Abstract
Hibernating ground squirrels maintain homeostasis despite extreme physiological challenges. In winter, these circannual hibernators fast for months while cycling between prolonged periods of low blood flow and body temperature, known as torpor, and short interbout arousals (IBA), where more typical mammalian parameters are rapidly restored. Here we examined the kidney proteome for changes that support the dramatically different physiological demands of the hibernator's year. We identified proteins in 150 two-dimensional gel spots that altered by at least 1.5-fold using liquid chromatography and tandem mass spectrometry. These data successfully classified individuals by physiological state and revealed three dynamic patterns of relative protein abundance that dominated the hibernating kidney: 1) a large group of proteins generally involved with capturing and storing energy were most abundant in summer; 2) a select subset of these also increased during each arousal from torpor; and 3) 14 spots increased in torpor and early arousal were enriched for plasma proteins that enter cells via the endocytic pathway. Immunohistochemistry identified α2-macroglobulin and albumin in kidney blood vessels during late torpor and early arousal; both exhibited regional heterogeneity consistent with highly localized control of blood flow in the glomeruli. Furthermore, albumin, but not α2-macroglobulin, was detected in the proximal tubules during torpor and early arousal but not in IBA or summer animals. Taken together, our findings indicate that normal glomerular filtration barriers remain intact throughout torpor-arousal cycles but endocytosis, and hence renal function, is compromised at low body temperature during torpor and then recovers with rewarming during arousal.
Keywords: cold ischemia, ictidomys, renal transplant, spermophilus, tridecemlineatus
mammalian hibernators, including the 13-lined ground squirrels studied here, become heterotherms in winter. Their heterothermy derives from repeated cycling between dramatically distinct states of torpor and arousal, where torpor is characterized by body temperature (Tb) hovering near ambient, often at or even below 4°C. During torpor, hibernators undergo profound physiological changes, reducing their heart rate from a summertime level of 200–300 beats/min to 3–5 beats/min and their respiratory rate from 100–200 breaths/min to 4–6 breaths/min (6). Arousal is characterized by rapid elevation of metabolic, heart, and respiratory rates to at least summer levels and rewarming to near 37°C using strictly endogenous mechanisms of heat production. The animals maintain this euthermic interbout arousal (IBA) period for ∼12 h before entering a new bout of torpor (Fig. 1). The hibernator's ability to survive sequential cycles of prolonged periods of torpor that are terminated by rapid reperfusion and rewarming provides a natural model of successful organ preservation throughout what is effectively cold storage followed by warm reperfusion (6, 34).
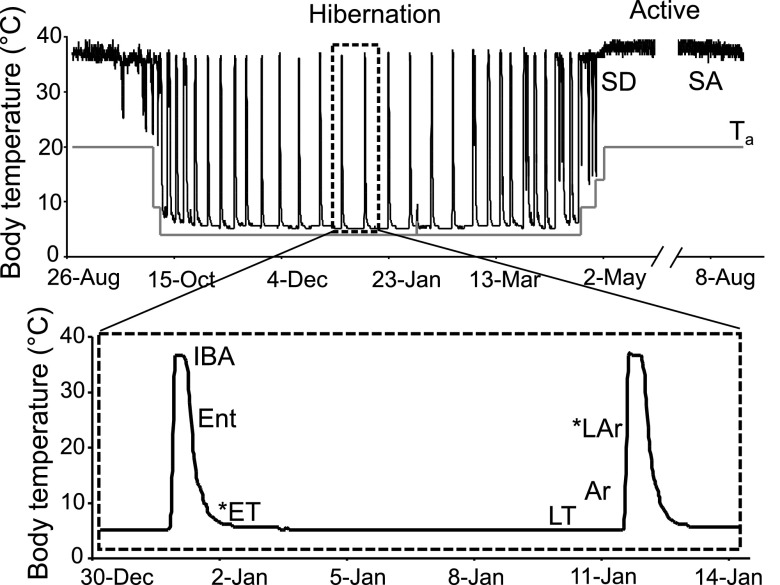
Fig. 1.
Body temperature (Tb) changes over time in a laboratory-housed 13-lined ground squirrel. Top: ∼1 yr; black line plots Tb, gray line plots ambient temperature. Hibernation is the heterothermic period. The boxed region of the Tb trace is expanded in the bottom panel. Abbreviations indicate group physiological status at time of kidney collection. For difference gel electrophoresis (DiGE), animals in 6 distinct physiological states were analyzed, including 2 groups from the homeothermic period (May-Sept.), spring dark (SD), March-April, while still housed in hibernaculum (n = 5) with Tb continuously euthermic (35–37°C) for 11–20 days following spontaneous exit from winter heterothermy (note that the animal in this trace did not spontaneously end heterothermy but rather waited until the ambient temperature was increased to switch to homeothermy), and summer active (SA), first week in August, euthermic (n = 6, Tb= 35–37°C). There were also 4 groups from the winter heterothermic period (collected between Dec.-Feb.): interbout aroused (IBA), euthermic 3–4 h after an arousal (n = 5, Tb = 35–37°C); entrance (Ent), entrance into torpor (n = 5), Tb dropping to between 23 and 27°C following an IBA; late torpor (LT), 80–95% of torpor bout length, based on previous bout (n = 6, Tb = 5–7°C); and arousing (Ar), spontaneously arousing from torpor with Tb elevated to 7.0–13°C (n = 6). We included 2 additional groups of winter hibernators, indicated with *, for Western blots and A414 measurements only: early torpor (ET), 10% of torpor bout length (Tb = 5–7°C), based on length of the previous torpor bout; and late arousing (LAr), spontaneously arousing from torpor with Tb elevated to 18–25°C during rewarming.
Much like kidneys undergoing cold storage during organ preservation, renal function is thought to effectively cease during the torpor phase of winter hibernation (28, 52). Arterial pressure to (52) and regional blood flow within (5) the kidney are greatly reduced, precluding glomerular filtration. Urine formation ceases during torpor and the early stages of arousal (38). The reduced arterial pressure and the ensuing loss of glomerular filtration in torpor are reversed during each of the natural arousal periods that punctuate the hibernation season. Remarkably, in spite of repeated cycles between low blood flow at cold body temperature and rapid restoration of flow with rewarming during arousal, the hibernating kidney does not appear to acquire structural damage that compromises function (30, 57). This outcome contrasts sharply with the severe damage typically seen in nonhibernating mammals, including human donor kidneys subjected to prolonged cold storage followed by transplantation. Human donor kidneys are cooled to 4°C following procurement to prevent injury. The beneficial effects of cooling the kidney to be transplanted are limited however, and deceased-donor kidneys cannot be maintained at 4°C indefinitely. The risk of donor kidney failure after transplant increases progressively as the duration of cold ischemia increases (43). For every 6 h increment stored cold the risk of delayed graft function increases by 23% (43). Donor kidney failure is much more common in kidneys stored cold for >36 h (24, 33) and is associated with worse long-term graft survival compared with kidneys that function immediately (24). Tubular cell death during human kidney transplantation is apoptotic during cold preservation (31, 42). During warm ischemia-reperfusion at implantation, both apoptotic and necrotic cell death occur (48). In the case of natural torpor-arousal cycles in hibernators, however, kidneys from 13-lined ground squirrels that are subjected to cold hypoperfusion of several days duration and subsequent warm perfusion are completely protected from tubular cell apoptosis and necrosis and maintain glomerular filtration rate and distal tubular function (30).
To gain insight into the molecular strategies employed by hibernators to maintain kidney integrity and function throughout their repeated natural cycles between prolonged cold hypoperfusion and restoration of warm perfusion, we applied a quantitative proteomics screening method to identify protein differences among kidneys from six stages of the circannual rhythm of hibernation. We hypothesized that the different physiological demands of hibernating kidney are met in part by reproducible changes in the proteome. The specific patterns of change revealed by this sampling strategy should reveal specific proteins underlying both seasonal (58) and winter-stage specific functional differences (38), as has been observed for liver and plasma metabolites and skeletal and heart muscle proteomes (11, 22, 27, 39, 40, 46). Thus, the specific goals of this study were to identify the proteins that 1) distinguish summer homeotherms from winter heterotherms and 2) discriminate among the winter stages of the torpor-arousal cycles. Identification of protein alterations underlying natural torpor-arousal cycles in hibernation suggests unique strategies for therapeutic approaches to minimize the injury caused by clinical cold ischemia and warm reperfusion of donor organs.
MATERIALS AND METHODS
Animals.
Ictidomys tridecemlineatus (13-lined ground squirrels) were purchased from the captive breeding program at the University of Wisconsin, Oshkosh. Animals were kept in standard housing (18–21°C, 14 h light-10 h dark, Purina cat chow and water ad libitum) until early October when they were moved to the hibernaculum, which was kept in constant darkness. The temperature of the hibernaculum was lowered stepwise from 18 to 4°C over a 2 wk period, and food and water were withdrawn, usually within 1 wk, as animals exhibited multiday bouts of torpor determined by Tb. Food and water were returned in the spring as torpor bouts shortened prior to collection of the spring dark (SD) samples. All animals except those in the summer active (SA) group were surgically implanted abdominally with both data-loggers (iButton, Embedded Data Systems) and radiotelemeters (VM-FH disks, Minimitter) for Tb measurements and then monitored remotely (Meta-Meter, Sable Systems) until tissues were collected during specific stages as noted in Fig. 1. All animal protocols were approved by the University of Colorado Institutional Animal Care and Use Committee.
Tissue collection.
Animals were euthanized under isoflurane anesthesia via cardiac puncture exsanguination and then perfused with at least six blood volumes (≥100 ml) through a 23-gauge needle delivering ice-cold isotonic saline at 17 ml/min to clear the vasculature before dissection. Tissues (e.g., kidney, plasma) were frozen in liquid nitrogen and stored at −80°C until use for protein or RNA extraction. Blood was mixed with 10 μl/ml citrate buffer (74 mM sodium citrate, 38 mM citric acid, 135 mM glucose), and cells removed by 10 min centrifugation at 10,000 g at 4°C. The supernatant was recovered as plasma and stored in small aliquots to avoid repeated freeze-thaw cycles. Urine was collected directly from the bladder after exsanguination and before perfusion using a tuberculin syringe. Fixed tissues were collected for immunohistochemistry (IHC) from separate animals perfused as above except for the use of cold 0.1 M phosphate pH 7.2 for clearing the blood (≥100 ml) followed by 40 ml of 10% formalin. Kidney slices from these animals were incubated in 10% formalin overnight at 4°C and then paraffin embedded.
Quantitative analysis of two-dimensional gel spots and protein identification.
The details for preparation of protein extracts, dye labeling, and two-dimensional (2D) gels were as described previously (12, 22, 27). This experiment evaluated protein spots from 17 analytical gels, each imaged for Cy3, Cy5, and Cy2, plus four Sypro Ruby stained gels for spot picking. All 55 gel images were imported into DeCyder 2D 7.0 software (GE Healthcare). The Cy2 image with the greatest number of spots was defined as the master gel, and then we matched the other 16 Cy2 images to it, first by identifying 250 “landmark” spots and then using the automated feature for spot matching. Spot intensity data were exported, and the pixel volume for each Cy3 and Cy5 spot on each gel was normalized to its corresponding Cy2 spot value using R. These normalized values were used for all further analyses. Matched spots were filtered for presence in at least three animals in at least two groups and then analyzed by ANOVA followed by Benjamini-Hochberg multiple test correction (2, as implemented in R, 51). Only spots with q < 0.05 and fold-change ≥1.5 between any pair of animal groups were considered for attempted protein identification; however, not all of these significantly changing spots were clearly resolved on the Sypro Ruby-stained gels, and thus not all could be processed for protein identification.
Spots matched to Sypro Ruby gel images were robotically picked and digested in gel with trypsin. The eluted peptides were analyzed by liquid chromatography and tandem mass spectrometry (LC-MS/MS) as described (22, 27). Each full MS scan from 300–2,200 m/z was followed by MS/MS scans of the four highest peaks with a dynamic exclusion of 30 s. Spectral data were analyzed with Spectrum Mill MS Proteomics Workbench, using an in-house compiled database (April 2010) comprising all National Center for Biotechnology Information mammal sequences plus Ensembl predicted protein sequences for 13-lined ground squirrel and Arctic ground squirrel sequences as described (47); the database had 792,655 entries. Orthologous proteins from the numerous species represented in this database were collated into unique IDs using ExtracTags and evaluated for reporting as described (37).
Additional data analyses.
After determining protein spots that were significantly changing by ANOVA, we defined pairwise differences by Tukey (51). A method for classification by machine learning, Random Forests (RF), was used to separate individuals and to identify biomarkers. This method is a classifier consisting of an ensemble of tree-structures (4) and works by unsupervised bootstrapping to separate samples based on their protein spot intensity profiles. Only spots that were present in all 33 individuals examined (418/2,073) were included in the RF clustering. We used the R software implementation of RF, setting the number of trees to 50,000 (51). Box plots and k-means clustering (26) were also done using R (51); we set the number of clusters = 5 for the k-means analysis, which gave four similar pattern clusters and a fifth with all remaining protein spots (cluster 4, Fig. 4). Gene enrichment analysis was performed using the Database for Annotation, Visualization and Integrated Discovery (DAVID) webserver (29) using high stringency for functional annotation clustering. Only clusters with at least one entry having q < 0.05 (Benjamini) were reported; clusters with strictly structural motifs were excluded. All other plots and statistical analyses (t-test or analysis of variance followed by Tukey post hoc test to identify differing groups) were done in KaleidaGraph (version 4.1.0, Synergy Software), with α set to 0.05.
Western blot analysis.
Proteins (20 μg kidney or 1 μg of plasma) were fractionated by SDS-PAGE through 4–15% gradient gels (Bio-Rad) and then transferred to Immobilon-FL membranes (Millipore). The membrane was divided into thirds by cutting at the 95 and 34 kDa size markers, and the appropriate region was blocked with Tris-buffered saline containing either 5% nonfat milk for α2-macroglobulin (A2M, top, 50) and albumin (ALB, middle; Bethyl Laboratories A110-125), or with 5% BSA for apolipoprotein A-1 (APOA1, bottom; Rockland Immunochemicals 600-101-109, Gilbertsville, PA), to which antibody at 1:1,000 dilution was added. After overnight incubation at 4°C, blots were washed and the appropriate Cy5-conjugated secondary antibody was bound for visualization (1:500, Jackson ImmunoResearch). Fluorescence images were captured using a Typhoon 9400 and analyzed with ImageQuant TL (GE). Pixel volumes were normalized to the lowest band intensity for each antibody.
Urine ALB and total protein.
Urine total protein and ALB were measured using an ACE Alfa Wasserman clinical autoanalyzer (West Caldwell, NJ). The reportable range is 0–14 g/dl for total protein and 0–7 g/dl for ALB; all ground squirrel samples were within this range.
RT-PCR.
Total RNA was isolated (TRIzol, Invitrogen) from 100 mg of both liver and kidney; quantity and quality were determined with Nanodrop (Thermo Scientific) spectroscopy and Bioanalyzer (Agilent), respectively; the RNA integrity number was at least 8 for all samples. RNA was DNase treated (RQ1 DNase kit, Promega) and requantified, and 500 ng were used for cDNA synthesis (High Capacity cDNA Reverse Transcription Kit, Applied Biosystems). The cDNA product was diluted 1:10, and 3 μl used as template for PCR amplification to assess the presence of four RNAs: A2M, APOA1, ALB, and 18S rRNA. The 25 μl PCR reactions containing template, primers [400 nM each of forward and reverse, A2MF: 5′-AGAATTCCCATGGTGGCTTCTCCT3′, A2MR: 5′-ACTGTTGGTAAGGAGACCTGCTGT3′, or using primers described previously (13)], 200 μM dNTPs, 1× buffer, 1.5 mM MgCl2, and 25 units enzyme (Taq DNA Polymerase Recombinant, Invitrogen) were incubated for 4 min at 95°C, followed by 17–35 cycles of 15 s at 95°C, 1 min at 56°C and 2 min at 72°C, and a final extension for 7 min at 72°C. PCR products (4 μl) were separated by agarose gel electrophoresis (2% 3:1 NuSieve; BMA Products, Rockland, ME), and visualized with SYBR Safe DNA gel stain (Invitrogen) using a Typhoon 9400 fluorescent scanner (GE Healthcare). No products were obtained in the no reverse transcriptase and no template controls. All reagents were used according to manufacturer's instructions.
IHC.
Kidney sections (4 μm) were deparaffinized and processed for IHC on a Leica Bond Instrument using the Bond Refine hrp/dab detection kit (Leica). Primary antibodies (see Western blot analysis above) were diluted 1:1,200 (A2M) and 1:200 (ALB); slides from all individuals were stained simultaneously for each antibody. Sections were counterstained with hematoxylin. Sections were scored blind for immunostaining in glomeruli. Images were captured on an Olympus BX51 microscope equipped with a four megapixel Macrofire digital camera (Optronics, Goleta, CA) using the PictureFrame Application 2.3 (Optronics). All images were cropped and assembled using Photoshop CS2 (Adobe Systems, Mountain View, CA).
RESULTS
Kidney protein differences among 13-lined ground squirrels in six stages of the hibernator's year (Fig. 1) were assessed using 2-D difference gel electrophoresis (DiGE) (Fig. 2). Matched protein spots (2,073) in individual samples were quantified relative to the internal reference standard and analyzed for significant differences based on physiological status (Fig. 1); 564 protein spots differed among groups (q < 0.05, Supplemental Table S1).1 The greatest number of differences (366) separated the late torpor (LT) hibernators from the August SA animals, and smallest number (18) separated IBA from Ent hibernators. Within winter, the arousing (Ar) animals were noteworthy for having a relatively large number of protein spot differences from animals in the other three hibernation groups, IBA, entrance (Ent), and LT (215, 86, and 238, respectively). Due to the large number of kidney protein spot differences, we applied an additional filter that only those with at least 1.5-fold change between any two groups were selected for attempted protein identification. Of these we obtained unambiguous protein identifications for 150 protein spots (Supplemental Table S2). As with the full set of significant spots, the greatest number of differences in the subset of uniquely identified proteins (119) occurred between SA and LT. Additionally, there were approximately twice as many changes on average between any pair of summer and winter groups than within either summer or winter. Arousing hibernators remained the most distinct of the winter groups (Table 1).
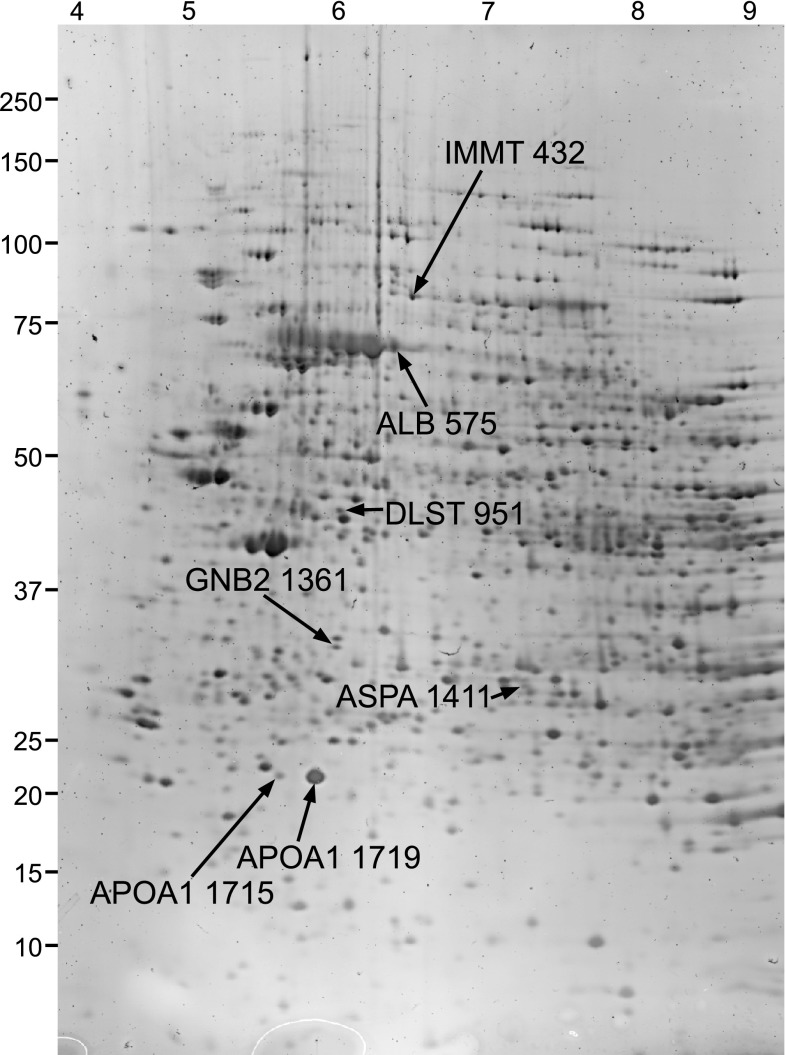
Fig. 2.
Two-dimensional (2D) gel of 13-lined ground squirrel kidney proteins. The approximate isoelectric value and molecular mass in kDa (left) are indicated. Proteins were visualized in the pooled reference standard after staining with SYPRO Ruby. Seven protein spots are highlighted, indicated by their official gene symbol and master numbers.
Table 1.
Number of pairwise protein spot differences among hibernation groups
| SD | SA | IBA | Ent | LT | Ar | |
|---|---|---|---|---|---|---|
| SD | 38 | 63 | 29 | 84 | 55 | |
| SA | 88 | 107 | 76 | 119 | 72 | |
| IBA | 154 | 300 | 4 | 6 | 55 | |
| Ent | 78 | 155 | 18 | 18 | 26 | |
| LT | 225 | 366 | 41 | 57 | 69 | |
| Ar | 171 | 201 | 215 | 86 | 239 |
Numbers of protein spots with P < 0.05 (Tukey, Supplemental Table S1) between pairs by physiological status (defined in Fig. 1); lower left, beneath diagonal: all 564 significantly different (ANOVA q < 0.05) spots; above diagonal: the 150 uniquely identified protein spots with fold change in at least one pairwise comparison ≥1.5. Winter hibernation group comparisons are in boldface.
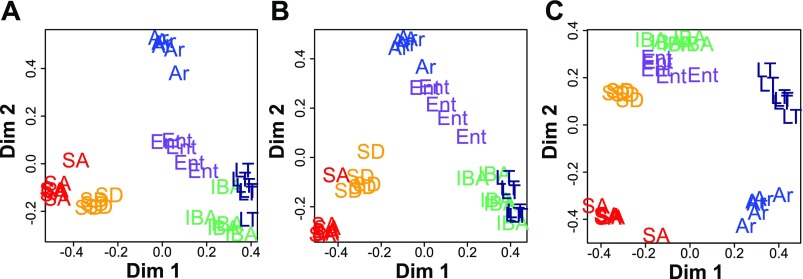
The unsupervised decision tree analysis algorithm, RF, was applied to the kidney spot intensities to test separation among individuals and identify top contributors to that separation. Using all of the protein spots, this analysis classified ground squirrels remarkably well into clusters based on physiological state; all of the individuals from both groups of homeotherms, SA and SD, were well-separated from the winter heterotherms IBA, Ent, LT, and Ar, with Ar distinct from the other winter groups (Fig. 3A). A second RF analysis, using the 89 protein spots with unambiguous unique IDs that were present on all gels (out of 150, Supplemental Tables S2 and S3), similarly divided individuals in the two summer groups from those in the four winter groups. The second RF also further separated Ent and Ar winter animals from those in IBA and LT in addition to separating Ar and Ent from each other (Fig. 3B). Importantly, the similarity between the results obtained using all vs. just the identified spots (compare Fig. 3A with 3B) demonstrates that the latter were not an inappropriately biased selection but, rather, faithfully represented the dominant patterns of change associated with the full complement of ground squirrel kidney proteins evaluated. We repeated RF analyses using the top 5–9 classifiers from the results obtained in Fig. 3B and found that the top seven protein spots provided the best separation of animals by hibernation stage with the fewest number of proteins (Fig. 3C). The result was similar to that using the full dataset (compare Fig. 3A with 3C), with improved separation and clustering of all individuals based on their physiological status.
Fig. 3.
Random forests (RF) clustering of individual samples based on protein spot abundance. The results of RF analysis using 418 (of 2,073) protein spots that were matched on all gels (A), the 89 (of 150) protein spots with unique IDs that were present on all gels (B), and the top 7 spots from the analysis in B (C). These proteins, which most strongly separate individuals from different physiological states and cluster those within states, are apolipoprotein A-I (APOA1, 2 spots), mitofilin (IMMT), dihydrolipoamide S-succinyltransferase (DLST), aspartoacylase (ASPA), albumin (ALB), and guanine nucleotide binding protein (GNB2). These proteins are indicated in Fig. 2. Stages are as defined in Fig. 1 with n = 5 for SD, IBA, and Ent, and n = 6 for SA, LT, and Ar.
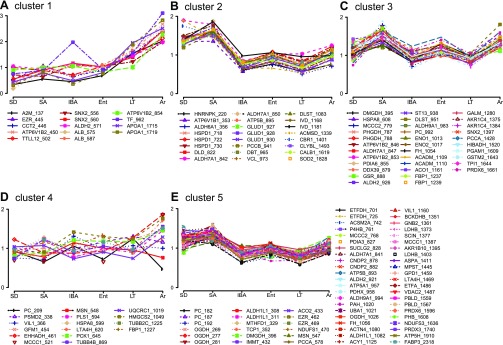
We next applied a k-means clustering analysis to the 150 uniquely identified protein spots (Supplemental Table S2) to gain a global understanding of changes in kidney function among hibernation states. The patterns of change were well represented by five clusters (Fig. 4). All of the top seven RF classifiers in Fig. 3C were contained in clusters 1, 3, and 5, which together included the majority of the identified protein spots (110/150, 73%; Fig. 4, A, C, E). The remaining 40 spots were captured in two additional clusters, 2 and 4 (Fig. 4, B and D). Two features dominated the k-means clusters: 1) increased abundance from LT to Ar, and 2) decreased abundance from SA to IBA, with clusters 1 and 2 representing the simplest versions of these two patterns, respectively. Cluster 1 proteins were lowest in the two homeothermic groups, SD and SA, then typically increased in LT to reach their greatest relative abundance in Ar. Cluster 2 contained the proteins that were seasonally elevated in SD and SA and then reduced throughout winter hibernation. Proteins in clusters 3 and 5 were similar to cluster 2 in that their relative abundance generally decreased between SA and IBA. In contrast to cluster 2, however, in cluster 3, and to a lesser extent cluster 5, protein abundance also increased during the arousal phase of hibernation to levels approaching or even exceeding SD and SA. Only three proteins were elevated in kidney in the four heterothermic states relative to the two homeothermic states, 3-hydroxy-3-methylglutaryl-CoA synthase (HMGCS2), phosphoenolpyruvate carboxykinase 1 (PCK1), and villin 1 (VIL1); all of these were in cluster 4. The proteins in each k-means cluster were evaluated for enriched functional pathways using DAVID. Proteins enriched in clusters 2–5 were predominantly involved in metabolism; unexpectedly, cluster 1 was significantly enriched in plasma proteins (Table 2).
Fig. 4.
K-means clustering of protein abundance changes. Five clusters containing the 150 uniquely identified protein spots: cluster 1, n = 14 (A); cluster 2, n = 24 (B); cluster 3, n = 34 (C); cluster 4, n = 16 (D), and cluster 5, n = 62 spots (E). Mean values for each state are plotted. The top 7 RF classifying proteins from Fig. 3C are found in clusters 1 (APOA1 and ALB), 3 (DLST), and 5 (IMMT, ASPA, and GNB2).
Table 2.
Gene enrichment analyses of kidney protein spot abundance patterns
| k-Means Cluster | Functional Annotation Cluster | Cluster Enrichment | Annotations, n | Top Enrichment | Proteins, n |
|---|---|---|---|---|---|
| 1 | cytoplasmic membrane-bound vesicle | 3.8 | 4 | 14.5 | 5 |
| plasma | 2.0 | 9 | 82.7 | 4 | |
| 2 | mitochondrion | 6.4 | 6 | 29.8 | 17 |
| coenzyme binding | 5.0 | 3 | 23.9 | 6 | |
| lipoic acid binding | 3.1 | 3 | 309.1 | 3 | |
| generation of precursor metabolites and energy | 2.2 | 4 | 13.5 | 5 | |
| 3 | hexose metabolism | 6.8 | 3 | 22.9 | 8 |
| mitochondrion | 6.7 | 4 | 14.1 | 11 | |
| propanoate metabolism | 4.0 | 3 | 37.0 | 5 | |
| TCA cycle | 2.7 | 9 | 94.8 | 3 | |
| aldehyde dehydrogenase | 2.6 | 13 | 167.9 | 3 | |
| nicotinamide metabolism | 2.4 | 7 | 37.6 | 3 | |
| cell redox homeostasis | 1.3 | 4 | 47.4 | 3 | |
| 4 | mitochondrion | 3.1 | 4 | 13.6 | 6 |
| gluconeogenesis | 2.6 | 13 | 202.5 | 3 | |
| actin binding | 2.0 | 3 | 96.2 | 3 | |
| 5 | membrane-enclosed lumen | 7.9 | 3 | 3.8 | 23 |
| amino acid catabolism | 6.9 | 4 | 29.6 | 8 | |
| mitochondrial membrane | 6.0 | 8 | 15.9 | 12 | |
| TCA cycle | 3.2 | 7 | 3.2 | 4 | |
| aldehyde dehydrogenase | 2.8 | 16 | 101.4 | 4 | |
| electron transport | 2.5 | 3 | 38.5 | 4 | |
| nucleotide binding | 2.4 | 5 | 2.5 | 17 | |
| oxidative phosphorylation | 1.6 | 46 | 48.1 | 5 |
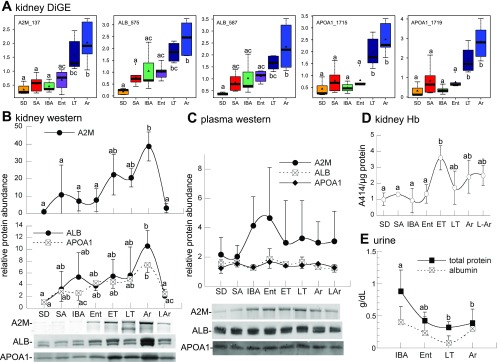
An increased relative abundance of kidney protein spots in LT and Ar was observed most strongly in cluster 1, but cluster 3 proteins also increased in Ar compared with the other winter states. An increase at this time, when Tb is still <13°C, was surprising in light of the fact that protein synthesis is depressed at the low Tb of torpor (54). Moreover, five of the Ar-increased spots in cluster 1 contained highly abundant plasma proteins that are typically synthesized in liver rather than in kidney: ALB and APOA1 each in two spots, transferrin (TF), and A2M. We asked whether any of the remaining five proteins in this group were also found in plasma by querying the human plasma protein database. Only one, mitochondrial aldehyde dehydrogenase (ALDH2), has been reported in the plasma, with estimated concentrations 4–5 orders of magnitude less than ALB, APOA1, TF, or A2M (15).
Inspection of the relative abundances of A2M, ALB, and APOA1 in kidney reveals significant increases for all of them in both LT and Ar compared with either homeothermic state (SD or SA), with maximum levels in Ar (Fig. 5A). This result was verified by Western blotting and extended to include two new groups from winter heterothermy, early torpor (ET) and late arousing (LAr, Fig. 1). Remarkably, the relative abundance of all three proteins quickly decreased as the animals continued to rewarm, reaching their winter nadir by the time Tb increased to 23–27°C during LAr (Fig. 5B).
Fig. 5.
Analysis of plasma proteins. Small letters on graphs distinguish significantly different groups (Tukey, P < 0.05). A: box plots of kidney protein spot intensities in 6 physiological stages determined by DiGE. Plots show mean (▲), median (thick horizontal line), center 25th–75th percentile (colored boxes), 0 and 100th percentile (capped thin lines), and outliers (○) for each group of protein abundance measurements. B: Western blot images and corresponding quantitative analysis of A2M, ALB, and APOA1 in kidney, adding ET and LAr (Fig. 1). Western blots confirm that all 3 proteins differ among stages: A2M P = 0.0013; ALB P = 0.03; APOA1 P = 0.0005. C: no significant differences were detected for any of these 3 proteins in plasma. D: plot of relative hemoglobin (Hb) as measured by A414/μg total protein in kidney extracts. Kidney Hb varies (P = 0.004). E: urine total protein and ALB. Total protein varied (P = 0.0047), but no change was detected in ALB. B–D show means ± SD for n = 3; E is for n = 5 for IBA and LT, 3 for Ent, and 6 for Ar.
Plasma from the same individuals was examined by Western blot to determine whether the dynamic pattern of these three proteins in kidney simply mirrored their changing abundance in plasma. None differed significantly in plasma during the torpor-arousal cycle, although A2M exhibited a trend for elevated levels in winter animals compared with summer, particularly in IBA and Ent (Fig. 5C), as reported for other ground squirrel species (10, 49, 50). Consistent with the poor correlation between plasma and kidney abundance of these three proteins, kidney hemoglobin (estimated using sample absorbance at 414 nm) was also poorly correlated (Fig. 5D). Thus, the elevated abundance of these plasma proteins within kidney during Ar is not a simple reflection of their abundance in the blood. Total urine protein and ALB were also measured to evaluate proteinuria; neither changed significantly during the torpor-arousal cycle (Fig. 5E).
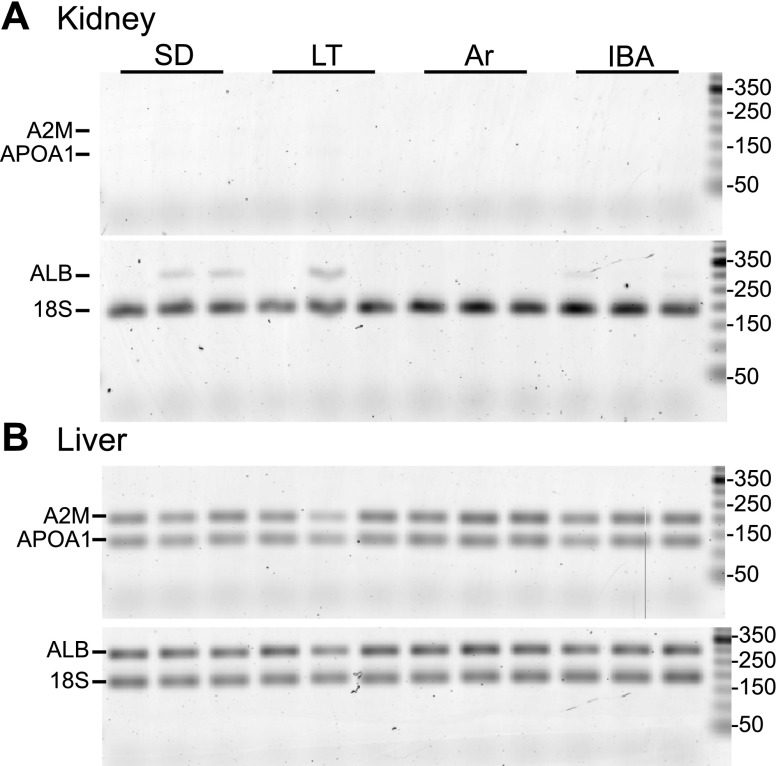
The expression of A2M, APOA1, and ALB in kidney was evaluated by RT-PCR using RNA isolated from a subset of kidneys analyzed by DiGE (Fig. 6). All three of these mRNAs were readily detected in every liver RNA sample along with 18S rRNA. In kidney, however, only 18S RNA was strongly amplified in every sample, whereas A2M and APOA1 were not detected, and a weak signal for ALB (compare the band intensity of ALB to 18S in kidney vs. liver) was observed in four of the 12 samples. Because these included two SA, one LT, and one IBA but excluded all of the Ar individuals, the pattern does not correlate with the DiGE results, and we conclude that local synthesis of ALB, A2M, and APOA1 does not account for the marked increase of these proteins in kidney during LT and Ar seen by DiGE.
Fig. 6.
Expression of A2M, APOA1, and ALB occurs primarily in the liver. We assessed 4 RNAs for presence in kidney (A) and liver (B) from 12 ground squirrels (n = 3 in SD, LT, Ar, and IBA) by RT-PCR. Reactions were combined after the final PCR step into 1 gel lane/individual for A2M and APOA1, and for ALB and 18S. The expected sizes of PCR products are: A2M, 199 bp; APOA1, 134; ALB, 293 bp; 18S rRNA 187 bp. Marker sizes are indicated on the right in bp.
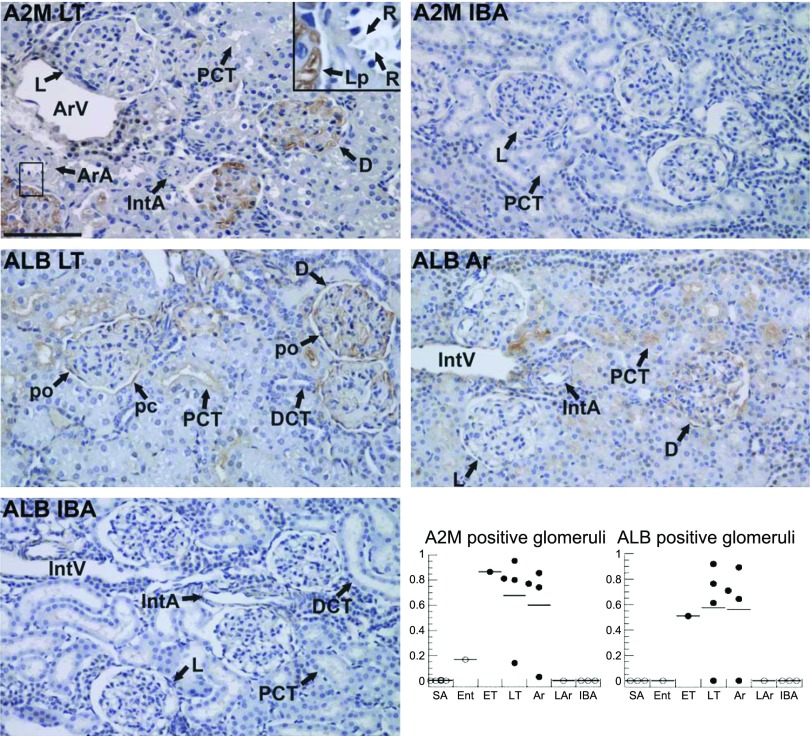
We next used IHC to localize A2M and ALB in kidney sections. The experiment was initially done with seven individuals (3 LT, 3 IBA, and 1 Ar; data not shown) and then expanded to at least 17 individuals with identical outcomes. Consistent with the results of DiGE and Western analyses, A2M and ALB are readily detected in most sections from ET, LT, and early Ar but less so or not at all in SA, Ent, LAr, and IBA animals. A2M conspicuously stained glomeruli in the renal cortex, where it was strictly confined to within the blood vessels. It is noteworthy that some glomeruli stained darkly, whereas other, often immediately adjacent, glomeruli stained only lightly or not at all (Fig. 7). In addition, A2M staining is not strictly correlated with blood contamination per se, because red blood cells (RBCs) can be found throughout the sections in vessels lacking A2M; likewise A2M staining can be seen on what appears to be the walls of glomerular capillaries in the absence of RBCs (inset in A2M LT panel, Fig. 7). ALB also stained some closely juxtaposed glomeruli much more strongly than others in sections from the same torpid and Ar animals, although there were generally fewer glomeruli stained with ALB than A2M. In contrast to the results obtained with the larger A2M, however, we also observed ALB staining in Bowman's space, where it appeared on the surface of the podocytes and parietal cells, as well as in the most proximal portion of many proximal convoluted tubules during LT and Ar, including in two animals where ALB was not detected in glomeruli (see plot). Furthermore, the majority of the ALB staining was not detected within the proximal tubular cells themselves but rather was on their luminal surfaces suggesting albumin was not simply taken up and retained within these cells. Significantly, no ALB staining was detected in the distal convoluted tubules of torpid or Ar animals, and there was no glomerular, proximal, or distal tubular staining for ALB during IBA, Ent, LAr, or SA (Fig. 7).
Fig. 7.
Localization of plasma proteins and visualization of their dynamics in kidney during torpor-arousal cycles by immunohistochemistry. Micrographs show representative sections of renal cortex from the indicated hibernation stage (Fig. 1) stained with antibodies to α2-macroglobulin (A2M) or ALB. All sections were counterstained with hematoxylin and imaged at the same magnification; the scale bar in top left panel indicates 100 μm. D and L point to dark and light-staining glomeruli, respectively; Ar A, afferent arteriole; DCT, distal convoluted tubule; Int A, interlobular artery; IntV, interlobular vein; Lp, glomerular capillary loop; pc, parietal cell; PCT, proximal convoluted tubule; po, podocyte; R, red blood cell. Graphs plot the fraction of glomeruli stained with the indicated antibody from different individuals from 7 stages (Fig. 1); the line marks the mean for each group. Significant differences among stages for the proportion of glomeruli stained were found for both A2M and ALB (P = 0.004 and 0.04 respectively; significant pairwise differences for A2M were found between LT and Ar, SA and IBA in addition to Ar and SA). Testing for temperature dependence by grouping all samples with Tb > 18°C (“warm” includes SA, Ent, LAr and IBA, n = 8 for ALB and n = 10 for A2M, ○) and those with Tb < 13°C (“cold,” includes ET, LT, Ar, n = 9, ●), finds significant differences based on Tb for both A2M (P = 0.0004) and ALB (P = 0.001).
DISCUSSION
This study was based on the hypothesis that the profound physiological changes undertaken by ground squirrels transitioning from summer homeothermy to the winter heterothermy of hibernation, as well as the transitions between torpor and arousal during winter heterothermy would be supported by changes in the kidney proteome. We identified 150 proteins that differed by at least 1.5-fold among six physiological stages, providing insight into kidney function throughout the circannual and torpor-arousal cycles. As with other “omic” datasets investigating at least five stages of the yearly cycle from circannual hibernators (11, 22, 27, 40, 56), the renal protein signature clearly distinguished summer homeotherms from winter heterotherms. The strong separation of Ar from the other winter groups, however, is so far unique to kidney, as is the paucity of proteins that were seasonally elevated in all of the winter hibernation stages.
The majority of kidney proteins were most abundant during SA, a pattern most strongly represented by the proteins in k-means cluster 2 (Fig. 4B), which were also relatively high in SD and lower in all of the winter states. This group was enriched in proteins involved with the “generation of precursor metabolites and energy” (Table 2). This pattern is more complex than would be expected from proteins simply responding to feeding and fasting; the increase between SD and SA likely reflects the intense period of capturing and storing dietary resources as animals prepare for the long fast of hibernation. Specifically, the kidney of summer animals appears to be especially metabolically active with increased abundance of enzymes involved in the uptake of energy from amino acids. Enzymes used for catabolism of essential amino acids including the branched chain and aromatics [e.g., the E2 subunit of dihydrolipoamide branched chain transacylase (DBT) and aminocarboxymuconate semialdehyde decarboxylase (ACMSD)] decreased in winter, facilitating preservation of these irreplaceable building blocks throughout the long winter fast. This finding is consistent with those of previous studies of heart proteins (22) and plasma (11) and liver (40) metabolites from multiple physiological stages throughout the hibernator's year, as well as from protein changes evident in liver between SA and Ent hibernators (14, 44). Several enzymes involved with amino acid catabolism remained low in kidney in the spring animals, including methylcrotonoyl-CoA carboxylase (MCCC2) in cluster 3 and phenylalanine hydroxylase (PAH) in cluster 5. Additionally, the function “amino acid catabolism” was enriched in cluster 5 (Table 2).
Proteins grouped in k-means cluster 3 were likewise generally highest in SA but also increased during Ar. Significantly, this cluster was enriched for hexose metabolism, including the two gluconeogenic enzymes fructose-1,6-bisphosphatase and pyruvate carboxylase, as well as triosephosphate isomerase, which is required for the use of glycerol in gluconeogenesis. The third gluconeogenic protein that is not also part of glycolysis, PCK1 in cluster 4, was one of the rare winter-elevated proteins in kidney, as was the pivotal enzyme for ketone body synthesis, HMGCS2 (also in cluster 4). Renal gluconeogenesis is stimulated by ketone bodies and fatty acids (32); medium and long chain free fatty acids are transiently increased in the circulation during early arousal from torpor (11). Taken together these findings emphasize the importance of renal gluconeogenesis in the torpor-arousal cycles of hibernation (16, 19, 23). Finally, k-means cluster 3 also contained three proteins with a role in cell redox homeostasis, peroxiredoxin 3 and 6 and glutathione reductase, consistent with a protective role for these proteins during arousal.
Another pattern identified in the changing kidney proteome of hibernation was one of increased abundance by LT and greatest abundance in Ar. The large increase (4- to 9-fold; Supplemental Table S2, Fig. 4A) of several protein spots during early Ar, while Tb was still <13°C, was unexpected, because both mRNA and protein synthesis are markedly depressed at low Tb in hibernators (53, 54). Thus, this pattern of increased abundance in torpor and early arousal is likely due to factors other than gene expression, such as changes in posttranslational modification, subcellular localization, or tissue level distribution. The gene enrichment analysis of k-means cluster 1, the most striking example of this pattern, revealed just two categories, cytoplasmic membrane enclosed vesicle and plasma. The former category is consistent with changes in subcellular localization, as is the fact that the nonplasma proteins in this cluster are cytoskeletal or multisubunit enzyme components. In addition, the observed enrichment of plasma proteins in kidney is consistent with tissue level redistribution, because their corresponding mRNAs are abundant in liver, but not in kidney (Fig. 6).
One explanation for recovery of plasma proteins in a proteomic screen of any organ is blood contamination; however, all of the kidneys used here were collected from animals that were first exsanguinated and then extensively perfused through the heart (∼6 whole body blood volumes) at a constant flow rate using a mechanical pump prior to dissection or fixation. Typically, ∼20% of the outflow from the mammalian heart enters the kidneys, implying that adequate saline was used to wash out blood proteins in patent vessels. However, torpid ground squirrels have low blood pressure and heart rates that could potentially decrease the efficacy of clearing the blood in specific stages when these methods are applied. During entrance into torpor, heart rate and blood pressure are already both dramatically decreased, whereas they are increased during arousal (35). These factors might imply that poor perfusion leading to retention of blood in the tissue would be most likely during Ent, ET, and LT, rather than early or late in the arousal process, contrary to the increased plasma proteins observed in kidney in this DiGE experiment. An additional factor to consider, however, is that blood is shunted during hibernation such that kidney in particular experiences a dramatic decline in blood flow in torpor, which persists late into arousal (5), a pattern more consistent with our findings. However, our data show the plasma proteins elevated in LT and early Ar were not well correlated with blood contamination; this is apparent from the relative hemoglobin content of the kidney extracts (Fig. 5D) as well as the fact that several plasma proteins at least as abundant as A2M were not recovered by DiGE, e.g., immunoglobulins. The observed specificity for hibernation stage and proteins recovered strongly argues against simple blood contamination due to perfusion artifacts and for a bona fide biological basis for these increased plasma proteins during torpor and early arousal in hibernation physiology.
Of particular interest in kidney are physiological changes during hibernation that impact renal function, for which two of the elevated proteins, A2M and ALB, serve as convenient probes because of their size. A2M is a 720 kDa homotetramer that is much too large to cross the glomerular filtration barrier. Because A2M was only observed in the vasculature, the filtration barriers were not severely compromised either during hibernation or as a result of our perfusion procedures. A2M therefore specifically provides information regarding the renal microvasculature during hibernation. First, we noted that the interlobar, arcuate, and interlobular arteries were largely devoid of RBCs and did not stain with A2M during torpor or early arousal, indicating that the mechanical perfusion of these vessels was effective. The patchwork distribution of A2M staining in these stages suggests that either some glomeruli were cleared of blood during the perfusion and others were not, or some glomeruli retained A2M preferentially despite adequate perfusion. Additional work beyond the scope of this study is needed to test the predictions of these alternative hypotheses. But, interestingly, A2M is a broad spectrum protease inhibitor that changes conformation when it engages a protease; the conformational change (which also occurs when A2M carries other ligands including growth factors, 36) enables interaction with its cell surface receptor and rapid clearance from the circulation (21). It is reasonable to postulate that receptor-mediated endocytosis slows sufficiently at the low Tb of hibernation, specifically in the stages where we observe increased A2M in the kidney, to capture these complexes that would otherwise be processed via endocytosis too rapidly to detect (20, 55). The temperature dependence of A2M retention in vessels observed by IHC is consistent with this interpretation, as is the apparent staining of the vessel walls and lack of a one-to-one correspondence between RBC (or hemoglobin, Fig. 5D) presence and A2M staining (Fig. 7). Remarkably, glomerular tufts immediately adjacent to each other showed differences in the presence/absence of A2M; this observation suggests that vasoconstriction, whether it functions to prevent wash-out by saline or to prevent the entry of A2M complexes and thus engagement with their cell surface receptors (or perhaps a combination of the two) occurs in the afferent arterioles near the affected renal corpuscle rather than in or prior to the interlobular artery. Because adjacent glomeruli (which presumably have afferent arterioles originating from the same interlobular artery) show dramatically different retention of blood protein during torpor and early arousal, it appears that only a subset of filtration units are shut down, while others maintain function. Finally, the patchwork nature of the staining suggests that the site of constriction is likely to be the afferent arterioles, near or at the juxtaglomerular apparatus.
ALB has a vascular pattern of distribution similar to that observed for A2M. However, ALB was also found within the renal corpuscle Bowman space (urinary space) where it coated the luminal surface of the parietal cells and podocytes and in the immediately adjoining proximal convoluted tubule. Therefore, ALB localization extends the ability of these experiments to probe renal physiology during hibernation beyond the vasculature, to the filtration barrier and the proximal convoluted tubules (PCT). In nonhibernating mammals, ALB is partially filtered by the glomerulus (25) and reabsorbed in the PCT by receptor-mediated endocytosis (1, 3, 7, 8, 17), the only known process for tubular protein clearance (25). There is no significant reabsorption of ALB distal to the proximal tubule (17). Within the PCT, ALB is either transported to lysosomes where it is degraded into amino acids (17) or rapidly transported through the PCT cell in large transcytosis vesicles and then disgorged intact to the peritubular capillaries (9, 45). In hibernating ground squirrels, ALB staining was seen within some glomeruli and their adjacent PCT during LT and Ar. Moreover, ALB was not observed in the distal tubules and was confined to the vasculature within the medulla, indicating that ALB was reabsorbed in the proximal tubule before reaching the loops of Henle. This finding is consistent with our inability to detect albuminuria (Fig. 5E) as well as with observations in nonhibernating animals (17). During Ent, LAr, IBA, and SA, ALB was not observed in vessels (e.g., arcuate artery, afferent arterioles or the interlobular artery or vein; Fig. 7) or tubules by IHC, consistent with the Western blotting results. Taken together, these observations suggest that ALB is present in the glomeruli, Bowman's space, and PCT lumen during torpor and early Ar but is not transported or degraded. During IBA, rewarming and reperfusion occur and presumably enable receptor-mediated endocytosis followed by lysosomal degradation or rapid transport of ALB to the peritubular capillaries. Thus, ALB provides a useful biomarker of changes in proximal tubular function during torpor-arousal cycles.
Lack of perfusion prior to tissue collection could explain elevated winter levels of A2M, ALB, APOA1, or TF recovered in proteomics screens of liver and intestine (10, 14, 37, 47). However, retained blood is less likely to explain the elevated levels of these plasma proteins in heart (22) or skeletal muscle (ALB, APOA1, and TF 27), also observed using tissues collected from extensively perfused animals; the possibility of local synthesis was not examined. To our knowledge, this is the first study to directly address and rule out both blood contamination and local synthesis across multiple stages of hibernation as explanations for an observed elevation of a particular plasma protein in an organ. We predict that these plasma proteins will in fact accumulate wherever they encounter their receptors due to the temperature sensitivity of receptor-mediated endocytosis (20, 55). Such accumulation is of practical value for the ability to trap endocytic intermediates under natural conditions (i.e., to address the controversy regarding ALB filtration, degradation, and reabsorption in the kidney; Refs. 18, 41, 45) and of potential functional value for cell survival at low temperature or during rewarming.
Human donor kidneys subjected to prolonged cold ischemia are most vulnerable to injury during warm reperfusion upon transplantation, when both apoptosis and necrosis occur (48). We recently showed that proximal tubule brush border injury is significantly increased during IBA, yet, despite brush border injury, renal function and distal tubular function repeatedly normalize prior to the next bout of torpor (30). The current study extends these findings and provides direct evidence for a loss of at least one proximal tubule function during torpor, i.e., ALB reabsorption, which recovers rapidly during arousal, as Tb rises >18°C. Because numerous aspects of kidney function depend upon energy-consuming transport mechanisms, kidney function must necessarily be compromised at the low Tb of torpor. Based on new findings in this work, it is tempting to speculate that protein ligands, like A2M, APOA1, ALB, or TF and the numerous small molecules that they carry, bound at surface receptors, help to stabilize function and prevent injury in the rewarming kidney during arousal from torpor by instantly delivering nutrients and signaling molecules when sufficient rewarming is achieved to restore endocytosis.
In conclusion, we have demonstrated that the kidney proteome significantly changes between winter heterothermy and summer homeothermy with a summer increase in proteins specifically associated with capturing and storing energy for the upcoming winter fast. Within winter, metabolic activity resumes in arousal, and gluconeogenesis and redox homeostasis are enhanced. We also provide evidence for maintenance of the filtration barrier during torpor, variable and dynamic regional control of blood circulation through the glomeruli, and altered dynamics of PCT reabsorption of ALB during the torpor-arousal cycle. Remarkably, among these widely varying conditions renal function is preserved throughout winter hibernation despite a hiatus during prolonged cold exposure and the potential for ischemic damage during warm reperfusion. As the details of the mechanisms underlying the hibernator's unique ability to suspend and restore function through torpor-arousal cycles become known, they will inform novel approaches to enhance kidney preservation and transplant outcomes.
GRANTS
This work was supported by National Institutes of Health Grants HL-089049 to S. L. Martin, K08 DK-069512 to A. Jani, R01GM-083649 to A. Karimpour-Fard, R01LM-008111 and R01LM-009254 to L. E. Hunter, and P30 CA-046934 to the Mass Spectrometry and Proteomics Core Facility of the Univ. Colorado Cancer Center.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.J., D.J.O., and S.L.M. interpreted results of experiments; A.J., D.J.O., R.L.R., and S.L.M. drafted manuscript; A.J., D.J.O., A.K.-F., L.E.E., R.L.R., and S.L.M. edited and revised manuscript; A.J., D.J.O., A.K.-F., L.E.E., R.L.R., L.E.H., and S.L.M. approved final version of manuscript; D.J.O., A.K.-F., and S.L.M. analyzed data; D.J.O., A.K.-F., L.E.E., R.L.R., and S.L.M. prepared figures; L.E.E. and S.L.M. conception and design of research; L.E.E. and R.L.R. performed experiments.
Supplementary Material
ACKNOWLEDGMENTS
We thank K. Grabek and A. Hindle for a critical review of the manuscript.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1. Amsellem S, Gburek J, Hamard G, Nielsen R, Willnow TE, Devuyst O, Nexo E, Verroust PJ, Christensen EI, Kozyraki R. Cubilin is essential for albumin reabsorption in the renal proximal tubule. J Am Soc Nephrol 21: 1859–1867, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res 125: 279–284, 2001. [DOI] [PubMed] [Google Scholar]
- 3. Birn H, Christensen EI. Renal albumin absorption in physiology and pathology. Kidney Int 69: 440–449, 2006. [DOI] [PubMed] [Google Scholar]
- 4. Breiman L. Random forests. Mach Learn 45: 5–32, 2001. [Google Scholar]
- 5. Bullard RW, Funkhouser GE. Estimated regional blood flow by rubidium 86 distribution during arousal from hibernation. Am J Physiol 203: 266–270, 1962. [DOI] [PubMed] [Google Scholar]
- 6. Carey HV, Andrews MT, Martin SL. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev 83: 1153–1181, 2003. [DOI] [PubMed] [Google Scholar]
- 7. Christensen EI, Birn H. Megalin and cubilin: synergistic endocytic receptors in renal proximal tubule. Am J Physiol Renal Physiol 280: F562–F573, 2001. [DOI] [PubMed] [Google Scholar]
- 8. Christensen EI, Gburek J. Protein reabsorption in renal proximal tubule-function and dysfunction in kidney pathophysiology. Pediatr Nephrol 19: 714–721, 2004. [DOI] [PubMed] [Google Scholar]
- 9. Comper WD, Hilliard LM, Nikolic-Paterson DJ, Russo LM. Disease-dependent mechanisms of albuminuria. Am J Physiol Renal Physiol 295: F1589–F1600, 2008. [DOI] [PubMed] [Google Scholar]
- 10. Epperson LE, Dahl T, Martin SL. Quantitative analysis of liver protein expression during hibernation in the golden-mantled ground squirrel. Mol Cell Proteomics 3: 920–933, 2004. [DOI] [PubMed] [Google Scholar]
- 11. Epperson LE, Karimpour-Fard A, Hunter LE, Martin SL. Metabolic cycles in a circannual hibernator. Physiol Genomics 43: 799–807, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Epperson LE, Martin SL. Proteomic strategies to investigate adaptive processes. In: Methods in Animal Proteomics, edited by Eckersall PD, Whitfield PD. Ames, IA: Wiley-Blackwell, 2011, p. 189–209. [Google Scholar]
- 13. Epperson LE, Martin SL. Quantitative assessment of ground squirrel RNA levels in multiple stages of hibernation. Physiol Genomics 10: 93–102, 2002. [DOI] [PubMed] [Google Scholar]
- 14. Epperson LE, Rose JC, Carey HV, Martin SL. Seasonal proteomic changes reveal molecular adaptations to preserve and replenish liver proteins during ground squirrel hibernation. Am J Physiol Regul Integr Comp Physiol 298: R329–R340, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Farrah T, Deutsch EW, Omenn GS, Campbell DS, Sun Z, Bletz JA, Mallick P, Katz JE, Malmström J, Ossola R, Watts JD, Lin B, Zhang H, Moritz RL, Aebersold RH. A high-confidence human plasma proteome reference set with estimated concentrations in PeptideAtlas. Mol Cell Proteomics 10: M110.–006353., 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Galster W, Morrison P. Gluconeogenesis in arctic ground squirrels between periods of hibernation. Am J Physiol 228: 325–330, 1975. [DOI] [PubMed] [Google Scholar]
- 17. Gekle M. Renal tubule albumin transport. Annu Rev Physiol 67: 573–594, 2005. [DOI] [PubMed] [Google Scholar]
- 18. Gekle M. Renal albumin handling: a look at the dark side of the filter. Kidney Int 71: 479–481, 2007. [DOI] [PubMed] [Google Scholar]
- 19. Gerich JE, Meyer C, Woerle HJ, Stumvoll M. Renal gluconeogenesis: its importance in human glucose homeostasis. Diabetes Care 24: 382–391, 2001. [DOI] [PubMed] [Google Scholar]
- 20. Goldstein JL, Anderson RG, Brown MS. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature 279: 679–685, 1979. [DOI] [PubMed] [Google Scholar]
- 21. Gonias SL, Pizzo SV. Chemical and structural modifications of α2-macroglobulin: effects on receptor binding and endocytosis studied in an in vivo model. Ann NY Acad Sci 421: 457–471, 1983. [DOI] [PubMed] [Google Scholar]
- 22. Grabek KR, Karimpour-Fard A, Epperson LE, Hindle AG, Hunter LE, Martin SL. Multistate proteomics analysis reveals novel strategies used by a hibernator to precondition the heart and conserve ATP for winter heterothermy. Physiol Genomics 43: 1263–1275, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Green CJ, Brosnan JT, Fuller BJ, Lowry M, Stubbs M, Ross BD. Effect of hibernation on liver and kidney metabolism in 13-lined ground squirrels. Comp Biochem Physiol B 79: 167–171, 1984. [DOI] [PubMed] [Google Scholar]
- 24. Halloran PF, Hunsicker LG. Delayed graft function: state of the art, November 10–11, 2000. Summit meeting, Scottsdale, Arizona, USA. Am J Transplant 1: 115–120, 2001. [PubMed] [Google Scholar]
- 25. Haraldsson B. Tubular reabsorption of albumin: it's all about cubilin. J Am Soc Nephrol 21: 1810–1812, 2010. [DOI] [PubMed] [Google Scholar]
- 26. Hartigan JA, Wong MA. A k-means clustering algorithm. Appl Stat 28: 100–108, 1979. [Google Scholar]
- 27. Hindle AG, Karimpour-Fard A, Epperson LE, Hunter LE, Martin SL. Skeletal muscle proteomics: carbohydrate metabolism oscillates with seasonal and torpor-arousal physiology of hibernation. Am J Physiol Regul Integr Comp Physiol 301: R1440–R1452, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hong SK. Renal function during hypothermia and hibernation. Am J Physiol 188: 137–150, 1957. [DOI] [PubMed] [Google Scholar]
- 29. Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4: 44–57, 2009. [DOI] [PubMed] [Google Scholar]
- 30. Jani A, Epperson E, Martin J, Pacic A, Ljubanovic D, Martin SL, Edelstein CL. Renal protection from prolonged cold ischemia and warm reperfusion in hibernating squirrels. Transplantation 92: 1215–1221, 2011. [DOI] [PubMed] [Google Scholar]
- 31. Jani A, Ljubanovic D, Faubel S, Kim J, Mischak R, Edelstein CL. Caspase inhibition prevents the increase in caspase-3, -2, -8 and -9 activity and apoptosis in the cold ischemic mouse kidney. Am J Transplant 4: 1246–1254, 2004. [DOI] [PubMed] [Google Scholar]
- 32. Krebs HA, Speake RN, Hems R. Acceleration of renal gluconeogenesis by ketone bodies and fatty acids. Biochem J 94: 712–720, 1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee CM, Carter JT, Alfrey EJ, Ascher NL, Roberts JP, Freise CE. Prolonged cold ischemia time obviates the benefits of 0 HLA mismatches in renal transplantation. Arch Surg 135: 1016–1019, 2000. [DOI] [PubMed] [Google Scholar]
- 34. Lindell SL, Klahn SL, Piazza TM, Mangino MJ, Torrealba JR, Southard JH, Carey HV. Natural resistance to liver cold ischemia-reperfusion injury associated with the hibernation phenotype. Am J Physiol Gastrointest Liver Physiol 288: G473–G480, 2005. [DOI] [PubMed] [Google Scholar]
- 35. Ma YL, Zhu X, Rivera PM, Toien O, Barnes BM, LaManna JC, Smith MA, Drew KL. Absence of cellular stress in brain after hypoxia induced by arousal from hibernation in Arctic ground squirrels. Am J Physiol Regul Integr Comp Physiol 289: R1297–R1306, 2005. [DOI] [PubMed] [Google Scholar]
- 36. Mantuano E, Mukandala G, Li X, Campana WM, Gonias SL. Molecular dissection of the human α2-macroglobulin subunit reveals domains with antagonistic activities in cell signaling. J Biol Chem 283: 19904–19911, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Martin SL, Epperson LE, Rose JC, Kurtz CC, Ane C, Carey HV. Proteomic analysis of the winter-protected phenotype of hibernating ground squirrel intestine. Am J Physiol Regul Integr Comp Physiol 295: R316–R328, 2008. [DOI] [PubMed] [Google Scholar]
- 38. Moy R. Renal function in the hibernating ground squirrel Spermophilus columbianus. Am J Physiol 220: 747–753, 1971. [DOI] [PubMed] [Google Scholar]
- 39. Nelson CJ, Otis JP, Carey HV. Global analysis of circulating metabolites in hibernating ground squirrels. Comp Biochem Physiol Part D Genomics Proteomics 5: 265–273, 2010. [DOI] [PubMed] [Google Scholar]
- 40. Nelson CJ, Otis JP, Martin SL, Carey HV. Analysis of the hibernation cycle using LC-MS based metabolomics in ground squirrel liver. Physiol Genomics 37: 43–51, 2009. [DOI] [PubMed] [Google Scholar]
- 41. Norden AGW, Unwin RJ. Is the albumin retrieval hypothesis a paradigm shift for nephrology? J Am Soc Nephrol 23: 569–571, 2012. [DOI] [PubMed] [Google Scholar]
- 42. Oberbauer R, Rohrmoser M, Regele H, Muhlbacher F, Mayer G. Apoptosis of tubular epithelial cells in donor kidney biopsies predicts early renal allograft function. J Am Soc Nephrol 10: 2006–2013, 1999. [DOI] [PubMed] [Google Scholar]
- 43. Ojo AO, Wolfe RA, Held PJ, Port FK, Schmouder RL. Delayed graft function: risk factors and implications for renal allograft survival. Transplantation 63: 968–974, 1997. [DOI] [PubMed] [Google Scholar]
- 44. Rose JC, Epperson LE, Carey HV, Martin SL. Seasonal liver protein differences in a hibernator revealed by quantitative proteomics using whole animal isotopic labeling. Comp Biochem Physiol 6: 163–170 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Russo LM, Sandoval RM, McKee M, Osicka TM, Collins AB, Brown D, Molitoris BA, Comper WD. The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: retrieval is disrupted in nephrotic states. Kidney Int 71: 504–513, 2007. [DOI] [PubMed] [Google Scholar]
- 46. Serkova NJ, Rose JC, Epperson LE, Carey HV, Martin SL. Quantitative analysis of liver metabolites in three stages of the circannual hibernation cycle in 13-lined ground squirrels by NMR. Physiol Genomics 31: 15–24, 2007. [DOI] [PubMed] [Google Scholar]
- 47. Shao C, Liu Y, Ruan H, Li Y, Wang H, Kohl F, Goropashnaya AV, Fedorov VB, Zeng R, Barnes BM, Yan J. Shotgun proteomic analysis of hibernating arctic ground squirrels. Mol Cell Proteomics 9: 313–326, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Solez K, Racusen LC, Marcussen N, Slatnik I, Keown P, Burdick JF, Olsen S. Morphology of ischemic acute renal failure, normal function, and cyclosporine toxicity in cyclosporine-treated renal allograft recipients. Kidney Int 43: 1058–1067, 1993. [DOI] [PubMed] [Google Scholar]
- 49. Srere HK, Belke D, Wang LCH, Martin SL. α2-Macroglobulin gene expression is independent of acute phase response during hibernation in ground squirrels. Am J Physiol Regul Integr Comp Physiol 268: R1507–R1512, 1995. [DOI] [PubMed] [Google Scholar]
- 50. Srere HK, Wang LCH, Martin SL. Central role for differential gene expression in mammalian hibernation. Proc Natl Acad Sci USA 89: 7119–7123, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Team RDC. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing, 2006. [Google Scholar]
- 52. Tempel GE, Musacchia XJ, Jones SB. Mechanisms responsible for decreased glomerular filtration in hibernation and hypothermia. J Appl Physiol 42: 420–425, 1977. [DOI] [PubMed] [Google Scholar]
- 53. van Breukelen F, Martin SL. Reversible depression of transcription during hibernation. J Comp Physiol 172: 355–361, 2002. [DOI] [PubMed] [Google Scholar]
- 54. van Breukelen F, Martin SL. Translational initiation is uncoupled from elongation at 18°C during mammalian hibernation. Am J Physiol Regul Integr Comp Physiol 281: R1374–R1379, 2001. [DOI] [PubMed] [Google Scholar]
- 55. Vasile E, Simionescu M, Simionescu N. Visualization of the binding, endocytosis, and transcytosis of low-density lipoprotein in the arterial endothelium in situ. J Cell Biol 96: 1677–1689, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yan J, Barnes BM, Kohl F, Marr TG. Modulation of gene expression in hibernating arctic ground squirrels. Physiol Genomics 32: 170–181, 2008. [DOI] [PubMed] [Google Scholar]
- 57. Zancanaro C, Malatesta M, Mannello F, Vogel P, Fakan S. The kidney during hibernation and arousal from hibernation. A natural model of organ preservation during cold ischaemia and reperfusion. Nephrol Dial Transplant 14: 1982–1990, 1999. [DOI] [PubMed] [Google Scholar]
- 58. Zatzman ML. Renal and cardiovascular effects of hibernation and hypothermia. Cryobiology 21: 593–614, 1984. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.