Abstract
The US Food and Drug Administration (FDA) recently approved two novel immunotherapy agents, sipuleucel-T and ipilimumab, which showed a survival benefit for patients with metastatic prostate cancer and melanoma, respectively. The mechanisms by which these agents provide clinical benefit are not completely understood. However, knowledge of these mechanisms will be crucial for probing human immune responses and tumour biology in order to understand what distinguishes responders from non-responders. The following next steps are necessary: first, the development of immune-monitoring strategies for the identification of relevant biomarkers; second, the establishment of guidelines for the assessment of clinical end points; and third, the evaluation of combination therapy strategies to improve clinical benefit.
The concept of cancer immune surveillance dates back decades1–3 and is based on the hypothesis that the immune system can suppress the development or progression of spontaneous malignancies. Recent data in murine models confirm this original concept and clearly define immune evasion by aberrant cells as playing an important part in the development and progression of tumours4,5. In an attempt to re-engage the immune system in its fight against cancer, cancer immunotherapy focuses on the development of agents that can activate the immune system to recognize and kill tumour cells.
Cancer immunotherapy encompasses diverse strategies that range from activating innate and adaptive immune effector mechanisms to neutralizing inhibitory and suppressive mechanisms (BOX 1). Strategies to stimulate effector immune cells include vaccination with tumour antigens, treatment with cytokines (for example, interleukin 2 (IL-2) or interferon-α (IFNα)) or enhancement of antigen presentation (for example, by stimulation of Toll-like receptors 7, 8 or 9, administration of dendritic cells or the use of a CD40-targeted agonistic antibody). Further stimulatory strategies include the use of antibodies targeting the tumour necrosis factor receptor superfamily (TNFRSF) members 41BB (also known as TNFRSF9 or CD137), OX40 (also known as TNFRSF4 or CD134) or glucocorticoid-induced TNFR-related protein (GITR; also known as TNFRSF18) in order to provide co-stimulatory signals to enhance T cell activity, and adoptive cellular therapy (ACT) as a means to administer immune cells directly to patients. Strategies to neutralize immune suppressor mechanisms include chemotherapy (for example, cyclophosphamide), the use of antibodies (for example, CD25-targeted antibodies) in an attempt to deplete regulatory T cells and the use of antibodies against immune-checkpoint molecules (for example, cytotoxic T lymphocyte-associated protein 4 (CTLA4)-targeted antibodies and programmed cell death 1 (PD1)-targeted antibodies). Many of these strategies were recently reviewed6–8.
Box 1 | Types of immune responses and regulatory mechanisms.
Innate immune response
Nonspecific
Lacks memory
Comprised of inflammatory cytokines, the complement system and phagocytes, such as macrophages, neutrophils and dendritic cells
Adaptive immune response
Highly specific
Development of memory cells
Comprised of B and T lymphocytes, specifically CD8+ cytotoxic T lymphocytes and CD4+ T helper lymphocytes (TH1 and TH2 cells)
Activation of T cell responses
Antigen-presenting cells (APCs), such as dendritic cells, can take up foreign antigens and process the antigens, which are then bound to major histocompatibility complex (MHC) molecules for presentation to T cells
T cells interact with MHC and MHC-bound antigen through the T cell receptor; the signalling that results from this interaction is known as signal 1
T cells become activated in the presence of signal 1 and co-stimulatory signals, which are known as signal 2
Activated T cells can directly kill tumour cells that express the antigen for which the T cell has specificity
Activated T cells can kill indirectly by producing cytokines that act to initiate apoptotic pathways in tumour and/or surrounding stromal cells
Activated T cells can also kill indirectly by secreting cytokines to recruit other cells, such as macrophages; these recruited cells act in a nonspecific manner to destroy surrounding tumour and/or stromal cells
Regulation and suppression of T cell responses
Regulatory mechanisms can be intrinsic to T cells; examples are inhibitory immune-checkpoint molecules, such as cytotoxic T lymphocyte-associated protein 4 (CTLA4) and programmed cell death 1 (PD1)
Regulatory mechanisms can also be extrinsic to T cells; examples are certain cytokines (such as IL-10), regulatory T cells and myeloid-derived suppressor T cells (MDSCs)
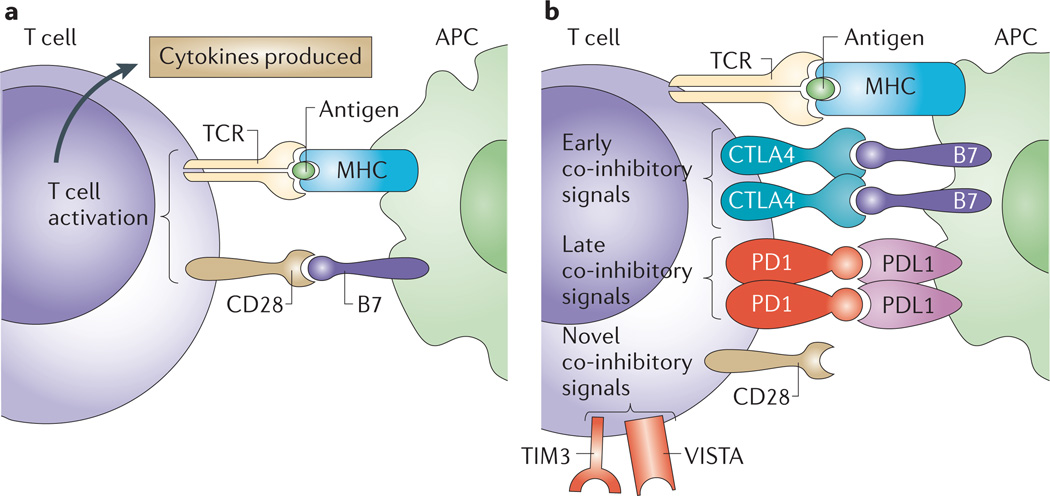
Successful T cell-based active immunotherapy requires not only the expression of antigens by cancer cells but also the successful and sustained mobilization of sufficient numbers of effector T cells that recognize these antigens in order to eliminate the tumour. T cell activation is initiated by stimulation of the antigen receptor (T cell receptor (TCR)) with major histocompatibility complex (MHC) molecules, which present peptides that are derived from tumour antigens. For productive activation, this must be accompanied by co-stimulatory signals mediated by the binding of CD28 on the T cell surface to B7 proteins (such as CD80 or CD86) on the antigen-presenting cell (APC) (FIG. 1a). These two signals allow T cells to begin to proliferate, to acquire effector functions and eventually to migrate. TCR signalling also induces the production of the CD28 homologue CTLA4, which is a T cell-specific molecule that has a higher binding affinity for B7, thereby outcompeting CD28 and eventually inhibiting T cell activity (FIG. 1b). CTLA4 restricts T cell activity in order to minimize damage to normal tissues. Other inhibitory molecules such as PD1 are also expressed on T cells after T cell activation, thus providing signals that control T cell responses. T cell activity may be enhanced in cancer patients to elicit clinical benefit by using tumour-specific antigens to stimulate T cell responses or antibodies that block inhibitory immune-checkpoint molecules such as CTLA4 or PD1 (FIG. 2).
Figure 1. Basic mechanisms of T cell stimulation and inhibition.
a | T cell activation begins with interaction of the T cell receptor (TCR) on a T cell with major histocompatibility complex (MHC) bound to antigen on an antigen-presenting cell (APC). This is known as signal 1, but appropriate activation of the T cell requires additional signals that are provided by the interaction between CD28 and B7 (signal 2). b | T cell activation is limited by cytotoxic T lymphocyte-associated protein 4 (CTLA4), which is upregulated on activated T cells, where it outcompetes CD28 for binding to B7 on an APC. Additional regulation of T cell activity is also provided by later inhibitory signals through other molecules such as programmed cell death 1 (PD1), which binds to PD1 ligand 1 (PDL1). Other regulators of T cell activation have recently been characterized and may have important roles; these regulators include T cell immunoglobulin and mucin domain-containing protein 3 (TIM3; also known as HAVCR2) and V-domain immunoglobulin suppressor of T cell activation (VISTA)60,61.
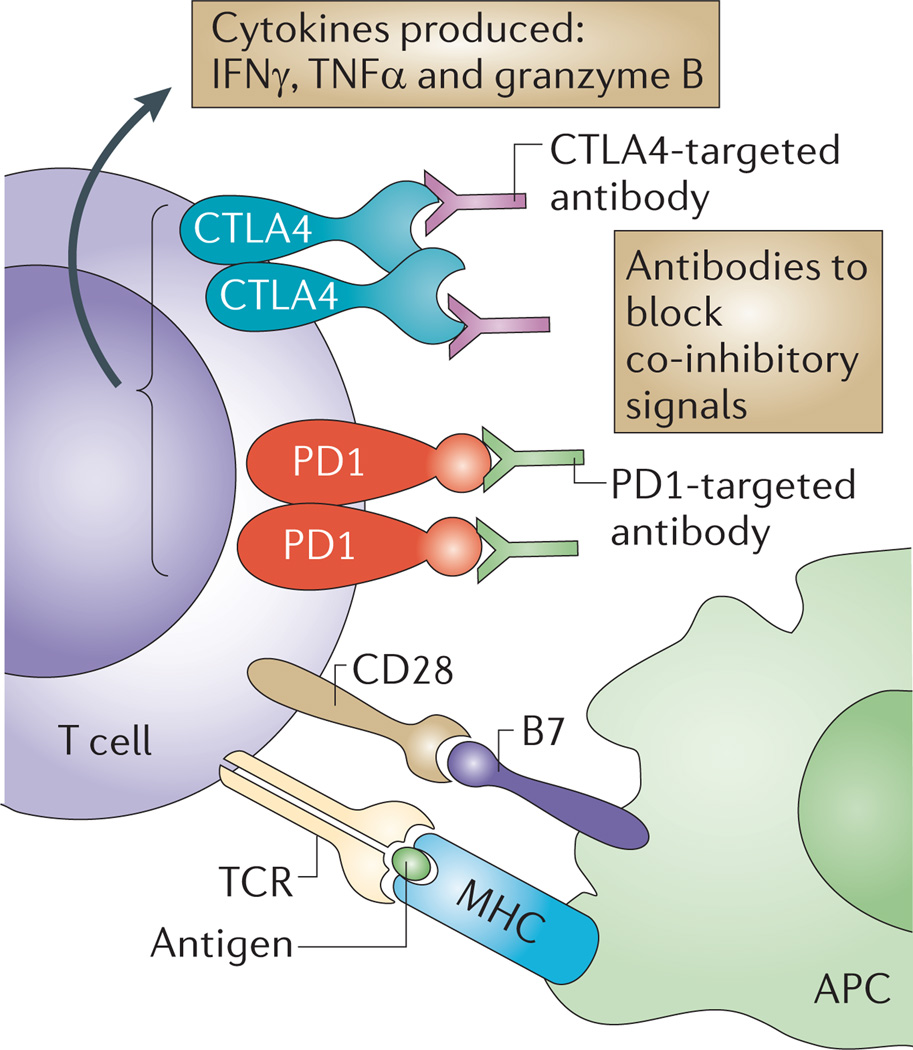
Figure 2. Current therapies that induce effector T cell functions.
Strategies to maintain activated tumour-specific T cells include the use of blocking monoclonal antibodies, such as anti-bodies targeting either cytotoxic T lymphocyte-associated protein 4 (CTLA4) or programmed cell death 1 (PD1), to neutralize co-inhibitory receptors. Therefore, these antibodies that block intrinsic inhibitory immune checkpoints allow a sustained T cell response, including an increased production of cytokines, such as tumour necrosis factor-α (TNFα), interferon-γ (IFNγ) and granzyme B. APC, antigen-presenting cell; MHC, major histocompatibility complex; TCR, T cell receptor.
Two immunotherapeutic approaches have recently shown overall survival benefit in randomized Phase III clinical trials for patients with metastatic disease. These are sipuleucel-T, which consists of autologous peripheral blood mononuclear cells (PBMCs) that have been pulsed ex-vivo with a fusion protein of prostatic acid phosphatase (PAP) and granulocyte macrophage colony stimulating factor (GM-CSF) before re-infusion into the patients, and ipilimumab, an antagonistic CTLA4-targeted antibody that acts to overcome the T cell inhibitory pathways that are elicited by CTLA4. Other immunotherapy strategies, including ACT whereby T cells are administered to patients9,10 and high-dose IL-2 (REF. 11), have also shown antitumour responses in clinical trials. However, ACT using T cells — as opposed to sipuleucel-T, which consists of PBMCs — remains an investigational therapy that is currently in Phase I/II trials. Additionally, high-dose IL-2, although approved by the US Food and Drug Administration (FDA) in 1992 on the basis of data from multiple Phase II trials, is lacking overall survival data as a monotherapy in Phase III trials. There are promising Phase I/II data with ACT using T cells, including clinical responses with genetically engineered T cells12–15, and such results will continue to move the field of cancer immunotherapy forwards. There was also a recent Phase III trial that showed improved clinical responses with a combination strategy of high-dose IL-2 and a peptide vaccine, compared to high-dose IL-2 alone16, which will clearly provide momentum for additional combination immunotherapy strategies. However, it should be pointed out that in-patient hospitalization is required for both high-dose IL-2 and ACT using T cells, owing to the toxicities that are associated with the administration of these therapies. Conversely, both sipuleucel-T and ipilimumab are administered in the outpatient clinic, with minimal toxicities at the time of administration, but toxicities may occur later, especially in the setting of ipilimumab therapy, which may require management with immunosuppressive agents such as corticosteroids. Owing to the recent Phase III data with sipuleucel-T and ipilimumab, these agents received FDA approval in 2010 and 2011 as standard-of-care therapies for the treatment of patients with metastatic prostate cancer and melanoma, respectively. It is expected that other immunotherapy strategies will also show clinical efficacy that will lead to FDA approval in the near future; however, this Perspective article focuses on sipuleucel-T and ipilimumab and discusses steps that can be taken to build on the clinical successes of these novel immunotherapy agents.
Immunotherapy can improve survival
Sipuleucel-T and PROSTVAC
In a Phase III randomized, controlled study in 512 patients with metastatic castration-resistant prostate cancer (CRPC), treatment with sipuleucel-T was shown to increase median overall survival by 4.1 months (25.8 versus 21.7 months; P = 0.032)17. These data led to the FDA approval of sipuleucel-T for patients with CRPC. Another immunotherapy strategy that targets prostate cancer is PROSTVAC; PROSTVAC-F and PROSTVAC-V are fowlpox- and vaccinia-based viral vaccines, respectively, that are thought to stimulate T cell responses against prostate-specific antigen (PSA). Recently, a Phase II randomized, controlled study in 125 patients with metastatic CRPC reported an 8.5 month improvement in median overall survival for patients who were treated with PROSTVAC (25.1 versus 16.6 months; P = 0.006)18. These Phase II data are encouraging, and plans are underway for a Phase III trial with PROSTVAC.
Although sipuleucel-T and PROSTVAC were both shown to improve median overall survival, the exact mechanism of action of these agents remains unclear. Sipuleucel-T and PROSTVAC are thought to enhance T cell responses against the PAP and PSA tumour antigens, respectively, that are expressed by prostate cancers. For sipuleucel-T, it has been postulated that the autologous PBMCs that are taken from patients include APCs, such as dendritic cells, that can engulf the PAP–GM-CSF fusion protein, process the protein to peptide antigens, present the peptide antigens to T cells in the context of MHC and co-stimulatory molecules and propagate the activation of T cells that are specific for the PAP antigen. Similarly, it is speculated that after the vaccination of patients with PROSTVAC, the vaccine is taken up by APCs, and the PSA protein that is encoded by the vaccine is processed for presentation to T cells, thereby leading to the activation of T cells that are specific for PSA.
Antibodies targeting CTLA4
In a Phase III randomized, controlled trial in 676 patients with metastatic melanoma, treatment with ipilimumab improved the median overall survival by 3.7 months (10.1 versus 6.4 months; P = 0.003)19. Ipilimumab functions to block the inhibitory signals that are mediated by the CTLA4 molecule that is expressed on T cells, thereby permitting enhanced T cell responses20–22. A different CTLA4-targeted antibody, tremelimumab, has also been tested in Phase I/II trials in patients with metastatic melanoma23,24. A Phase III clinical trial with tremelimumab in patients with melanoma, who were randomized to either tremelimumab or standard-of-care therapies (dacarbazine or temozolomide), was conducted, but the trial was halted when an interim analysis failed to show a benefit for tremelimumab25. Potential reasons that the trial failed are speculative but include the fact that tremelimumab was dosed at 15 mg kg−1 every 3 months (as opposed to 3 mg kg−1 every 3 weeks for ipilimumab) and that some patients in the tremelimumab trial who were randomized to standard-of-care therapies may have also participated in some of the ongoing Phase I/II trials of ipilimumab, leading to unintentional crossover. An updated analysis of these data was reported in abstract form and suggested a trend towards an overall survival benefit for patients who were treated with tremelimumab26. In patients, both ipilimumab and tremelimumab have been shown to enhance the frequency of CD4+ and CD8+ T cells in the peripheral blood as well as antibody responses to antigens that are present on tumour cells27–31; however, as will be discussed in more detail below, the biological changes or mechanisms that lead to clinical benefit are still under investigation.
Clinical outcomes of early Phase I and II trials of CTLA4-targeted antibodies were recently reviewed32. In one study, patients with metastatic melanoma received different doses of ipilimumab (0.3, 3 or 10 mg kg−1), and the results suggested an increase in response rates with higher doses33. The Phase III trial tested the efficacy of ipilimumab alone and ipilimumab in combination with a peptide vaccine that is comprised of gp100 (also known as melanocyte protein PMEL), an antigen that is known to be expressed in melanoma19. Ipilimumab was given four times at 3 mg kg−1 (one dose every 3 weeks), and patients with a documented clinical benefit could receive additional doses (termed ‘maintenance–re-induction’ doses). An additional cohort of patients received the gp100 vaccine alone, which was equivalent to a placebo: the peptide vaccine by itself had not provided measurable clinical benefit to patients with metastatic melanoma in a previous study34. However, it was speculated that the combination of gp100 vaccine plus ipilimumab might provide additive or synergistic benefits over ipilimumab alone, as was recently noted in a study combining high-dose IL-2 and gp100 (REF. 16). The Phase III trial with ipilimumab showed a median overall survival of 10.1 months for patients who were treated with ipilimumab alone and 10.0 months for patients treated with both ipilimumab and the gp100 vaccine. By comparison, patients who were treated with the gp100 vaccine alone had a median overall survival of 6.4 months. Treatment with ipilimumab alone also led to a 36% reduction in the risk of progression compared to treatment with gp100 alone (hazard ratio = 0.64; P < 0.001) and a significant difference in the best overall response rate (10.9%) and the disease control rate (28.5%) compared to treatment with gp100 alone (1.5% and 11.0%, respectively). A remarkable aspect of this trial was that approximately 45% of ipilimumab-treated patients were alive after 1 year, 24% of patients were alive after 2 years, and some patients had a durable clinical benefit that lasted for the 4.5 years of follow-up. This was a dramatic improvement in the survival of patients with metastatic melanoma compared to a previously published meta-analysis that indicated that only 25% of patients with metastatic melanoma lived for 1 year35. These data led to the approval of ipilimumab by the FDA in March 2011 for first- or second-line treatment of unresectable stage III or stage IV melanoma.
A second randomized Phase III trial was reported recently. This trial confirmed the findings of the first trial by showing an overall survival of 11.2 months for patients who were treated with a combination of ipilimumab and standard dacarbazine chemotherapy, versus 9.1 months for patients who received dacarbazine alone36. This second trial administered ipilimumab at a dose of 10 mg kg−1; however, only 36.8% of patients completed all four doses of the treatment. Additional data will be needed to clarify whether four doses are sufficient for clinical benefit or whether additional (maintenance) doses are necessary. In the first Phase III trial only 17.2% of patients received at least one maintenance dose19, and in the second Phase III trial only 7% of patients received at least one maintenance dose36. Data from the published Phase III trials, as well as data from the published Phase II dose-ranging study, indicate that ipilimumab is better-tolerated at 3 mg kg−1 compared with 10 mg kg−1, and that maintenance dosing may not be necessary for either regimen.
Other agents
Antibodies against other inhibitory immune-checkpoint molecules on T cells are now being tested in clinical trials. Phase II data with a PD1-targeted antibody were reported in abstract form and showed a 37% objective response rate in a trial involving patients with melanoma, renal cell carcinoma, prostate cancer, non-small-cell lung cancer or colorectal cancer, with most responses occurring in patients with metastatic melanoma or renal cell carcinoma37. Ongoing clinical trials using this PD1-targeted antibody will provide additional data regarding the potential for this immunotherapy agent to provide survival benefit for patients with cancer.
Assessment of clinical responses
Active immunotherapy generates antitumour effects by enhancing tumour-specific T cell responses. Because this is an indirect process that relies on affecting immune responses rather than the direct killing of tumour cells (which is seen when using standard agents such as chemotherapy), antitumour responses may be challenging to assess. Responses to immunotherapy may take longer to become detectable by radiographic imaging, and the assessment of responses might be difficult, if not misleading, by standard methods, such as Response Evaluation Criteria in Solid Tumours (RECIST) or modified World Health Organization (mWHO) criteria. Although responses to chemotherapy are usually apparent after two cycles (approximately 6–8 weeks) of treatment, clinical responses to immunotherapy agents can range from weeks to months38. These differences in response kinetics may explain why treatment with sipuleucel-T improved the median overall survival but did not affect progression-free survival (PFS) (3.7 months with sipuleucel-T versus 3.6 months with placebo; P = 0.63). Similarly, the PROSTVAC Phase II study, which was designed with a primary end point of PFS, showed a difference in median overall survival but failed to meet its primary end point of PFS.
Following ipilimumab treatment, prolonged periods of stable disease followed by tumour regression have been observed in some patients, indicating that, for immunotherapy agents, a longer period of time may be required before clinical benefit can be detected by imaging studies. In an initial randomized Phase II trial of 217 patients who were treated with ipilimumab at various doses33 and an open-label study of 155 patients who were treated with ipilimumab at 10 mg kg−1 (REF. 39), ipilimumab showed unique response characteristics in some patients. These responses included slow or delayed regression over months, or even initial progressive disease followed by stable disease or a response, which has led to discussions regarding the need for a novel set of response criteria for immunotherapies. The heterogeneity in the kinetics of clinical responses to ipilimumab was analysed across multiple Phase II trials. A subset (10–20%) of the total pooled population of 227 patients who had radiographic progression at the 12-week imaging time point — as indicated by either the enlargement of index lesions or the appearance of new lesions — went on to achieve stable disease or even objective responses without other anticancer therapies. Importantly, patients with these unique delayed responses showed similar overall survival when compared with patients who had responded, according to mWHO criteria, 12 weeks after the initiation of therapy. Therefore, continued observation might be advisable in patients with stable or even progressive disease, who have asymptomatic radiographic progression and who have maintained a baseline performance status, which indicates a lack of clinical deterioration. A new set of criteria for response to cancer immunotherapy was therefore proposed and termed the immune-related response criteria (irRC)40. By this definition, the response is measured as the sum of the largest diameters of all lesions, but the appearance of new lesions is not a cause for automatic categorization as progression, provided that there is no decrease in the performance status and that any increase in the overall size measurements of existing and new lesions is <30%. Patients without responses by RECIST or mWHO criteria, but who show irRC responses, may show delayed benefit from immunotherapy, and 4–6 weeks of further observation should be considered as a realistic option in the absence of a symptomatic decline in the performance status.
This dissociation between survival benefit and other clinical end points has also been observed for other therapeutic agents. For example, anti-angiogenic agents (such as bevacizumab or sorafenib), which are not directly cytotoxic to cancer cells, have been shown to prolong survival without significantly increasing objective response rates by RECIST criteria41–43. In the case of immune modulators such as ipilimumab, response-based end points are not informative because they use imaging studies in which data are collected at pre-determined time points. Some patients may respond quickly, while others may progress and then respond only after the immune response reaches a level that is sufficient for disease control. Some investigators have hypothesized that the initial disease progression may be due to immune infiltration, but this may not explain all instances in which tumours enlarge before stabilizing or regressing. Until we identify surrogate biomarkers that correlate with clinical outcomes, it is clear that overall survival needs to be used as the ‘gold standard’ end point in immunotherapy trials, with incorporation of revised response criteria, such as the irRC. Owing to the prolonged time and expensive costs that are associated with conducting clinical trials in which the primary end point is overall survival, it is imperative that we focus our efforts on identifying successful intermediary correlates.
The next steps for improving immunotherapy strategies include further elucidation of the mechanisms that are responsible for clinical benefit in subsets of cancer patients, the identification of relevant biomarkers for response and toxicity and the translation of preclinical data to the clinic for combination therapies that may provide greater benefit.
Immune-monitoring strategies
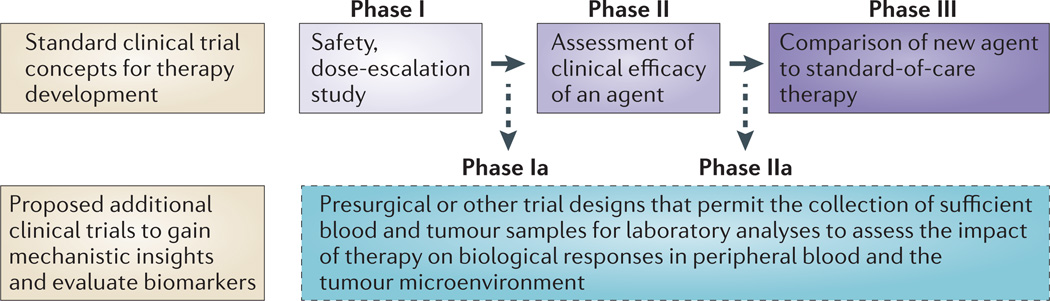
Designs of clinical trials with immunomodulatory agents need to take into consideration the immunological end points that can be used to determine whether the agents have had an effect on their targets. Therefore, although standard Phase I dose-escalation studies to assess toxicity may be warranted, it is also important to implement Phase Ia or Phase IIa trials with appropriate doses of the agent to assess biological end points (FIG. 3). We also support the concept of adaptive trial design whereby real-time data analysis during a trial allows for immediate modifications of the immune-monitoring strategies. Furthermore, given our improved understanding of the tumour microenvironment and the numerous immunosuppressive factors that may prevent effective antitumour immune responses, it is necessary to consider the immune monitoring of samples from both tumour tissues and peripheral blood.
Figure 3. Clinical trial concepts.
The standard paradigm for clinical trial design involves Phase I studies to assess the safety of an agent or therapy, Phase II studies to assess the clinical efficacy of an agent or therapy and Phase III studies to compare the agent or therapy with already established standard-of-care agents or therapies. We propose including Phase Ia and Phase IIa studies as a part of the standard paradigm for cancer immunotherapy trials, thereby allowing the evaluation of biological and immunological responses to an agent or therapy in both peripheral blood and tumour tissues.
Data from tumour tissues
The examination of tumour tissues may provide information regarding the baseline immune status that could help to select certain patients for specific immunotherapy agents or may shed light on relevant immunological markers that could be used when monitoring peripheral blood samples. For example, a signature of genes — those that were differentially expressed in pretreatment tumour tissue samples from patients who showed a favourable versus unfavourable clinical outcome after combination immunotherapy with peptide vaccines plus IL-12, — predicted the response to a dendritic-cell-based vaccine in one study44. Similarly, gene expression profiling on biopsy specimens that were taken before treatment led to the identification of a set of genes that are associated with clinical benefit from a vaccine consisting of a melanoma-associated antigen, MAGEA3 (REF. 44). Our group has conducted presurgical clinical trials in order to investigate immunological end points in tumour tissues and peripheral blood cells obtained from patients who were treated with ipilimumab. We found a significantly increased frequency of T cells expressing the inducible co-stimulator (ICOS) marker in tumour tissues after treatment with the antibody27,28. Then, in a small retrospective study, we found that a sustained increase in ICOS-expressing T cells in peripheral blood was associated with clinical benefit in patients with metastatic melanoma who were treated with ipilimumab29. These studies highlight the importance of analysing tumour tissues as well as peripheral blood samples in order to identify potential surrogates for clinical outcomes.
Immune-monitoring assays
The ideal assays for immune monitoring should be sensitive, specific, reliable, simple and should yield data that correlate with clinical outcomes. Assays that allow the simultaneous measurement of multiple parameters might also be advantageous. Because many existing immunemonitoring assays incorporate phenotypic and functional aspects of different subsets of cells, one should keep in mind that the selection of phenotypic and functional assays relies on having prior knowledge of these areas of immunology to inform the selection of these assays; therefore, these are knowledge-driven analyses. However, a central disadvantage of this knowledge-driven approach is that the result will be only as good as the body of knowledge, so genes that are not known to be involved in the phenotype or function cannot be considered. An alternative to knowledge-based identification of immune correlates is a data-driven approach, in which genome-wide analyses of gene expression are carried out on different subsets of cells, such as T and B cells. Subsequently, the correlates between patterns of gene expression and the phenotype or function of interest are sought45. Because the ideal assay or assays for identifying the immunological events that correlate with clinical benefit remain to be established, we need to re-examine our current immune-monitoring strategies and design novel assays, including gene expression-based assays, that may provide more relevant data.
Most cancer immunotherapy trials have used vaccines and have commonly relied on the evaluation of T cell responses in peripheral blood samples using tetramers of peptide–MHC class I complexes to detect antigen-specific T cells, and have used enzyme-linked immunosorbent spot (ELISPOT) or flow cytometric assays to detect cytokine production by antigen-specific T cells (BOX 2). Other assays that have been used include the measurement of serum cytokines and humoral responses by an enzyme-linked immunosorbent assay (ELISA). However, most often these approaches have not been shown to provide immunological data that correlate with clinical outcomes. For example, in a study in which 120 patients with measurable stage IV melanoma were treated with a multi-peptide vaccine, the ELISPOT assay was used to measure immune responses46. Multi-peptide vaccination elicited positive T cell responses in some patients. However, a positive immune response, as detected by ELISPOT assays, did not correlate with clinical benefit (that is, no correlation with a complete response, a partial response or stable disease). Although 12 patients with clinical benefit (as shown by a complete response, a partial response or stable disease) did have positive immune responses, 17 patients with clinical benefit did not have positive immune responses. These investigators reported that “carefully trained technologists, using standard operating procedures and validated reagents” performed the ELISPOT assays, so the failure of the assay results to correlate with clinical outcome can probably not be attributed to improper performance. Additional analyses showed that the stage of disease at diagnosis was the most significant predictor of overall survival (P = 0.002). Conversely, another study found that ELISPOT responses did correlate with disease-free survival in patients who underwent surgical resection of stage II–IV melanomas and then received a multi-peptide vaccine. However, subset analyses based on the stage of disease were not performed, and it remains unclear whether the stage of disease before surgical resection contributed to the observed differences in disease-free survival for these patients47.
Box 2 | Immune-monitoring assays.
Immune-monitoring strategies currently encompass multiple assays. These include: antigen-specific T cell assays, such as the enzyme-linked immunosorbent spot (ELISPOT) assay, which assesses the production of cytokines (such as interferon-γ (IFNγ)) by T cells in response to a specific antigen; and the tetramer assay, which uses a tetramer of major histocompatibility complex (MHC) molecules that are bound to a specific antigen to detect antigen-specific T cells by flow cytometry. Flow cytometry assays can also be used to measure, in a population of cells, the frequency of expression of specific proteins — such as the activation markers CD69, human leukocyte antigen DR proteins (HLA-DR) and inducible co-stimulator (ICOS) — that are expressed by T cells. In contrast to the cellular assays, an enzyme-linked immunosorbent assay (ELISA) can be used to measure antibody responses in the serum and/or plasma to specific antigens. Novel immune-monitoring strategies are now evolving to include the evaluation of immune responses in both peripheral blood and tumour tissues. Novel assays are also being developed to assess signalling pathways in T cells, changes in gene expression and microRNA profiles and tumour and/or stromal cell expression of genes encoding proteins that have roles in eliciting tumour cell death in response to cytokine signals.
The ELISPOT assay definitely has a role in immunological monitoring in vaccine trials, in which the assessment of responses to the administered antigen is both appropriate and necessary to assess the success of the vaccination. However, because the ELISPOT assay examines only a single T cell antigen and is performed on peripheral blood samples, it seems unlikely that this assay will provide data that correlate with clinical outcome for vaccine studies. Recently, the importance of harmonization and standardization of immune monitoring assays, such as the ELISPOT assay, has been emphasized in an attempt to establish a framework for conducting large, prospective clinical trials48–52. This is commended, but it is important to acknowledge that there is not yet an immunological assay or biomarker that correlates with clinical outcome. For large multi-institutional trials that may require an immunological assay to be carried out according to pre-defined criteria, in order to permit an accurate evaluation of the data, it may be best to consider a centralized monitoring site rather than attempting to standardize a particular assay across multiple institutions. Given that we currently lack an appropriate assay that correlates with clinical outcome, pre-occupation with harmonization and standardization of assays may be premature. In a recent review that discussed similar issues in the HIV field, the authors pointed out that an ELISPOT assay was used to show T cell responses to a vaccine, but although the ELISPOT results showed that the vaccine stimulated T cell responses, the vaccine failed to protect volunteers from HIV or to reduce viral loads after infection53. These results call into question whether the ELISPOT assay is an appropriate method to evaluate clinically beneficial immune responses and suggest that newer and better measures of T cell function are needed. Immunological concepts in cancer and HIV greatly overlap, and we should pay attention to lessons from the HIV field. Moreover, it is as yet unclear whether the assessment of antigen-specific T cells will be useful in trials of agents that block the inhibitory immune checkpoints or enhance co-stimulation. Trials using these strategies may be best assessed by tracking changes that are related to: T cell activation, such as increased PI3K signalling; the expression of activation markers, such as CD69, human leukocyte antigen DR (HLA-DR) proteins, and ICOS; the trafficking of T cells to tumour tissues; and memory T cell development.
We suggest that, rather than focusing our efforts on the harmonization of assays of questionable relevance, we should concentrate on the development of novel immune-monitoring strategies that encompass a series of integrated assays that measure multiple biological events, including those within tumour tissues, in order to obtain laboratory data that correlate with clinical outcome. Novel assays that are currently being explored include: the analysis of signalling pathways (such as the PI3K pathway) that provide signals that are crucial for the function of activated T cells, gene expression signatures, micro-RNA signatures and the gene methylation statuses of multiple cell subsets from both peripheral blood and tumour tissues. Some studies are also taking into account that although an immunotherapy strategy may lead to T cell activation and the production of cytokines such as IFNγ, tumour cell death might only occur if the tumour and/or stromal cells express the IFNγ receptor or are able to mediate downstream signals through the Janus kinase (JAK)–signal transducer and activator of transcription (STAT) pathway. For example, it has been shown that LNCaP prostate cancer cells are insensitive to IFNα and IFNγ in in vitro assays owing to epigenetic silencing of JAK1 (REF. 54). It is important to consider these types of issues in immunotherapy clinical trials. We need to accept that novel immune-monitoring strategies will not necessarily be harmonized or standardized when they are first reported. We refer readers to “Guidelines for the development and incorporation of biomarker studies in early clinical trials of novel agents”55, which describes the differences between biomarkers that are being used to make patient-related decisions (and therefore require validated assays) versus exploratory biomarkers that are hypothesis-generating. The field of cancer immunotherapy is still in the arena of exploratory biomarkers and, therefore, we need to first identify relevant biomarkers and assays before we can routinely implement validated assays.
Future plans: combination therapy
It is obvious from both preclinical models and clinical trials that combinatorial approaches may be required for optimally effective and broadly applicable cancer immunotherapy. The scientific literature provides evidence for many potentially useful combinations, including: vaccines that allow for the improved priming of T cells and improved antigen presentation; methods that lead to enhanced T cell number and function, such as ACT; agonistic TNFR-targeted antibodies and cytokines; the suppression of inhibitory immune checkpoints using antibodies against CTLA4 and PD1; and lymphodepletion or other means of suppressing the inhibitory effects of regulatory T cells.
Recent work in cancer genomics has suggested another strategy for optimizing immunotherapy approaches. Sequencing the genomes of human breast and colon carcinomas showed that there were almost 100 missense mutations per tumour56. Analysis of these mutations using epitope-prediction algorithms suggested that a large fraction of these mutations might create neo-antigens with the potential to be recognized by the immune system57. Although these are largely random and vary from tumour to tumour, their presence suggests that agents that kill tumour cells without suppressing immune responses could be used to prime T cell responses, which could then be expanded and sustained by immune-checkpoint blockade58. Thus radiation, some chemotherapies, hormone ablation and therapies that target signalling pathways in tumour cells can be viewed as immunosupportive vaccines that would liberate multiple neo-antigens that, in combination with immune-checkpoint blockade, might result in a multipronged and effective immune attack.
Currently, we lack predictive diagnostic biomarkers to rationally choose combinations of immunotherapy for individual patients or cancer types. In addition, the timing or sequence of immunomodulatory interventions might be important because the kinetics of immune reactions have a crucial functional role. Therefore, preclinical data for promising combinations are necessary, with subsequent clinical trials, to evaluate the efficacy of novel combinations. Combination therapies are currently being tested in a few ongoing clinical trials including: ipilimumab plus radiation therapy in a randomized Phase III trial enrolling patients with metastatic prostate cancer (ClinicalTrials.gov identifier NCT00861614); ipilimumab plus leuprolide acetate hormonal therapy in a Phase IIa study in patients with localized prostate cancer (NCT01194271); ipilimumab plus androgen-deprivation therapy in a Phase II study in patients with metastatic prostate cancer (NCT01377389); and ipilimumab plus a PD1-targeted antibody in a trial in patients with metastatic melanoma (NCT01024231). An abstract that was presented at the 2011 American Society of Clinical Oncology (ASCO) annual meeting reported results from a Phase I trial of ipilimumab plus bevacizumab, an antibody that targets vascular endothelial growth factor A (VEGFA), in patients with melanoma; these preliminary data indicated that 14 out of 21 patients had partial responses and/or stable disease lasting >6 months59. There are also combination clinical trials with sipuleucel-T that are in the early stages of development and that are expected to begin patient accrual within the next year. The data from these trials are eagerly awaited and will help to guide the design of future combination studies.
Conclusion
Basic immunology has advanced our understanding of the complex mechanisms of immune regulation, and this knowledge has been translated into the clinic with two novel agents, a CTLA4-targeted antibody (ipilimumab) and sipuleucel-T, both of which showed clinical benefit in Phase III trials. CTLA4-targeted antibody therapy is the first of its kind and has opened a new field in immunotherapy that is based on the targeting of inhibitory pathways and immune checkpoints. The dramatic antitumour responses that were seen in some patients as a result of treatment with ipilimumab provide, for the first time, a significant subset of patients who could be investigated for potential biomarkers that correlate with clinical outcome. Next-generation immunotherapy agents, such as PD1-targeted antibodies, have also led to tumour regression in some patients. We must conduct careful studies in treated patients so that we can gain knowledge to develop even more effective therapies. The era of mechanistic studies in patients is upon us, and it is imperative that we apply the same innovative strategies that were used to identify relevant genes and pathways in murine models to experimental clinical trials that are aimed at understanding human immune responses. We are optimistic that the field of cancer immunotherapy will continue to move forwards as we build on recent successes.
Acknowledgements
The authors’ research work was supported by the Howard Hughes Medical Institute (for J.P.A.), the Ludwig Center for Cancer Immunotherapy (for P.S., J.D.W. and J.P.A.), a Prostate Cancer Foundation Challenge Award in Immunology (to P.S. and J.P.A.). P.S. also acknowledges support from an M. D. Anderson Cancer Center Physician Scientist Award, a Doris Duke Charitable Foundation Clinical Scientist Development Award, a Clinical Investigator Award from the Cancer Research Institute, a Melanoma Research Alliance Young Investigator Award, an American Cancer Society Mentored Research Scholar Grant and a US Department of Defense Prostate Cancer Idea Development Award.
Footnotes
Competing interests statement
The authors declare competing financial interests. See Web version for details.
DATABASES
ClinicalTrials.gov: http://clinicaltrials.gov NCT00861614 | NCT01024231 | NCT01194271 | NCT01377389
National Cancer Institute Drug Dictionary: http://www.cancer.gov/drugdictionary bevacizumab | cyclophosphamide | dacarbazine | ipilimumab | leuprolide acetate | MAGEA3 | PROSTVAC-F | PROSTVAC-V | sipuleucel-T | sorafenib | temozolomide | tremelimumab
FURTHER INFORMATION
Padmanee Sharma’s homepage: http://faculty.mdanderson.org/Padmanee_Sharma
Jedd D. Wolchok’s homepage: http://www.mskcc.org/prg/prg/bios/608.cfm
James P. Allison’s homepage: http://www.mskcc.org/mskcc/html/50929.cfm
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
Contributor Information
Padmanee Sharma, Departments of Genitourinary Medical Oncology and Immunology, University of Texas M. D. Anderson Cancer Center, Box 0018-7, 1515 Holcombe Boulevard, Houston, Texas 77030, USA; and also at the Ludwig Center for Cancer Immunotherapy, Memorial Sloan–Kettering Cancer Center, 1275 York Avenue, New York, New York 10065, USA..
Klaus Wagner, Division of Medicine, University of Texas M. D. Anderson Cancer Center, Box 0018-7, 1515, Holcombe Boulevard, Houston, Texas 77030, USA..
Jedd D. Wolchok, Ludwig Center for Cancer Immunotherapy, Memorial Sloan–Kettering Cancer Center, 1275 York Avenue, New York, New York 10065, USA. Division of Melanoma Medical Oncology, Memorial Sloan–Kettering Cancer Center, 1275 York Avenue, New York, New York 10065, USA.
James P. Allison, Ludwig Center for Cancer Immunotherapy, Memorial Sloan–Kettering Cancer Center, 1275 York Avenue, New York, New York 10065, USA. Departments of Immunology and the Howard Hughes Medical Institute, Memorial Sloan–Kettering Cancer Center, 1275 York Avenue, New York, New York 10065, USA.
References
- 1.Burnet FM. Immunological aspects of malignant disease. Lancet. 1967;1:1171–1174. doi: 10.1016/s0140-6736(67)92837-1. [DOI] [PubMed] [Google Scholar]
- 2.Burnet FM. The concept of immunological surveillance. Prog. Exp. Tumor Res. 1970;13:1–27. doi: 10.1159/000386035. [DOI] [PubMed] [Google Scholar]
- 3.Thomas L. On immunosurveillance in human cancer. Yale. J. Biol. Med. 1982;55:329–333. [PMC free article] [PubMed] [Google Scholar]
- 4.Kaplan DH, et al. Demonstration of an interferon-γ-dependent tumor surveillance system in immunocompetent mice. Proc. Natl Acad. Sci. USA. 1998;95:7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shankaran V, et al. IFNγ and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. [PubMed] [Google Scholar]
- 6.Lasaro MO, Ertl HC. Targeting inhibitory pathways in cancer immunotherapy. Curr. Opin. Immunol. 2010;22:385–390. doi: 10.1016/j.coi.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schreibelt G, et al. Toll-like receptor expression and function in human dendritic cell subsets: implications for dendritic cell-based anti-cancer immunotherapy. Cancer Immunol. Immunother. 2010;59:1573–1582. doi: 10.1007/s00262-010-0833-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Speiser DE, Romero P. Molecularly defined vaccines for cancer immunotherapy, and protective T cell immunity. Semin. Immunol. 2010;22:144–154. doi: 10.1016/j.smim.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr. Opin. Immunol. 2009;21:233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudley ME, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fyfe G, et al. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J. Clin. Oncol. 1995;13:688–696. doi: 10.1200/JCO.1995.13.3.688. [DOI] [PubMed] [Google Scholar]
- 12.Robbins PF, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J. Clin. Oncol. 2011;29:917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kershaw MH, et al. A phase I study of adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin. Cancer Res. 2006;12:6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morgan RA, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartzentruber D, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N. Engl. J. Med. 2011;364:2119–2127. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kantoff PW, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 18.Kantoff PW, et al. Overall survival analysis of a phase II randomized controlled trial of a proxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J. Clin. Oncol. 2010;28:1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 21.Van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic lymphocyte-associated antigen 4 (CTLA4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J. Exp. Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quezada SA, et al. CTLA4-blockade and GMCSF combination immunotherapy alters the intra-tumor balance of effector and regulatory T cells. J. Clin. Invest. 2006;116:1935–1945. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirkwood JM, et al. Phase II trial of tremelimumab (CP675206) in patients with advanced refractory or relapsed melanoma. Clin. Cancer Res. 2010;16:1042–1048. doi: 10.1158/1078-0432.CCR-09-2033. [DOI] [PubMed] [Google Scholar]
- 24.Camacho LH, et al. Phase I/II trial of tremelimumab in patients with metastatic melanoma. J. Clin. Oncol. 2009;27:1075–1081. doi: 10.1200/JCO.2008.19.2435. [DOI] [PubMed] [Google Scholar]
- 25.Ribas A. Clinical development of the anti-CTLA4 antibody tremelimumab. Semin. Oncol. 2010;37:450–454. doi: 10.1053/j.seminoncol.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Marshall MA, Ribas A, Huang B. Evaluation of baseline serum C-reactive protein (CRP) and benefit from tremelimumab compared to chemotherapy in first-line melanoma. J. Clin. Oncol. 2010;28(Suppl.) Abstract 2609. [Google Scholar]
- 27.Liakou CI, et al. CTLA4 blockade increases IFNγ-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc. Natl Acad. Sci. USA. 2008;105:14987–14992. doi: 10.1073/pnas.0806075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen H, et al. Anti-CTLA4 therapy results in higher CD4+ICOShi T cell frequency and IFN-γ levels in both nonmalignant and malignant prostate tissues. Proc. Natl Acad. Sci. USA. 2009;106:2729–2734. doi: 10.1073/pnas.0813175106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carthon BC, et al. Preoperative CTLA4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin. Cancer Res. 2010;16:2861–2871. doi: 10.1158/1078-0432.CCR-10-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vonderheide RH, et al. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells. Clin. Cancer Res. 2010;16:3485–3494. doi: 10.1158/1078-0432.CCR-10-0505. [DOI] [PubMed] [Google Scholar]
- 31.Yuan J, et al. CTLA4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proc. Natl Acad. Sci. USA. 2008;105:20410–20415. doi: 10.1073/pnas.0810114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peggs KS, Quezada SA, Sharma P, Allison JP. Cancer Medicine. 8th edn. Shelton, Connecticut: People’s Medical Publishing House; 2010. Cancer immunotherapy; pp. 175–189. [Google Scholar]
- 33.Wolchok JD, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 34.Salgaller M, Marincola F, Cormier J, Rosenberg S. Immunization against epitopes in the human melanoma antigen gp100 following patient immunization with synthetic peptides. Cancer Res. 1996;56:4749–4757. [PubMed] [Google Scholar]
- 35.Korn EL, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J. Clin. Oncol. 2008;26:527–534. doi: 10.1200/JCO.2007.12.7837. [DOI] [PubMed] [Google Scholar]
- 36.Robert C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 37.Sznol M, et al. Safety and antitumor activity of biweekly MDX1106 (Anti-PD1, BMS936558/ONO4538) in patients with advanced refractory malignancies. J. Clin. Oncol. 2010;28(Suppl.) Abstract 2506. [Google Scholar]
- 38.Saenger YM, Wolchok JD. The heterogeneity of the kinetics of response to ipilimumab in metastatic melanoma: patient cases. Cancer Immun. 2008;8:1. [PMC free article] [PubMed] [Google Scholar]
- 39.O’Day S, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann. Oncol. 2010;21:1712–1717. doi: 10.1093/annonc/mdq013. [DOI] [PubMed] [Google Scholar]
- 40.Wolchok JD, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin. Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 41.Kane RC, et al. Sorafenib for the treatment of advanced renal cell carcinoma. Clin. Cancer Res. 2006;12:7271–7278. doi: 10.1158/1078-0432.CCR-06-1249. [DOI] [PubMed] [Google Scholar]
- 42.Spira D, et al. Comparison of different tumor response criteria in patients with hepatocellular carcinoma after systemic therapy with the multikinase inhibitor sorafenib. Acad. Radiol. 2010;18:89–96. doi: 10.1016/j.acra.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki C, et al. Radiologic measurements of tumor response to treatment: practical approaches and limitations. Radiographics. 2008;28:329–344. doi: 10.1148/rg.282075068. [DOI] [PubMed] [Google Scholar]
- 44.Gajewski TF, Louahed J, Brichard VG. Gene signature in melanoma associated with clinical activity: a potential clue to unlock cancer immunotherapy. Cancer J. 2010;16:399–403. doi: 10.1097/PPO.0b013e3181eacbd8. [DOI] [PubMed] [Google Scholar]
- 45.Haining WN, Wherry EJ. Integrating genomic signatures for immunologic discovery. Immunity. 2010;32:152–161. doi: 10.1016/j.immuni.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 46.Kirkwood JM, et al. Immunogenicity and antitumor effects of vaccination with peptide vaccine +/− granulocyte-monocyte colony-stimulating factor and/or IFNα2b in advanced metastatic melanoma: Eastern Cooperative Oncology Group Phase II Trial E1696. Clin. Cancer Res. 2009;15:1443–1451. doi: 10.1158/1078-0432.CCR-08-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Slingluff CL, et al. Immunologic and clinical outcomes of a randomized phase II trial of two multipeptide vaccines for melanoma in the adjuvant setting. Clin. Cancer Res. 2007;13:6386–6395. doi: 10.1158/1078-0432.CCR-07-0486. [DOI] [PubMed] [Google Scholar]
- 48.Britten CM, Janetzki S, van der Burg SH, Gouttefangeas C, Hoos A. Toward the harmonization of immune monitoring in clinical trials: quo vadis? Cancer Immunol. Immunother. 2008;57:285–288. doi: 10.1007/s00262-007-0379-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Janetzki S, et al. “MIATA”-minimal information about T cell assays. Immunity. 2009;31:527–528. doi: 10.1016/j.immuni.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Janetzki S, Cox JH, Oden N, Ferrari G. Standardization and validation issues of the ELISPOT assay. Methods Mol. Biol. 2005;302:51–86. doi: 10.1385/1-59259-903-6:051. [DOI] [PubMed] [Google Scholar]
- 51.Janetzki S, et al. Results and harmonization guidelines from two large-scale international Elispot proficiency panels conducted by the Cancer Vaccine Consortium (CVC/SVI) Cancer Immunol. Immunother. 2008;57:303–315. doi: 10.1007/s00262-007-0380-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moodie Z, et al. Response definition criteria for ELISPOT assays revisited. Cancer Immunol. Immunother. 2010;59:1489–1501. doi: 10.1007/s00262-010-0875-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koup RA, Graham BS, Douek DC. The quest for a T cell-based immune correlate of protection against HIV: a story of trials and errors. Nature Rev. Immunol. 2011;11:65–70. doi: 10.1038/nri2890. [DOI] [PubMed] [Google Scholar]
- 54.Dunn GP, Sheehan KC, Old LJ, Schreiber RD. IFN unresponsiveness in LNCaP cells due to the lack of JAK1 gene expression. Cancer Res. 2005;65:3447–3453. doi: 10.1158/0008-5472.CAN-04-4316. [DOI] [PubMed] [Google Scholar]
- 55.Dancey JE, et al. Guidelines for the development and incorporation of biomarker studies in early clinical trials of novel agents. Clin. Cancer Res. 2010;16:1745–1755. doi: 10.1158/1078-0432.CCR-09-2167. [DOI] [PubMed] [Google Scholar]
- 56.Sjöblom T, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314:268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 57.Segal NH. Epitope landscape in breast and colorectal cancer. Cancer Res. 2008;68:889–892. doi: 10.1158/0008-5472.CAN-07-3095. [DOI] [PubMed] [Google Scholar]
- 58.Peggs KS, Segal NH, Allison JP. Targeting immunosupportive cancer therapies: accentuate the positive, eliminate the negative. Cancer Cell. 2007;12:192–199. doi: 10.1016/j.ccr.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 59.Hodi FS, et al. A phase I trial of ipilimumab plus bevacizumab in patients with unresectable stage III or stage IV melanoma. J. Clin. Oncol. 2011;29(Suppl.) Abstract 8511. [Google Scholar]
- 60.Ngiow S, et al. Anti-TIM3 antibody promotes T cell IFNγ-mediated antitumor immunity and suppresses established tumors. Cancer Res. 2011;71:3540–3551. doi: 10.1158/0008-5472.CAN-11-0096. [DOI] [PubMed] [Google Scholar]
- 61.Wang L, et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J. Exp. Med. 2011;208:577–592. doi: 10.1084/jem.20100619. [DOI] [PMC free article] [PubMed] [Google Scholar]