Abstract
We recently reported that integrin αvβ3 is necessary for vascular barrier protection in mouse models of acute lung injury and peritonitis. Here, we used mass spectrometric sequencing of integrin complexes to isolate the novel β3-integrin binding partner IQGAP1. Like integrin β3, IQGAP1 localized to the endothelial cell-cell junction after sphingosine-1-phosphate (S1P) treatment, and IQGAP1 knockdown prevented cortical actin formation and barrier enhancement in response to S1P. Furthermore, knockdown of IQGAP1 prevented localization of integrin αvβ3 to the cell-cell junction. Similar to β3-null animals, IQGAP1-null mice had increased pulmonary vascular leak compared with wild-type controls 3 days after intratracheal LPS. In an Escherichia coli pneumonia model, IQGAP1 knockout mice had increased lung weights, lung water, and lung extravascular plasma equivalents of 125I-labeled albumin compared with wild-type controls. Taken together, these experiments indicate that IQGAP1 is necessary for S1P-mediated vascular barrier protection during acute lung injury and is required for junctional localization of the barrier-protective integrin αvβ3.
Keywords: S1P, cortical actin, proteomics
vascular leak of protein and fluid into the extravascular space is a hallmark of inflammation and contributes to the severe organ injury seen in acute lung injury and sepsis (26). During acute inflammation, edemagenic agonists such as thrombin and histamine disrupt junctional interactions among endothelial cells, leading to extravasation of fluid and protein via a paracellular route (11, 25). Vascular leak is normally counteracted by the barrier formed by endothelial cell-cell contacts, interactions that are stabilized by the cellular cytoskeleton and, in particular, the polymerization of actin subjacent to the plasma membrane (8). This cytoskeleton structure, known as cortical actin, can be modulated by a number of agonists, including hyperosmolar sucrose, angiopoietin-1, and the lipid sphingosine-1-phosphate (S1P; 9, 14, 20).
Multiple studies have shown a barrier-protective effect of S1P or S1P analogs administered during acute lung injury (13, 16, 17). Moreover, in vivo vascular barrier function during treatment with edemagenic agonists requires endogenous, intravascular S1P (4). The S1P receptor S1P1 signals intracellularly via the second messenger Gi to activate the small GTPase Rac1 and downstream effectors that promote the formation of cortical actin, stabilizing cell-cell contacts. S1P thus increases barrier function of endothelial monolayers as measured by transendothelial electrical resistance (TER) (8). Evidence of the importance of S1P-induced cortical actin in barrier enhancement was established by studies in which inhibitors of actin polymerization prevented the barrier-enhancing effect of S1P (9).
We recently reported that blockade of integrin αvβ3 prevents the barrier-protective effect of S1P added to thrombin-treated endothelial monolayers (21). Moreover, integrin β3 null mice have higher vascular leak and mortality in LPS models of acute lung injury and sepsis, respectively. As part of these studies, we found that S1P caused assembly of β3-containing complexes at the cell-cell junction, and blockade of αvβ3 prevented S1P-induced cortical actin formation. These data motivated the question of how β3 integrin-containing focal complexes regulate endothelial barrier function downstream of S1P, and in particular what integrin binding partners mediate the barrier strengthening and actin organizing effects of the integrin.
Here, taking a proteomic approach to discovery of potential intracellular effectors of the vascular barrier function of αvβ3, we performed immunoprecipitation of β3 complexes from human pulmonary artery endothelial cells (HPAEC) for mass spectrometric discovery of β3 integrin binding partners. By this method, we identified the novel β3 binding partner IQGAP1, a 190-kDa scaffold protein that has been linked to multiple cellular pathways including MAP kinase signaling and GTPase activation (3). Of note, Berdyshev et al. (2) recently found that IQGAP1 relocated to the endothelial cell periphery after S1P treatment, and small interfering RNA knockdown of IQGAP1 inhibited endothelial migration in response to S1P. However, whether IQGAP1 is required for the barrier-protective effects of S1P remains untested.
At baseline, IQGAP1 knockout mice are normal except for gastric hyperplasia (12). However, study of these mice in the context of in vivo inflammatory injury has not been reported. Given the discovery of IQGAP1 as a binding partner of integrin β3, and the previous finding that S1P-induced migration required IQGAP1, we hypothesized that IQGAP1 is necessary for vascular barrier protection in experimental models of acute lung injury and for S1P-induced barrier protection in endothelial monolayers.
MATERIALS AND METHODS
Immunoprecipitation.
Immunoprecipitation intended for mass spectrometry was performed as follows (10): anti-β3-integrin antibody made by our laboratory (Axum-2) or anti-major histocompatibility complex (MHC) I antibody (Biolegend, clone W6/32) was coupled to tosyl-activated paramagnetic Dynabeads M-450 (4.5-μm diameter; Invitrogen) as described in the manufacturer's protocol. Beads were then combined with HPAEC (6 beads per cell, 30 million total cells) that had been trypsinized and suspended in endothelial basal medium-2 (EBM-2, Lonza). Beads and cells were incubated with 70 rotations/min for 1 h at 4°C. Samples were washed twice in PBS. dimethyl 3,3′-dithiobispropionimidate·2 HCl (DTBP) cross-linker (Thermo Fisher Scientific) in PBS (pH = 8) was added to bead-bound cells to a final concentration of 3 mM, and samples were incubated for 45 min at 37°C with 70 rotations/min. Cross-linker was quenched with 25 mM Tris·HCl before isolation of bead-bound cells on a magnet. Samples were washed with CSK (cytoskeletal) buffer [10 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (pH 6.8), 50 mM NaCl, 150 mM sucrose, 3 mM MgCl2, and 1 mM MnCl2] supplemented with 20 mM Tris·HCl (pH 8.5). Cells were lysed in CSK+ buffer [CSK buffer supplemented with 0.5% (wt/vol) Triton X-100 and protease and phosphatase inhibitor cocktail (Thermo)] for 30 min on ice with sonication (Branson Sonifier 250, four 5-s continuous pulses at amplitude 2, Danbury, CT). After cell lysis, beads were washed five times with CSK+ buffer, and affinity-purified proteins were eluted from beads with reducing sample buffer [50 mM Tris·HCl (pH 6.8), 10% (wt/vol) glycerol, 4% (wt/vol) SDS, 0.004% (wt/vol) bromophenol blue, and 8% (vol/vol) β-mercaptoethanol]. Protein samples were separated from beads with a magnet, resolved by SDS-PAGE, and analyzed by Coomassie staining.
Other samples were analyzed by immunoprecipitation by the following protocol: after overnight serum starvation, confluent HPAEC were lysed in 1% Triton X-100, 25 mM Tris·HCl, 125 mM NaCl, and protease and phosphatase inhibitor cocktail (Thermo). In some cases, cells were pretreated with S1P (0.5 μM) for 5 min prior to lysis. Lysates of equal protein concentration were precleared with Sepharose beads (Sigma) and then incubated with IQGAP1 antibody (Millipore, clone AF4) for 1 h at 4°C with rotation (70 rpm). Protein G Sepharose beads were then added with incubation for a further 1 h. Samples were washed six times with 1 ml of lysis buffer and eluted with reducing sample buffer as above, resolved by SDS-PAGE, and analyzed by immunoblot with IQGAP1 antibody (Millipore, clone AF4) or anti-integrin β3 antibody (Axum-4) generated in our laboratory.
GST-integrin protein binding assay.
A glutathione S-transferase (GST) fusion protein containing the β3 cytoplasmic domain (amino acids 742–788) was generated by cloning PCR-amplified sequences into the bacterial expression plasmid pGEX-4T-1 (Amersham Biosciences). Plasmids for GST-β3 and GST were used to transform DH5α bacteria (Invitrogen) under ampicillin selection, and transformed cells were cultured to log phase in 400 ml of YT medium followed by addition of 0.1 mM isopropyl-β-d-1-thiogalactopyranoside (Sigma) for 3 h. Cultures were pelleted by centrifugation, resuspended in PBS with protease inhibitor (Roche) and 100 μM PMSF, and sonicated (Branson Sonifier 250, 2 30-s pulses with 30% duty cycle at amplitude 7, Danbury, CT). Then 1% Triton X-100 was added to the solution with gentle agitation at 4° for 30 min. The solution was then centrifuged for 15 min at 10,000 rpm in a JA-20 rotor (Beckman Centrifuge, J2-21M) at 4°. GST and GST-β3 were purified by incubation with glutathione-Sepharose beads (Amersham), which were then used as bait for capture of recombinant IQGAP1, made by in vitro transcription and translation (IVTT). Plasmid containing full-length wild-type IQGAP1 (gift of Dr. David B. Sacks) was used as a template for IVTT with the T7 RNA polymerase transcription/translation system by using rabbit reticulocyte lysate (Promega) and l-[35S]methionine (AG1094; Amersham Biosciences) to produce 35S-labeled proteins according to the manufacturer's recommendations. Equally divided volumes of five pooled 40-μl in vitro translation reactions were incubated with 1.0 nmol of bead-bound GST or GST-β3 cytoplasmic domain fusion protein, in binding buffer (PBS with 1% Triton X-100) at 4°C for 2 h. The beads were washed five times in 1 ml of binding buffer, resuspended in 40 μl 2 × Laemmli sample buffer, and heated at 95°C for 5 min. Bound proteins were separated by SDS-PAGE (10%) under reducing conditions. Gels were fixed in 50% methanol and 10% acetic acid for 30 min, soaked in a fluorographic reagent (Amplify; Amersham Biosciences), dried, and developed by autoradiography.
Reversed-phase liquid chromatography-electrospray tandem mass spectrometry analysis.
Gels were cut along the lanes in 10 pieces, and proteins were digested in-gel with trypsin as described previously (19). The extracted digests were vacuum evaporated and resuspended in 10 μl of 0.1% formic acid in water. The digests were separated by nano-flow liquid chromatography using a 75 μm × 150 mm reverse phase 1.7 μm BEH 130 C18 column (Waters) at a flow rate of 350 nl/min in a NanoAcquity Ultra performance UPLC system (Waters). Solvent A was 0.1% formic acid in water and solvent B was 0.1% formic acid in acetonitrile. Following equilibration of the column in 5% solvent B, approximately one-half of each digest (5 μl) was injected, then the organic content of the mobile phase was increased linearly to 40% over 30 min, and then to 50% in 1 min. The liquid chromatography eluate was coupled to a hybrid linear ion trap-Orbitrap mass spectrometer (LTQ-Orbitrap, Thermo Scientific, San Jose, CA) equipped with a nanoelectrospray ion source. Spraying was from an uncoated 15-μm inner diameter spraying needle (New Objective, Woburn, MA). Peptides were analyzed in positive ion mode and in information-dependent acquisition mode to automatically switch between mass spectrometry (MS) and tandem mass spectrometry (MS/MS) acquisition. MS spectra were acquired in profile mode using the Orbitrap analyzer in the mass-to-charge ratio (m/z) range between 300 and 1,800. For each MS spectrum, the six most intense multiple charged ions over a threshold of 1,000 counts were selected to perform collision-induced dissociation (CID) experiments. Product ions were analyzed on the linear ion trap in centroid mode. The CID collision energy was automatically set to 25%. A dynamic exclusion window of 0.5 Da was applied that prevented the same m/z from being selected for 180 s after its acquisition.
In some cases samples were analyzed using the same chromatographic setting in a hybrid linear ion trap-Fourier transform mass spectrometer (LTQ-FT, Thermo Scientific, San Jose, CA) equipped with a nanoelectrospray ion source. Spraying was from an uncoated 15-μm-inner diameter spraying needle (New Objective, Woburn, MA). Peptides were analyzed in positive ion mode and in information-dependent acquisition mode to automatically switch between MS and MS/MS acquisition. MS spectra were acquired in profile mode by using the ion cyclotron resonance analyzer in the m/z range between 310 and 1,600. For each MS spectrum, the five most intense multiple charged ions over a threshold of 200 counts were selected to perform CID experiments. Product ions were analyzed on the linear ion trap in profile mode. CID collision energy was automatically set to 35%. A dynamic exclusion window of 1 Da was applied that prevented the same m/z from being selected for 60 s after its acquisition.
Peak lists were generated by use of Mascot Distiller version 2.1.0.0 (Matrix Science, Boston, MA). Parameters for MS processing were set as follows: peak half-width, 0.02; data points per dalton, 100. Parameters for MS/MS data were set as follows: peak half-width, 0.02; data points per dalton, 100. The peak list was searched against the human subset of the UniProtKB database as of January 11, 2011 (containing 194452 entries) using in-house ProteinProspector version 5.8.0 (a public version is available on line). Peptide tolerance in searches was 30 ppm for precursor and 0.6 Da for product ions, respectively. Peptides containing two miscleavages were allowed. Carbamidomethylation of cysteine, acetylation of the NH2 terminus of the protein, pyroglutamate formation from NH2-terminal glutamine, and oxidation of methionine were allowed as variable modifications. The number of modifications was limited to two per peptide. Hits were considered significant when two or more peptide sequences matched a protein entry and the Prospector score was above the significance level. A minimal ProteinProspector protein score of 20, a peptide score of 15, a maximum expectation value of 0.05, and a minimal discriminant score threshold of 0.0 were used for initial identification criteria. For identifications based on one peptide sequence with high scores, the MS/MS spectrum was reinterpreted manually by matching all the observed fragment ions to a theoretical fragmentation obtained by use of MS Product (Protein Prospector; 5).
Immunoblot.
Cultured cells were lysed with 1% Triton X-100 [25 mM Tris·HCl, 125 mM NaCl, and protease and phosphatase inhibitor cocktail (Thermo)]. Equal amounts of protein were resolved by SDS-PAGE under reducing conditions, blotted onto polyvinylidene difluoride membranes, blocked for 1 h at room temperature with 3% nonfat milk, and probed for IQGAP1 (Millipore, clone AF4) and Crk (BD Transduction Laboratories) at room temperature for 1 h. Horseradish peroxidase-conjugated anti-mouse antibodies were used as secondary antibodies (Santa Cruz).
Immunocytochemistry.
HPAEC were maintained in culture using EGM-2 media (Lonza) and used for experiments between passages 5 and 10. Cells were seeded on glass coverslips (Fisher) coated with 0.1% gelatin (Sigma) at a density of 60,000 cells/coverslip. At 24 h, cells were in some cases serum starved for 1 h and then treated with integrin β3 blocking antibody (Axum 2) made in our laboratory or mouse IgG control (Jackson Laboratories); in other cases the cells were simply serum starved for 2 h. Cells were then treated with 0.5 μM S1P (Sigma, stock solution 1 mg/ml methanol diluted 1:5,270 in serum-free medium) or PBS for 5 min, washed with PBS (Cellgro), and fixed in 4% paraformaldehyde (Electron Microscopy Sciences, 16% stock solution diluted in PBS) for 10 min at room temperature. Cells were then washed three times in PBS before permeabilization in 0.5% Triton X-100 (vol/vol in PBS) for 5 min. Cells were blocked in 1% BSA with 10% serum for 1 h followed by 1 h incubation with IQGAP1 antibody (H-106; Santa Cruz) or integrin β3 antibody made in our laboratory (Axum 8). Cells were PBS washed and incubated with fluorophore-tagged secondary antibody (Invitrogen) followed by PBS wash. In some cases, cells were stained for 20 min with 1:40 rhodamine phalloidin (Invitrogen) followed by PBS wash. Coverslips were mounted in Prolong Gold mounting medium (Sigma) and imaged with a Leica DM5000B inverted microscope equipped for immunofluorescence.
shRNA.
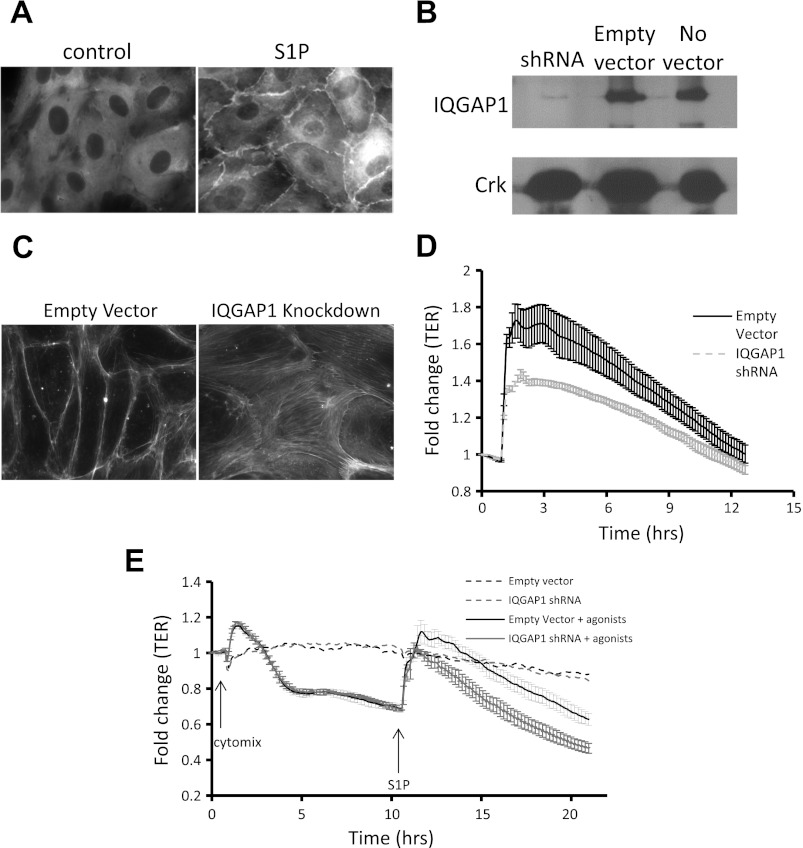
PsicoOligomaker 1.5 (web.mit.edu/jacks-lab) was used to select short hairpin RNA (shRNA) targets from the 3′ untranslated region of human IQGAP1 mRNA and to generate corresponding forward and reverse oligos for annealing and ligation into the green fluorescent protein (GFP)-containing expression vector PSicoR (Addgene). Ligation products found by sequencing to contain the shRNA construct and empty vector control were submitted for lentivirus production by the University of California, San Francisco (UCSF) Lentiviral RNAi Core (Dr. Michael McManus). HPAEC were inoculated with 2 plaque-forming units/cell (multiplicity of infection = 2) virus with 8 μg/ml polybrene (Sigma) for 72 h, sorted based on GFP expression (FACS Aria, BD Biosciences), and cultured for 10 days to ensure adequate knockdown prior to use in experiments. The IQGAP1 3′ untranslated region target GCAAAGACCTAGCCAACAA was selected for functional experiments based on efficient knockdown by immunoblot in HPAEC (Fig. 2B).
Fig. 2.
Effects of S1P on cellular localization of IQGAP1 and on barrier function after IQGAP1 knockdown. A: HPAEC were grown to confluence on glass coverslips, serum starved for 2 h, treated with S1P (0.5 μM) or vehicle for 5 min, and fixed and labeled with IQGAP1 antibody (×40). B: Western blot demonstrating short hairpin RNA (shRNA) knockdown of IQGAP1 in HPAEC. C: HPAEC were infected with lentivirus, sorted for green fluorescent protein (GFP) positivity marking viral infection, grown to confluence on glass coverslips, serum starved for 2 h, treated with S1P (0.5 μM) or vehicle for 5 min, and stained with rhodamine phalloidin for visualization of filamentous actin (×40). D: HPAEC were sorted for GFP marking shRNA infection and were seeded in a 96-well plate for measurement of transendothelial electrical resistance (TER) increase in response to S1P (0.5 μM) or vehicle by electrical cell-substrate impedance sensing (ECIS). Data for either condition are means for 6 wells normalized to baseline prior to addition of S1P (± SE). P < 0.01 for peak difference. E: HPAEC were sorted for GFP marking shRNA infection and were seeded in a 96-well plate for measurement of TER increase in response to cytomix (a combination of TNF-α, IL-1β, and IFN-γ, each at 50 ng/ml) followed by S1P (0.5 μM) as indicated by arrows. P < 0.05 for peak difference after S1P treatment. Results depicted in A, B, and C are representative of 3 experiments.
TER.
Measurements of TER were performed by use of an electrical cell-substrate impedance sensing system (ECIS) (Applied BioPhysics, Troy, NY). HPAEC were grown to confluence in 96-well-format printed circuit board wells containing gold microelectrodes in series with a large gold counterelectrode (1 cm2) connected to a phase-sensitive lock-in amplifier. A noninvasive constant current of less than 2 μA was applied across the electrodes by the Z-theta system at 4,000 Hz, and TER was monitored continuously including during treatment with S1P (Sigma) and cytomix (IFN-γ, TNF-α, and IL-1β, R&D Systems). Values from each microelectrode were pooled at discrete time points and plotted vs. time as means ± SE.
Animals.
IQGAP1 and wild-type controls (strain 129J) were the kind gift of Wadie Bahou (SUNY Stony Brook) and were maintained in the UCSF barrier facility. Eight- to 10-wk-old, sex-matched mice were used for all studies. Generation and genotyping procedures of IQGAP1 mice were described previously (12). Anesthesia was induced with an intraperitoneal injection of a mixture of ketamine (90 mg/kg) and xylazine (10 mg/kg) or by inhalation of 3 l/min isoflurane. The Committee on Animal Research of the University of California, San Francisco approved all the protocols, and all procedures conformed to National Institutes of Health guidelines.
LPS lung injury.
After induction of anesthesia, 100 μl of a 1 mg/ml aqueous solution of LPS (List Biological Laboratories) was instilled via an intratracheal P10 catheter (Fisher). Three days later mice were injected with 75 mg/kg aqueous Evans blue dye solution (12.5 mg/ml, retroorbital) and euthanized after 2 h. The lung was perfused with 20 U/ml heparin in PBS by placement of an angiocath in the right ventricle, with left atrial puncture for effluent. A simultaneous 1-ml sample of venous blood was drawn from the inferior vena cava, heparinized with 2 μl of 20 U/ml aqueous heparin, and centrifuged at 14,000 rpm with a tabletop centrifuge for 5 min; the plasma supernatant was saved for analysis. The lung was removed en bloc and placed in 1 ml formamide for 3 days. Absorbance at 620 nm of the formamide and a 1:50 dilution of plasma in formamide were measured and expressed as a quotient of lung Evans blue/plasma Evans blue. In some cases, instead of injection of Evans blue, mice were euthanized at 3 days for bronchoalveolar lavage (BAL), with measurement of leukocyte number by manual counting and BAL protein by BCA colorimetric protein assay (Thermo).
Acute E. coli pneumonia mouse model.
Escherichia coli serotype K1 was originally isolated from the blood of a patient with biliary sepsis. The methods used to passage, store, amplify, and quantify the bacteria have been previously described (22). E. coli 107 CFU in 25 μl of PBS were instilled by direct visualization into trachea after induction of anesthesia by ketamine-xylazine as indicated above. Immediately before exposure to E. coli, mice received 0.05 μCi 125I-labeled albumin via right jugular vein. Mice were monitored for 5 h and euthanized to measure excess lung water and lung vascular permeability to protein. As previously described (22, 23), the lungs were removed, counted in a gamma counter (Packard), weighed, and homogenized (after addition of 1 ml distilled water). The blood was collected through puncture of the right ventricle. The homogenate was weighed and a fraction was centrifuged (12,000 rpm, 8 min) for assay of hemoglobin concentration in the supernatant. Another fraction of homogenate, supernatant, and blood was weighed and then desiccated in an oven (60°C for 24 h). Excess lung water calculation was based on a standard formula. Lung extravascular plasma equivalents (index of lung vascular permeability to protein) were calculated as the counts of 125I-labeled albumin in the blood-free lung tissue divided by the counts of 125I-labeled albumin in the plasma. In some cases, BAL leukocytes were measured by a Coulter counter (Z1 series; Beckman Coulter).
Statistics.
Differences between groups were compared by unpaired t-tests. Each n represents isolation from a single mouse. A P value ≤ 0.05 was considered significant. Data are reported as means ± SE.
RESULTS
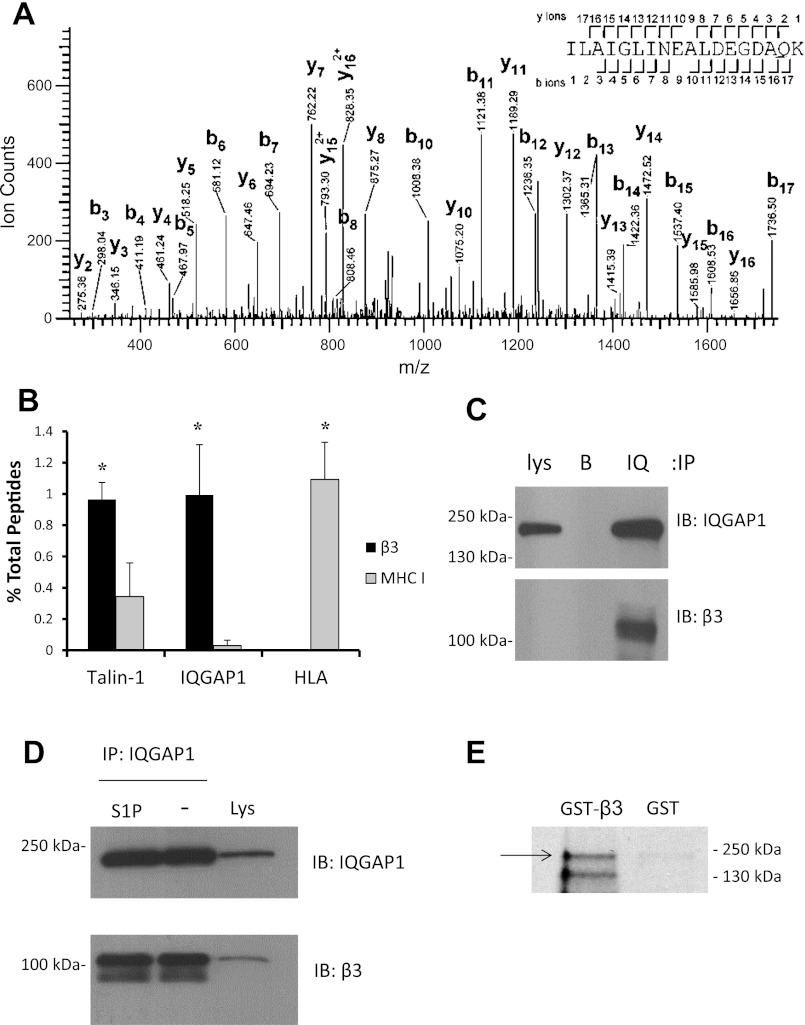
To identify novel binding partners of endothelial integrin β3, we employed a recently described immunoprecipitation protocol for high-yield isolation of cytoskeletally engaged integrin complexes (10). After antibody capture of the extracellular domain of β3 integrin in intact cells, samples were chemically cross-linked, lysed, and sonicated for separation of integrin complexes from the associated cytoskeletal network. Analysis of peptide mass spectra from these integrin β3 immunoprecipitates from HPAEC revealed the novel binding partner IQGAP1 (Fig. 1A). For this proteomic screening immunoprecipitation, an irrelevant cell surface protein (MHC I) was used as a control for pull-down of nonspecific cytoskeletal proteins. Quantitative comparison with results for MHC I revealed that IQGAP1 was a specific integrin partner (Fig. 1B), with abundance similar to that of talin-1, a well-characterized integrin binding partner. As expected, human leukocyte antigen, present in MHC complexes, was absent from β3 immunoprecipitates (Fig. 1B). The discovery of IQGAP1 in cytoskeletally derived integrin β3 immunoprecipitates was confirmed by traditional coimmunoprecipitation of β3 with antibody to IQGAP1 from HPAEC lysates (Fig. 1C), and integrin β3 was found to associate with IQGAP1 in equal quantities with and without S1P pretreatment (Fig. 1D). Finally, a GST-β3 cytoplasmic domain fusion protein was found to bind recombinant full-length IQGAP1, demonstrating direct binding between integrin β3 and IQGAP1 (Fig. 1E).
Fig. 1.
Proteomic screen of novel integrin binding partners. A: confluent human pulmonary artery endothelial cells (HPAEC) were serum starved overnight, treated with saline for 5 min, immunoprecipitated as described in materials and methods for β3 or major histocompatibility complex (MHC) I control, and submitted for 1D SDS-PAGE. Individual lanes were sliced and submitted for sample preparation and mass spectrometric (MS) analysis. The data presented is a collision-induced dissociation tandem MS (MS/MS) obtained in a LTQ-Orbitrap XL from a precursor ion with mass-to-charge ratio (m/z) = 942.0156+2, observed in the tryptic digests of β3 integrin immunoprecipitations. This ion corresponds to a peptide spanning residues I539 to K556 of human Ras GTPase-activating-like protein IQGAP1 (theoretical m/z = 942.0097+2; error 6.3 ppm). Sequence ions are labeled. Expectation value for this peptide is 1.1E-7. B: relative abundance of 3 representative proteins identified by tandem mass spectrometry of protein complexes immunoprecipitated with either β3 integrin antibody or MHC I control. The data represent means (± SE) for 4 (for MHC I) or 6 (for β3) experiments of protein abundance quantified as the number of peptides identified for a given protein divided by the total peptide count for all proteins identified in the immunoprecipitate. *P < 0.05. C: immunoprecipitation (IP) of IQGAP1 from HPAEC lysates (lys) followed by immunoblot for IQGAP1 and integrin β3. B, beads alone; IQ, IQGAP1. D: immunoprecipitation of IQGAP1 and immunoblot (IB) of IQGAP1 and integrin β3, with and without sphingosine-1-phosphate (S1P) treatment. E: glutathione S-transferase (GST) pull-down assay with the β3 cytoplasmic domain fused to GST, or GST alone, incubated with wild-type IQGAP1 protein produced by in vitro transcription and translation (IVTT) of full-length IQGAP1 plasmid DNA in the presence of [35S]methionine. Bound proteins were separated by 10% SDS-PAGE and analyzed by autoradiography. Arrow indicates 190-kDa band corresponding to full-length IQGAP1. The second, lower-molecular-weight band is expected to have resulted from cleavage of IQGAP1 by proteases contained within the reticulocyte lysate used for IVTT.
We next confirmed the finding (2, 24) that IQGAP1 localizes to the cell-cell junction in response to S1P treatment in HPAEC (Fig. 2A). Furthermore, IQGAP1 knockdown (Fig. 2B) prevented the formation of cortical actin after S1P treatment (Fig. 2C). To test the role of IQGAP1 in endothelial barrier function, we measured the effect of S1P on TER in endothelial cells with knockdown of IQGAP1. Knockdown of IQGAP1 caused a 40% reduction in the peak TER elevation after S1P compared with empty shRNA vector control (Fig. 2D). This blunting of TER elevation in response to S1P due to IQGAP1 knockdown was also observed when cells were pretreated with cytomix, a barrier-disruptive agonist (Fig. 2E).
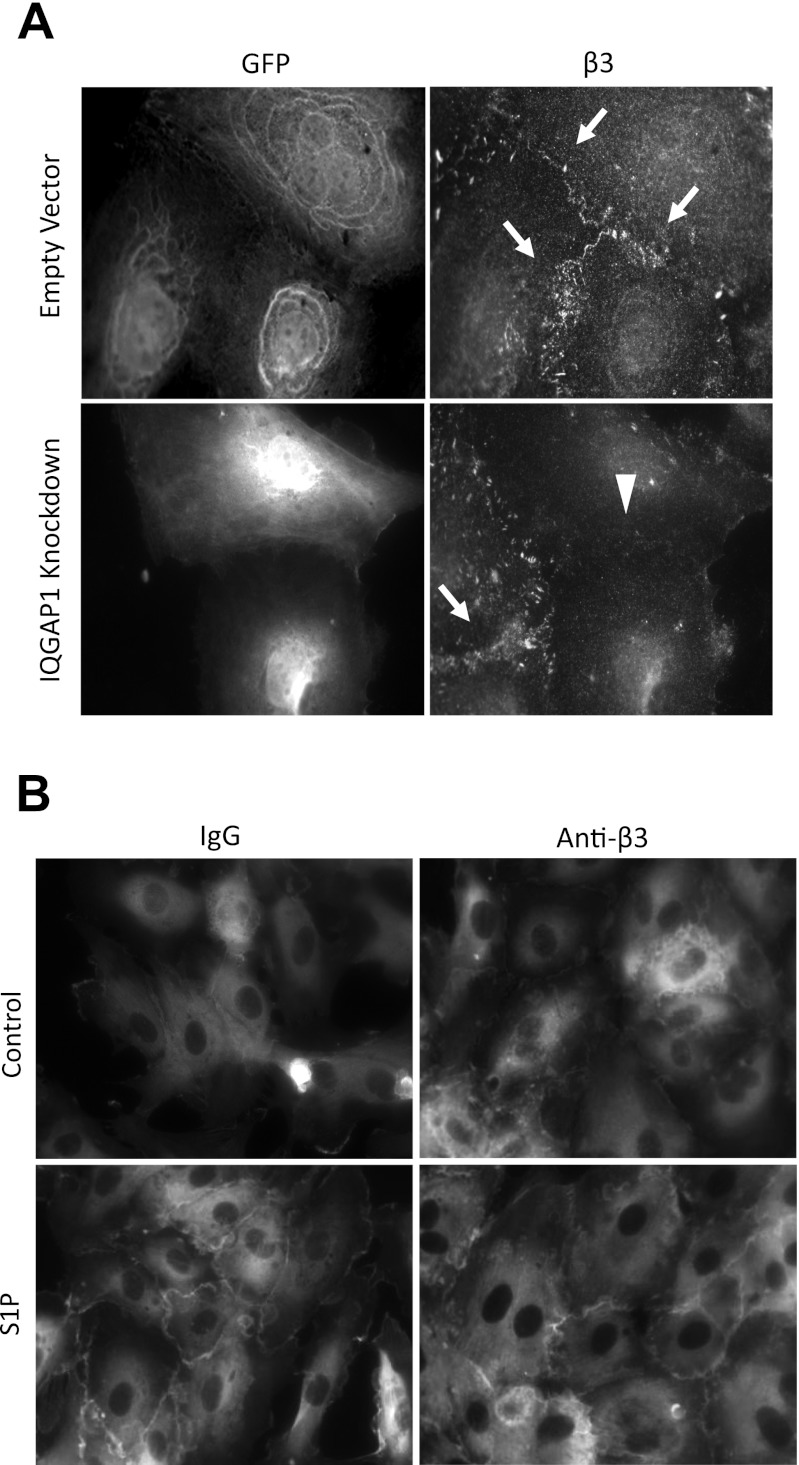
We previously showed that S1P causes relocalization of integrin β3 to the cell-cell junction, where it organizes cortical actin (21). Recent data suggest that IQGAP1 functions as a chaperone for junctional localization of protein complexes in epithelial cells (27). Thus we hypothesized that IQGAP1 would be necessary for junctional relocalization of integrin β3 in response to S1P. shRNA knockdown of IQGAP1 prevented junctional localization of integrin β3 (Fig. 3A). Conversely, S1P-induced junctional relocalization of IQGAP1 was not inhibited by antibody blockade of integrin β3 (Fig. 3B).
Fig. 3.
Effect of IQGAP1 knockdown on junctional localization of integrin β3. A: HPAEC were infected with shRNA virus, grown to confluence, serum starved for 2 h, and treated with S1P for 5 min (0.5 μM). Adjacent panels are the same microscopic field (×63) viewed with alternative filters for dual immunofluorescent staining of GFP and integrin β3. GFP positivity marks only cells infected with either shRNA virus for IQGAP1 knockdown or virus containing empty shRNA vector. β3 staining is seen at cell-cell junctions (arrows) except in cells with IQGAP1 knockdown (arrowhead). B: IQGAP1 immunofluorescence (×40). HPAEC were grown to confluence and serum starved for 1 h, followed by treatment with either blocking antibody to integrin β3 or mouse IgG control, for 1 h. Coverslips were then treated with either S1P (0.5 μM) or vehicle for 5 min. Results are representative of 3 experiments.
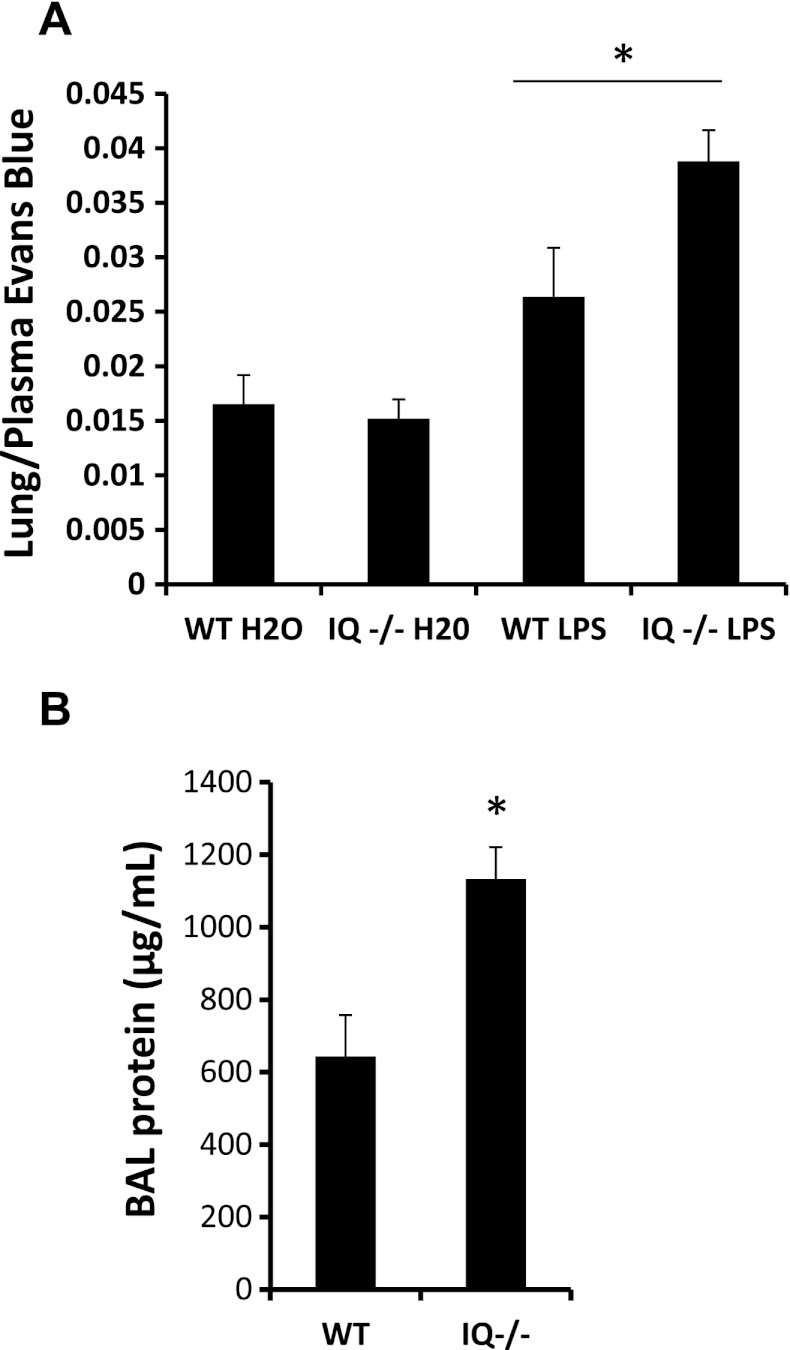
Having demonstrated a role for IQGAP1 in cytoskeletal organization, barrier function, and junctional targeting of the integrin β3, we tested the in vivo relevance of IQGAP1. Like β3 knockout mice (21), IQGAP1 knockout mice had increased lung Evans blue extravasation, normalized for plasma concentration, 3 days after intratracheal instillation of 100 μg LPS (Fig. 4A). BAL protein concentration was also increased in the knockout compared with wild-type mice at 3 days (Fig. 4B), confirming increased lung vascular permeability to protein. BAL leukocyte counts for wild type and IQGAP1 knockout, respectively, were 506 × 104 cells (80.6 × 104 cells, SE, n = 5) and 526 × 104 cells (35.1 × 104 cells, SE, n = 5), and the difference between wild type and knockout was not statistically significant.
Fig. 4.
Effect of absence of IQGAP1 on intratracheal LPS-induced pulmonary vascular leak in mice. A: pulmonary Evans blue extravasation 3 days after intratracheal LPS or water (100 μg; n = 7 for all conditions except IQGAP1 H2O-treated, where n = 6). B: bronchoalveolar lavage (BAL) protein at 3 days after LPS (n = 5 in either group). WT, wild type. *P < 0.05.
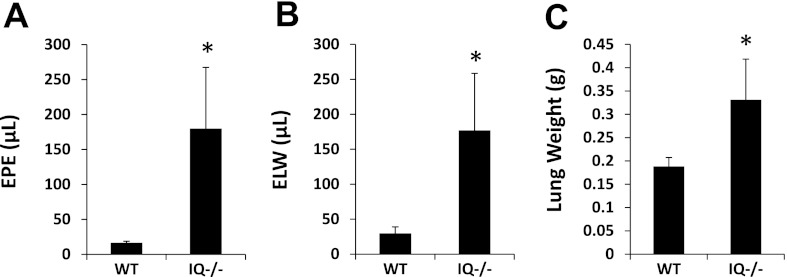
Given that LPS is a constituent of the cell wall of gram-negative bacteria, we next tested the role of IQGAP1 in gram-negative pneumonia, a more clinically relevant model than intratracheal LPS. Mice received intratracheal instillation of pathogenic E. coli originally isolated from patients (23). Given the lethality of this model, mice were euthanized at the earlier time point of 5 h. Similar to intratracheal LPS, E. coli pneumonia in IQGAP1 knockout mice caused increased pulmonary vascular leak of protein (Fig. 5A) and a significant increase in extravascular lung water accumulation (Fig. 5, B and C) compared with wild type. BAL leukocyte counts for wild type and IQGAP1 knockout, respectively, were 23.3 × 104 cells (8.0 × 104 cells, SE, n = 3) and 30.0 × 104 cells (3.20 × 104 cells, SE, n = 4) at baseline, and 35.2 × 104 cells (6.66 × 104 cells, SE, n = 5) and 34.5 × 104 cells (6.28 × 104 cells, SE, n = 4) at 5 h after tracheal instillation of E. coli; the differences between wild type and knockout at either time point were not statistically significant.
Fig. 5.
Effect of absence of IQGAP1 on intratracheal E. coli-induced pulmonary vascular leak. Mice were euthanized at 5 h after intratracheal inoculation of bacteria. A: lung extravascular plasma equivalents (EPE). B: excess lung water (ELW). C: lung weight (n = 4 IQGAP1 knockout; n = 5 wild type). *P ≤ 0.05.
DISCUSSION
Acute lung injury is characterized by high-permeability edema due to an increase in protein permeability across the endothelial and epithelial barriers of the lung. Vascular barrier function is dependent on the fidelity of endothelial cell-cell junctions, and these junctions are stabilized by the actin cytoskeleton, a dynamic structure that is modulated by both mechanical and chemical stimuli during acute inflammatory illness. Several recent studies have revealed an important role for integrins in actin cytoskeletal organization (7), and in particular integrin αvβ3 was shown to be barrier protective during VEGF-induced dermal edema (18). Recently, we confirmed this vascular barrier-protective role of αvβ3, in models of both acute lung injury and bacterial sepsis (21). Furthermore, we showed that αvβ3 integrin is rapidly recruited to the cell-cell junction after treatment of cells with S1P, and junctional αvβ3 is necessary for S1P-induced barrier protection and cortical actin formation in endothelial monolayers.
Our goal in these experiments was to identify a functionally relevant effector of these integrin-related phenomena in endothelium. Using a mass spectrometric approach for screening of immunoprecipitated β3 integrin complexes, we identified the novel β3 integrin binding partner IQGAP1. Functional studies confirmed a phenotypic congruence with the observed β3 integrin effects: knockdown of IQGAP1 decreased the barrier-protective effect of S1P, consistent with the effect of antibody blockade of integrin αvβ3. Like αvβ3 blockade, IQGAP1 knockdown inhibited the formation of cortical actin in response to S1P. Furthermore, drawing a link to the barrier-protective mechanism of integrin β3, IQGAP1 knockdown prevented junctional localization of β3 after S1P treatment, a result suggesting that the barrier-protective function of IQGAP1 is due at least in part to its role in trafficking of integrin β3 to the junction. Moreover, we found that the lung endothelial barrier-protective function of IQGAP1 is functionally significant in vivo, similar to integrin β3: IQGAP1 knockout mice had a significant increase in pulmonary vascular leak in response to both intratracheal LPS and live E. coli. However, in contrast to inhibition of αvβ3 integrin, knockdown of IQGAP1 did not increase the in vitro effects of edemagenic agonists on barrier properties of cultured endothelial cells. Like inhibition of αvβ3, IQGAP1 knockdown did impair the in vitro protective effects of S1P. These results suggest that some of the barrier-protective effects of αvβ3 integrin are independent of IQGAP1. Since loss of S1P itself has been previously shown to increase the magnitude of permeability responses in multiple in vivo models, we suspect that the enhanced permeability responses we observed in IQGAP1 knockout mice could be explained by a reduction in the normally protective effects of S1P or other barrier-enhancing agonists generated in vivo in the setting of vascular leak.
These experiments have established the likely importance of IQGAP1 in regulating pulmonary vascular permeability. However, this study did not address whether IQGAP1 has additional important functions in regulating endothelial barrier function, beyond its role in trafficking the αvβ3 integrin to cell-cell junctions. IQGAP1 is an actin cross-linking protein (3, 15) and also binds the GTPases Rac1 and Cdc42, which are known to modulate actin cytoskeletal dynamics (1). One previous study reported a role for IQGAP1 in angiopoietin-1-induced barrier enhancement in vitro (6), a pathway that has not clearly been linked to integrin function. Therefore, future studies will need to examine further the functional relevance of the integrin binding property of IQGAP1, both in vivo and in endothelial monolayers, for instance by employing mutagenesis to abrogate integrin binding.
In summary, we have found that IQGAP1 is necessary for S1P-induced barrier enhancement in endothelium and for pulmonary vascular barrier protection during acute lung injury from both endotoxin and live bacteria. Furthermore, our data suggest that assembly of the barrier enhancing integrin αvβ3 at the junction is dependent on intracellular trafficking by IQGAP1.
GRANTS
This work was supported by a UCSF Cardiovascular Research Institute training grant NIH T32 HL007185, NIH grant 1F32HL099026-01A1 awarded to M. Bhattacharya, NIH grant 5K08HL083097-03 awarded to G. Su, a Parker B. Francis Career Award to X. Su, HL51854 and NIAID A1053194 awarded to M. A. Matthay, and NIH grants R01 HL053949 and U19AI077439-04 awarded to D. Sheppard. The UCSF Mass Spectrometry Facility (A. L. Burlingame, Director) was supported by the Biomedical Research Technology Program of the National Center for Research Resources, NIH NCRR RR001614, and 1S10RR019934.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
REFERENCES
- 1. Bashour AM, Fullerton AT, Hart MJ, Bloom GS GS. IQGAP1, a Rac- and CDC42-binding protein, directly binds and crosslinks actin filaments. J Cell Biol 137: 1555–1566, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berdyshev EV, Gorshkova I, Usatyuk P, Kalari S, Zhao Y, Pyne NJ, Pyne S, Sabbadini RA, Garcia JG, Natarajan V. Intracellular S1P generation is essential for S1P-induced motility of human lung endothelial cells: role of sphingosine kinase 1 and S1P lyase. PLoS One 6: e16571, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brandt DT, Grosse R. Get to grips: steering local actin dynamics with IQGAPs. EMBO Rep 8: 1019–1923, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Camerer E, Regard JB, Cornelissen I, Srinivasan Y, Duong DN, Palmer D, Pham TH, Wong JS, Pappu R, Coughlin SR. Sphingosine-1-phosphate in the plasma compartment regulates basal and inflammation-induced vascular leak in mice. J Clin Invest 119: 1871–1879, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clauser KR, Baker PR, Burlingame AL. Role of accurate mass measurement (+/- 10 ppm) in protein identification strategies employing MS or MS/MS and database searching. Anal Chem 71: 2871–2873, 1999 [DOI] [PubMed] [Google Scholar]
- 6. David S, Ghosh CC, Mukherjee A, Parikh SM. Angiopoietin-1 requires IQ domain GTPase-activating protein 1 to activate Rac1 and promote endothelial barrier defense. Arterioscler Thromb Vasc Biol 31: 2643–2652, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Delon I, Brown NH. Integrins and the actin cytoskeleton. Curr Opin Cell Biol 19: 43–50, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol 91: 1487–1500, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. . Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest 108: 689–701, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Humphries JD, Byron A, Bass MD, Craig SE, Pinney JW, Knight D, Humphries MJ. Proteomic analysis of integrin-associated complexes identifies RCC2 as a dual regulator of Rac1 and Arf6. Sci Signal 2: ra51, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Komarova Y, Malik AB. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu Rev Physiol 72: 463–493, 2010 [DOI] [PubMed] [Google Scholar]
- 12. Li S, Wang Q, Chakladar A, Bronson RT, Bernards A. Gastric hyperplasia in mice lacking the putative Cdc42 effector IQGAP1. Mol Cell Biol 20: 697–701, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu HB, Cui NQ, Wang Q, Li DH, Xue XP. Sphingosine-1-phosphate and its analogue FTY720 diminish acute pulmonary injury in rats with acute necrotizing pancreatitis. Pancreas 36: e10–e15, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Mammoto T, Parikh SM, Mammoto A, Gallagher D, Chan B, Mostoslavsky G, Ingber DE, Sukhatme VP. Angiopoietin-1 requires p190 RhoGAP to protect against vascular leakage in vivo. J Biol Chem 282: 23910–23918, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Mateer SC, Morris LE, Cromer DA, Benseñor LB, Bloom GS. Actin filament binding by a monomeric IQGAP1 fragment with a single calponin homology domain. Cell Motil Cytoskeleton 4: 231–241, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Mathew B, Jacobson JR, Berdyshev E, Huang Y, Sun X, Zhao Y, Gerhold LM, Siegler J, Evenoski C, Wang T, Zhou T, Zaidi R, Moreno-Vinasco L, Bittman R, Chen CT, Lariviere PJ, Sammani S, Lussier YA, Dudek SM, Natarajan V, Weichselbaum RR, Garcia JG. Role of sphingolipids in murine radiation-induced lung injury: protection by sphingosine 1-phosphate analogs. FASEB J 25: 3388–3400, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, Pearse D, Tuder RM, Garcia JG. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med 169: 1245–1251, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Robinson SD, Reynolds LE, Wyder L, Hicklin DJ, Hodivala-Dilke KM. Beta3-integrin regulates vascular endothelial growth factor-A-dependent permeability. Arterioscler Thromb Vasc Biol 24: 2108–2114, 2004 [DOI] [PubMed] [Google Scholar]
- 19. Rosenfeld J, Capdeveille J, Guillemot JC, Ferrara F. In-gel digestion of proteins for internal sequence analysis after one- or two-dimensional gel electrophoresis. Anal Biochem 203: 173–179, 1992 [DOI] [PubMed] [Google Scholar]
- 20. Safdar Z, Wang P, Ichimura H, Issekutz AC, Quadri S, Bhattacharya J. Hyperosmolarity enhances the lung capillary barrier. J Clin Invest 112: 1541–1549, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Su G, Atakilit A, Li JT, Wu N, Bhattacharya M, Zhu J, Shieh JE, Li E, Chen R, Sun S, Su CP, Sheppard D. Absence of integrin αvβ3 enhances vascular leak in mice by inhibiting endothelial cortical actin formation. Am J Respir Crit Care Med 185: 58–66, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Su X, Camerer E, Hamilton JR, Coughlin SR, Matthay MA. Protease-activated receptor-2 activation induces acute lung inflammation by neuropeptide-dependent mechanisms. J Immunol 175: 2598–605, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Su X, Looney M, Robriquet L, Fang X, Matthay MA. Direct visual instillation as a method for efficient delivery of fluid into the distal airspaces of anesthetized mice. Exp Lung Res 30: 479–493, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Usatyuk PV, He D, Bindokas V, Gorshkova IA, Berdyshev EV, Garcia JG, Natarajan V. Photolysis of caged sphingosine-1-phosphate induces barrier enhancement and intracellular activation of lung endothelial cell signaling pathways. Am J Physiol Lung Cell Mol Physiol 300: L840–L850, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vandenbroucke E, Mehta D, Minshall R, Malik AB. Regulation of endothelial junctional permeability. Ann NY Acad Sci 1123: 134–145, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Ware LB, Matthay MA. Clinical practice. Acute pulmonary edema. N Engl J Med 353: 2788–2796, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Wickström SA, Lange A, Hess MW, Polleux J, Spatz JP, Krüger M, Pfaller K, Lambacher A, Bloch W, Mann M, Huber LA, Fässler R. Integrin-linked kinase controls microtubules dynamics required for plasma membrane targeting of caveolae. Dev Cell 19: 574–588, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]