the degradation of extracellular proteoglycans. Jmj expression in the endocardium negatively regulates trabeculation by repressing Notch.
A study by Delgado-Olguin et al. used a MEF2c-ANF::Cre to inactivate Ezh2 from E7.5 on in cardiac progenitors of the second heart field (SHF, also known as the anterior heart field or AHF) and their derivatives. Interestingly, Ezh2SHF mice did not exhibit overt defects in cardiac morphogenesis; instead, such animals survived to adulthood but developed cardiac hypertrophy and fibrosis in the SHF-derived right ventricle (Delgado-Olguin et al., 2012) (Fig. 6). Six1 was identified as the main effector of Ezh2 function in cardiac hypertrophy. Removing one copy of Six1 rescued the hypertrophy and fibrosis phenotypes caused by SHF-specific deletion of Ezh2 (Delgado-Olguin et al., 2012). Six1 is normally expressed in progenitors in the SHF at E7.5-E8.5, but is down-regulated quickly upon differentiation (Guo et al., 2011). In Ezh2SHF hearts, Six1 expression persists throughout cardiogenesis and in the adult myocardium. An interesting question is whether Ezh2 (and PRC2 activity) plays an initiating or maintenance role in the developmental silencing of Six1 and other cardiac targets. In Drosophila, the developmental silencing of Hox genes can be divided into initiating and maintenance phases, which require distinct regulatory elements, and PcG activity is specifically required during the maintenance phase (Ringrose and Paro, 2007). Thus we might expect that the activity of mammalian PRC2 is continuously required, throughout adulthood, to keep Six1 in a silent state. In other words, Six1 repression may have been initiated in Ezh2SHF hearts but was not maintained. Alternatively, mammalian PRC2 may be needed transiently for initiating Six1 silencing but becomes dispensable afterwards. Differentiating between these two scenarios will impact on the design of therapies that target the PRC2 pathway. It is worth noting that the adult heart predominantly expresses Ezh1 instead of Ezh2 (Sdek et al., 2011). However, Ezh1 function was not sufficient to repress Six1 in adult Ezh2SHF hearts (Delgado-Olguin et al., 2012). This may be due to functional divergence between Ezh1 and Ezh2. Although Ezh1 has been shown to methylate H3K27 and complement Ezh2 in ES cells and skin tissue (Ezhkova et al., 2011; Shen et al., 2008), two studies have highlighted important differences between the functions of the two homologues, including a transcriptional activating role for Ezh1 (Margueron et al., 2008; Mousavi et al., 2012).
Figure 6. Opposing roles of Ezh2 and Brg1 in the regulation of hypertrophic response.
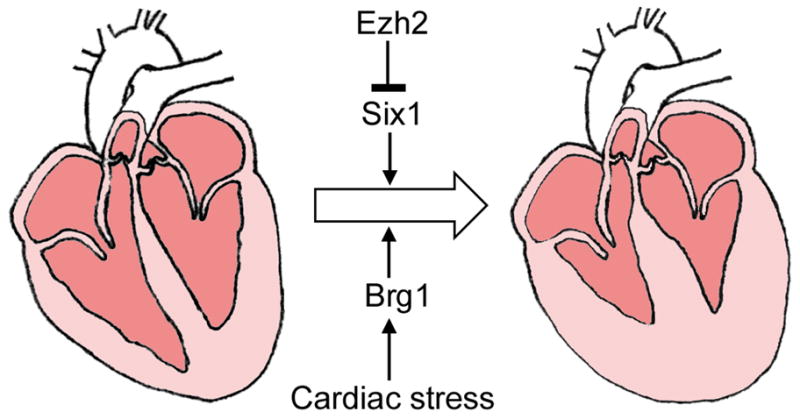
The adult heart responds to stress, such as pressure overload or β-adrenergic stimulation, by hypertrophic growth of cardiomyocytes. This results in thickened myocardial walls and smaller ventricular chamber(s). The PcG protein Ezh2 represses cardiac hypertrophy through a Six1-dependent pathway. The TrxG protein Brg1 is required for development of the hypertrophic response.
In addition to methylating H3K27, Ezh2 was also found to methylate the cardiac transcription factor GATA4 and inhibit GATA4 activity both in vivo and in vitro (He et al., 2012b) (Fig. 7). This makes GATA4 the first known non-histone substrate of PRC2 and reveals a novel mechanism by which PRC2 may regulate transcription. Future experiments are needed to elucidate the relative contribution by H3K27 methylation versus GATA4 methylation toward PRC2-mediated gene silencing of GATA4 target genes. Moreover, it is possible that GATA4 is not an isolated example and PRC2 has other non-histone substrates that remain to be identified.
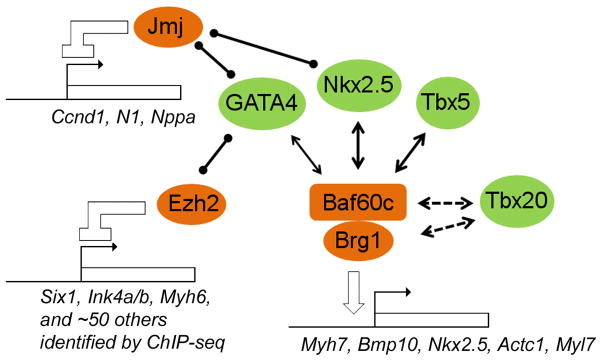
Figure 7. Known interactions between PcG/TrxG proteins and cardiac transcription factors.
The TrxG complex BAF physically interacts with cardiac transcription factors GATA4, Nkx2.5 and Tbx5 and potentiates their activity on target promoters. A genetic interaction between Brg1 and the transcription factor Tbx20 has been shown, but it is unclear whether Tbx20 physically interacts with Brg1, Baf60c, or other subunit(s) of BAF. GATA4 also physically interacts with the PcG protein Ezh2, which methylates GATA4 and inhibits its activity. Jmj interacts with both GATA4 and Nkx2.5 and inhibits their activities through an as-yet unknown mechanism.
PRC2 accessory component: Jumonji/Jarid2
Jumonji (Jmj, also known as Jarid2) is the founding member of the Jumonji family that consists of 27 proteins in humans. Many of Jumonji family proteins are predicted to be histone lysine demethylases (KDM) (reviewed in Cloos et al., 2008). However, Jmj itself is predicted to be enzymatically inactive because it carries several amino acid substitutions in the cofactor Fe(II) binding region that is essential for KDM activity (Klose et al., 2006). Jmj is associated with PRC2 in ES cells and several other cell types and tissue (Landeira et al., 2010; Li et al., 2010a; Pasini et al., 2010; Peng et al., 2009; Shen et al., 2009). Consistent with this association, Jmj and PRC2 co-localize at many chromatin regions and each promotes the efficient recruitment of the other to target chromatin. However, it is uncertain whether Jmj enhances or inhibits the HMTase activity of PRC2, and it is possible that Jmj can do both depending on its protein level relative to PRC2. Finally, Landeira et al. showed that Jmj is required for maintaining PRC2 target promoters in a “primed” state, bound by RNA polymerase II phosphorylated at Ser 5 (the “poised” RNA pol II) and ready to be activated when differentiation cues are received.
Jmj plays critical roles in cardiac development. Jmj expression in the ventricles initiates in the trabecular layer at E10.5 and expands into the compact layer by E12.5; it is broadly expressed in both layers by E18.5 and in postnatal stages (Toyoda et al., 2003). The effect of Jmj mutation is highly influenced by genetic background. Jmj−/− in a C3H/He background died by around E11.5 with hyper-proliferation in the trabecular layer, which could have prevented blood circulation and caused lethality (Takeuchi et al., 1999). On the other hand, Jmj−/− embryos in a C57BL/6 background survived to perinatal stages and exhibited double-outlet right ventricle (DORV), ventricular septal defects (VSD) and severe noncompaction of the ventricular wall (Lee et al., 2000).
While the molecular mechanism of Jmj function during cardiac development is far from well-understood, a number of studies have shed light on it in various ways. First of all, Jmj interacts with two important cardiac transcription factors, Nkx2.5 and GATA4, and inhibits the activation of Nkx2.5 and GATA4 target promoters (Kim et al., 2004). This may explain why Jmj−/− hearts failed to down-regulate atrial natriuretic factor (ANF, encoded by the Nppa gene), which is a known target of Nkx2.5 and GATA4 (Lee et al., 2000). Secondly, Jmj regulates cardiomyocyte cell cycle in two ways (Fig. 5A): on the one hand, it represses cyclin D1 expression (and hence inhibits Rb phosphorylation); on the other hand, it interacts with Rb and functions as an Rb co-repressor (Toyoda et al., 2003; Jung et al., 2005). Both will result in repression of E2F target genes, which are required for cell cycle progression. Jmj is able to bind to the cyclin D1 promoter in transfected cells and recruit H3K9 methylation activity (Shirato et al., 2009), thus directly repressing cyclin D1. Thirdly, in the endocardium, Jmj is a negative regulator of Notch signaling, which serves as a mitogenic signal to the myocardium and regulates the trabeculation process (Fig. 5) (for a review of Notch signaling in trabeculation, see High and Epstein, 2008). Jmj is associated with a conserved region in the Notch1 locus, and Notch1 protein level is significantly elevated in Jmj−/− hearts (Mysliwiec et al., 2011). Remarkably, an endothelial-specific knockout of Jmj, which deleted Jmj in the endocardium and other cells of the endothelial lineage, recapitulated most of the cardiac phenotypes of Jmj−/− mice (Mysliwiec et al., 2011). This suggests that Notch and possibly other signals originating in the endocardium mediate a significant portion of Jmj function, in a cell non-autonomous manner. Finally, Jmj is required for the expression of differentiation markers, such as Myh6 (encoding α-MHC), α-cardiac actin, and actinin, in fetal cardiomyocytes (Takeuchi et al., 1999; Nakajima et al., 2011). However, this may be an indirect effect stemming from Jmj function in regulating proliferation versus differentiation. Increased expression of cyclin D in Jmj−/− hearts causes hyper-proliferation and prevents the expression of GATA4, which is a transcriptional activator of these differentiation genes (Nakajima et al., 2011).
Polycomb Repressor Complex 1 (PRC1) core component: Phc1
Phc1 (also known as Rae28) is a mammalian homolog of the Drosophila PcG protein Polyhomeotic (Ph). Phc1 and Ph are components of the mammalian and Drosophila PRC1 complex, respectively. Phc1 mutant embryos are defective in an early and important step of cardiac morphogenesis - cardiac looping - which takes place between E8.5 and E9.5 (Shirai et al., 2002). While the mutant heart is able to form chambers, it displays VSD and other cardiac abnormalities (Takihara et al., 1997; Shirai et al., 2002). These defects appear to stem from a loss of expression of the cardiac transcription factor Nkx2.5 (Shirai et al., 2002). Phc1 is not required for the initiation of Nkx2.5 expression, but is required for its continued expression. Given that PcG proteins generally function to silence genes, regulation of Nkx2.5 by Phc1 may be indirect, but the exact mechanism remains to be determined.
Interestingly, transgenic studies showed that while ubiquitous expression of a Phc1 transgene could restore Nkx2.5 expression and rescue cardiac morphogenesis defects in Phc1−/− embryos, cardiomyocyte-specific expression could not (Shirai et al., 2002; Koga et al., 2002). This suggests that normal cardiac morphogenesis requires the function of Phc1 in a cell population other than cardiomyocytes. Cardiomyocyte-specific over-expression of Phc1 neither rescued congenital heart defects in Phc1−/− embryos nor disrupted cardiac morphogenesis in embryos of wild-type background. However, continued expression of Phc1 in adult cardiomyocytes is deleterious and leads to disorganization of sarcomeres, cardiomyocyte apoptosis, chamber dilation and heart failure (Koga et al., 2002). Because PcG proteins function in multi-subunit complexes, the activity of a complex – such as PRC1 – is likely influenced by both subunit composition and stoichiometry. Thus, it is difficult to predict whether constitutive expression of Phc1 in adult cardiomyocytes would boost or interfere with PRC1 activity. Nonetheless, we can conclude from the Phc1 transgenic studies that the fine regulation of PcG activity in adult stages is essential for the maintenance of cardiomyocyte function. It awaits future studies to decipher the molecular function(s) of PRC1 in the adult heart.
BAF complex core component: Brg1/Smarca4
The Drosophila TrxG protein Brm is a core component of the BRM complex, which mediates ATP-dependent chromatin remodeling (Papoulas et al., 1998). In humans, the BRG1-associated-factor (BAF) complex shares multiple conserved subunits with the Drosophila BRM complex (Wang et al., 1996). The ATPase core of hBAF can be either hBRG1 (also known as SMARCA4) or hBRM (SMARCA2), both of which are homologous to Drosophila Brm.
In the mouse, Brg1 is widely expressed in embryonic heart, and its expression in different regions appears to serve different roles. Brg1 expression in the endocardium is crucial for trabeculation in the ventricular myocardium (Stankunas et al., 2008) (Fig. 5B). During the process of trabeculation, signaling between the endocardium and the myocardium induces myocardial cells to form finger-like projections, or trabeculae. Trabeculation is a temporally regulated process that initiates at ~E9.0, slows down around E12.5 and completes by E14.5. An appropriate degree of trabeculation is critical for normal contraction and hemodynamics of the embryonic heart, and is essential for the survival of the embryo. The extracellular matrix between endocardium and myocardium, known as cardiac jelly, plays important roles in trabeculation by affecting the diffusion and function of signaling molecules and by providing a microenvironment that supports the extensive cellular movements needed for trabeculae formation (for a comprehensive review of the role of ECM in cell signaling, see Kim et al., 2011). Brg1 is required for the repression of ADAMTS1, a secreted matrix metalloproteinase that degrades Versican and possibly other proteoglycans in the cardiac jelly, and thereby terminates the trabeculation process (Stankunas et al., 2008). When Brg1 is deleted in the endocardium, ADAMST1 becomes de-repressed prematurelly, resulting in early degradation of cardiac jelly and hypo-trabeculation. A small-molecule inhibitor of ADAMST1 can rescue the hypo-trabeculation phenotype in cultured Brg1 mutant embryos, suggesting that the main function of Brg1 in the trabeculation process is to regulate ADAMST1.
Brg1 is also expressed throughout the embryonic myocardium, and this expression is required for normal proliferation and differentiation of cardiomyocytes (Hang et al., 2010). Myocardium-specific deletion of Brg1 resulted in significantly reduced cardiomyocyte proliferation, reduced expression of Bmp10 (a cardiomyocyte growth factor) and increased expression of p57kip2 (a cyclin-dependent kinase inhibitor) (Fig. 5A). In addition, Brg1−/− cardiomyocytes exhibited premature formation of organized sarcomeres, elevated expression of the “adult” MHC isoform α-MHC, and reduced expression of β-MHC (encoded by the Myh7 gene), the “fetal” isoform that is primarily expressed by embryonic hearts.
In addition to being required for heart development, Brg1 also has roles in heart disease in the adult. With the exception of a small number of non-cardiomyocyte cells, the adult heart does not express Brg1. However, Brg1 can be reactivated by stress signals. The reactivation of Brg1 is essential for the development of hypertrophy in TAC-operated mice (Hang et al., 2010) (Fig. 6). The adult murine heart predominantly expresses α-MHC. One of the hallmark events during the hypertrophic process is α/β-MHC isoform switching: the re-activation of Myh7, and often a concurrent repression of Myh6. Consistent with its role in the embryonic heart, Brg1 is required for Myh7 activation and Myh6 repression. This may partially explain why Brg1 is required for hypertrophy, though additional mechanism(s) may also be at work.
While the recruitment of PcG proteins to target chromatin sites is thought to involve specialized regulatory elements (reviewed in Ringrose and Paro, 2007) and/or long non-coding RNA (Kotake et al., 2011; Pandey et al., 2008; Rinn et al., 2007; Plath et al., 2003; Zhao et al., 2008), a large number of studies suggest that transcription factors play important roles in the recruitment of BAF and other chromatin remodeling complexes (reviewed in Peterson and Workman, 2000; Sudarsanam and Winston, 2000). Consistent with this view, Brg1 and the BAF complex functionally interact with multiple cardiac transcription factors (Lickert et al., 2004; Lou et al., 2011; Takeuchi and Bruneau, 2009; Takeuchi et al., 2011) (Fig. 7). In the mouse, Brg1 genetically interacts with Tbx5, Nkx2.5 and Tbx20 (Takeuchi et al., 2011). Double heterozygotes of Brg1 and any of these transcription factors die before E14.5 and exhibit various cardiac morphogenic defects, demonstrating mutual genetic enhancement between mutations in Brg1 and in these transcription factors. Functional interaction has also been observed between BAF complex and GATA4 in both mouse and zebrafish (Lickert et al., 2004; Lou et al., 2011; Takeuchi and Bruneau, 2009; Takeuchi et al., 2011).
BAF complex core component: Baf60c/Smarcd3
Baf60c (also known as Smarcd3) is another core component of the BAF complex. Its yeast homolog has been shown to be essential for the activity of the SWI/SNF complex, which is the yeast counterpart of BAF (Cairns et al., 1996). There are three Baf60 paralogues in the mammalian genome, Baf60a, b and c. Among the three, Baf60c is the only one that is expressed in the developing heart (Lickert et al., 2004).
In contrast to the broad expression of Brg1, the expression pattern of Baf60c is highly tissue-specific. When Baf60c expression initiates at ~E7.5, it is restricted to the cardiac crescent. It continues to be expressed at high levels in the heart tube and chambered heart. By E9.5, expression is also detected in the somites, dorsal neural tube and limb bud. Using transgenic mice expressing siRNA against Baf60c, Lickert et al. showed that Baf60c was particularly important for development of the outflow tract (OFT), right ventricle (RV) and atrium, all of which are derivatives of the SHF (Lickert et al., 2004).
When Baf60c and GATA4 were co-transfected into wild-type E6.5–E8.75 mouse embryos, the early cardiac marker Actc1 was ectopically induced in normally non-cardiogenic mesoderm tissues (Takeuchi and Bruneau, 2009). Addition of Tbx5 to the transfection mixture allowed further differentiation into beating cardiomyocytes. These results suggest that Baf60c may have a central function in the specification of cardiac fate in addition to its later role in cardiac morphogenesis. This function was not uncovered in the siBaf60c model, possibly because RNAi did not completely eliminate Baf60c expression (Lickert et al., 2004).
Although Brg1 and Baf60c are both core components of the BAF complex, Brg1- and Baf60c-deficient mice exhibited distinct, albeit overlapping, phenotypes. Both are required for trabeculation (Lickert et al., 2004; Stankunas et al., 2008). On the other hand, neither epicardium-specific nor myocardium-specific deletion of Brg1 exhibited gross defects in the SHF (Stankunas et al., 2008; Hang et al., 2010). The disparity in Brg1- and Baf60c- deficiency phenotypes may result from differences in the timing of Brg1 or Baf60c loss in the respective mouse models: a role for Brg1 in SHF development may have been missed in the conditional knockouts, in which deletion of Brg1 occurs after E9.5. Alternatively, it may be due to the distinct roles that the two proteins play within the BAF complex. In reporter assays, Baf60c potentiates the activity of Tbx5, Nkx2.5, and GATA4 by promoting the interaction between these cardiac transcription factors and Brg1 (Lickert et al., 2004) (Fig. 7). This suggests that Baf60c functions as a bridge between the BAF complex and select cardiac transcription factors. In addition to bringing chromatin remodeling activity contained in Brg1, Baf60c may allow its partner transcription factors to access other activities via the BAF complex, such as histone H2B ubiquitinase activity (Li et al., 2010b) or interaction with the basal transcription machinery (Cho et al., 1998; Lemieux and Gaudreau, 2004; Neish et al., 1998; Wilson et al., 1996). Thus, different target genes may exhibit individual requirements for Brg1 and Baf60c, depending on whether their activation requires chromatin remodeling and/or other activities mediated by BAF.
Baf60c not only is important for the recruitment of Brg1 and other BAF-associated activities, but also is required for detectable binding of GATA4 to two of its target loci (Takeuchi and Bruneau, 2009). A role for BAF in the recruitment of its interacting transcription factors has been previously observed. For example, SWI/SNF (the yeast counterpart of BAF) stimulates nucleosome binding by transcription factors Sp1, USF, and NF-κB in vitro (Utley et al. 1997). In vivo, SWI/SNF is required for the efficient binding of GAL4 to low affinity, nucleosomal sites, but not for GAL4 binding to high affinity sites or nucleosome-free low affinity sites (Burns and Peterson, 1997). It has been proposed that BAF may be recruited by a transcription factor binding to a high affinity site, and in turn promotes the recruitment of other transcription factors that bind to weaker sites. Alternatively, BAF may interact with the latter transcription factor in solution before both are recruited to the target site (Peterson and Workman, 2000; Sudarsanam and Winston, 2000). Thus, Baf60c may be recruited by another factor and permits subsequent GATA4 binding, or it may be co-recruited with GATA4 and stabilizes GATA4-chromatin association that is otherwise weak or transient. Whether the recruitment of Baf60c/BAF is dependent on GATA4 or any of its interacting transcription factors needs to be directly tested by future experiments.
Perspectives
During embryonic development, the process of heart morphogenesis involves multiple groups of cells whose specification, proliferation, migration, differentiation and interaction must be precisely regulated both temporally and spatially. During adult life, cardiomyocytes need to maintain their identity and function for many years while having the capacity to respond to physiological changes. PcG and TrxG proteins are well suited for the regulation of both embryonic heart development and adult heart function. By creating repressive and activating chromatin structures, respectively, PcG and TrxG proteins play unique roles in the maintenance of lineage identity and cellular memory. The studies reviewed here have demonstrated important functions for several PcG and TrxG proteins in the heart. However, many questions remain to be answered. While we know PcG and TrxG proteins play critical roles in multiple steps of cardiac development, there are conspicuous gaps in our knowledge: we still know nothing about their function in the development of cardiac neural crest and the proepicardium, both of which make essential contributions to the heart (reviewed in Gittenberger-de Groot et al., 2010; Stoller and Epstein, 2005). While PcG and TrxG proteins have been shown to regulate the hypertrophic response in the adult heart, we need a deeper understanding of their functional mechanisms to address whether cardiac hypertrophy involves a modification of “cellular memory” and whether the normal cellular memory, once modified, can be restored. While functional interactions between PcG/TrxG proteins and cardiac transcription factors have been reported, we are still a long way from integrating PcG and TrxG function into the overall picture of cardiac transcriptional regulation. Finally, to translate findings from basic research to medicine, we need to address whether mutations in human PcG and TrxG genes are associated with heart disease. As we gain a better knowledge of the roles and functional mechanisms of PcG and TrxG proteins in the heart, we will move closer to harnessing epigenetic mechanisms in the prevention and/or treatment of heart disease.
Supplementary Material
Acknowledgments
I apologize for omitting many references on heart development, heart function, and the general mechanisms of PcG and TrxG function in this highly focused review. I thank Teresa Orenic, Andrea Marion, Farida Khan and Honghu Quan for comments. I thank the National Heart, Lung and Blood Institute (NHLBI) for funding.
Abbreviations
- AHF
Anterior heart field
- ANF
atrial natriuretic factor
- BAF
BRG1-associated-factor
- CHD
congenital heart defects
- DORV
double-outlet right ventricle
- ES cells
embryonic stem cells
- H3K4
histone H3 lysine 4
- H3K4me3
trimethylated histone H3 lysine 4
- H3K27
histone H3 lysine 27
- H3K27me3
trimethylated histone H3 lysine 27
- HDAC
histone deacetylase
- HMTase
histone methyltransferase
- Hox genes
homeotic genes
- KDM
lysine demethylases
- LV
left ventricle
- OFT
outflow tract
- PcG
Polycomb Group
- PRC1
Polycomb Repressor Complex 1
- PRC2
Polycomb Repressor Complex 2
- PR-DUB
Polycomb Repressor Deubiquitinase
- RV
right ventricle
- SHF
second heart field
- TAC
tranverse aortic constriction
- TrxG
Trithorax Group
- uH2A
mono-ubiquitinated H2A
- VSD
ventricular septal defect
References Cited
- Aalfs JD, Kingston RE. What does “chromatin remodeling” mean? Trends in biochemical sciences. 2000;25:548–555. doi: 10.1016/s0968-0004(00)01689-3. [DOI] [PubMed] [Google Scholar]
- Aries A, Paradis P, Lefebvre C, Schwartz RJ, Nemer M. Essential role of GATA-4 in cell survival and drug-induced cardiotoxicity. Proc Natl Acad Sci USA. 2004;101:6975–6980. doi: 10.1073/pnas.0401833101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balza RO, Jr, Misra RP. Role of the serum response factor in regulating contractile apparatus gene expression and sarcomeric integrity in cardiomyocytes. J Biol Chem. 2006;281:6498–6510. doi: 10.1074/jbc.M509487200. [DOI] [PubMed] [Google Scholar]
- Berger SL. Histone modifications in transcriptional regulation. Current opinion in genetics & development. 2002;12:142–148. doi: 10.1016/s0959-437x(02)00279-4. [DOI] [PubMed] [Google Scholar]
- Bernstein BE, Kamal M, Lindblad-Toh K, Bekiranov S, Bailey DK, Huebert DJ, McMahon S, Karlsson EK, Kulbokas EJ, 3rd, Gingeras TR, Schreiber SL, Lander ES. Genomic maps and comparative analysis of histone modifications in human and mouse. Cell. 2005;120:169–181. doi: 10.1016/j.cell.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Bracken AP, Helin K. Polycomb group proteins: navigators of lineage pathways led astray in cancer. Nature Reviews Cancer. 2009;9:773–784. doi: 10.1038/nrc2736. [DOI] [PubMed] [Google Scholar]
- Brand T. Heart development: molecular insights into cardiac specification and early morphogenesis. Dev Biol. 2003;258:1–19. doi: 10.1016/s0012-1606(03)00112-x. [DOI] [PubMed] [Google Scholar]
- Bruneau BG. Transcriptional regulation of vertebrate cardiac morphogenesis. Circ Res. 2002;90:509–519. doi: 10.1161/01.res.0000013072.51957.b7. [DOI] [PubMed] [Google Scholar]
- Bultman S, Gebuhr T, Yee D, La Mantia C, Nicholson J, Gilliam A, Randazzo F, Metzger D, Chambon P, Crabtree G, Magnuson T. A Brg1 null mutation in the mouse reveals functional differences among mammalian SWI/SNF complexes. Mol Cell. 2000;6:1287–1295. doi: 10.1016/s1097-2765(00)00127-1. [DOI] [PubMed] [Google Scholar]
- Burns LG, Peterson CL. The yeast SWI-SNF complex facilitates binding of a transcriptional activator to nucleosomal sites in vivo. Molecular and cellular biology. 1997;17:4811–4819. doi: 10.1128/mcb.17.8.4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai C-L, Liang X, Shi Y, Chu P-H, Pfaff SL, Chen J, Evans S. sl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Developmental Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns BR, Levinson RS, Yamamoto KR, Kornberg RD. Essential role of Swp73p in the function of yeast Swi/Snf complex. Genes Dev. 1996;10:2131–2144. doi: 10.1101/gad.10.17.2131. [DOI] [PubMed] [Google Scholar]
- Chamberlain SJ, Yee D, Magnuson T. Polycomb repressive complex 2 is dispensable for maintenance of embryonic stem cell pluripotency. Stem Cells. 2008;26:1496–1505. doi: 10.1634/stemcells.2008-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung P, Allis CD, Sassone-Corsi P. Signaling to chromatin through histone modifications. Cell. 2000;103:263–271. doi: 10.1016/s0092-8674(00)00118-5. [DOI] [PubMed] [Google Scholar]
- Cho H, Orphanides G, Sun X, Yang XJ, Ogryzko V, Lees E, Nakatani Y, Reinberg D. A human RNA polymerase II complex containing factors that modify chromatin structure. Molecular and cellular biology. 1998;18:5355–5363. doi: 10.1128/mcb.18.9.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KL, Yutzey KE, Benson DW. Transcription factors and congenital heart defects. Annu Rev Physiol. 2006;68:97–121. doi: 10.1146/annurev.physiol.68.040104.113828. [DOI] [PubMed] [Google Scholar]
- Cloos PAC, Christensen J, Agger K, Helin K. Erasing the methyl mark: histone demethylases at the center of cellular differentiation and disease. Genes Dev. 2008;22:1115–1140. doi: 10.1101/gad.1652908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway SJ, Firulli B, Firulli AB. A bHLH code for cardiac morphogenesis. Pediatric Cardiology. 2010;31:318–24. doi: 10.1007/s00246-009-9608-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini DL, Arruda EP, Agarwal P, Kim K-H, Zhu Y, Zhu W, Lebel M, Cheng CW, Park CY, Pierce SA, Guerchicoff A, Pollevick GD, Chan TY, Kabir MG, Cheng SH, Husain M, Antzelevitch C, Srivastava D, Gross GJ, Hui C, Backx PH, Bruneau BG. The homeodomain transcription factor Irx5 establishes the mouse cardiac ventricular repolarization gradient. Cell. 2005;123:347–358. doi: 10.1016/j.cell.2005.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cripps RM, Olson EN. Control of cardiac development by an evolutionarily conserved transcriptional network. Dev Biol. 2002;246:14–28. doi: 10.1006/dbio.2002.0666. [DOI] [PubMed] [Google Scholar]
- Czermin B, Melfi R, McCabe D, Seitz V, Imhof A, Pirrotta V. Drosophila enhancer of Zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal polycomb sites. Cell. 2002;111:185–196. doi: 10.1016/s0092-8674(02)00975-3. [DOI] [PubMed] [Google Scholar]
- Daubresse G, Deuring R, Moore L, Papoulas O, Zakrajsek I, Waldrip WR, Scott MP, Kennison JA, Tamkun JW. The Drosophila kismet gene is related to chromatin-remodeling factors and is required for both segmentation and segment identity. Development. 1999;126:1175–1187. doi: 10.1242/dev.126.6.1175. [DOI] [PubMed] [Google Scholar]
- Delgado-Olguín P, Huang Y, Li X, Christodoulou D, Seidman CE, Seidman JG, Tarakhovsky A, Bruneau BG. Epigenetic repression of cardiac progenitor gene expression by Ezh2 is required for postnatal cardiac homeostasis. Nature Genetics. 2012;44:343–347. doi: 10.1038/ng.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimri GP, Martinez J-l, Jacobs JJL, Keblusek P, Itahana K, Lohuizen MV, Campisi J, Wazer DE, Band V. The Bmi-1 oncogene induces telomerase activity and immortalizes human mammary epithelial cells. Cancer Res. 2002;62:4736–4745. [PubMed] [Google Scholar]
- Eskeland R, Leeb M, Grimes GR, Kress C, Boyle S, Sproul D, Gilbert N, Fan Y, Skoultchi AI, Wutz A, Bickmore Wa. Ring1B compacts chromatin structure and represses gene expression independent of histone ubiquitination. Mol Cell. 2010;38:452–464. doi: 10.1016/j.molcel.2010.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezhkova E, Lien W-H, Stokes N, Pasolli HA, Silva JM, Fuchs E. EZH1 and EZH2 cogovern histone H3K27 trimethylation and are essential for hair follicle homeostasis and wound repair. Genes Dev. 2011;25:485–498. doi: 10.1101/gad.2019811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H-Y, Narlikar GJ, Kingston RE. Noncovalent modification of chromatin: different remodeled products with different ATPase domains. Cold Spring Harbor symposia on quantitative biology. 2004;69:183–192. doi: 10.1101/sqb.2004.69.183. [DOI] [PubMed] [Google Scholar]
- Fischle W, Wang Y, Jacobs SA, Kim Y, Allis CD, Khorasanizadeh S. Molecular basis for the discrimination of repressive methyl-lysine marks in histone H3 by Polycomb and HP1 chromodomains. Genes Dev. 2003;17:1870–1881. doi: 10.1101/gad.1110503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis NJ, Kingston RE, Woodcock CL. Chromatin compaction by a polycomb group protein complex. Science. 2004;306:1574–1577. doi: 10.1126/science.1100576. [DOI] [PubMed] [Google Scholar]
- Gittenberger-de Groot AC, Winter EM, Poelmann RE. Epicardium-derived cells (EPDCs) in development, cardiac disease and repair of ischemia. Journal of Cellular and Molecular Medicine. 2010;14:1056–1060. doi: 10.1111/j.1582-4934.2010.01077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser S, Schaft J, Lubitz S, Vintersten K, van der Hoeven F, Tufteland KR, Aasland R, Anastassiadis K, Ang SL, Stewart AF. Multiple epigenetic maintenance factors implicated by the loss of Mll2 in mouse development. Development. 2006;133:1423–1432. doi: 10.1242/dev.02302. [DOI] [PubMed] [Google Scholar]
- Glatt S, Alfieri C, Müller CW. Recognizing and remodeling the nucleosome. Current Opinion in Structural Biology. 2011;21:335–41. doi: 10.1016/j.sbi.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Gould A. Functions of mammalian Polycomb group and trithorax group related genes. Current Opinion in Genetics & Development. 1997;7:488–494. doi: 10.1016/s0959-437x(97)80075-5. [DOI] [PubMed] [Google Scholar]
- Guo C, Sun Y, Zhou B, Adam RM, Li X, Pu WT, Morrow BE, Moon A, Li X. A Tbx1-Six1/Eya1-Fgf8 genetic pathway controls mammalian cardiovascular and craniofacial morphogenesis. The Journal of clinical investigation. 2011;121:1585–1595. doi: 10.1172/JCI44630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greulich F, Rudat C, Kispert A. Mechanisms of T-box gene function in the developing heart. Cardiovascular Research. 2011;91:212–22. doi: 10.1093/cvr/cvr112. [DOI] [PubMed] [Google Scholar]
- Han P, Hang CT, Yang J, Chang C-P. Chromatin remodeling in cardiovascular development and physiology. Circ Res. 2011;108:378–396. doi: 10.1161/CIRCRESAHA.110.224287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang CT, Yang J, Han P, Cheng H-L, Shang C, Ashley E, Zhou B, Chang C-P. Chromatin regulation by Brg1 underlies heart muscle development and disease. Nature. 2010;466:62–67. doi: 10.1038/nature09130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KH, Bracken AP, Pasini D, Dietrich N, Gehani SS, Monrad A, Rappsilber J, Lerdrup M, Helin K. A model for transmission of the H3K27me3 epigenetic mark. Nat Cell Biol. 2008;10:1291–1300. doi: 10.1038/ncb1787. [DOI] [PubMed] [Google Scholar]
- Hargreaves DC, Crabtree GR. ATP-dependent chromatin remodeling: genetics, genomics and mechanisms. Cell Research. 2011;21:396–420. doi: 10.1038/cr.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He A, Ma Q, Cao J, von Gise A, Zhou P, Xie H, Zhang B, Hsing M, Christodoulou D, Cahan P, Daley GQ, Kong SW, Orkin SH, Seidman CE, Seidman JG, Pu WT. Polycomb Repressive Complex 2 Regulates Normal Development of the Mouse Heart. Circ Res. 2012a;110:406–415. doi: 10.1161/CIRCRESAHA.111.252205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He A, Shen X, Ma Q, Cao J, von Gise AV, Zhou P, Wang G, Marquez VE, Orkin SH, Pu WT. PRC2 directly methylates GATA4 and represses its transcriptional activity. Genes Dev. 2012b;26:37–42. doi: 10.1101/gad.173930.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess JL. MLL: a histone methyltransferase disrupted in leukemia. Trends in Molecular Medicine. 2004;10:500–507. doi: 10.1016/j.molmed.2004.08.005. [DOI] [PubMed] [Google Scholar]
- High FA, Epstein JA. The multifaceted role of Notch in cardiac development and disease. Nature Reviews Genetics. 2008;9:49–61. doi: 10.1038/nrg2279. [DOI] [PubMed] [Google Scholar]
- Hueber SD, Lohmann I. Shaping segments: Hox gene function in the genomic age. BioEssays. 2008;30:965–979. doi: 10.1002/bies.20823. [DOI] [PubMed] [Google Scholar]
- Ingham PW. A gene that regulates the bithorax complex differentially in larval and adult cells of Drosophila. Cell. 1984;37:815–823. doi: 10.1016/0092-8674(84)90416-1. [DOI] [PubMed] [Google Scholar]
- Jacobs JJ, van Lohuizen M. Cellular memory of transcriptional states by Polycomb-group proteins. Seminars in cell & developmental biology. 1999;10:227–235. doi: 10.1006/scdb.1999.0304. [DOI] [PubMed] [Google Scholar]
- Jung J, Kim T-G, Lyons GE, Kim H-RC, Lee Y. Jumonji regulates cardiomyocyte proliferation via interaction with retinoblastoma protein. J Biol Chem. 2005;280:30916–30923. doi: 10.1074/jbc.M414482200. [DOI] [PubMed] [Google Scholar]
- Kennison JA. The Polycomb and trithorax group proteins of Drosophila: trans-regulators of homeotic gene function. Annu Rev Genet. 1995;29:289–303. doi: 10.1146/annurev.ge.29.120195.001445. [DOI] [PubMed] [Google Scholar]
- Kennison JA, Tamkun JW. Dosage-dependent modifiers of polycomb and antennapedia mutations in Drosophila. Proc Natl Acad Sci USA. 1988;85:8136–8140. doi: 10.1073/pnas.85.21.8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-H, Turnbull J, Guimond S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. The Journal of Endocrinology. 2011;209:139–151. doi: 10.1530/JOE-10-0377. [DOI] [PubMed] [Google Scholar]
- Kim T-G, Chen J, Sadoshima J, Lee Y. Jumonji represses atrial natriuretic factor gene expression by inhibiting transcriptional activities of cardiac transcription factors. Molecular and cellular biology. 2004;24:10151–10160. doi: 10.1128/MCB.24.23.10151-10160.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klymenko T, Papp B, Fischle W, Köcher T, Schelder M, Fritsch C, Wild B, Wilm M, Muller J. A Polycomb group protein complex with sequence-specific DNA-binding and selective methyl-lysine-binding activities. Genes Dev. 2006;20:1110–1122. doi: 10.1101/gad.377406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodo K, Yamagishi H. A decade of advances in the molecular embryology and genetics underlying congenital heart defects. Circulation Journal. 2011;75:2296–304. doi: 10.1253/circj.cj-11-0636. [DOI] [PubMed] [Google Scholar]
- Koga H, Kaji Y, Nishii K, Shirai M, Tomotsune D, Osugi T, Sawada A, Kim JY, Hara J, Miwa T, Yamauchi-Takihara K, Shibata Y, YT Overexpression of Polycomb-group gene rae28 in cardiomyocytes does not complement abnormal cardiac morphogenesis in mice lacking rae28 but causes dilated cardiomyopathy. Lab Invest. 2002;82:375–385. doi: 10.1038/labinvest.3780432. [DOI] [PubMed] [Google Scholar]
- Kotake Y, Nakagawa T, Kitagawa K, Suzuki S, Liu N, Kitagawa M, Xiong Y. Long non-coding RNA ANRIL is required for the PRC2 recruitment to and silencing of p15(INK4B) tumor suppressor gene. Oncogene. 2011;30:1956–1962. doi: 10.1038/onc.2010.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku M, Koche RP, Rheinbay E, Mendenhall EM, Endoh M, Mikkelsen TS, Presser A, Nusbaum C, Xie X, Chi AS, Adli M, Kasif S, Ptaszek LM, Cowan CA, Lander ES, Koseki H, Bernstein BE. Genomewide analysis of PRC1 and PRC2 occupancy identifies two classes of bivalent domains. PLoS Genet. 2008;4:e1000242. doi: 10.1371/journal.pgen.1000242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon H, Imbalzano AN, Khavari PA, Kingston RE, Green MR. Nucleosome disruption and enhancement of activator binding by a human SW1/SNF complex. Nature. 1994;370:477–481. doi: 10.1038/370477a0. [DOI] [PubMed] [Google Scholar]
- Landeira D, Sauer S, Poot R, Dvorkina M, Mazzarella L, Jørgensen HF, Pereira CF, Leleu M, Piccolo FM, Spivakov M, Brookes E, Pombo A, Fisher C, Skarnes WC, Snoek T, Bezstarosti K, Demmers J, Klose RJ, Casanova M, Tavares L, Brockdorff N, Merkenschlager M, Fisher AG. Jarid2 is a PRC2 component in embryonic stem cells required for multi-lineage differentiation and recruitment of PRC1 and RNA Polymerase II to developmental regulators. Nature cell biology. 2010;12:618–624. doi: 10.1038/ncb2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Song AJ, Baker R, Micales B, Conway SJ, Lyons GE. Jumonji, a nuclear protein that is necessary for normal heart development. Circ Res. 2000;86:932–938. doi: 10.1161/01.res.86.9.932. [DOI] [PubMed] [Google Scholar]
- Lemieux K, Gaudreau L. Targeting of Swi/Snf to the yeast GAL1 UAS G requires the Mediator, TAF IIs, and RNA polymerase II. The EMBO Journal. 2004;23:4040–4050. doi: 10.1038/sj.emboj.7600416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine SS, King IFG, Kingston RE. Division of labor in Polycomb group repression. Trends Biochem Sci. 2004;29:478–485. doi: 10.1016/j.tibs.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Li G, Margueron R, Ku M, Chambon P, Bernstein BE, Reinberg D. Jarid2 and PRC2, partners in regulating gene expression. Genes Dev. 2010a;24:360–380. doi: 10.1101/gad.1886410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XS, Trojer P, Matsumura T, Treisman JE, Tanese N. Mammalian SWI/SNF--a subunit BAF250/ARID1 is an E3 ubiquitin ligase that targets histone H2B. Molecular and cellular biology. 2010b;30:1673–1688. doi: 10.1128/MCB.00540-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lickert H, Takeuchi JK, Von Both I, Walls JR, McAuliffe F, Adamson SL, Henkelman RM, Wrana JL, Rossant J, Bruneau BG. Baf60c is essential for function of BAF chromatin remodelling complexes in heart development. Nature. 2004;432:107–112. doi: 10.1038/nature03071. [DOI] [PubMed] [Google Scholar]
- Lou X, Deshwar AR, Crump JG, Scott IC. Smarcd3b and Gata5 promote a cardiac progenitor fate in the zebrafish embryo. Development. 2011;138:3113–3123. doi: 10.1242/dev.064279. [DOI] [PubMed] [Google Scholar]
- Mallo M, Wellik DM, Deschamps J. Hox genes and regional patterning of the vertebrate body plan. Dev Biol. 2010;344:7–15. doi: 10.1016/j.ydbio.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margueron R, Li G, Sarma K, Blais A, Zavadil J, Woodcock CL, Dynlacht BD, Reinberg D. Ezh1 and Ezh2 maintain repressive chromatin through different mechanisms. Mol Cell. 2008;32:503–518. doi: 10.1016/j.molcel.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margueron R, Reinberg D. Chromatin structure and the inheritance of epigenetic information. Nature Reviews Genetics. 2010;11:285–296. doi: 10.1038/nrg2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margueron R, Justin N, Ohno K, Sharpe ML, Son J, Drury WJ, Voigt P, Martin SR, Taylor WR, De Marco V, Pirrotta V, Reinberg D, Gamblin SJ. Role of the polycomb protein EED in the propagation of repressive histone marks. Nature. 2009;461:762–767. doi: 10.1038/nature08398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulley DJ, Black BL. Transcription factor pathways and congenital heart disease. Current Topics in Developmental Biology. 2012;100:253–77. doi: 10.1016/B978-0-12-387786-4.00008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden DG, Barbosa AC, Richardson JA, Schneider MD, Srivastava D, Olson EN. The Hand1 and Hand2 transcription factors regulate expansion of the embryonic cardiac ventricles in a gene dosage-dependent manner. Development. 2005;132:189–201. doi: 10.1242/dev.01562. [DOI] [PubMed] [Google Scholar]
- Mesbah K, Harrelson Z, Théveniau-Ruissy M, Papaioannou VE, Kelly RG. Tbx3 is required for outflow tract development. Circ Res. 2008;103:743–750. doi: 10.1161/CIRCRESAHA.108.172858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen TS, Ku M, Jaffe DB, Issac B, Lieberman E, Giannoukos G, Alvarez P, Brockman W, Kim TK, Koche RP, Lee W, Mendenhall EM, O’Donovan A, Presser A, Russ C, Xie X, Meissner A, Wernig M, Jaenisch R, Nusbaum C, Lander ES, Bernstein BE. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007;448:553–560. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne TA, Briggs SD, Brock HW, Martin ME, Gibbs D, Allis CD, Hess JL. MLL Targets SET Domain Methyltransferase Activity to Hox Gene Promoters. Mol Cell. 2002;10:1107–1117. doi: 10.1016/s1097-2765(02)00741-4. [DOI] [PubMed] [Google Scholar]
- Mousavi K, Zare H, Wang AH, Sartorelli V. Polycomb Protein Ezh1 Promotes RNA Polymerase II Elongation. Mol Cell. 2011;45:255–262. doi: 10.1016/j.molcel.2011.11.019. [DOI] [PubMed] [Google Scholar]
- Müller J, Hart CM, Francis NJ, Vargas ML, Sengupta A, Wild B, Miller EL, O’Connor MB, Kingston RE, Simon JA. Histone Methyltransferase Activity of a Drosophila Polycomb Group. Repressor Complex Cell. 2002;111:197–208. doi: 10.1016/s0092-8674(02)00976-5. [DOI] [PubMed] [Google Scholar]
- Muller J, Kassis JA. Polycomb response elements and targeting of Polycomb group proteins in Drosophila. Current Opinion in Genetics & Development. 2006;16:476–484. doi: 10.1016/j.gde.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Müller J, Verrijzer P. Biochemical mechanisms of gene regulation by polycomb group protein complexes. Current opinion in genetics & development. 2009;19:150–8. doi: 10.1016/j.gde.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Mysliwiec MR, Bresnick EH, Lee Y. Endothelial Jarid2/Jumonji is required for normal cardiac development and proper Notch1 expression. J Biol Chem. 2011;286:17193–17204. doi: 10.1074/jbc.M110.205146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Inagawa M, Uchida C, Okada K, Tane S, Kojima M, Kubota M, Noda M, Ogawa S, Shirato H, Sato M, Suzuki-Migishima R, Hino T, Satoh Y, Kitagawa M, Takeuchi T. Coordinated regulation of differentiation and proliferation of embryonic cardiomyocytes by a jumonji (Jarid2)-cyclin D1 pathway. Development. 2011;138:1771–1782. doi: 10.1242/dev.059295. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Mori T, Tada S, Krajewski W, Rozovskaia T, Wassell R, Dubois G, Mazo A, Croce CM, Canaani E. ALL-1 is a histone methyltransferase that assembles a supercomplex of proteins involved in transcriptional regulation. Mol Cell. 2002;10:1119–1128. doi: 10.1016/s1097-2765(02)00740-2. [DOI] [PubMed] [Google Scholar]
- Neish AS, Anderson SF, Schlegel BP, Wei W, Parvin JD. Factors associated with the mammalian RNA polymerase II holoenzyme. Nucleic Acids Research. 1998;26:847–853. doi: 10.1093/nar/26.3.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemer M. Genetic insights into normal and abnormal heart development. Cardiovasc Pathol. 2008;17:48–54. doi: 10.1016/j.carpath.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Nightingale KP, O’Neill LP, Turner BM. Histone modifications: signalling receptors and potential elements of a heritable epigenetic code. Current opinion in genetics & development. 2006;16:125–136. doi: 10.1016/j.gde.2006.02.015. [DOI] [PubMed] [Google Scholar]
- O’Carroll D, Erhardt S, Pagani M, Barton SC, Surani MA, Jenuwein T. The polycomb-group gene Ezh2 is required for early mouse development. Molecular and cellular biology. 2001;21:4330–4336. doi: 10.1128/MCB.21.13.4330-4336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani K, Dimmeler S. Epigenetic regulation of cardiovascular differentiation. Cardiovascular Research. 2011;90:404–412. doi: 10.1093/cvr/cvr019. [DOI] [PubMed] [Google Scholar]
- Oka T, Maillet M, Watt AJ, Schwartz RJ, Aronow BJ, Duncan SA, Molkentin JD. Cardiac-specific deletion of Gata4 reveals its requirement for hypertrophy, compensation, and myocyte viability. Circ Res. 2006;98:837–845. doi: 10.1161/01.RES.0000215985.18538.c4. [DOI] [PubMed] [Google Scholar]
- Oka T, Xu J, Molkentin JD. Re-employment of evelopmental transcription factors in adult heart disease. Semin Cell Dev Biol. 2007;18:117–131. doi: 10.1016/j.semcdb.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EN. Gene regulatory networks in the evolution and development of the heart. Science. 2006;313:1922–1927. doi: 10.1126/science.1132292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan G, Tian S, Nie J, Yang C, Ruotti V, Wei H, Jonsdottir GA, Stewart R, JAT Whole-genome analysis of histone H3 lysine 4 and lysine 27 methylation in human embryonic stem cells. Cell Stem Cell. 2007;1:299–312. doi: 10.1016/j.stem.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Pandey RR, Mondal T, Mohammad F, Enroth S, Redrup L, Komorowski J, Nagano T, Mancini-Dinardo D, Kanduri C. Kcnq1ot1 antisense noncoding RNA mediates lineage-specific transcriptional silencing through chromatin-level regulation. Mol Cell. 2008;32:232–246. doi: 10.1016/j.molcel.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Papoulas O, Beek SJ, Moseley SL, McCallum CM, Sarte M, Shearn A, Tamkun JW. The Drosophila trithorax group proteins BRM, ASH1 and ASH2 are subunits of distinct protein complexes. Development. 1998;125:3955–3966. doi: 10.1242/dev.125.20.3955. [DOI] [PubMed] [Google Scholar]
- Parlakian A, Charvet C, Escoubet B, Mericskay M, Molkentin JD, Gary-Bobo G, De Windt LJ, Ludosky MA, Paulin D, Daegelen D, Tuil D, Li Z. Temporally controlled onset of dilated cardiomyopathy through disruption of the SRF gene in adult heart. Circulation. 2005;112:2930–2939. doi: 10.1161/CIRCULATIONAHA.105.533778. [DOI] [PubMed] [Google Scholar]
- Pasini D, Bracken AP, Hansen JB, Capillo M, Helin K. The polycomb group protein Suz12 is required for embryonic stem cell differentiation. Molecular and cellular biology. 2007;27:3769–3779. doi: 10.1128/MCB.01432-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasini D, Cloos PaC, Walfridsson J, Olsson L, Bukowski J-P, Johansen JV, Bak M, Tommerup N, Rappsilber J, Helin K. JARID2 regulates binding of the Polycomb repressive complex 2 to target genes in ES cells. Nature. 2010;464:306–310. doi: 10.1038/nature08788. [DOI] [PubMed] [Google Scholar]
- Peng JC, Valouev A, Swigut T, Zhang J, Zhao Y, Sidow A, Wysocka J. Jarid2/Jumonji coordinates control of PRC2 enzymatic activity and target gene occupancy in pluripotent cells. Cell. 2009;139:1290–1302. doi: 10.1016/j.cell.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson CL, Workman JL. Promoter targeting and chromatin remodeling by the SWI/SNF complex. Current Opinion in Genetics & Development. 2000;10:187–192. doi: 10.1016/s0959-437x(00)00068-x. [DOI] [PubMed] [Google Scholar]
- Phelan ML, Schnitzler GR, Kingston RE. Octamer transfer and creation of stably remodeled nucleosomes by human SWI-SNF and its isolated ATPases. Molecular and cellular biology. 2000;20:6380–6389. doi: 10.1128/mcb.20.17.6380-6389.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirrotta V. Chromatin complexes regulating gene expression in Drosophila. Current opinion in genetics & development. 1995;5:466–472. doi: 10.1016/0959-437x(95)90050-q. [DOI] [PubMed] [Google Scholar]
- Plath K, Fang J, Mlynarczyk-Evans SK, Cao R, Worringer KA, Wang H, de la Cruz CC, Otte AP, Panning B, Zhang Y. Role of histone H3 lysine 27 methylation in X inactivation. Science. 2003;300:131–135. doi: 10.1126/science.1084274. [DOI] [PubMed] [Google Scholar]
- Reisman DN, Sciarrotta J, Bouldin TW, Weissman BE, Funkhouser WK. The expression of the SWI/SNF ATPase subunits BRG1 and BRM in normal human tissues. Applied Immunohistochemistry & Molecular Morphology. 2005;13:66–74. doi: 10.1097/00129039-200503000-00011. [DOI] [PubMed] [Google Scholar]
- Ribeiro I, Kawakami Y, Büscher D, Raya A, Rodríguez-León J, Morita M, Rodríguez Esteban C, Izpisúa Belmonte JC. Tbx2 and Tbx3 regulate the dynamics of cell proliferation during heart remodeling. PLoS One. 2007;2:e398. doi: 10.1371/journal.pone.0000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley P, Anson-Cartwright L, Cross JC. The Hand1 bHLH transcription factor is essential for placentation and cardiac morphogenesis. Nature Genetics. 1998;18:271–275. doi: 10.1038/ng0398-271. [DOI] [PubMed] [Google Scholar]
- Ringrose L, Paro R. Polycomb/Trithorax response elements and epigenetic memory of cell identity. Development. 2007;134:223–232. doi: 10.1242/dev.02723. [DOI] [PubMed] [Google Scholar]
- Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, Goodnough H, Helms JA, Farnham PJ, Chang HY. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risebro CA, Smart N, Dupays L, Breckenridge R, Mohun TJ, Riley PR. Hand1 regulates cardiomyocyte proliferation versus differentiation in the developing heart. Development. 2006;133:4595–4606. doi: 10.1242/dev.02625. [DOI] [PubMed] [Google Scholar]
- Scheuermann JC, de Ayala Alonso AG, Oktaba K, Ly-Hartig N, McGinty RK, Fraterman S, Wilm MMT, Müller J. Histone H2A deubiquitinase activity of the Polycomb repressive complex PR-DUB. Nature. 2010;465:243–247. doi: 10.1038/nature08966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuettengruber B, Martinez A-M, Iovino N, Cavalli G. Trithorax group proteins: switching genes on and keeping them active. Nature Reviews Molecular Cell Biology. 2011;12:799–814. doi: 10.1038/nrm3230. [DOI] [PubMed] [Google Scholar]
- Schmitges FW, Prusty AB, Faty M, Stützer A, Lingaraju GM, Aiwazian J, Sack R, Hess D, Li L, Zhou S, Bunker RD, Wirth U, Bouwmeester T, Bauer A, Ly-Hartig N, Zhao K, Chan H, Gu J, Gut H, Fischle W, Müller J, Thomä NH. Histone Methylation by PRC2 Is Inhibited by Active Chromatin Marks. Mol Cell. 2011;42:330–341. doi: 10.1016/j.molcel.2011.03.025. [DOI] [PubMed] [Google Scholar]
- Schneider R, Bannister AJ, Myers FA, Thorne AW, Crane-Robinson C, Kouzarides T. Histone H3 lysine 4 methylation patterns in higher eukaryotic genes. Nat Cell Biol. 2004;6:73–77. doi: 10.1038/ncb1076. [DOI] [PubMed] [Google Scholar]
- Sdek P, Zhao P, Wang Y, Huang C-J, Ko CY, Butler PC, Weiss JN, Maclellan WR. Rb and p130 control cell cycle gene silencing to maintain the postmitotic phenotype in cardiac myocytes. The Journal of Cell Biology. 2011;194:407–423. doi: 10.1083/jcb.201012049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao A, Raible F, Mollaaghababa R, Guyon JR, Wu CT, Bender W, Kingston RE. Stabilization of chromatin structure by PRC1, a Polycomb complex. Cell. 1999;98:37–46. doi: 10.1016/S0092-8674(00)80604-2. [DOI] [PubMed] [Google Scholar]
- Shen X, Kim W, Fujiwara Y, Simon MD, Liu Y, Mysliwiec MR, Yuan G-C, Lee Y, Orkin SH. Jumonji modulates polycomb activity and self-renewal versus differentiation of stem cells. Cell. 2009;139:1303–1314. doi: 10.1016/j.cell.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Liu Y, Hsu Y-J, Fujiwara Y, Kim J, Mao X, Yuan G-C, Orkin SH. EZH1 mediates methylation on histone H3 lysine 27 and complements EZH2 in maintaining stem cell identity and executing pluripotency. Mol Cell. 2008;32:491–502. doi: 10.1016/j.molcel.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai M, Osugi T, Koga H, Kaji Y, Takimoto E, Komuro I, Hara J, Miwa T, Yamauchi-Takihara K, Takihara Y. The Polycomb-group gene Rae28 sustains Nkx2.5/Csx expression and is essential for cardiac morphogenesis. J Clin Invest. 2002;110:177–184. doi: 10.1172/JCI14839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirato H, Ogawa S, Nakajima K, Inagawa M, Kojima M, Tachibana M, Shinkai Y, Takeuchi T. A jumonji (Jarid2) protein complex represses cyclin D1 expression by methylation of histone H3-K9. The Journal of Biological Chemistry. 2009;284:733–739. doi: 10.1074/jbc.M804994200. [DOI] [PubMed] [Google Scholar]
- Simon J. Locking in stable states of gene expression: transcriptional control during Drosophila development. Current Opinion in Cell Biology. 1995;7:376–385. doi: 10.1016/0955-0674(95)80093-x. [DOI] [PubMed] [Google Scholar]
- Singh R, Hoogaars WM, Barnett P, Grieskamp T, Rana MS, Buermans H, Farin HF, Petry M, Heallen T, Martin JF, Moorman AFM, ‘t Hoen PAC, Kispert A, Christoffels VM. Tbx2 and Tbx3 induce atrioventricular myocardial development and endocardial cushion formation. Cellular and Molecular Life Sciences. 2011 doi: 10.1007/s00018-011-0884-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankunas K, Hang CT, Tsun Z-Y, Chen H, Lee NV, Wu JI, Shang C, Bayle JH, Shou W, Iruela-Arispe ML, Chang C-P. Endocardial Brg1 represses ADAMTS1 to maintain the microenvironment for myocardial morphogenesis. Developmental Cell. 2008;14:298–311. doi: 10.1016/j.devcel.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoller JZ, Epstein JA. Cardiac neural crest. Semin Cell Dev Biol. 2005;16:704–715. doi: 10.1016/j.semcdb.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Stoller JZ, Huang L, Tan CC, Huang F, Zhou DD, Yang J, Gelb BD, Epstein JA. Ash2l interacts with Tbx1 and is required during early embryogenesis. Experimental Biology and Medicine. 2010;235:569–576. doi: 10.1258/ebm.2010.009318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl G, Akam M. Altered distributions of Ultrabithorax transcripts in extra sex combs mutant embryos of Drosophila. The EMBO Journal. 1985;4:3259–3264. doi: 10.1002/j.1460-2075.1985.tb04075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudarsanam P, Winston F. The Swi/Snf family:nucleosome-remodeling complexes and transcriptional control. Trends in Genetics. 2000;16:345–351. doi: 10.1016/s0168-9525(00)02060-6. [DOI] [PubMed] [Google Scholar]
- Surface LE, Thornton SR, Boyer LA. Polycomb group proteins set the stage for early lineage commitment. Cell Stem Cell. 2010;7:288–298. doi: 10.1016/j.stem.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Takeuchi JK, Bruneau BG. Directed transdifferentiation of mouse mesoderm to heart tissue by defined factors. Nature. 2009;459:708–711. doi: 10.1038/nature08039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi JK, Lou X, Alexander JM, Sugizaki H, Delgado-Olguín P, Holloway AK, Mori AD, Wylie JN, Munson C, Zhu Y, Zhou Y-Q, Yeh R-F, Henkelman RM, Harvey RP, Metzger D, Chambon P, Stainier DYR, Pollard KS, Scott IC, Bruneau BG. Chromatin remodelling complex dosage modulates transcription factor function in heart development. Nature Communictions. 2011;2:187. doi: 10.1038/ncomms1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi T, Kojima M, Nakajima K, Kondo S. jumonji gene is essential for the neurulation and cardiac development of mouse embryos with a C3H/He background. Mechanism of Development. 1999;86:29–38. doi: 10.1016/s0925-4773(99)00100-8. [DOI] [PubMed] [Google Scholar]
- Takihara Y, Tomotsune D, Shirai M, Katoh-Fukui Y, Nishii K, Motaleb MA, Nomura M, Tsuchiya R, Fujita Y, Shibata Y, Higashinakagawa T, Shimada K. Targeted disruption of the mouse homologue of the Drosophila polyhomeotic gene leads to altered anteroposterior patterning and neural crest defects. Development. 1997;124:3673–3682. doi: 10.1242/dev.124.19.3673. [DOI] [PubMed] [Google Scholar]
- Toko H, Zhu W, Takimoto E, Shiojima I, Hiroi Y, Zou Y, Oka T, Akazawa H, Mizukami M, Sakamoto M, Terasaki F, Kitaura Y, Takano H, Nagai T, Nagai R, Komuro I. Csx/Nkx2–5 is required for homeostasis and survival of cardiac myocytes in the adult heart. J Biol Chem. 2002;277:24735–24743. doi: 10.1074/jbc.M107669200. [DOI] [PubMed] [Google Scholar]
- Tomotsune D, Shirai M, Takihara Y, Shimada K. Regulation of Hoxb3 expression in the hindbrain and pharyngeal arches by rae28, a member of the mammalian Polycomb group of genes. Mechanism of Development. 2000;98:165–169. doi: 10.1016/s0925-4773(00)00457-3. [DOI] [PubMed] [Google Scholar]
- Toyoda M, Shirato H, Nakajima K, Kojima M, Takahashi M, Kubota M, Suzuki-Migishima R, Motegi Y, Yokoyama M, Takeuchi T. jumonji downregulates cardiac cell proliferation by repressing cyclin D1 expression. Developmental Cell. 2003;5:85–97. doi: 10.1016/s1534-5807(03)00189-8. [DOI] [PubMed] [Google Scholar]
- Trainor PA, Krumlauf R. Hox genes, neural crest cells and branchial arch patterning. Current Opinion in Cell Biology. 2001;13:698–705. doi: 10.1016/s0955-0674(00)00273-8. [DOI] [PubMed] [Google Scholar]
- Utley RT, Côté J, Owen-Hughes T, Workman JL. SWI/SNF stimulates the formation of disparate activator-nucleosome complexes but is partially redundant with cooperative binding. The Journal of Biological Chemistry. 1997;272:12642–12649. doi: 10.1074/jbc.272.19.12642. [DOI] [PubMed] [Google Scholar]
- Vallaster M, Vallaster CD, Wu SM. Epigenetic mechanisms in cardiac development and disease. Acta Biochimica et Biophysica Hungarica. 2012;44:92–102. doi: 10.1093/abbs/gmr090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lohuizen M. Functional analysis of mouse Polycomb group genes. Cellular and Molecular Life Sciences. 1998;54:71–79. doi: 10.1007/s000180050126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Weerd JH, Koshiba-Takeuchi K, Kwon C, Takeuchi JK. Epigenetic factors and cardiac development. Cardiovascular Research. 2011;91:203–211. doi: 10.1093/cvr/cvr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt RGAB, Otte AP, Rubin MA, Chinnaiyan AM. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419:624–629. doi: 10.1038/nature01075. [DOI] [PubMed] [Google Scholar]
- Vincent SD, Buckingham ME. How to make a heart: the origin and regulation of cardiac progenitor cells. Current topics in developmental biology. 2010;90:1–41. doi: 10.1016/S0070-2153(10)90001-X. [DOI] [PubMed] [Google Scholar]
- Voncken JW, Roelen BAJ, Roefs M, de Vries S, Verhoeven E, Marino S, Deschamps J, van Lohuizen M. Rnf2 (Ring1b) deficiency causes gastrulation arrest and cell cycle inhibition. Proc Natl Acad Sci USA. 2003;100:2468–2473. doi: 10.1073/pnas.0434312100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wang L, Erdjument-Bromage H, Vidal M, Tempst P, Jones RS, Zhang Y. Role of histone H2A ubiquitination in Polycomb silencing. Nature. 2004;431:873–878. doi: 10.1038/nature02985. [DOI] [PubMed] [Google Scholar]
- Wang J, Xu N, Feng X, Hou N, Zhang J, Cheng X, Chen Y, Zhang Y, Yang X. Targeted disruption of Smad4 in cardiomyocytes results in cardiac hypertrophy and heart failure. Circ Res. 2005;97:821–828. doi: 10.1161/01.RES.0000185833.42544.06. [DOI] [PubMed] [Google Scholar]
- Wang W, Côté J, Xue Y, Zhou S, Khavari PA, Biggar SR, Muchardt C, Kalpana GV, Goff SP, Yaniv M, Workman JL, Crabtree GR. Purification and biochemical heterogeneity of the mammalian SWI-SNF complex. The EMBO Journal. 1996;15:5370–5382. [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Chao DM, Imbalzano AN, Schnitzler GR, Kingston RE, Young RA. RNA polymerase II holoenzyme contains SWI/SNF regulators involved in chromatin remodeling. Cell. 1996;84:235–244. doi: 10.1016/s0092-8674(00)80978-2. [DOI] [PubMed] [Google Scholar]
- Wirrig EE, Yutzey KE. Transcriptional regulation of heart valve development and disease. Cardiovascular Pathology. 2011;20:162–7. doi: 10.1016/j.carpath.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama A, Wang Z, Wysocka J, Sanyal M, Aufiero DJ, Kitabayashi I, Herr W, Cleary ML. Leukemia proto-oncoprotein MLL forms a SET1-like histone methyltransferase complex with menin to regulate Hox gene expression. Mol Cell Biol. 2004;24:5639–5649. doi: 10.1128/MCB.24.13.5639-5649.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Chai J, Azhar G, Sheridan P, Borras AM, Furr MC, Khrapko K, Lawitts J, Misra RP, Wei JY. Early postnatal cardiac changes and premature death in transgenic mice overexpressing a mutant form of serum response factor. J Biol Chem. 2001;276:40033–40040. doi: 10.1074/jbc.M104934200. [DOI] [PubMed] [Google Scholar]
- Zhao J, Sun BK, Erwin JA, Song JJ, Lee JT. Polycomb proteins targeted by a short repeat RNA to the mouse X chromosome. Science. 2008;322:750–756. doi: 10.1126/science.1163045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XD, Han X, Chew JL, Liu J, Chiu KP, Choo A, Orlov YL, Sung WK, Shahab A, Kuznetsov VA, Bourque G, Oh S, Ruan Y, Ng HH, Wei CL. Whole-genome mapping of histone H3 Lys4 and 27 trimethylations reveals distinct genomic compartments in human embryonic stem cells. Cell Stem Cell. 2007;1:286–298. doi: 10.1016/j.stem.2007.08.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.