Abstract
The Dahl salt-sensitive (S) rat is a widely studied model of salt-sensitive hypertension and develops proteinuria, glomerulosclerosis, and renal interstitial fibrosis. An earlier genetic analysis using a population derived from the S and spontaneously hypertensive rat (SHR) identified eight genomic regions linked to renal injury in the S rat and one protective locus on chromosome 11. The “protective” locus in the S rat was replaced with the SHR genomic segment conferring “susceptibility” to kidney injury. The progression of kidney injury in the S.SHR(11) congenic strain was characterized in the present study. Groups of S and S.SHR(11) rats were followed for 12 wk on either a low-salt (0.3% NaCl) or high-salt (2% NaCl) diet. By week 12 (low-salt), S.SHR(11) demonstrated a significant decline in kidney function compared with the S. Blood pressure was significantly elevated in both strains on high salt. Despite similar blood pressure, the S.SHR(11) exhibited a more significant decline in kidney function compared with the S. The decline in S.SHR(11) kidney function was associated with more severe kidney injury including tubular loss, immune cell infiltration, and tubulointerstitial fibrosis compared with the S. Most prominently, the S.SHR(11) exhibited a high degree of medullary fibrosis and a significant increase in renal vascular medial hypertrophy. In summary, genetic modification of the S rat generated a model of accelerated renal disease that may provide a better system to study progression to renal failure as well as lead to the identification of genetic variants involved in kidney injury.
Keywords: proteinuria, renal injury, chronic kidney disease, animal model, renal vessels
the prevalence of chronic kidney disease (CKD) in the United States has increased since the late 1980s and affects more than 26 million people (3). This trend is partly due to aging of the population as well as an increased prevalence of hypertension, diabetes, and obesity (3). Blood pressure (BP), in conjunction with environmental and genetic factors, strongly influences the progression of CKD (33, 34). Thus, identification of factors that modulate the onset and progression of kidney disease in the setting of hypertension may lead to the development of more effective therapies for CKD.
Rats are extensively used as a model system to study the pathophysiology and genetics of kidney disease and hypertension because the nature and type of renal injury observed closely mimic the human disease. Furthermore, because CKD is strongly influenced by genetic factors (1, 6, 7, 29) a number of inbred rat strains have been used to examine the role of genetics of CKD (2, 17, 20, 25–27, 30). In particular, the Dahl salt-sensitive (S) rat (4, 5) is a widely studied model of salt-sensitive hypertension but is also considered a useful model of hypertension-related CKD since it develops proteinuria, glomerulosclerosis, interstitial fibrosis, and progressive loss of renal function (11, 31).
Previously, a genetic analysis for proteinuria was performed using a population derived from the S and spontaneously hypertensive rat (SHR) (8, 10). In contrast to the S, the SHR does not develop significant levels of proteinuria or glomerular damage. Therefore, the genetic cross allowed identification of genetic loci that influenced proteinuria on genetic backgrounds (S or SHR) permissive for hypertension, but differing in susceptibility to renal injury. Linkage analysis identified quantitative trait loci (QTL) or regions linked to proteinuria, albuminuria, and renal injury on rat chromosomes 1, 2, 6, 8, 9, 10, 11, 13, and 19 (8). The S genotype was associated elevated proteinuria and renal injury for almost all proteinuria QTL. However, a single locus (located on chromosome 11) was associated with decreased proteinuria and renal injury in the S rat. That is, the S allele was “protective,” while the SHR allele conferred “susceptibility,” despite overall the SHR strain being highly resistant to develop renal injury.
The aim of the present study was to confirm the linkage analysis by generating a congenic strain by replacement of the protective locus on S rat chromosome 11 with the SHR genomic segment conferring susceptibility to renal injury. The new S.SHR(11) congenic model was extensively characterized for BP, proteinuria, histological kidney injury, and renal function. The S.SHR(11) strain demonstrated earlier onset and more severe kidney injury compared with the S rat and may provide an improved model to study progression to renal failure as well as a means to begin substitution mapping and gene identification of the underlying causative genetic variants.
MATERIAL AND METHODS
Animals and Study Design
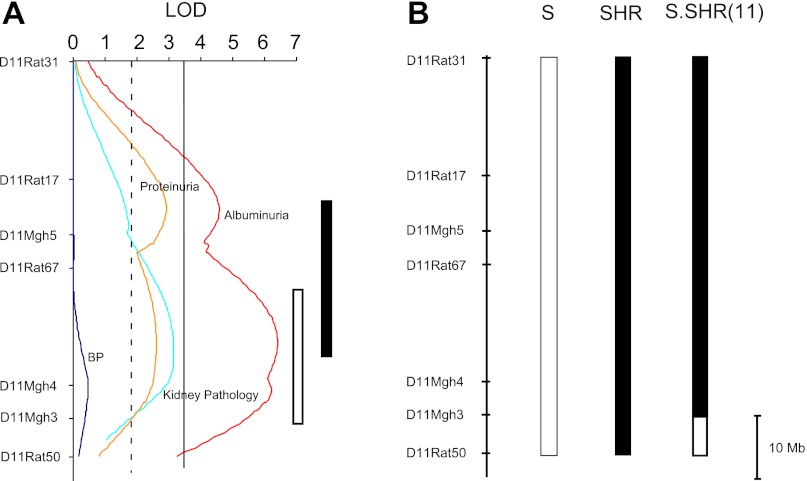
All experiments were approved by the Institutional Animal Care and Use Committee. The S and the SHR inbred strains were maintained in institutional animal facility. The S.SHR(11) congenic strain was developed as previously described using the speed congenic approach (10). The breeding scheme ensured that the mitochondrial genome for S.SHR(11) was derived from the S. The S.SHR(11) congenic was previously studied at a single time point and only for proteinuria (10). Detailed information on the location of the QTL and the extent of SHR donor genome transferred to the S genetic background is presented in Fig. 1. Animals used for this studied were provided either low-salt diet [TD7034, 0.3% NaCl (0.1% Na, 0.2% Cl)] and/or high-salt diet (TD94217, 2.0% NaCl) from Harlan Teklad (Madison, WI).
Fig. 1.
Summary of linkage analysis and congenic strain. A: representative logarithm of the odds (LOD) plots for proteinuria, albuminuria, blood pressure (week 8), and kidney pathology (week 16) from F1(S × SHR) × S population (n = 276) raised on 0.3% NaCl diet (8). The total length of rat chromosome 11 is 88 Mb (http://www.ensembl.org). The linkage map/LOD plot spans ∼78 Mb; ∼5 Mb on either side of 1st and last marker is not accounted for in the original linkage analysis. The horizontal dashed line at LOD = 1.9 is the threshold for suggestive significance, and the solid line at LOD = 3.3 is the threshold for significance. The open bar to the right represents the 95% confidence interval for the quantitative trait loci (QTL) at week 8. The solid bar represents the most probable location of the kidney injury QTL based on peak LOD plots from all time points (week 8, 12, and 16). B: S.SHR(11) congenic strain. The open bar represents the S genome. The black bar designates SHR genome. The open region on the end of the congenic segment represents the recombination interval.
Protocol 1: time course measurement of proteinuria and cardiovascular parameters.
At 4 wk of age, groups of age-matched male S and SHR(11) rats (n = 28 per group) were weaned to the low-salt diet. At 6 wk of age, a subset of each group (n = 14 per group) were placed on the high-salt diet. Animals were studied for renal and cardiovascular traits at 6, 9, and 12 wk of age. Rats were kept in metabolic cages (Lab Products, Seaford, DE) for 24 h with free access to water at week 6, 9, and 12. Total urine protein was determined as previously described (9, 10). No significant correlation was observed between proteinuria and body weight. Thus, it was not necessary to normalize total urine protein for differences in body weight and it is reported as mg/24 h. At week 12, animals were placed under anesthesia for measurement of mean arterial pressure (MAP) (described under protocol 2). The animals were subsequently euthanized. Organ weights were measured. Kidney samples and other tissue were processed for histological examination. Serum samples were obtained from cardiac puncture to measure blood parameters. Measurement of serum creatinine (SCr) and blood urea nitrogen (BUN) was performed using an Alfa Wassermann ACE automated chemistry analyzer.
BP was also collected using a telemetry system (Data Sciences International, St. Paul, MN) in a separate group of animals (n = 8, per group). At 7 wk of age, animals were anesthetized using 2–3% isoflurane gas and a transmitter was surgically implanted in each animal as previously described (9). Readings for each BP parameter was collected for a 24 h period (5 min time intervals for 10 s) at 9 and 12 wk of age.
Protocol 2: BP and renal hemodynamic parameters.
At 4 wk of age, groups of age-matched male S (n = 5) and S.SHR(11) (n = 6) rats were weaned to the low-salt diet. At 6 wk of age animals were placed on the high-salt diet (2.0% NaCl; TD7034, Harlan Teklad). At week 12, MAP, renal blood flow (RBF), and glomerular filtration rate (GFR) were measured in each group. Rats were anesthetized with ketamine (30 mg/kg im) and Inactin (50 mg/kg ip) and placed on a heating table to maintain body temperature at 37°C. Cannulas were placed into the femoral artery for measurement of MAP and in the femoral vein for infusion of 2% BSA in a 0.9% NaCl solution at 100 μl/min iv. A catheter was inserted into the left ureter for the collection of urine and FITC-labeled inulin (2 mg/ml; Sigma, St. Louis, MO) for the measurement of GFR. RBF was measured using an ultrasound flow probe (Transonic System, Ithaca, NY) on the renal artery. After a 30 min equilibration period, urine and plasma samples were collected for 30 min. At the end of each experiment, the left kidney was removed and weighed and the concentration of inulin in urine and plasma samples was determined using a plate reader fluorometer (16).
Protocol 3: survival study and assessment at advanced age.
For low-salt survival study (0.3% NaCl), male S, SHR, and S.SHR(11) rats (n = 12 per group) were maintained on the low-salt diet from weaning. For the high-salt study (2% NaCl), male S, S.SHR(11), and SHR rats (n = 12 per group) were raised on low-salt diet until 6 wk of age and placed on high-salt diet (n = 12, per group) for duration of the study. Animals were examined twice daily and were euthanized if they displayed signs of distress (e.g., shaking, lethargy, persistent recumbency, or tachypnea). An additional group of animals (n = 20 per group) were raised to evaluate cardiovascular and renal parameters at the point that approximated median survival (50% mortality). At ∼40 wk of age, animals that were still alive [S, n = 12 and S.SHR(11), n = 11] were evaluated for BP, proteinuria, renal function, and degree of renal injury. Urine was collected for 24 h for determination of proteinuria. Rats were subsequently anesthetized with isoflurane for placement of catheter into the femoral artery. The catheter was tunneled subcutaneously under the skin and emerged at the base of the neck. After 24 h, MAP was measured in conscious freely moving rats for 10–15 min. The animals were then euthanized for collection of serum and tissue.
Protocol 4: renal ischemia-reperfusion injury.
Groups of S and S.SHR(11) rats were exposed to renal ischemia-reperfusion (IR) injury at week 8. Briefly, rats were anesthetized with ketamine and were placed on a heated surgical table to maintain body temperature at 37°C. Bilateral renal ischemia was induced by clamping the renal pedicles for 30 min as previously described (24). The clamps were removed, and the rats were allowed to recover for 24 h at which time the rats were euthanized under isoflurane to harvest tissue for histology and collection of blood for measurement of SCr.
Histological Assessment
Tissue was fixed in zinc formalin and embedded in paraffin, cut into 4 μm sections, and stained with hematoxylin and eosin (H&E) and Masson's trichrome. Two central longitudinal sections from each kidney were examined under light microscopy. Glomerular injury was assessed using a semiquantitative scoring system in 20 randomly selected images (H&E at ×40) as previously described (21, 28). Tubulointerstitial injury was determined by evaluation of slides stained with Masson's trichrome to quantify the percent fibrosis (blue staining) compared with background in 20 randomly selected images as previously described (21, 28). For protocol 3, tubulointerstitial injury was determined by evaluation of slides stained with Masson's trichrome. Whole kidney sections were scanned at ×40 magnification using a Hamamatsu NanoZoomer HT (Hamamatsu Photonics KK, Hamamatsu City, Japan) digital scanner. The areas of medulla and cortex of the kidney sections were marked manually based on the absence or presence of glomeruli and type of tubules present. The percentage of connective tissue was obtained by dividing the area of bright blue stained tissue by total area of tissue (red and purple colors) using Visiomorph and Microimager software (Visiopharm). Vessel media area was calculated by measuring the outer circumference of the vessel minus the inner circumference of the lumen (20 random images at ×40 per animal)(28). Immunostaining was performed on unstained cut sections and processed for detection by DAB (Ultravision LPValue Detection System, Thermo Scientific) using 1° antibodies directed at α-smooth muscle actin (SMA) or CD-68/ED-1 (Santa Cruz Biotechnology). Slides processed for CD-68 were counterstained with methyl green. Images were captured using Nikon 55i microscope with DS-Fi1 5-Meg Color C digital camera (Nikon, Melville, NY) and analyzed using Nis-Elements image analysis software (version 3.03, Nikon Instruments).
Statistical Analysis
S and S.SHR(11) phenotypic data (proteinuria, BP, etc.) were evaluated by t-test (SPSS, Chicago, IL) and P < 0.05 was considered to be statistically significant or by one-analysis of variance followed by correction for multiple comparisons where noted. All data are presented as means ± SE. Survival was evaluated by the Kaplan-Meier method (Prism 5; GraphPad, La Jolla, CA).
RESULTS
Proteinuria, Renal Function, and BP
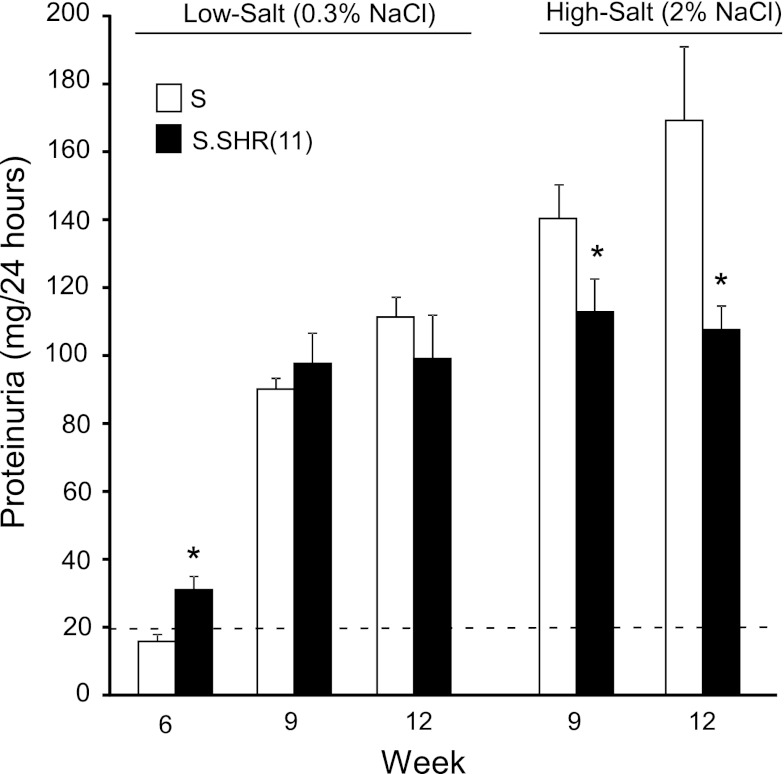
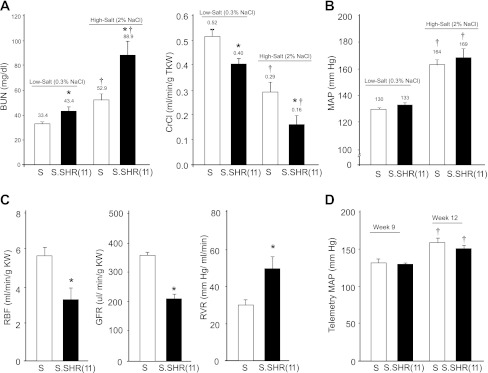
The time course for proteinuria in each group of rats is presented in Fig. 2. On a low-salt diet there was a significant increase in proteinuria observed in the S.SHR(11) compared with the S at week 6 (P < 0.001). Proteinuria did not differ between groups at weeks 9 or 12 when the rats were fed a low-salt diet. In contrast, proteinuria was significantly lower in the S.SHR(11) compared with the S at weeks 9 and 12 on a high-salt (2% NaCl) diet (P < 0.01) and did not significantly differ from low-salt animals at the same time points (week 9 and 12). However, measures of renal function were significantly different in the S.SHR(11) compared with the S including BUN and creatinine clearance (CrCl) (Fig. 3A). The difference in renal function was accentuated by exposure to the high-salt diet. Reduced renal function (measured by CrCl) in the S.SHR(11) was confirmed by FITC-inulin measured GFR and RBF measurement on animals raised on high salt (Fig. 3C). GFR was 220 ± 16.1 μl·min−1·g kidney weight−1 in the S.SHR(11) compared with 346 ± 30.1 in the S rat. Renal vascular resistance was significantly elevated in the S.SHR(11) compared with the S.
Fig. 2.
Assessment of proteinuria in S and S.SHR(11) rats raised on either a low (0.3% NaCl) or high-salt (2% NaCl) diet. The dashed line represents the generally accepted threshold of when urine protein excretion is outside the normal range (>20 mg/24 h) (10). Week 6, n = 28 animals per group; week 9 and 12, n = 14 per group. *Significantly different between S and S.SHR(11) at P < 0.05.
Fig. 3.
Cardiovascular and renal parameters in S and S.SHR(11) rats at week 12. Rats were raised on low salt (0.3% NaCl) until 6 wk of age and either continued on low-salt diet or were placed on high salt (2% NaCl) for 6 wk. A: blood urea nitrogen (BUN) and creatinine clearance (CrCl). B: mean arterial pressure (MAP). The average value is denoted above each graph. Parameters were determined using n = 14 per group, except for MAP, n = 8 per group. C: renal hemodynamic parameters: renal blood flow (RBF), glomerular filtration rate (GFR), and renal vascular resistance (RVR) in S and S.SHR(11) rats at week 12 on high salt. D: telemetry-measured blood pressure. For renal hemodynamic parameters, n = 6 (S) and n = 5 [S.SHR(11)]; n = 8 per group for telemetry. *P < 0.05 between groups. †P < 0.05 same strain different age/treatment.
Notably, despite the significant differences in renal function between the S and S.SHR(11), no detectable difference in MAP was observed between strains at week 12 on either low-salt or high-salt diet (Fig. 3B). This was consistent with BP measured by telemetry at week 9 and 12 (Fig. 3D). No significant difference in heart weight (data not shown) was observed between the S and S.SHR(11) on either diet, further suggesting that BP load did not differ between the strains. S.SHR(11) animals raised on high salt did exhibit significantly larger kidney weight [13.9 mg/g body weight (BW)] compared with S (12.0 mg/g BW), but no difference was observed on low-salt diet (week 12).
Histological Assessment of Renal Injury
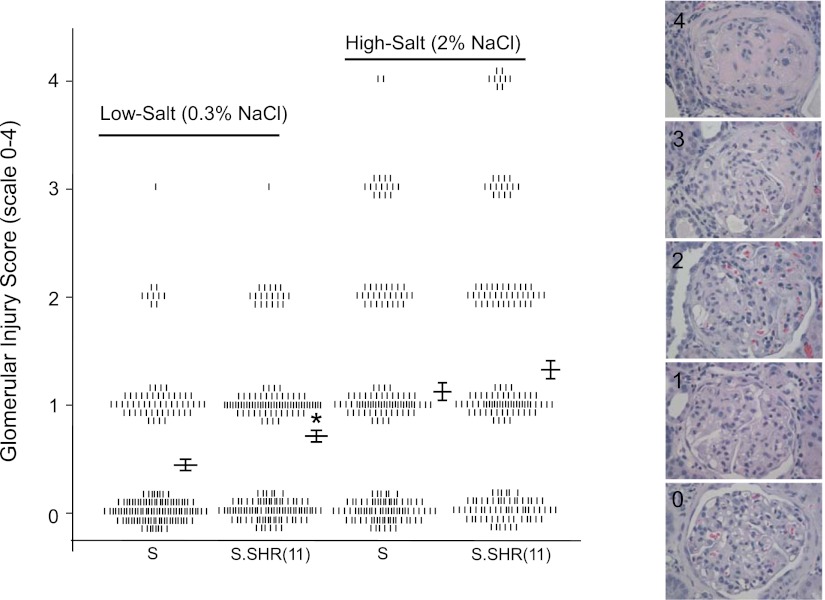
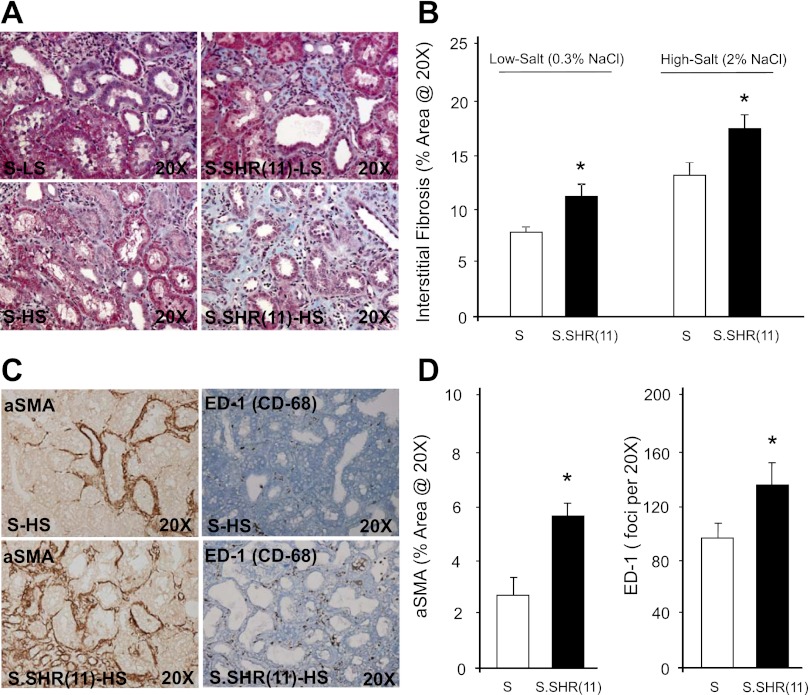
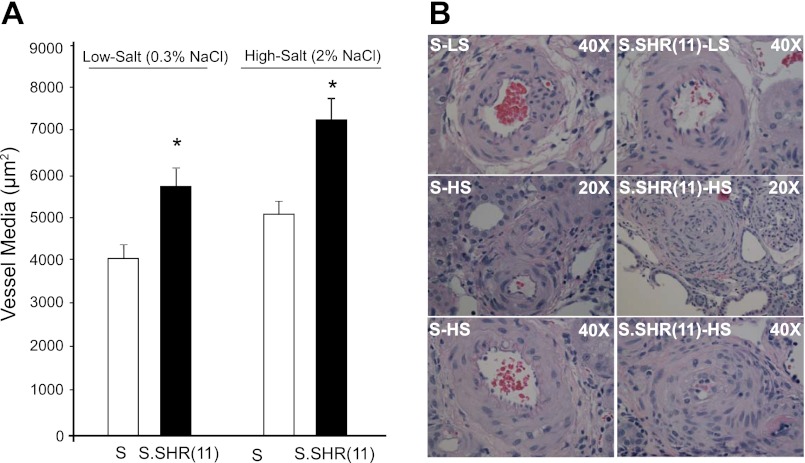
Kidneys from S and S.SHR(11) rats were examined and evaluated for glomerular, interstitial, and vascular damage at week 12 after exposure to either a low-salt or high-salt diet. Representative glomerular images from rats at week 12 are shown in Fig. 4. The degree of glomerular injury was slightly higher in S.SHR(11) compared with S on a low-salt diet. Significant glomerular injury was observed on a high-salt diet, but this injury was similar between strains [S = 1.1 ± 0.14; S.SHR(11) = 1.3 ± 0.14] (Fig. 4). Representative tubulointerstitial images from rats at week 12 are presented in Fig. 5A. The extent of interstitial fibrosis was significantly increased in the S.SHR(11) compared with the S under both low-salt and high-salt conditions. For high salt, the percent fibrosis was 12.9 ± 1.22% compared with 17.1 ± 1.29% in S and S.SHR(11), respectively (Fig. 5B). This was confirmed by increased α-SMA expression and macrophage infiltration in the S.SHR(11) compared with the S (Fig. 5, C and D). Renal arteriolar medial hypertrophy was significantly increased in S.SHR(11) compared with the S under both low-salt (5,702 ± 466 μm2 vs. 4,071 ± 342 μm2, P < 0.01) and high-salt conditions (6,921 ± 473 μm2 vs. 5,129 ± 243 μm2, P < 0.001) (Fig. 6). No difference in arteriolar media area was observed between strains in other organs, including the heart and brain (data not shown).
Fig. 4.
Histological evaluation of glomerular injury in S and S.SHR(11) rats at week 12. Individual glomeruli were scored on a scale from 0 (normal) to 4 (global sclerosis). Glomerular scores are denoted by individual tick marks. Representative images [×40 hematoxylin and eosin (H&E)] of individual glomeruli demonstrating normal to significant abnormalities including mesangial expansion, glomerulosclerosis, etc. are shown in the right panel. A minimum of 20 randomly selected glomeruli (H&E at ×40) were assessed for degree of glomerulosclerosis and mesangial expansion for each animal (n = 8, per group). *Significantly different between S and S.SHR(11) at P < 0.05.
Fig. 5.
Histological evaluation of tubulointerstitial injury in S and S.SHR(11) rats at week 12. A: representative light microscopy images of tubule-interstitial region (×20 Masson's trichrome). B: percent interstitial fibrosis kidney. Images demonstrate mild to severe tubule atrophy, immune cell infiltration, and/or fibrosis. C: representative immunohistochemical images of α-smooth muscle actin (α-SMA) and CD-68 (ED-1; macrophage) for S and S.SHR(11) raised on high salt. D: percent α-SMA staining and quantitation of ED-1 foci. The S.SHR(11) demonstrated significantly greater tubulointerstitial injury including increased fibrosis, α-SMA staining, and macrophage infiltration compared with the S. n = 8 per group. *Significantly different strains at P < 0.05.
Fig. 6.
Morphometric analysis of vessel medial hypertrophy injury in S and S.SHR(11) rats at week 12. A: measurement of vessel medial hypertrophy between S and S.SHR(11) on low and high salt (20 random images per animal, n = 8 animals per group). B: representative light microscopy images of renal vessels (×40 H&E). Vessel media (μm2) was calculated by measuring the outer circumference of the vessel minus the inner circumference of the lumen. *Significantly different between S and S.SHR(11) at P < 0.05.
Survival and Assessment of Renal Injury at Advanced Age
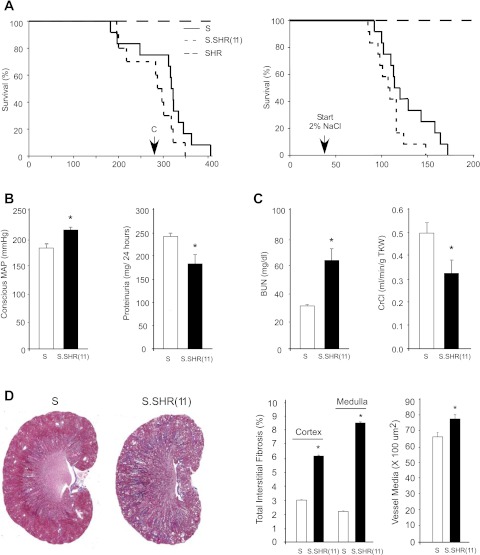
Survival of S, S.SHR(11), and SHR rats was assessed following exposure to either a low-salt or high-salt diet. Animals were raised on low salt until 6 wk of age and then maintained on a low-salt diet or switched to a high-salt diet for duration of experiment. On a low-salt diet, the median survival of S.SHR(11) rats (292 days) was less, but not significantly different from the median survival (320 days) for S rats (Fig. 7A). On a high-salt diet, the median survival of S.SHR(11) rats was 108 days and did not significantly differ from the median survival of the S rats (117 days) (Fig. 7A). No SHR rats died during the course of the experiment on either low or high salt. In a separate study, conscious BP was significantly different in S.SHR(11) compared with S (215 ± 6.6 vs. 183 ± 5.9, P < 0.01) as was proteinuria for rats that lived through the median survival period (∼40 wk) (Fig. 7B). Renal function, as measured by BUN and CrCl was significantly decreased in S.SHR(11) compared with the S (Fig. 7C). Renal interstitial fibrosis in both the cortex and medulla was significantly greater in the S.SHR(11). Most interesting was that medullary fibrosis was greater than that observed in the cortex of S.SHR(11) kidney, whereas fibrosis in the medulla was less than observed in the cortex of the S (Fig. 7D). Renal vessel hypertrophy was significantly increased at this time point in the S.SHR(11) compared with the S, consistent with studies in younger animals (Fig. 6).
Fig. 7.
Survival, blood pressure, and assessment of renal injury in S and S.SHR(11) at advanced age. A: survival study for animals raised on low salt (0.3% NaCl) or high salt (2% NaCl). Animals were raised on low salt until 6 wk of age and then placed on 2% NaCl diet for duration of experiment. B: blood pressure and proteinuria at ∼40 wk of age (∼50% mortality). See material and methods for details. This time point is denoted by “C” on survival curve in A. C: renal function measures. D: representative full-section kidney images of S and S.SHR(11) raised on low salt and morphometric analysis of fibrosis and vessel wall thickening. Percent kidney interstitial fibrosis in cortex and medulla measured on whole kidney sections (see material and methods for details). The percentage of fibrotic tissue was obtained by dividing the area of bright blue-stained tissue by total area. The S.SHR(11) exhibits greater tubular dilation and fibrosis compared with the S at the same age. For survival study, n = 12 per group were evaluated. Proteinuria and renal parameters measured in n = 12 (S) and n = 11 [S.SHR(11)]. Blood pressure was measured in conscious freely moving rats for 10–15 min prior to euthanasia. *Significantly different between S and S.SHR(11) at P < 0.05.
Renal IR Injury in CKD
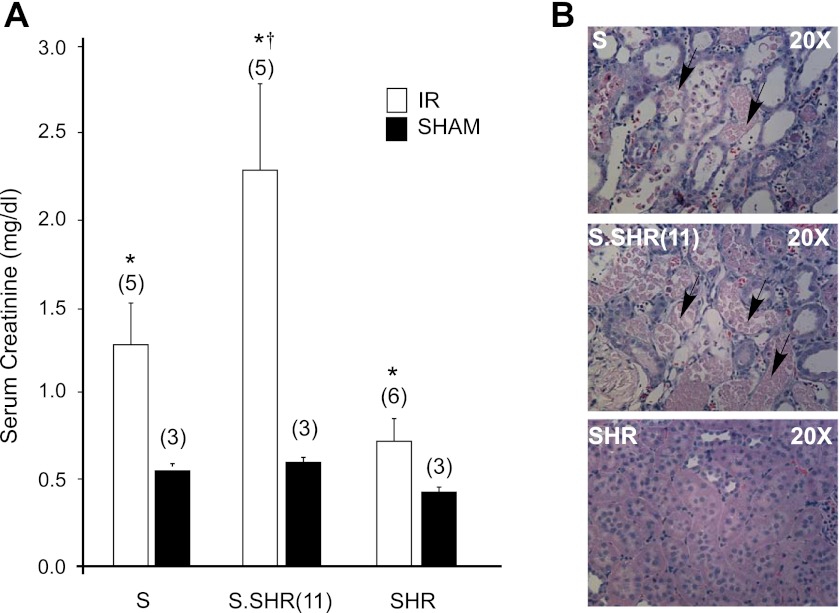
Given the increased susceptibility of the S.SHR(11) to both medullary and vascular injury, the effect of IR injury was evaluated between strains (Fig. 8). The S rat exhibited a significant increase in SCr (1.3 ± 0.24 mg/dl) compared with the SHR (0.7 ± 0.13 mg/dl), but S.SHR(11) animals exhibited a twofold increase (2.3 ± 0.4 mg/dl) over the S. These findings were supported by the substantial increase in tubular necrosis observed in the S.SHR(11) compared with the S and SHR (Fig. 8). Little to no tubular injury was observed in the SHR.
Fig. 8.
Renal ischemia-reperfusion (IR) injury in S, S.SHR(11), and SHR rats at week 8. A: measurement of serum creatinine. B: representative light microscopy images (×40 H&E) of tubule-interstitial region. Necrotic tubules are denoted by an arrow. The S.SHR(11) animals exhibit significantly greater renal injury. Numbers in parenthesis indicate number of rats studied in each group. *P < 0.05 between IR and Sham for each group. †P < 0.05 between S.SHR(11) and either S or SHR.
DISCUSSION
The S rat exhibits progressive kidney injury that is characterized by significant glomerulosclerosis, interstitial fibrosis, and progressive loss of renal function (9, 10). The progression of kidney injury is similar to that exhibited by humans with CKD, especially those who also exhibit hypertension. This underscores the significance of the current work because a model that more rapidly develops kidney injury, in the context of hypertension, could be of great benefit to understanding both the pathophysiology and genetic causes of kidney disease.
The S.SHR(11) strain demonstrated an early increase in proteinuria (week 6), which was not evident at later time points (week 9 or 12) on low or high salt compared with the S. Taken alone, this suggests that there was no significant acceleration of kidney injury in the S.SHR(11) model. However, upon examination of renal function measurements (e.g., SCr, BUN, and GFR) it became evident that the S.SHR(11) strain exhibited a marked reduction in GFR. Therefore, it is likely that progression of proteinuria in the S.SHR(11) was attenuated by the substantial decrease in GFR, especially after exposure to a high-salt diet. Clinically, in a variety of kidney diseases the magnitude of proteinuria is usually strongly associated with an increase in the risk of progression of renal failure (32). Several studies have demonstrated that clinical outcomes are worse in patients with early heavy proteinuria compared with those with moderately reduced GFR and no proteinuria (12). The results of the current study are consistent with this clinical finding, where an early increase in proteinuria was associated with reduced renal function in the S.SHR(11) later in life.
Histologically, the S.SHR(11) demonstrated substantially more interstitial fibrosis and vascular hypertrophy, with little difference in glomerular pathology. This suggests that the significant decline in renal function exhibited by the S.SHR(11), and underlying genetic cause is likely not associated with glomerular injury. The evaluation of cortical and medullary fibrosis by assessment of whole kidney sections demonstrated that medullary fibrosis was more severe than that observed in cortex. This indicates that the causative genetic event that promotes renal injury and reduced renal function may originate in the renal medulla. The role of medullary injury in the initiation and progression of kidney injury has been examined in several models (14, 19, 22). These studies suggest that reduced blood flow (ischemia), angiotensin II, and/or oxidative stress (among others) may play an important role in kidney injury and ultimately lead to renal failure. The vascular difference, which appears to be kidney specific, may also be important to the decline in kidney function in the model. It has been suggested that the damage to the renal microvasculature, along with deterioration of the angiogenic response, may constitute an early stage in progressive renal injury (15). While the precise molecular mechanism is not clear, the severity of damage in the medulla and the renal vasculature in the S.SHR(11) may play an important causative role in declining renal function.
Renal IR injury is a frequent cause of acute kidney injury (AKI) and contributes to significant increases in morbidity and mortality in hospitalized patients (35). Previous studies have shown that pre-existing kidney disease is an important predictor of AKI (13, 18). Reduced medullary blood flow and increased renal vascular resistance are critical factors in the pathogenesis of AKI due to IR injury (23), both of which are exhibited in the S.SHR(11) compared with the S. The IR injury significantly impacted renal function (based on SCr) and the development of more severe tubular necrosis in the S.SHR(11). These findings suggest that reduced medullary blood flow may be a key pathophysiological determinant of the increased risk of AKI in patients with CKD.
Despite the clear increase in kidney injury and reduced kidney function in the S.SHR(11), there was only a trend for increased mortality in the S.SHR(11). This is not particularly surprising since the S rat already exhibits significant hypertension, which leads to other cardiovascular complications and death from heart failure and stroke in addition to renal failure. BP measured on animals at median survival was observed to be significantly elevated in the S.SHR(11), whereas BP was similar to the S at week 12 irrespective of salt-loading. This suggests that increased BP is not the driving factor that promotes increased kidney injury and more rapid decline in kidney function observed in the S.SHR(11). However, hypertension developed as a consequence of the progressive injury and significant decline in kidney function (∼50% reduced renal function) exhibited by the S.SHR(11).
The precise mechanism of injury in this model remains to be determined, but the data do suggest that the mode of injury could be related to the predisposition of the S.SHR(11) to develop medullary and/or vascular injury in the presence of similar BP. The S.SHR(11) strain may have some defect in myogenic response that promotes pressure-related injury even in the presence of similar systemic BP. This could also explain the increased susceptibility to IR injury. The elucidation of the mechanism will require further studies and will be strengthened by identification of the underlying genetic cause. The genomic substitution from the SHR rat encompasses a large part of the entire chromosome, and there are a large number of genes or genetic variants that could explain the increased susceptibility of the S.SHR(11). The 95% confidence interval of the QTL spans ∼15 Mb and contains >100 genes. Thus, the identification of the actual disease causing variants will require additional substitution analysis (i.e., congenic substrains), gene expression studies, genome sequencing, and functional analysis of gene variants.
In conclusion, the S.SHR(11) congenic strain exhibits accelerated kidney disease compared with the already susceptible S rat and is characterized by increased tubulointerstitial fibrosis, vascular hypertrophy, and a more rapid decline in kidney function. The detailed physiological analysis of the S.SHR(11) congenic has provided a potential mechanism associated with the QTL as well as a strong basis from which to pursue additional studies to identify the actual genetic variants that underlie the QTL. Aside from utility of the congenic strain for genetic studies, it could also provide a novel system to study progression of renal failure because it rapidly develops severe kidney injury (in the context of hypertension).
GRANTS
M. R. Garrett is supported by National Institutes of Health (NIH) Grant HL-094446 and Robert M. Hearin Foundation. K. R. Regner is supported by NIH Grant DK-090123 and funds from the Clinical and Translational Science Institute of Southeastern Wisconsin. A. C. Harmon is supported by NIH Grant 1T32HL-105324.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.R.R., A.C.H., J.M.W., C.S., A.J., P.B.K., A.L.-G., S.M.W., and M.R.G. performed experiments; K.R.R., A.C.H., J.M.W., C.S., A.J., P.B.K., A.L.-G., S.M.W., and M.R.G. analyzed data; K.R.R., A.C.H., and M.R.G. interpreted results of experiments; K.R.R. and M.R.G. edited and revised manuscript; K.R.R., A.C.H., J.M.W., C.S., A.J., P.B.K., A.L.-G., S.M.W., and M.R.G. approved final version of manuscript; M.R.G. conception and design of research; M.R.G. prepared figures; M.R.G. drafted manuscript.
ACKNOWLEDGMENTS
The authors acknowledge Jonathan Lee for animal surgeries and blood pressure measurements.
REFERENCES
- 1. Bergman S, Key BO, Kirk KA, Warnock DG, Rostant SG. Kidney disease in the first-degree relatives of African-Americans with hypertensive end-stage renal disease. Am J Kidney Dis 27: 341–346, 1996 [DOI] [PubMed] [Google Scholar]
- 2. Brown DM, Provoost AP, Daly MJ, Lander ES, Jacob HJ. Renal disease susceptibility and hypertension are under independent genetic control in the fawn-hooded rat. Nat Genet 12: 44–51, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. Jama 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Dahl LK, Heine M, Tassinari L. Effects of chronic excess salt ingestion: evidence that genetic factors play an important role in the susceptibility to experimental hypertension. J Exp Med 115: 1173–1190, 1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dahl LK, Heine M, Tassinari L. Role of genetic factors in susceptibility to experimental hypertension due to chronic excess salt ingestion. Nature 194: 480–482, 1962 [DOI] [PubMed] [Google Scholar]
- 6. Ferguson R, Grim CE, Opgenorth TJ. A familial risk of chronic renal failure among blacks on dialysis? J Clin Epidemiol 41: 1189–1196, 1988 [DOI] [PubMed] [Google Scholar]
- 7. Freedman BI, Spray BJ, Tuttle AB, Buckalew VM., Jr The familial risk of end-stage renal disease in African Americans. Am J Kidney Dis 21: 387–393, 1993 [DOI] [PubMed] [Google Scholar]
- 8. Garrett MR, Dene H, Rapp JP. Time-course genetic analysis of albuminuria in Dahl salt-sensitive rats on low-salt diet. J Am Soc Nephrol 14: 1175–1187, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Garrett MR, Gunning WT, Radecki T, Richard A. Dissection of a genetic locus influencing renal function in the rat and its concordance with kidney disease loci on human chromosome 1q21. Physiol Genomics 30: 322–334, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garrett MR, Joe B, Yerga-Woolwine S. Genetic linkage of urinary albumin excretion in Dahl salt-sensitive rats: influence of dietary salt and confirmation using congenic strains. Physiol Genomics 25: 39–49, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Hampton JA, Bernardo DA, Khan NA, Lacher DA, Rapp JP, Gohara AF, Goldblatt PJ. Morphometric evaluation of the renal arterial system of Dahl salt-sensitive and salt-resistant rats on a high salt diet. Lab Invest 60: 839–846, 1989 [PubMed] [Google Scholar]
- 12. Hemmelgarn BR, Manns BJ, Lloyd A, James MT, Klarenbach S, Quinn RR, Wiebe N, Tonelli M. Relation between kidney function, proteinuria, and adverse outcomes. JAMA 303: 423–429, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Hsu CY, Ordonez JD, Chertow GM, Fan D, McCulloch CE, Go AS. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int 74: 101–107, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kriegel AJ, Liu Y, Cohen B, Usa K, Liu Y, Liang M. MiR-382 targeting of kallikrein 5 contributes to renal inner medullary interstitial fibrosis. Physiol Genomics 44: 259–267, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lerman LO, Chade AR. Angiogenesis in the kidney: a new therapeutic target? Curr Opin Nephrol Hypertens 18: 160–165, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lorenz JN, Gruenstein E. A simple, nonradioactive method for evaluating single-nephron filtration rate using FITC-inulin. Am J Physiol Renal Physiol 276: F172–F177, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Matsuyama M, Ogiu T, Kontani K, Yamada C, Kawai M, Hiai H, Nakamura T, Shimizu F, Toyokawa T, Kinoshita Y. Genetic regulation of the development of glomerular sclerotic lesions in the BUF/Mna rat. Nephron 54: 334–337, 1990 [DOI] [PubMed] [Google Scholar]
- 18. Mittalhenkle A, Stehman-Breen CO, Shlipak MG, Fried LF, Katz R, Young BA, Seliger S, Gillen D, Newman AB, Psaty BM, Siscovick D. Cardiovascular risk factors and incident acute renal failure in older adults: the Cardiovascular Health Study. Clin J Am Soc Nephrol 3: 450–456, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mori T, O'Connor PM, Abe M, Cowley AW. Enhanced superoxide production in renal outer medulla of Dahl salt-sensitive rats reduces nitric oxide tubular-vascular cross-talk. Hypertension 49: 1336–1341, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Murayama S, Yagyu S, Higo K, Ye C, Mizuno T, Oyabu A, Ito M, Morita H, Maeda K, Serikawa T, Matsuyama M. A genetic locus susceptible to the overt proteinuria in BUF/Mna rat. Mamm Genome 9: 886–888, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Packard M, Saad Y, Gunning WT, Gupta S, Shapiro J, Garrett MR. Investigating the effect of genetic background on proteinuria and renal injury using two hypertensive strains. Am J Physiol Renal Physiol 296: F839–F846, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Polichnowski AJ, Lu L, Cowley AW. Renal injury in angiotensin II+l-NAME-induced hypertensive rats is independent of elevated blood pressure. Am J Physiol Renal Physiol 300: F1008–F1016, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Regner KR, Roman RJ. Role of medullary blood flow in the pathogenesis of renal ischemia-reperfusion injury. Curr Opin Nephrol Hypertens 21: 33–38, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Regner KR, Zuk A, Van Why SK, Shames BD, Ryan RP, Falck JR, Manthati VL, McMullen ME, Ledbetter SR, Roman RJ. Protective effect of 20-HETE analogues in experimental renal ischemia reperfusion injury. Kidney Int 75: 511–517, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schulz A, Litfin A, Kossmehl P, Kreutz R. Genetic dissection of increased urinary albumin excretion in the Munich Wistar Frömter rat. J Am Soc Nephrol 13: 2706–2714, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Schulz A, Standke D, Kovacevic L, Mostler M, Kossmehl P, Stoll M, Kreutz R. A major gene locus links early onset albuminuria with renal interstitial fibrosis in the MWF rat with polygenetic albuminuria. J Am Soc Nephrol 14: 3081–3089, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Shiozawa M, Provoost AP, van Dokkum RP, Majewski RR, Jacob HJ. Evidence of gene-gene interactions in the genetic susceptibility to renal impairment after unilateral nephrectomy. J Am Soc Nephrol 11: 2068–2078, 2000 [DOI] [PubMed] [Google Scholar]
- 28. Solberg Woods LC, Stelloh C, Regner KR, Schwabe T, Eisenhauer J, Garrett MR. Heterogeneous stock rats: a new model to study the genetics of renal phenotypes. Am J Physiol Renal Physiol 298: F1484–F1491, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Spray BJ, Atassi NG, Tuttle AB, Freedman BI. Familial risk, age at onset, and cause of end-stage renal disease in white Americans. J Am Soc Nephrol 5: 1806–1810, 1995 [DOI] [PubMed] [Google Scholar]
- 30. Stella P, Cusi D, Duzzi L, Bianchi G. Relations between hypertension and glomerulosclerosis in first-generation hybrid rats of the Milan strains. Child Nephrol Urol 11: 6–9, 1991 [PubMed] [Google Scholar]
- 31. Sterzel RB, Luft FC, Gao Y, Schnermann J, Briggs JP, Ganten D, Waldherr R, Schnabel E, Kriz W. Renal disease and the development of hypertension in salt-sensitive Dahl rats. Kidney Int 33: 1119–1129, 1988 [DOI] [PubMed] [Google Scholar]
- 32. Stevens LA, Greene T, Levey AS. Surrogate end points for clinical trials of kidney disease progression. Clin J Am Soc Nephrol 1: 874–884, 2006 [DOI] [PubMed] [Google Scholar]
- 33. Tylicki L, Rutkowski B, Horl WH. Multifactorial determination of hypertensive nephroangiosclerosis. Kidney Blood Press Res 25: 341–353, 2002 [DOI] [PubMed] [Google Scholar]
- 34. USRDS United States Renal Data System 2003 Annual Data Report. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Disease, 2005 [Google Scholar]
- 35. Waikar SS, Liu KD, Chertow GM. Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol 3: 844–861, 2008 [DOI] [PubMed] [Google Scholar]