Abstract
Recent evidence suggests that a rapid, automatic face-detection system is supported by subcortical structures including the amygdala, pulvinar, and superior colliculus. Early emerging abnormalities in these structures may be related to reduced social orienting in children with autism, and subsequently, to aberrant development of cortical circuits involved in face processing. Our objective was to determine whether functional abnormalities in the subcortical face processing system are present in adults with autism spectrum disorders (ASD) during supraliminal fearful face processing. Participants included twenty-eight individuals with ASD and 25 controls group-matched on age, IQ, and behavioral performance. The ASD group met diagnostic criteria on the ADI-R, ADOS-G, and DSM-IV. Both the ASD and control groups showed significant activation in bilateral fusiform gyri. The control group exhibited additional significant responses in the right amygdala, right pulvinar, and bilateral superior colliculi. In the direct group comparison, the controls showed significantly greater activation in the left amygdala, bilateral fusiform gyrus, right pulvinar, and bilateral superior colliculi. No brain region showed significantly greater activation in the ASD group compared to the controls. Thus, basic rapid face identification mechanisms appear to be functional in ASD. However, individuals with ASD failed to engage the subcortical brain regions involved in face detection and automatic emotional face processing, suggesting a core mechanism for impaired socioemotional processing in ASD. Neural abnormalities in this system may contribute to early emerging deficits in social orienting and attention, the putative precursors to abnormalities in social cognition and cortical face processing specialization.
Autism spectrum disorders (ASD) are characterized by social deficits, communication deficits, and restricted, repetitive, and stereotyped patterns of behavior(American Psychiatric Association, 1994). Of the triad of symptoms, social dysfunction alone diagnostically differentiates ASDs from other neurodevelopmental disorders. Thus, understanding the neural basis of social deficits may be critical to identifying autism’s etiology(Schultz, 2005).
Face perception has been a main focus of research in autism because it is a fundamental component of social cognition. Abnormal face perception in autism spectrum disorders (ASD) includes poor memory for faces (Boucher et al., 1998), difficulty evaluating trustworthiness (Adolphs et al., 2001), reduced inversion effect (Hobson et al., 1988), reduced attention to the eyes (Dalton et al., 2005; Klin et al., 2002; Neumann et al., 2006; Spezio et al., 2007; Sterling et al., 2008), and abnormal emotional perception (Ashwin et al., 2006b; Celani et al., 1999; Hobson et al., 1988; Tantam et al., 1989).
Despite well-characterized behavioral abnormalities, the neural circuitry underlying deficits in face perception in autism remain controversial. One unresolved topic is whether individuals with ASD have reduced neural sensitivity to faces or abnormally localized regions of face sensitivity. Several investigators have hypothesized that the fusiform face area is underdeveloped in autism because of the limited of experience with faces that is a feature of this disorder. Consistent with this view, the earliest functional imaging studies of face processing in autism showed clear evidence of reduced activation in the fusiform face area (Critchley et al., 2000; Hubl et al., 2003; Pierce et al., 2001; Schultz et al., 2000) combined with increased activation outside of the lateral fusiform gyrus (Pierce et al., 2001; Schultz et al., 2000). Subsequent studies replicated the finding of reduced fusiform activation (Dalton et al., 2005; Grelotti et al., 2005; Humphreys et al., 2008; Koshino et al., 2007; Piggot et al., 2004; Pinkham et al., 2008; Wang et al., 2004), however, many others did not(Ashwin et al., 2006a; Bird et al., 2006; Dapretto et al., 2006; Hadjikhani et al., 2004; Hadjikhani et al., 2006; Kleinhans et al., 2008; Pelphrey et al., 2007; Pierce et al., 2001; Pierce and Redcay, 2008; Wicker et al., 2008). One possible explanation for the inconsistencies in fusiform face area activation is differences in eye-gaze patterns in ASD. As noted above, eye-tracking research has identified a pattern of reduced attention to the eyes when individuals with ASD view pictures of faces. The relevance of this characteristic to fMRI studies of ASD was identified by Dalton et al(2005), who reported a direct correlation between time spent fixating on the eyes and activation in the fusiform gyrus and amygdala. This relationship between atypical eye-gaze patterns and reduced fusiform activation has also been demonstrated in non-affected individuals(Morris et al., 2007a). Consequently, Hadjikhani et al(2004; 2006) and others have emphasized that reduced fusiform activation in ASD may be secondary to differences in visual attention to face stimuli. As such, it appears to be possible to elicit normal levels of fusiform activation in individuals with ASD through experimental manipulations such as directing participants to fixate on the eye region(Hadjikhani et al., 2004; Hadjikhani et al., 2006) or by including pictures of personally familiar individuals(Pierce et al., 2004; Pierce and Redcay, 2008).
Although a much greater understanding of the neural abnormalities related to face processing in ASD has been obtained, little neuroimaging research has addressed the putative precursor to face processing abnormalities, i.e. why individuals with ASD do not preferentially attend to faces in the first place. Typically developing children are born with a preference for looking at faces (Simion et al., 2007). The development of face specialization appears to be driven by innate perceptual biases and fine-tuning of the visual system by experience (Simion et al., 2007). The developmental process of face specialization in infants with autism is largely unknown because clinical diagnoses are often not made until the 2nd year of life. However, at one year children with autism look less at faces (Osterling and Dawson, 1994; Osterling et al., 2002) and at two years, autistic toddlers spend less time fixating on eyes and more time fixating on mouths than typically developing toddlers (Jones et al., 2008). Dawson and colleagues have proposed that face processing impairments are secondary to a primary impairment in social motivation, which is mediated by amygdala dysfunction and results in failure to attend to socially relevant stimuli including faces (Dawson et al., 2005).
A limitation of traditional face processing studies that focus on cortical dysfunction is that development of face specificity in the fusiform gyrus, superior temporal gyrus, and inferior frontal lobe is a slow, experience driven process and unlikely to explain deficits in social orienting, etc. that are apparent in toddlers with autism. Instead, we hypothesize that the neural abnormalities underlying the abnormal development of face perception and reduced social orienting in ASD may begin at the level of rapid face detection. Johnson (2005) described a low frequency face detector system consisting of the superior colliculi, pulvinar, and amygdala. In this system, face information is conveyed with less than 100 ms latencies (Braeutigam et al., 2001; Eimer and Holmes, 2002; Pourtois et al., 2005; Streit et al., 2003). Johnson (2005) further proposed that a disruption of this subcortical face processing route, through its involvement in social orienting, could account for some of the social deficits present in autism. Thus, the current study used a rapid (23 ms) face processing paradigm to test the integrity of the subcortical face processing system in ASD. We predicted that the ASD group would demonstrate reduced activation in the superior colliculus, pulvinar, and the amygdala. Fusiform face area activation has been reported during subliminal face processing (Morris et al., 2007b). Thus, we hypothesized that fusiform activation to faces would be present in both groups with no significant differences based on our previous study (Kleinhans et al., 2008). Other cortical regions involved in face processing (e.g., temporal lobes, frontal lobes) were not included in the study because they have not been implicated in rapid face detection (Johnson, 2005).
MATERIALS AND METHODS
Participants
Thirty-one adults with an autism spectrum disorder and 25 typically developing controls participated in the fMRI protocol. Three individuals with ASD were excluded for excessive motion. All participants had FSIQ and VIQ ≥ 80. The included ASD group (n=28) was composed of 11 individuals with autistic disorder, 15 individuals with Asperger’s disorder, and 2 individuals with pervasive developmental disorder-not otherwise specified. Diagnoses were confirmed with the Autism Diagnostic Interview- Revised (ADI-R, Lord et al., 1994), the Autism Diagnostic Observation Schedule (ADOS, Lord et al., 2000), and clinical judgment based on all available information and DSM-IV criteria (American Psychiatric Association, 1994). The ASD and control groups did not significantly differ on age, verbal IQ, performance IQ, or full-scale IQ. Clinical and demographic information is reported in table 1. Control participants were screened for current and past psychiatric disorders, history of a developmental learning disability, and contraindications to MR imaging.
Table 1.
Charateristics of included participants
| ASD (n=28) | Control (n = 25) | ||||
|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | p value | |
| Age | 23.57 | (6.60) | 23.32 | (5.15) | .90 |
| Full scale IQ | 113.3 | (14.22) | 112.05 | (15.17) | .79 |
| Verbal IQ | 114.2 | (14.89) | 111.94 | (14.74) | .64 |
| Performance IQ | 109.3 | (16.05) | 109.16 | (16.22) | .98 |
| SADS | 16.6 | (6.96) | 2.76 | (4.10) | .00 |
| Autism Quotient (AQ) | 28.2 | (6.10) | 12.12 | (5.39) | .00 |
| ADOS subscales | |||||
| Communication | 3.68 | (1.31) | |||
| Social | 8.68 | (2.80) | |||
| ADI-R subscales | |||||
| Communication | 13.54 | (5.01) | |||
| Social | 16.82 | (5.26) | |||
| Repetitive behavior | 5.54 | (2.80) | |||
This study was approved by the University of Washington Human Subjects Institutional Review Board. Informed written consent was obtained from all study participants.
Behavioral Pretest
Prior to acquiring the fMRI scan, all participants were administered a behavioral pretest to determine the fastest stimuli presentation rate that could be validly used for the fMRI experiment. Eighty pictures were shown: 40 neutral faces and 40 houses. All faces were obtained from the MacBrain Face Stimulus Set. Face stimuli were cropped to 500×500 pixels and converted into grayscale images. The behavioral pre-test was administered in a testing room on a PC using Presentation® software. The computer monitor refresh rate was set to 85 Hz and four picture presentation rates pseudorandomly ordered throughout the experiment were included: 11.7 ms, 23.4 ms, 35.1 ms, and 58.5 ms. Participants were shown a picture of a face or a house immediately followed by a masking scrambled image. The scrambled image was created for each picture (i.e., faces and houses) by dividing each picture into 25×25 squares and randomly scrambling the squares using Matlab®. After each picture-mask presentation, a prompt appeared on the screen asking the participants to indicate with a button press whether a face or a house had been presented. Participants were given as much time as they needed to respond and instructed to guess even if they did not perceive anything. Directly following the participant’s response, a fixation cross appeared in the center of the screen for 2 seconds followed by the next picture-mask pair. Ten of each stimulus type was shown per presentation rate. This pretest utilized a forced-choice paradigm (Did you see a face or a house?) rather than the more common query asking for a yes/no response (e.g., Did you see a face?) in order to minimize the negative response bias associated with that approach (Pessoa et al., 2005).
fMRI Data Acquisition
Structural and functional MRI were performed on a 1.5T Signa MR imaging system (General Electric, Waukesha). FMRI series were collected using a echo-planar pulse sequence (TR/TE 3000/30msec, 21 slices; 6mm thick with 1mm gap, 64×64 matrix, 90 volumes total per run). The fMRI scan lasted 4 minutes 30 seconds. An SPGR was collected for fMRI registration and anatomical localization (TR= 33 milliseconds, TE= minimum, flip angle =30°, field of view =24 cm, 256 × 256 matrix, scan thickness =1.5 mm, acquisition plane= coronal plane).
fMRI task stimuli
Pictures of fearful faces, houses and scrambled mask images were used as visual stimuli throughout the fMRI task. Fearful faces were selected in order to maximize amygdala activation. All stimuli were prepared as described in the Behavioral Pretest section. The fearful faces were obtained from the MacBrain Face Stimulus Set. House stimuli included photographs provided by A. Ishai, N. Kanwisher and M. Eimer and house stimuli developed by our laboratory. The house pictures were edited for extraneous details in the environment. Stimuli were projected onto a screen positioned at the foot of the bore. The projector had a refresh rate of 85 Hz. Presentation® software was used to control the stimuli presentation timing.
fMRI task
The fMRI task included 78 pictures of individuals with a fearful facial expression and 98 houses. The task was described to the participants as a series of images that looked like a flashing checkerboard. Participants were instructed to attend to the pictures at all times and to press the button each time a fixation cross appeared. The presence of faces and houses was not mentioned to the participants. The 38 fixation events, consisting of a fixation cross appearing for 500 ms in the center of the screen, occurred pseudo randomly throughout the experiment at an average rate of 1 per 7.157 sec (ISI range = 2 sec – 20 sec).
A block-design was used to present four different stimulus blocks: fearful faces masked by a scrambled image (“F”; 30 sec per block), houses masked by a scrambled image (“H”; 30 sec per block), pairs of scrambled images (“S”; 18 sec per block) (see Figure 1) and a blank screen (“B”; 9 sec per block), which was presented at the beginning, end, and middle of the experiment to provide participants a respite from the flashing stimuli. The order of the blocks was as follows: BSFHFSHBSHFB. The 3 volumes acquired during the first “B” block were not included in the statistical analyses. During the Fear and House blocks, pictures were presented for 23 ms then backwards masked with their own scrambled image. The 23 ms rate was selected based on the results of the behavioral pre-test (see Results section below). A scrambled image mask was selected instead of a neutral face mask in order to guard against confounding supraliminal emotional face processing impairments with known impairments in neutral face processing in ASD. The duration of the visual mask varied (range 63 ms- 150 ms) in order to jitter the inter-stimulus interval between the mask and next picture. Two picture-mask pairs were presented per second; 60 pictures were presented per 30 second block. During the scramble blocks, two scrambled images were presented in succession with the same timing parameters as the stimuli in the Fear and House blocks.
Figure 1.
Example stimuli and presentation times. Each target was presented for 23 ms then masked with the scrambled image.
Post scan debriefing
Immediately upon exiting the scanner, each participant was questioned by NMK about the rapidly presented stimuli. First, the participant was verbally asked, “Were you able to see the faces and the houses?” If the participant answered no, the questioning was terminated. If the participant responded “yes,” the examiner asked, “Were you able to see the facial expressions?” If the participant responded no, the questioning was terminated. However, the if the participant responded “yes,” s/he was asked, “what type of facial expressions did you see?” All answers were written down.
fMRI Processing and Statistical Analysis
FMRI data analyses were performed using the FMRIB Software Library version 3.3 (FSL; http://www.fmrib.ox.ac.uk/fsl/). The following preprocessing steps were applied: the first three volumes were discarded; motion correction was conducted using MCFLIRT (Jenkinson et al., 2002); nonbrain structures were removed using BET (Smith, 2002); data were spatially smoothed using a Gaussian kernel of FWHM 5 mm and temporally smoothed using a high-pass filter sigma = 96 s. Time series statistical analyses were carried out using FMRIB's Improved Linear Model (FILM) with local autocorrelation correction (Woolrich et al., 2001). Individual FMRI data were registered to the high resolution SPGR and then warped to the MNI152 standard image with an affine transformation using FLIRT (FMRIB’s Linear Image Registration Tool) and resampled to 2 mm3 voxels.
Analysis of group-wise effects were conducted using FLAME (FMRIB's Local Analysis of Mixed Effects), a method for modeling and estimating the random-effects component of the measured inter-session mixed-effects variance. This method allows inferences to be made about the wider population from which the sessions/subjects were drawn. Two analyses were conducted 1) Fear > Scramble; 2) House > Scramble. Seven a-priori regions of interest (ROI) were defined on MNI152 and tested separately for each statistical analysis. The brain regions included R/L amygdala; R/L lateral fusiform gyrus; R/L pulvinar; and the superior colliculi. The right and left amygdala ROIs were hand drawn within our laboratory on the standard brain using a previously validated method (Honeycutt et al., 1998) and the right and left fusiform gyrus were hand and drawn on the standard brain within our laboratory, based on atlas images (Duvernoy, 1999). The right and left pulivinar masks were defined by the Talairach Daemon. No mask was used for the Superior colliculus; the cluster location was determined by visual inspection in consultation with the Talairach atlas (Talairach and Tournoux, 1988).
Statistical corrections for multiple comparisons were conducted using cluster-thresholding based on Markov Chain Monte Carlo sampling for each ROI. All fMRI results were corrected for multiple comparisons using this method.
RESULTS
Behavioral Testing
Behavioral Pre-test
Independent samples t-tests were used to test for group differences in performance on the behavioral pretest. Performance (number of errors) was tested for each presentation rate separately. The maximum number of errors per presentation rate was 20; thus chance performance (i.e., 50% correct) was 10 errors. Because this was a forced-choice design, a response of either face or house was obtained for every trial. The ASD group performed significantly worse that the control group at the 11.7 ms presentation rate (ASD errors mean = 6.57, SD = 3.10; Control errors mean = 5.44, SD = 2.10; p = .007). No significant between group differences were observed at the slower present rates [23.4 ms: ASD errors mean = 2.43, SD 1.60, Control errors mean = 2.32, SD = 1.70; p .831. 35.1 ms: ASD errors mean = 0.61, SD 0.87, Control errors mean = 1.04, SD = 1.06; p = .471. 58.5 ms: ASD errors mean = 0.18, SD = 0.48, Control errors mean = 0.48, SD = 1.23; p=.064]. In order to match both groups on performance, the presentation rate for the fMRI experiment was set at 23.4 ms. Follow-up testing was conducted to determine if there were within- group or between-group differences at the 23.4 ms presentation rate in the ability to perceive faces compared to houses. There were no significant between group differences in number of errors for the face stimuli (ASD mean = .143, SD = .591, Control mean = .250, SD = .610; p = .523) or the house stimuli (ASD mean = 2.3, SD = 1.441, Control mean = 2.167, SD = 1.606; p = .716). However, paired t-tests showed that both groups were significantly worse at detecting the houses than the neutral faces [ASD mean difference = −2.179, SD = 1.541; p <.0001; Control mean difference = −1.917, SD = 1.717; p < .0001]. These data suggest that different levels of awareness were present for the face stimuli compared to the house stimuli. Further inspection of the behavioral data revealed that differences in performance between the faces and houses persisted at all presentation rates except for the 58.5 ms rate. The use of fearful faces in the fMRI experiment was expected to exacerbate the performance difference between faces and houses. Because or main purpose was to investigate the rapid subcortical face processing system, we determined that the best solution was to present the stimuli at the fastest rate that the ASD and control groups were matched on faces (i.e., 23.4 ms). The house processing condition was included for exploratory purposes; thus comparisons to the fearful faces results should be interpreted with caution and a direct statistical comparison was not reported because we felt the results were not valid or interpretable. Because our participants performed above chance on the behavioral pre-test, indicating a rudimentary level of awareness, we consider our experiment to be assessing supraliminal visual processing. However, when considering these finding in the context of the larger literature on face processing, it is important to note that most similar paradigms in the literature, including those by Rauch et al (see e.g., 2000) upon which this paradigm is based, consider presentation rates below 30 ms to be subliminal.
fMRI Behavioral Performance
Between-group differences on the fMRI behavioral task were tested using independent samples t-tests. There were 38 events throughout the experiment, with no significant between group differences in performance (ASD hits: mean =37.86, Control hits: mean = 37.88, p = .893; ASD false alarms: mean = .38, Control false alarms: mean = .21, p = .285).
Post-scan debriefing results
Data were obtained from all study participants except one control. In the ASD group, 26/28 (93%) participants reported being able to see the faces; of those, 14/26 (54%) reported seeing facial expressions at least occasionally. Of the control participants, 21/24 (88%) reported being able to see the faces and 14/21 (67%) reported seeing facial expressions at least occasionally. Responses that were typical for both groups included: surprised, shocked, angry, excited. Very few participants used the terms “scared” or” fearful”.
fMRI Results
Fear > Scramble
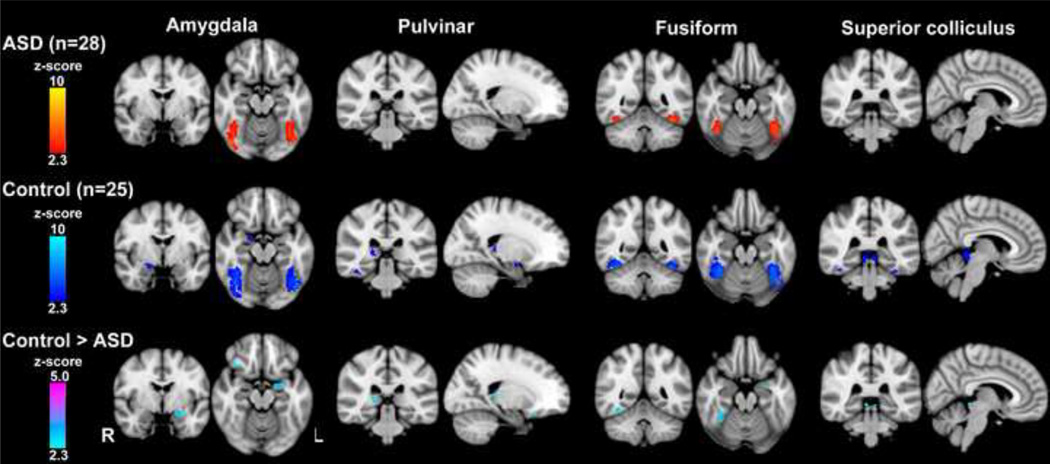
Both the ASD and control groups showed significant activation in the bilateral fusiform gyrus. Paralleling previous studies (Das et al., 2005; Liddell et al., 2005; Morris et al., 1999) the control group exhibited additional clusters of significant activation in the right amygdala, right pulvinar, and bilateral superior colliculi. In the direct group comparison, the controls showed significantly greater activation in the left amygdala, right and left fusiform gyrus, right pulvinar, and bilateral superior colliculi. No brain region showed significantly greater activation in the ASD group compared to the controls. (see Table 2, Figure 2).
Table 2.
Clusters of significant fMRI activation for supraliminal fearful face processing.
| Fear > Scramble | side | location | No. of voxels | z max | x (mm) | y (mm) | z ( mm) |
|---|---|---|---|---|---|---|---|
| ASD (n=28) | |||||||
| L | fusiform | 382 | 5.09 | −38 | −50 | −20 | |
| R | fusiform | 228 | 4.45 | 40 | −58 | −20 | |
| Control (n=25) | |||||||
| L | amygdalaa | 14 | 2.75 | −22 | −4 | −14 | |
| R | amygdala | 26 | 3.25 | 22 | −2 | −14 | |
| L | fusiform | 582 | 6.07 | −34 | −44 | −22 | |
| R | fusiform | 682 | 6.76 | 36 | −50 | −22 | |
| R | pulvinar | 23 | 3.46 | 18 | −32 | 4 | |
| R,L | superior colliculusa | 163 | 3.3 | 4 | −40 | −12 | |
| ASD > Control | |||||||
| none | |||||||
| Control > ASD | |||||||
| L | amygdala | 52 | 3.49 | −22 | −4 | −16 | |
| L | fusiform | 85 | 3.68 | −28 | −36 | −20 | |
| R | fusiform | 205 | 4.07 | 34 | −46 | −24 | |
| R | pulvinar | 15 | 2.74 | 20 | −34 | 4 | |
| R,L | superior colliculusa | 23 | 2.83 | 6 | −38 | −6 |
Note. Data were analyzed using mixed effects and cluster threshold corrected (p < .05, corrected for multiple comparisons) unless otherwise noted.
p < .05, uncorrected for multiple comparisons.
Figure 2.
This is a composite picture of all the ROI-basedsignificant clusters of activation for the contrast fearful face > scramble. The contrast ASD > control is not pictured because there were no significant clusters of activation. Clusters were thresholded at p < .05, corrected for multiple comparisons.
House > Scramble
The ASD group had no significant clusters of activation. The control group exhibited significant activation in the right and left medial fusiform gyrus. In the direct group comparison, controls evidenced significantly greater activation in the right amygdala compared to ASD (see Table 3).
Table 3.
Clusters of significant fMRI activation for supraliminal house processing.
| House > Scramble | side | location | No. of voxels | z max | x (mm) | y (mm) | z (mm) |
|---|---|---|---|---|---|---|---|
| ASD (n=28) | |||||||
| none | |||||||
| Control (n=25) | |||||||
| L | fusiform | 209 | 4.79 | −32 | −44 | −10 | |
| R | fusiform | 402 | 4.49 | 36 | −46 | −20 | |
| ASD > Control | |||||||
| none | |||||||
| Control > ASD | |||||||
| R | amygdala | 17 | 2.98 | 22 | −4 | −12 |
Note. Data were analyzed using mixed effects and cluster threshold corrected (p < .05, corrected for multiple comparisons).
DISCUSSION
This study explored the neural correlates of rapid face processing in individuals with ASD. Participants viewed supraliminally (23.4 ms) presented faces depicting fear masked by a scrambled image during fMRI imaging. Consistent with our hypotheses, substantially reduced activation was observed in subcortical face processing brain networks in the ASD group. The control group activated bilateral fusiform gyrus, bilateral superior colliculi, right amygdala, and right pulvinar in response to the masked fearful faces. In contrast, the ASD group only showed significant activation in the bilateral fusiform gyrus. Significantly greater activation in the controls compared to the ASD group was observed in the left amygdala, bilateral fusiform, right pulvinar, and superior colliculi. Note that the cluster of activation that is labeled “superior colliculi” may include inferior colliculi activation as well. Our scan parameters and behavioral task were not designed to differentiate the two structures. This is a limitation of the current study.
We did not predict reduced fusiform face area activation based on the results of our previous study of neutral faces (Kleinhans et al., 2008), which did not find any difference in overall activation in the fusiform or the amygdala. It is possible that fusiform activation appeared more normal previously because of the limited task demands and neutral stimuli type that were utilized. Individuals with autism may need longer exposure to a faces to in order to reach the same level of BOLD activation as typical controls. Furthermore, given that the previous study used neutral faces and the current study used fearful faces, it may be that fusiform activation is not modulated by the emotional valence of a face to the same degree as a neurotypical adult. We propose that reduced fusiform face area activation in ASD may be directly related to reduced amygdala activation. This theory is consistent with known structural and functional connectivity between these brain regions in healthy controls (Ishai et al., 2004) and previous studies showing abnormal connectivity between these regions (Conturo et al., 2008; Kleinhans et al., 2008) in ASD.
We also tested the neural correlates of rapid house processing for exploratory purposes. However, it is important to note that ability to correctly identify the house stimuli at 23.4 ms was significantly worse than the ability to identify the fearful face stimuli in both groups. Because of this confound, we did not directly test the fearful face stimuli to the house stimuli. The ASD group did not show any significant activation to the rapidly presented house stimuli compared to scrambled images, while the control group had significant activation in the bilateral fusiform gyrus. In the direct group comparison, the control group showed significantly greater activation than the ASD group in the right amygdala. Further inspection of the data revealed that the group difference in the amygdala is related to differences in response to the scramble-scramble stimuli, as both groups showed greater activation to the scramble-scrambled images compared to masked houses. We are unsure how to interpret this result, as we did not have any a-prior hypotheses regarding amygdala activation to scrambled images.
These findings are notable considering that behavioral testing conducted outside of the scanner showed nearly identical ability to discriminate between neutral faces and houses presented at this rapid rate. However, the distinct patterns of activation between the groups suggest that the stimuli were not being processed in the same manner or with the same depth. During the processing of fearful faces, the ASD group activation was restricted to the fusiform gyri, which plays a role primarily limited to face identification (Johnson, 2005). The controls, in contrast, activated the fusiform and subcortical structures (pulvinar, superior colliculi, and amygdala) involved in face detection and emotional processing (Johnson, 2005). Although our behavioral paradigm was not complex enough to probe subtle levels of processing, previous behavioral studies provide supporting evidence that subliminal stimuli are processed abnormally in autism (Hall et al., 2007; Kamio et al., 2006). Johnson (2005) proposed that the subcortical rapid face detection system is the starting point for the development of face specialization. Rapid face detection follows a series of steps: the retina sends direct projections to the superior colliculus, which project to the pulvinar in the thalamus, which in turn project to the amygdala (LeDoux, 1996). Face information is transmitted in under 100 ms through this subcortical system (Braeutigam et al., 2001; Eimer and Holmes, 2002; Pourtois et al., 2005; Streit et al., 2003). Unlike the cortical pathway, which is sensitive to high spatial frequency images of faces (Livingstone and Hubel, 1988; Merigan and Maunsell, 1993), the subcortical pathway processes low spatial frequency information and is thought to be involved in social orienting to faces (Adolphs and Tranel, 2003; Vuilleumier et al., 2003). Abnormalities in social orienting and attention have been assessed in ASD using experimental paradigms and eye-tracking studies. Typically developing individuals fixate on the eyes more frequently than other facial features (Haith et al., 1977; Janik et al., 1978). In contrast, individuals with autism display aberrant eye tracking patterns throughout the lifespan. By two years of age, toddlers with autism fail to show a preference for looking at the eye region (Jones et al., 2008). Further, those children who spend the least amount of time fixating on the eyes display the most socially impaired behavior (Jones et al., 2008). Adults with autism viewing still images of faces (Neumann et al., 2006; Sterling et al., 2008) and naturalistic social scenes (Klin et al., 2002) still show reduced attention to eyes. Because we did not use a neutral face as a mask and utilized only one emotional expression, we cannot discern whether the current findings are specific to emotional face processing or present to faces in general. It is possible that the neural abnormalities observed in this study maybe present in response to emotionally salient stimuli generally.
The observed BOLD signal differences, particularly in structures such as the amygdala, may be mediated by grey matter abnormalities. Amygdalar abnormalities have been widely documented and include reduced numbers of neurons (Schumann and Amaral, 2006), volumetric deviations from age-matched controls (Aylward et al., 1999; Munson et al., 2006; Nacewicz et al., 2006; Pierce et al., 2001; Schumann et al., 2004; Sparks et al., 2002), biochemical alterations (Endo et al., 2007; Gabis et al., 2008; Otsuka et al., 1999; Page et al., 2006), and impaired activation. The thalamus has not undergone the same scrutiny as the amygdala; however some clues exist to the structural integrity of this brain region in ASD. Most studies have not found volumetric differences in the thalamus (Hardan et al., 2006; Hardan et al., 2008a; Hardan et al., 2008b; Haznedar et al., 2006; Tsatsanis et al., 2003)but see(Waiter et al., 2004)); however, the expected linear relationship between thalamic volume and whole brain volume is not present in adults with ASD (Hardan et al., 2006; Hardan et al., 2008a; Hardan et al., 2008b; Tsatsanis et al., 2003) suggesting abnormal connections between the thalamus and cortical structures. In addition, biochemical alterations consisting of reduced n-acetyl aspartate, phosphocreatine and creatine, and choline-containing compounds have been reported in children with autism (Hardan et al., 2008b). One study has reported regionally variations in glucose metabolic mapping in the thalamus in ASD (Haznedar et al., 2006) with reduced metabolic activity in the ventral thalamus and increased metabolic activity in the pulvinar in the ASD group relative to the controls (Haznedar et al., 2006). Studies of the superior colliculi in ASD are extremely limited. One volumetric study of the midbrain, which included the superior and inferior colliculi found no difference in a midline measurement (Hsu et al., 1991) in autism. However, a previous fMRI study by our group found reduced functional connectivity between the fusiform face area and the superior colliculi during face processing in ASD (Kleinhans et al., 2008).
We propose that early-emerging deficits in social orienting and attention and social deficits that persist throughout the lifespan in ASD may be associated with neural abnormalities in the subcortical face processing system. The majority of fMRI studies of face processing in ASD have focused on cortical structures, particularly the fusiform gyrus and the superior temporal gyrus. However, the discrepancy between the timing of the emergence of social deficits and the development of cortical structures necessitates that the foundation of such impairments must be driven by earlier developing neural circuitry. For example, studies of typical brain development indicate that temporal lobe grey matter volume (Giedd et al., 1999) and cortical thickness (Shaw et al., 2008) peak in late childhood to early adolescence. Cortical thickness of the inferior temporal lobe, which includes the fusiform gyrus, is largest at approximately 11 years of age (Shaw et al., 2008). The superior temporal gyrus, which is associated with eye gaze(Johnson, 2005) and the interpretation of actions and social intentions of others (Pelphrey et al., 2004), reaches full maturity even later at approximately 15 years of age (Shaw et al., 2008). Typical adult face processing networks continue to develop through late childhood and adolescence (Aylward et al., 2005). For example, a developmental fMRI study of face processing, Aylward et al (2005) found that adolescents between 12–14 showed greater fusiform activation to faces compared to houses. This face-specific fusiform activation was not observed in the younger sample (ages 8–10), suggesting the cortical face-specificity may not fully develop until adolescence is reached. Together, this literature renders it unlikely that abnormalities in the brain networks involved in higher order social processing drive the rudimentary deficits in social orienting and attention present in infants and toddlers with ASD.
Are abnormalities in the subcortical face processing system specific to autism? ASD appears to be the only neuropsychiatric disorders to show hypoactivation across the entire subcortical face processing system. Other psychiatric disorders including generalized anxiety disorder (Monk et al., 2008), post-traumatic stress disorder (Bryant et al., 2008; Rauch et al., 2000), and major depression (Sheline et al., 2001) show significantly increased amygdalar activation in response to masked faces. This pattern of amygdala hyperarousal has been interpreted to be related to hypervigilance for threatening stimuli (Monk et al., 2008). Individuals with schizophrenia, like those with ASD, show reduced amygdala activation to masked emotional faces (Das et al., 2007); however, activation in the superior colliculus and pulvinar is significantly increased compared to controls. Notably, individuals with ASD and schizophrenia share a deficit in emotional processing and have atypically reduced attention to the eyes of facial expressions (Das et al., 2007). Thus, it appears that widespread hypoactivation of the subcortical face processing system may be unique to ASD and related to deficits in social orienting and emotional evaluation. More comprehensive research across a variety of neuropsychiatric disorders will be necessary to confirm this proposal.
We found striking hypoactivation of the subcortical face processing system in high functioning individuals with ASD related to supraliminal face detection. This system is critical to automatic social orienting and perceptual processing of emotionally-relevant stimuli. The lack of engagement of this system may be the proximal cause the neurobiological basis of face processing impairments in ASD related to a putative lack of experience with faces. A review of the literature further suggests that this pattern of dysfunction of the subcortical face processing system may be specific to individuals with ASD.
Supplementary Material
Figure 3.
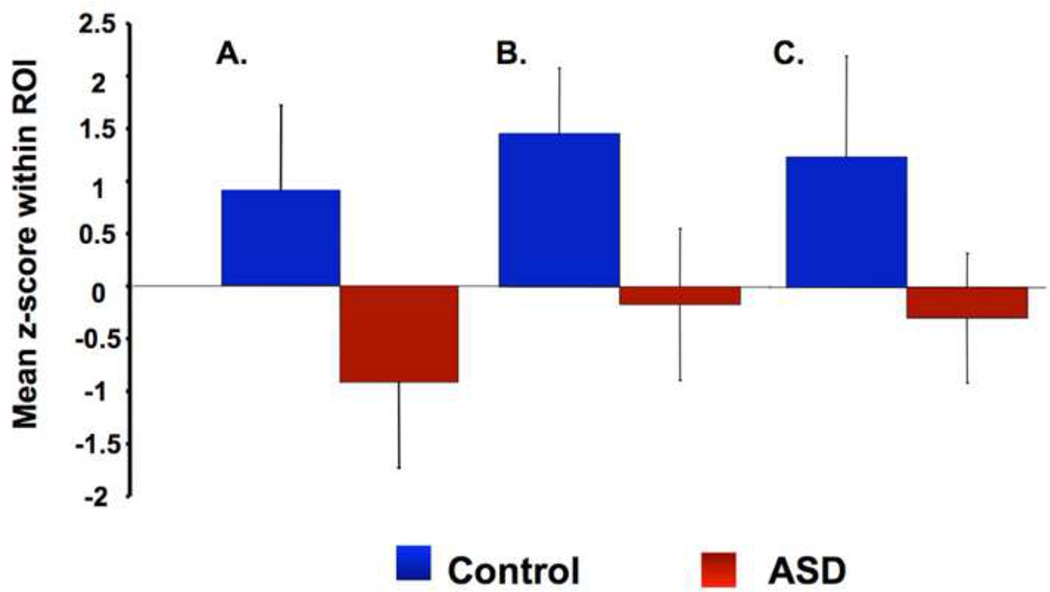
Bar graph of average activation for the contrast fearful face > scramble within the A) Left Amygdala, B) Right Pulvinar, and C) Superior Colliculi. The Controls are shown in blue and the ASD group is shown in red.
ACKNOWLEDGEMENTS
This work was supported by NICHD (U19 HD34565) and NIMH (U54MH066399). The development of the MacBrain Face Stimulus Set was overseen by Nim Tottenham (tott0006@tc.umn.edu) and supported by the John D. and Catherine T. MacArthur Foundation Research Network on Early Experience and Brain Development. We would like to thank Dr. Heracles Panagiotides for help discussions of the subcortical face processing system, Dr. Sara Webb for her input on the experimental design, and Dr. Neva Corrigan for help with stimuli preparation and MATLAB programming.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of this manuscript were presented at 7th International Meeting for Autism Research, London, England, 2008.
References
- Adolphs R, Sears L, Piven J. Abnormal processing of social information from faces in autism. J Cogn Neurosci. 2001;13:232–240. doi: 10.1162/089892901564289. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D. Amygdala damage impairs emotion recognition from scenes only when they contain facial expressions. Neuropsychologia. 2003;41:1281–1289. doi: 10.1016/s0028-3932(03)00064-2. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Ashwin C, Baron-Cohen S, Wheelwright S, O'Riordan M, Bullmore ET. Differential activation of the amygdala and the 'social brain' during fearful face-processing in Asperger Syndrome. Neuropsychologia. 2006a doi: 10.1016/j.neuropsychologia.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Ashwin C, Chapman E, Colle L, Baron-Cohen S. Impaired recognition of negative basic emotions in autism: a test of the amygdala theory. Soc Neurosci. 2006b;1:349–363. doi: 10.1080/17470910601040772. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Minshew NJ, Goldstein G, Honeycutt NA, Augustine AM, Yates KO, Barta PE, Pearlson GD. MRI volumes of amygdala and hippocampus in non-mentally retarded autistic adolescents and adults. Neurology. 1999;53:2145–2150. doi: 10.1212/wnl.53.9.2145. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Park JE, Field KM, Parsons AC, Richards TL, Cramer SC, Meltzoff AN. Brain activation during face perception: evidence of a developmental change. J Cogn Neurosci. 2005;17:308–319. doi: 10.1162/0898929053124884. [DOI] [PubMed] [Google Scholar]
- Bird G, Catmur C, Silani G, Frith C, Frith U. Attention does not modulate neural responses to social stimuli in autism spectrum disorders. Neuroimage. 2006;31:1614–1624. doi: 10.1016/j.neuroimage.2006.02.037. [DOI] [PubMed] [Google Scholar]
- Boucher J, Lewis V, Collis G. Familiar face and voice matching and recognition in children with autism. J Child Psychol Psychiatry. 1998;39:171–181. [PubMed] [Google Scholar]
- Braeutigam S, Bailey AJ, Swithenby SJ. Task-dependent early latency (30–60 ms) visual processing of human faces and other objects. Neuroreport. 2001;12:1531–1536. doi: 10.1097/00001756-200105250-00046. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Kemp AH, Felmingham KL, Liddell B, Olivieri G, Peduto A, Gordon E, Williams LM. Enhanced amygdala and medial prefrontal activation during nonconscious processing of fear in posttraumatic stress disorder: an fMRI study. Hum Brain Mapp. 2008;29:517–523. doi: 10.1002/hbm.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celani G, Battacchi MW, Arcidiacono L. The understanding of the emotional meaning of facial expressions in people with autism. J Autism Dev Disord. 1999;29:57–66. doi: 10.1023/a:1025970600181. [DOI] [PubMed] [Google Scholar]
- Conturo TE, Williams DL, Smith CD, Gultepe E, Akbudak E, Minshew NJ. Neuronal fiber pathway abnormalities in autism: an initial MRI diffusion tensor tracking study of hippocampo-fusiform and amygdalo-fusiform pathways. J Int Neuropsychol Soc. 2008;14:933–946. doi: 10.1017/S1355617708081381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Daly EM, Bullmore ET, Williams SC, Van Amelsvoort T, Robertson DM, Rowe A, Phillips M, McAlonan G, Howlin P, Murphy DG. The functional neuroanatomy of social behaviour: changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain. 2000;123(Pt 11):2203–2212. doi: 10.1093/brain/123.11.2203. [DOI] [PubMed] [Google Scholar]
- Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, Alexander AL, Davidson RJ. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8:519–526. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dapretto M, Davies MS, Pfeifer JH, Scott AA, Sigman M, Bookheimer SY, Iacoboni M. Understanding emotions in others: mirror neuron dysfunction in children with autism spectrum disorders. Nat Neurosci. 2006;9:28–30. doi: 10.1038/nn1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P, Kemp AH, Flynn G, Harris AW, Liddell BJ, Whitford TJ, Peduto A, Gordon E, Williams LM. Functional disconnections in the direct and indirect amygdala pathways for fear processing in schizophrenia. Schizophr Res. 2007;90:284–294. doi: 10.1016/j.schres.2006.11.023. [DOI] [PubMed] [Google Scholar]
- Das P, Kemp AH, Liddell BJ, Brown KJ, Olivieri G, Peduto A, Gordon E, Williams LM. Pathways for fear perception: modulation of amygdala activity by thalamo-cortical systems. Neuroimage. 2005;26:141–148. doi: 10.1016/j.neuroimage.2005.01.049. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, McPartland J. Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Dev Neuropsychol. 2005;27:403–424. doi: 10.1207/s15326942dn2703_6. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. The Human Brain Surface, Blood Supply, and Three-Dimensional Sectional Anatomy. Second ed. New York: Springer-Verlag Wien; 1999. [Google Scholar]
- Eimer M, Holmes A. An ERP study on the time course of emotional face processing. Neuroreport. 2002;13:427–431. doi: 10.1097/00001756-200203250-00013. [DOI] [PubMed] [Google Scholar]
- Endo T, Shioiri T, Kitamura H, Kimura T, Endo S, Masuzawa N, Someya T. Altered chemical metabolites in the amygdala-hippocampus region contribute to autistic symptoms of autism spectrum disorders. Biol Psychiatry. 2007;62:1030–1037. doi: 10.1016/j.biopsych.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Gabis L, Wei H, Azizian A, DeVincent C, Tudorica A, Kesner-Baruch Y, Roche P, Pomeroy J. 1H-magnetic resonance spectroscopy markers of cognitive and language ability in clinical subtypes of autism spectrum disorders. J Child Neurol. 2008;23:766–774. doi: 10.1177/0883073808315423. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Grelotti DJ, Klin AJ, Gauthier I, Skudlarski P, Cohen DJ, Gore JC, Volkmar FR, Schultz RT. fMRI activation of the fusiform gyrus and amygdala to cartoon characters but not to faces in a boy with autism. Neuropsychologia. 2005;43:373–385. doi: 10.1016/j.neuropsychologia.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, Chabris CF, Clark J, Steele S, McGrath L, Vangel M, Aharon I, Feczko E, Harris GJ, Tager-Flusberg H. Activation of the fusiform gyrus when individuals with autism spectrum disorder view faces. Neuroimage. 2004;22:1141–1150. doi: 10.1016/j.neuroimage.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Joseph RM, Snyder J, Tager-Flusberg H. Abnormal activation of the social brain during face perception in autism. Hum Brain Mapp. 2006 doi: 10.1002/hbm.20283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haith MM, Bergman T, Moore MJ. Eye contact and face scanning in early infancy. Science. 1977;198:853–855. doi: 10.1126/science.918670. [DOI] [PubMed] [Google Scholar]
- Hall GB, West CD, Szatmari P. Backward masking: evidence of reduced subcortical amygdala engagement in autism. Brain Cogn. 2007;65:100–106. doi: 10.1016/j.bandc.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Hardan AY, Girgis RR, Adams J, Gilbert AR, Keshavan MS, Minshew NJ. Abnormal brain size effect on the thalamus in autism. Psychiatry Res. 2006;147:145–151. doi: 10.1016/j.pscychresns.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Hardan AY, Girgis RR, Adams J, Gilbert AR, Melhem NM, Keshavan MS, Minshew NJ. Brief report: abnormal association between the thalamus and brain size in Asperger's disorder. J Autism Dev Disord. 2008a;38:390–394. doi: 10.1007/s10803-007-0385-1. [DOI] [PubMed] [Google Scholar]
- Hardan AY, Minshew NJ, Melhem NM, Srihari S, Jo B, Bansal R, Keshavan MS, Stanley JA. An MRI and proton spectroscopy study of the thalamus in children with autism. Psychiatry Res. 2008b;163:97–105. doi: 10.1016/j.pscychresns.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haznedar MM, Buchsbaum MS, Hazlett EA, LiCalzi EM, Cartwright C, Hollander E. Volumetric analysis and three-dimensional glucose metabolic mapping of the striatum and thalamus in patients with autism spectrum disorders. Am J Psychiatry. 2006;163:1252–1263. doi: 10.1176/ajp.2006.163.7.1252. [DOI] [PubMed] [Google Scholar]
- Hobson RP, Ouston J, Lee A. What's in a face? The case of autism. Br J Psychol. 1988;79(Pt 4):441–453. doi: 10.1111/j.2044-8295.1988.tb02745.x. [DOI] [PubMed] [Google Scholar]
- Honeycutt NA, Smith PD, Aylward E, Li Q, Chan M, Barta PE, Pearlson GD. Mesial temporal lobe measurements on magnetic resonance imaging scans. Psychiatry Res. 1998;83:85–94. doi: 10.1016/s0925-4927(98)00035-3. [DOI] [PubMed] [Google Scholar]
- Hsu M, Yeung-Courchesne R, Courchesne E, Press GA. Absence of magnetic resonance imaging evidence of pontine abnormality in infantile autism. Arch Neurol. 1991;48:1160–1163. doi: 10.1001/archneur.1991.00530230068024. [DOI] [PubMed] [Google Scholar]
- Hubl D, Bolte S, Feineis-Matthews S, Lanfermann H, Federspiel A, Strik W, Poustka F, Dierks T. Functional imbalance of visual pathways indicates alternative face processing strategies in autism. Neurology. 2003;61:1232–1237. doi: 10.1212/01.wnl.0000091862.22033.1a. [DOI] [PubMed] [Google Scholar]
- Humphreys K, Hasson U, Avidan G, Minshew N, Behrmann M. Cortical patterns of category-selective activation for faces, places and objects in adults with autism. 2008;1:52–63. doi: 10.1002/aur.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishai A, Pessoa L, Bikle PC, Ungerleider LG. Repetition suppression of faces is modulated by emotion. Proc Natl Acad Sci U S A. 2004;101:9827–9832. doi: 10.1073/pnas.0403559101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janik SW, Wellens AR, Goldberg ML, Dell'osso LF. Eyes as the center of focus in the visual examination of human faces. Percept Mot Skills. 1978;47:857–858. doi: 10.2466/pms.1978.47.3.857. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister PR, Brady JM, Smith S. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Subcortical face processing. Nat Rev Neurosci. 2005;6:766–774. doi: 10.1038/nrn1766. [DOI] [PubMed] [Google Scholar]
- Jones W, Carr K, Klin A. Absence of preferential looking to the eyes of approaching adults predicts level of social disability in 2-year-old toddlers with autism spectrum disorder. Arch Gen Psychiatry. 2008;65:946–954. doi: 10.1001/archpsyc.65.8.946. [DOI] [PubMed] [Google Scholar]
- Kamio Y, Wolf J, Fein D. Automatic processing of emotional faces in high-functioning pervasive developmental disorders: An affective priming study. J Autism Dev Disord. 2006;36:155–167. doi: 10.1007/s10803-005-0056-z. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Richards T, Sterling L, Stegbauer KC, Mahurin R, Johnson LC, Greenson J, Dawson G, Aylward E. Abnormal functional connectivity in autism spectrum disorders during face processing. Brain. 2008;131:1000–1012. doi: 10.1093/brain/awm334. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch Gen Psychiatry. 2002;59:809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Koshino H, Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. fMRI Investigation of Working Memory for Faces in Autism: Visual Coding and Underconnectivity with Frontal Areas. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. The Emotional Brain. New York: Simon & Schuster; 1996. [Google Scholar]
- Liddell BJ, Brown KJ, Kemp AH, Barton MJ, Das P, Peduto A, Gordon E, Williams LM. A direct brainstem-amygdala-cortical 'alarm' system for subliminal signals of fear. Neuroimage. 2005;24:235–243. doi: 10.1016/j.neuroimage.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Livingstone M, Hubel D. Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science. 1988;240:740–749. doi: 10.1126/science.3283936. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook E, Leventhal B, DiLavore P, Pickles A, Rutter M. The Autism Diagnostic Observation Schedule--Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism & Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Merigan WH, Maunsell JH. How parallel are the primate visual pathways? Annu Rev Neurosci. 1993;16:369–402. doi: 10.1146/annurev.ne.16.030193.002101. [DOI] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM, Chen G, McClure-Tone EB, Ernst M, Pine DS. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry. 2008;65:568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JP, Pelphrey KA, McCarthy G. Controlled scanpath variation alters fusiform face activation. 2007a:31–38. doi: 10.1093/scan/nsl023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JP, Pelphrey KA, McCarthy G. Face processing without awareness in the right fusiform gyrus. Neuropsychologia. 2007b;45:3087–3091. doi: 10.1016/j.neuropsychologia.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ. A subcortical pathway to the right amygdala mediating "unseen" fear. Proc Natl Acad Sci U S A. 1999;96:1680–1685. doi: 10.1073/pnas.96.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson J, Dawson G, Abbott R, Faja S, Webb SJ, Friedman SD, Shaw D, Artru A, Dager SR. Amygdalar volume and behavioral development in autism. Arch Gen Psychiatry. 2006;63:686–693. doi: 10.1001/archpsyc.63.6.686. [DOI] [PubMed] [Google Scholar]
- Nacewicz BM, Dalton KM, Johnstone T, Long MT, McAuliff EM, Oakes TR, Alexander AL, Davidson RJ. Amygdala volume and nonverbal social impairment in adolescent and adult males with autism. Arch Gen Psychiatry. 2006;63:1417–1428. doi: 10.1001/archpsyc.63.12.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann D, Spezio ML, Piven J, Adolphs R. Looking you in the mouth: abnormal gaze in autism resulting from impaired top-down modulation of visual attention. 2006:194–202. doi: 10.1093/scan/nsl030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterling J, Dawson G. Early recognition of children with autism: a study of first birthday home videotapes. J Autism Dev Disord. 1994;24:247–257. doi: 10.1007/BF02172225. [DOI] [PubMed] [Google Scholar]
- Osterling JA, Dawson G, Munson JA. Early recognition of 1-year-old infants with autism spectrum disorder versus mental retardation. Dev Psychopathol. 2002;14:239–251. doi: 10.1017/s0954579402002031. [DOI] [PubMed] [Google Scholar]
- Otsuka H, Harada M, Mori K, Hisaoka S, Nishitani H. Brain metabolites in the hippocampus-amygdala region and cerebellum in autism: an 1H-MR spectroscopy study. Neuroradiology. 1999;41:517–519. doi: 10.1007/s002340050795. [DOI] [PubMed] [Google Scholar]
- Page LA, Daly E, Schmitz N, Simmons A, Toal F, Deeley Q, Ambery F, McAlonan GM, Murphy KC, Murphy DGM. In Vivo 1H-Magnetic Resonance Spectroscopy Study of Amygdala-Hippocampal and Parietal Regions in Autism. 2006:2189–2192. doi: 10.1176/appi.ajp.163.12.2189. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G. Grasping the intentions of others: the perceived intentionality of an action influences activity in the superior temporal sulcus during social perception. J Cogn Neurosci. 2004;16:1706–1716. doi: 10.1162/0898929042947900. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G, Labar KS. Perception of dynamic changes in facial affect and identity in autism. Soc Cogn Affect Neurosci. 2007;2:140–149. doi: 10.1093/scan/nsm010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, S J, LG, U Visual awareness and the detection of fearful faces. Emotion. 2005;5(243–247) doi: 10.1037/1528-3542.5.2.243. [DOI] [PubMed] [Google Scholar]
- Pierce K, Haist F, Sedaghat F, Courchesne E. The brain response to personally familiar faces in autism: findings of fusiform activity and beyond. Brain. 2004;127:2703–2716. doi: 10.1093/brain/awh289. [DOI] [PubMed] [Google Scholar]
- Pierce K, Muller RA, Ambrose J, Allen G, Courchesne E. Face processing occurs outside the fusiform 'face area' in autism: evidence from functional MRI. Brain. 2001;124:2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- Pierce K, Redcay E. Fusiform function in children with an autism spectrum disorder is a matter of "who". Biol Psychiatry. 2008;64:552–560. doi: 10.1016/j.biopsych.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggot J, Kwon H, Mobbs D, Blasey C, Lotspeich L, Menon V, Bookheimer S, Reiss AL. Emotional attribution in high-functioning individuals with autistic spectrum disorder: a functional imaging study. J Am Acad Child Adolesc Psychiatry. 2004;43:473–480. doi: 10.1097/00004583-200404000-00014. [DOI] [PubMed] [Google Scholar]
- Pinkham AE, Hopfinger JB, Pelphrey KA, Piven J, Penn DL. Neural bases for impaired social cognition in schizophrenia and autism spectrum disorders. Schizophr Res. 2008;99:164–175. doi: 10.1016/j.schres.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourtois G, Thut G, Grave de Peralta R, Michel C, Vuilleumier P. Two electrophysiological stages of spatial orienting towards fearful faces: early temporo-parietal activation preceding gain control in extrastriate visual cortex. Neuroimage. 2005;26:149–163. doi: 10.1016/j.neuroimage.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Schultz RT. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int J Dev Neurosci. 2005;23:125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, Skudlarski P, Lacadie C, Cohen DJ, Gore JC. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome. Arch Gen Psychiatry. 2000;57:331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Amaral DG. Stereological analysis of amygdala neuron number in autism. J Neurosci. 2006;26:7674–7679. doi: 10.1523/JNEUROSCI.1285-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH, Lammers CR, Reiss AL, Amaral DG. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J Neurosci. 2004;24:6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN, Wise SP. Neurodevelopmental Trajectories of the Human Cerebral Cortex. 2008:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Simion F, Leo I, Turati C, Valenza E, Dalla Barba B. How face specialization emerges in the first months of life. Prog Brain Res. 2007;164:169–185. doi: 10.1016/S0079-6123(07)64009-6. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks BF, Friedman SD, Shaw DW, Aylward EH, Echelard D, Artru AA, Maravilla KR, Giedd JN, Munson J, Dawson G, Dager SR. Brain structural abnormalities in young children with autism spectrum disorder. Neurology. 2002;59:184–192. doi: 10.1212/wnl.59.2.184. [DOI] [PubMed] [Google Scholar]
- Spezio ML, Adolphs R, Hurley RS, Piven J. Analysis of face gaze in autism using "Bubbles". Neuropsychologia. 2007;45:144–151. doi: 10.1016/j.neuropsychologia.2006.04.027. [DOI] [PubMed] [Google Scholar]
- Sterling L, Dawson G, Webb S, Murias M, Munson J, Panagiotides H, Aylward E. The Role of Face Familiarity in Eye Tracking of Faces by Individuals with Autism Spectrum Disorders. J Autism Dev Disord. 2008 doi: 10.1007/s10803-008-0550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit M, Dammers J, Simsek-Kraues S, Brinkmeyer J, Wolwer W, Ioannides A. Time course of regional brain activations during facial emotion recognition in humans. Neurosci Lett. 2003;342:101–104. doi: 10.1016/s0304-3940(03)00274-x. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers, Inc.; 1988. [Google Scholar]
- Tantam D, Monaghan L, Nicholson H, Stirling J. Autistic children's ability to interpret faces: a research note. J Child Psychol Psychiatry. 1989;30:623–630. doi: 10.1111/j.1469-7610.1989.tb00274.x. [DOI] [PubMed] [Google Scholar]
- Tsatsanis KD, Rourke BP, Klin A, Volkmar FR, Cicchetti D, Schultz RT. Reduced thalamic volume in high-functioning individuals with autism. Biol Psychiatry. 2003;53:121–129. doi: 10.1016/s0006-3223(02)01530-5. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Distinct spatial frequency sensitivities for processing faces and emotional expressions. Nat Neurosci. 2003;6:624–631. doi: 10.1038/nn1057. [DOI] [PubMed] [Google Scholar]
- Waiter GD, Williams JH, Murray AD, Gilchrist A, Perrett DI, Whiten A. A voxel-based investigation of brain structure in male adolescents with autistic spectrum disorder. Neuroimage. 2004;22:619–625. doi: 10.1016/j.neuroimage.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Wang AT, Dapretto M, Hariri AR, Sigman M, Bookheimer SY. Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 2004;43:481–490. doi: 10.1097/00004583-200404000-00015. [DOI] [PubMed] [Google Scholar]
- Wicker B, Fonlupt P, Hubert B, Tardif C, Gepner B, Deruelle C. Abnormal cerebral effective connectivity during explicit emotional processing in adults with autism spectrum disorder. 2008:135–143. doi: 10.1093/scan/nsn007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.