Abstract
Spinal Muscular Atrophy (SMA) is the leading genetic cause of infantile death and caused by the loss of functional Survival Motor Neuron 1(SMN1). The remaining copy gene, SMN2, is unable to rescue from disease because the primary gene product lacks the final coding exon, exon 7, due to an alternative splicing event. While SMNΔ7 is a rapidly degraded protein, exon 7 is not specifically required in a sequence-specific manner to confer increased functionality to this truncated protein. Based upon this molecular observation, aminoglycosides have been examined to artificially elongate the C-terminus of SMNΔ7 by “read-through” of the stop codon. An SMNΔ7 read-through event benefits intermediate mouse models of SMA. Here we demonstrate that delivery of a read-through inducing compound directly to the CNS can partially lessen the severity of a severe model of SMA (Smn−/−; SMN2+/+), albeit not to the extent seen in the less severe model. This further demonstrates the utility of read-through inducing compounds in SMA.
Keywords: Spinal Muscular Atrophy (SMA), Survival Motor Neuron (SMN), read-through, aminoglycoside
1. Introduction
Spinal Muscular Atrophy (SMA) is the number one autosomal recessive cause of infantile death. This disease has a carrier frequency of 1:35, affecting every one in 6,000 live births [6]. SMA is caused by the lack of a functional copy of the gene Survival Motor Neuron 1 (SMN1) [10]. In humans, there is a nearly identical copy gene to SMN1, termed SMN2 [22]. While this gene rescues embryonic lethality, it does not fully compensate for the loss of SMN1 due to an alternative splicing event [12, 19]. This alternative splicing event is caused by a C to T transition within exon 7, causing the majority of SMN2 transcripts to exclude this exon (SMNΔ7), compared to SMN1 which exclusively produces a full-length transcript [12, 19]. SMNΔ7 protein is highly unstable and is degraded at least two-fold faster than full-length SMN [2, 11]. The loss of full-length SMN protein causes the death of the α–motor neurons and the subsequent atrophy of the voluntary muscle groups. It is currently unknown why motor neurons are particularly susceptible to the loss of this ubiquitously expressed protein, however, two not necessarily mutually exclusive hypotheses suggest that snRNP biogenesis and/or axonal RNA transport underlie the SMA-associated activity [4, 26, 27, 29].
Drugs such as Indoprofen [13], Aminoglycosides [25] and proteosome inhibitors [2], were examined in SMA as a means to stabilize SMNΔ7 protein. SMN exon 7 is clearly important since its absence leads to SMA development. Within SMN exon 7 there is a cytoplasmic localization signal that can readily transport SMN exons 1–6 to the cytoplasm [28]. Interestingly, however, heterologous sequences can seemingly compensate for this signal in vitro in regards to SMN protein localization and can partially restore functionality in several cell-based assays [7, 9, 15, 25]. Knowing that heterologous sequences can at least partially restore functionality to SMN 7 protein aminoglycosides have been examined in SMA. The mode of action for these compounds was presumed to inhibit the recognition of the normal SMNΔ7 translational stop codon, thereby allowing the incorporation of an extended and a more functional C-terminus [7, 9, 15, 25]. Translational read-through of SMNΔ7 would result in the elongation of the truncated protein by an additional 5 amino acids. Using a SMNΔ7 C-terminal read-through assay it was demonstrated that these compounds could indeed promote a read-through of the exon 8 stop codon[7, 15]. Treatments using FDA-approved aminoglycosides and derivative structures have increased SMN protein in Type I primary SMA patient fibroblasts (3813s) in culture, as shown both by gem numbers and Western blotting [7, 18, 25]. Aminoglycoside treatments of an SMA mouse model have shown some benefits, albeit not on lifespan, by inter-peritoneal and subcutaneous administration [7, 17]. However, we have previously demonstrated that a novel aminoglycoside (TC007) can increase SMN protein in 3813s in vitro by stabilizing the SMN protein [18] and significantly lessens the severity of a moderate SMA animal model when administered directly to the central nervous system (CNS) [16]. CNS-delivery of TC007 to the Smn−/−; SMN2+/+; SMNΔ7 model significantly increased lifespan and gross-motor function. This was due to significantly increased SMN protein in the brain and spinal cord after administration, leading to an increased survival of the motor neurons even at end-stage of disease. The increased benefit of direct CNS administration of TC007, compared to subcutaneous, is logical as it is unknown how readily TC007 can pass the blood-brain-barrier. Since SMA presents in different severities, it is extremely important to investigate potential therapeutic compounds in various animal model that reflect the different types of SMA. This then may relate to a compound’s efficacy in the varying SMA patient population. Therefore, we here examine the utility of direct CNS delivery of TC007 to a more severe model of SMA as a means of investigating the use of therapeutics in this model as well as expanding the potential application for aminoglycosides in SMA models.
2. Materials and methods
2.1 Animals and drug treatment
All animal experiments were carried out in accordance with protocols approved by the Animal Care and Use Committee of the University of Missouri. SMA model mice were used that were lacking both copies of the murine Smn, and contained a human SMN2 transgene [21] (Smn−/−; SMN2+/+) on a FVB/N background (Jackson labs). Mice were genotyped according to Jackson labs and litters were excluded based upon pup number to control for variation induced by amount of care by the mother mouse (litters smaller than 3 pups or more than 8). TC007 was initially resuspended in dH20, further diluted in PBS, and administered by intracerebral ventricular injection (ICV) (1 μl/gram of body weight) on post-natal days 1 and 3 (N=17). PBS(vehicle N=13) was injected as a negative control. ICV injections were performed as previously described [5, 23]. Briefly described, the mice are anesthetized using hypothermia before being bilaterally injected in the intracerebral ventricles using a pulled-glass needle through the skull. The pups are then warmed on a heating-pad and re-scented with nest material before being returned to their cage. To assess gross motor function, righting reflex (as described in [3]) was measured starting at post-natal day 5. Briefly described, every day each pup was placed on its back and timed while given a maximum of 60 seconds to place all four paws on the ground stably.
2.2 Statistical analyses
Analysis was performed as previously described [24]. Briefly, error bars on graphs represent Standard Error of the Mean (S.E.M.). Significance of lifespan between TC007- and vehicle-treated mice determined by Mantel-Cox test. All other significance indicated was calculated by Student’s T Test of p<0.05 or greater and is indicated in the figure legends.
3. Results
3.1 Severe SMA mice benefit from TC007 ICV injections:no significant increase in lifespan
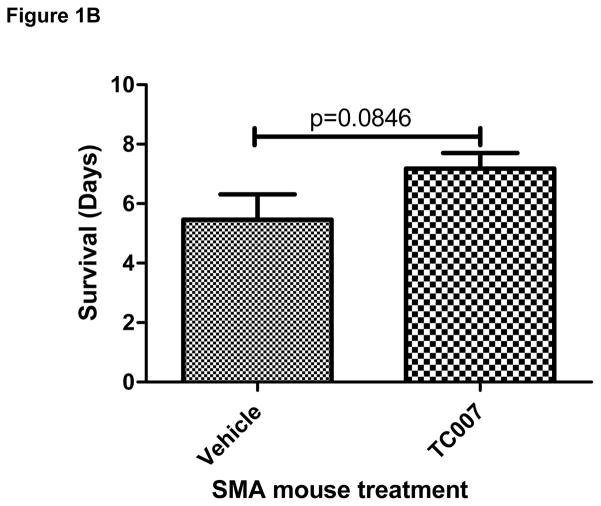
It has been recently demonstrated in an “intermediate” mouse model of SMA (Smn−/−; SMN2+/+; SMNΔ7) that G418 can increase gross-motor function presumably by promoting SMN read-through [7]. Direct CNS administration of the novel aminoglycoside TC007 to a moderate-severity SMA model mouse not only can increase gross-motor function, but also extend lifespan by increasing endogenous SMN levels [16]. To examine the utility of this therapeutic strategy in a more severe model of disease, we examined the effects of TC007 in the more severe model of SMA in which mice live ~5 days and are significantly smaller and weaker than their unaffectedlittermates [20]. These mice lack mouse Smn, but have two copies of human SMN2 transgene (Smn−/−; SMN2+/+) [20]. SMA animals were given [30 mg/kg] TC007 via intracerebral ventricular (ICV) injections, or PBS as vehicle-treated, on post-natal days (p) 1 and 3. TC007-treated mice (n=17) lived on average ~31% longer (from 5.462 ± 0.8520 to 7.176 ± 0.5302 days) than vehicle-treated mice (n=13) (Fig. 1). While thismean increase in lifespan is statistically insignificant, it does appearthat the compound may assist in prevention of the earliest deaths, ratherthan an overall extension of life. However, since the overall lifespan differences showed no significance (p=0.1554), it is possible thatbecause this model initiates disease progression extremely early it is less receptive to therapeutic intervention.
Fig. 1.
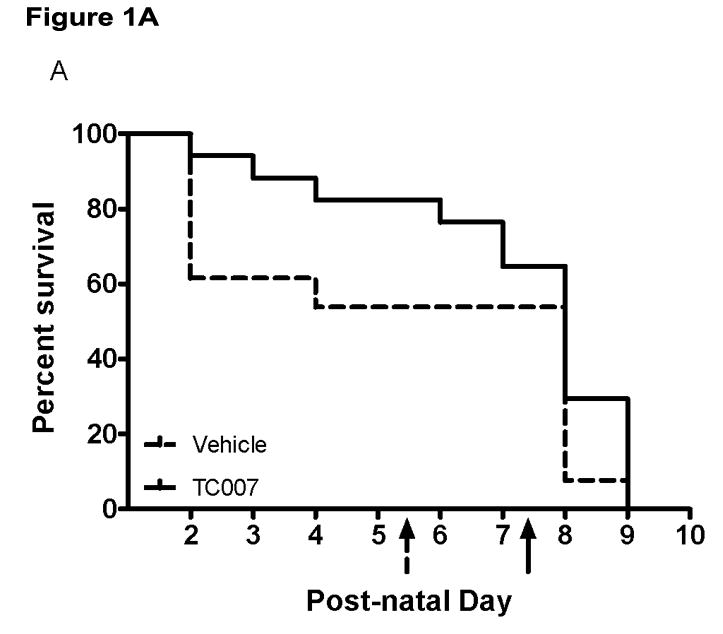
Lifespan of severe SMA mice with CNS administration of TC007 at p1 and p3 not significantly increased. (A) Kaplan-Meyer survival curve shown with TC007-treated SMA mice (solid black), and vehicle-treated SMA mice (dotted black). Arrows on X-axis indicates average lifespan of the SMA mice. (Mantel-Cox test p=0.1554; Vehicle-treated N=13, TC007-treated N=17) (B) Average lifespan of TC007-treated versus vehicle-treated SMA pups (Student’s T Test p=0.0846 and bars represent SEM).
3.2 Severe SMA mice benefit from TC007 ICV injections:no significant increase in gross motor function but significant increase in body weight
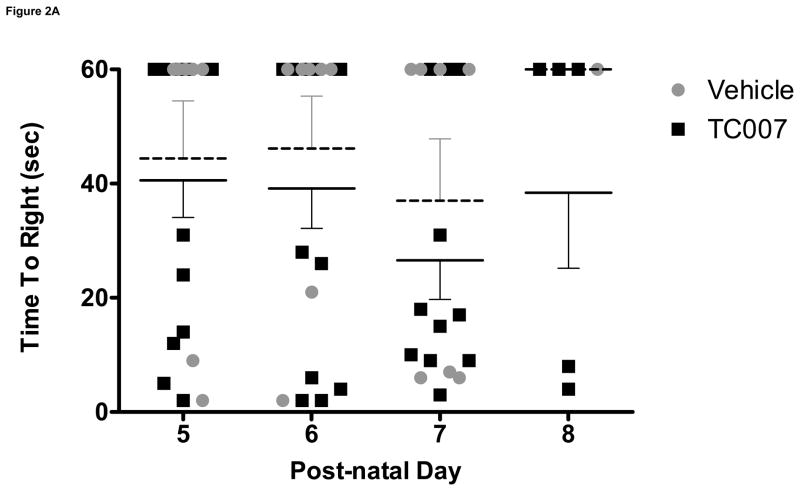
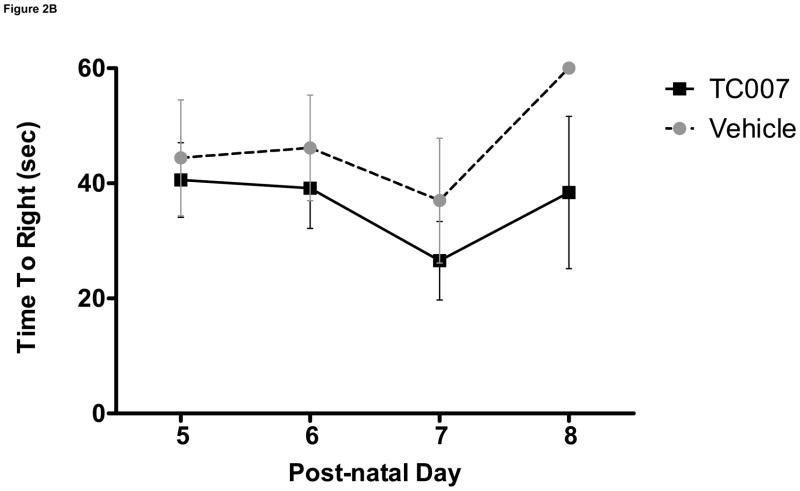
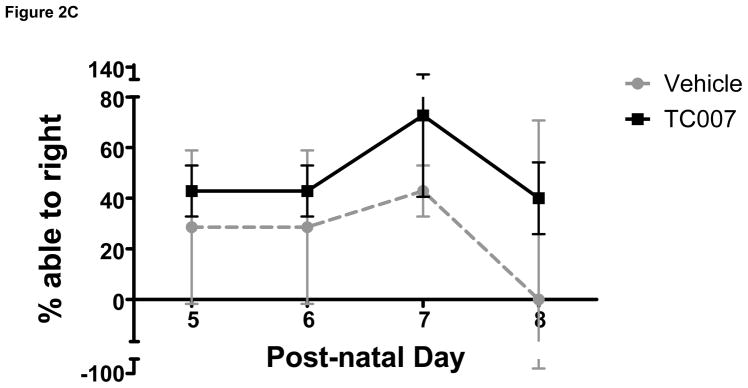
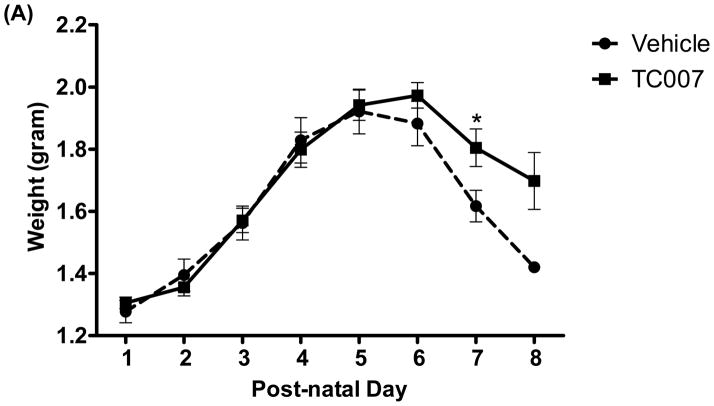
To determine whether quality-of-life measures are impacted following TC007 delivery, the pups were examined from p5 until death in terms of gross motor function. “Time to right” (TTR) is the time it takes for a mouse to right itself onto its four legs from its back has been widely used as a test for gross motor function in SMA mice. On average, TC007-treated pups had no significant difference in motor function than vehicle-treated pups, as determined by TTR (Fig. 2). While more TC007-treated pups right on average (Fig. 2C), and they also were able to right faster (Fig. 2B), this difference did not reach significance. As an additional measure of total quality of life, the weights of TC007- versus vehicle-treated mice were collected daily and plotted (Fig. 3). TC007-treated mice did however weigh significantly more at end-stages of disease (Fig. 3). This could be due to the small increase in gross motor function, seen by the TTR testing, denoting the ability of these severe mice to feed better.
Fig. 2.
Gross-motor function of severe SMA TC007-treated mice not significantly increased as compared to vehicle-treated (Student’s T Test). (A) Each grey circle (vehicle) or black square (TC007) represents an individual mouse plotted per day, post-natal days 5 through 8 (N for each day TC007: 14, 14, 11, 5 and vehicle: 7, 7, 7, 1 respectively). Lines represent the average of each group (TC007: black bar, vehicle: grey dotted bar) and bars represent SEM. (B) TC007-treated mice (line) on average not significantly different than vehicle-treated (dotted) littermates. Error bars represent SEM. (C) TC007-treated (black line) mice are not significantly different in ability to right on average than vehicle-treated (grey dotted). Bars represent Standard Deviation.
Fig. 3.
TC007-treated pups weigh significantly more at end-stages. The weights of the TC007-treated pups (squares) were significantly larger (as indicated on graph) at end-stages than vehicle-treated (circles). Asterisk represents (*) p=0.0460 by Student’s T Test and error bars represent SEM.
4. Discussion and conclusion
It has been shown that SMNΔ7 protein has severely reduced functionality when compared to full-length SMN [1, 15], assumedly due mostly to its instability [2] and inability to interact with members of the Gemin complex [2], known to be crucial in SMN’s most well-studied role in UsnRNP biogenesis. Therefore, it is completely logical that if SMNΔ7 could regain its stability to self-associate and associate with its complex, it would regain its functionality. This thought is the basis of this study. Since SMNΔ7 is the primary product of SMN2, and therefore an ideal target for therapeutic intervention, we postulated that if aminoglycosides could induce a read-through event of the stop-codon. This would thereby rescuing stability of the protein by adding an artificial C-terminus which would restore functionality to the protein and rescue from disease. We have shown just this in vitro, that a read-through even can denote stability and functionality to the SMNΔ7 protein [15]. Additionally, we have previously shown in a moderate-severity SMA model that TC007 administered to the CNS increases total SMN protein without altering total SMN RNA levels or exon 7 inclusion [16]. This would indicate that the elevated protein is due to an increased stability of the protein. The increased SMN protein lead to an increased motor neuron count, lifespan and gross motor function of the animals [16].
In conclusion, SMNΔ7 read-through therapy in the severe SMA mouse model results in only modest benefits, compared to the less severe Δ7 SMA model [16]. TC007 treatment results in an insignificant, but trending towards a slight increase in gross motor function leads to a significant increase in the weight of the pups. While we did not examine SMN protein levels after ICV injections of TC007 in this model, we assume, based upon previous data seen in the less severe model [16], that any benefit seen is due to an increase in SMN. Importantly, direct delivery of TC007 did not appear to result in general toxicity, although this it is difficult to draw extensive conclusions based upon the relatively limited exposure to the compound. This model may still be amenable for therapeutic analysis, however, it may require more potent SMN-inducing compounds. Additionally, the less severe model has not only a hSMN2transgene, but also has a cDNA SMNΔ7 transgene. Therefore, in terms of aread -through therapy this cDNA presents a very strong target for intervention. The lack of this additional transgene in this model may account for the lessened response to the therapy. Alternatively, it has been shown in the less severe Δ7 SMA model that there exists a limited therapeutic window’, where the ability to rescue from disease decreases as severity of the phenotype increases [14]. Therefore, in this model disease progression may have already proceeded to a stage that makes therapeutic intervention intractable in neonates and therefore, even gene replacement strategies may not rescue the severe SMA phenotype.
Furthermore, in this study we directly administered TC007 directly to the CNS via an ICV injection. This was in effort to avoid the blood-brain-barrier (BBB) and exact the treatment directly to where it could be taken up by the motor neurons, as this compound has not been shown to cross the BBB. It has since been demonstrated that systemic administration of therapeutics has additional benefits to ICV injections alone [8]. Therefore, a drug which retains the capabilities to induce a read-through event, with the combined ability to cross the BBB, might be the ideal therapeutic for SMA.
Highlights.
SMNΔ7 stop-codon read-through stabilizes the protein and increases functionality
Aminoglycosides can be used to induce read-through events in SMNΔ7
CNS-administration of aminoglycosides slightly benefits a severe SMA mouse model
Acknowledgments
Role of funding sources
The funding sources had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
We would like to thank John Marston for assistance with the mouse colony. This work was supported by grants from FightSMA [C.L.L.] and [C.W.C., AI053138].
Abbreviations
- SMA
Spinal Muscular Atrophy
- SMN
Survival Motor Neuron
- CNS
Central Nervous System
- ICV
intracerebral ventricular injection
- BBB
blood-brain-barrier
Footnotes
Disclosure Statement
The authors declare that they have no conflicts of interest. V.B. Mattis conducted animal behavioral testing, managed literature search, undertook statistical analysis and data interpretation, and co-wrote the manuscript. C.W. Chang developed and manufactured the novel aminoglycoside TC007. C.L. Lorson supervised the study design and interpretation of data, and co-wrote the manuscript. All authors contributed to and have approved the final article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bowerman M, Shafey D, Kothary R. Smn depletion alters profilin II expression and leads to upregulation of the RhoA/ROCK pathway and defects in neuronal integrity. J Mol Neurosci. 2007;32:120–131. doi: 10.1007/s12031-007-0024-5. [DOI] [PubMed] [Google Scholar]
- 2.Burnett BG, Munoz E, Tandon A, Kwon DY, Sumner CJ, Fischbeck KH. Regulation of SMN protein stability. Mol Cell Biol. 2009;29:1107–1115. doi: 10.1128/MCB.01262-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butchbach ME, Edwards JD, Burghes AH. Abnormal motor phenotype in the SMNDelta7 mouse model of spinal muscular atrophy. Neurobiol Dis. 2007;27:207–219. doi: 10.1016/j.nbd.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carrel TL, McWhorter ML, Workman E, Zhang H, Wolstencroft EC, Lorson C, Bassell GJ, Burghes AH, Beattie CE. Survival motor neuron function in motor axons is independent of functions required for small nuclear ribonucleoprotein biogenesis. J Neurosci. 2006;26:11014–11022. doi: 10.1523/JNEUROSCI.1637-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coady TH, Baughan TD, Shababi M, Passini MA, Lorson CL. Development of a single vector system that enhances trans-splicing of SMN2 transcripts. PLoS ONE. 2008;3:e3468. doi: 10.1371/journal.pone.0003468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldkotter M, Schwarzer V, Wirth R, Wienker TF, Wirth B. Quantitative analyses of SMN1 and SMN2 based on real-time lightCycler PCR: fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. Am J Hum Genet. 2002;70:358–368. doi: 10.1086/338627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heier CR, DiDonato CJ. Translational readthrough by the aminoglycoside geneticin (G418) modulates SMN stability in vitro and improves motor function in SMA mice in vivo. Hum Mol Genet. 2009;18:1310–1322. doi: 10.1093/hmg/ddp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hua Y, Sahashi K, Rigo F, Hung G, Horev G, Bennett CF, Krainer AR. Peripheral SMN restoration is essential for long-term rescue of a severe spinal muscular atrophy mouse model. Nature. 2011;478:123–126. doi: 10.1038/nature10485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hua Y, Zhou J. Modulation of SMN nuclear foci and cytoplasmic localization by its C-terminus. Cell Mol Life Sci. 2004;61:2658–2663. doi: 10.1007/s00018-004-4300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lefebvre S, Burglen L, Reboullet S, Clermont O, Burlet P, Viollet L, Benichou B, Cruaud C, Millasseau P, Zeviani M, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–165. doi: 10.1016/0092-8674(95)90460-3. [DOI] [PubMed] [Google Scholar]
- 11.Lorson CL, Androphy EJ. An exonic enhancer is required for inclusion of an essential exon in the SMA-determining gene SMN. Hum Mol Genet. 2000;9:259–265. doi: 10.1093/hmg/9.2.259. [DOI] [PubMed] [Google Scholar]
- 12.Lorson CL, Hahnen E, Androphy EJ, Wirth B. A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc Natl Acad Sci U S A. 1999;96:6307–6311. doi: 10.1073/pnas.96.11.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lunn MR, Root DE, Martino AM, Flaherty SP, Kelley BP, Coovert DD, Burghes AH, Man NT, Morris GE, Zhou J, Androphy EJ, Sumner CJ, Stockwell BR. Indoprofen upregulates the survival motor neuron protein through a cyclooxygenase-independent mechanism. Chem Biol. 2004;11:1489–1493. doi: 10.1016/j.chembiol.2004.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lutz CM, Kariya S, Patruni S, Osborne MA, Liu D, Henderson CE, Li DK, Pellizzoni L, Rojas J, Valenzuela DM, Murphy AJ, Winberg ML, Monani UR. Postsymptomatic restoration of SMN rescues the disease phenotype in a mouse model of severe spinal muscular atrophy. J Clin Invest. 2011;121:3029–3041. doi: 10.1172/JCI57291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mattis VB, Bowerman M, Kothary R, Lorson CL. A SMNDelta7 read-through product confers functionality to the SMNDelta7 protein. Neurosci Lett. 2008;442:54–58. doi: 10.1016/j.neulet.2008.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattis VB, Ebert AD, Fosso MY, Chang CW, Lorson CL. Delivery of a read-through inducing compound, TC007, lessens the severity of a spinal muscular atrophy animal model. Hum Mol Genet. 2009;18:3906–3913. doi: 10.1093/hmg/ddp333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mattis VB, Fosso MY, Chang CW, Lorson CL. Subcutaneous administration of TC007 reduces disease severity in an animal model of SMA. BMC Neurosci. 2009;10:142. doi: 10.1186/1471-2202-10-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mattis VB, Rai R, Wang J, Chang CW, Coady T, Lorson CL. Novel aminoglycosides increase SMN levels in spinal muscular atrophy fibroblasts. Hum Genet. 2006;120:589–601. doi: 10.1007/s00439-006-0245-7. [DOI] [PubMed] [Google Scholar]
- 19.Monani UR, Lorson CL, Parsons DW, Prior TW, Androphy EJ, Burghes AH, McPherson JD. A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Hum Mol Genet. 1999;8:1177–1183. doi: 10.1093/hmg/8.7.1177. [DOI] [PubMed] [Google Scholar]
- 20.Monani UR, Sendtner M, Coovert DD, Parsons DW, Andreassi C, Le TT, Jablonka S, Schrank B, Rossol W, Prior TW, Morris GE, Burghes AH. The human centromeric survival motor neuron gene (SMN2) rescues embryonic lethality in Smn(−/−) mice and results in a mouse with spinal muscular atrophy. Hum Mol Genet. 2000;9:333–339. doi: 10.1093/hmg/9.3.333. [DOI] [PubMed] [Google Scholar]
- 21.Monani UR, Sendtner M, Coovert DD, Parsons DW, Andreassi C, Le TT, Jablonka S, Schrank B, Rossoll W, Prior TW, Morris GE, Burghes AH. The human centromeric survival motor neuron gene (SMN2) rescues embryonic lethality in Smn(−/−) mice and results in a mouse with spinal muscular atrophy. Hum Mol Genet. 2000;9:333–339. doi: 10.1093/hmg/9.3.333. [DOI] [PubMed] [Google Scholar]
- 22.Parsons DW, McAndrew PE, Iannaccone ST, Mendell JR, Burghes AH, Prior TW. Intragenic telSMN mutations: frequency, distribution, evidence of a founder effect, and modification of the spinal muscular atrophy phenotype by cenSMN copy number. Am J Hum Genet. 1998;63:1712–1723. doi: 10.1086/302160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Passini MA, Dodge JC, Bu J, Yang W, Zhao Q, Sondhi D, Hackett NR, Kaminsky SM, Mao Q, Shihabuddin LS, Cheng SH, Sleat DE, Stewart GR, Davidson BL, Lobel P, Crystal RG. Intracranial delivery of CLN2 reduces brain pathology in a mouse model of classical late infantile neuronal ceroid lipofuscinosis. J Neurosci. 2006;26:1334–1342. doi: 10.1523/JNEUROSCI.2676-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rose FF, Jr, Mattis VB, Rindt H, Lorson CL. Delivery of recombinant follistatin lessens disease severity in a mouse model of Spinal Muscular Atrophy. Hum Mol Genet. 2008 doi: 10.1093/hmg/ddn426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolstencroft EC, Mattis V, Bajer AA, Young PJ, Lorson CL. A non-sequence-specific requirement for SMN protein activity: the role of aminoglycosides in inducing elevated SMN protein levels. Hum Mol Genet. 2005;14:1199–1210. doi: 10.1093/hmg/ddi131. [DOI] [PubMed] [Google Scholar]
- 26.Workman E, Saieva L, Carrel TL, Crawford TO, Liu D, Lutz C, Beattie CE, Pellizzoni L, Burghes AH. A SMN missense mutation complements SMN2 restoring snRNPs and rescuing SMA mice. Hum Mol Genet. 2009 doi: 10.1093/hmg/ddp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H, Xing L, Rossoll W, Wichterle H, Singer RH, Bassell GJ. Multiprotein complexes of the survival of motor neuron protein SMN with Gemins traffic to neuronal processes and growth cones of motor neurons. J Neurosci. 2006;26:8622–8632. doi: 10.1523/JNEUROSCI.3967-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang HL, Pan F, Hong D, Shenoy SM, Singer RH, Bassell GJ. Active transport of the survival motor neuron protein and the role of exon-7 in cytoplasmic localization. J Neurosci. 2003;23:6627–6637. doi: 10.1523/JNEUROSCI.23-16-06627.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Z, Lotti F, Dittmar K, Younis I, Wan L, Kasim M, Dreyfuss G. SMN deficiency causes tissue-specific perturbations in the repertoire of snRNAs and widespread defects in splicing. Cell. 2008;133:585–600. doi: 10.1016/j.cell.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]