Abstract
Objective
To determine factors regulating human aortic smooth muscle cells (HASMC) supported tissue factor-induced thrombin generation.
Methods and Results
The addition of nonlipidated tissue factor and Ca2+ to HASMCs maintained in reptilase-treated platelet-poor plasma resulted in the robust formation of thrombin after a lag phase of approximately 6 minutes. Pretreatment with low concentrations of α-thrombin before the addition of tissue factor and Ca2+ accelerated the rate of thrombin generation (time to reach half of peak thrombin was reduced by [mean±SD] 42.0±2.2%; P<0.05) but had no effect on the amount of peak thrombin generated. Protease-activated receptor (PAR) 3 activating peptides (APs) or PAR-4 APs accelerated thrombin generation without affecting peak thrombin levels (time to half of peak thrombin decreased by 17.4±5.6% and 21.7±3.5%; P<0.05 with PAR-3 AP and PAR-4 AP, respectively). The addition of PAR-3 AP and PAR-4 AP together had an additive effect, with a reduction in time to half of peak thrombin of 43.9±4.0%. PAR-3 AP or PAR-4 AP enhanced tissue factor–induced factor Xa production and phosphatidylserine exposure on the surface of HASMCs. PAR-1 activation had no effect on thrombin generation, factor Xa production, or phosphatidylserine exposure.
Conclusion
Low concentrations of α-thrombin accelerate tissue factor–induced thrombin generation on the surface of HASMCs, and this effect is mediated by PAR-3 and PAR-4.
Keywords: thrombin, protease activated receptor, smooth muscle
Cardiovascular disease resulting from the formation of an arterial thrombus remains a leading cause of mortality and morbidity in the Western world. Advances in anticoagulant and antiplatelet therapies have reduced cardiovascular events during acute coronary syndromes and percutaneous coronary interventions, yet thrombotic events still occur despite treatment with the most potent inhibitors of the coagulation system that are available.1 In addition, recent studies have highlighted the important adverse impact of bleeding complications on clinical outcomes, providing more impetus for an understanding of optimal anticoagulation at the site of vascular injury.2
Arterial injury that disrupts the endothelium at sites of atherosclerotic plaques enables plasma to come into contact with tissue factor–bearing cells.3,4 This results in the production of small amounts of thrombin with virtually little or no platelet participation, a reaction described as the initiation phase of coagulation.5,6 This small amount of thrombin is crucial in regulating the coagulation response by controlling the timing and magnitude of further thrombin production during the priming and propagation phase. Previous results showed that thrombin generation occurs on the surface of human aortic smooth muscle cells (HASMCs) after treatment with tissue factor and Ca2+; however, factors that regulate the kinetics of thrombin generation within the initiation phase are largely unknown.7
Studies in platelets found that activation of protease-activated receptor (PAR) 4, but not PAR-1, reduced time to peak thrombin without affecting maximal thrombin generated.8 Other studies found that activation of PAR-4 caused a left shift in the dose-response curve of collagen-induced thrombin generation, providing further evidence that PAR-4 plays a role in regulating platelet thrombin generation.9 HASMCs express functionally active PAR-1, PAR-3, and PAR-410,11; however, studies on the role of PARs in SMCs have focused primarily on contraction and growth responses,12,13 with little information on the role of PARs in SMC-supported thrombin generation. Because the rate at which thrombin is generated on the surface of vascular SMCs at arterial injury plays an important role in vascular thrombosis and arterial repair, the objective of these studies was to examine the hypothesis that PARs regulate the kinetics of tissue factor–induced thrombin generation in HASMCs.
Methods
Thrombin and Factor Xa Assays
Thrombin generation was assayed as previously described.7 Briefly, HASMCs from passage 5 to 7 were grown in 24-well tissue culture plates in Dulbecco-modified Eagle medium supplemented with 10% FCS, 1% penicillin-streptomycin, and SMC proliferation medium at a seeding density of 8×103 to 10×103 cells/cm2. After reaching 70% to 80% confluence, HASMCs were washed with ×1 PBS followed by the addition of 500 µL of reptilase-treated platelet-poor plasma (PPP) per well for 1.5 hours. Fresh-frozen PPP was obtained from the New York Blood Center and was prepared within 3 hours from fresh blood collected from healthy voluntary donors; this blood was anticoagulated with sodium citrate, centrifuged at 2000 rpm for 10 minutes at 22°C, centrifuged again at 5000 rpm for 10 minutes at 4°C, and then frozen at −20°C. Nonlipidated recombinant tissue factor (final concentration, 0.6 pmol/L) and Ca2+ (final concentration, 0.5 mmol/L) were added. At various points, 15 µL of plasma from each well was added to 90 µL of 3.8% sodium citrate in 96-well plates and was used as a stop solution. Thrombin and factor Xa were measured using the chromogenic substrates S-2238 and S-2765, respectively. Standard curves were generated using known amounts of thrombin and factor Xa. Factor X–deficient PPP was obtained commercially.
Phosphatidylserine Exposure
Phosphatidylserine (PS) on the surface of HASMCs was detected using a commercially available fluorescein isothiocyanate kit (Annexin V), as described by Siow et al.14 Images were captured using a Leica DM Inverted Microscope and the level of fluorescence was quantified using open source image analysis software (ImageJ; available at: http://rsbweb.nih.gov/ij/).
PAR-3 Small and Interfering RNA Transfection
HASMCs were plated at the seeding density of 0.1×106 in 12-well plates and grown overnight in antibiotic-free complete medium at 37°C with 5% CO2. A total of 50 µL of 2 µmol/L PAR-3 specific siRNA (On-TARGETplus) or scrambled siRNA were added to each well together with 1.0 µL of the transfection agent (DharmaFECT) in serum-free medium. The cells were incubated with antibiotic-free complete medium for 24 hours at 37°C with 5% CO2 and then reinfected every 24 hours for 7 days. Western blotting was performed as previously described.15
Reagents
α-Thrombin, δ-thrombin, and factor Xa were purchased from Hematologic Technologies, Inc. PAR-1–activating peptide (AP) (SFLLRN-NH2), PAR-2 AP (SLIGRL-NH2), and PAR-1 antagonist (N3-cyclopropyl-7-[[4-(1-methylethyl)phenyl]methyl]-7H-pyrrolo[3,2-f]quinazoline-1,3-diamine dihydrochloride) were obtained from Tocris Bioscience. Antibodies directed against PAR-1 (ATAP2), PAR-3, and PAR-4 were purchased from Santa Cruz Biotechnology, Inc; and PAR-4 antagonist (tcY-NH2) was purchased from BioTrend. PAR-3 AP (TFRGAP) and PAR-4 AP (GYPGQV) were obtained from BaChem, Inc; and a scrambled PAR-1 peptide (FSLLRN) was obtained from AnaSpec. A monoclonal anti–tissue factor–blocking antibody (4509) was purchased from American Diagnostica Inc. Anti–phosphorylated extracellular signal–regulated kinase (ERK) and anti–ERK antibodies were obtained from Cell Signaling Technology.
Results
Pretreatment With α-Thrombin Accelerates HASMC-Supported Tissue Factor–Induced Thrombin Generation, and This Effect Requires the Catalytic Activity of Thrombin
The addition of tissue factor and Ca2+ to a monolayer of HASMCs maintained in reptilase-treated PPP resulted in robust thrombin generation, as previously described (Figure 1A). Minimal thrombin was generated in the absence of HASMCs. Previously, it was shown that platelets and platelet microparticles do not contribute to thrombin generation in this system.7
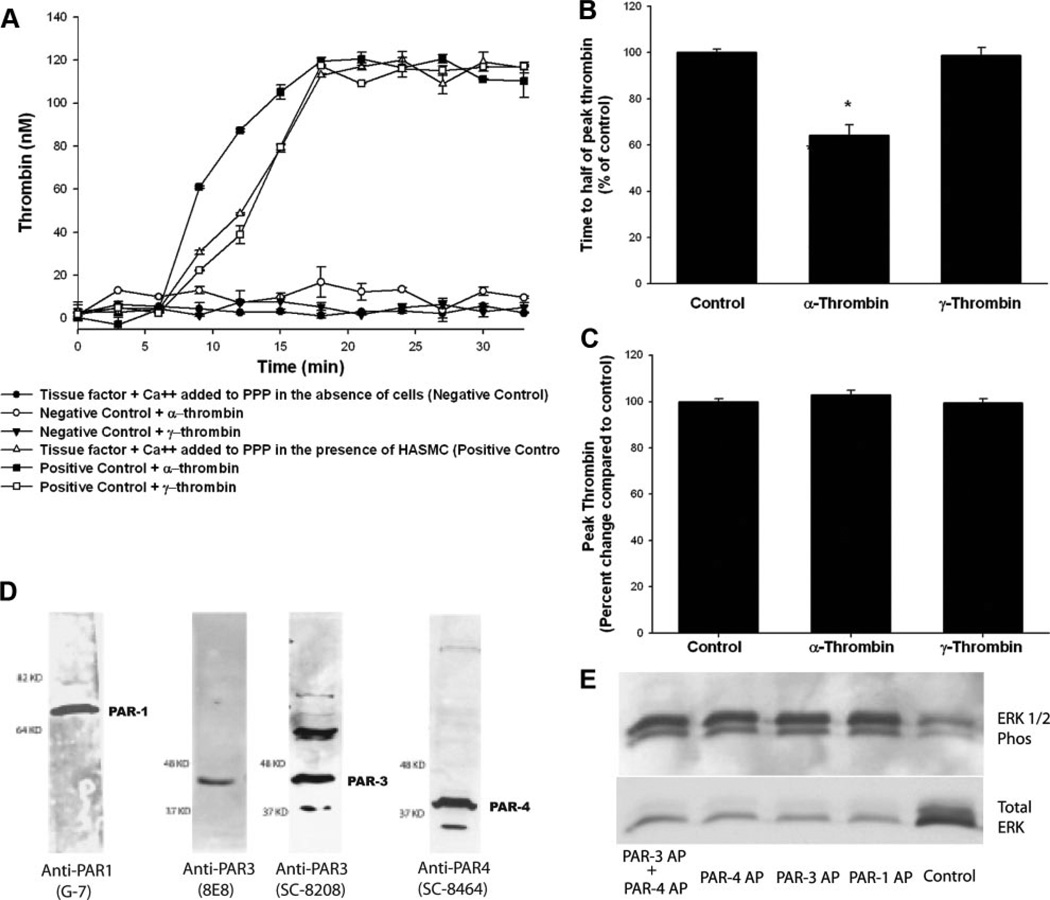
Figure 1.
Catalytic activity of α-thrombin is required for reduction in time to half of peak thrombin, and HASMCs express functionally active PAR-1, PAR-3, and PAR-4. HASMCs were growth arrested, washed, and maintained in reptilase-treated PPP. α-Thrombin or γ-thrombin (10.7 nmol/L each) was added before the addition of tissue factor and Ca2+. Experimental wells that were not exposed to any additional treatment served as positive controls, whereas wells that did not have any cells but that were treated with tissue factor and Ca2+ served as negative controls. A, A representative experiment. B and C, Results of 4 independent experiments. D, PAR-1, PAR-3, and PAR-4 were detected using specific monoclonal or polyclonal antibodies, as described in the Methods section. E, Growth-arrested HASMCs were treated with PAR-1 AP, PAR-3 AP, or PAR-4 AP (200 µmol/L each); and ERK 1/2 phosphorylation (Phos) was determined 15 minutes later. *P<0.05.
Treatment with low concentrations of α-thrombin before the addition of tissue factor and Ca2+ resulted in an increase in the rate of thrombin generation. Time to reach half of peak thrombin was reduced by 42.0±2.2% with the addition of 1.5 U/mL (10.7 nmol/L) of α-thrombin (P<0.05; Figure 1B). γ-Thrombin, which lacks the catalytic activity of α-thrombin, had no effect on the time to reach half of peak thrombin compared with control (decrease of 1.2±3.2%; P=1.0). The amount of peak thrombin generated was similar among all 3 groups (117.9±2.0 nmol/L for positive control, 119.4±1.3 nmol/L for α-thrombin, and 117.1±1.5 nmol/L for γ-thrombin; P=0.27; Figure 1C). Data are provided as mean±SD.
HASMCs Express Functionally Active PAR-1, PAR-3, and PAR-4
Because the catalytic domain of thrombin was necessary for thrombin-mediated acceleration of tissue factor–induced thrombin generation on the surface of HASMCs, we next sought to determine which PARs were involved in this response. The HASMCs used in this study expressed PAR-1, PAR-3, and PAR-4 (Figure 1D). These receptors were functionally active, as determined by ERK phosphorylation elicited by treatment with APs that mimic the “tethered ligand” exposed after thrombin cleavage of PAR (SFLLRN, TFRGAP, and GYPGQV were used as PAR-1, PAR-3, and PAR-4 APs, respectively; Figure 1E). These results are consistent with prior studies showing that SFLLRN, TFRGAP, and GYPGQV elicit ERK 1/2 phosphorylation in human vascular SMCs isolated from the saphenous vein.10,11
PAR-1 Does Not Mediate α-Thrombin Acceleration of HASMC-Supported Tissue Factor–Induced Thrombin Generation
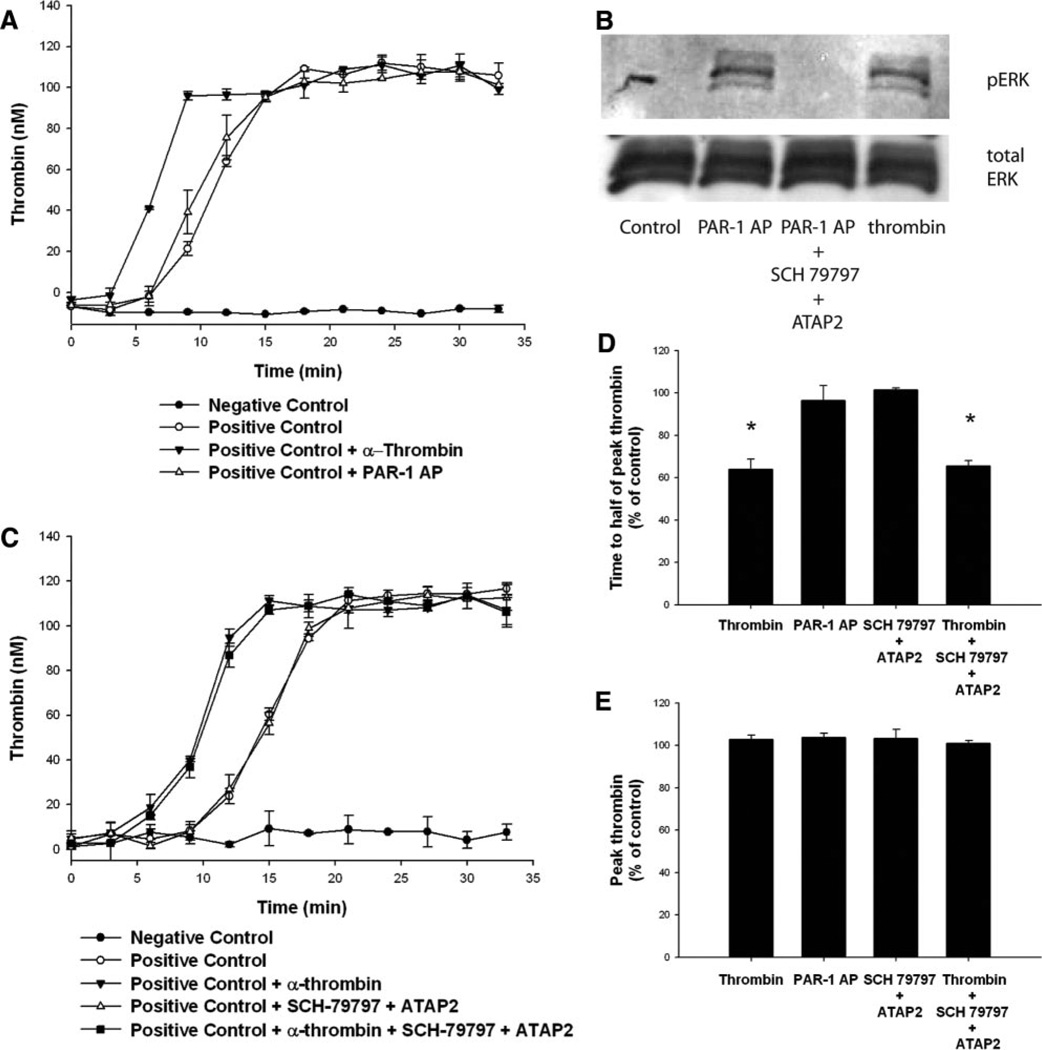
PAR-1 is the most widely studied PAR in vascular cells; it mediates growth responses.12,13 Treatment of HASMCs with varying concentrations of PAR-1 AP (SFLLRN) did not alter the kinetics of thrombin generation (difference in time to half of peak thrombin between positive control and PAR-1 AP pretreatment was a decrease of 3.7±7.1%; P=0.83; Figure 2A). Antagonism of PAR-1 with the combination of a peptide antagonist (SCH-79797) and a blocking antibody (ATAP2) at concentrations that prevented PAR-1 AP-induced ERK 1/2 phosphorylation (Figure 2B) did not inhibit α-thrombin–induced acceleration of tissue factor–induced thrombin generation when compared with control (increase of 1.6±0.8%; P=0.75; Figure 2C–2E).
Figure 2.
PAR-1 activation does not affect thrombin generation in HASMCs. HASMCs were growth arrested, washed, and maintained in reptilase-treated PPP. α-Thrombin (10.7 nmol/L) or PAR-1 AP (200 µmol/L), with or without PAR-1 antagonists (SCH-79797 plus ATAP-2), was added. A and C, Tissue factor and Ca2+ were added and thrombin generation was assayed at the indicated times. Experimental wells that were not exposed to any additional treatment served as positive controls, whereas wells that did not have any cells but that were treated with tissue factor and Ca2+ served as negative controls. B, ERK phosphorylation (pERK) was determined 15 minutes after treatment, as described in the Methods section. A through C, Representative data from 4 independent experiments. D and E, The results of 4 independent experiments. *P<0.05.
Activation of PAR-3 or PAR-4 Causes Acceleration of HASMC-Supported Tissue Factor–Induced Thrombin Generation
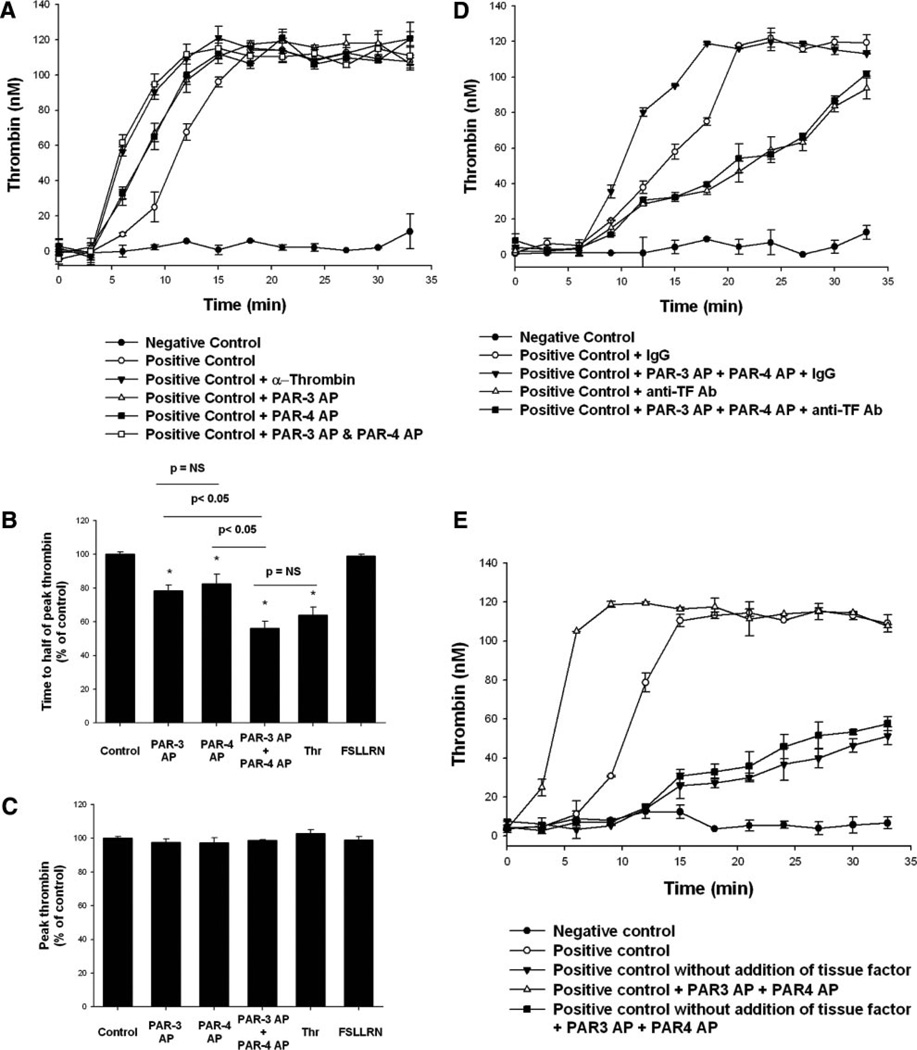
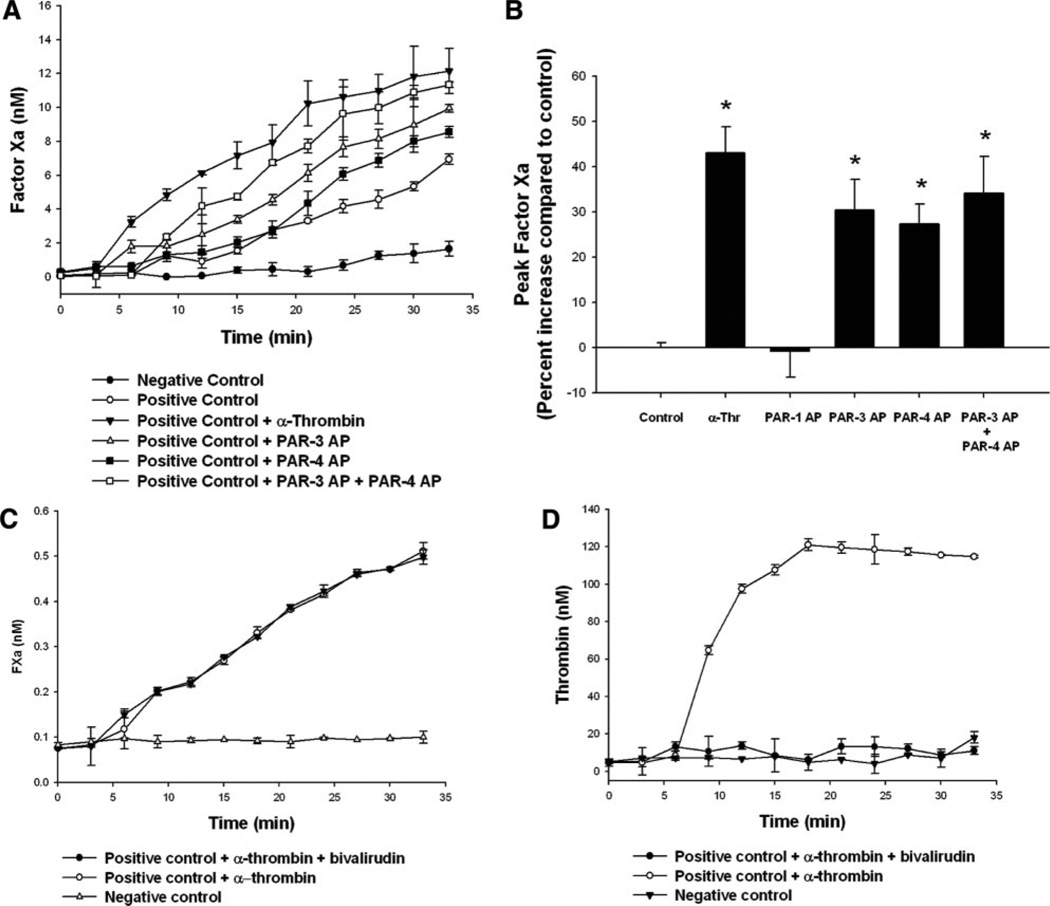
In contrast to the results observed with PAR-1 AP, pretreatment with either PAR-4 AP or PAR-3 AP reduced the time to half of peak thrombin (decrease of 17.4±5.6% [P<0.05] and decrease of 21.7±3.5% [P<0.05], respectively; Figure 3A and 3B, respectively). When PAR-3 and PAR-4 APs were added simultaneously, the time to half of peak thrombin was reduced more than with either AP alone (decrease of 43.9±4.0%; P<0.05 versus positive control, PAR-3 AP, or PAR-4 AP). PAR-3 AP, PAR-4 AP, and the combination of PAR-3 AP plus PAR-4 AP had no effect on peak thrombin generated (Figure 3C).
Figure 3.
Activation of PAR-3 or PAR-4 causes acceleration of HASMC-supported tissue factor–induced thrombin generation. HASMCs were treated with PAR-3 AP (200 µmol/L), PAR-4 AP (200 µmol/L), PAR-3 AP plus PAR-4 AP, α-thrombin (10.7 nmol/L), or a scrambled PAR-1 peptide (FSLLRN; 200 µmol/L) for 15 minutes before nonlipidated tissue factor and Ca2+ were added. Thrombin was assayed at indicated points, as described in the Methods section. Experimental wells that were not exposed to any additional treatment served as positive controls, whereas wells that did not have any cells but that were treated with tissue factor and Ca2+ served as negative controls. A, A representative experiment. B and C, The results of 4 independent experiments. D, HASMCs were pretreated for 15 minutes with anti–tissue factor antibody or nonspecific IgG (1 µg/mL), with or without PAR-3 AP plus PAR-4 AP, before the addition of tissue factor and Ca2+. E, Exogenous tissue factor was only added to the groups specified in the label. *P<0.05 vs control; there were no statistically significant differences in the data presented in C.
To test the effects of activation of PAR-3 and PAR-4 on thrombin generation in the absence of tissue factor–dependent events, we studied the kinetics of thrombin generation in HASMCs treated with exogenous tissue factor plus a tissue factor–blocking monoclonal antibody (in the absence of exogenous tissue factor). The addition of tissue factor–blocking antibody resulted in inhibition of tissue factor–induced thrombin generation and inhibition of PAR-3 AP–and PAR-4 AP–mediated acceleration of thrombin generation (Figure 3D). The addition of PAR-3 AP and PAR-4 AP in the absence of exogenous tissue factor had no effect on thrombin generation (Figure 3E).
Simultaneous Inhibition of Both PAR-3 and PAR-4 Is Required to Prolong the Initiation Phase of Thrombin Generation
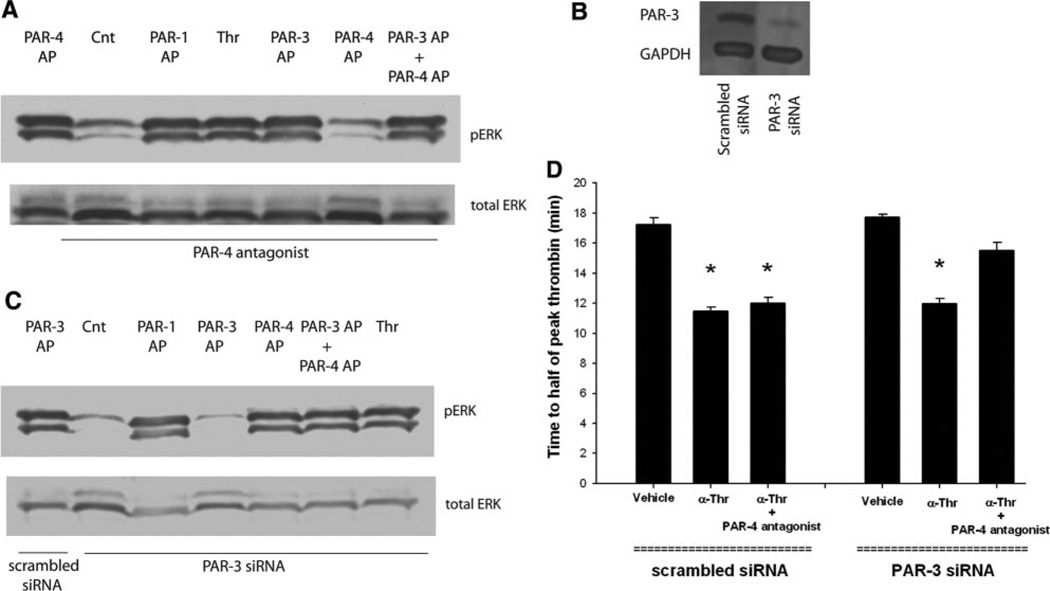
A PAR-4 antagonist (tcY-NH2) that successfully inhibited the activity of the receptor in human platelets16 abolished PAR-4 AP-induced ERK 1/2 phosphorylation in HASMCs (Figure 4A). Successful inhibition of PAR-3 function with peptide or antibody antagonists in HASMCs has not been reported. However, inhibition of PAR-3 function in human vascular cells using an siRNA approach has been successful17; thus, we used this strategy. HASMCs were transfected with PAR-3–specific siRNA, and Western blotting confirmed successful knockdown of PAR-3 (Figure 4B). ERK 1/2 phosphorylation provided functional evidence that levels of PAR-3 were significantly reduced (Figure 4C).
Figure 4.
Antagonism of both PAR-3 and PAR-4 is required to inhibit the effect of α-thrombin on the kinetics of tissue factor–induced thrombin (Thr) generation. HASMCs were pretreated with PAR-4 antagonist (tY-NH2) for 15 minutes before the addition of PAR-1 AP (200 µmol/L), PAR-3 AP (200 µmol/L), PAR-4 AP (200 µmol/L), or α-thrombin (10.7 nmol/L). A, ERK phosphorylation (pERK) was determined 15 minutes later. B, HASMCs were transfected with PAR-3 siRNA or scrambled siRNA, and effective knockdown of PAR-3 was confirmed with Western blotting performed using a monoclonal antibody directed against PAR-3. C, These cells were treated with α-thrombin, PAR-1 AP, PAR-3 AP, or PAR-4 AP at the concentrations indicated; and ERK phosphorylation was determined 15 minutes later. Transfected cells were treated with vehicle or α-thrombin (10.7 nmol/L), with or without PAR-4 antagonist (tc-NH2). D, The results of 4 independent experiments. *P<0.05 vs control (Cnt).
Treatment with a PAR-4 antagonist had no effect on α-thrombin acceleration of HASMC-supported tissue factor–induced thrombin generation (Figure 4D). Similarly, a marked reduction in PAR-3 expression using siRNA did not affect the kinetics of HASMC-supported tissue factor–induced thrombin generation. Time to half of peak thrombin was 11.5±0.3 minutes for HASMC treated with α-thrombin, 12.0±0.4 minutes for HASMC treated with α-thrombin +PAR-4 antagonist and 11.9±0.4 minutes in HASMC that did not express PAR-3 receptors treated with α-thrombin (P=0.21 for comparison between groups). In contrast, the inhibition of both PAR-4 and PAR-3 prevented α-thrombin acceleration of HASMC-supported tissue factor–induced thrombin generation (Figure 4D). Time to half of peak thrombin for PAR-3 knockdown cells treated with PAR-4 antagonist before the addition of α-thrombin was 15.5±0.5 minutes (P<0.05 versus treatment with α-thrombin).
PAR-3 AP and PAR-4 AP Enhanced Tissue Factor–Induced Factor Xa Production
We next sought to determine whether α-thrombin effects on acceleration of HASMC-supported tissue factor–induced thrombin generation occurred before or after factor Xa generation. Pretreatment with low concentrations of α-thrombin or with PAR-3 AP or PAR-4 AP resulted in an increase in the amount of factor Xa generated compared with control (increase of 43.1±5.6%, 30.4±6.7%, 27.3±4.5%, and 34.1±8.1% with 1.5 U/mL of α-thrombin, 200 µmol/L of PAR-3 AP, 200 µmol/L of PAR-4 AP, or 200 µmol/L of both PAR-3 and PAR-4, respectively; P<0.05 versus control; Figure 5A and 5B). PAR-1 AP did not affect the amount of factor Xa generated within 33 minutes of exposure to tissue factor and Ca2+ (decrease of 0.9±5.7%, P=0.88; Figure 5B).
Figure 5.
Activation of PAR-3 or PAR-4 enhances factor Xa production. HASMCs were growth arrested, washed, and maintained in reptilase-treated PPP. α-Thrombin (10.7 nmol/L), PAR-1 AP (200 µmol/L), PAR-3 AP (200 µmol/L), or PAR-4 AP (200 µmol/L) was added 15 minutes before the addition of tissue factor and Ca2+. Experimental wells that were not exposed to any additional treatment served as positive controls, whereas wells that did not have any cells but that were treated with tissue factor and Ca2+ served as negative controls. A, A representative experiment. B, The results of 4 independent experiments. C and D, Bivalirudin (50 µg/mL) was added to the samples before adding the samples to the chromogenic substrate. Factor Xa or thrombin levels were measured as described in the Methods section. *P<0.05 vs control.
Thrombin is able to cleave some chromogenic substrates used to determine factor Xa levels, although the substrate (S-2765) used in these experiments is reportedly highly specific for factor Xa. Evidence that thrombin was not cleaving S-2765 and, thus, giving falsely elevated levels of factor Xa is provided by data that bivalirudin, which directly binds to the catalytic site of α-thrombin,9 had no effect on cleavage of S-2765 (Figure 5C) at a concentration sufficient to completely suppress all tissue factor–induced thrombin activity, as assayed by cleavage of S-2238 (Figure 5D).
PAR-3 and PAR-4 Activation Enhanced Exposure of PS on the Surface of HASMCs
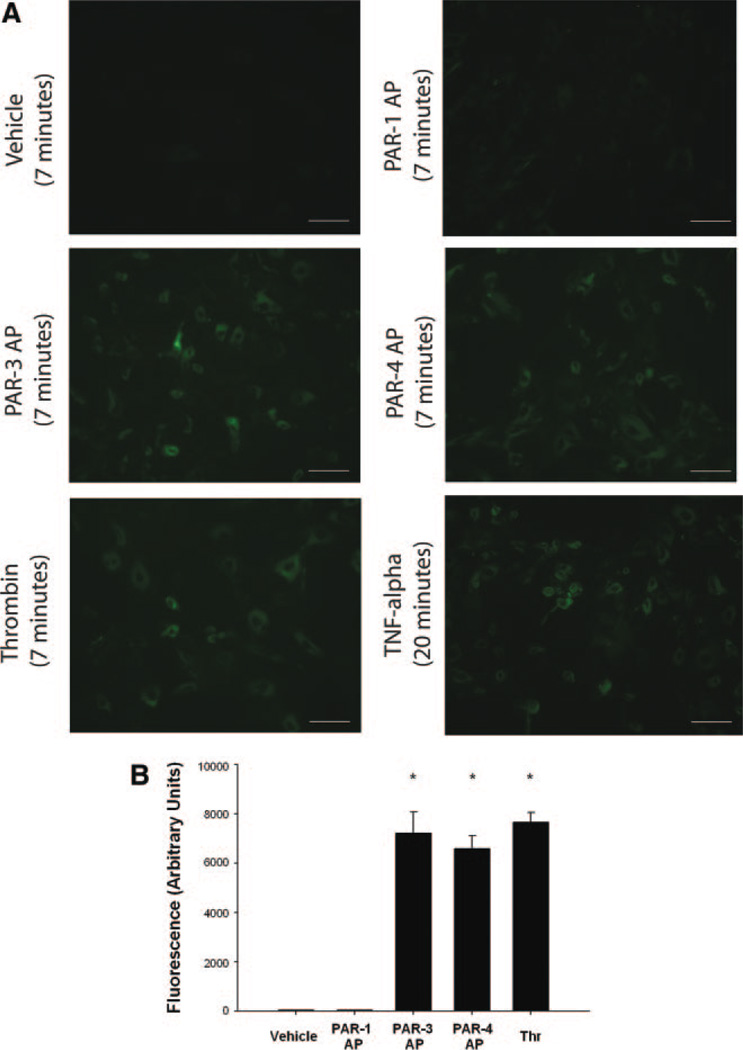
PS is an anionic aminophospholipid that, when exposed on the cell surface, plays an essential role in the assembly of coagulation factor complexes, including the tenase and prothrombinase complexes, necessary for thrombin generation in HASMCs.7,18 Treatment with α-thrombin, PAR-3 AP, or PAR-4 AP resulted in a marked increase in PS exposure beginning as early as 3 minutes after treatment (data not shown). When assayed 7 minutes after treatment, PS levels on the cell surface had increased approximately 300-fold compared with treatment with vehicle (Figure 6A and 6B). In contrast, treatment with PAR-1 AP had no effect on PS exposure. These data were consistent from 6 independent experiments.
Figure 6.
Activation of PAR-3 or PAR-4 enhances phosphatidylserine exposure on the surface of HASMC. HASMC were growth arrested, washed and maintained in reptilase-treated PPP. Cells were treated with vehicle, α-thrombin (10.7 nM), PAR-1 AP (200 µM), PAR-3 AP (200 µM), PAR-4 AP (200 µM) or TNF-α (10 ng/ml). Seven minutes after treatment (20 minutes after treatment with TNF-α) the cells were washed, incubated in FITC labeled Annexin V and washed again. The intensity of the fluorescence signal was measured using ImageJ (scale bar=50 µM). Representative photomicrographs are shown in A and data from six independent experiments are shown in B. (*P<0.05 compared to control).
PAR-3 and PAR-4 Activation Accelerated Thrombin Generation Downstream of Factor Xa Production
PS on the cell surface enhances factor Xa binding to factor Va and the formation of the prothrombinase complex, which is essential for efficient thrombin generation. Thus, we examined whether PAR-3 and PAR-4 signaling influenced thrombin generation downstream of factor Xa generation. To study this question, PPP derived from an individual deficient in factor X was used. Previously, it was shown that thrombin is not generated when factor X–deficient plasma is used in the current system.7
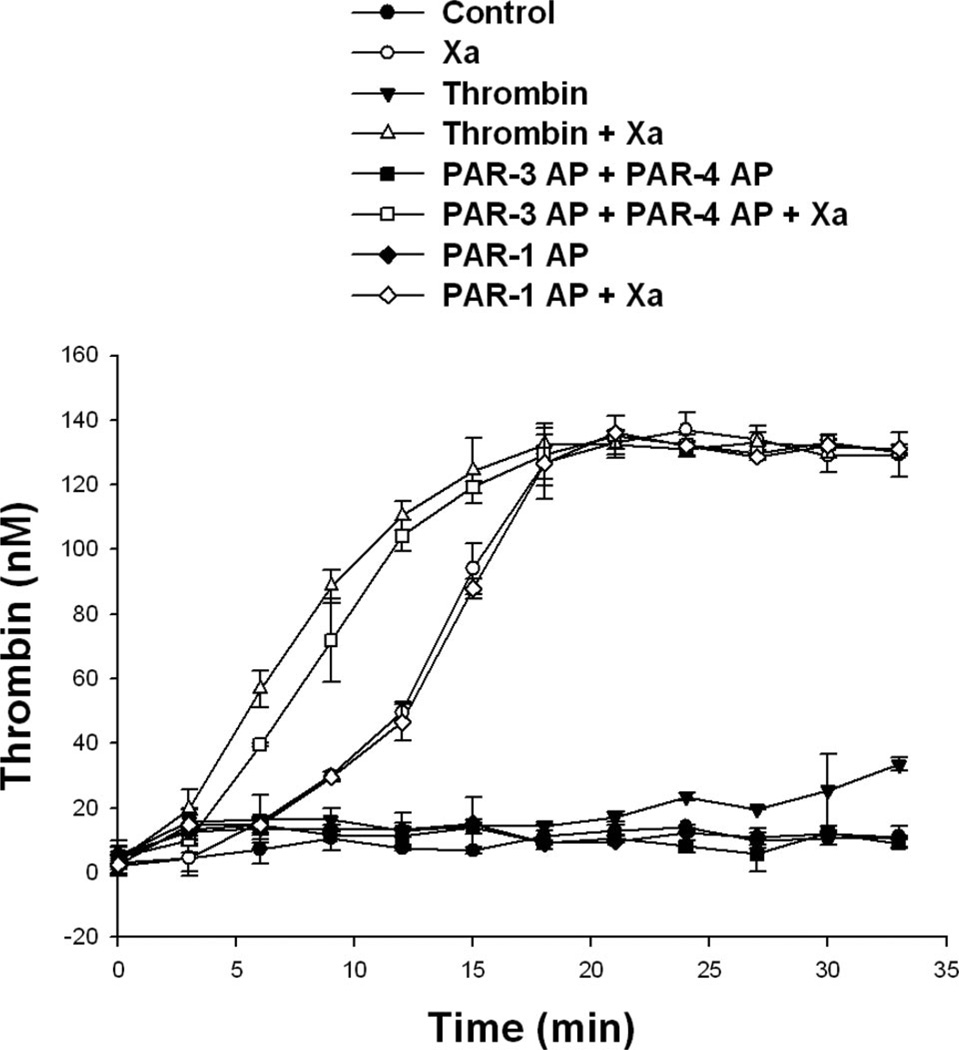
The addition of purified factor Xa along with tissue factor and Ca2+ to a monolayer of HASMCs maintained in reptilase-treated PPP that was deficient in factor X resulted in robust thrombin generation (Figure 7). Treatment with low concentrations of α-thrombin or PAR-3 AP plus PAR-4 AP resulted in an acceleration of thrombin generation in HASMCs maintained in factor X–deficient plasma when factor Xa was added at time 0 (Figure 7). In contrast, PAR-1 AP had no effect on the kinetics of thrombin generation in this system. No thrombin was generated in the absence of exogenous factor Xa in any of the groups.
Figure 7.
PAR-3 AP plus PAR-4 AP cause acceleration of HASMC-supported tissue factor–induced thrombin generation via mechanisms downstream of factor Xa production. HASMCs were growth arrested, washed, and maintained in reptilase-treated PPP derived from an individual deficient in factor X. PAR-3 AP (200 µmol/L) plus PAR-4 AP (200 µmol/L), PAR-1 AP (200 µmol/L), or α-thrombin (1.5 U/mL) was added 15 minutes before nonlipidated tissue factor, Ca2+ with or without factor Xa (10 pmol/L). Thrombin was assayed at indicated points, as described in the Methods section. Representative data from 4 independent experiments are shown.
Discussion
The results of these studies show that tissue factor–induced thrombin generation on the surface of HASMCs is accelerated by low concentrations of α-thrombin and that this effect is primarily, if not solely, mediated via PAR-3 and PAR-4. PAR-3 and PAR-4 provide a redundant pathway in the early phase of thrombin generation in that activation of either PAR-3 or PAR-4 reduces the time to half of peak thrombin, whereas blockage of the effects of thrombin on the kinetics of the reaction requires successful inhibition of both receptors. The biological events responsible for a shift in the kinetics of thrombin generation are worthy of investigation because the rate of thrombin generation is important for timely hemostasis and, thus, the mechanisms that dictate the rate of clot formation are of interest in drug development.
Results observed in HASMCs whereby PAR-3 and PAR-4 independently contribute to acceleration of thrombin production mimic the redundancy in platelets provided by PAR-1 and PAR-4. Human platelets express PAR-1 and PAR-4 but not PAR-3.19,20 The activation of either PAR-1 or PAR-4 in human platelets can trigger secretion and aggregation.21 Successful inhibition of thrombin-induced platelet activation required blocking both PAR-1 and PAR-4, whereas inhibition of PAR-4 alone has no impact on platelet activation and inhibition of PAR-1 was only able to block platelet activation at low thrombin concentrations.22 Our data in HASMCs showed that activation of either PAR-3 or PAR-4 reduced the time to half of peak thrombin with an additive effect when both receptors were activated simultaneously. Analogous to the platelet model, inhibition of both PAR-3 and PAR-4 was required to block α-thrombin–induced acceleration in thrombin generation in HASMCs.
Potential mechanisms for interactions between different types of PARs on HASMCs are provided by studies in platelets. Thrombin activates platelets by cleaving PAR-1 and PAR-4, which signal with different kinetics and appear to have distinct functions in platelet aggregation. PAR-1 and PAR-4 evoke 2 distinct waves of intracellular calcium in which the signal from PAR-1 is brief and fast and the signal from PAR-4 is slower in onset but more prolonged.23 PAR-1 also serves to enhance the response of PAR-4 to thrombin on the surface of human platelets by forming stable heterodimeric complexes that assist in the activation and cleavage of PAR-4.24 The effects of interactions of PAR-1 and PAR-4 on platelet-mediated thrombin generation were reported by Thomas et al,25 who found that thrombin treatment of human platelets resulted in PAR-1– and PAR-4–dependent production of phosphatidylethanolamine or phosphatidylcholine-esterified 12S-HETE. Esterified HETEs remained cell associated, with HETE-phosphatidylethanolamines migrating to the outside of the plasma membrane, where they enhanced tissue factor–dependent thrombin generation.
Activation of PAR-3 and PAR-4 increased the amount and rate of tissue factor, and Ca2+ stimulated factor Xa generation on the surface of HASMCs. Furthermore, the effects of PAR-3 and PAR-4 activation on factor Xa generation were abolished by antibodies against tissue factor. PAR-3 and PAR-4 signaling also accelerated thrombin generation via mechanisms downstream of factor Xa generation. These findings are consistent with the cell-based thrombin generation model proposed by Monroe et al.5 During the initiation phase of cell-based coagulation, model factor Xa formed by factor VIIa/TF binds to factor Va on the cell surface and converts a small amount of prothrombin to thrombin. The small amount of thrombin accelerates further thrombin generation by activation of factor V and factor VIII. Once activated, factor V moves into a protected complex with factor Xa, resulting in a burst of thrombin generation, triggering the propagation phase.5 In the current studies, preincubation with low concentrations of α-thrombin, PAR-3 AP, or PAR-4 AP resulted in a faster rate of thrombin generated in the early phase of the reaction without affecting the amount of peak thrombin generated. Our observations demonstrate that PAR-3 and PAR-4 play a role in the initiation phase of the thrombin generation reaction.
Our data show that α-thrombin, PAR-3 AP, and PAR-4 AP rapidly increase cell surface expression of PS in HASMCs. Regulation of PS in HASMCs is, thus, different from human platelets in which externalization of PS is via PAR-1 and inhibition of PAR-1 attenuates PS expression.26 In HASMCs, PAR-1 AP failed to increase PS exposure and activation of PAR-1 had no effect on the kinetics of tissue factor–induced thrombin generation. Our results demonstrate that increased cell surface expression of PS is one mechanism whereby activation of PAR-3 or PAR-4 could accelerate tissue factor–induced thrombin generation in HASMCs.
PAR-3 and PAR-4 signaling enhanced thrombin generation both before and after factor Xa generation, raising the possibility that increased PS expression enhances formation of the tenase and prothrombinase complexes. Consistent with this hypothesis, Ananyeva et al27 reported that treatment of SMCs with oxidized low-density lipoprotein enhanced PS translocation to the outer membrane, enhanced tenase and prothrombinase complex activity, and resulted in a 10-fold increase in thrombin generation and a 6-fold increase in factor Xa production. Flynn et al18 showed that apoptotic SMCs have increased thrombin generation activity, which is mediated in part by the translocation of PS from the inner to the outer leaflet of the cellular membrane. Taken together, these studies show that treatment with specific agonists (eg, thrombin or oxidized low-density lipoprotein) or apoptosis results in translocation of PS to the outer surface of SMCs, resulting in enhanced thrombin generation.
The present studies add to the literature on the functional role of PAR-3 and PAR-4 in SMCs. Bretschneider and colleagues reported that PAR-310 and PAR-411 were expressed on cultured SMCs from the human saphenous vein and that these receptors mediated calcium transients and ERK 1/2 phosphorylation. In the current studies, a novel function for PAR-3 and PAR-4 on SMCs has been identified, in which activation of these receptors accelerates tissue factor–induced thrombin generation.
Footnotes
Disclosures
None.
References
- 1.Bonaca MP, Steg PG, Feldman LJ, Canales JF, Ferguson JJ, Wallentin L, Califf RM, Harrington RA, Giugliano RP. Antithrombotics in acute coronary syndromes. J Am Coll Cardiol. 2009;54:969–984. doi: 10.1016/j.jacc.2009.03.083. [DOI] [PubMed] [Google Scholar]
- 2.Manoukian SV. Predictors and impact of bleeding complications in percutaneous coronary intervention, acute coronary syndromes, and ST-segment elevation myocardial infarction. Am J Cardiol. 2009;104:9C–15C. doi: 10.1016/j.amjcard.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 3.Butenas S, Orfeo T, Mann KG. Tissue factor in coagulation: Which? Where? When? Arterioscler Thromb Vasc Biol. 2009;29:1989–1996. doi: 10.1161/ATVBAHA.108.177402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mackman N. Triggers, targets and treatments for thrombosis. Nature. 2008;451:914–918. doi: 10.1038/nature06797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monroe DM, Hoffman M, Roberts HR. Platelets and thrombin generation. Arterioscler Thromb Vasc Biol. 2002;22:1381–1389. doi: 10.1161/01.atv.0000031340.68494.34. [DOI] [PubMed] [Google Scholar]
- 6.Mann KG, Butenas S, Brummel K. The dynamics of thrombin formation. Arterioscler Thromb Vasc Biol. 2003;23:17–25. doi: 10.1161/01.atv.0000046238.23903.fc. [DOI] [PubMed] [Google Scholar]
- 7.Pathak A, Zhao R, Monroe DM, Roberts HR, Sheridan BC, Selzman CH, Stouffer GA. Thrombin generation in vascular tissue. J Thromb Haemost. 2006;4:60–67. doi: 10.1111/j.1538-7836.2005.01630.x. [DOI] [PubMed] [Google Scholar]
- 8.Vretenbrant K, Ramstrom S, Bjerke M, Lindahl TL. Platelet activation via PAR4 is involved in the initiation of thrombin generation and in clot elasticity development. Thromb Haemost. 2007;97:417–424. [PubMed] [Google Scholar]
- 9.Dorsam RT, Tuluc M, Kunapuli SP. Role of protease-activated and ADP receptor subtypes in thrombin generation on human platelets. J Thromb Haemost. 2004;2:804–812. doi: 10.1111/j.1538-7836.2004.00692.x. [DOI] [PubMed] [Google Scholar]
- 10.Bretschneider E, Spanbroek R, Lotzer K, Habenicht AJ, Schror K. Evidence for functionally active protease-activated receptor-3 (PAR-3) in human vascular smooth muscle cells. Thromb Haemost. 2003;90:704–709. doi: 10.1160/TH03-04-0203. [DOI] [PubMed] [Google Scholar]
- 11.Bretschneider E, Kaufmann R, Braun M, Nowak G, Glusa E, Schror K. Evidence for functionally active protease-activated receptor-4 (PAR-4) in human vascular smooth muscle cells. Br J Pharmacol. 2001;132:1441–1446. doi: 10.1038/sj.bjp.0703947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNamara CA, Sarembock IJ, Gimple LW, Fenton JW, Coughlin SR, Owens GK. Thrombin stimulates proliferation of cultured rat aortic smooth muscle cells by a proteolytically activated receptor. J Clin Invest. 1993;91:94–98. doi: 10.1172/JCI116206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patterson C, Stouffer GA, Madamanchi NR, Runge MS. New tricks for old dogs: nonthrombotic effects of thrombin in vessel wall biology. Circ Res. 2001;88:987–997. doi: 10.1161/hh1001.091447. [DOI] [PubMed] [Google Scholar]
- 14.Siow RC, Richards JP, Pedley KC, Leake DS, Mann GE. Vitamin C protects human vascular smooth muscle cells against apoptosis induced by moderately oxidized LDL containing high levels of lipid hydroperoxides. Arterioscler Thromb Vasc Biol. 1999;19:2387–2394. doi: 10.1161/01.atv.19.10.2387. [DOI] [PubMed] [Google Scholar]
- 15.Sajid M, Zhao R, Pathak A, Smyth SS, Stouffer GA. Alphav beta3 integrin antagonists inhibit thrombin-induced proliferation and focal adhesion formation in smooth muscle cells. Am J Physiol Cell Physiol. 2003;285:C1330–C1338. doi: 10.1152/ajpcell.00475.2002. [DOI] [PubMed] [Google Scholar]
- 16.Harper MT, Sage SO. Actin polymerisation regulates thrombin-evoked Ca(2+) signalling after activation of PAR-4 but not PAR-1 in human platelets. Platelets. 2006;17:134–142. doi: 10.1080/09537100500441218. [DOI] [PubMed] [Google Scholar]
- 17.McLaughlin JN, Patterson MM, Malik AB. Protease-activated receptor-3 (PAR3) regulates PAR1 signaling by receptor dimerization. Proc Natl Acad Sci U S A. 2007;104:5662–5667. doi: 10.1073/pnas.0700763104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flynn PD, Byrne CD, Baglin TP, Weissberg PL, Bennett MR. Thrombin generation by apoptotic vascular smooth muscle cells. Blood. 1997;89:4378–4384. [PubMed] [Google Scholar]
- 19.Coughlin SR. Protease-activated receptors in hemostasis, thrombosis and vascular biology. J Thromb Haemost. 2005;3:1800–1814. doi: 10.1111/j.1538-7836.2005.01377.x. [DOI] [PubMed] [Google Scholar]
- 20.Leger AJ, Covic L, Kuliopulos A. Protease-activated receptors in cardio-vascular diseases. Circulation. 2006;114:1070–1077. doi: 10.1161/CIRCULATIONAHA.105.574830. [DOI] [PubMed] [Google Scholar]
- 21.Kahn ML, Zheng YW, Huang W, Bigornia V, Zeng D, Moff S, Farese RV, Jr, Tam C, Coughlin SR. A dual thrombin receptor system for platelet activation. Nature. 1998;394:690–694. doi: 10.1038/29325. [DOI] [PubMed] [Google Scholar]
- 22.Kahn ML, Nakanishi-Matsui M, Shapiro MJ, Ishihara H, Coughlin SR. Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin. J Clin Invest. 1999;103:879–887. doi: 10.1172/JCI6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Covic L, Gresser AL, Kuliopulos A. Biphasic kinetics of activation and signaling for PAR1 and PAR4 thrombin receptors in platelets. Biochemistry. 2000;39:5458–5467. doi: 10.1021/bi9927078. [DOI] [PubMed] [Google Scholar]
- 24.Leger AJ, Jacques SL, Badar J, Kaneider NC, Derian CK, Andrade-Gordon P, Covic L, Kuliopulos A. Blocking the protease-activated receptor 1–4 heterodimer in platelet-mediated thrombosis. Circulation. 2006;113:1244–1254. doi: 10.1161/CIRCULATIONAHA.105.587758. [DOI] [PubMed] [Google Scholar]
- 25.Thomas CP, Morgan LT, Maskrey BH, Murphy RC, Kühn H, Hazen SL, Goodall AH, Hamali HA, Collins PW, O’Donnell VB. Phospholipid-esterified eicosanoids are generated in agonist-activated human platelets and enhance tissue factor-dependent thrombin generation. J Biol Chem. 2010;285:6891–6903. doi: 10.1074/jbc.M109.078428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keuren JF, Wielders SJ, Ulrichts H, Hackeng T, Heemskerk JW, Deckmyn H, Bevers EM, Lindhout T. Synergistic effect of thrombin on collagen-induced platelet procoagulant activity is mediated through protease-activated receptor-1. Arterioscler Thromb Vasc Biol. 2005;25:1499–1505. doi: 10.1161/01.ATV.0000167526.31611.f6. [DOI] [PubMed] [Google Scholar]
- 27.Ananyeva NM, Kouiavskaia DV, Shima M, Saenko EL. Intrinsic pathway of blood coagulation contributes to thrombogenicity of atherosclerotic plaque. Blood. 2002;99:4475–4485. doi: 10.1182/blood-2001-11-0140. [DOI] [PubMed] [Google Scholar]