Abstract
Eating is necessary for survival, gives great pleasure and can be perturbed leading to undernutrition, overnutrition and eating disorders. The development of feeding in humans relies on complex interplay between homeostatic mechanisms; neural reward systems; and child motor, sensory and socio-emotional capability. Furthermore, parenting, social influences and the food environment influence the development of eating behavior. The rapid expansion of new knowledge in this field, from basic science to clinical and community-based research, is expected to lead to urgently needed research in support of effective, evidence-based prevention and treatment strategies for undernutrition, overnutrition and eating disorders in early childhood. Using a biopsychosocial approach, this review covers current knowledge of the development of eating behavior from the brain to the individual child, taking into account important contextual influences.
Key Terms: eating, feeding behavior, child development, social environment, parenting
INTRODUCTION
Human eating behavior develops rapidly from infancy to school age. Normal development should lead to adequate but not excessive weight gain during childhood and healthy eating behaviors throughout the life-course. We review multilevel influences on eating behavior including neural mechanisms, individual child development, parent-child interaction and social influences. This review brings together the biological basis of energy regulation; current understanding of sensory determinants; research related to social influences; and eating behavioral processes that influence the development of food acceptance and appetite regulation. Exciting advances in neurosciences inform our understanding of the complexities of eating for energy balance and for pleasure. The developmental focus of this article is on the prenatal to school-age periods and does not include adolescent and adult eating disorders or treatment approaches to infant, toddler and school-age eating problems.
ENERGY REGULATION
Biological basis
Physiological signals can induce or suppress feeding. The neurophysiology of eating regulation involves the hypothalamus and brain stem, gastrointestinal system, pancreas, and adipose tissue through neuroendocrine feedback loops (Figure 1). During periods of energy deficit, ghrelin, a peptide, is released from the stomach signaling the hypothalamic arcuate nucleus to release agouti-related peptide, neuropeptide Y and orexin, for appetite stimulation. [1] After eating, pancreatic insulin, intestinal peptide YY, and leptin from adipocytes decrease the release of appetite-stimulating peptides and orexin from the arcuate nucleus, inhibiting appetite stimulation. [2, 3] In the face of energy surplus, cholecystokinin and leptin influence the release of pro-opiomelanocortin and cocaine-amphetamine regulated transcript neurons from the arcuate nucleus to inhibit appetite. [4–6] Leptin increases the desire to eat when levels are low and decreases hunger when levels are high. [2] Adipocytes also release adiponectin with increasing levels in response to fasting and decreasing levels in case of obesity. [7]
Figure 1.
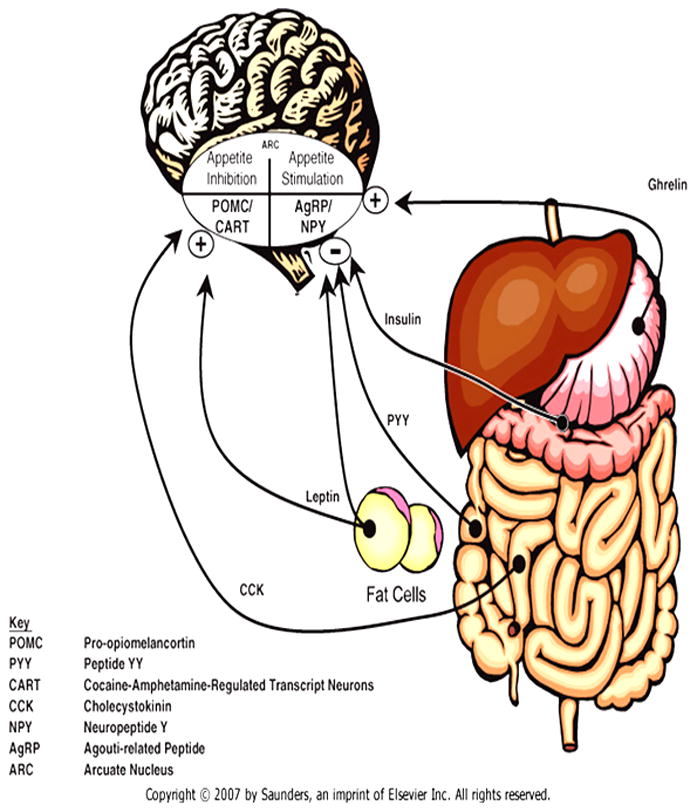
Control of appetite
Citation/Permissions: Reprinted from Nelson Textbook of Pediatrics, 19th Edition, Figure 44-2, page no. 182, Copyright 2011, with permission from Elsevier.
Sophisticated biological systems exist to promote energy balance, avoiding starvation whenever possible and decreasing likelihood for obesity. Recent research confirms the existence of individual differences in sensitivity to energy balance and suggests that gene-environment interactions could influence body weight homeostasis. [8] Furthermore, humans can override these systems. [9–11] For example, during famine, people can focus on a variety of activities until they become debilitated. In the opposite extreme, when palatable food is available in excess, many people consume more than they need. [12] On a day-to-day basis, human eating behavior is strongly influenced by features independent of energy need, especially characteristics of available food and the environment. [13] Flavor, odor, texture, temperature and food presentation are important determinants. Furthermore, social factors may be even more important than the sensory cues of the foods themselves in influencing the development of eating behavior. [14–18]
Infant Regulation of Energy Intake
Breastfed infants benefit from a positive feedback loop that relates suckling to milk production.[19, 20] In the first months of life, formula fed infants are also capable of self-regulating milk intake. Under experimental conditions, 3-day old infants responded negatively to the odor of their formula within several hours of a feeding, but not if more time had lapsed. [21] This suggests that newborns are less interested in feeding when satiated. In a study of 7- to 14-week-old infants, milk volume intake related to time since the last feeding. [17] The link between time elapsed and milk intake was unexpected, as newborn experimental animals maximize intake of available milk. Exactly what the infant senses as a correlate of time since last feeding remains unclear but could include energy expended, sensation of gastric filling, or desire to suckle. Breastfed infants reduce milk volume intake with pacifier use, suggesting a role for suckling in self-regulation of intake. [22, 23] It is also possible that hormonal mechanisms increase pleasure related to feeding after some time has passed. Whether infants self-regulate their intake for energy balance or energy excess is not known, but is one of the most important scientific questions related to early feeding and risk for obesity. Even in adults, it is not clear whether energy is regulated around a set point, settling point, or a different model that better explains gene-by-environment interaction. [24] Behavioral and basic research testing drivers of infant satiety and satisfaction, bringing together longitudinal studies of different feeding strategies with careful measurement of possible regulators promise to begin to solve this important puzzle. [25–30]
REWARD MECHANISMS RELATED TO EATING
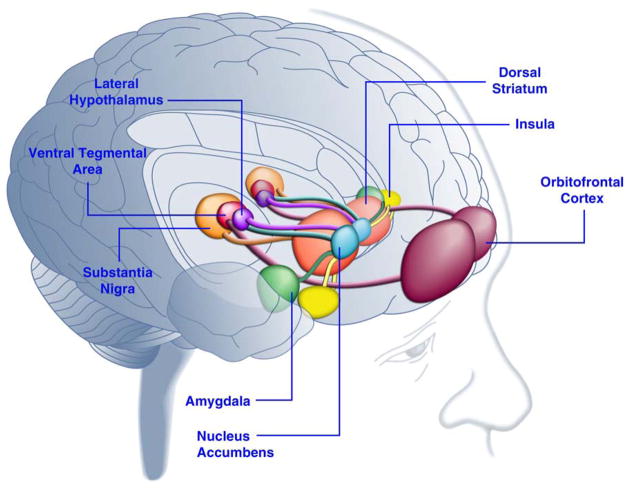
Recent advances in neuroscience relating eating behavior to brain reward systems hold promise for understanding disorders of under-eating and overeating (Figure 2). [31] Brain-based reward systems work in concert with hormonal regulators of energy balance. [32, 33] While early development of reward systems remain incompletely understood, it seems likely that pleasure and reward come into play in young children. Humans are unlikely to overeat bland foods, but palatable foods are frequently eaten in excess of energy requirements. [34–38] Delicious foods are known to enhance mood by activating reward systems.[39] The striatum, insula, anterior cingulate cortex and midbrain regions including the ventral tegmental area and substantia nigra are active in representing reward in response to food. [31] Dopamine is involved in reward response to palatable food consumption. [40] In addition, the orbito-frontal cortex encodes specific types of reward stimuli including various aspects of food: odor; visual input; temperature, viscosity, astringency and fat texture; and taste. [41–44] Neurons respond strongly at the beginning of an eating episode and become less responsive as satiety for a specific food is reached. Behavioral research suggests that this phenomenon is already present in 2.5- to 5-year-old children. [45] As interest diminishes for one food, neurons remain capable of a reward response to other foods. Having a variety of foods available may lead to increased consumption. [46] This may have had evolutionary advantage in ensuring intake of a variety of nutrients. In the current era, it could be one factor related to population risk for obesity. Similarities between overeating in obesity and excessive drug use in addiction suggest that both may relate to a change from “liking to wanting”. [43] Novel research strategies are bringing energy homeostasis and reward homeostasis together in new models of appetite regulation to disentangle “liking” from “wanting”. [24, 47–50]
Figure 2.
Areas of the Human Brain Activated in Response to Palatable Food or Food-Associated Cues
Citation/permissions: Reprinted from Neuron, Vol 69, Paul J. Kenny, Reward Mechanisms in Obesity: New Insights and Future Directions, page no. 665, Copyright 2011, with permission from Elsevier.
DEVELOPMENT OF SENSORY PREFERENCES – GENES AND EXPOSURE
Recognition of tastes and odors develops before birth with fetal exposure, as the fetus swallows amniotic fluid, flavored by the mother’s diet including aromatic compounds from garlic, anise and onion. [51–54] Infants exposed to carrot juice in the mother’s diet during the third trimester of pregnancy or lactation were more likely to prefer infant cereal made with carrot juice compared to those without early life exposure. [55] There is considerable interest in prenatal programming of taste preferences as it could lead to interventions to influence early acceptance of nutritious foods. [55, 56] Longitudinal studies of infant taste preferences could be designed by enrolling infants born to mothers participating in randomized controlled trials testing interventions to promote healthier diets diets during pregnancy. [57–59]
After birth, breastfed infants are still exposed to flavors from the maternal diet. [55] Months before the first tastes of baby foods or non-milk beverages, these exposures establish and modify taste preferences in the infant, at least in the short term. Early in life, most infants and children prefer sweet and salty flavors [60–62], preferences that are enhanced by increased exposure and decrease slightly by adulthood. [63–66] Bitter flavors, such as those in vegetables, are often rejected when first experienced, but liking increases with exposure. For example, infancy exposure to soy and hydrolyzed protein formulas was associated with accepting these formulas 4 years later. [67] During early childhood, repeated exposure to a variety of foods is associated with initially non-preferred foods. [68] In some cultures, children are deliberately exposed to strong flavors. For example in Mexico, they are given food flavored with chili peppers at gradually increasing strength. [69, 70] Learning to like initially unpalatable foods may be part of a process of socialization. [71] Taste preferences relate to prior experience, developmental stage, and genes that influence flavor perception. [72] A well-studied example is the ability to taste the bitter compound, 6-n-propylthiouracil (PROP), mediated in part by the TAS2R38 gene. [73] Children who do not taste PROP may be more likely to accept vegetables. Insensitivity to, PROP, occurs in approximately 30% of Europeans and varies worldwide. [74] The ability to taste PROP is associated with perceiving bitter foods as less palatable, lower vegetable intake, more food aversions, and risk for childhood obesity. [75–82]
EARLY DEVELOPMENT
During the first 2 years, infants progress through three developmental feeding periods: the nursing period, the transitional feeding period, and the modified adult feeding period. Feeding development typically proceeds based on well-described neuro-developmental milestones (Table 1).
Table 1.
| Gestational age (weeks) | |
|
| |
| 16–20 | Swallows amniotic fluid |
| 18–24 | True suckling |
| 34–37 | Coordinated suck and swallow |
|
| |
| Postnatal age (months) | |
|
| |
| 2–4 | Loss of extrusion reflex |
| 10–12 | Pincer grasp |
| 8–18 | Self-feeding develops |
| 9–12 | Cup drinking |
| 18–24 | Precise up and down tongue movements |
| 24–26 | Circulatory jaw rotation |
Nursing Period
Dependence on milk feeding characterizes the nursing period (birth to 6 months). The full term neonate possesses primitive reflexes, including sucking and rooting, allowing feeding during first hours of life. [83] Another primitive reflex, gagging, protects the airway and sometimes interferes with suckling. [84] The small volume of colostrum decreases the chance of choking and regurgitation. As coordinated sucking and swallowing rapidly improve, the volume of transitional milk gradually increases over the first 4 to 10 days. Most full term infants are accomplished “nursers” within 2 weeks. Competent infant sucking is necessary for the dyadic process of breastfeeding. Therefore, breastfeeding is perturbed when the infant is sleepy or neurologically impaired.
Breastfeeding mothers experience important physiological changes related to infant feeding, including a surge in oxytocin and prolactin production by the fourth postpartum day. [85, 86] Oxytocin promotes maternal-infant bonding and prolactin inhibits sexual interest. Both hormones remain high during lactation and are highest during exclusive breastfeeding. Oxytocin and prolactin contribute to maternal-infant responsiveness and decreased attention to other interests. The reciprocal process of breastfeeding is the prototype of dyadic maternal-infant feeding. [87] Social feeding behaviors, holding, rocking, stroking, and visual engagement, occur during breast- and bottle-feeding. Fatigue, distraction, stress and depression can disrupt adaptive feeding interaction. [88]
Complementary feeding
Complementary foods, nutritionally inferior to human milk or iron-fortified infant formula, are frequently offered to infants less than 6-months old. In developing countries, this practice can be a major cause of malnutrition. [89] By 6 months, infant caloric and iron needs typically exceed the capacity of breastfeeding, beginning the “transitional feeding period”. Between 6 and 12 months, infants gradually increase consumption of complementary foods as calories continue to come predominantly from milk. Current U.S. recommendations for infant feeding include: exclusive breastfeeding for 6 months; using infant formulas to supplement breastfeeding, if necessary; and introduction of complementary foods at 4 to 6 months with breastfeeding and complementary foods continuing to 12 months. [90] World Health Organization recommendations are for exclusive breastfeeding for 6 months followed by complementary foods and breastfeeding until 2 years.[91] Feeding practices during this period have varied widely by history, culture and custom. In the U.S., feeding practices changed radically from the early 1900s to the 1960s. At the turn of the last century, supplemental foods were not routinely provided until the end of the first year. Concern about malnutrition including rickets led to the development of precooked infant cereal. The initial product, introduced in 1930, was made of wheat, oatmeal, corn meal, bone meal, dried brewer’s yeast, and powdered alfalfa leaf, fortified with reduced iron, providing minerals and vitamins A, B1, B2, D, and E. [92] By 1950, most pediatricians recommended complementary foods for infants beginning at 3 to 8 weeks. [93] This trend toward very early feeding was reversed over the next 2 decades. [94] The fact that most infants accommodate to varying cultural and historical feeding patterns and achieve adequate growth is a testimony to infant adaptability and resilience.
Development
Recommendations to begin complementary feeding at 6 months are based on current understanding of nutritional needs and infant neurodevelopmental capacity (Table 1). Important considerations include the ability of the infant to accept non-liquid food without choking, gagging or tongue thrusting. One primitive reflex, the extrusion reflex, protects the infant from choking by a tongue thrust in response to food placed on the tongue. This reflex gradually diminishes over the first 2 to 4 months. The development of neck stability, sitting and reaching are also important milestones related to feeding. Six-month-old infants sit with support and indicate interest and disinterest in feeding. Near the end of the first year, infants become capable of handling more textured foods and partial self-feeding. The timing of cup feeding varies by context. For example, in Australia, public health messages encourage cup feeding as early as 6 months, because of bottle feeding-related tooth decay. [95]
Modified adult diet
In the second year, toddlers consume a diet that resembles their families’ preferences. Introduction of a variety of nutritious foods and flavors is important during both the transitional and modified adult periods as younger toddlers are initially more accepting of novel foods compared to preschool children, who may be reluctant to try new foods. The reluctance to try new foods is low at weaning and rapidly rises to a peak between 2 and 6 years, with considerable variability. [96] Neophobia is often more pronounced for eating than it is in other developmental domains. [97–100] It is possible that food neophobia provided selective advantage, protecting individuals from eating unfamiliar, potentially toxic plants. [101] Highly neophobic children eat fewer vegetables, fruits, and meats compared to others. Some children, such as those with autistic spectrum disorder, are at greater risk for nutritional problems because of selective food intake. [102]
Cultural influences
Cultural norms for caring for infants and toddlers influence feeding practices. [103] Customs related to clothing, carrying, sleeping, holding and placing the infant when not held, play a role in feeding. [104] In some cultures, mothers are expected to chew food for their infants and toddlers while in other cultures this practice is considered unsanitary and possibly harmful. [105] Toddlers may be placed in a feeding chair, fed in the arms of the parent, or allowed to walk from person to person at the family table. [106] Cultural feeding practices often persist in first generation immigrants, perhaps more so when grandparents from the country of origin are involved. [106] Furthermore, authoritarian feeding practices have been noted to increase in first generation immigrants and lessen during the second generation. The diversity of feeding practices by culture reinforces the concept that infants and toddlers, as a group, can adapt to vastly differing approaches to feeding. However, individual vulnerabilities may lead to feeding perturbations even when the feeding practices are culturally acceptable.
Many families do not follow expert feeding recommendations. [107] Given the historical context, clinicians might accept some variation for infants who are gaining appropriate weight with positive affect during feeding. However, certain culturally-appropriate feeding strategies might increase risk for excess or inadequate weight gain, or feeding problems. [103] A current concern, the dramatic increase in childhood obesity over the last 40 years, occurred during a period of stable recommendations related to infant feeding. As formula feeding and early complementary feeding are associated with more rapid infant growth and risk for obesity, cultural norms at odds with current infant feeding recommendations could be one factor in obesity risk. [108–116] Cultural influences on infant feeding practices have been documented in the medical literature for more than 35 years and there is a vast literature related to culturally-tailored interventions to promote breastfeeding initiation and longer breastfeeding duration. [117–121] Research aimed at developing culturally-tailored approaches to optimizing early life growth and feeding development is needed. Culturally tailored interventions to promote healthful toddler feeding are similarly important. [122]
The amount of toddler feeding independence allowed or encouraged varies greatly by culture and often relates to how independence and interdependence are valued. [106] In the U.S., toddlers are expected to self-feed early, but in some cultures, toddlers may be consistently fed by adults until much later. Infants depend on the caretaker’s capacity to understand their cues related to hunger and satiety. The dyadic nature of feeding continues into the transitional period. As infants approach the end of the first year, they are increasingly able to express their wants and needs and gradually express increasing desire for independence in many activities, including feeding. Strategies to allow increasing self-feeding as competence develops may prevent mealtime distress and, in some cases, feeding disturbance.
PARENT-CHILD INTERACTION
Temperament
Infant and child temperament may influence parental feeding strategies and, in some cases, may be an independent factor related to eating behavior. Infant-caretaker interaction difficulties or poor “fit” can lead to failure to thrive. [123] The caretaker may be unable to understand the infant’s cues and the infant may not adequately express wants and needs. Difficult infants are more prone to develop feeding problems. [124–128] Early food refusal may predate weaning problems, and less positive maternal perceptions of parenting. [129] In several observational studies infants perceived to have more difficult temperaments were less likely to be exclusively breastfed in the first 6 months and more likely to receive early complementary feeding. [107] In school age children, a number of published studies have found no cross-sectional relationship between temperament and eating behavior. However, a Canadian study of 81 sibling pairs concluded that shyness was correlated with increased risk for food neophobia. [130] Another study from Australia, found that infant temperamental characteristics were associated with increased risk for eating disorder symptoms at 12 to 13 years. [131] While these studies, as a whole, do not support infant temperament as a strong predictor of short term or long term eating behaviors, they do suggest that infant temperament plays a role in parent-child interaction related to feeding. Including observational repeated measures of infant temperament and caretaker-infant interaction in longitudinal studies would shed led on the relationships between infant temperament, parenting practices and eating behaviors across childhood.
Parenting styles
Parenting styles, defined by how demanding and responsive parents are in relationship to child behaviors, are important environmental determinants of child emotional maturity, self regulation and behavioral inhibition. [132–136] Research on the influence of parenting on child outcomes have relied on 4 parental prototypes—described as authoritative, authoritarian, permissive, and rejecting/neglecting—developed by Baumrind almost four decades ago. [137] According to this schema, parents are classified according to how they reconcile the joint needs of children for nurturance and limit setting. Authoritative parents are demanding and responsive, and are characterized by high levels of control and warmth. They monitor the child’s behavior and convey clear standards without resorting to intrusive or restrictive approaches. Parents categorized as authoritarian are demanding and directive with low levels of responsiveness. They exhibit high levels of control (similar to authoritative parents), but in contrast show lower levels of warmth. Permissive parents are less likely to be demanding and to require mature behavior, but exhibit high levels of responsiveness. They tend to be lenient and avoid confrontation. The fourth prototype, parents who exhibit a rejecting/neglecting parenting style, are neither demanding nor responsive. Using this construct, children exposed to authoritative parenting show the highest levels of self efficacy, self-discipline, and emotional maturity. Recent research on eating behavior has examined parenting style as a potential environmental determinant of children’s eating behavior. Indulgent or permissive parental feeding style has been positively related to child BMI in preschool children from low-income U.S. and Australian samples and in middle class U.S. first graders. [138, 139] Permissive parenting has also been associated with lower monitoring of unhealthy dietary intake in school-age U.S. children. [140, 141] Parenting characterized by low responsiveness was also a risk for obesity and for emotional eating (associated with disregulated eating and risk for obesity) in U.S. first graders, sampled from the longitudinal NICHD Early Child Care Study. [138] In both U.S. and British samples—sampled from a large longitudinal study and small observational cohort, respectively—authoritative parenting and warmth are protective against emotional eating and risk for obesity. [138, 140]
Restriction, control and monitoring
Parental control of child feeding has been extensively studied relative to highly directive feeding, including restriction. [142, 143] It has become clear that it is important to differentiate between overt and covert parental control of children’s eating behavior and food choices. Overt control includes both restricting, and pressure to eat. Covert control includes strategies such as purchasing only healthy foods for the home and avoiding stores and restaurants that sell unhealthy foods. [144] Overt control can be detected by the child, while covert control involves management that the child may not recognize. Parental eating behaviors also play a role in covert control [144, 145], as parents model healthy eating behaviors for their children. Most parents use some overt and some covert controlling strategies. [146] Furthermore, they may be controlling in some situations but not in others. Parents report changing strategies as the child’s needs evolve. Because child feeding involves a bidirectional process, parent and child characteristics are both instrumental in determining parental control of child eating. Parental control is also influenced by family and cultural norms related to diet and to child eating behavior. Controlling or restrictive feeding practices play an important role in the development of feeding behavior. [14, 142] A large body of research shows that overtly restricting access to certain foods or to desired quantities of food may be counterproductive and associated with increased caloric intake and disinhibited eating. For example, girls exposed to higher levels of maternal restriction at 5 years, were more likely to exhibit disinhibited eating, measured experimentally in the laboratory as “eating in the absence of hunger” at age 7 and 9 years. The relationship was stronger if they were overweight. [14, 145, 147, 148] The investigators hypothesized that overt restricting and controlling could blunt ability to self-regulate caloric balance and energy needs and lead to varying degrees of uncontrolled eating, weight gain, obesity, and risk for eating disorders. In this longitudinal study, child obesity was not a precondition of restriction. In other studies, parental restriction has been shown to develop in relationship to perceiving the child as overweight or tending to overeat, or maternal body image. [149] The important work of Birch has been based largely on a longitudinal cohort of approximately 200 white, middle class girls from non-urban Pennsylvania, and is best understood in that context. [150] Birch and her research team have continued to investigate these relationships as the participants age using both laboratory-based to naturalistic settings. [142] The paradigm of eating in the absence of hunger has been used in research in a variety of different contexts, including Latino adolescents and urban samples. [34, 151, 152]
Other aspects of parental control merit specific mention, including rewards, prompts to eat, intrusiveness and monitoring. More than 20 years ago, Birch showed that using rewards to promote consumption of healthy foods, or to “clean your plate” resulted in declining preference for those healthy foods. [153] In another study, mothers with less education were more likely to prompt their 3- to 6-year olds to eat. This was especially true if the child was younger and the food was novel. While obese mothers were not more likely to prompt their child to eat than non-obese mothers, [154] when prompted, their children were more likely to accept the food (compliant) than children whose mothers were not obese. This research highlights the complexity of the dyadic interaction related to feeding and suggests that unmeasured variables (i.e. mother-child relationship, genes) may promote the child to eat more in certain settings. Conversely, high maternal promotion of feeding has also been noted to adversely influence growth in infants with failure to thrive, in certain settings. [155] More aberrant parenting behaviors, including intrusiveness, have been associated with infant eating disorders. [156] Intrusive feeding patterns manifest when parents are unresponsive to the child’s feeding cues, in favor of their own perceptions or needs. Examples include insistence on finishing the last bite, coercion and, in the, extreme, force-feeding. To date studies related to pressure and intrusiveness in infant feeding are mostly cross sectional making it difficult to infer temporal precedence.
Parental monitoring of child intake, one of three factors assessing parental control attitudes and practices in Birch’s validation of the Child-Feeding Questionnaire, [157] was included in the construct of parental control that was associated with increased risk for disinhibited eating. However, recent research, assessing monitoring as a separate construct, suggests that parental monitoring can, in fact, positively influence children’s growth and eating behavior. Monitoring has been related to appropriate weight gain and to weight loss in the case of obesity. [158, 159] Recent research using the NICHD Early Child Care Study showed that parental dietary monitoring of 3rd grade girls was not related to increased risk for eating disorders, 3 years later, [160] supporting the distinction between restricion and monitoring. Furthermore, evidence-based weight loss programs include monitoring and control, and these programs do not promote eating disorders in obese children (who are known to be at increased risk). [146, 161]
In summary, a vast body of work on parenting behaviors related to child eating suggests that most parents use some overt or covert strategies related to promoting what they perceive as optimal eating behavior and diet. In the current obesogenic environment, control and monitoring of children’s food environment seems essential. However, some strategies can be counterproductive, including rewarding the consumption of certain foods, some overt controlling practices and intrusive feeding strategies.
SOCIAL INFLUENCES ON ENERGY INTAKE AND EATING BEHAVIOR
Eating is among the most social of human activities. [162] Experimental evidence suggests social influences on energy regulation in infants. In an elegant experiment with 7- to 14-week-old infants, volume of intake was related to social interaction. Infants who were held, compared to infants fed in an infant seat, had intake linearly related to time since the last feeding. [17] However, when a novel feeder visually engaged with the infant, there was a lower association between volume and time since last feeding. The investigators concluded that “social influences exert strong immediate impacts on suckling”. Suckling provided nutrition and an opportunity to obtain social information about the feeder. Disregulation of volume from time since last feeding, did not occur if the mother was the feeder and socially engaged with the infant during the feeding.
Social facilitation, increased behavior based on the sight or sound of others engaged in the same behavior, has been demonstrated as a determinant of eating behavior in primates, and human children and adults. [15, 163–173] Social facilitation is believed to be based on either increased arousal or time extension. In an experimental study, preschool children showed increased consumption, when groups were large and snack time was longer (≥11 minutes). [15] Children in larger groups initiated the snack more rapidly and ate slightly faster. Increased consumption only achieved significance when there was adequate time, as eating rates were marginally different. When the children socialized during the meal, their consumption was lower. Adult prompting had no effect on amount consumed. [15]
Adult testimony and modeling promote children’s acceptance and liking of novel foods. Children as young as 18-months old recognize that they may not like the same foods adults do, but are more likely to try foods that adults recommend. [174] By 3 years, children understand that adult testimony is not always reliable, yet still tend to try foods based on adult recommendations. Later, children are more likely to remember these flavors, and children over 4-years old have increased odds of “liking” the flavor. [175] Testimony regarding palatability is likely to be trusted by 3- to 6-year olds especially when absolute terms (“great”, “delicious”) instead of relative terms (“better”, “more”) are used. [174] Adult modeling is a remarkably effective method of persuading preschool children to eat novel foods. [96, 174] Acceptance of a novel food is enhanced if offered by a familiar adult, who is eating food identical in shape, size and color. [96] This differentiates human children from juvenile primates who accept novel foods based on social facilitation alone.
Social suggestion can also modify children’s food preferences. In 1938, social psychologist, Karl Duncker, studied the efficacy of social suggestion in influencing British preschool child behaviors and endorsed preferences. [176] The children tasted and rank-ordered preferences for six different foods. One week later, they were exposed to a classmate’s preferences. Most children changed their rank order to match their classmate’s, especially if he/she was slightly older, or particularly admired. Change in food preference was likely to persist for 2 months if there were multiple exposures over several weeks. In another experiment, children who listened to a social story in which the hero “saved his people” by providing an unpalatable food, were likely to choose to eat the unpalatable food over chocolate. (They had tasted both foods before). The preference was prolonged by hearing the story again. It appears that social suggestion is a powerful modifier of children’s food preferences. This becomes important with increasing peer influence during adolescence and young adulthood, often resulting in preferences for less healthy foods. [177]Furthermore, the power of social suggestion is not lost on industries that successfully influence children’s food preferences and choices, the effects being strongest for the least healthy foods. [176, 178, 179] In summary, behavioral research suggests that social factors from family, peers and larger social groups strongly influence food selection and development of preferences.
CONCLUSION
While there are many unanswered questions related to the development of eating behavior, we are in a period of rapid expansion of our understanding of the intricate and sophisticated processes of energy regulation and reward regulation. Advances in neurosciences and genomics promise to elucidate individual differences in energy regulation, taste preferences, and reward seeking behavior related to food. Future research promises to change the way we think about energy and reward regulation by addressing many questions including the following. How does exposure increase liking? To what extent do specific sensory cues and social influences account for acceptance and liking of foods? Is food an addictive substance for some people? Furthermore, behavioral research is shedding light on how feeding experiences, parent-child interactions and larger social influences modify eating behavior. Understanding the development of eating behavior is important as many clinical and public health problems, including failure to thrive, eating disorders and obesity, are better understood when development is taken into account. Complex biological, developmental, psychological and social systems drive the development and maintenance of eating behaviors. Increasing understanding of each level will inform prevention, intervention and treatment of eating behavior related conditions.
Acknowledgments
Support provided by Award Number R01HL088530 (PI: Gahagan) from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute or the National Institutes of Health.
Footnotes
Disclosure: No conflicts of interest to report.
REFERENCES CITED
- 1.Inui A, Asakawa A, Bowers CY, Mantovani G, Laviano A, Meguid MM, et al. Ghrelin, appetite, and gastric motility: the emerging role of the stomach as an endocrine organ. FASEB J. 2004;18:439–56. doi: 10.1096/fj.03-0641rev. [DOI] [PubMed] [Google Scholar]
- 2.Margetic S, Gazzola C, Pegg GG, Hill RA. Leptin: a review of its peripheral actions and interactions. Int J Obes Relat Metab Disord. 2002;26:1407–33. doi: 10.1038/sj.ijo.0802142. [DOI] [PubMed] [Google Scholar]
- 3.Konner AC, Klockener T, Bruning JC. Control of energy homeostasis by insulin and leptin: targeting the arcuate nucleus and beyond. Physiol Behav. 2009;97:632–8. doi: 10.1016/j.physbeh.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 4.Mutt V. Historical perspectives on cholecystokinin research. Ann N Y Acad Sci. 1994;713:1–10. doi: 10.1111/j.1749-6632.1994.tb44046.x. [DOI] [PubMed] [Google Scholar]
- 5.Dockray GJ. Cholecystokinin and gut-brain signalling. Regulatory Peptides. 2009;155:6–10. doi: 10.1016/j.regpep.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Elmer PJ, Obarzanek E, Vollmer WM, Simons-Morton D, Stevens VJ, Young DR, et al. Effects of Comprehensive Lifestyle Modification on Diet, Weight, Physical Fitness, and Blood Pressure Control: 18-Month Results of a Randomized Trial. Ann Intern Med. 2006;144:485–95. doi: 10.7326/0003-4819-144-7-200604040-00007. [DOI] [PubMed] [Google Scholar]
- 7.Gustafson B. Adipose tissue, inflammation and atherosclerosis. J Atheroscler Thromb. 2010;17:332–41. doi: 10.5551/jat.3939. [DOI] [PubMed] [Google Scholar]
- 8.Bouchard C. Gene-environment interactions in the etiology of obesity: defining the fundamentals. Obesity (Silver Spring) 2008;16:S5–S10. doi: 10.1038/oby.2008.528. [DOI] [PubMed] [Google Scholar]
- 9.Kempson KM, Palmer-Keenan D, Sadani PS, Ridlen S, Scotto Rosato N. Food management practices used by people with limited resources to maintain food sufficiency as reported by nutrition educators. J Am Diet Assoc. 2002;102:1795–9. doi: 10.1016/s0002-8223(02)90385-8. [DOI] [PubMed] [Google Scholar]
- 10.Olson CM. Nutrition and health outcomes associated with food insecurity and hunger. J Nutr. 1999;129:521S–524S. doi: 10.1093/jn/129.2.521S. [DOI] [PubMed] [Google Scholar]
- 11.Hamelin AM, Habicht JP, Beaudry M. Food insecurity: consequences for the household and broader social implications. J Nutr. 1999;129:525S–528S. doi: 10.1093/jn/129.2.525S. [DOI] [PubMed] [Google Scholar]
- 12.Wammes B, French S, Brug J. What young Dutch adults say they do to keep from gaining weight: self-reported prevalence of overeating, compensatory behaviours and specific weight control behaviours. Public Health Nutr. 2007;10:790–8. doi: 10.1017/S1368980007258537. [DOI] [PubMed] [Google Scholar]
- 13.Drewnowski A. Obesity, diets, and social inequalities. Nutr Rev. 2009;67:S36–9. doi: 10.1111/j.1753-4887.2009.00157.x. [DOI] [PubMed] [Google Scholar]
- 14.Fisher JO, Birch LL. Restricting access to palatable foods affects children’s behavioral response, food selection, and intake. Am J Clin Nutr. 1999;69:1264–72. doi: 10.1093/ajcn/69.6.1264. [DOI] [PubMed] [Google Scholar]
- 15.Lumeng JC, Hillman KH. Eating in larger groups increases food consumption. Arch Dis Child. 2007;92:384–7. doi: 10.1136/adc.2006.103259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rozin P, Kennel K. Acquired preferences for piquant foods by chimpanzees. Appetite. 2007;4:69–77. doi: 10.1016/s0195-6663(83)80003-8. [DOI] [PubMed] [Google Scholar]
- 17.Lumeng JC, Patil N, Blass EM. Social influences on formula intake via suckling in 7 to 14-week-old-infants. Dev Psychobiol. 2007;49:351–61. doi: 10.1002/dev.20221. [DOI] [PubMed] [Google Scholar]
- 18.Addessi E, Galloway AT, Visalberghi E, Birch LL. Specific social influences on the acceptance of novel foods in 2–5-year-old children. Appetite. 2005;45:264–71. doi: 10.1016/j.appet.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Dewey KG, Lonnerdal B. Infant self-regulation of breast milk intake. Acta Paediatr Scand. 1986;75:893–8. doi: 10.1111/j.1651-2227.1986.tb10313.x. [DOI] [PubMed] [Google Scholar]
- 20.Hartmann PE, Atwood CS, Cox DB, Daly SEJ. Endocrine and autocrine strategies for the control of lactation in women and sows. In: Wilde CJ, Peaker M, Knight CH, editors. Intercellular Signaling in the Mammary Gland. New York, NY: Plenum Publishing Company Limited; 1994. pp. 203–26. [Google Scholar]
- 21.Soussignan R, Schaal B, Marlier L. Olfactory alliesthesia in human neonates: prandial state and stimulus familiarity modulate facial and autonomic responses to milk odors. Dev Psychobiol. 1999;35:3–14. doi: 10.1002/(sici)1098-2302(199907)35:1<3::aid-dev2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 22.Victora CG, Tomasi E, Olinto MTA, Barros FC. Use of pacifiers and breastfeeding duration. The Lancet. 1993;341:404–6. doi: 10.1016/0140-6736(93)92991-2. [DOI] [PubMed] [Google Scholar]
- 23.Barros FC, Victora CG, Semer TC, Filho ST, Tomasi E, Weiderpass E. Use of Pacifiers is Associated With Decreased Breast-Feeding Duration. Pediatrics. 1995;95:497–9. [PubMed] [Google Scholar]
- 24.Speakman JR, Levitsky DA, Allison DB, Bray MS, de Castro JM, Clegg DJ, et al. Set points, settling points and some alternative models: theoretical options to understand how genes and environments combine to regulate body adiposity. Dis Model Mech. 2001;4:733–45. doi: 10.1242/dmm.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Savino F, Grassino EC, Fissore MF, Guidi C, Liguori SA, Silvestro L, et al. Ghrelin, motilin, insulin concentration in healthy infants in the first months of life: relation to fasting time and anthropometry. Clin Endocrinol (Oxf) 2006;65:158–62. doi: 10.1111/j.1365-2265.2006.02561.x. [DOI] [PubMed] [Google Scholar]
- 26.Cripps RL, Martin-Gronert MS, Ozanne SE. Fetal and perinatal programming of appetite. Clin Sci (Lond) 2005;109:1–11. doi: 10.1042/CS20040367. [DOI] [PubMed] [Google Scholar]
- 27.Cripps RL, Martin-Gronert MS, Archer ZA, Hales CN, Mercer JG, Ozanne SE. Programming of hypothalamic neuropeptide gene expression in rats by maternal dietary protein content during pregnancy and lactation. Clin Sci (Lond) 2009;117:85–93. doi: 10.1042/CS20080393. [DOI] [PubMed] [Google Scholar]
- 28.Plagemann A. Perinatal nutrition and hormone-dependent programming of food intake. Horm Res. 2006;65:83–9. doi: 10.1159/000091511. [DOI] [PubMed] [Google Scholar]
- 29.Soto PL, Grandy DK, Hursh SR, Katz JL. Behavioral economics of food reinforcement and the effects of prefeeding, extinction, and eticlopride in dopamine D2 receptor mutant mice. Psychopharmacology (Berl) 2011;215:775–84. doi: 10.1007/s00213-011-2173-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cramer CP, Blass EM. Mechanisms of control of milk intake in suckling rats. Am J Physiol. 1983;245:R154–9. doi: 10.1152/ajpregu.1983.245.2.R154. [DOI] [PubMed] [Google Scholar]
- 31.Kenny PJ. Reward mechanisms in obesity: new insights and future directions. Neuron. 2011;69:664–79. doi: 10.1016/j.neuron.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lutter M, Nestler EJ. Homeostatic and hedonic signals interact in the regulation of food intake. J Nutr. 2009;139:629–32. doi: 10.3945/jn.108.097618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 34.Shomaker LB, Tanofsky-Kraff M, Zocca JM, Courville A, Kozlosky M, Columbo KM, et al. Eating in the absence of hunger in adolescents: intake after a large-array meal compared with that after a standardized meal. Am J Clin Nutr. 2010;92:697–703. doi: 10.3945/ajcn.2010.29812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang GJ, Volkow ND, Telang F, Jayne M, Ma J, Rao M, et al. Exposure to appetitive food stimuli markedly activates the human brain. Neuroimage. 2004;21:1790–7. doi: 10.1016/j.neuroimage.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 36.Sunday SR, Sanders SA, Collier G. Palatability and meal patterns. Physiol Behav. 1983;30:915–8. doi: 10.1016/0031-9384(83)90257-3. [DOI] [PubMed] [Google Scholar]
- 37.Zheng H, Lenard NR, Shin AC, Berthoud HR. Appetite control and energy balance regulation in the modern world: reward-driven brain overrides repletion signals. Int J Obes (Lond) 2009;33:S8–13. doi: 10.1038/ijo.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang GJ, Volkow ND, Thanos PK, Fowler JS. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. J Addict Dis. 2004;23:39–53. doi: 10.1300/J069v23n03_04. [DOI] [PubMed] [Google Scholar]
- 39.Macht M, Mueller J. Immediate effects of chocolate on experimentally induced mood states. Appetite. 2007;49:667–74. doi: 10.1016/j.appet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Cornelius JR, Tippmann-Peikert M, Slocumb NL, Frerichs CF, Silber MH. Impulse control disorders with the use of dopaminergic agents in restless legs syndrome: a case-control study. Sleep. 2010;33:81–7. [PMC free article] [PubMed] [Google Scholar]
- 41.Rolls ET. Taste, olfactory and food texture reward processing in the brain and obesity. Int J Obes (Lond) 2011;35:550–61. doi: 10.1038/ijo.2010.155. [DOI] [PubMed] [Google Scholar]
- 42.Small DM. Taste representation in the human insula. Brain Struct Funct. 2010;214:551–61. doi: 10.1007/s00429-010-0266-9. [DOI] [PubMed] [Google Scholar]
- 43.Berridge KC. ‘Liking’ and ‘wanting’ food rewards: brain substrates and roles in eating disorders. Physiol Behav. 2009;97:537–50. doi: 10.1016/j.physbeh.2009.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dagher A. The neurobiology of appetite: hunger as addiction. Int J Obes (Lond) 2009;33:S30–3. doi: 10.1038/ijo.2009.69. [DOI] [PubMed] [Google Scholar]
- 45.Birch LL, Deysher M. Caloric compensation and sensory specific satiety: evidence for self regulation of food intake by young children. Appetite. 1986;7:323–31. doi: 10.1016/s0195-6663(86)80001-0. [DOI] [PubMed] [Google Scholar]
- 46.Rolls BJ, Rowe EA, Rolls ET, Kingston B, Megson A, Gunary R. Variety in a meal enhances food intake in man. Physiology & Behavior. 1981;26:215–221. doi: 10.1016/0031-9384(81)90014-7. [DOI] [PubMed] [Google Scholar]
- 47.Havermans RC. You Say it’s Liking, I Say it’s Wanting …”. On the difficulty of disentangling food reward in man. Appetite. 2001;57:286–94. doi: 10.1016/j.appet.2011.05.310. [DOI] [PubMed] [Google Scholar]
- 48.Epstein LH, Leddy JJ, Temple JL, Faith MS. Food reinforcement and eating: a multilevel analysis. Psychol Bull. 2007;133:884–906. doi: 10.1037/0033-2909.133.5.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Epstein LH, Temple JL, Neaderhiser BJ, Salis RJ, Erbe RW, Leddy JJ. Food reinforcement, the dopamine D2 receptor genotype, and energy intake in obese and nonobese humans. Behav Neurosci. 2007;121:877–86. doi: 10.1037/0735-7044.121.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJ, Cull JG, et al. The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. J R Soc Med. 1996;89:396–400. doi: 10.1177/014107689608900711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mennella JA, Johnson A, Beauchamp GK. Garlic ingestion by pregnant women alters the odor of amniotic fluid. Chem Senses. 1995;20:207–9. doi: 10.1093/chemse/20.2.207. [DOI] [PubMed] [Google Scholar]
- 52.Mennella JA, Beauchamp GK. Maternal Diet Alters the Sensory Qualities of Human Milk and the Nursling’s Behavior. Pediatrics. 1991;88:737–44. [PubMed] [Google Scholar]
- 53.Schaal B, Marlier L, Soussignan R. Human foetuses learn odours from their pregnant mother’s diet. Chem Senses. 2000;25:729–37. doi: 10.1093/chemse/25.6.729. [DOI] [PubMed] [Google Scholar]
- 54.Mennella J, Beauchamp GK. The ontogeny of human flavor perception. In: Beauchamp G, Bartoshuk L, editors. Tasting and Smelling. 2. San Diego, CA: Academic Press; 1997. pp. 199–216. [Google Scholar]
- 55.Mennella JA, Jagnow CP, Beauchamp GK. Prenatal and Postnatal Flavor Learning by Human Infants. Pediatrics. 2001;107:e88. doi: 10.1542/peds.107.6.e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cooke L, Fildes A. The impact of flavour exposure in utero and during milk feeding on food acceptance at weaning and beyond. Appetite. 2011 doi: 10.1016/j.appet.2011.05.317. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 57.Jackson RA, Stotland NE, Caughey AB, Gerbert B. Improving diet and exercise in pregnancy with Video Doctor counseling: a randomized trial. Patient Educ Couns. 2011;83:203–9. doi: 10.1016/j.pec.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 58.Skouteris H, Hartley-Clark L, McCabe M, Milgrom J, Kent B, Herring SJ, et al. Preventing excessive gestational weight gain: a systematic review of interventions. Obes Rev. 2010;11:757–68. doi: 10.1111/j.1467-789X.2010.00806.x. [DOI] [PubMed] [Google Scholar]
- 59.Kinnunen TI, Pasanen M, Aittasalo M, Fogelholm M, Hilakivi-Clarke L, Weiderpass E, et al. Preventing excessive weight gain during pregnancy - a controlled trial in primary health care. Eur J Clin Nutr. 2007;61:884–91. doi: 10.1038/sj.ejcn.1602602. [DOI] [PubMed] [Google Scholar]
- 60.Ventura AK, Mennella JA. Innate and learned preferences for sweet taste during childhood. Curr Opin Clin Nutr Metab Care. 2011;14:379–84. doi: 10.1097/MCO.0b013e328346df65. [DOI] [PubMed] [Google Scholar]
- 61.Birch LL. Development of food preferences. Annu Rev Nutr. 1999;19:41–62. doi: 10.1146/annurev.nutr.19.1.41. [DOI] [PubMed] [Google Scholar]
- 62.Schwartz C, Issanchou S, Nicklaus S. Developmental changes in the acceptance of the five basic tastes in the first year of life. Br J Nutr. 2009;102:1375–85. doi: 10.1017/S0007114509990286. [DOI] [PubMed] [Google Scholar]
- 63.Desor J, Greene L, Maller O. Preferences for sweet and salty in 9- to 15-year-old and adult humans. Science. 1975;190:686–7. doi: 10.1126/science.1188365. [DOI] [PubMed] [Google Scholar]
- 64.Desor JA, Beauchamp GK. Longitudinal changes in sweet preferences in humans. Physiol Behav. 1987;39:639–41. doi: 10.1016/0031-9384(87)90166-1. [DOI] [PubMed] [Google Scholar]
- 65.De Graaf C, Zandstra EH. Sweetness intensity and pleasantness in children, adolescents, and adults - The genesis of sweet preference. Physiol Behav. 1999;67:513–20. doi: 10.1016/s0031-9384(99)00090-6. [DOI] [PubMed] [Google Scholar]
- 66.Beauchamp GK, Cowart BJ, Moran M. Developmental changes in salt acceptability in human infants. Dev Psychobiol. 1986;19:17–25. doi: 10.1002/dev.420190103. [DOI] [PubMed] [Google Scholar]
- 67.Julie AM, Gary KB. Flavor experiences during formula feeding are related to preferences during childhood. Early human development. 2002;68:71–82. doi: 10.1016/s0378-3782(02)00008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Birch LL. The role of experience in children’s food acceptance patterns. J Am Diet Assoc. 1987;87:S36–40. [PubMed] [Google Scholar]
- 69.Rozin P, Schiller D. The nature and acquisition of a preference for chili pepper by humans. Motiv Emot. 1980;4:77–101. [Google Scholar]
- 70.Mennella JA, Turnbull B, Ziegler PJ, Martinez H. Infant Feeding Practices and Early Flavor Experiences in Mexican Infants: An Intra-Cultural Study. J Am Diet Assoc. 2005;105:908–915. doi: 10.1016/j.jada.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 71.Wardle J. Parental influences on children’s diets. Proc Nutr Soc. 1995;54:747–58. doi: 10.1079/pns19950074. [DOI] [PubMed] [Google Scholar]
- 72.Mennella JA, Pepino MY, Reed DR. Genetic and environmental determinants of bitter perception and sweet preferences. Pediatrics. 2005;115:e216–22. doi: 10.1542/peds.2004-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Duffy VB, Davidson AC, Kidd JR, Kidd KK, Speed WC, Pakstis AJ, et al. Bitter receptor gene (TAS2R38), 6-n-propylthiouracil (PROP) bitterness and alcohol intake. Alcohol Clin Exp Res. 2004;28:1629–37. doi: 10.1097/01.ALC.0000145789.55183.D4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guo SW, Reed DR. The genetics of phenylthiocarbamide perception. Ann Hum Biol. 2001;28:111–42. doi: 10.1080/03014460151056310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fischer R, Griffin F, England S, Garn SM. Taste thresholds and food dislikes. Nature. 1961;23:1328. doi: 10.1038/1911328a0. [DOI] [PubMed] [Google Scholar]
- 76.Keller KL, Steinmann L, Nurse RJ, Tepper BJ. Genetic taste sensitivity to 6-n-propylthiouracil influences food preference and reported intake in preschool children. Appetite. 2002 Feb;38:3–12. doi: 10.1006/appe.2001.0441. [DOI] [PubMed] [Google Scholar]
- 77.Drewnowski A, Henderson SA, Hann CS, Berg WA, Ruffin MT. Genetic taste markers and preferences for vegetables and fruit of female breast care patients. J Am Diet Assoc. 2000;100:191–7. doi: 10.1016/S0002-8223(00)00061-4. [DOI] [PubMed] [Google Scholar]
- 78.Dinehart ME, Hayes JE, Bartoshuk LM, Lanier SL, Duffy VB. Bitter taste markers explain variability in vegetable sweetness, bitterness, and intake. Physiol Behav. 2006;87:304–13. doi: 10.1016/j.physbeh.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 79.Turnbull B, Matisoo-Smith E. Taste sensitivity to 6-n-propylthiouracil predicts acceptance of bitter-tasting spinach in 3–6-y-old children. Am J Clin Nutr. 2002;76:1101–5. doi: 10.1093/ajcn/76.5.1101. [DOI] [PubMed] [Google Scholar]
- 80.Lumeng JC, Cardinal TM, Sitto JR, Kannan S. Ability to taste 6-n-propylthiouracil and BMI in low-income preschool-aged children. Obesity (Silver Spring) 2008;16:1522–8. doi: 10.1038/oby.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Glanvill EV, Kaplan AR. Food Preference and Sensitivity of Taste for Bitter Compounds. Nature. 1965;205:851. [Google Scholar]
- 82.Tepper BJ, Keller KL, Ullrich NV. Genetic variation in taste and preferences for bitter and pungent foods: Implications for chronic disease risk. Challenges in Taste Chemistry and Biology. 2004;867:60–74. [Google Scholar]
- 83.Diamant NE. Development of esophageal function. Am Rev Respir Dis. 1985;131:S29–32. doi: 10.1164/arrd.1985.131.S5.S29. [DOI] [PubMed] [Google Scholar]
- 84.Bu’Lock F, Woolridge MW, Baum JD. Development of co-ordination of sucking, swallowing and breathing: ultrasound study of term and preterm infants. Dev Med Child Neurol. 1990;32:669–78. doi: 10.1111/j.1469-8749.1990.tb08427.x. [DOI] [PubMed] [Google Scholar]
- 85.Uvnäs-Moberg K, Widström A-M, Werner S, Matthiesen A-S, Winberg J. Oxytocin and prolactin levels in breast-feeding women. Correlation with milk yield and duration of breast-feeding. Acta Obstetricia et Gynecologica Scandinavica. 1990;69:301–6. doi: 10.3109/00016349009036151. [DOI] [PubMed] [Google Scholar]
- 86.McNeilly AS, Robinson IC, Houston MJ, Howie PW. Release of oxytocin and prolactin in response to suckling. Br Med J (Clin Res Ed) 1983;286:257–9. doi: 10.1136/bmj.286.6361.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gahagan S. Infant feeding processes and disorders. In: Wolraich ML, Drotar DD, Dworkin PH, Perrin EC, editors. Developmental-behavioral pediatrics: Evidence and practice. Philadelphia, PA: Mosby Elsevier; 2008. p. 757. [Google Scholar]
- 88.Kehler HL, Chaput KH, Tough SC. Risk factors for cessation of breastfeeding prior to six months postpartum among a community sample of women in Calgary, Alberta. Can J Public Health. 2009;100:376–380. doi: 10.1007/BF03405274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dewey KG. The challenges of promoting optimal infant growth. J Nutr. 2001;131:1879–80. doi: 10.1093/jn/131.7.1879. [DOI] [PubMed] [Google Scholar]
- 90.American Academy of Pediatrics. Breastfeeding and the use of human milk. Pediatrics. 2005;115:496–506. doi: 10.1542/peds.2004-2491. [DOI] [PubMed] [Google Scholar]
- 91.World Health Organization. Global Strategy for Infant and Young Child Feeding [WHO Web site] 2003 Available at: http://whqlibdoc.who.int/publications/2003/9241562218.pdf.
- 92.Tisdall FF, Drake TG, Summerfeldt P, Brown A. A new whole wheat irradiated biscuit, containing vitamins and mineral elements. Can Med Assoc J. 1930;22:166–70. [PMC free article] [PubMed] [Google Scholar]
- 93.Butler AM, Wolman IJ. Trends in the early feeding of supplementary foods to infants; an analysis and discussion based on a nationwide survey. Quart Rev Pediatr. 1954;9:63–85. [Google Scholar]
- 94.Fomon SJ. What are infants fed in the United States? Pediatrics. 1975;56:350–4. [PubMed] [Google Scholar]
- 95.Channel BH. [Accessed January 18, 2011];Dental care: preventing infant tooth decay, fact sheet [State Government of Victoria, Australia Web site] 2010 Available at: http://www.betterhealth.vic.gov.au/bhcv2/bhcarticles.nsf/pages/Dental_care_preventing_infant_tooth_decay.
- 96.Addessi E, Galloway AT, Visalberghi E, Birch LL. Specific social influences on the acceptance of novel foods in 2–5-year-old children. Appetite. 2005;45:264–271. doi: 10.1016/j.appet.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 97.Birch LL, McPhee L, Shoba BC, Pirok E, Steinberg L. What kind of exposure reduces children’s food neophobia?: Looking vs. tasting. Appetite. 1987;9:171–8. doi: 10.1016/s0195-6663(87)80011-9. [DOI] [PubMed] [Google Scholar]
- 98.Birch LL, Marlin DW. I don’t like it; I never tried it: effects of exposure on two-year-old children’s food preferences. Appetite. 1982;3:353–60. doi: 10.1016/s0195-6663(82)80053-6. [DOI] [PubMed] [Google Scholar]
- 99.Sullivan S, Birch LL. Pass the sugar, pass the salt: experience dictates preference. Developmental Psychology. 1990;26:546–51. [Google Scholar]
- 100.Galloway AT, Lee Y, Birch LL. Predictors and consequences of food neophobia and pickiness in young girls. J Am Diet Assoc. 2003;103:692–8. doi: 10.1053/jada.2003.50134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cashdan E. Adaptiveness of food learning and food aversions in children. Soc Sci Inform. 1998;37:613–632. [Google Scholar]
- 102.Cermak SA, Curtin C, Bandini LG. Food selectivity and sensory sensitivity in children with autism spectrum disorders. J Am Diet Assoc. 2010;110:238–46. doi: 10.1016/j.jada.2009.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Evans A, Seth JG, Smith S, Harris KK, Loyo J, Spaulding C, et al. Parental feeding practices and concerns related to child underweight, picky eating, and using food to calm differ according to ethnicity/race, acculturation, and income. Matern Child Health J. 2009 doi: 10.1007/s10995-009-0526-6. epub. [DOI] [PubMed] [Google Scholar]
- 104.Lozoff B, Brittenham G. Infant care: cache or carry. J Pediatr. 1979;95:478–83. doi: 10.1016/s0022-3476(79)80540-5. [DOI] [PubMed] [Google Scholar]
- 105.Pelto GH, Zhang Y, Habicht JP. Premastication: the second arm of infant and young child feeding for health and survival? Matern Child Nutr. 2010;6:4–18. doi: 10.1111/j.1740-8709.2009.00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gahagan S, Sturm L. Cross Cultural Issues in Provider-Parent Relationships. In: Kessler DB, Dawson P, editors. Failure to Thrive and Pediatric Undernutrition: A Transdisciplinary Approach. 26. 13. Vol. 1. Baltimore, MD: Brookes Publishing Company; 1999. pp. 354–6. [Google Scholar]
- 107.Wasser H, Bentley M, Borja J, Davis Goldman B, Thompson A, Slining M, et al. Infants perceived as “fussy” are more likely to receive complementary foods before 4 months. Pediatrics. 2011;127:229–37. doi: 10.1542/peds.2010-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kramer MS, Barr RG, Leduc DG, Boisjoly C, McVey-White L, Pless IB, et al. Determinants of weight and adiposity in the first year of life. J Pediatr. 1985;106:10–4. doi: 10.1016/s0022-3476(85)80456-x. [DOI] [PubMed] [Google Scholar]
- 109.Baker JL, Michaelsen KF, Rasmussen KM, Sorensen TI. Maternal prepregnant body mass index, duration of breastfeeding, and timing of complementary food introduction are associated with infant weight gain. Am J Clin Nutr. 2004;80:1579–88. doi: 10.1093/ajcn/80.6.1579. [DOI] [PubMed] [Google Scholar]
- 110.Ong KK, Emmett PM, Noble S, Ness A, Dunger DB. Dietary energy intake at the age of 4 months predicts postnatal weight gain and childhood body mass index. Pediatrics. 2006;117:e503–8. doi: 10.1542/peds.2005-1668. [DOI] [PubMed] [Google Scholar]
- 111.Schack-Nielsen L, Sorensen T, Mortensen EL, Michaelsen KF. Late introduction of complementary feeding, rather than duration of breastfeeding, may protect against adult overweight. Am J Clin Nutr. 2010;91:619–27. doi: 10.3945/ajcn.2008.27078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hediger ML, Overpeck MD, Kuczmarski RJ, Ruan WJ. Association between infant breastfeeding and overweight in young children. JAMA. 2001;285:2453–60. doi: 10.1001/jama.285.19.2453. [DOI] [PubMed] [Google Scholar]
- 113.Arenz S, Ruckerl R, Koletzko B, von Kries R. Breast-feeding and childhood obesity—a systematic review. Int J Obes Relat Metab Disord. 2004;28:1247–56. doi: 10.1038/sj.ijo.0802758. [DOI] [PubMed] [Google Scholar]
- 114.Harder T, Bergmann R, Kallischnigg G, Plagemann A. Duration of breastfeeding and risk of overweight: a meta-analysis. Am J Epidemiol. 2005;162:397–403. doi: 10.1093/aje/kwi222. [DOI] [PubMed] [Google Scholar]
- 115.Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Effect of infant feeding on the risk of obesity across the life course: a quantitative review of published evidence. Pediatrics. 2005;115:1367–77. doi: 10.1542/peds.2004-1176. [DOI] [PubMed] [Google Scholar]
- 116.Singhal A, Lanigan J. Breastfeeding, early growth and later obesity. Obes Rev. 2007;8:s51–4. doi: 10.1111/j.1467-789X.2007.00318.x. [DOI] [PubMed] [Google Scholar]
- 117.Jelliffe DB, Jelliffe EF. Cultural interaction and child nutrition (toward a curvilinear compromise?) Basic Life Sci. 1976;7:263–73. doi: 10.1007/978-1-4684-2883-4_23. [DOI] [PubMed] [Google Scholar]
- 118.Faraz A. Clinical recommendations for promoting breastfeeding among Hispanic women. J Am Acad Nurse Pract. 2010;22:292–9. doi: 10.1111/j.1745-7599.2010.00510.x. [DOI] [PubMed] [Google Scholar]
- 119.Ludington-Hoe SM, McDonald PE, Satyshur R. Breastfeeding in African-American women. J Natl Black Nurses Assoc. 2002;13:56–64. [PubMed] [Google Scholar]
- 120.Pak-Gorstein S, Haq A, Graham EA. Cultural influences on infant feeding practices. Pediatr Rev. 2009;30:e11–21. doi: 10.1542/pir.30-3-e11. [DOI] [PubMed] [Google Scholar]
- 121.Dewey KG. Cross-cultural patterns of growth and nutritional status of breast-fed infants. Am J Clin Nutr. 1998;67:10–7. doi: 10.1093/ajcn/67.1.10. [DOI] [PubMed] [Google Scholar]
- 122.Bender M, Clark MJ. Cultural adaptation for ethnic diversity: a review of obesity interventions for preschool children. CJHP. In press. [PMC free article] [PubMed] [Google Scholar]
- 123.Satter E. The Feeding Relationship. In: Kessler DB, Dawson P, editors. Failure to Thrive and Pediatric Undernutrition a Transdisciplinary Approach. Baltimore, MD: Paul H. Brookes Publishing Co, Inc; 1999. pp. 122–123. [Google Scholar]
- 124.Hagekull B, Bohlin G, Rydell AM. Maternal sensitivity, infant temperament, and the development of early feeding problems. IMHJ. 1997;18:92–106. [Google Scholar]
- 125.Farrow C, Blissett J. Maternal cognitions, psychopathologic symptoms, and infant temperament as predictors of early infant feeding problems: a longitudinal study. Int J Eat Disord. 2006;39(2):128–34. doi: 10.1002/eat.20220. [DOI] [PubMed] [Google Scholar]
- 126.Haycraft E, Farrow C, Meyer C, Powell F, Blissett J. Relationships between temperament and eating behaviours in young children. Appetite. 2011;56:689–92. doi: 10.1016/j.appet.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 127.Hittner JB, Faith MS. Typology of emergent eating patterns in early childhood. Eat Behav. 2011;12:242–8. doi: 10.1016/j.eatbeh.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 128.Faith MS, Hittner JB. Infant temperament and eating style predict change in standardized weight status and obesity risk at 6 years of age. Int J Obes (Lond) 2010;34:1515–23. doi: 10.1038/ijo.2010.156. [DOI] [PubMed] [Google Scholar]
- 129.Lindberg L, Bohlin G, Hagekull B, Thunström M. Early food refusal: Infant and family characteristics. Inf Mental Hlth J. 1994;15:262–277. [Google Scholar]
- 130.Pliner P, Loewen ER. Temperament and Food Neophobia in Children and their Mothers. Appetite. 1997;28:239–254. doi: 10.1006/appe.1996.0078. [DOI] [PubMed] [Google Scholar]
- 131.Martin GC, Wertheim EH, Prior M, Smart D, Sanson A, Oberklaid F. A longitudinal study of the role of childhood temperament in the later development of eating concerns. Int J Eat Disord. 2000;27:150–62. doi: 10.1002/(sici)1098-108x(200003)27:2<150::aid-eat3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 132.Darling N, Steinberg L. Parenting style as context - an integrative model. Psychol Bull. 1993;113:487–496. [Google Scholar]
- 133.Steinberg L, Lamborn SD, Darling N, Mounts NS, Dornbusch SM. Over-time changes in adjustment and competence among adolescents from authoritative, authoritarian, indulgent, and neglectful families. Child Dev. 1994;65:754–770. doi: 10.1111/j.1467-8624.1994.tb00781.x. [DOI] [PubMed] [Google Scholar]
- 134.Steinberg L, Elmen JD, Mounts NS. Authoritative parenting, psychosocial maturity, and academic success among adolescents. Child Dev. 1989;60:1424–36. doi: 10.1111/j.1467-8624.1989.tb04014.x. [DOI] [PubMed] [Google Scholar]
- 135.Baumrind D. The influence of parenting style on adolescent competence and substance use. J Early Adolesc. 1991;11:56–95. [Google Scholar]
- 136.Baumrind D, Larzelere RE, Owens EB. Effects of preschool parents’ power assertive patterns and practices on adolescent development. Parenting: Science & Practice. 2010;10:157–201. [Google Scholar]
- 137.Baumrind D. The Development of Instrumental Competence through Socialization. In: Pick AD, editor. Minnesota Symposia on Child Psychology. 1. Vol. 7. Minneapolis, MN: University of Minnesota Press; 1973. pp. 3–46. [Google Scholar]
- 138.Rhee KE, Lumeng JC, Appugliese DP, Kaciroti N, Bradley RH. Parenting styles and overweight status in first grade. Pediatrics. 2006;117:2047–54. doi: 10.1542/peds.2005-2259. [DOI] [PubMed] [Google Scholar]
- 139.Wake M, Nicholson JM, Hardy P, Smith K. Preschooler obesity and parenting styles of mothers and fathers: Australian national population study. Pediatrics. 2007;120:e1520–7. doi: 10.1542/peds.2006-3707. [DOI] [PubMed] [Google Scholar]
- 140.Blissett J, Haycraft E. Are parenting style and controlling feeding practices related? Appetite. 2008;50:477–85. doi: 10.1016/j.appet.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 141.Topham GL, Hubbs-Tait L, Rutledge JM, Page MC, Kennedy TS, Shriver LH, et al. Parenting styles, parental response to child emotion, and family emotional responsiveness are related to child emotional eating. Appetite. 2011;56:261–4. doi: 10.1016/j.appet.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 142.Anzman SL, Rollins BY, Birch LL. Parental influence on children’s early eating environments and obesity risk: implications for prevention. Int J Obes. 2010;34:1116–1124. doi: 10.1038/ijo.2010.43. [DOI] [PubMed] [Google Scholar]
- 143.Birch LL, Fisher JO. Development of eating behaviors among children and adolescents. Pediatrics. 1998;101:539–49. [PubMed] [Google Scholar]
- 144.Ogden J, Reynolds R, Smith A. Expanding the concept of parental control: a role for overt and covert control in children’s snacking behaviour? Appetite. 2006;47:100–6. doi: 10.1016/j.appet.2006.03.330. [DOI] [PubMed] [Google Scholar]
- 145.Faith MS, Berkowitz RI, Stallings VA, Kerns J, Storey M, Stunkard AJ. Parental feeding attitudes and styles and child body mass index: prospective analysis of a gene-environment interaction. Pediatrics. 2004;114:e429–36. doi: 10.1542/peds.2003-1075-L. [DOI] [PubMed] [Google Scholar]
- 146.Brown KA, Ogden J, Vogele C, Gibson EL. The role of parental control practices in explaining children’s diet and BMI. Appetite. 2008;50:252–9. doi: 10.1016/j.appet.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 147.Johnson SL, Birch LL. Parents’ and children’s adiposity and eating style. Pediatrics. 1994;94:653–61. [PubMed] [Google Scholar]
- 148.Birch LL, Fisher JO, Davison KK. Learning to overeat: maternal use of restrictive feeding practices promotes girls’ eating in the absence of hunger. Am J Clin Nutr. 2003;78:215–20. doi: 10.1093/ajcn/78.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rhee KE, Coleman SM, Appugliese DP, Kaciroti NA, Corwyn RF, Davidson NS, et al. Maternal feeding practices become more controlling after and not before excessive rates of weight gain. Obesity (Silver Spring) 2009;17:1724–9. doi: 10.1038/oby.2009.54. [DOI] [PubMed] [Google Scholar]
- 150.Francis LA, Birch LL. Maternal influences on daughters’ restrained eating behavior. Health Psychol. 2005;24:548–54. doi: 10.1037/0278-6133.24.6.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fisher JO, Cai G, Jaramillo SJ, Cole SA, Comuzzie AG, Butte NF. Heritability of hyperphagic eating behavior and appetite-related hormones among Hispanic children. Obesity (Silver Spring) 2007;15:1484–95. doi: 10.1038/oby.2007.177. [DOI] [PubMed] [Google Scholar]
- 152.Kral TV, Moore RH, Stunkard AJ, Berkowitz RI, Stettler N, Stallings VA, et al. Adolescent eating in the absence of hunger and relation to discretionary calorie allowance. J Am Diet Assoc. 2010;110:1896–900. doi: 10.1016/j.jada.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Birch LL, McPheee L, Shoba BC, Steinberg L, Krehbiel R. “Clean up your plate”: effects of child feeding practices on the conditioning of meal size. Learn Motiv. 1987;18:301–317. [Google Scholar]
- 154.Lumeng JC, Burke LM. Maternal prompts to eat, child compliance, and mother and child weight status. J Pediatr. 2006;149:330–335. doi: 10.1016/j.jpeds.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 155.Wright CM, Parkinson KN, Drewett RF. How does maternal and child feeding behavior relate to weight gain and failure to thrive? Data from a prospective birth cohort. Pediatrics. 2006;117:1262–9. doi: 10.1542/peds.2005-1215. [DOI] [PubMed] [Google Scholar]
- 156.Feldman R, Keren M, Gross-Rozval O, Tyano S. Mother-child touch patterns in infant feeding disorders: relation to maternal, child, and environmental factors. J Am Acad Child Adolesc Psychiatry. 2004;43:1089–97. doi: 10.1097/01.chi.0000132810.98922.83. [DOI] [PubMed] [Google Scholar]
- 157.Birch LL, Fisher JO, Grimm-Thomas K, Markey CN, Sawyer R, Johnson SL. Confirmatory factor analysis of the Child Feeding Questionnaire: a measure of parental attitudes, beliefs and practices about child feeding and obesity proneness. Appetite. 2001;36:201–10. doi: 10.1006/appe.2001.0398. [DOI] [PubMed] [Google Scholar]
- 158.Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self-regulation program for maintenance of weight loss. N Engl J Med. 2006;355:1563–71. doi: 10.1056/NEJMoa061883. [DOI] [PubMed] [Google Scholar]
- 159.Boutelle KN, Kirschenbaum DS. Further support for consistent self-monitoring as a vital component of successful weight control. Obes Res. 1998;6:219–24. doi: 10.1002/j.1550-8528.1998.tb00340.x. [DOI] [PubMed] [Google Scholar]
- 160.Rhee KE, Appugliese DP, Prisco A, Kaciroti NA, Corwyn RF, Bradley RH, et al. Controlling maternal feeding practices associated with decreased dieting behavior in sixth-grade children. J Am Diet Assoc. 2010;110:619–23. doi: 10.1016/j.jada.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Epstein LH, Paluch RA, Saelens BE, Ernst MM, Wilfley DE. Changes in eating disorder symptoms with pediatric obesity treatment. J Pediatr. 2001;139:58–65. doi: 10.1067/mpd.2001.115022. [DOI] [PubMed] [Google Scholar]
- 162.Rozin P. The Importance Of Social Factors In Understanding The Acquisition Of Food Habits. In: Capaldi ED, Powley TL, editors. Taste, experience, and feeding. 16. 1. Vol. 13. Washington, DC: American Psychological Association; 1990. pp. 255–269. [Google Scholar]
- 163.Hermans RC, Engels RC, Larsen JK, Herman CP. Modeling of palatable food intake. The influence of quality of social interaction. Appetite. 2009;52:801–4. doi: 10.1016/j.appet.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 164.Visalberghi E, Valente M, Fragaszy D. Social context and consumption of unfamiliar foods by capuchin monkeys (Cebus apella) over repeated encounters. Am J Primatol. 1998;45:367–80. doi: 10.1002/(SICI)1098-2345(1998)45:4<367::AID-AJP4>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 165.Feunekes GI, de Graaf C, van Staveren WA. Social facilitation of food intake is mediated by meal duration. Physiol Behav. 1995;58:551–8. doi: 10.1016/0031-9384(95)00087-y. [DOI] [PubMed] [Google Scholar]
- 166.de Castro JM. Social facilitation of duration and size but not rate of the spontaneous meal intake of humans. Physiology & Behavior. 1990;47:1129–1135. doi: 10.1016/0031-9384(90)90363-9. [DOI] [PubMed] [Google Scholar]
- 167.Redd M, de Castro JM. Social facilitation of eating: effects of social instruction on food intake. Physiol Behav. 1992;52:749–54. doi: 10.1016/0031-9384(92)90409-u. [DOI] [PubMed] [Google Scholar]
- 168.de Castro JM, Brewer EM. The amount eaten in meals by humans is a power function of the number of people present. Physiol Behav. 1992;51:121–5. doi: 10.1016/0031-9384(92)90212-k. [DOI] [PubMed] [Google Scholar]
- 169.Drewett RF. The social facilitation of food intake. Arch Dis Child. 2007;92:377. doi: 10.1136/adc.2006.108332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pliner P, Bell R, Hirsch ES, Kinchla M. Meal duration mediates the effect of “social facilitation” on eating in humans. Appetite. 2006;46:189–198. doi: 10.1016/j.appet.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 171.Koh J, Pliner P. The effects of degree of acquaintance, plate size, and sharing on food intake. Appetite. 2009;52:595–602. doi: 10.1016/j.appet.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 172.Salvy SJ, Vartanian LR, Coelho JS, Jarrin D, Pliner PP. The role of familiarity on modeling of eating and food consumption in children. Appetite. 2008;50:514–8. doi: 10.1016/j.appet.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hermans RC, Larsen JK, Herman CP, Engels RC. Effects of social modeling on young women’s nutrient-dense food intake. Appetite. 2009;53:135–8. doi: 10.1016/j.appet.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 174.Lumeng JC, Cardinal TM, Jankowski M, Kaciroti N, Gelman SA. Children’s use of adult testimony to guide food selection. Appetite. 2008;51:302–10. doi: 10.1016/j.appet.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lumeng JC, Cardinal TM. Providing information about a flavor to preschoolers: effects on liking and memory for having tasted it. Chem Senses. 2007;32:505–13. doi: 10.1093/chemse/bjm019. [DOI] [PubMed] [Google Scholar]
- 176.Duncker K. Experimental modification of children’s food preferences through social suggestion. JASP. 1939;33:489–507. [Google Scholar]
- 177.Ali MM, Amialchuk A, Heiland FW. Weight-Related Behavior among Adolescents: The Role of Peer Effects. PLoS One. 2011;6:e21179. doi: 10.1371/journal.pone.0021179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Roberto CA, Baik J, Harris JL, Brownell KD. Influence of licensed characters on children’s taste and snack preferences. Pediatrics. 2010;126:88–93. doi: 10.1542/peds.2009-3433. [DOI] [PubMed] [Google Scholar]
- 179.Jones SC, Mannino N, Green J. ‘Like me, want me, buy me, eat me’: relationship-building marketing communications in children’s magazines. Public Health Nutrition. 2010;13:2111–8. doi: 10.1017/S1368980010000455. [DOI] [PubMed] [Google Scholar]