Abstract
Increased cell proliferation and migration, of several cell types are key components of vascular remodeling observed in pulmonary hypertension (PH). Our previous data demonstrate that adventitial fibroblasts isolated from pulmonary arteries of chronically hypoxic hypertensive calves (termed PH-Fibs) exhibit a “constitutively activated” phenotype characterized by high proliferative and migratory potential. Osteopontin (OPN) has been shown to promote several cellular activities including growth and migration in cancer cells. We thus tested the hypothesis that elevated OPN expression confers the “activated” highly proproliferative and promigratory/invasive phenotype of PH-Fibs. Our results demonstrate that, both in vivo and ex vivo, PH-Fibs exhibited increased expression of OPN, as well as its cognate receptors, αVβ3 and CD44, compared with control fibroblasts (CO-Fibs). Augmented OPN expression in PH-Fibs corresponded to their high proliferative, migratory, and invasive properties and constitutive activation of ERK1/2 and AKT signaling. OPN silencing via small interfering RNA or sequestering OPN production by specific antibodies led to decreased proliferation, migration, invasion, and attenuated ERK1/2, AKT phosphorylation in PH-Fibs. Furthermore, increasing OPN levels in CO-Fibs via recombinant OPN resulted in significant increases in their proliferative, migratory, and invasive capabilities to the levels resembling those of PH-Fibs. Thus our data suggest OPN as an essential contributor to the activated (highly proliferative, migratory, and proinvasive) phenotype of pulmonary adventitial fibroblasts in hypoxic PH.
Keywords: vascular remodeling, inflammation, matricellular protein, extracellular matrix protein, vascular proliferation
all forms of chronic pulmonary hypertension (PH) are characterized by structural and fibroproliferative changes in both small and large pulmonary arteries (PAs) through a process termed vascular remodeling. Remodeling can affect all layers of the vessel wall and involves changes in the phenotype and functional behavior of each of the principal cell types in the vessel wall. However, one of the most consistent findings in experimental models of PH, as well as in models of vascular injury and hypertension in the systemic circulation, is early and dramatic adventitial remodeling, characterized by fibroblast proliferation, migration, and differentiation (5, 33, 53–55, 58, 64, 65). Although the mechanisms involved in these responses remain obscure, the possibility that they are coordinated through interactions with surrounding extracellular matrix (ECM) proteins is one possible mechanism (55, 58).
The ECM is a dynamic structure continually changing in response to pathophysiological stimuli. ECM provides signals to adjacent cells via cell surface receptors such as integrins, which ultimately regulate proliferation, migration, intracellular signaling, and differentiation state of the cells. Components of ECM include basic structural proteins such as collagen and elastin, specialized proteins such as fibronectin and proteoglycans, and matricellular proteins (3, 39, 55, 58). The latter are receiving a great deal of attention in cancer and tissue remodeling because of their marked upregulation during tissue injury/repair and their ability to exert regulatory functions through direct binding to cell surface receptors, other matrix proteins, and soluble extracellular factors such as growth factors and cytokines. Matricellular proteins include osteopontin (OPN), thrombospondin-1/2 (TSP-1/2), tenascin-C/X (TNC/TNX), periostin, and osteonectin (secreted protein acid and rich in cysteine, SPARC). These proteins are abundantly expressed during development; however, in adulthood their production is mainly restricted to wound healing and tissue remodeling (7, 25, 34, 36). Among these proteins, OPN is currently receiving the most attention in cancer and tissue repair (7, 43, 61). OPN was first described as a phosphoprotein secreted by malignant epithelial cells (50) but has now been shown to be secreted by many cell types, including osteoclasts, lymphocytes, macrophages, vascular smooth muscle cells, and cancer-associated fibroblasts. Extensive research has demonstrated the involvement of OPN in malignant transformation and tumor progression, wherein OPN acts through several mechanisms including increased cell proliferation, migration, and invasion, (1, 59). Recent studies have demonstrated marked upregulation of OPN in the setting of tissue remodeling in various systemic organs and diseases, including cardiovascular diseases (15). Circulating levels of OPN have been suggested to be one of the biomarkers of various diseases including cancer, asthma, cardiac remodeling following myocardial infarction, pulmonary fibrosis, and PH (32, 57). OPN has also been shown to be upregulated in the lungs and pulmonary arteries of rats and mice with monocrotaline- and hypoxia-induced PH and to be decreased by treatments with fluoxetine and atrial natriuretic peptide (5, 35, 60). Little, however, is known regarding the role of OPN in modulating the phenotype of pulmonary adventitial fibroblasts, the cells that undergo the earliest and most dramatic phenotypic and functional changes in the setting of PH.
We hypothesized that OPN would be an important contributing factor to the increased proliferative, migratory, and proinvasive phenotype of pulmonary adventitial fibroblasts in hypoxia-induced PH and that modulating OPN expression would alter phenotypic and functional characteristics of adventitial fibroblasts isolated from pulmonary arteries of chronically hypoxic hypertensive calves (PH-Fibs). Our approach was, first, to evaluate in vivo lung tissue- and cell-specific OPN expression in the setting of PH, and, further, to determine OPN effects on proliferation, migration, and invasion in cultured pulmonary adventitial fibroblasts by utilizing molecular [small interfering RNA (siRNA) and recombinant protein], as well as immunological (neutralizing antibody) approaches to alter OPN expression.
METHODS
Animal Model
The neonatal calf model of severe hypoxic pulmonary hypertension has been described previously (12) and includes the development of PA pressure equal to or exceeding systemic pressure accompanied by remarkable PA remodeling with medial and adventitial thickening, as well as perivascular inflammation (24, 56). One-day-old male Holstein calves were purchased from a local dairy farm. The experimental group (n = 7) was exposed to hypobaric hypoxia [barometric pressure (Pb) = 445 mmHg] for 2 wk, whereas age-matched controls (n = 6) were kept at ambient Denver altitude (Pb = 640 mmHg). Standard veterinary care was used following institutional guidelines, and the procedure was approved by the Institutional Animal Care and Use Committee approved (Dept. of Physiology, School of Veterinary Medicine, Colorado State Univ., Ft. Collins, CO).
Human Specimens
Frozen sections of lung tissue from human subjects with idiopathic pulmonary arterial hypertension (iPAH) (n = 5) and controls (n = 4) were used for immunocytochemical analyses (see Acknowledgments).
Isolation of Bovine Distal Pulmonary Artery Adventitial Fibroblasts
Fibroblasts were isolated from the adventitia of distal pulmonary arteries with external diameter of 670–1,340 μm (means 994 ± 25 μm) from chronically hypoxic hypertensive and normotensive control calves by using an explant techniques as previously described (13). Fibroblasts isolated from the explants were expanded in complete DMEM with 10% calf serum (CS). Fibroblast phenotypes were characterized by immunofluorescence analysis using mesenchymal, smooth muscle, and monocyte/macrophage markers. Cells utilized for study were shown to lack cell type-specific expression of smooth muscle markers (smooth muscle myosin), endothelial markers (von Willebrand factor), and monocytic markers (CD45, CD14, CD68) as previously shown (28).
Reagents and Antibodies
DMEM media was purchased from Cellgro (Manassas, VA), neonatal CS was from Gemini Bio-Products (Sacramento, CA), and bovine serum albumin was from Sigma Aldrich (St. Louis, MO). Protease inhibitor mixture was obtained from Calbiochem (San Diego, CA). OPN-specific rabbit polyclonal antibodies (used at 1:50 dilution) were from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal antibodies against human αVβ3 integrin/vitronectin receptor (cross-reacting with bovine species) and against bovine CD44 were from United States Biological (Swampscott, MA) and from VMRD (Pullman, WA), respectively. Rabbit polyclonal antibodies against both total and phospho-ERK1/2 (Tyr202/Thr204) and against total and phospho-AKT (Ser473) were from Cell Signaling Technology (Danvers, MA). Recombinant bovine osteopontin was purchased from R&D Systems (Minneapolis, MN).
Immunofluorescent Analysis of OPN Expression
Immunostaining with OPN-specific primary antibodies was performed via biotin-streptavidin system via a previously described method (13), and stained sections/cells were examined via a Zeiss fluorescent microscope with an AxioVision digital imaging system (Carl Zeiss MicroImaging,, Thompsonwood, NY).
Quantitative Real-Time PCR
Total cellular RNA from each sample (total calf lung; or from CO and PH-fibroblasts) was extracted by use of RNeasy Mini Kit (Qiagen, Hilden, Germany). Complementary DNA was synthesized from 1 μg of total cellular RNA using iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). Quantitative real-time RT-PCR was performed in triplicate with the iCycler My iQ with iQ SYBR Green Supermix (Bio-Rad). Primers were designed with Primer 3 Software (44). Gene expression was normalized to the housekeeping gene hypoxanthine-xanthine phosphoribosyl transferase (HPRT). The sequences for primers are listed in Table 1. Results are presented as expression relative to HPRT by the delta cycle threshold method (48).
Table 1.
Bovine primer sequences for real-time PCR
| Gene | Forward 5′-3′ | Reverse 5′-3′ |
|---|---|---|
| Osteopontin | CAGAGTCCAGATGCCACAGA | GGAAAGCTCGCTACTGTTGG |
| CD44 | TTCCCCGGATCCACCCCAAT | GCAGGTCCGTGACCGATGTA |
| CD29 | ACCCCAAGTTCCAAGGGCCA | AAGGCTCGGCACTGAACACA |
| MMP-2 | GCTGTGTACGAAGACCCACA | AGCATCCACCTTCTGGACAC |
| MMP-9 | TAGCACGCACGACATCTTTC | GAAGGTCACGTAGCCCACAT |
| HPRT | CTGGCTCGAGATGTGATGAA | CAACAGGTCGGCAAAGAACT |
Table 2.
Osteopontin siRNA sequences
| Sequence Used | |
|---|---|
| OPN-siRNA-1 | CGCCAAAGGCCAAGGAUAAUU |
| OPN-siRNA-2 | CCAAAGAACUCACGCCAAAUU |
| OPN-siRNA-3 | CAAUGAAAGCCCUGAGCAAUU |
Gene Silencing
Cells were seeded in six-well plates at 2.4 × 105 cells per well and grown to 80% confluence. OPN siRNA (target sequence: 5′-CAAUGAAAGCCCUGAGCAAUU-3′) custom designed and synthesized by Dharmacon (Lafayette, CO) from the bovine accession number M66236 was used at 50 nM. Different OPN siRNAs used are listed in Table 1. Nonsilencing siRNA scrambled siGENOME Negative no. 3 (cat. no. D-001210-03) was used as a negative control (Dharmacon) at 50 nM. siRNAs were delivered to the cells with DharmaFECT1 transfection reagent (Dharmacon) for 48–72 h at a ratio of 1:2 siRNA to transfection reagent according to manufacturer's instructions.
Cell Proliferation and Viability Assays
Cell proliferation was quantified either by cell count using 0.4% Trypan blue exclusion, or via a colorimetric method based on the metabolic reduction of 3-(4,5-dimethylthiozol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) dye to formozan as described earlier (26). Briefly, fibroblasts were plated onto 24-multiwell plates at 2 × 103 cells/well. The next day, cells were rinsed with PBS, and 0.5 ml of 0.1% CS-DMEM was added. Cell counts or MTT assays were performed at 24, 48, and 72 h.
DNA Synthesis Analysis
DNA synthesis analysis was determined by measuring [3H]thymidine incorporation in growth-arrested fibroblasts. Briefly, fibroblasts were plated at 20 × 103 cells/well onto 24-multiwell plates in 10% CS-DMEM, rinsed with PBS after 24 h, and serum-free DMEM was added for 72 h. [3H]thymidine (0.5 μCi/ml) was added to each well. After additional 24-h incubation, measurement of [3H]thymidine incorporation was performed in total cell lysates by using a liquid scintillation counter (Beckman LS 6500).
Cell Migration Assays
Boyden chamber migration assay.
Migration assays were carried out as described earlier (14). Briefly, fibroblasts (1.0 × 105 cells/well) were plated in 200 μl of serum-free DMEM in permeable cell culture inserts (8.0 μM pore size, Costar, Milpitas, CA) precoated with 0.1% gelatin (Sigma Aldrich). Cells remaining on the upper surface of the filter were wiped off, and migrated cells were fixed with methanol for 15 min and stained with 0.2% crystal violet in 2% ethanol (vol/vol) for a minimum of 15 min. Cells that had migrated through the filter were photographed under ×40 magnification of a phase-contrast microscope in six random fields.
Scrape migration assay.
Cell migration was also assessed by using a “scrape” assay in complete DMEM with 5 μM hydroxyurea to inhibit cell proliferation as described earlier (13). Briefly, cells (3 × 105) were plated in a six-well plate and were allowed to attach for 6 h, after which a 2-mm scrape was performed (that time point was considered 0 h). The borders of the wound (scrape) were labeled with a permanent marker (on the bottom of the plate), and the number of cells migrating past the drawn line (into the scrape area), were counted at 24 and 48 h. Cell migration analysis was also performed in the presence of bovine rOPN (R&D Systems) as well as with anti-OPN antibodies at concentrations indicated in the text and figure legends.
Cell Invasion Assays
The invasion assay was performed in Boyden Transwell chambers as described previously (67). Filters were coated with 1 μg/ml growth factor-reduced Matrigel (BD Biosciences, Palo Alto, CA). Fibroblasts were placed in chambers in serum-free DMEM medium for 48 h after the respective treatments (anti-OPN siRNA or nonspecific scrambled siRNA for 72 h), and 10% CS was added to the lower chamber. Cells that penetrated the membrane were fixed with cold methanol for 15 min and stained with 0.2% crystal violet in 2% ethanol (vol/vol) for a minimum of 15 min. Cells that penetrated through the membrane were photographed using phase-contrast microscope in six random fields and were counted separately. For three-dimensional (3D) culture studies fibroblasts were grown in growth factor-reduced Matrigel for 9 days and fluorescence staining was performed as described (8). Quantification of filopodia-like structures was performed via the ibidi website for tube formation image analysis: http://www.ibidi.com (Munich, Germany).
Western Blot Analysis
Fibroblasts (untreated controls, scrambled- and siRNA-treated) were washed twice with ice-cold PBS and lysed with Tris·HCl buffer (40 mM pH 7.5, 4°C), containing 0.1% Triton X-100, 0.25 M sucrose, 3 mM EGTA, 3 mM EDTA, 50 μM β-mercaptoethanol, 1 mM PMSF, and complete protease inhibitor cocktail (Calbiochem, San Diego, CA). Cell lysates were centrifuged at 7,500 g for 10 min at 4°C. Equivalent amounts of total cell protein were subjected to 10% SDS-PAGE as described earlier (2). Proteins were transferred to polyvinylidene fluoride (PVDF) membranes and probed with appropriate antibodies as indicated in the figure legends. After washing with Tris-buffered saline-Tween solution, membranes were incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. Immunoreactive bands were detected by ECL (Renaissance, NEN Life Science Products, Boston, MA) followed by exposure to Hyperfilm. In all experiments equivalent ample loading and transfer were verified by staining PVDF membrane with Ponceau stain and probing with antibodies against β-actin (Sigma).
Statistical Analysis
Data are presented as means ± SE. Student's t-test and one-way ANOVA were used for statistical analysis. A value of P < 0.05 was considered statistically significant.
RESULTS
OPN Expression Is Augmented in PH-Associated Adventitial Remodeling In Vivo
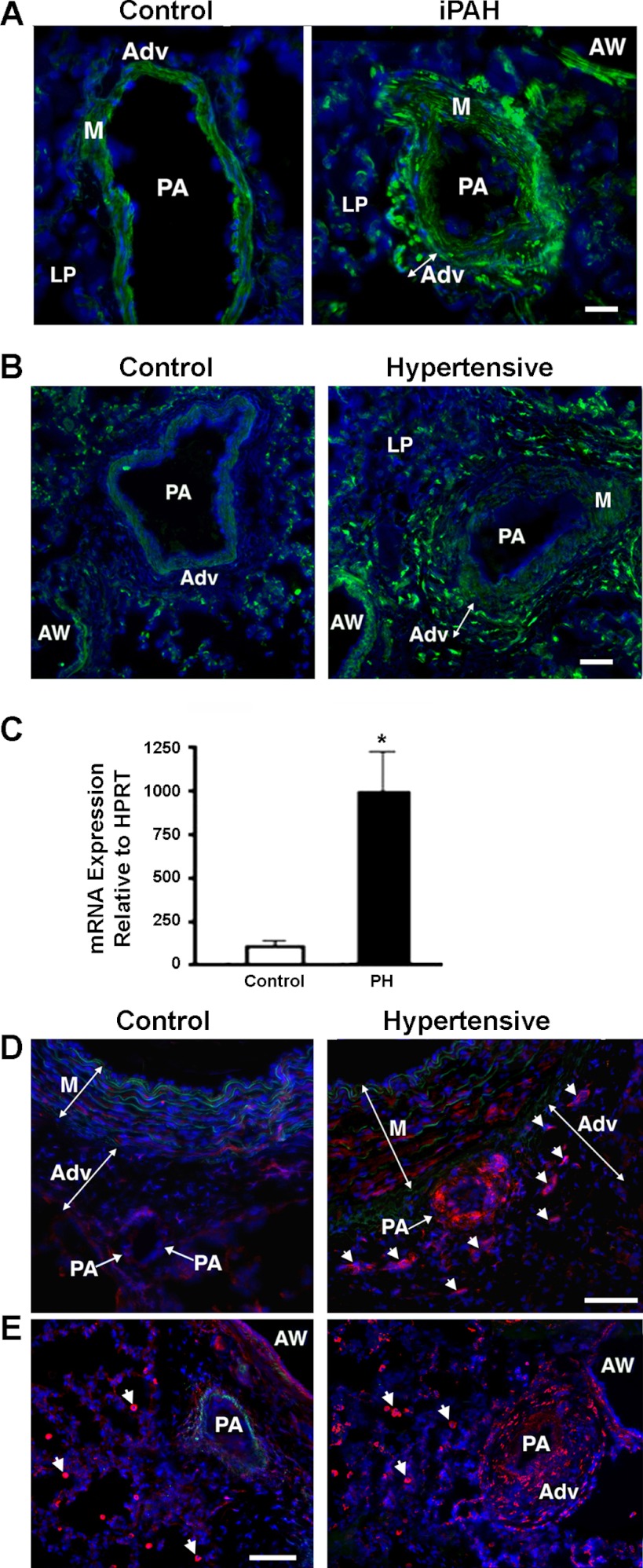
Since circulating levels of OPN have been suggested to be a biomarker of PH, we first assessed OPN expression in lung cryosections from human subjects with iPAH and observed markedly increased vascular OPN expression compared with controls (Fig. 1A). Pulmonary adventitial cells in iPAH lung tissue displayed the highest immunoreactivity, although cells in the vascular media and lung parenchyma also showed positive staining, yet at a lower level. Next, we analyzed cellular distribution of OPN protein in the lung specimens of chronically hypoxic hypertensive calves and demonstrated markedly augmented OPN expression in the pulmonary perivascular adventitia, compared with controls (Fig. 1B). Quantification of OPN expression in the whole lung extracts demonstrated eightfold increases in mRNA levels in hypertensive calves compared with age-matched controls (Fig. 1C). Cells in the lung parenchyma as well as in PA media also showed reactivity for OPN, although at much lower levels.
Fig. 1.
Osteopontin (OPN) expression is upregulated in hypoxia-induced pulmonary hypertension (PH) (in vivo assessment). A–C: pulmonary hypertension-associated vascular remodeling is characterized by upregulation of OPN. A: in human subjects with idiopathic pulmonary arterial hypertension (iPAH, right), marked upregulation of OPN expression is observed in vascular adventitia, compared with control specimens (Control, left). B: upregulation of OPN protein expression is observed in vascular adventitia (Adv) of chronically hypoxic hypertensive calves (right) compared with normotensive controls (left). Lung cryosections in A and B were labeled with OPN-specific antibodies (green fluorescence) and counterstained with 4,6-diamidino-2-phenylindole (DAPI) (cell nuclei, blue). C: OPN mRNA expression is upregulated in whole lung extracts from neonatal calves with chronic hypoxia-induced PH compared with age-matched control calves. HPRT, hypoxanthine-xanthine phosphoribosyl transferase. D and E: upregulated expression of the OPN receptors αVβ3 integrin (D) and CD44 (E) is observed in pulmonary vascular adventitia of chronically hypertensive calves (right) compared with normoxic controls (left). In chronically hypoxic hypertensive calves, adventitia of a smaller-size vessel (PA with an arrow) as well as cells in the adventitia (arrowheads) of a larger elastic intralobar pulmonary artery (PA) demonstrate intense positive staining for αVβ3 integrin (red fluorescence). Vascular media (M) also show increase in reactivity. In control normoxic animals, both vascular adventitia and media display minimal expression of OPN. Number of specimens analyzed: CO and PH calves (n = 7, each group); human subjects (controls, n = 4; iPAH, n = 5). Scale bars in A and C = 50 μM; in D and E = 100 μM. AW, airway; LP, lung parenchyma.
Concurrent markedly increased levels of OPN expression in the PA adventitia, augmented expression of OPN receptors αVβ3 and CD44 in pulmonary vascular adventitia of chronically hypoxic hypertensive animals was also documented (Fig. 1, D and E).
Fibroblasts Isolated from Pulmonary Arterial Adventitia of Hypertensive Calves Maintain Augmented OPN Expression in Culture
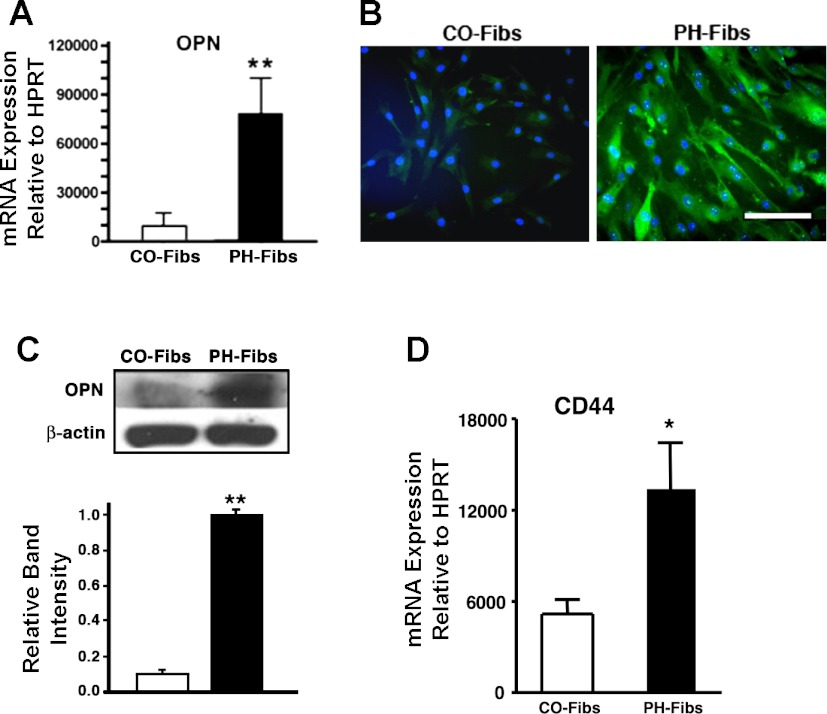
Fibroblasts isolated from distal pulmonary arteries of chronically hypertensive calves (termed PH-Fibs) exhibited elevated expression of OPN, compared with fibroblasts isolated from control normotensive animals (CO-Fibs) at both the mRNA and protein levels, as determined by quantitative RT-PCR, immunofluorescent staining, and Western blotting analyses (Fig. 2, A, B, and C, respectively). The elevated OPN expression was maintained by PH-Fibs over numerous passages in culture.
Fig. 2.
Pulmonary adventitial fibroblasts isolated from severely hypertensive calves (PH-Fibs) express markedly higher levels of OPN and OPN receptor CD44 than control fibroblasts (CO-Fibs). A: quantitative real-time PCR demonstrates that PH-Fibs express 8-fold higher OPN mRNA levels than CO-Fibs. Data presented are relative OPN transcript abundance to HPRT. B and C: at the protein level, as shown by immunofluorescent staining (B) and Western blotting (C), OPN expression levels are markedly higher in PH-Fibs compared with CO-Fibs. Scale bar in B = 50 μM. C: Western blotting (top) was quantified by densitometric scanning analysis (bottom); data are representative of at least 3 independent experiments. D: RT-PCR analysis demonstrates that PH-Fibs express OPN receptor CD44 at levels 2.58 ± 0.59-fold higher than those in CO-Fibs. Data presented are relative OPN transcript abundance to HPRT. *P < 0.05, **P < 0.01.
Concurrently, expression of CD44, known as one potential OPN receptors, was significantly upregulated in PH-Fibs compared with Co-Fibs [(2.58 ± 0.59)-fold difference] (Fig. 2D). Analysis of another potential OPN receptor, CD29 (β1-integrin) in CO- and PH-Fibs showed no differences in expression between cell types (not shown).
Elevated OPN Expression Contributes to Increased Proliferation of PH-Fibs
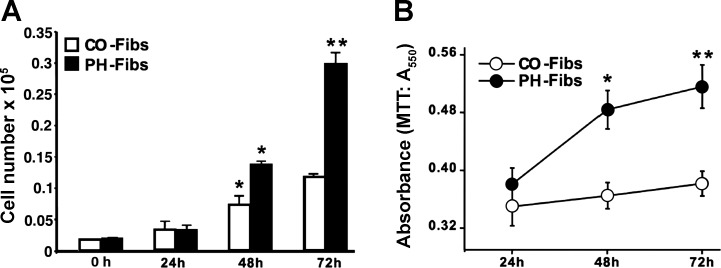
PH-Fibs demonstrated elevated proliferative potential under serum-deprived (0.1% CS) conditions (assessed by both cell counts and MTT assay) compared with CO-Fibs. As shown in Fig. 3, A and B, in 72 h, PH-Fibs doubled in number compared with CO-Fibs.
Fig. 3.
PH-Fibs proliferate at markedly higher rates than CO-Fibs. A and B: cell proliferation under serum-free conditions was assessed by cell number counts (A) and 3-(4,5-dimethylthiozol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (B) over a 72-h period (see methods). Results are expressed as means ± SE; *P < 0.05; **P < 0.01.
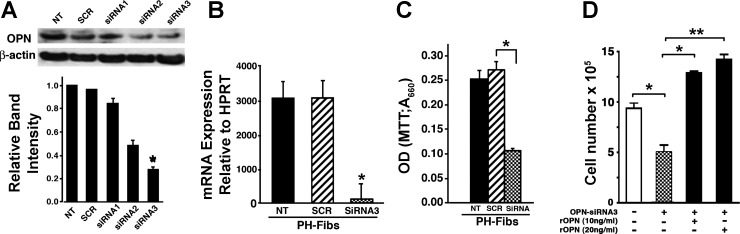
To evaluate the role of OPN in regulating augmented PH-Fibs proliferation, we first tested knockdown of OPN expression by siRNA. Screening of three different siRNAs against OPN was carried out and the effect of OPN knockdown was monitored both at mRNA and protein levels. As shown in Fig. 4, A and B, OPN-siRNA-3 was most effective in silencing OPN in PH-Fibs at the mRNA and protein levels. PH-Fibs were then treated with OPN-siRNA-3 and/or scrambled siRNA (50 nM, 72 h) and the rates of proliferation were measured by both the MTT method and cell counts (see methods). OPN-specific siRNA-3 treatment led to a marked decrease in proliferation of PH-Fibs, whereas the scrambled siRNA had no effect (Fig. 4C). Addition of recombinant OPN (rOPN) to siRNA-treated PH-Fibs restored and actually increased their proliferative potential (Fig. 4D).
Fig. 4.
Silencing of OPN by small interfering RNA (siRNA) in PH-Fibs results in attenuated cell proliferation. A and B: different siRNAs (siRNA 1–3) were tested for their ability to block OPN protein expression in PH-Fibs and siRNA-3 was chosen as the best one. As shown by both Western blotting (A) and real-time PCR analysis (B), treatment of PH-Fibs with siRNA-3 (50 nM, 48 h for RT-PCR, 72 h for Western blotting) mediated significant downregulation of OPN expression. C: siRNA3-mediated OPN silencing in PH-Fibs resulted in decreased cell proliferation as shown by MTT assay (72-h time point). D: addition of recombinant OPN (rOPN; 10 or 20 ng/ml) to siRNA-treated PH-Fibs restored and even elevated their proliferative potential, as determined by cell count assessment. Data shown are representative of at least 3 independent experiments. NT, not treated; SCR, treated with scrambled RNA; siRNA 1–3, treated with siRNA 1, 2, or 3. *P < 0.05; **P < 0.01.
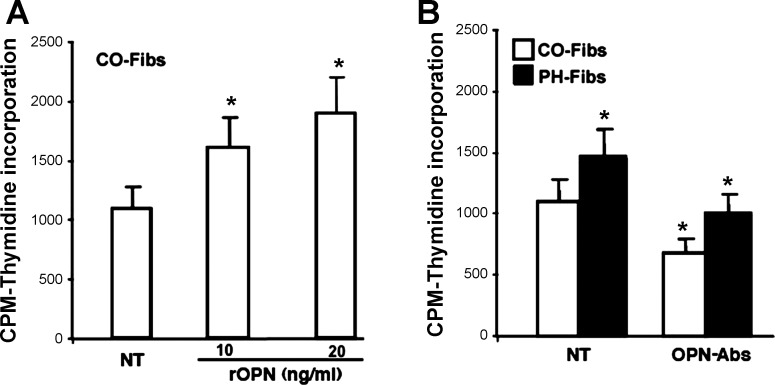
Since CO-Fibs exhibited low levels of OPN expression (shown in Fig. 2, A–C) and low proliferative rates (Fig. 3, A and B), we next tested whether upregulation of OPN expression in CO-Fibs would enhance their proliferative phenotype. CO-Fibs were treated with 10 or 20 ng/ml of rOPN for 24 h and demonstrated increased rates of DNA synthesis in a dose-dependent manner (Fig. 5A).
Fig. 5.
Modulation of OPN expression in PH- and CO-Fibs results in alterations of their proproliferative capabilities. A: overexpression of OPN in CO-Fibs by treatment with rOPN (10 or 20 ng/ml, 24 h) resulted in augmented DNA synthesis. B: treatment of PH-Fibs and CO-Fibs with OPN-specific antibodies (7.5 μg/ml, 24 h) resulted in significantly attenuated DNA synthesis in both cell types. OPN-Abs, treated with OPN-specific antibodies. Number of cell populations analyzed: PH-Fibs, n = 5; CO-Fibs, n = 4. Results are expressed as mean ± SE; *P < 0.05.
Furthermore, treatment of PH-Fibs and CO-Fibs with OPN-specific antibodies led to a decrease in rate of DNA synthesis of both cell types. Importantly, levels of DNA synthesis in PH-Fibs were decreased to the basal level of untreated CO-Fibs, whereas DNA synthesis level in CO-Fibs lowered even further (Fig. 5B).
OPN Contributes to Augmented Migratory and Proinvasive Capabilities of PH-Fibs
Next, we examined the role of OPN in the migratory and proinvasive capabilities of PH-Fibs using the scrape assay, 3D Matrigel- and 2-dimensional (2D) Transwell Boyden chamber assays.
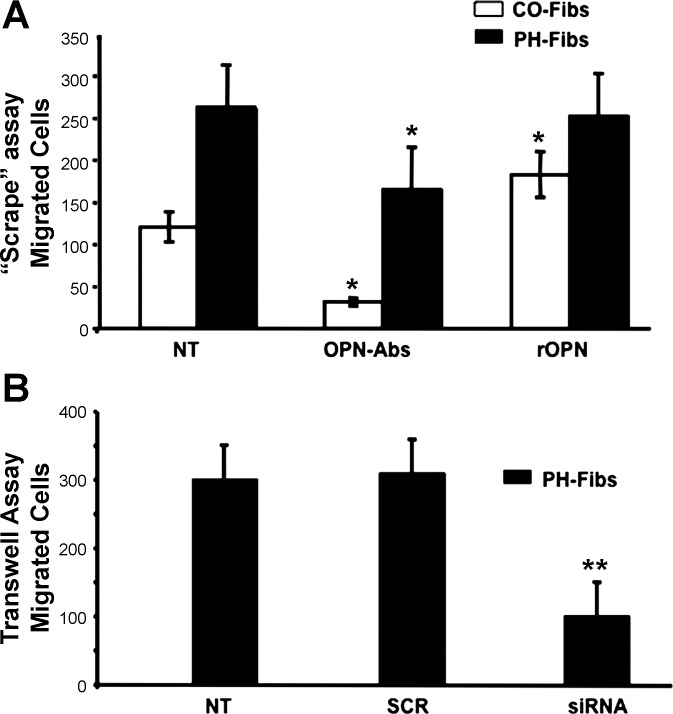
In a scrape (“wound”) assay under basal unstimulated conditions, PH-Fibs migrated at rates approximately threefold higher than CO-Fibs (Fig. 6A, NT). Addition of OPN-specific antibodies (7.5 μg/ml) markedly attenuated the migratory capabilities of both CO- and PH-Fibs (Fig. 6A, OPN-Abs). Of note, whereas addition of rOPN to PH-Fibs did not significantly affect their already high migratory capabilities, the low migratory capabilities of CO-Fibs were significantly augmented with addition of rOPN (Fig. 6A, rOPN).
Fig. 6.
Elevated migratory capabilities of PH-Fibs are attenuated upon downregulation of OPN expression. A: in a “scrape” assay (performed with addition of hydroxyurea to inhibit cell proliferation; see methods), sequestering OPN production by treatment with OPN-specific antibodies (7.5 μg/ml, 48 h) resulted in significantly attenuated migratory capabilities of both CO- and PH-Fibs (OPN-Abs). Addition of rOPN augmented migratory potential of CO-Fibs; however, it did not affect PH-Fibs. Number of cell populations analyzed: PH-Fibs, n = 5; CO-Fibs, n = 4. B: in a Boyden Transwell chamber assay under serum-free conditions, OPN knockdown by siRNA-3 (50 nM, 48 h) resulted in markedly attenuated migration of PH-Fibs. Data represent mean ± SE from 3 independent experiments. Number of PH-Fibs populations analyzed, n = 3; *P < 0.01 vs. NT cells.
In a Transwell Boyden chamber assay, PH-Fibs were treated with OPN-siRNA-3 or scrambled siRNA (50 nM, 48 h) and the numbers of migrating cells were counted. OPN-siRNA-3 treatment of PH-Fibs resulted in a significant decrease in the number of migrating cells compared with untreated cells, whereas scrambled siRNA did not affect migration (Fig. 6B).
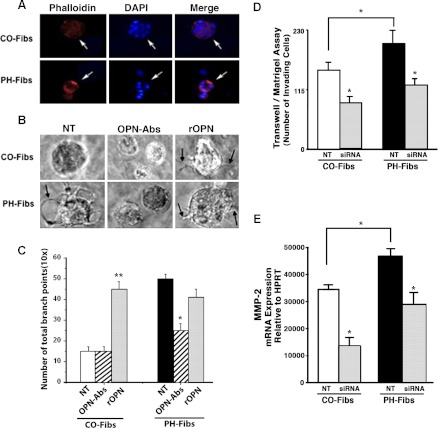
The role of OPN in proinvasive capabilities of PH-Fibs was tested by using both 3D growth factor-reduced Matrigel assay and 2D Transwell Boyden chamber assay approaches under serum-free conditions. Increased intra-Matrigel invasion of PH-Fibs compared with that of CO-Fibs (defined by the increased number of filopodia-like structures, a hallmark of cell invasion into Matrigel) was observed (Fig. 7). Addition of OPN-specific antibodies attenuated the increased invasiveness of PH-Fibs (Fig. 7, B and C, OPN-Abs), whereas addition of rOPN to CO-Fibs resulted in their increased invasiveness (i.e., appearance of the filopodia-like structures) (Fig. 7, B and C, rOPN).
Fig. 7.
OPN expression levels modulate proinvasive capabilities of PH- and CO-Fibs in 3D Matrigel assays. A: cells were plated onto growth factor-reduced Matrigel in serum-free medium. After 9 days, immunostaining for actin (via Alexa-594-conjugated phalloidin, red) to define cell morphology, and for cell nuclei (DAPI, blue) was performed. B: in 3D growth factor-reduced Matrigel cultures PH-Fibs generated visible filopodia-like structures, a hallmark of cell invasion into Matrigel (arrow in NT image), as opposed to CO-Fibs. In the presence of OPN-specific antibodies (7.5 μg/ml), generation of filopodia in PH-Fibs was inhibited. Addition of rOPN (20 ng/ml) resulted in generation of filopodia (arrows) not only in PH-Fibs, but even in CO-Fibs that did not occur under NT conditions. C: quantification of filopodia-like structures (the number of branch points) was performed via ibidi software analysis: http://www.ibidi.com (Munich, Germany). **P < 0.01, *P < 0.05. D: in Matrigel-coated Boyden chamber assay of cell invasion (see methods), siRNA3-mediated knockdown of OPN expression resulted in reduced invasion through Matrigel (used in a growth factor-reduced form) in both CO- and PH-Fibs. Data represent mean ± SE from 3 different experiments. *P < 0.05 vs. NT cells. E: under NT conditions, MMP-2 expression in PH-Fibs was significantly higher than in CO-Fibs. Treatment of both CO- and PH-Fibs with OPN-siRNA (50 nM, 48 h) resulted in significant decrease of MMP-2 expression (6-fold in CO-Fibs and 4-fold in PH-Fibs) compared with respective NT cells.
In the Transwell Boyden chamber assay, in which membranes were coated with Matrigel in its growth factor-reduced form (to exclude contribution of cell proliferation), invasiveness of untreated PH-Fibs was significantly higher than that of CO-Fibs (Fig. 7D, NT). Silencing of OPN with siRNA3 led to a significant decrease in the invasive capabilities of PH-Fibs, as well as to decreased basal levels in CO-Fibs (Fig. 7D, siRNA).
Cellular invasion is known to depend, at least in part, on cooperation between adhesion and proteolytic mechanisms. Since, on one hand, our data show upregulation of OPN receptors αVβ3 and CD44, and, on another hand, CD44 and matrix metalloproteinases (MMPs) have been shown to regulate fibroblast invasion and MMP-2 was shown to bind directly to αVβ3, we investigated the status of MMP expression, specifically MMP-2 and MMP-9 in highly proinvasive PH-Fibs and their control counterpart, CO-Fibs. Under basal unstimulated conditions, increased levels of MMP-2 expression (Fig. 7E, NT), but not MMP-9 (not shown) were observed in PH-Fibs compared with CO-Fibs. Treatment with OPN-siRNA-3 led to decreases in the MMP-2 mRNA levels in both CO- and PH-Fibs (Fig. 7E, siRNA). No significant differences were observed in the levels of MMP-9 expression between PH-Fibs and CO-Fibs (data not shown).
OPN Contributes to Constitutively Activated ERK and AKT Signaling Pathways in PH-Fibs
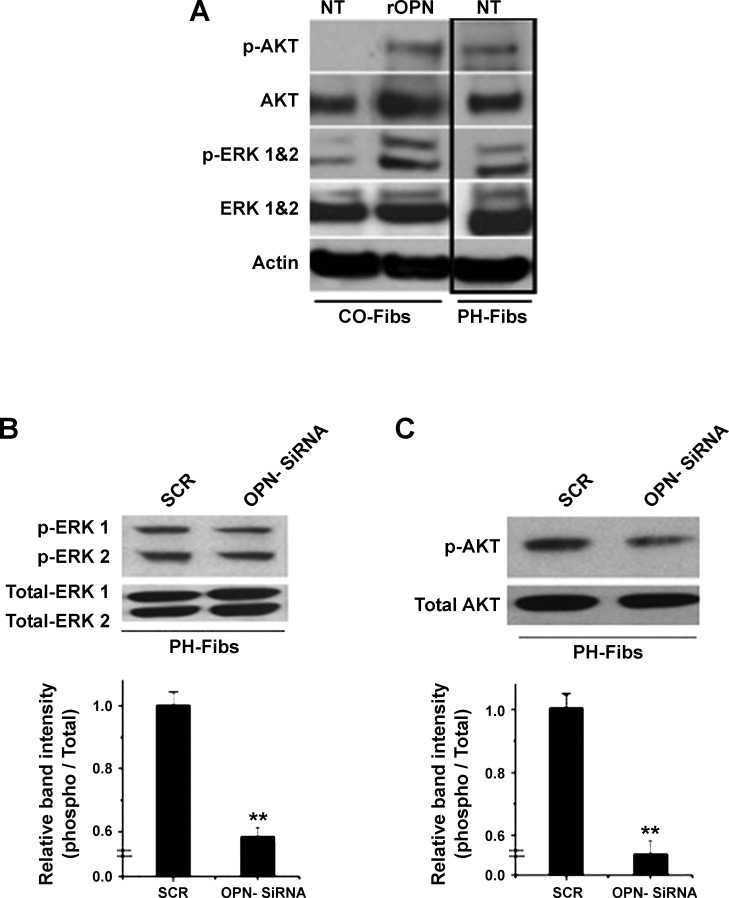
Since PH-Fibs exhibited high proliferative, migratory, and proinvasive capabilities under serum-free conditions, and this phenotype was maintained over numerous passages in culture (i.e., exhibited a “constitutively” activated phenotype), we next investigated the status of ERK1/2 and AKT signaling pathways that are known to contribute, at least in part, to cell proliferation, migration, and invasion (11, 41, 59). Under basal, serum-free conditions, PH-Fibs had markedly elevated levels of phospho-AKT and phospho-ERK1/2 compared with CO-Fibs (Fig. 8A, NT). However, treatment of CO-Fibs with rOPN (40 ng/ml, 2 h) resulted in the activation of both signaling pathways (Fig. 8A, rOPN). Treatment of PH-Fibs with OPN-siRNA3 for 72 h resulted in significant decreases of ERK1/2 and AKT phosphorylation (Fig. 8, B and C).
Fig. 8.
OPN expression controls activation state of ERK1/2 and AKT signaling pathways in CO- and PH-Fibs. A: under serum-free conditions, PH-Fibs exhibited constitutively activated [phosphorylated (p-)] ERK1/2 and AKT signaling pathways (PH-Fibs, NT lane). In contrast, under serum-free conditions, CO-Fibs exhibited low- to not- phosphorylated states of ERK1/2 and AKT, respectively (CO-Fibs, NT lane). Treatment of CO-Fibs with rOPN (20 ng/ml, 6 h) resulted in profound activation (phosphorylation) of both ERK1/2 and AKT signaling pathways (CO-Fibs, rOPN lane). B and C: treatment of PH-Fibs, maintained under serum-free conditions, with OPN-siRNA-3 (50 nM, 72 h) resulted in significant downregulation of both phospho-ERK1/2 (B) and phospho-AKT (C) compared with scrambled oligos (SCR). Data represent mean ± SE from 3 different experiments. **P < 0.01.
DISCUSSION
Both the in vivo and in vitro data of the present study demonstrate that PH-associated pulmonary vascular remodeling is characterized by markedly increased OPN expression in the pulmonary artery adventitia and by the emergence of adventitial fibroblasts (termed here PH-Fibs) that exhibit markedly increased OPN expression compared with control fibroblasts (CO-Fibs). PH-Fibs, isolated from calves with severe hypoxia-induced PH, exhibited high proliferative, migratory, and proinvasive capabilities, as well as constitutively activated ERK1/2 and AKT signaling pathways that correlated with high OPN expression. Decreasing expression levels of OPN (by either silencing OPN mRNA expression by siRNA or sequestering OPN production by specific antibodies) led to functional modulation of PH-Fibs toward a phenotype with attenuated proliferative, migratory, and invasive capabilities and attenuated levels of ERK1/2 and AKT phosphorylation, i.e., similar to that of CO-Fibs. Importantly, we showed that when slowly growing CO-Fibs were treated with rOPN, their DNA synthesis markedly increased to the levels resembling those of PH-Fibs. Thus our study supports the hypothesis that OPN directly contributes to the constitutively activated phenotype of PH-Fibs and that decreasing OPN expression levels in these cells results in altering their phenotype toward that of CO-Fibs.
PH is characterized by marked fibroproliferative changes in the pulmonary artery wall. The earliest and most dramatic proliferative changes are observed in the adventitia, where the fibroblast is considered by many to be the “sentinel” cell. In a variety of other vascular diseases, adventitial remodeling and augmented proliferation of adventitial fibroblasts have also been documented (53, 54). Although the mechanisms involved in the fibroblast responses remain obscure, the possibility that they are coordinated through interactions with ECM proteins is increasingly appreciated. OPN is a pleiotropic matricellular protein that is known to be involved in an array of biological processes including remodeling, metastasis, scarring, and inflammation (4, 7, 40, 41, 43, 61). Several in vivo and in vitro studies suggested an important role of OPN in mesenchymal cell proliferation in vascular remodeling (31, 32, 37). In patients with iPAH, increased OPN gene expression has been reported in the whole lung extracts, and elevated levels of OPN have been detected in plasma, compared with control subjects (32). Furthermore, upregulation of OPN mRNA expression in response to hypoxia has been demonstrated in lungs of rats and mice (5, 22, 23, 29). However, these studies did not pinpoint a specific cell type responsible for increased OPN expression/production in the hypoxic lung. A recent study from our laboratory, using laser capture microdissection techniques, showed pulmonary artery-specific upregulation of OPN along with other ECM proteins and cytokines/chemokines in the setting of chronic hypoxic PH, thus suggesting development of a pulmonary artery-specific fibroproliferative and proinflammatory microenvironment that potentially contributes to PH-associated vascular remodeling (5). In the present study we further demonstrated that, in vivo, the most dramatic upregulation of OPN in the pulmonary circulation was observed in the vascular adventitia and that PH-Fibs exhibited elevated OPN expression levels compared with low expression levels in CO-Fibs. Yet upregulating OPN expression in CO-Fibs with rOPN was sufficient to significantly augment their proproliferative capabilities.
Similar observations were made in cultured lung fibroblasts, where treatment with exogenous OPN has been shown to promote both cell proliferation and migration (38). Of note, silencing/sequestering already-low OPN expression/production levels in CO-Fibs resulted in further decreases in their proliferative and migratory capabilities, suggesting that a certain basal level of OPN expression exists in CO-Fibs that is essential for the growth and survival responses in these cells.
In several disease states (pulmonary fibrosis, myocardial infarction, experimental models of renal injury, certain forms of PH), tissue fibroblasts have been shown to display highly migratory and even proinvasive properties similar to those of metastatic cancer cells (30, 42, 66, 68). Vascular adventitial fibroblasts with an activated phenotype have been shown to invade vascular matrix and migrate to the medial and even intimal vessel wall layers (47, 51, 52). Data of the present study demonstrate that modulating OPN expression in PH-Fibs and/or CO-Fibs resulted in alteration of migratory and proinvasive phenotype of these cells. Proinvasive cell behavior is known to depend, at least in part, on cooperation between adhesion and proteolytic mechanisms. In the present study we show upregulation of OPN and its receptors αVβ3 and CD44 in vivo, in adventitia of hypertensive calves, and in PH-Fibs in vitro. Since cooperation/functional interaction of CD44, αVβ3, and MMPs have been shown to regulate fibroblast invasion (16, 21, 46), we tested the relationship between OPN expression and that of gelatinases MMP-2 and MMP-9. Our in vitro data demonstrate that the augmented proinvasive potential of PH-Fibs was regulated by OPN at least in part via MMP-2. These data are consistent with previous in vivo studies showing that downregulation of OPN by fluoxetine is associated with decreases in the expression of MMP-2 and with amelioration of vascular remodeling and inflammation in the monocrotaline rat model of PH (60).
In tumor cells, cellular processes, including proliferation, migration, and invasion, are known to be regulated, at least in part, by ERKs and AKT signaling pathways that have been shown to be activated by OPN (11, 41, 59). The present study demonstrates that pulmonary vascular remodeling in chronic hypoxic PH is associated with emergence of adventitial fibroblasts (PH-Fibs) with constitutively activated ERK1/2 and AKT signaling that is dependent on OPN expression levels. Thus our findings in a noncancerous cell type suggest that OPN contributes to enhanced proliferative, migratory, and proinvasive potential of a distinct adventitial fibroblast phenotype in the setting of hypoxic PH.
Our studies demonstrate upregulation of OPN and its receptors (αVβ3 and CD44) in the PA adventitia and in isolated fibroblasts from chronically hypoxic hypertensive calves. OPN promotes cell signaling, migration, and invasion via its interaction with two families of receptors: integrins and CD44 [note: OPN does not bind a standard form of CD44 (hyaluronic acid receptor) but does bind various isoforms of CD44 generated by alternative splicing] (45, 63). A well-recognized OPN receptor, αVβ3 integrin, has been shown to modulate several cellular functional modalities including activation of signal transduction, secretion of MMP-2, and cell motility/invasion (6, 16, 21, 46, 49, 69). CD44 has been correlated with cell metastatic/invasion potential of cancer cells (10, 18, 27, 62) and fibroblasts (30), and loss of CD44 expression in transformed cells resulted in impaired potential to form tumor nodules (19). Both CD44 and αVβ3 OPN receptors were suggested to play a role in distribution of MMP molecules on the surface of invasive cells and cooperate in cellular migration and invasion potential (20). Thus the data of the present study, demonstrating coordinately elevated expression levels of OPN receptors CD44 (shown at mRNA and protein levels both in vivo and in vitro) and αVβ3 (shown at protein level in vivo), as well as MMP-2 in PH-Fibs compared with their control counterpart CO-Fibs, suggest that αVβ3 and CD44 receptors cooperate in OPN-induced high proliferative, migratory, and invasive potential of PH-Fibs. Elucidating the OPN/αVβ3/CD44/MMP-2 interactions/pathways contributing to constitutively activated proproliferative and promigratory invasive PH-Fibs phenotype could facilitate better understanding of and potential molecular targets for therapy aimed at vascular remodeling in pulmonary hypertension just as it has in cancer (7, 17, 61).
In conclusion, our study suggests that elevated OPN expression contributes to enhanced proliferative, migratory, and proinvasive capabilities of a newly emerging adventitial fibroblast phenotype in the setting of hypoxic PH. When considered in the context of the previous data, demonstrating that OPN plasma levels correlate with degree of PH in human patients and that abrogating OPN expression ameliorates PH in experimental animal models, the present study provides additional information on the role of OPN in promoting a microenvironment in the pulmonary arteries conducive to arterial remodeling in chronic hypoxic PH. These data suggest that targeting fibroblast-OPN interactions could be useful in abrogating hypoxia-induced vascular remodeling.
GRANTS
This work was supported in part by a NIH Specialized Centers of Clinically Oriented Research (SCCOR) Grant HL-084923-05 (K. R. Stenmark) and a NIH Program Project Grant HL-014985-36, NHLBI Grant 5T32HL007171-34 (K. R. Stenmark), and NHLBI-HL 086783-01A2 (E. V. Gerasimovskaya).
AUTHOR CONTRIBUTIONS
AA, KRS are responsible for conception and design of the research. AA, ML, MGF, BK, EVG, SRR, BAM, MAF, RT performed the experiments. AA, KRS analyzed the data. AA, MGF, EVG, BM, KRS drafted, revised, and edited the manuscript. AA, ML, MGF, BK, EVG, SRR, BAM, MAF, RT, BOM, KRS approved the final version of the manuscript.
DISCLOSURES
No conflicts of interest, financial or otherwise are declared by the author(s).
ACKNOWLEDGMENTS
The authors are thankful to Danielle Burke for help with the primers, to Stephen Hofmeister and Marcia McGowan for outstanding help in preparing the manuscript, and to Drs. Dale Brown and Michael Yeager for critical review of the manuscript.
Lung tissues from idiopathic PAH patients and control subjects were provided by the Pulmonary Hypertension Breakthrough Initiative (PHBI), which is funded by the Cardiovascular Medical Research Education Fund (CMREF). The tissues were procured at the Transplant Procurement Centers at Stanford University, University of California, San Diego, Vanderbilt University and Allegheny General Hospital.
REFERENCES
- 1. Angelucci A, Festuccia C, Gravina GL, Muzi P, Bonghi L, Vicentini C, Bologna M. Osteopontin enhances the cell proliferation induced by the epidermal growth factor in human prostate cancer cells. Prostate 59: 157–166, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Anwar A, Dehn D, Siegel D, Kepa JK, Tang LJ, Pietenpol JA, Ross D. Interaction of human NAD(P)H:quinone oxidoreductase 1 (NQO1) with the tumor suppressor protein p53 in cells and cell-free systems. J Biol Chem 278: 10368–10373, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Archer S, Rich S. Primary pulmonary hypertension: a vascular biology and translational research “Work in progress”. Circulation 102: 2781–2791, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature 432: 332–337, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burke DL, Frid MG, Kunrath CL, Karoor V, Anwar A, Wagner BD, Strassheim D, Stenmark KR. Sustained hypoxia promotes the development of a pulmonary artery-specific chronic inflammatory microenvironment. Am J Physiol Lung Cell Mol Physiol 297: L238–L250, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chellaiah M, Fitzgerald C, Filardo EJ, Cheresh DA, Hruska KA. Osteopontin activation of c-src in human melanoma cells requires the cytoplasmic domain of the integrin alpha v-subunit. Endocrinology 137: 2432–2440, 1996 [DOI] [PubMed] [Google Scholar]
- 7. Chong HC, Tan CK, Huang RL, Tan NS. Matricellular proteins: a sticky affair with cancers. J Oncol 2012: 351089, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Debnath J, Walker SJ, Brugge JS. Akt activation disrupts mammary acinar architecture and enhances proliferation in an mTOR-dependent manner. J Cell Biol 163: 315–326, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Denhardt DT, Lopez CA, Rollo EE, Hwang SM, An XR, Walther SE. Osteopontin-induced modifications of cellular functions. Ann NY Acad Sci 760: 127–142, 1995 [DOI] [PubMed] [Google Scholar]
- 10. Desai B, Rogers MJ, Chellaiah MA. Mechanisms of osteopontin and CD44 as metastatic principles in prostate cancer cells. Mol Cancer 6: 18, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fong YC, Liu SC, Huang CY, Li TM, Hsu SF, Kao ST, Tsai FJ, Chen WC, Chen CY, Tang CH. Osteopontin increases lung cancer cells migration via activation of the alphavbeta3 integrin/FAK/Akt and NF-kappaB-dependent pathway. Lung Cancer 64: 263–270, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Frid MG, Aldashev AA, Dempsey EC, Stenmark KR. Smooth muscle cells isolated from discrete compartments of the mature vascular media exhibit unique phenotypes and distinct growth capabilities. Circ Res 81: 940–952, 1997 [DOI] [PubMed] [Google Scholar]
- 13. Frid MG, Li M, Gnanasekharan M, Burke DL, Fragoso M, Strassheim D, Sylman JL, Stenmark KR. Sustained hypoxia leads to the emergence of cells with enhanced growth, migratory, and promitogenic potentials within the distal pulmonary artery wall. Am J Physiol Lung Cell Mol Physiol 297: L1059–L1072, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gerasimovskaya EV, Woodward HN, Tucker DA, Stenmark KR. Extracellular ATP is a pro-angiogenic factor for pulmonary artery vasa vasorum endothelial cells. Angiogenesis 11: 169–182, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Giachelli CM, Liaw L, Murry CE, Schwartz SM, Almeida M. Osteopontin expression in cardiovascular diseases. Ann NY Acad Sci 760: 109–126, 1995 [DOI] [PubMed] [Google Scholar]
- 16. Godefroy E, Moreau-Aubry A, Diez E, Dreno B, Jotereau F, Guilloux Y. alpha v beta3-dependent cross-presentation of matrix metalloproteinase-2 by melanoma cells gives rise to a new tumor antigen. J Exp Med 202: 61–72, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hahnel A, Wichmann H, Greither T, Kappler M, Wurl P, Kotzsch M, Taubert H, Vordermark D, Bache M. Prognostic impact of mRNA levels of osteopontin splice variants in soft tissue sarcoma patients. BMC Cancer 12: 131, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harrison GM, Davies G, Martin TA, Mason MD, Jiang WG. The influence of CD44v3-v10 on adhesion, invasion and MMP-14 expression in prostate cancer cells. Oncol Rep 15: 199–206, 2006 [PubMed] [Google Scholar]
- 19. Hart IR, Birch M, Marshall JF. Cell adhesion receptor expression during melanoma progression and metastasis. Cancer Metastasis Rev 10: 115–128, 1991 [DOI] [PubMed] [Google Scholar]
- 20. Hofmann UB, Westphal JR, Van Muijen GN, Ruiter DJ. Matrix metalloproteinases in human melanoma. J Invest Dermatol 115: 337–344, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Hofmann UB, Westphal JR, Waas ET, Becker JC, Ruiter DJ, van Muijen GN. Coexpression of integrin alpha(v)beta3 and matrix metalloproteinase-2 (MMP-2) coincides with MMP-2 activation: correlation with melanoma progression. J Invest Dermatol 115: 625–632, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Hoshikawa Y, Matsuda Y, Suzuki S, Okada Y, Tabata T, Matsumura Y, Kondo T. Osteopontin may be responsible for pulmonary vascular remodeling. Chest 128, Suppl 6: 621S, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Hoshikawa Y, Nana-Sinkam P, Moore MD, Sotto-Santiago S, Phang T, Keith RL, Morris KG, Kondo T, Tuder RM, Voelkel NF, Geraci MW. Hypoxia induces different genes in the lungs of rats compared with mice. Physiol Genomics 12: 209–219, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Jeffery TK, Morrell NW. Molecular and cellular basis of pulmonary vascular remodeling in pulmonary hypertension. Prog Cardiovasc Dis 45: 173–202, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Klingel K, Kandolf R. Osteopontin: a biomarker to predict the outcome of inflammatory heart disease. Semin Thromb Hemost 36: 195–202, 2010 [DOI] [PubMed] [Google Scholar]
- 26. Kumar B, Koul S, Khandrika L, Meacham RB, Koul HK. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res 68: 1777–1785, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Lesley J, Hyman R, English N, Catterall JB, Turner GA. CD44 in inflammation and metastasis. Glycoconj J 14: 611–622, 1997 [DOI] [PubMed] [Google Scholar]
- 28. Li M, Riddle SR, Frid MG, El Kasmi KC, McKinsey TA, Sokol RJ, Strassheim D, Meyrick B, Yeager ME, Flockton AR, McKeon BA, Lemon DD, Horn TR, Anwar A, Barajas C, Stenmark KR. Emergence of fibroblasts with a proinflammatory epigenetically altered phenotype in severe hypoxic pulmonary hypertension. J Immunol 187: 2711–2722, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li P, Oparil S, Feng W, Chen YF. Hypoxia-responsive growth factors upregulate periostin and osteopontin expression via distinct signaling pathways in rat pulmonary arterial smooth muscle cells. J Appl Physiol 97: 1550–1558; discussion 1549, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Li Y, Jiang D, Liang J, Meltzer EB, Gray A, Miura R, Wogensen L, Yamaguchi Y, Noble PW. Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. J Exp Med 208: 1459–1471, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liaw L, Lombardi DM, Almeida MM, Schwartz SM, deBlois D, Giachelli CM. Neutralizing antibodies directed against osteopontin inhibit rat carotid neointimal thickening after endothelial denudation. Arterioscler Thromb Vasc Biol 17: 188–193, 1997 [DOI] [PubMed] [Google Scholar]
- 32. Lorenzen JM, Nickel N, Kramer R, Golpon H, Westerkamp V, Olsson KM, Haller H, Hoeper MM. Osteopontin in patients with idiopathic pulmonary hypertension. Chest 139: 1010–1017, 2011 [DOI] [PubMed] [Google Scholar]
- 33. Majesky MW, Dong XR, Hoglund V, Mahoney WM, Jr, Daum G. The adventitia: a dynamic interface containing resident progenitor cells. Arterioscler Thromb Vasc Biol 31: 1530–1539, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Matsui Y, Morimoto J, Uede T. Role of matricellular proteins in cardiac tissue remodeling after myocardial infarction. World J Biol Chem 1: 69–80, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nakanishi K, Tajima F, Itoh H, Nakata Y, Osada H, Hama N, Nakagawa O, Nakao K, Kawai T, Takishima K, Aurues T, Ikeda T. Changes in atrial natriuretic peptide and brain natriuretic peptide associated with hypobaric hypoxia-induced pulmonary hypertension in rats. Virchows Arch 439: 808–817, 2001 [DOI] [PubMed] [Google Scholar]
- 36. Okamoto H, Imanaka-Yoshida K. Matricellular proteins: new molecular targets to prevent heart failure. Cardiovasc Ther 2011. doi: 10.1111/j.1755-5922.2011.00276.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 37. Panda D, Kundu GC, Lee BI, Peri A, Fohl D, Chackalaparampil I, Mukherjee BB, Li XD, Mukherjee DC, Seides S, Rosenberg J, Stark K, Mukherjee AB. Potential roles of osteopontin and alphaVbeta3 integrin in the development of coronary artery restenosis after angioplasty. Proc Natl Acad Sci USA 94: 9308–9313, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pardo A, Gibson K, Cisneros J, Richards TJ, Yang Y, Becerril C, Yousem S, Herrera I, Ruiz V, Selman M, Kaminski N. Up-regulation and profibrotic role of osteopontin in human idiopathic pulmonary fibrosis. PLoS Med 2: e251, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rabinovitch M. Pathobiology of pulmonary hypertension. Extracellular matrix. Clin Chest Med 22: 433–449, viii, 2001 [DOI] [PubMed] [Google Scholar]
- 40. Rangaswami H, Bulbule A, Kundu GC. Nuclear factor-inducing kinase plays a crucial role in osteopontin-induced MAPK/IkappaBalpha kinase-dependent nuclear factor kappaB-mediated promatrix metalloproteinase-9 activation. J Biol Chem 279: 38921–38935, 2004 [DOI] [PubMed] [Google Scholar]
- 41. Rangaswami H, Bulbule A, Kundu GC. Osteopontin: role in cell signaling and cancer progression. Trends Cell Biol 16: 79–87, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Riches K, Morley ME, Turner NA, O'Regan DJ, Ball SG, Peers C, Porter KE. Chronic hypoxia inhibits MMP-2 activation and cellular invasion in human cardiac myofibroblasts. J Mol Cell Cardiol 47: 391–399, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rodrigues LR, Teixeira JA, Schmitt FL, Paulsson M, Lindmark-Mansson H. The role of osteopontin in tumor progression and metastasis in breast cancer. Cancer Epidemiol Biomarkers Prev 16: 1087–1097, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132: 365–386, 2000 [DOI] [PubMed] [Google Scholar]
- 45. Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science 238: 491–497, 1987 [DOI] [PubMed] [Google Scholar]
- 46. Samanna V, Wei H, Ego-Osuala D, Chellaiah MA. Alpha-V-dependent outside-in signaling is required for the regulation of CD44 surface expression, MMP-2 secretion, and cell migration by osteopontin in human melanoma cells. Exp Cell Res 312: 2214–2230, 2006 [DOI] [PubMed] [Google Scholar]
- 47. Sartore S, Chiavegato A, Faggin E, Franch R, Puato M, Ausoni S, Pauletto P. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: from innocent bystander to active participant. Circ Res 89: 1111–1121, 2001 [DOI] [PubMed] [Google Scholar]
- 48. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3: 1101–1108, 2008 [DOI] [PubMed] [Google Scholar]
- 49. Seftor RE, Seftor EA, Gehlsen KR, Stetler-Stevenson WG, Brown PD, Ruoslahti E, Hendrix MJ. Role of the alpha v beta 3 integrin in human melanoma cell invasion. Proc Natl Acad Sci USA 89: 1557–1561, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Senger DR, Wirth DF, Hynes RO. Transformed mammalian cells secrete specific proteins and phosphoproteins. Cell 16: 885–893, 1979 [DOI] [PubMed] [Google Scholar]
- 51. Shi Y, O'Brien JE, Jr, Ala-Kokko L, Chung W, Mannion JD, Zalewski A. Origin of extracellular matrix synthesis during coronary repair. Circulation 95: 997–1006, 1997 [DOI] [PubMed] [Google Scholar]
- 52. Sobin SS, Tremer HM, Hardy JD, Chiodi HP. Changes in arteriole in acute and chronic hypoxic pulmonary hypertension and recovery in rat. J Appl Physiol 55: 1445–1455, 1983 [DOI] [PubMed] [Google Scholar]
- 53. Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res 99: 675–691, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Stenmark KR, Gerasimovskaya E, Nemenoff RA, Das M. Hypoxic activation of adventitial fibroblasts: role in vascular remodeling. Chest 122: 326S–334S, 2002 [DOI] [PubMed] [Google Scholar]
- 55. Stenmark KR, Nozik-Grayck E, Gerasimovskaya EV, Anwar A, Li M, Riddle SR, Frid MG. The adventitia: essential role in pulmonary vascular remodeling. Comp Physiol 1: 141–161, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tuder RM, Yun JH, Bhunia A, Fijalkowska I. Hypoxia and chronic lung disease. J Mol Med 85: 1317–1324, 2007 [DOI] [PubMed] [Google Scholar]
- 57. Vij R, Noth I. Peripheral blood biomarkers in idiopathic pulmonary fibrosis. Transl Res 159: 218–227, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wagenseil JE, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiol Rev 89: 957–989, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wai PY, Kuo PC. Osteopontin: regulation in tumor metastasis. Cancer Metastasis Rev 27: 103–118, 2008 [DOI] [PubMed] [Google Scholar]
- 60. Wang Y, Han DD, Wang HM, Liu M, Zhang XH, Wang HL. Downregulation of osteopontin is associated with fluoxetine amelioration of monocrotaline-induced pulmonary inflammation and vascular remodelling. Clin Exp Pharmacol Physiol 38: 365–372, 2011 [DOI] [PubMed] [Google Scholar]
- 61. Weber GF. The cancer biomarker osteopontin: combination with other markers. Cancer Genomics Proteomics 8: 263–288, 2011 [PubMed] [Google Scholar]
- 62. Weber GF. The metastasis gene osteopontin: a candidate target for cancer therapy. Biochim Biophys Acta 1552: 61–85, 2001 [DOI] [PubMed] [Google Scholar]
- 63. Weber GF, Ashkar S, Glimcher MJ, Cantor H. Receptor-ligand interaction between CD44 and osteopontin (Eta-1). Science 271: 509–512, 1996 [DOI] [PubMed] [Google Scholar]
- 64. Welsh DJ, Peacock AJ, MacLean M, Harnett M. Chronic hypoxia induces constitutive p38 mitogen-activated protein kinase activity that correlates with enhanced cellular proliferation in fibroblasts from rat pulmonary but not systemic arteries. Am J Respir Crit Care Med 164: 282–289, 2001 [DOI] [PubMed] [Google Scholar]
- 65. Welsh DJ, Scott P, Plevin R, Wadsworth R, Peacock AJ. Hypoxia enhances cellular proliferation and inositol 1,4,5-triphosphate generation in fibroblasts from bovine pulmonary artery but not from mesenteric artery. Am J Respir Crit Care Med 158: 1757–1762, 1998 [DOI] [PubMed] [Google Scholar]
- 66. White ES, Lazar MH, Thannickal VJ. Pathogenetic mechanisms in usual interstitial pneumonia/idiopathic pulmonary fibrosis. J Pathol 201: 343–354, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Woo MM, Salamanca CM, Minor A, Auersperg N. An improved assay to quantitate the invasiveness of cells in modified Boyden chambers. In Vitro Cell Dev Biol Anim 43: 7–9, 2007 [DOI] [PubMed] [Google Scholar]
- 68. Xie Y, Sakatsume M, Nishi S, Narita I, Arakawa M, Gejyo F. Expression, roles, receptors, and regulation of osteopontin in the kidney. Kidney Int 60: 1645–1657, 2001 [DOI] [PubMed] [Google Scholar]
- 69. Yang Y, Dang D, Atakilit A, Schmidt B, Regezi J, Li X, Eisele D, Ellis D, Ramos DM. Specific alpha v integrin receptors modulate K1735 murine melanoma cell behavior. Biochem Biophys Res Commun 308: 814–819, 2003 [DOI] [PubMed] [Google Scholar]
- 70. Zhang A, Liu Y, Shen Y, Xu Y, Li X. Osteopontin silencing by small interfering RNA induces apoptosis and suppresses invasion in human renal carcinoma Caki-1 cells. Med Oncol 27: 1179–1184, 2010 [DOI] [PubMed] [Google Scholar]