Abstract
Acid aspiration, a common cause of acute lung injury, leads to alveolar edema. Increase in lung vascular permeability underlies this pathology. To define mechanisms, isolated rat lungs were perfused with autologous blood. Hydrochloric acid and rhodamine-dextran 70 kDa (RDx70) were coinstilled into an alveolus by micropuncture. RDx70 fluorescence was used to establish the spatial distribution of acid. Subsequently, FITC-dextran 20 kDa (FDx20) was infused into microvessels for 60 min followed by a 10-min HEPES-buffered saline wash. During the infusion, FITC fluorescence changes were recorded to quantify the ratio of peak to postwash fluorescence. The ratio, termed normalized fluorescence, was low for acid compared with buffer instillation both in microvessels abutting acid-treated alveoli and those located more than 700 μm away. In contrast, the normalized fluorescence was similar to buffer controls when a higher molecular weight tracer (FITC-dextran 70 kDa) was infused instead of FDx20, suggesting that normalized FDx20 fluorescence faithfully represented microvascular permeability. Inhibiting endothelial connexin43 (Cx43) gap junction communication with Gap27 blunted the acid-induced reduction in normalized fluorescence, although scrambled Gap27 did not have any effect. The blunting was evident not only in microvessels away from the site of injury, but also in those abutting directly injured alveoli. Thus the new fluorescence-based method reveals that acid increases microvascular permeability both at acid-instilled and away sites. Inhibiting endothelial Cx43 blocked the permeability increase even at the direct injury sites. These data indicate for the first time that Cx43-dependent mechanisms mediate acid-induced increases in microvascular permeability. Cx43 may be a therapeutic target in acid injury.
Keywords: acid injury, blood-perfused lung, FITC-dextran, fluorescence, gap junctions
acute lung injury and acute respiratory distress syndrome continue to extract a high mortality rate (18). Aspiration of acid, a common cause of acute lung injury, initiates vascular permeability increases and alveolar edema, resulting in compromised pulmonary gas exchange. Direct determinations of acid-induced increases in microvascular permeability and relevant mechanisms remain inadequate.
Recent studies suggest that intercellular gap junctions may play a role in pulmonary vascular responses. Endothelial connexin43 (Cx43)-containing gap junctions mediate the spatial spread of proinflammatory responses in pulmonary microvessels (21). It was reported recently that intrabronchial instillation of acid into a small region of the lung induced spatially extensive lung injury, as determined by increases in lung water (20). Lung water increases at the remote sites, but not at the acid-instilled region, were blunted by pretreating microvessels with the gap junctional blocker glycyrrhetinic acid. However, pretreating alveolar epithelial gap junctions with glycyrrhetinic acid had no effect. Thus vascular gap junctions may serve as conduits for the spatial expansion of acid injury. However, the specific role of Cx43 gap junctions in this expansion remains unclear.
It has been recently reported that conditional deletion of endothelial-specific Cx43 in combination with global deletion of Cx40 disrupts alveolocapillary septa and causes progressive hemorrhage in lungs (4, 15). However, in contrast, endothelial-specific deletion of Cx43 blunts pulmonary vascular permeability increases induced by thrombin perfusion (21). Although it emerges from these reports that Cx43 gap junctions may be relevant in vascular permeability increases, the nature of this role remains unclear. Moreover, it remains undefined whether Cx43 gap junctions play a role during the more extensive vascular permeability increases that accompany acid injury.
Although current strategies for the determination of changes in pulmonary vascular permeability, such as gravimetric methods, or postexperiment quantification of fluorophore-labeled or radiolabeled probe extravasation, generate valuable global data, methods for direct and dynamic elucidation of microvessel-specific mechanisms remain limited. In this regard, a novel fluorescence-based method was developed and characterized to determine permeability changes specifically in lung microvessels. By this method, changes in fluid flux across the microvascular barrier were determined in response to a focal alveolar acid instillation by using an isolated blood-perfused rat lung model. The data reveal a novel role for endothelial Cx43-containing gap junctions as mediators of microvascular permeability at sites of direct acid injury.
METHODS
Isolated blood perfused lung preparation.
Anesthetized male Sprague-Dawley rats weighing 250–400 g were used to prepare isolated blood-perfused lungs as reported (22). Briefly, lungs were excised and continuously pump perfused with autologous blood at 37°C. Perfusion rate was maintained at 12 ml/min. The pulmonary artery and left atrial pressures were constant at 10 and 3 cmH2O, respectively. Lungs were held at a constant inflation pressure of 5 cmH2O. Animals were maintained by the University of Tennessee Lab Animal Care Unit and had free access to food and water. For experiments, animals were used as per protocols approved by the Institutional Animal Care and Use Committee of the University of Tennessee Health Science Center.
Agents and fluorophores.
Hydrochloric acid (HCl) was from Fisher Scientific (Pittsburgh, PA). HCl concentrations used in these experiments were 0.1, 0.05, and 0.025 N. The gap junction inhibitor 18α-glycyrrhetinic acid (GA; 5 μM and 20 μM) was from Sigma-Aldrich (St. Louis, MO). Gap peptide (Gap27; 190 μM) that inhibits Cx43-dependent gap junction communication and scrambled gap peptide (sc-Gap27; 190 μM) were used as reported (21) and were custom generated by Genemed Synthesis (San Antonio, TX). FITC-dextran 10 kDa (FDx10; 0.5 mg/ml), FITC-dextran 20 kDa (FDx20; 0.5 mg/ml), FITC-dextran 70 kDa (FDx70; 0.5 mg/ml), and rhodamine-dextran 70 kDa (RDx70; 0.5 mg/ml) were from Sigma.
Experiment procedure.
A PE-10 (Becton-Dickinson, Sparks, MD) catheter was advanced through the left atrial cannula till it met resistance. HEPES-buffered Ringer's solution (HBS; pH = 7.4) was infused through the microcatheter to establish blood-free conditions in microvessels of a small region of the lung, as previously reported (22). The microcatheter was also used to infuse agents and fluorophores into the cleared microvessels. HBS was continuously infused through the catheter, except where stated. By micropuncture, an oil drop was instilled into an alveolus within the cleared region as a marker. Subsequently, in a nearby alveolus, HCl+RDx70 in saline was instilled by micropuncture. During the instillation, RDx70 fluorescence was collected with an Olympus BX61WI microscope (Center Valley, PA) equipped with a ×20 objective (0.5 NA) coupled to ×0.5 camera adapter, a filter wheel (Lambda10–3; Sutter Instruments, Novato, CA), and appropriate interference filters. The fluorescence was captured via an ImagEM CCD camera (Hammamatsu Photonics, Bridgewater, NJ) controlled by the image-processing software Metamorph (Molecular Devices, Sunnyvale, CA). The captured images were used to determine the spatial distribution of the instilled acid. In some experiments, HCl was mixed with FDx10 and then instilled. The spatial distribution of the instilled acid was then determined by capturing FDx10 fluorescence. Immediately following the instillation, the HBS infusion into microvessels was stopped and FDx20 infusion started. Changes in microvessel FITC fluorescence were captured at defined intervals using appropriate interference filters (excitation 495 nm and emission 510 nm). After 1 h, FDx20 infusion was stopped and HBS infusion was resumed to wash off the microvascular FDx20. At the end of the experiment, the catheter was withdrawn and blood flow was restored to the region. All data were analyzed with Metamorph.
Extended distance and expanded duration experiments.
To establish responses in vessels located at longer distances from the site of acid instillation, the lung was positioned accordingly and the FDx20 infusion was initiated. The FDx20 fluorescence in the distant vessels were captured and recorded. An image of the acid instilled site and the adjoining vessels was also captured to facilitate the distance measurements. To quantify the decay of FDx20 fluorescence by HBS wash over a longer duration, HBS infusion was maintained up to 30 min after FDx20 infusion was stopped.
Inhibitor treatments.
To inhibit endothelial gap junction communication, the inhibitors were infused into microvessels prior to alveolar acid instillation. The pretreatment duration for GA was 10 min, whereas that for Gap27 was 1 h, as reported previously (3, 5, 20, 21). Since the inhibitory effect of Gap27 is lost when the peptide is washed off (21), Gap27 infusion was maintained even after acid instillation, by mixing it with FDx20.
Experimental groups.
The various treatment groups in this study are given below with the number of lungs per group in parenthesis: a) 0.1 N HCl instillation (8), b) 0.05 N HCl instillation (4), c) 0.025 N HCl instillation (6), d) saline instillation (8), e) 0.1 N HCl instillation with Gap27 pretreatment (4), f) 0.1 N HCl instillation with scGap27 pretreatment (3), g) 0.1 N HCl instillation with microvessel 5 μM GA pretreatment (6), h) 0.1 N HCl instillation with microvessel 20 μM GA pretreatment (2), i) 0.1 N HCl instillation with alveolar GA pretreatment (2), j) 0.1 N HCl instillation followed by FDx70 infusion instead of FDx20 (4), and k) vascular 0.1 N HCl instillation (3). In addition, the following experiment groups were done to determine responses at longer distances from the acid-instilled site: l) 0.1 N HCl instillation (3), m) 0.05 N HCl instillation (3), and n) saline instillation (3). Furthermore, the following groups of experiments were done to establish the responses at 25 min post-HBS wash: o) 0.1 N HCl instillation (4), p) 0.05 N HCl instillation (3), q) saline instillation (3), r) 0.1 N HCl instillation with Gap27 pretreatment (2), and s) 0.1 N HCl instillation with microvessel 20 μM GA pretreatment (2).
Statistics.
All data are reported as means ± SE. Multiple groups were compared with Kruskal-Wallis one-way ANOVA on ranks followed by pairwise multiple comparisons by Dunn's method.
RESULTS
Focal alveolar acid instillation.
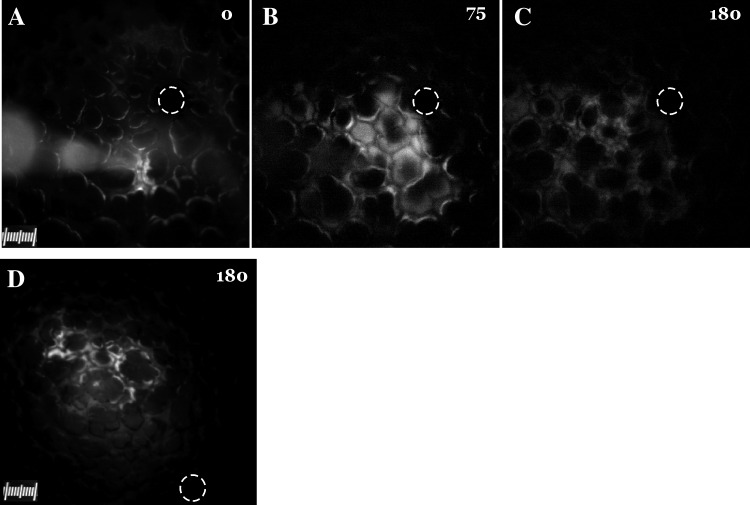
As the major objective of this study was to determine microvessel responses, acid delivery was restricted to alveoli in a focal region of the lung. Toward this, HCl+RDx70 was instilled by micropuncture into a single alveolus over several seconds. The spatial spread of the instilled mixture was then determined by visualizing RDx70 fluorescence. RDx70 fluorescence images captured at different time points during instillation revealed that the spread of acid was restricted to alveoli that surrounded the micropunctured alveolus (Fig. 1, A and B). At the end of the instillation, the micropipette was withdrawn, to allow recovery of both the micropunctured and the surrounding alveoli (Fig. 1C), as previously reported (27). The spatial spread of acid was evident only during instillation of the acid. After the micropipette was withdrawn, no further spread of fluorescence was detectable. The alveolar recovery to the air-filled condition suggested that the target alveoli were not irreversibly damaged by the instillation procedure. Postrecovery, RDx70 fluorescence was still detectable in the alveolar wall liquid (AWL) of the instilled alveoli (Fig. 1C), which further facilitated identification of the instilled region.
Fig. 1.
Alveolar acid instillation in an isolated blood-perfused rat lung. A–C: images show instillation of rhodamine-dextran (RDx) + 0.05 N HCl into alveoli at start (0 s; A) and end (75 s; B) and after alveolar recovery from instillation (180 s; C). The pipette tip and the body of the pipette are visible in A. D: image shows spatial spread of FITC-dextran 10 kDa (FDx10) fluorescence 180 s after instillation of FDx10+0.1 N HCl into alveoli. Circles show site of oil marker instilled into an alveolus.
To exclude a possible effect of tracer size in estimating the extent of acid spread, HCl+FDx10 was instilled into an alveolus. FDx10 fluorescence images reveal that the spread was limited to alveoli surrounding the micropunctured alveolus (Fig. 1D). This suggests that the spread of acid was independent of tracer size. In addition, no further spread of the FDx10 fluorescence was detectable for up to 5 min after the end of acid instillation (data not shown).
Acid augmented the magnitude of FDx20 fluorescence increases and the residual fluorescence.
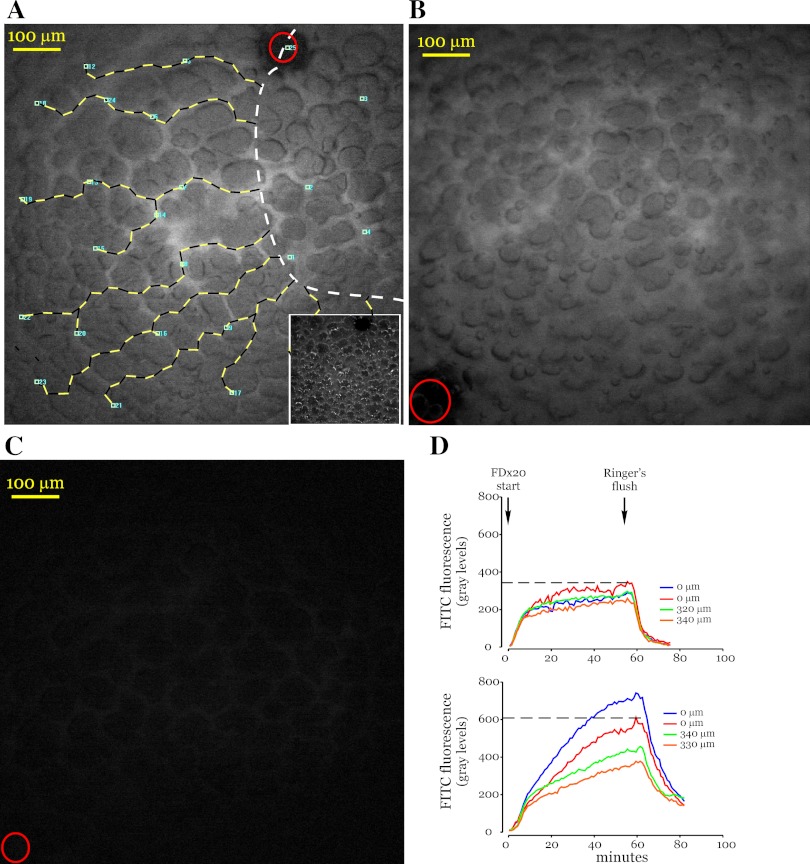
To determine acid-induced changes in microvascular permeability, FDx20 was instilled into microvessels and FDx20 fluorescence increase was continuously imaged. The images revealed that FDx20 fluorescence increased in all vessels, in both acid-instilled and acid-free regions (Fig. 2A), suggesting that the vessels within the field of view were patent. One hour after the start of the FDx20 infusion, the infusion was stopped and HBS was started. This caused the FDx20 fluorescence to decline rapidly, although the residual fluorescence at 10 min after the start of HBS wash was still high (Fig. 2B). In contrast, for control saline instillations, the residual fluorescence was lower (Fig. 2C), suggesting that acid instillation increased residual fluorescence.
Fig. 2.
Vascular infusion of FITC-dextran. A: image shows peak FITC-dextran 20 kDa (FDx20) fluorescence in a lung microvascular network. Small blue squares (5 μm × 5 μm) were randomly placed fluorescence sampling sites on microvessels. Numbers next to the squares identify measurement sites. Microvessels were identified by using both the fluorescence image and the corresponding bright-field image (inset). No microvessel had more than 1 sampling site. The lines with alternating black and yellow segments were drawn along vessels from the outer boundary of the acid-instilled region (dashed white line) to the fluorescence measurement sites and provide distance information. Both black and yellow segments are 20 μm each, with shorter 10-μm segments interspersed as needed. Note that only a part of the acid-instilled region lies within the image. Red circle indicates site of oil drop marker. B and C: images show residual FDx20 fluorescence following 10 min of Ringer's wash in acid- (B) and saline- (C) instilled lungs. Red circles in images show site of oil drop marker. D: graphs show changes in FDx20 fluorescence for multiple measurement sites. The 0-μm tracings are fluorescence data from measurement sites that lie within the acid-instilled regions, and others are from sites at the indicated distances from the outer boundary of the acid-instilled region. Top, saline instillation; bottom, acid instillation. Dashed lines are representative and mark peak fluorescence for red 0-μm tracings only.
To analyze the fluorescence response further, the vascular fluorescence increase was quantified at sampling sites randomly placed in both the acid-instilled and acid-free regions (Fig. 2A). The distance of the sampling sites in the acid-free region was determined from the outer boundary of the acid-instilled region by a line drawn along the vessels (Fig. 2A). At all sampling sites, the fluorescence increase was rapid during the first few minutes of FDx20 infusion and then transitioned to a slower increase during the reminder of the infusion period (Fig. 2D). The slower phase of the increase displayed marked heterogeneity. For saline instillation (top graph in Fig. 2D), the increase was small and similar in both the instilled and free regions and resulted in similar peak fluorescence (dashed lines in Fig. 2D). In contrast, for acid instillation (bottom graph in Fig. 2D), the increase was larger overall, suggesting that acid induces a greater increase in FDx20 fluorescence compared with saline. In addition, for sites located within the acid-instilled region the increase was greater compared with that for sites in the acid-free region. Thus, even within individual lungs, acid induces a site-dependent increase in FDx20 fluorescence and, hence, a heterogeneous distribution of peak fluorescence. About 1 h after the start of the FDx20 infusion, the fluorescence increase appeared to plateau at most sampling sites. Hence this time point was selected to initiate the Ringer's wash (dashed lines in Fig. 2D).
Acid reduced normalized fluorescence in microvessels both at acid-instilled and away sites.
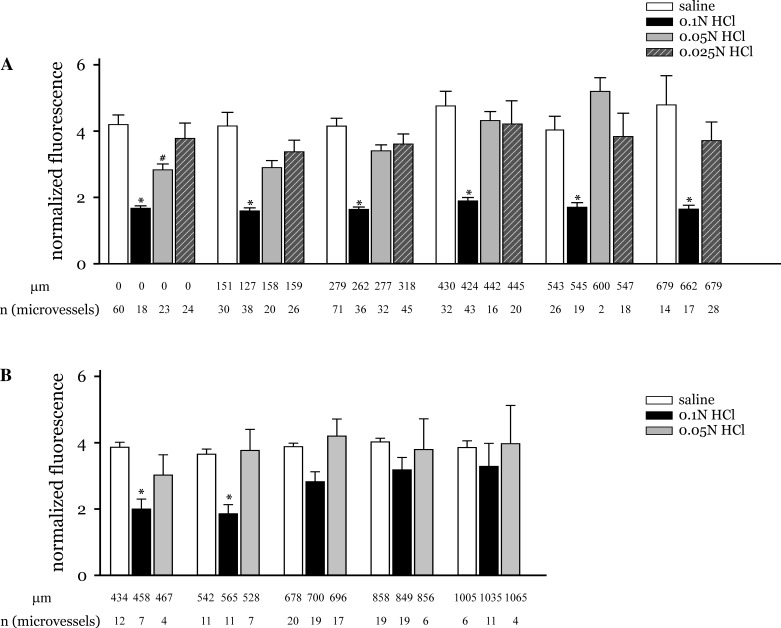
Both peak and residual fluorescence varied among treatments and vessels. Hence, to minimize the heterogeneity, the normalized fluorescence was quantified as the ratio of peak to residual fluorescence at 10 min (Fpeak/Fresidual). Fpeak/Fresidual was significantly different in vessels of acid- and saline-instilled lungs (Fig. 3A). In acid-instilled lungs, Fpeak/Fresidual was low, indicating that the residual fluorescence magnitude declined little from the corresponding peak fluorescence magnitude. In contrast, in saline-instilled lungs, Fpeak/Fresidual was significantly higher, indicating a lower residual compared with peak fluorescence. Acid-induced changes in Fpeak/Fresidual were concentration dependent. Thus, in lungs instilled with 0.025 N HCl, the normalized fluorescence in vessels was similar to that in saline instilled lungs.
Fig. 3.
HCl dose response. A: graph shows changes in normalized fluorescence with distance from the edge of the instilled region for the indicated HCl concentrations and saline. The measurement sites were grouped on the basis of following distance ranges: within instilled region, edge of instilled region-200 μm, 200–400 μm, 400–500 μm, 500–600 μm, and 600–800 μm. Note that the data marked 0 μm are from measurement sites that lie within the instilled region. B: graph shows changes in normalized fluorescence at longer distances from the edge of the instilled region for the indicated HCl concentrations and saline. The measurement sites were grouped on the basis of following distance ranges: 400–500 μm, 500–600 μm, 600–800 μm, 800–1,000 μm, and 1,000–1,200 μm. Mean distance of all measurement sites within a group is shown below each group (in μm). Number of measurement sites for each group is indicated as n. *P < 0.05 compared with saline responses at all distances groups shown.
In addition, the data revealed that the normalized fluorescence in vessels located within the acid-instilled region was similar to that in vessels located up to 700 μm from the boundary of the instilled region (Fig. 3A). Thus acid initiated microvascular responses not only within the region of acid instillation, but also in regions that were acid free.
To determine the spatial extent of the microvascular responses induced by the acid, the normalized fluorescence was determined in vessels located up to 1,200 μm. At these longer distances Fpeak/Fresidual in 0.1 N HCl-instilled lungs was higher and closer to responses in saline-instilled lungs (Fig. 3B). Fpeak/Fresidual in 0.05 N HCl instilled lungs was similar to responses in saline-instilled lungs in these remote vessels.
To determine whether microvessel subtype played a role in the observed responses, normalized fluorescence was calculated separately for capillaries and venules in both acid-instilled and acid-free regions. The data indicated that the responses were similar in both microvessel subtypes (data not shown). Hence in this study the responses from both microvessel subtypes were pooled.
Gap junction inhibition blunted the acid-induced reduction in normalized fluorescence.
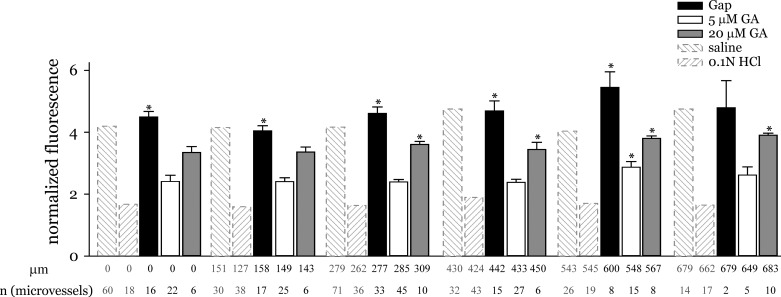
The gap junction inhibitor GA increased Fpeak/Fresidual (Fig. 4), suggesting that GA pretreatment augmented the decrease in residual fluorescence compared with the GA-untreated condition. The inhibition was dose dependent. At lower concentrations, the GA effect was significant only in microvessels located more than 500 μm from the site of acid instillation. At a higher concentration of 20 μM, GA increased Fpeak/Fresidual even in microvessels located more than 200 μm from the acid instillation site, suggesting a concentration dependent effect. Furthermore, in 0.05 N HCl instilled lungs, 5 μM GA increased Fpeak/Fresidual in all regions, including the acid-instilled region, to levels in saline instilled lungs (data not shown). The peptide gap junction inhibitor Gap27 caused a significant increase in Fpeak/Fresidual (Fig. 4). This suggests that with Gap27 pretreatment the residual fluorescence was significantly lower compared with the peak fluorescence. Interestingly, Gap27 treatment increased Fpeak/Fresidual at both the acid-instilled and acid-free regions. This indicates that inhibiting gap junction communication with gap peptides blunted the acid-induced microvascular responses even in microvessels located in the acid-treated region. Moreover, the normalized fluorescence increased even further at distances greater than 500 μm from the edge of the acid-instilled region, although the increase was not significant. Yet the trend suggests that gap peptides protected against the spatial spread of acid responses.
Fig. 4.
Effect of gap junction inhibition. Graph shows the effect of gap junction inhibitors on changes in normalized fluorescence. Microvessels were pretreated with gap junction inhibitors prior to acid instillation as described in methods. Saline and HCl responses without any inhibitor are included for comparison. Measurement sites were grouped as in Fig. 3 legend. Mean distance and number of measurement sites are shown below each group. *P < 0.05 compared with acid responses at all distance groups shown. GA, glycyrrhetinic acid; Gap, gap peptide gap27.
Control experiments show that the roles of Gap27 and FDx20 are specific.
Scrambled Gap27 pretreatment did not result in any significant change in Fpeak/Fresidual from acid-alone responses (Fig. 5). This suggests that the scrambled peptide, lacking only the inhibitory properties, did not affect the acid-induced responses. Thus we interpret that the blunting effect observed with Gap27 was due to its specific inhibition of interendothelial communication via Cx43 gap junctions.
Fig. 5.
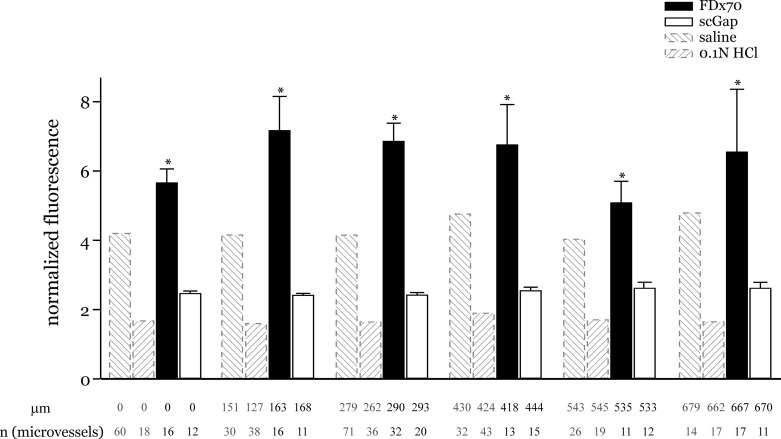
Fluorescence and inhibitor controls. Graph shows acid-induced normalized fluorescence responses separately to infusions of a higher molecular weight dextran (FDx70) and pretreatment with scrambled gap peptide (ScGap). Saline and HCl responses included for comparison. Measurement sites were grouped as in Fig. 3 legend. Mean distance and number of measurement sites are shown below each group. *P < 0.05 compared with acid responses at all distance groups shown. FDx70, FITC-dextran 70 kDa; ScGap, scrambled gap peptide scGap27.
To determine whether the observed responses were possibly a result of free FITC released from FDx20, FDx20 was substituted with FDx70 and the acid instillation experiments were repeated. With FDx70, the washoff was rapid, resulting in negligible residual fluorescence. Thus Fpeak/Fresidual was significantly high, and even higher than that obtained with FDx20 in saline-instilled lungs (Fig. 5). The large differences in the results between FDx20 and FDx70 indicate that the tracers likely did not contain free FITC. Moreover, the larger size of FDx70 likely restricted the tracer to the microvascular lumen and thus allowed the tracer to be washed off rapidly. Thus it can be interpreted that FDx20 likely crossed the microvascular barrier and, hence, both Fpeak and Fresidual determined with FDx20 infusions are indicative of microvascular barrier permeability.
Acid-induced microvascular responses were independent of epithelial gap junction mechanisms.
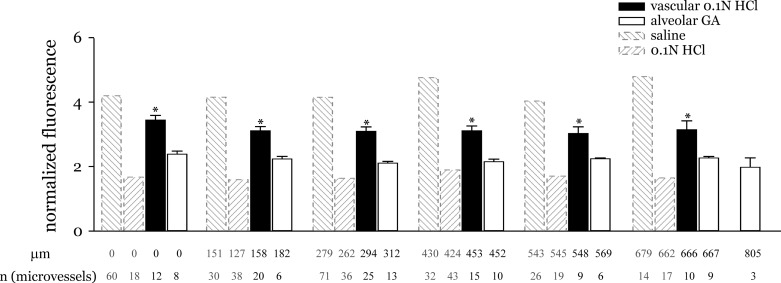
To determine whether alveolar gap junctions played a role, an alveolus was micropunctured and GA was instilled in it. GA filled the punctured alveolus and the surrounding alveoli. After 10 min and verification that the instilled alveoli had recovered, a different alveolus in the GA-treated region was micropunctured and HCl+RDx70 was instilled. Alveolar GA did not increase Fpeak/Fresidual above acid-alone responses both at the acid-instilled and away microvessels, suggesting that the acid-induced responses were independent of alveolar gap junction-dependent mechanisms (Fig. 6).
Fig. 6.
Controls for alternate communication pathways. Graph shows normalized fluorescence responses separately to microvessel instillation of HCl and inhibition of gap junctions of alveolar epithelium with GA pretreatment. Responses to alveolar HCl and saline instillation included for comparison. Measurement sites were grouped as in Fig. 3 legend. Mean distance and number of measurement sites are shown below each group. *P < 0.05 compared with acid responses at all distance groups shown.
Capillary acid instillation does not mimic alveolar acid-induced response.
To exclude the possibility that the instilled acid entered the adjoining microvessels and directly initiated the observed endothelium-dependent responses, acid was instilled directly into microvessels followed by FDx20 infusion as before. Capillary acid-induced Fpeak/Fresidual responses were similar to that for saline instillations, suggesting that the observed alveolar acid instillation responses are unaffected by any possible acid leak from alveoli to capillaries (Fig. 6). Thus the alveolar acid-induced microvascular responses were possibly due to an acid-induced alveolar epithelium to microvascular endothelial cross talk at the instilled region, followed by an endothelial gap-junction mediated mechanism that augmented the response in acid-free regions.
Characterization of the temporal increases in microvascular FDx20 fluorescence.
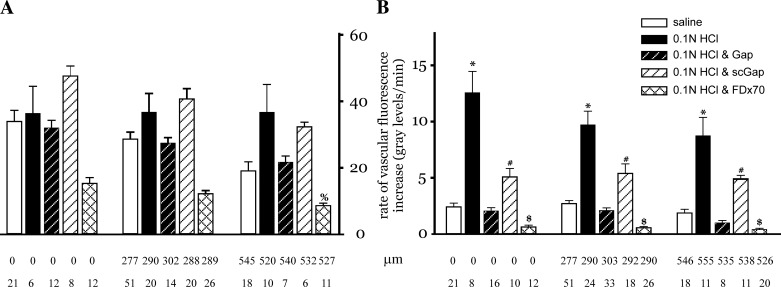
To correlate the microvascular fluorescence changes with acid-induced permeability responses, the rate of FDx20 fluorescence increase during the initial rapid phase was compared with the subsequent slow phase across all treatments conditions. The initial rapid phase increase rate was similar across treatments (Fig. 7A). In contrast, the slow phase rates were smaller and heterogeneous (Fig. 7B). Among treatments, the rates during the slow phase FDx20 increase were highest for acid-only instillation. For gap peptide pretreatment conditions, the rates were lower and similar to that for saline instillation. The smallest rates were evident when FDx70 was infused instead of FDx20. Since the rates represent changes in the quantity of the fluorescent tracer at the measurement site, the dual-phase increase in fluorescence suggests two separate events, both contributing to the increase. Since the first phase initiates within a few minutes of the start of fluorescent tracer infusion, is rapid, and is similar across treatments, this increase represents the tracer filling the microvessel lumen. In contrast, since the second phase is slower and varies across treatments, with the acid instillation having the largest increase and FDx70 infusion having the smallest increase, it is likely that this increase represents fluorescent tracer leakage into the perivascular region. Thus the slow phase increase represents the FDx20 flux across the microvascular barrier. This flux is greatest for acid-only instillation and low for gap peptide treatment, suggesting that inhibition of Cx43 gap junction communication inhibits acid-induced increases in fluid flux across the microvascular barrier.
Fig. 7.
Rate of fluorescence increase. Bar charts show rate of the fluorescence increase during the rapid phase (A) and slow phase of the increase (B). The rapid phase rate was calculated as the change in fluorescence over 3 min, beginning 1 min after the start of the fluorescence increase. The slow phase rate was calculated over 10 min, with the initial value determined at 15 min after the start of the fluorescence increase. The measurement sites were grouped on the basis of the following distance ranges: within instilled region, 200–400 μm, and 500–600 μm. Note that the data marked 0 μm are from measurement sites that lie within the instilled region. Mean distance of all measurement sites within a group is shown below each group (in μm). Number of measurement sites for each group is indicated as n. %P < 0.05 compared with all treatment groups at 0-μm distance only. *P < 0.05 compared with saline, Gap, and FDx70 treatment groups at all 3 distance sites. #P < 0.05 compared with Gap and FDx70 treatment groups at all 3 distance sites. $P < 0.05 compared with all treatment groups at all 3 distance sites.
Characterization decay of FDx20 fluorescence at extended time periods and peak FDx20 fluorescence.
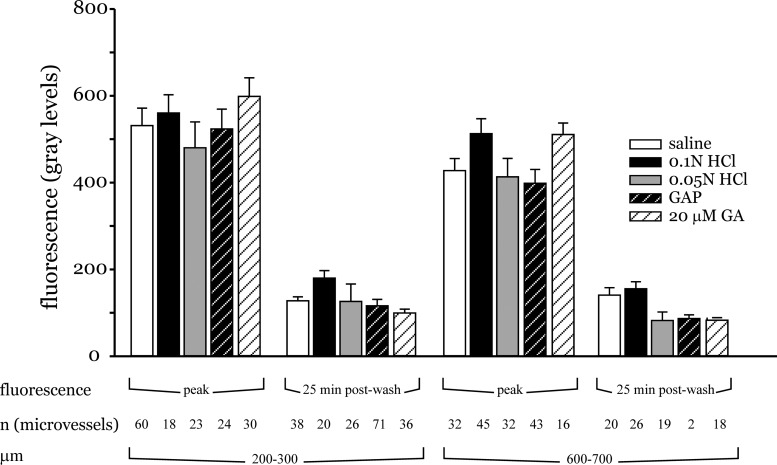
To better elucidate the mechanisms underlying the vascular FDx20 fluorescence changes, the peak fluorescence and decay times were compared for different treatment conditions at two different distances from the site of acid instillation. The mean FDx20 peak fluorescence from all experiments within a treatment group was similar across all treatment groups both in vessels located 200 and 600 μm from the acid-instilled region (Fig. 8). Similarly, the FDx20 residual fluorescence determined 25 min after initiating the Ringer's wash was also similar at the two distances for all the treatment conditions (Fig. 8). Thus, by separately comparing the peak and decay fluorescence, the differences among the different treatment groups was less evident.
Fig. 8.
Peak and decay time analysis. Bar charts show peak and residual FDx20 fluorescence determined 25 min after the start of HEPES-buffered saline wash. The measurement sites were grouped at the distance ranges shown. Number of measurement sites for each group is indicated as n.
DISCUSSION
The results from this study show that 1) acid instillation by micropuncture allows for focal acid delivery restricted to a few alveoli, 2) permeability changes in single microvessels can be faithfully determined by a novel fluorescence method, 3) focal acid instillation increased microvascular permeability at both the acid-instilled and acid-free regions, 4) inhibiting endothelial Cx43-containing gap junctions blocked the acid-induced permeability responses at the acid-instilled region, and 5) Cx43 inhibition blocked the spatial expansion of the acid response.
Methodological considerations.
Micropuncture instillation of acid into alveoli allowed control over the delivery and limited the instillation to a few alveoli. By spatially limiting the instillation, it was possible to 1) ensure that the responses were microvessel specific and 2) simultaneously visualize the instilled and free regions within the field of view of our microscope. Although liquid-filled alveoli were clearly identifiable under bright-field illumination, as previously reported (27), RDx70 fluorescence allowed identifying even partially filled alveoli and thus determining the correct extent of the acid distribution. In addition, RDx70 made feasible the postrecovery identification of instilled alveoli via AWL fluorescence as reported (17). Since instilled alveoli recovered within a short duration, AWL fluorescence became necessary for their identification during imaging. The instillations of acid mixed with FDx10 revealed that the estimate of the spatial spread of acid was independent of tracer size. Furthermore, the absence of any detectable spread of the fluorescence after the instillation period suggested that the acid did not have a direct effect on vessels spatially remote from the site of instillation.
Circulating neutrophils have been implicated as the primary mediator of endothelial injury and barrier compromise in acid aspiration (7, 14). In this study, the imaged microvessels were made blood-free prior to acid instillation, thus excluding neutrophil-dependent responses from the present findings. Folkesson and Matthay (6) reported that under neutrophil-depleted conditions, lung vascular permeability estimates using the tracer 131I-albumin were similar to saline controls. These observations are in agreement with the present findings for FDx70, which is similar in size to albumin. However, for FDx20, the fluorescence increases were markedly different for acid and saline instillations. This difference suggests that alveolar acid instillation increases permeability for small molecules similar to FDx20, but not for larger albumin-sized molecules. In addition, recent data show that microvessels located in lung regions made blood free by the microcatheter method were free of any residual leukocytes (12). Thus it can be interpreted that alveolar acid instillation directly elicits microvascular endothelial responses.
Increase in vascular FDx20 fluorescence was biphasic. Because the initial rapid increase in fluorescence was similar across treatments, this phase likely indicates vascular filling with FDx20. In contrast, the subsequent slow phase fluorescence increase was steeper for acid instillation than for saline, suggesting that acid induced a larger FDx20 flux across the microvessel barrier and thus a greater accumulation for FDx20 in the perivascular space. This possibility is further supported by a small slow phase slope elicited by acid instillation coupled with infusion of FDx70, a larger vascular tracer with lower transvascular permeability and higher vessel retention (25). The results of the FDx70 experiments also preclude free FITC as the cause for the acid-induced FDx20 fluorescence increase. Thus acid-induced FDx20 fluorescence increase represents changes in microvascular permeability to acid. Fpeak/Fresidual was used primarily to minimize the effect of inter-vessel variations in FDx20. As evident from comparing the Fpeak and Fresidual separately across experiment groups, the intervessel variations may obscure the acid-induced responses. Thus Fpeak/Fresidual may better represent changes in microvascular permeability to alveolar instillations.
The spread of the acid-induced microvascular responses were evident in microvessels location more than 700 μm from the site of acid instillation. At distances in the 1,000-μm range, the acid-induced responses were closer to saline controls suggesting a spatial limit to the spread of acid-induced microvascular responses. Although the present experimental setup limited determinations of responses at even longer distances, the trend of the response suggested that the acid-induced permeability increase will likely decline to saline control levels in the 1,500-μm range. Since the average size of the acid-instilled region was in the 500-μm range, the responses appear to spread approximately three times this size. These data are similar to that reported recently, wherein lung water increases spread up to 3 cm in response to acid instillation in a 1-cm-sized region (20).
Gap27 inhibition of acid-induced permeability increases.
The data show for the first time that inhibiting Cx43 gap junctions blunted the permeability increases in microvessels that abut acid treated alveoli. Pretreating microvessels with Cx43 gap peptide, Gap27, prior to acid instillation limited the slope of FDx20 fluorescence increase to saline instillation levels. This suggests that Gap27 blunted the fluid flux across microvessels. As reported previously, Gap27 blunted thrombin-induced increases in microvascular filtration coefficient and the spatial spread of cytosolic Ca2+ in the microvessels (21). In addition, reports suggest that Gap27 decreases intercellular communication, including those across the myoendothelial junctions (10). Thus the observed reductions in microvascular permeability with Gap27 suggest reduced interendothelial communication as the primary mechanism for limiting endothelial barrier damage. However, because the reductions in microvascular permeability were evident even in microvessels that abut directly injured alveoli, other Cx43-dependent mechanisms may also be involved. Thus the relevant mechanisms that result in microvascular barrier strengthening in response to inhibiting Cx43 gap junctions and thus limit acid injury need further investigation.
GA vs. Gap27 effects on acid responses.
Although the peptide inhibitor blunted the acid-induced endothelial barrier compromise even at the site of acid instillation, the effect of GA was less pronounced. At the concentration reported to block endothelial Cx43 channels (5), GA blocked the acid-induced responses in vessels located more than 200 μm from the acid-instilled site. In a recent report, GA pretreatment did not inhibit acid-induced lung water increases at the site of direct injury (20). The present findings that vascular GA significantly blunted acid-induced reduction in normalized fluorescence only in microvessels located outside the acid-treated region are in agreement with these reported data. The reason for the difference in the blunting response between GA and Gap27 at the direct injury site is not clear and may require further consideration.
Mechanisms underlying spatial spread of acid injury.
In patients who have aspirated gastric contents, the clinical presentations include chest radiographs showing mottled densities in the lower lobes (2) that spread rapidly. The present study mimics these observations. Focal acid instillation increased microvascular permeability in both acid-instilled and acid-free regions of the lung. This suggests that the acid instillation initiated responses even in microvessels that did not abut treated alveoli. These data are similar to recent findings (20) in which intrabronchial instillation of acid in a small lung region increased lung water at sites more than 2 cm away from the acid-instilled region. These remote increases were blocked by inhibiting interendothelial gap junctional communication. In the present study, since vascular infusions of Cx43 gap peptide blocked the permeability increase in acid-free regions, it can be interpreted that endothelial Cx43 gap junctions communicated proinflammatory signals to the remote microvessels.
An increase in interstitial fluid content caused by elevated microvascular permeability may initiate removal of the fluid via lung lymphatics (8). A possible effect of FDx20 clearance via interstitial pathways from sites at or closer to the acid-instilled region would be an elevated level of FDx20 fluorescence at sites farther away from the acid instillation site compared with sites that are closer. However, determinations of the 25-min residual FDx20 fluorescence at sites 400 μm apart reveals that the distant sites have similar or lower fluorescence compared with that at sites nearer to the site of acid instillation. Thus it is less likely that interstitial conductance played a role in the clearance of FDx20.
Another primary mechanism of interstitial edema clearance is resorption into the vasculature (8). Thus FDx20 may be resorbed into microvessels during the HBS wash. Because the acid-induced increase in microvascular permeability may persist several hours (6), it is likely that the resorption will be faster at sites adjacent to the site of acid instillation than at distant sites, as the permeability will be higher at the adjacent sites. Thus at longer durations after the initiation of the HBS wash the residual fluorescence may be similar and low at all sites. Estimates of residual fluorescence at 10 min post wash limit the resorption-induced loss of FDx20 fluorescence. On this basis, it can be interpreted that the present findings establish the role of Cx43 gap junction in acid-induced microvascular permeability responses.
Inhibiting alveolar gap junction communication failed to block the acid-induced microvascular permeability responses. Although alveolar epithelial gap junctions mediate proinflammatory responses among adjacent alveoli (9), the present data reveal that they do not play a role in acid-induced responses in remote microvessels. These data are similar to recent findings that alveolar GA did not inhibit lung water increases at sites away from the direct injury site (20). Thus the mechanism of this spatially extensive response could evolve via cross talk between the alveolar and capillary compartments, as suggested before for other inflammatory mechanisms. Although second messengers such as ATP and cPLA2 have been implicated in the cross talk (13, 16), the specific messenger involved in acid-injury needs elucidated. It is possible that the cross talk initiates activation of endothelial cells at the site of acid instillation that result in second messenger generation and their spatial spread via endothelial Cx43 gap junctions.
Cx43 and microvascular barrier permeability.
The data suggest a novel role for endothelial Cx43 in mediating microvascular barrier permeability. Previous studies show that Cx43 gap junctions mediate thrombin-induced vascular permeability responses (21). Since the data in the earlier studies were derived from whole lung experiments, microvessel-specific interpretations remain difficult. Other indirect evidence of connexin-dependent endothelial barrier regulation includes Cx40-facilitated neutrophil migration-associated changes in permeability of human umbilical vein endothelial cell monolayers (28). In addition, lungs lacking both endothelial Cx43 and Cx40 become progressively hemorrhagic (4, 15). Thus, whereas these studies allude to a role for Cx43, the present study provides direct evidence that Cx43 is involved in endothelial barrier regulation in pulmonary microvessels.
Endothelial mechanisms that lead to microvascular barrier strengthening under Cx43-inhibited conditions require further studies. In cardiomyocytes, Cx43 has been shown to associate with the tight junction protein zonula occludens-1 (ZO-1) and through ZO-1 to the actin cytoskeleton (26). Furthermore, Cx43 may also be associated with cadherins at the intercellular membrane (11). Current findings in macrophages also indicate that Cx43 may be necessary for actin rearrangement (1). Because both cadherins and ZO-1, in tandem with the actin cytoskeleton, are involved in the regulation of the endothelial barrier (19, 23, 24), the exact interactions among these junctional molecules that underlie the present findings need further investigation.
In conclusion, the data show that focal alveolar acid instillation increases endothelial barrier permeability in both local and remote microvessels. Inhibition of Cx43 gap junctions blocked both the local and remote permeability increase. Thus inhibiting Cx43 gap junctions could be therapeutic in the treatment of acid injury.
GRANTS
This work was funded by HL75503 to K. Parthasarathi.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
The author sincerely thanks Drs. Christopher M. Waters and A. P. Naren for comments on the manuscript.
REFERENCES
- 1. Anand RJ, Dai S, Gribar SC, Richardson W, Kohler JW, Hoffman RA, Branca MF, Li J, Shi XH, Sodhi CP, Hackam DJ. A role for connexin43 in macrophage phagocytosis and host survival after bacterial peritoneal infection. J Immunol 181: 8534– 8543, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bartlett JG, Gorbach SL. The triple threat of aspiration pneumonia. Chest 68: 560– 566, 1975 [DOI] [PubMed] [Google Scholar]
- 3. Boitano S, Evans WH. Connexin mimetic peptides reversibly inhibit Ca2+ signaling through gap junctions in airway cells. Am J Physiol Lung Cell Mol Physiol 279: L623– L630, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Chatterjee S, Baeter S, Bhattacharya J. Endothelial and epithelial signaling in the lung. Am J Physiol Lung Cell Mol Physiol 293: L517– L519, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Faigle M, Seessle J, Zug S, El Kasmi KC, Eltzschig HK. ATP release from vascular endothelia occurs across Cx43 hemichannels and is attenuated during hypoxia. PLoS One 3: e2801, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Folkesson HG, Matthay MA. Inhibition of CD18 or CD11b attenuates acute lung injury after acid instillation in rabbits. J Appl Physiol 82: 1743– 1750, 1997 [DOI] [PubMed] [Google Scholar]
- 7. Folkesson HG, Matthay MA, Hebert CA, Broaddus VC. Acid aspiration-induced lung injury in rabbits is mediated by interleukin-8-dependent mechanisms. J Clin Invest 96: 107– 116, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fukue M, Serikov VB, Jerome EH. Recovery from increased pressure or increased leakiness edema in perfused sheep lungs. J Appl Physiol 77: 184– 189, 1994 [DOI] [PubMed] [Google Scholar]
- 9. Ichimura H, Parthasarathi K, Lindert J, Bhattacharya J. Lung surfactant secretion by interalveolar Ca2+ signaling. Am J Physiol Lung Cell Mol Physiol 291: L596– L601, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Isakson BE, Duling BR. Heterocellular contact at the myoendothelial junction influences gap junction organization. Circ Res 97: 44– 51, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Jongen WM, Fitzgerald DJ, Asamoto M, Piccoli C, Slaga TJ, Gros D, Takeichi M, Yamasaki H. Regulation of connexin 43-mediated gap junctional intercellular communication by Ca2+ in mouse epidermal cells is controlled by E-cadherin. J Cell Biol 114: 545– 555, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kandasamy K, Sahu G, Parthasarathi K. Real-time imaging reveals endothelium-mediated leukocyte retention in LPS-treated lung microvessels. Microvasc Res 83: 323– 331, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kiefmann R, Islam MN, Lindert J, Parthasarathi K, Bhattacharya J. Paracrine purinergic signaling determines lung endothelial nitric oxide production. Am J Physiol Lung Cell Mol Physiol 296: L901– L910, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Knight PR, Druskovich G, Tait AR, Johnson KJ. The role of neutrophils, oxidants, and proteases in the pathogenesis of acid pulmonary injury. Anesthesiology 77: 772– 778, 1992 [DOI] [PubMed] [Google Scholar]
- 15. Koval M, Billaud M, Straub AC, Johnstone SR, Zarbock A, Duling BR, Isakson BE. Spontaneous lung dysfunction and fibrosis in mice lacking connexin 40 and endothelial cell connexin 43. Am J Pathol 178: 2536– 2546, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuebler WM, Parthasarathi K, Wang PM, Bhattacharya J. A novel signaling mechanism between gas and blood compartments of the lung. J Clin Invest 105: 905– 913, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lindert J, Perlman CE, Parthasarathi K, Bhattacharya J. Chloride-dependent secretion of alveolar wall liquid determined by optical-sectioning microscopy. Am J Respir Cell Mol Biol 36: 688– 696, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matthay MA, Zimmerman GA. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am J Respir Cell Mol Biol 33: 319– 327, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev 86: 279– 367, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Parthasarathi K, Bhattacharya J. Localized acid instillation by a wedged-catheter method reveals a role for vascular gap junctions in spatial expansion of acid injury. Anat Rec (Hoboken) 2011. August 1 doi: 10.1002/ar.21460. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Parthasarathi K, Ichimura H, Monma E, Lindert J, Quadri S, Issekutz A, Bhattacharya J. Connexin 43 mediates spread of Ca2+-dependent proinflammatory responses in lung capillaries. J Clin Invest 116: 2193– 2200, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Parthasarathi K, Ichimura H, Quadri S, Issekutz A, Bhattacharya J. Mitochondrial reactive oxygen species regulate spatial profile of proinflammatory responses in lung venular capillaries. J Immunol 169: 7078– 7086, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Quadri SK, Bhattacharjee M, Parthasarathi K, Tanita T, Bhattacharya J. Endothelial barrier strengthening by activation of focal adhesion kinase. J Biol Chem 278: 13342– 13349, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Safdar Z, Wang P, Ichimura H, Issekutz AC, Quadri S, Bhattacharya J. Hyperosmolarity enhances the lung capillary barrier. J Clin Invest 112: 1541– 1549, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thorball N. FITC-dextran tracers in microcirculatory and permeability studies using combined fluorescence stereo microscopy, fluorescence light microscopy and electron microscopy. Histochemistry 71: 209– 233, 1981 [DOI] [PubMed] [Google Scholar]
- 26. Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Hori M, Tada M. Direct association of the gap junction protein connexin-43 with ZO-1 in cardiac myocytes. J Biol Chem 273: 12725– 12731, 1998 [DOI] [PubMed] [Google Scholar]
- 27. Wang PM, Ashino Y, Ichimura H, Bhattacharya J. Rapid alveolar liquid removal by a novel convective mechanism. Am J Physiol Lung Cell Mol Physiol 281: L1327– L1334, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Zahler S, Hoffmann A, Gloe T, Pohl U. Gap-junctional coupling between neutrophils and endothelial cells: a novel modulator of transendothelial migration. J Leukoc Biol 73: 118– 126, 2003 [DOI] [PubMed] [Google Scholar]