Not all synapses in the brain are the same. Some transmit signals from one neuron to the next more efficiently than others; the efficiency is referred to as synaptic strength. Currently the most widely accepted theory of memory is that memories are stored as patterns of synaptic strength. Long-term potentiation (LTP) is one way that the strength of synapses is changed and perhaps memories are encoded. Because of this, LTP is one of the most widely studied topics of neuroscience research. In particular, LTP in the mammalian hippocampus has been the focus of intensive research by many scientists, as well as the source of considerable controversy. Although a great deal of effort has been devoted to elucidating the mechanism of LTP expression in the CA1 region of hippocampus, other exciting questions concerning LTP are addressed by two papers in this issue of the Proceedings. Min et al. (1) investigate synapses in the dentate gyrus region of the hippocampus, and ask the question “Do synapses from the two input pathways with different properties exhibit the same type of LTP?” For synapses in CA1, Peterson et al. (2) raise the critical question “Are changes in synaptic strength graded or all-or-none?”
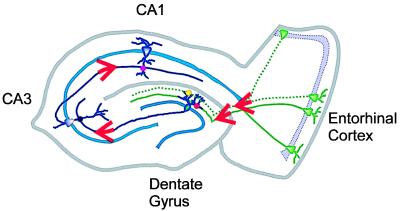
The hippocampus is a popular site for electrophysiology research because most of its primary cell types are separated into easily recognizable regions, and the axonal fiber tracts connecting these regions are relatively simple and well defined, as illustrated in Fig. 1. In the dentate gyrus the principal cell type is the granule cell, and the majority of granule cell input comes from a fiber tract called the perforant path. The perforant path contains axons that originate in the entorhinal cortex and provide a major sensory input to the hippocampus. The perforant path splits into two anatomically distinct pathways, called the medial perforant path and the lateral perforant path. These fibers form excitatory synapses on the middle third and outer third (respectively) of the granule cell dendritic tree. This anatomical organization enables the study of two different populations of synapses onto the same cell type using extracellular stimulation. Axons in the medial and lateral perforant pathways originate from different regions of the entorhinal cortex and carry different kinds of sensory inputs (3). The locations of the medial and lateral perforant path are shown in Fig. 1; the two types of synapses studied by Min et al. (1) are represented in red (medial) and yellow (lateral).
Figure 1.
Synaptic connections in the hippocampus and entorhinal cortex. Lateral perforant path (dotted green) and medial perforant path (solid green) provide inputs from the entorhinal cortex to the dentate gyrus of the hippocampus. Perforant path axons form synapses onto dentate granule cells (lateral shown in yellow, medial shown in red); these synapses are studied in Min et al. (1). Axons from the CA3 region of hippocampus form synapses onto cells in CA1 (shown in purple); these synapses are investigated in Peterson et al. (2).
The two types of synapses are similar in that they both release the neurotransmitter glutamate, and, like most cortical synapses, contain two types of postsynaptic glutamate receptors, NMDA and AMPA receptors. At these synapses, like others, neurotransmitter is released probabilistically; stimulating the synapse may cause either transmitter release or no transmitter release. But differences between the medial and lateral synapses also have been found. Many pharmacological manipulations produce markedly different results in the two populations (4–7). Physiological differences occur as well (8, 9). For example, synapses in the medial perforant path grow weaker (are less likely to release transmitter) on the second of two closely spaced stimuli, whereas synapses in the lateral pathway grow stronger with the same stimulus pair (10). Furthermore, the two pathways are different in terms of the stimulation needed to induce LTP (11).
In the current paper, Min et al. (1) address the important question of whether the differences between the inputs from these two pathways represent two distinct forms of LTP. They use a trick for estimating the probability that stimulation causes transmitter release, and see if the probability is increased by LTP. The trick is to add a drug that only blocks synapses that release transmitter, and see how quickly the synapses are blocked. The drug, MK-801, acts at NMDA receptors and irreversibly blocks synapses where transmitter has been released (12). The rate of MK-801 blocking therefore is related to transmitter release probability: faster blocking indicates an increase in release probability, whereas a slower rate indicates a decrease (13, 14).
A key finding of this paper is that LTP causes an significant increase in the MK-801 blocking rate in synapses from the medial path, whereas no change was observed in the blocking rate in the lateral path synapses (1). The authors conclude that LTP in the medial perforant path is accompanied by an increase in neurotransmitter release probability. Their interpretation of the results from the lateral path, however, is more complex. Although the LTP in the lateral path appears to be different, the authors point out that in the lateral path an increase in release probability after LTP still could be present, but difficult to detect using the MK-801 method. To explain this, they invoke the currently popular hypothesis of intersynaptic cross-talk.
To understand this possibility, we need to take a step back and review the meaning of “intersynaptic cross-talk.” Intersynaptic cross-talk refers to the idea that neurotransmitter released by one synapse may spill over and be detected by a neighboring synapse. This would involve transmitter diffusing out of the synaptic cleft and over to a neighboring cleft in a high enough concentration to activate postsynaptic receptors. The idea of intersynaptic cross-talk challenges the traditional assumption that a synapse is a private connection between an axon and a dendrite, and that all synapses act independently of each other. Excitatory synapses in cortex are on average, however, only separated from a neighboring synapse by 1 μm, and because glutamate can diffuse on the order of 1 μm2/msec, the idea of glutamate spillover is not unreasonable. NMDA receptors have an affinity for glutamate, which is two orders of magnitude greater than that of AMPA receptors (15). In addition, NMDA receptors are tonically activated by baseline levels of exogenous glutamate (16). As a result, NMDA receptors are more likely to be affected by glutamate spillover than AMPA receptors. Consequently, synaptic transmission involving AMPA receptors could have little or no cross-talk, whereas synaptic transmission mediated by NMDA receptors could involve much more cross-talk. Min et al. (1) measured a greater amount of transmitter sensed by the NMDA receptors than the AMPA receptors in the lateral perforant path synapses, which supports the idea of glutamate spillover selectively activating neighboring NMDA receptors in these synapses. This same result has been shown recently at the CA3 to CA1 synapse in hippocampus (shown in purple in Fig. 1) (17). However, in both of these systems this difference between transmitter sensed by NMDA and AMPA receptors is greatly reduced or absent when experiments are performed at physiological temperatures rather than room temperature (1, 18). In the medial perforant path there was no evidence of glutamate spillover onto neighboring synapses (1). Although at some synapses intersynaptic cross-talk seems theoretically possible, its existence at these synapses remains controversial (19–21).
If intersynaptic cross-talk does occur at lateral path synapses, as the authors suggest, then the trick of using the MK-801 blocking rate to detect changes in release probability does not work. LTP may, in fact, cause an increase in release probability at lateral perforant path synapses, in which case the medial and lateral path LTP mechanisms are not different.
Peterson et al. (2) explore a different feature of LTP at excitatory synapses between CA3 axons and CA1 pyramidal cells in the hippocampus (represented in purple in Fig. 1). They ask the novel question of whether potentiation at single synapses happens in a graded fashion, or is instead an all-or-none process. Although graded levels of LTP can be observed in populations of synapses, it is not known whether single synapses are also capable of multiple LTP levels. This important question has implications both for the mechanism underlying LTP and for theories of information storage and perhaps memory formation in the brain.
The authors use two protocols for inducing potentiation: one weaker, the other much stronger. When these two protocols are used successively to potentiate a population of synapses, the response is graded amounts of potentiation. The weak pairing produces a small, nonsaturating amount of potentiation, after which the strong pairing protocol is able to produce even more potentiation. What happens at the individual synapses? Do they also potentiate in a graded fashion? Or is there all-or-none potentiation of first some of the synapses with the weak stimulation and then additional synapses with the strong protocol?
Peterson et al. (2) repeat the experiment using minimal stimulation techniques to measure the response of a single synapse. The weak stimulus protocol was applied first to try to cause potentiation; it then was followed by the stronger protocol, to see whether potentiated synapses could exhibit further potentiation. Although some synapses failed to show potentiation in response to either protocol, the majority of cells exhibited potentiation in an all-or-none fashion. Either they were potentiated by the first pairing or by the second pairing, but not by both. The authors interpret these results as indicating that once potentiated, a synapse is not capable of showing further potentiation. In addition, synapses seem to have (at least) two threshold levels for the induction of potentiation: some were able to be potentiated by the weak protocol, whereas others required the stronger stimulus protocol to potentiate. Interestingly, different synapses also showed different amounts of potentiation, although on average the amount of potentiation observed was not different between the weak and strong protocols.
This exciting result suggests that at these synapses, information is stored in a discrete or digital manner rather than a graded or analog manner. The authors suggest that in the context of synaptic memories there exist only two stable states, “potentiated” and “not potentiated.” Because these synapses are also capable of long-term depression (LTD), which might or might not be a reversal of LTP, it appears that at least three states must be present: potentiated, neither potentiated nor depressed, and depressed. One complication to the idea of information being digitally encoded in synaptic strength is that several forms of short-term plasticity exist that transiently change synaptic strength at single synapses (22–28). This would lead to the appearance of many more apparent states depending on the history of use of the synapse. Two other exciting questions for future study: What determines the threshold for LTP at a single synapse? And what determines the magnitude of potentiation achieved?
It remains to be determined, however, as to whether the all-or-none potentiation observed in Peterson et al. (2) is actually LTP. One limitation of the work is that responses were not measured long enough after potentiation to determine whether it was actually a long-term effect. In light of the significance of the findings, additional experiments should help to clarify whether this all-or-none potentiation is in fact LTP.
The thought-provoking results presented in both Min et al. (1) and Peterson et al. (2) provide important links between the cellular physiology of long-term potentiation and the computational function of hippocampal synapses. Intersynaptic cross-talk provides a major challenge to traditional ideas about the computational importance of synapse specificity. And the existence of all-or-none potentiation as a form of digital signal storage may provide a critical clue for deciphering what information is being stored in the strength of synapses. It also raises the question of whether the postsynaptic cell can “read out” the digital level encoded in the synapse and make use of that information. If so, neural networks and computational models of central nervous system function will need to incorporate this fundamental fact about synaptic strength.
References
- 1.Min M-Y, Asztely F, Kokaia M, Kullmann D M. Proc Natl Acad Sci USA. 1998;95:4702–4707. doi: 10.1073/pnas.95.8.4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peterson C C H, Malenka R C, Nicoll R A, Hopfield J J. Proc Natl Acad Sci USA. 1998;95:4732–4737. doi: 10.1073/pnas.95.8.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Witter M P. Hippocampus. 1993;3:33–44. [PubMed] [Google Scholar]
- 4.Dahl D, Burgard E C, Sarvey J M. Exp Brain Res. 1990;83:172–177. doi: 10.1007/BF00232206. [DOI] [PubMed] [Google Scholar]
- 5.Kahle J S, Cotman C W. Brain Res. 1989;482:159–163. doi: 10.1016/0006-8993(89)90554-4. [DOI] [PubMed] [Google Scholar]
- 6.Bramham C R, Errington M L, Bliss T V. Brain Res. 1988;449:352–356. doi: 10.1016/0006-8993(88)91052-9. [DOI] [PubMed] [Google Scholar]
- 7.Koerner J F, Cotman C W. Brain Res. 1981;216:192–198. doi: 10.1016/0006-8993(81)91288-9. [DOI] [PubMed] [Google Scholar]
- 8.Abraham W C, McNaughton N. Brain Res. 1984;303:251–260. doi: 10.1016/0006-8993(84)91211-3. [DOI] [PubMed] [Google Scholar]
- 9.McNaughton B L, Barnes C A. J Comp Neurol. 1977;175:439–454. doi: 10.1002/cne.901750404. [DOI] [PubMed] [Google Scholar]
- 10.McNaughton B L. Brain Res. 1980;199:1–19. doi: 10.1016/0006-8993(80)90226-7. [DOI] [PubMed] [Google Scholar]
- 11.Colino A, Malenka R C. J Neurophysiol. 1993;69:1150–1159. doi: 10.1152/jn.1993.69.4.1150. [DOI] [PubMed] [Google Scholar]
- 12.Huettner J E, Bean B P. Proc Natl Acad Sci USA. 1988;85:1307–1311. doi: 10.1073/pnas.85.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hessler N A, Shirke A M, Malinow R. Nature (London) 1993;366:569–572. doi: 10.1038/366569a0. [DOI] [PubMed] [Google Scholar]
- 14.Rosenmund C, Clements J D, Westbrook G L. Science. 1993;262:754–757. doi: 10.1126/science.7901909. [DOI] [PubMed] [Google Scholar]
- 15.Patneau D K, Mayer M L. J Neurosci. 1990;10:2385–2399. doi: 10.1523/JNEUROSCI.10-07-02385.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sah P, Hestrin S, Nicoll R A. Science. 1989;246:815–818. doi: 10.1126/science.2573153. [DOI] [PubMed] [Google Scholar]
- 17.Kullmann D M, Erdemli G, Asztely F. Neuron. 1996;17:461–474. doi: 10.1016/s0896-6273(00)80178-6. [DOI] [PubMed] [Google Scholar]
- 18.Asztely F, Erdemli G, Kullmann D. Neuron. 1997;18:281–293. doi: 10.1016/s0896-6273(00)80268-8. [DOI] [PubMed] [Google Scholar]
- 19.Kullmann D, Asztely F. Trends Neurosci. 1998;21:8–14. doi: 10.1016/s0166-2236(97)01150-8. [DOI] [PubMed] [Google Scholar]
- 20.Barbour B, Häusser M. Trends Neurosci. 1997;20:377–384. doi: 10.1016/s0166-2236(96)20050-5. [DOI] [PubMed] [Google Scholar]
- 21.Malenka R, Nicoll R. Neuron. 1997;19:473–476. doi: 10.1016/s0896-6273(00)80362-1. [DOI] [PubMed] [Google Scholar]
- 22.Dobrunz L E, Huang E P, Stevens C F. Proc Natl Acad Sci USA. 1997;94:14843–14847. doi: 10.1073/pnas.94.26.14843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dobrunz L E, Stevens C F. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- 24.Murthy V N, Sejnowski T J, Stevens C F. Neuron. 1997;18:559–612. doi: 10.1016/s0896-6273(00)80301-3. [DOI] [PubMed] [Google Scholar]
- 25.Turner D A, Chen Y, Isaac J T, West M, Wheal H V. J Physiol. 1997;500:441–461. doi: 10.1113/jphysiol.1997.sp022032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Debanne D, Guérineau N C, Gähwiler B H, Thompson S M. J Physiol. 1996;491.1:163–176. doi: 10.1113/jphysiol.1996.sp021204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y, Chad J E, Wheal H V. Neurosci Lett. 1996;218:204–208. doi: 10.1016/s0304-3940(96)13149-9. [DOI] [PubMed] [Google Scholar]
- 28.Stevens C F, Wang Y. Neuron. 1995;14:795–802. doi: 10.1016/0896-6273(95)90223-6. [DOI] [PubMed] [Google Scholar]