Abstract
Objective
To investigate the association between serum perfluorooctanoic acid (PFOA) concentration and cardiovascular disease, as measured by homocysteine level and blood pressure in a representative sample of US adults.
Methods
A cross-sectional study of 2934 adults (≥20 years) who participated in the 2003–2004 and 2005–2006 National Health and Nutrition Examination Survey and had detectable levels of PFOA in their serum. The health effects analysed as potentially associated with PFOA exposure included homocysteine level and blood pressure.
Results
The geometric mean value (95% CI) of the study participants' serum PFOA concentration was 4.00 μg/l (95% CI 3.86 to 4.13). The homocysteine and systolic blood pressure were shown to increase significantly with an increase in the log-transformed serum PFOA concentration, after adjusting for potential confounding variables. Adjusted ORs comparing participants at the 80th versus the 20th percentiles were 2.62 for hypertension (95% CI 2.09 to 3.14), and a positive association was also evident in models based on quartiles or based on restricted cubic splines.
Conclusion
These findings suggest that background exposure to PFOA may continue a risk factor for the development of cardiovascular diseases.
Keywords: Perfluorooctanoic acid, homocysteine, blood pressure, perfluorooctane sulfonate, ageing, cardiovascular, lung function, neurobehavioural effects, smoking
What this paper adds.
Perfluorooctanoic acid (PFOA) is a man-made compound used in a wide range of industrial and consumer products, such as Teflon and Gore-Tex. It is speculated that PFOA exposure has several adverse effects on health, but little data are.
We examined the association between serum PFOA concentrations and homocysteine and blood pressure, using the 2003–2004 and 2005–2006 National Health and Nutrition Examination Survey data.
In a representative sample of US adults, higher serum PFOA concentrations were associated with high homocysteine and hypertension after adjusting for any potential confounding variables.
Given its widespread presence in the environment and the frequency of exposure in the general population, the potential health effects of background PFOA exposure should be considered.
Perfluorooctanoic acid (PFOA) is a man-made compound that the US Environmental Protection Agency has classified as a probable human carcinogen. Since the introduction of PFOA in the 1950s, its capacity to impart fire resistance and oil, stain, grease and water repellency has led to its widespread use in many industrial and consumer products, such as Teflon and Gore-Tex.1 PFOA does not break down readily and tends to persist and bio-accumulate in the environment and the body. In many countries, nearly everyone in the general population has detectable levels of PFOA in their serum.2–5
Although controversy exists regarding the risk to human health of PFOA,6–8 epidemiological studies have suggested that there are adverse health outcomes in populations with occupational or community exposure to PFOA9; in some studies, PFOA exposure has been linked to elevated cholesterol levels and hyperuricaemia.6 7 10 11 Two recent studies supported such effect of PFOA exposure among the general population, who are exposed to the relatively low doses of PFOA. Nelson et al12 showed that subjects exposed to the highest quartile of PFOA concentration had mean total cholesterol levels that were 9.8 mg/dl higher than those exposed to the lowest quartile. Shankar et al13 observed a positive association between increasing quartiles of serum PFOA and the odds (quartile 4 vs 1: OR=1.97, 95% CI 1.44 to 2.70) of hyperuricaemia in US adults. Considering that increases in cholesterol and uric acid are significant cardiovascular risk factors,14 15 this finding has led to speculation that PFOA affects the development of cardiovascular disease.
Cardiovascular disease is the most common cause of death in the USA.16 In addition to the classical risk factors, such as smoking, diabetes and obesity, there has been growing concern that environmental toxicants may pose a risk for the development of cardiovascular disease.17 18 Despite the observed associations reported above,6 7 10–13 whether PFOA levels are implicate in the development of cardiovascular disease is still unknown, which prevents any firm conclusions from being drawn.
Therefore, we hypothesized that an increased PFOA exposure is a risk factor for the development of cardiovascular disease. This study investigated the association between serum PFOA concentration and homocysteine level and blood pressure using the 2003–2004 and 2005–2006 National Health and Nutrition Examination Survey (NHANES) data.
Methods
Study population
Data were taken from the 2003–2004 and 2005–2006 NHANES. The NHANES, conducted by the Centers for Disease Control and Prevention, is a national representative survey of the non-institutionalised civilian US population. The study protocols and all the NHANES testing procedures were approved by the National Center for Health Statistics Institutional Review Board. Oral and written consents were obtained from all the participants.
Of 4191 subjects were found to have detectable levels of PFOA in their serum in the 2003–2004 and 2005–2006 NHANES. We restricted our analyses to 2934 participants (>20 years old) to focus on the health effects of PFOA on adults. We excluded 359 participants with missing values for the demographic variables (ie, income and education) and 276 participants who showed no information on health behaviour variables (ie, smoking and physical activity). For the hypertension analysis, 91 participants with missing blood pressure and certain health outcomes data were excluded, leaving 2208 individuals for the hypertensive analysis. For the homocysteine analysis, 36 participants had no data pertaining to certain health outcomes, leaving a total of 2263 individuals who were included for homocysteine.
Perfluorooctanoic acid
A subsample consisting of one-third of the survey participants (12 years or older) was randomly selected. The PFOA concentrations in the serum samples collected from each participant were analysed at the Environmental Health Sciences Laboratory in the CDC's National Center for Environmental Health, following standard protocols. An assay employing automated solid-phase extraction, followed by high-performance liquid chromatography, with turboionspray ionisation-tandem mass spectrometry was used. Detailed laboratory methods are available.3 The limit of PFOA detection was 0.1 μg/l. Any values below the detection limit were excluded from this study.
Health outcomes
Homocysteine was measured using a fluorescence polarization immunoassay from Abbott Diagnostics. In brief, dithiothreitol reduces mixed disulfides and homocysteine bound to albumin and to other small molecules to free thiol. S-adenosyl-homocysteine (SAH) hydrolase catalyses the conversion of homocysteine to SAH in the presence of added adenosine. In the subsequent steps, the specific monoclonal antibody and the fluoresceinated SAH analog tracer constitute the FPIA detection system.19 Total plasma homocysteine concentrations are calculated by the Abbott Axsym® using a machine-stored calibration curve.
Blood pressure (systolic and diastolic) was measured once after the subject had rested quietly in a sitting position for 5 min and again after the maximum inflation level was determined. The blood pressures were measured three to four times in the mobile examination centre, and the mean values of these measurements were calculated for this study. The examiners are certified in blood pressure measurement through a training programme from Shared Care Research and Education Consulting.
Other variables
Because serum perfluorooctane sulfonate (PFOS) levels were highly correlated with PFOA levels (r=0.61), the PFOS concentration was included as a log-transformed continuous variable in the final model. The following additional covariates were obtained from the questionnaire information: age (20–29, 30–39, 40–49, 50–59, 60–69, 70–79 or 80+ years), sex (male or female), race/ethnicity (non-Hispanic white, non-Hispanic black, Mexican–American or other), education (less than high school, high school diploma or college or more), annual household income (≤20 000 or > 20 000), cigarette smoking (current smoker, ex-smoker and never smoked), alcohol consumption in the past year (yes or no), exercise (performed or did not perform moderate or vigorous physical activity in the preceding 30 days) and total saturated fatty acid intake (1Q, <14.72 g; 2Q, 14.72–22.214 g; 3Q, 22.214–32.512 g and 4Q, >32.512 g). Obesity was defined as a body mass index (BMI) (calculated as the weight in kilograms divided by the height in metres squared) and included three categories: BMI <25, 25≤BMI<30 and BMI ≥30 kg/m2. Total cholesterol levels were categorised into four categories: 1Q, <155 mg/dl; 2Q, 155–179 mg/dl; 3Q, 179–210 mg/dl and 4Q, >210 g). Kidney function was evaluated by the estimated glomerular filtration rate (eGFR) based on the Chronic Kidney Disease Epidemiology Collaboration equation, and poor kidney function was defined as an eGFR <60 ml/min per 1.73 m2.20 Serum folate (1Q, <8.3 ng/ml; 2Q, 8.3–10.8 ng/ml; 3Q, 10.8–13.8 ng/ml; 4Q, 13.8–17.9 ng/ml and 5Q, >17.9 ng/ml) and serum vitamin B12 (1Q, <364 pg/ml; 2Q, 364–476 pg/ml; 3Q, 476–601 pg/ml; 4Q, 601–796 ng/ml and 5Q. >796 pg/ml) were also included as covariates with homocysteine. Serum folate and vitamin 12 levels were measured using the Bio-Rad Laboratories ‘Quantaphase II Folate/vitamin B12’ radioassay kit.21 The assay is performed by combining serum or a whole blood hemolysate sample with 125I-folate and 57Co-vitamin B12 in a solution containing dithiothreitol and cyanide.
Statistical analysis
To account for the complex sampling design, weighted estimates of the population parameters were computed using the NHANES Analytic and Reporting Guidelines. All the analyses were performed using the PROC SURVEY procedures in SAS V.9.2 (SAS Institute) and R (R Foundation for Statistical Computing).
The PFOA concentrations and homocysteine levels were log transformed to improve their levels of normality. We conducted linear regression analyses to evaluate the associations between the serum PFOA level and the measures of homocysteine and blood pressure (table 1). For the risk analysis of hypertension, a logistic regression was conducted to obtain the ORs and the corresponding 95% CIs comparing the 80th and 20th percentiles of the PFOA concentrations; another analysis estimated the ORs (95% CIs) for hypertension by comparing the highest and the lowest quartiles of the PFOA concentration (table 2). Herein, hypertension was defined as systolic blood pressure >140 mm Hg or diastolic blood pressure >90 mm Hg or as a self-reported medical diagnosis of hypertension. We also provided adjusted ORs (95% CIs) based on restricted cubic splines for the log-transformed PFOA concentration with three knots (figure 1). A sensitivity analysis of hypertension was conducted; participants who were >80 years old, pregnant, breast feeding or on dialysis were excluded from this analysis. Furthermore, we conducted the analysis with participants who were not taking antihypertensive medication.
Table 1.
Linear regression coefficients of homocysteine and blood pressure in the log-transformed serum PFOA concentration
| Homocysteine (n=2263) | Systolic blood pressure (n=2208) | Diastolic blood pressure (n=2208) | |||||||
| β | SE | p Value | β | SE | p Value | β | SE | p Value | |
| Model 1* | 0.050 | 0.011 | 0.0001 | 2.202 | 0.493 | 0.0001 | 0.784 | 0.433 | 0.081 |
| Model 2† | 0.041 | 0.011 | 0.001 | 2.198 | 0.504 | 0.0001 | 0.747 | 0.447 | 0.105 |
| Model 3‡ | 0.031 | 0.014 | 0.039 | 2.483 | 0.629 | 0.0004 | 0.746 | 0.448 | 0.106 |
Model 1 was adjusted for age, sex, race/ethnicity, education, income, smoking habits and alcohol use.
Model 2 was further adjusted for obesity status, total saturated fatty acid intake, physical activity, serum folate and vitamin B12 levels (only in regressions with homocysteine levels).
Model 3 was further adjusted for serum PFOS concentrations, total cholesterol and poor kidney function (assessed by estimated glomerular filtration rate).
Table 2.
ORs (95% CIs) of hypertension* by serum PFOA concentration
| 80th vs 20th percentiles of PFOA (2.4 vs 6.1 μg/l) | Quartile 1 (<2.6 μg/l) | Quartile 2 (2.7–3.9 μg/l) | Quartile 3 (4.0–5.5 μg/l) | Quartile 4 (>5.6 μg/l) | p Value for trend† | |
| Model 1‡ | 2.54 (2.01 to 3.06) | 1 (Reference) | 1.24 (0.89 to 1.74) | 1.63 (1.20 to 2.20) | 1.80 (1.35 to 2.41) | 0.0001 |
| Model 2§ | 2.63 (2.11 to 3.16) | 1 (Reference) | 1.22 (0.86 to 1.74) | 1.65 (1.20 to 2.27) | 1.82 (1.35 to 2.45) | <0.0001 |
| Model 3¶ | 2.62 (2.09 to 3.14) | 1 (Reference) | 1.21 (0.86 to 1.70) | 1.60 (1.15 to 2.22) | 1.71 (1.23 to 2.36) | 0.001 |
Hypertension was defined as systolic blood pressure >140 mm Hg or diastolic blood pressure >90 mm Hg or a self-reported medical diagnosis of hypertension.
p Value for trend based on log-transformed PFOA concentrations.
Model 1 was adjusted for age, sex, race/ethnicity, education, income, smoking habits and alcohol use.
Model 2 was further adjusted for obesity status, total saturated fatty acid intake and physical activity.
Model 3 was further adjusted for serum PFOS concentrations, total cholesterol and poor kidney function (measured by estimated glomerular filtration rate).
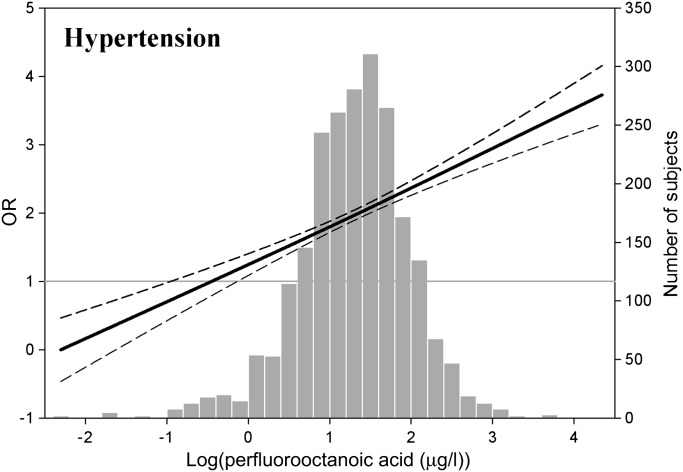
Figure 1.
Adjusted ORs for hypertension depending on the PFOA concentrations. Bold lines indicate the adjusted ORs based on restricted cubic splines for log-transformed PFOA concentration with three knots. Dotted lines indicate the corresponding 95% CIs. ORs were adjusted for age, sex, race/ethnicity, education, income, obesity, smoking status, alcohol intake, serum albumin levels, total saturated fatty acid intake, exercise, PFOS level, total cholesterol and poor kidney function. The bar histograms represent the frequency distribution of the log-transformed PFOA concentration.
All models for the PFOA concentration with homocysteine level and blood pressure were fitted with increasing degrees of adjustment. First, we adjusted for age, sex, race/ethnicity, education, income, smoking habits and alcohol use. Second, each model was adjusted for obesity status, total saturated fatty acid intake, physical activity and serum folate and vitamin B12 levels (only in regressions with homocysteine levels). Finally, the serum PFOS concentration, total cholesterol and poor kidney function (assessed by eGFR) were also adjusted for in each model.
Results
The geometric mean values (95% CI) of the study participants were 4.00 μg/l (3.86–4.13) for the serum PFOA concentration. Overall, adults who were ≥50 years old had a higher mean serum PFOA concentration than adults between 20 and 40 years of age. The mean PFOA concentration was higher in men, white participants, current smokers and drinkers, participants with high levels of education (college or more), an annual household income of ≥$20 000 and individuals who participated in moderate or vigorous exercise and had poor kidney function (assessed by eGFR <60 ml/min/1.73 m2). There was no regular trend connecting the PFOA concentration to total saturated fatty acid (highest in relation to 4Q, >32.512 g), total cholesterol (highest in relation to 2Q, 155–179 mg/dl), serum folate (highest in relation to 3Q, 12.2–16.6 ng/ml) and serum vitamin B12 (highest in relation to 1Q, <393 pg/ml).
In the linear regression analyses, the homocysteine (β=0.031, p=0.039) and systolic blood pressure (β=2.483, p=0.0004) were shown to significantly increase with an increasing log-transformed serum PFOA concentration, after adjusting for age, sex, serum albumin, race/ethnicity, education, annual household income, obesity, smoking, alcohol, exercise, serum folate and vitamin B12 (only in regressions with homocysteine levels), serum PFOS concentrations, total cholesterol and poor kidney function (assessed by eGFR) (table 1).
For the risk analysis, the adjusted ORs (95% CIs) comparing participants in the 80th versus the 20th percentiles of PFOA level were 2.62 for hypertension (95% CI 2.09 to 3.14), after adjusting for all potential confounding variables. When we compared the highest quartile with the lowest quartile of the PFOA level, the risk for hypertension (OR=1.71, 95% CI 1.23 to 2.36) showed significant increase after adjusting for confounding variables and the PFOS level (table 2). Positive associations with increasing log-transformed PFOA concentrations were observed in a model based on restricted cubic splines (figure 1).
For a sensitivity analysis of hypertension (n=2208), the analysis was restricted to 1894 participants after the exclusion of 314 participants who were >80 years old, pregnant, breast feeding or on dialysis. When compared the highest with the lowest quartile of the PFOA, the resulting OR (95% CIs) was 1.67 (1.18 to 2.38). Furthermore, when we restricted the analysis to 1415 participants who were not taking antihypertensive medication, the increased PFOA concentration noted when comparing the highest with the lowest quartile was associated with an increased risk of hypertension (OR=1.84, 95% CIs 1.07 to 3.18) after adjusting for socio-demographic variables, health risk factors, the serum PFOS level, total cholesterol and poor kidney function.
Discussion
In a representative sample of US adults, higher serum PFOA concentrations were associated with increases in homocysteinaemia and blood pressure after adjusting for any potential confounding variables. The associations were of moderate strength, showed a dose–response relationship and were independent of PFOS exposure. Our findings extend the hitherto sparse evidence involving the potential cardiovascular toxicity of PFOA in humans22–24 and suggest that background exposure to PFOA may be involved in the pathogenesis of cardiovascular disease in the general population. However, because this study is based on a cross-sectional design, it is impossible to establish causal relationships among the findings. Herein, it is still unknown whether higher serum PFOA contributes to high levels of homocysteine and hypertension or vice versa. Given that the prior evidence about the risks of PFOA is based on relatively low levels of exposure,12 13 25 the chronic, low-level exposure observed here could be causally tied to adverse health outcomes.
PFOA has recently received attention because of its widespread use, its capacity for bioaccumulation and the risk of accumulating toxicity. Although its potential sources are not fully defined, currently known sources of PFOA include drinking water, food or migration from food packaging and dust in homes.2 Once PFOA is taken up through the gut, it is not metabolised in the body. It is distributed to the kidneys, liver and blood by binding to proteins, and it has a long biological half-life in human.26 Importantly, PFOA is detectable in virtually everyone in the general population.3–5
Elevated homocysteine levels are associated with cardiovascular diseases such as atherosclerosis, myocardial infarction and peripheral arterial disease.27–29 Hypertension is also a well-known predictor of premature cardiovascular disease.30 In our study, increased odds of hyperhomocysteisnaemia and hypertension in relation to PFOA exposure may be consistent with earlier studies on its contribution to increased cardiovascular risks (eg, elevated cholesterol and uric acid)10–12 31; it is likely that PFOA exposure is linked to the development of cardiovascular disease. However, few studies have addressed cardiovascular toxicity of PFOA. Leonard et al22 found no convincing evidence for an increased risk of ischaemic heart disease mortality in workers exposed to PFOA. Sakr et al23 found no significant association between ischaemic heart disease mortality and the estimated cumulative exposure in workers; they only observed a positive trend after an exposure lag of 10 years. In a study using the NHANES data, Melzer et al32 reported that the serum PFOA concentration does not contribute to the risk of self-reported cardiovascular diseases. Thus, future studies are required to determine whether PFOA exposure may contribute to the initiation and development of cardiovascular events.
A mechanism by which PFOA might lead to higher homocysteine levels and blood pressure is unknown. However, some researchers have suggested its role in increasing oxidative stress in the liver and endothelial cells to account for PFOA-related health hazards.33 34 Homocysteine is formed by the demethylation of methionine. Gori et al35 demonstrated that non-compensated oxidative stress may cause increased homocystein by decreasing the synthesis of homocysteine methyl group donors, which are used to repair oxidative damage. Other studies showed that pre-treatment with antioxidant vitamins reduced homocysteinaemia, suggesting an oxidative mechanism.36 37 Oxidative stress has also been proposed as the cause of hypertension, suggesting that a resulting imbalance in nitric oxide and superoxide may reduce vasodilation.38 Thus, it is possible that oxidative stress caused by PFOA exposure provokes an increased demand for homocysteine methyl group donors and contributes to impaired vasodilation. Mechanistic studies would be essential to verify the suggested hypotheses or to discover new biological mechanisms of this process.
Although the strengths of the current study include the use of a representative sample of the general US adult population, rigorous methodology and the quality control procedures of NHANES, some limitations should be considered. The main limitation of our analyses is the cross-sectional design, which means that a definitive conclusion about the causality of PFOA levels and high homocysteine levels and hypertension cannot be drawn. In addition, although humans have a long PFOA half-life estimated that is estimated to be approximately 3.5 years,26 considering that human health risks are most likely associated with long-term and chronic exposure, the relevance of a single measurement of PFOA has been questioned. Thus, prospective studies are needed to monitor whether PFOA concentrations predict onset of diseases or changes in health parameters.
Conclusions
We found that increases in serum PFOA concentration were associated with a significantly increased risk of high homocysteine levels and hypertension in US adults. The association was independent of any other potential confounding variables including PFOS. Further studies to confirm the role of PFOA in cardiovascular disease onset are necessary.
Footnotes
Competing interests: None.
Ethics approval: Ethics approval was provided by the study protocol and by the Institutional Review Board, Ajou University School of Medicine.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Prevedouros K, Cousins IT, Buck RC, et al. Sources, fate and transport of perfluorocarboxylates. Environ Sci Technol 2006;40:32–44 [DOI] [PubMed] [Google Scholar]
- 2.Lau C, Anitole K, Hodes C, et al. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci 2007;99:366–94 [DOI] [PubMed] [Google Scholar]
- 3.Calafat AM, Kuklenyik Z, Reidy JA, et al. Serum concentrations of 11 polyfluoroalkyl compounds in the U.S. population: data from the national health and Nutrition examination survey (NHANES). Environ Sci Technol 2007;41:2237–42 [DOI] [PubMed] [Google Scholar]
- 4.Kannan K, Corsolini S, Falandysz J, et al. Perfluorooctanesulfonate and related fluorochemicals in human blood from several countries. Environ Sci Technol 2004;38:4489–95 [DOI] [PubMed] [Google Scholar]
- 5.Kubwabo C, Vais N, Benoit FM. A pilot study on the determination of perfluorooctanesulfonate and other perfluorinated compounds in blood of Canadians. J Environ Monit 2004;6:540–5 [DOI] [PubMed] [Google Scholar]
- 6.Sakr CJ, Kreckmann KH, Green JW, et al. Cross-sectional study of lipids and liver enzymes related to a serum biomarker of exposure (ammonium perfluorooctanoate or APFO) as part of a general health survey in a cohort of occupationally exposed workers. J Occup Environ Med 2007;49:1086–96 [DOI] [PubMed] [Google Scholar]
- 7.Sakr CJ, Leonard RC, Kreckmann KH, et al. Longitudinal study of serum lipids and liver enzymes in workers with occupational exposure to ammonium perfluorooctanoate. J Occup Environ Med 2007;49:872–9 [DOI] [PubMed] [Google Scholar]
- 8.Olsen GW, Zobel LR. Assessment of lipid, hepatic, and thyroid parameters with serum perfluorooctanoate (PFOA) concentrations in fluorochemical production workers. Int Arch Occup Environ Health 2007;81:231–46 [DOI] [PubMed] [Google Scholar]
- 9.Steenland K, Fletcher T, Savitz DA. Epidemiologic evidence on the health effects of perfluorooctanoic acid (PFOA). Environ Health Perspect 2010;118:1100–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steenland K, Tinker S, Shankar A, et al. Association of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) with uric acid among adults with elevated community exposure to PFOA. Environ Health Perspect 2010;118:229–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costa G, Sartori S, Consonni D. Thirty years of medical surveillance in perfluooctanoic acid production workers. J Occup Environ Med 2009;51:364–72 [DOI] [PubMed] [Google Scholar]
- 12.Nelson JW, Hatch EE, Webster TF. Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general U.S. population. Environ Health Perspect 2010;118:197–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shankar A, Xiao J, Ducatman A. Perfluoroalkyl chemicals and elevated serum uric acid in US adults. Clin Epidemiol 2011;3:251–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stamler J, Daviglus ML, Garside DB, et al. Relationship of baseline serum cholesterol levels in 3 large cohorts of younger men to long-term coronary, cardiovascular, and all-cause mortality and to longevity. JAMA 2000;284:311–18 [DOI] [PubMed] [Google Scholar]
- 15.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med 2008;359:1811–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thom T, Haase N, Rosamond W, et al. Heart disease and stroke statistics–2006 update: a report from the American heart association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2006;113:e85–151 [DOI] [PubMed] [Google Scholar]
- 17.Bhatnagar A. Environmental cardiology: studying mechanistic links between pollution and heart disease. Circ Res 2006;99:692–705 [DOI] [PubMed] [Google Scholar]
- 18.O'Toole TE, Conklin DJ, Bhatnagar A. Environmental risk factors for heart disease. Rev Environ Health 2008;23:167–202 [DOI] [PubMed] [Google Scholar]
- 19.Shipchandler MT, Moore EG. Rapid, fully automated measurement of plasma homocysteine in with the Abbott IMx® analyzer. Clin Chem 1995;41:991–4 [PubMed] [Google Scholar]
- 20.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39(2 Suppl 1):S1–266 [PubMed] [Google Scholar]
- 21.Instruction Manual, bio-Rad Quantaphase II Folate Radioassay Kit. Hercules, CA: Bio-Rad Laboratories, 1993 [Google Scholar]
- 22.Leonard RC, Kreckmann KH, Sakr CJ, et al. Retrospective cohort mortality study of workers in a polymer production plant including a reference population of regional workers. Ann Epidemiol 2008;18:15–22 [DOI] [PubMed] [Google Scholar]
- 23.Sakr CJ, Symons JM, Kreckmann KH, et al. Ischaemic heart disease mortality study among workers with occupational exposure to ammonium perfluorooctanoate. Occup Environ Med 2009;66:699–703 [DOI] [PubMed] [Google Scholar]
- 24.Lundin JI, Alexander BH, Olsen GW, et al. Ammonium perfluorooctanoate production and occupational mortality. Epidemiology 2009;20:921–8 [DOI] [PubMed] [Google Scholar]
- 25.Shankar A, Xiao J, Ducatman A. Perfluoroalkyl chemicals and chronic kidney disease in US adults. Am J Epidemiol 2011;174:893–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andersen ME, Butenhoff JL, Chang SC, et al. Perfluoroalkyl acids and related chemistries–toxicokinetics and modes of action. Toxicol Sci 2008;102:3–14 [DOI] [PubMed] [Google Scholar]
- 27.Boushey CJ, Beresford SA, Omenn GS, et al. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. JAMA 1995;274:1049–57 [DOI] [PubMed] [Google Scholar]
- 28.Verhoef P, Kok FJ, Kruyssen DA, et al. Plasma total homocysteine, B vitamins, and risk of coronary atherosclerosis. Arterioscler Thromb Vasc Biol 1997;17:989–95 [DOI] [PubMed] [Google Scholar]
- 29.Stampfer MJ, Malinow MR, Willett WC, et al. A prospective study of plasma homocyst(e)ine and risk of myocardial infarction in US physicians. JAMA 1992;268:877–81 [PubMed] [Google Scholar]
- 30.Wilson PW. Established risk factors and coronary artery disease: the Framingham Study. Am J Hypertens 1994;7:7S–12S [DOI] [PubMed] [Google Scholar]
- 31.Fletcher T, Savitz D, Steenland K; C8 Science Panel Status Report: Association of Perfluorooctanoic Acid (PFOA) and Perfluoroctanesulfonate (PFOS) with Lipids Among Adults in a Community with High Exposure to (PFOA). 2008. http://www.c8sciencepanel.org/pdfs/Status_Report_C8_and_lipids_Oct2008.pdf (accessed 31 Mar 2011). [Google Scholar]
- 32.Melzer D, Rice N, Depledge MH, et al. Association between serum perfluoroctanoic acid (PFOA) and thyroid disease in the NHANES study. Environ Health Perspect 2010;118:686–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panaretakis T, Shabalina IG, Grandér D, et al. Reactive oxygen species and mitochondria mediate the induction of apoptosis in human hepatoma HepG2 cells by the rodent peroxisome proliferator and hepatocarcinogen, perfluorooctanoic acid. Toxicol Appl Pharmacol 2001;173:56–64 [DOI] [PubMed] [Google Scholar]
- 34.Yao X, Zhong L. Genotoxic risk and oxidative DNA damage in HepG2 cells exposed to perfluorooctanoic acid. Mutat Res 2005;587:38–44 [DOI] [PubMed] [Google Scholar]
- 35.Gori AM, Corsi AM, Fedi S, et al. A proinflammatory state is associated with hyperhomocysteinemia in the elderly. Am J Clin Nutr 2005;82:335–41 [DOI] [PubMed] [Google Scholar]
- 36.Nappo F, De Rosa N, Marfella R, et al. Impairment of endothelial functions by acute hyperhomocysteinemia and reversal by antioxidant vitamins. JAMA 1999;281:2113–18 [DOI] [PubMed] [Google Scholar]
- 37.Chambers JC, McGregor A, Jean-Marie J, et al. Demonstration of rapid onset vascular endothelial dysfunction after hyperhomocysteinemia: an effect reversible with vitamin C therapy. Circulation 1999;99:1156–60 [DOI] [PubMed] [Google Scholar]
- 38.Ceriello A. Possible role of oxidative stress in the pathogenesis of hypertension. Diabetes Care 2008;31(Suppl 2):S181–4 [DOI] [PubMed] [Google Scholar]