Abstract
Proglucagon, which is encoded by the glucagon gene (Gcg), is the precursor of several peptide hormones, including glucagon and glucagon-like peptide 1 (GLP-1). Whereas glucagon stimulates hepatic glycogenolysis and gluconeogenesis, GLP-1 stimulates insulin secretion to lower blood glucose and also supports ß-cell proliferation and protection from apoptotic stimuli. Pregnancy is a strong inducer of change in islet function, however the roles of proglucagon-derived peptides in pregnancy are only partially understood. In the present study, we analyzed fertility and pregnancy-associated changes in homozygous glucagon-green fluorescent protein (gfp) knock-in mice (Gcggfp/gfp), which lack all the peptides derived from proglucagon. Female Gcggfp/gfp mice could deliver and raise Gcggfp/gfp pups to weaning and Gcggfp/gfp pups from Gcggfp/gfp dams were viable and fertile. Pregnancy induced ß-cell proliferation in Gcggfp/gfp mice as well as in control mice. However, serum insulin levels in pregnant Gcggfp/gfp females were lower than those in control pregnant females under ad libitum feeding, and blood glucose levels in pregnant Gcggfp/gfp females were higher after gestational day 12. Gcggfp/gfp females showed a decreased pregnancy rate and smaller litter size. The rate of successful breeding was significantly lower in Gcggfp/gfp females and was not improved by experience of breeding. Taken together, proglucagon-derived peptides are not required for pregnancy-associated ß-cell proliferation, however, are required for regulation of blood glucose levels and normal reproductive capacity. Gcggfp/gfp mice may serve as a novel model to analyze the effect of mild hyperglycemia during late gestational periods.
Introduction
Pregnancy is one of the strongest physiological stimuli that induce structural and functional changes in pancreatic islet ß-cells. Both ß-cell mass and insulin secretion are increased and the threshold for glucose-stimulated insulin secretion is decreased during pregnancy [1], [2], [3], [4], and insufficiency in such responses can lead to the development of gestational diabetes mellitus (GDM) [5], [6], [7]. Therefore, pathogenesis of type 2 diabetes mellitus (DM) and that of GDM overlaps, and the women who have a history of GDM are under high risk of developing type 2 DM in later life [7], [8].
The molecular mechanisms that are involved in altered ß-cell function during pregnancy are not fully understood. Previous studies have shown that prolactin (PRL), placental lactogens (PLs), and serotonin play important roles in the pregnancy-associated changes in ß-cell mass and function in rodents [4], [9], [10], [11]. On the other hand, involvement of glucagon-like peptide-1 (GLP-1) in the pregnancy-associated changes in ß-cell mass and function has not been investigated, although it is well established that GLP-1 supports β-cell proliferation and inhibits apoptosis of these cells [12], [13], [14], [15], [16].
Tissue-specific posttranslational processing of proglucagon produces multiple peptides that harbor apparently counteracting functions. Glucagon is produced in pancreatic islet α-cells through the cleavage of proglucagon by prohormone convertase 2 (Pcsk2) and it stimulates hepatic glycogenolysis and gluconeogenesis to increase blood glucose levels. GLP-1 is produced in intestinal L-cells through the cleavage of proglucagon by prohormone convertase 1/3 (Pcsk1) and is released in response to nutrient ingestion. Compared with glucagon and GLP-1, the physiological roles of other proglucagon-derived peptides including GLP-2, glicentin and oxyntomodulin are far less understood.
We have recently established a mouse model, in which the glucagon gene is disrupted by introduction of the cDNA for green fluorescentr protein (GFP; Gcggfp/+). Homozygous Gcggfp/gfp mice are born in the expected Mendelian ratio and appear grossly normal, despite the absence of proglucagon-derived peptides including glucagon and GLP-1 [16]. Interestingly, the blood glucose levels in adult Gcggfp/gfp mice are not significantly different from those in the control mice. Therefore, the Gcggfp/gfp mice make contrasts with two animal models defective in glucagon production or action, Pcsk2−/− and glucagon receptor-deficient (Gcgr−/−) mice, both of which display lower blood glucose levels and markedly elevated serum GLP-1 concentrations.
In the present study, we analyzed the fertility of Gcggfp/gfp mice to clarify the roles of proglucagon-derived peptides in pregnancy and pregnancy-associated changes in ß-cell mass and function.
Materials and Methods
Animals and Experimental Set up
Heterozygous Gcg-EGFP knock-in mice (Gcggfp/+) were generated as described previously [16]. In brief, the murine glucagon gene was disrupted by replacing the region of the glucagon gene that spans from exon 2 to exon 5 with GFP cDNA, a polyadenylation signal, and an expression cassette for neomycin resistance. Gcggfp/+ mice were backcrossed into the C57/BL6J background for more than 10 generations. Gcggfp/+ female mice were bred with Gcggfp/+ males to generate homozygous (Gcggfp/gfp) offspring. All mice were housed in specific pathogen-free barrier facilities at the Research Institute of Environmental Medicine, Nagoya University, and maintained on a 12-h light, 12-h dark cycle and constant temperature (23°C) with free access to certified chow (Lab Animal Diet MF; Oriental Yeast Co. Ltd., Tokyo, Japan) and distilled water. All procedures were performed in accordance with a protocol approved by the Nagoya University Institutional Animal Care and Use Committee. To obtain pregnant females, female mice aged 3–4 months were housed with male mice in all combinations of genotypes. The presence of a mucous vaginal plug indicated that mating had occurred during the previous night, and the time point was designated as gestational day (G) 0.5 ( = embryonic day (E) 0.5).
Blood Glucose and Serum Insulin Levels
Blood samples were obtained via tail bleeding and from severed neck vessels in adult female mice and neonates respectively. Fasting blood samples were collected following overnight fasting (16–18 hours). Glucose levels were determined using a Medisafe glucose meter (TERUMO, Tokyo, Japan). Serum insulin levels ware measured in duplicate using the High Sensitivity PLUS insulin kit (Morinaga-Seikagaku Co. Ltd., Yokohama, Japan) in accordance with the manufacturer’s instructions.
Oral Glucose Tolerance Test (OGTT)
After a 16-h period of fasting, a glucose load of 2 g/kg was administered to conscious mice orally. Blood samples were collected at 0, 15, 30, 60, 90, and 120 minutes after the administration of glucose.
Pancreatic Insulin Content
Mice were sacrificed by cervical dislocation and the entire pancreas was dissected free from other tissue immediately. Following the measurement of the weight, the pancreas was homogenized in 8 ml of acid ethanol (70% ethanol, 1.5%HCl). After overnight incubation at −20°C, the suspension was centrifuged at 2000 rpm for 15 minutes at 4°C. The supernatant was collected and neutralized. The extract was analyzed for insulin content [17].
Immunohistochemistry and Morphometry
Adult female mice were perfused intracardially with 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) and post-fixed for 4 hours in the same fixative. Embryos and pups were killed by decapitation, and the pancreases were dissected and fixed for 4 hours in the same fixative saline. Fixed tissues were cryoprotected with graded sucrose (10∼30%) in PBS followed by immersion into OCT compound (Sakura Co. Ltd., Tokyo, Japan) and were then frozen in liquid N2. Twenty micrometer sections were prepared using a Microm HM500 OM Microtome Cryostat (Carl Zeiss Japan, Tokyo, Japan).
The following primary antibodies were used: guinea pig polyclonal anti-insulin antibody (diluted to 1∶300; Abcam plc., Cambridge, UK), rabbit polyclonal anti-glucagon antibody (1∶200; Abcam, plc., Cambridge, UK), and rabbit polyclonal anti-serotonin antibody (1∶500; Incstar, Stillwater, Minn., USA). After incubation with primary antibodies at 4°C overnight, the sections were incubated with Cy3-, Alexa 568-, or Alexa 633-labeled species-specific anti-IgG antibodies (diluted to 1∶250∼500; Life Technologies Japan, Tokyo, Japan) for 1 h. The immunostained sections were examined with a Zeiss LSM 710 confocal laser-scanning microscope (Carl Zeiss Microscopy, Tokyo, Japan).
Quantitative analyses of ß-cell mass were performed on three to four sections of each pancreas at 200-µm intervals. Each section was scanned using a NanoZoomer 2.0 RS whole slide scanner (Hamamatsu Photonics, Hamamatsu City, Japan) to generate high resolution images at 20× magnification. Images were analyzed with Image-Pro Plus 6.1 software (Media Cybernetics, Silver Springs, MD) to measure the areas of insulin-positive cells as well as the total area of pancreatic sections. The ß-cell mass was calculated by multiplying the weight of pancreas by the average percentage area of insulin-positive cells relative to the total area of pancreatic sections. Results represent the average of three animals from each genotype.
Statistical Analysis
The quantitative data are presented as the means±SEM. The statistical significance of the differences between two groups was determined by Student’s t-test. Differences among more than two groups were calculated with one-way ANOVA followed by Turkey’s test, using IBM SPSS Statistics software Version 19. The deviation of a given genotype from the expected Mendelian ratio and fertility parameters were analyzed by the chi-square test using the same software. p<0.05 was considered to be statistically significant.
Results
We first analyzed the effect of parental Gcg genotype on pregnancy and breeding parameters. Female mice were mated with male mice in all combinations of genotypes. Given that no significant differences in fertility, blood glucose levels and body weights were observed between Gcggfp/+ and Gcg+/+ females (data not shown), we compared the data for Gcggfp/gfp mice with the combined data for Gcg+/+ and Gcggfp/+ mice as the control. Female mice were considered to be infertile if the presence of vaginal plugs was confirmed twice or more in a time interval of more than 4 weeks, and no subsequent successful pregnancy occurred. The proportion of infertile Gcggfp/gfp females was not significantly different from that of the infertile control females (7.4 percent vs. 4.4 percent, P = 0.48). The genotype of the male mice did not affect the pregnancy outcomes as indicated in Table 1.
Table 1. Genotype of offspring from multiple matings analyzed at P0.
| Genotype of pairs | Offspring | p | |||||||
| No. of pairs | Mean litter sizea | genotype ratio | |||||||
| female | male | genotype | n | observed | (expected) | ||||
| +/+ | x | gfp/+ | 10 | 6.5±0.7 | +/+ | 30 | 0.46 | (0.5) | 0.54 |
| gfp/+ | 35 | 0.54 | (0.5) | ||||||
| +/+ | x | gfp/gfp | 9 | 6.6±0.9 | gfp/+ | 59 | – | – | |
| gfp/+ | x | gfp/+ | 20 | 7.4±0.5 | +/+ | 43 | 0.29 | (0.25) | 0.43 |
| gfp/+ | 72 | 0.49 | (0.5) | ||||||
| gfp/gfp | 32 | 0.22 | (0.25) | ||||||
| gfp/+ | x | gfp/gfp | 9 | 7.0±0.8 | gfp/+ | 36 | 0.57 | (0.5) | 0.26 |
| gfp/gfp | 27 | 0.43 | (0.5) | ||||||
| gfp/gfp | x | gfp/+ | 16 | 3.8±0.6 | gfp/+ | 30 | 0.49 | (0.5) | 0.90 |
| gfp/gfp | 31 | 0.51 | (0.5) | ||||||
| gfp/gfp | x | gfp/gfp | 9 | 3.9±0.6 | gfp/gfp | 35 | – | – | |
Data were compared by the chi-square test.
Values are expressed as means±SEM.
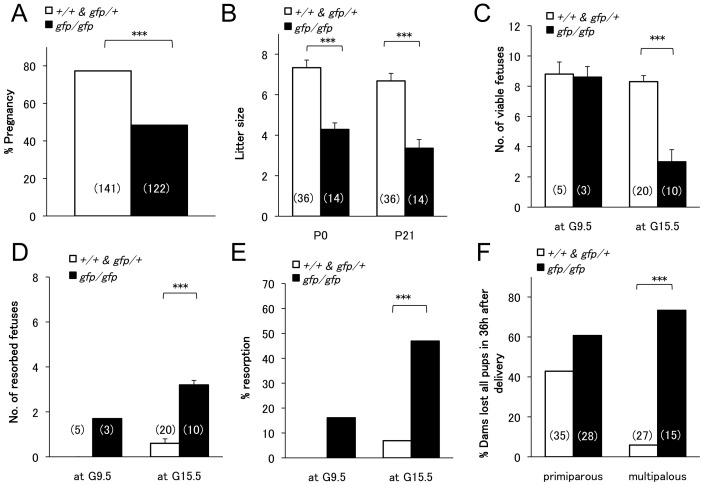
The pregnancy rate of Gcggfp/gfp females was significantly lower than that of the control females (Fig. 1A), but most of pregnant Gcggfp/gfp females could deliver at term. As Gcggfp/gfp females produced smaller litters than the control females (Fig. 1B), we sacrificed pregnant females at G9.5 or G15.5 to determine the gestational age at which emryos were lost. Although the number and survival rate of embyos in Gcggfp/gfp dams were not significantly different from those in control dams at G9.5, approximately half of the embryos in Gcggfp/gfp dams were resorbed at G15.5 (Fig. 1C–E). These results indicate that maternal proglucagon-derived peptides are required to fully sustain survival of embryos. We then compared the proportion of dams that had lost all pups within 36 hours after birth for both primiparous and multiparous dams. In most cases, postnatal survival of the litter followed an all-or-none pattern regardless of maternal genotype. At primiparas, no significant difference in neonatal survival was observed between the Gcggfp/gfp and control dams (Fig. 1F). However, neonatal survival was not improved by prior birthing experience in Gcggfp/gfp females, whereas most of the multiparous Gcg+/+ and Gcggfp/+ dams could raise their pups (Fig. 1F). These results suggest that proglucagon-derived peptides are involved in breeding behavior of the dams.
Figure 1. Fertility, litter size and survival of fetuses/pups in Gcggfp/gfp mice.
(A) Pregnancy rate in the control (open squares: Gcg+/+ and Gcggfp/+) and Gcggfp/gfp (closed squares) mice. Numbers in brackets indicate mating. The rate was not affected by genotype of the male (data not shown). ***p<0.01 by the chi-square test. (B) Litter size in the control and Gcggfp/gfp (closed squares) mice at postnatal day 0 and 21. Numbers in brackets indicate litters analyzed. Values are expressed as means±SEM. ***p<0.01 by one-way ANOVA and t-test. (C–E) Survival of fetuses. Female mice were sacrificed at gestational day 9.5 and 15.5. Numbers in brackets indicate number of pregnant females analyzed. Numbers of viable fetuses (C), resorbed fetuses (D) and % resorption (E) are shown. Values are expressed as means±SEM. ***p<0.01 by one-way ANOVA and t-test. (F) Rate of losing litters within 36 hours after delivery. Values are expressed as means±SEM. ***p<0.01 by one-way ANOVA and t-test.
Although Gcggfp/gfp females produced smaller numbers of pups per a litter and lost litters more frequently than the control females as described above, pups from Gcggfp/gfp dams that survived the first postnatal 36 hours grew as well as pups from control dams and the genotypes of the pups were in accordance with the expected Mendelian ratio (Table 1). At post partum day 0 (P0), no significant difference in blood glucose levels was observed among pups from Gcg+/+, Gcggfp/+ and Gcggfp/gfp dams (Table 2). Although the underlying mechanism is obscure, the mean body weight of Gcggfp/gfp pups from Gcggfp/gfp dams was greater than that of other types of pup (Table 2). Gcggfp/gfp pups that were born to Gcggfp/gfp dams survived and grew to adulthood and their fertility was not significantly different from that of Gcggfp/gfp pups born to Gcggfp/+ dams (data not shown).
Table 2. Body weight and blood glucose levels of neonates at P0.
| Genotype of neonates | Dam | N | Body weight (g) | Blood glucose (mM) |
| +/+ & gfp/+ | from gfp/+ | 108 | 1.37±0.03a | 1.89±0.09 |
| gfp/gfp | from gfp/+ | 21 | 1.37±0.02b | 1.76±0.16 |
| gfp/+ | from gfp/gfp | 14 | 1.32±0.04c | 1.96±0.25 |
| gfp/gfp | from gfp/gfp | 15 | 1.52±0.03abc | 2.16±0.24 |
Data were compared by one-way ANOVA.
Values are expressed as means±SEM.
Data in the same column with the same letter denote a significant difference between groups (p<0.01).
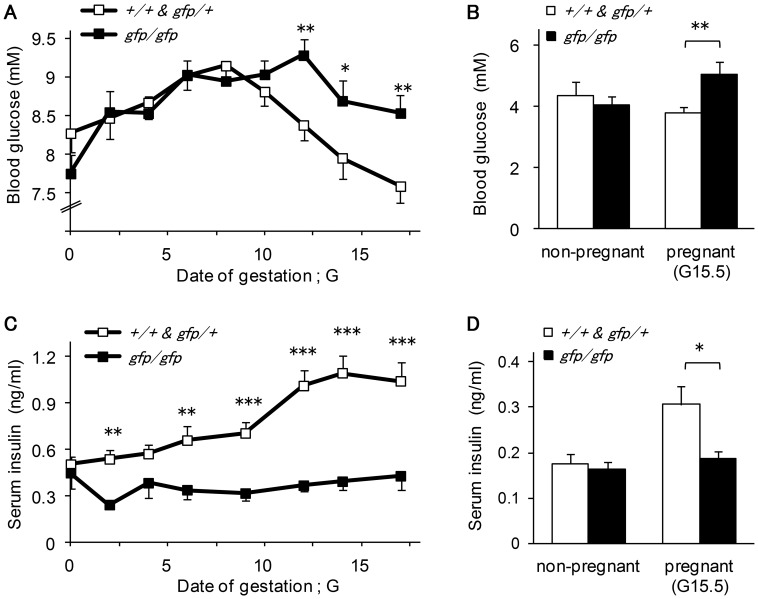
We have shown previously that blood glucose levels in Gcggfp/gfp females are not significantly different from those in the control females even after 16 hours of fasting [16]. Serum insulin levels in Gcggfp/gfp mice are significantly lower than those in control mice under ad libitum feeding, and are comparable after 16 hours of fasting [16]. To investigate the role of proglucagon-derived peptides in the regulation of blood glucose levels during pregnancy, blood glucose levels and serum insulin levels were measured in pregnant mice throughout gestation. During early gestation, blood glucose levels under ad libitum feeding increased gradually in both Gcggfp/gfp and control mice. However, blood glucose levels were significantly higher in Gcggfp/gfp mice than in control females during late gestation. In control females, blood glucose levels decreased progressively from mid to late gestation, and the levels at late gestation were lower than those before pregnancy. In contrast, pregnant Gcggfp/gfp mice maintained higher blood glucose levels during the period of mid gestation and the levels declined only slightly during late gestation (Figure 2A). Fasting blood glucose levels were also significantly higher in pregnant Gcggfp/gfp mice than in pregnant control females at G15.5 (Figure 2B). Serum insulin levels in pregnant Gcggfp/gfp mice were maintained at a lower level and no remarkable change was observed throughout gestation. In contrast, in pregnant control females, serum insulin levels increased steadily throughout the duration of pregnancy. Accordingly, the differences in serum insulin levels between Gcggfp/gfp and control mice under ad libitum feeding became more notable during late gestation (Figure 2C). Fasting serum insulin levels were also significantly lower in Gcggfp/gfp mice than in control females at G15.5 (Figure 2D). These results indicate that higher blood glucose levels in pregnant Gcggfp/gfp mice are attributable to the impairment of insulin secretion to meet the enhanced metabolic demand that accompanies pregnancy.
Figure 2. Blood glucose and serum insulin levels in pregnant Gcggfp/gfp mice.
Blood glucose levels (A) (n = 49–51) and serum insulin levels (C) (n = 8–14) in ad libitum-fed Gcggfp/gfp mice during pregnancy. Fasting blood glucose levels (B) (n = 9–11) and serum insulin levels (D) (n = 6–14) in non-pregnant Gcggfp/gfp and pregnant Gcggfp/gfp mice. Open squares: Gcg+/+ and Gcggfp/+; closed squares: Gcggfp/gfp. Values are expressed as means±SEM. *p<0/05, **p<0.01, ***p<0.001.
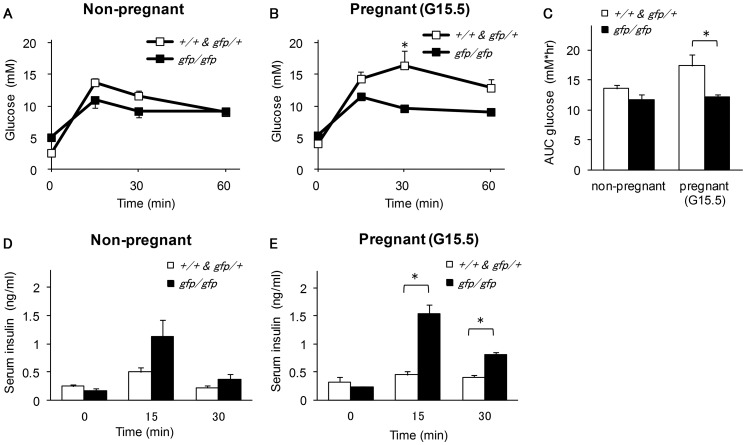
Given that the higher blood glucose levels and lower serum insulin concentration in pregnant Gcggfp/gfp mice suggested the involvement of proglucagon derived-peptides in the alteration of islet function during pregnancy, we performed OGTTs in non-pregnant and pregnant (G15.5) mice. Among the non-pregnant mice, there was no significant difference between Gcggfp/gfp and control mice in blood glucose levels and the area under the curve (Figures 3A and 3C). Although the difference did not reach statistical significance, serum insulin levels at 15 min after glucose injection were higher in non-pregnant Gcggfp/gfp mice than in control mice (Figure 3B). Despite the presence of higher blood glucose levels and lower insulin levels in pregnant Gcggfp/gfp mice under ad libitum feeding, these mice showed improved glucose tolerance on the OGTT as compared with pregnant control mice. Blood glucose levels at 30 min after glucose injection and the area under the curve were significantly lower in pregnant Gcggfp/gfp mice than in pregnant control mice, and serum insulin levels from 15 to 30 min after glucose injection were significantly higher in the former (Figures 3C–E).
Figure 3. Oral glucose tolerance test (OGTT) in non-pregnant and pregnant Gcggfp/gfp mice.
Blood glucose levels in non-pregnant Gcggfp/gfp mice (A) and pregnant Gcggfp/gfp mice (B) and the area under the curve for blood glucose levels(C) during OGTT. Serum insulin levels in non-pregnant Gcggfp/gfp mice (D) and pregnant Gcggfp/gfp mice (E) from 0 to 30 minutes following OGTT. Open squares: Gcg+/+ and Gcggfp/+ (n = 3–4); closed squares: Gcggfp/gfp (n = 3–5). Values are expressed as means±SEM. *p<0.05.
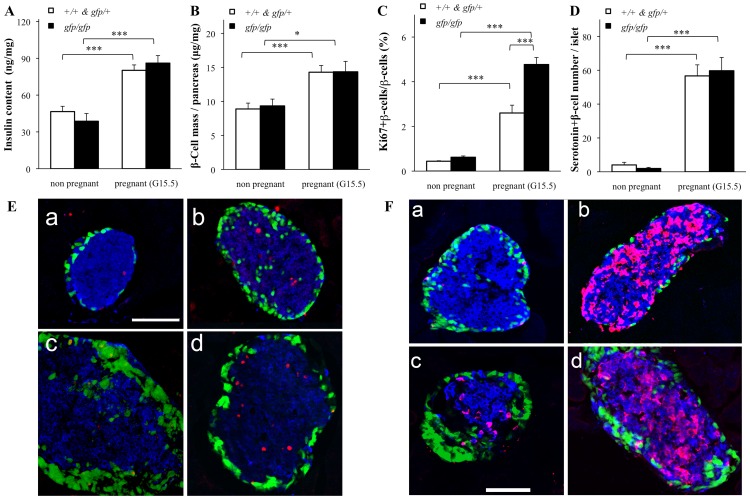
To investigate whether ß-cells proliferate during pregnancy in Gcggfp/gfp mice, the insulin content of the whole pancreas, ß-cell mass and the percentage of ß-cells positive for the proliferation marker Ki-67 in pancreatic sections were compared. In both in the Gcggfp/gfp and control mice, the pancreatic insulin content and the ß-cell mass in the pancreas increased significantly during pregnancy (Figures 4A and 4B) and no significant difference in these parameters was observed between the Gcggfp/gfp and control mice regardless of pregnant status. There was no significant difference in the number of Ki-67-positive ß-cells per islet between pregnant Gcggfp/gfp and pregnant control mice. However, the percentage of Ki-67-positive ß-cells per islet was significantly higher in pregnant Gcggfp/gfp mice than in pregnant control mice because the number of ß-cells per islet was lower in Gcggfp/gfp mice than in control mice (Figure 4C and 4E). Therefore, these results indicate that pregnancy-induced ß-cell proliferation in Gcggfp/gfp mice is, at least, not attenuated compared to that in control mice.
Figure 4. Pregnancy-induced ß-cell adaptations in Gcggfp/gfp mice.
A, Pancreatic insulin content was measured after a 16-h fasting period (n = 5–7). B, ß-Cell mass was calculated as the relative percentage area of ß-cells (defined as the percentage area of pancreatic sections that stained positive for insulin) multiplied by the weight of the pancreas (n = 3). C, The average number of ki-67-positive ß-cells per ß-cells (n = 3). D, The percentage of serotonin-positive ß-cells per islet (n = 3). Open squares: Gcg+/+ and Gcggfp/+; closed squares: Gcggfp/gfp. Values are expressed as means±SEM. *p<0.05, **p<0.01, ***p<0.001. E, Immunohistochemical-autofluorescent analyses for GFP (green), insulin (blue) and Ki 67 (red). Islets in non-pregnant Gcggfp/+ (a), pregnant Gcggfp/gfp (b), non-pregnant Gcggfp/+ (c) and pregnant Gcggfp/gfp (d) pancreas. F, Immunohistochemical-autofluorescent analyses for GFP (green), insulin (blue) and serotonin (magenta). Islets in non-pregnant Gcggfp/+ (a), pregnant Gcggfp/gfp (b), non-pregnant Gcggfp/+ (c) and pregnant Gcggfp/gfp (d) pancreas. Scale bars: 100 µm.
Recent studies have shown that inhibition of serotonin synthesis in ß-cells leads to the impairment of ß-cell mass expansion and glucose intolerance. Serotonin synthesis is induced by the signaling of PLs and increases during pregnancy [18], [19]. Therefore, we also analyzed the percentage area of ß-cells positive for serotonin in pancreatic sections from pregnant Gcggfp/gfp and control mice. The percentage area of serotonin-positive ß-cells was enhanced in both pregnant Gcggfp/gfp mice and control mice (Figure 4D and 4F). These results indicated that proglucagon-derived peptides were not required for pregnancy-induced ß-cell proliferation.
Discussion
In the present study, we observed that Gcggfp/gfp mice could deliver and raise Gcggfp/gfp pups to weaning despite the absence of proglucagon-derived peptides. This finding contrasted sharply with the results of a previous study in Gcgr−/− mice, in which Gcgr−/− pups born to Gcgr−/− mothers showed delayed maturation of islet cells and died within 24 h after birth [20]. Given that maternal hypoglycemia leads to fetal growth retardation [21], [22], [23], it is likely that the persistently lower blood glucose levels that is observed in Gcgr−/− mice is responsible for the growth failure of Gcgr−/− pups born to Gcgr−/− dams, rather than the absence of glucagon action. In Gcgr−/− mice, not only are glucagon levels increased markedly, but also GLP-1 levels. The lower blood glucose levels in Gcgr−/− mice can be attributed to the elevated GLP-1 levels, because blood glucose levels in Gcgr/GLP-1 receptor (Glp1r) double knockout mice are normal, as are those in Gcggfp/gfp mice [24].
In the present study, we also found that ß-cell proliferation was induced by pregnancy in Gcggfp/gfp mice as well as in control mice. Therefore, proglucagon-derived peptides are not required for pregnancy-induced ß-cell proliferation. GLP-1 has been shown to increase expression of the Pancreas duodenal homeobox 1 (Pdx-1) gene, thereby stimulating ß-cell proliferation [14]. However, Pdx-1 levels are not increased by PRL [25], which plays important roles in ß-cell proliferation during pregnancy [4], [9], [10], [11]. Several studies indicate that signaling pathways for PRL and those for GLP-1 are independent [19], [26] [27]. Our results are in agreement with these findings, which indicate that GLP-1 does not play a major role in pregnancy-induced ß-cell proliferation.
Pregnant Gcggfp/gfp mice showed higher blood glucose levels and lower insulin levels under ad libitum feeding than pregnant control mice in spite of comparable increases in ß-cell mass. Yet, as mentioned above, pregnant Gcggfp/gfp mice showed lower insulin levels and higher glucose levels under ad libitum feeding. And these results suggested that pregnant Gcggfp/gfp mice have a higher threshold for insulin secretion in response to a moderate elevation in blood glucose levels, and the absence of proglucagon-derived peptides, most likely GLP-1 is probably responsible for the altered threshold for insulin secretion. Nevertheless, after the oral administration of glucose, serum insulin levels were higher in pregnant Gcggfp/gfp mice than in pregnant control mice, which indicated that insulin secretion in response to a rapid elevation in blood glucose is enhanced in Gcggfp/gfp mice.
Gcggfp/gfp mice showed a decreased pregnancy rate and smaller litter size as compared with control mice. In addition, a higher rate of embryo resorption and smaller litter size were observed in Gcggfp/gfp females as compared with control females (Fig. 1). Given that the genotypes of the pups conformed with the expected Mendelian ratio, the resorption and/or growth failure of the fetuses were not affected by the fetal genotype. In Gcgr−/− mice, which also lose half of the fetuses during late gestation [20], it was demonstrated recently that expression of the genes for IGF-1, IGF-1 receptor and GLP-1 in the placenta was significantly down-regulated in Gcgr−/− mice [28]. As IGF-1 is a candidate for a regulator of placental nutrient transport [29], attenuated IGF-1 signaling is one of the possible causes of fetal loss caused by the absent action of glucagon. The cause of this partially defective fertility in Gcggfp/gfp mice is obscure, however, chronic hypoinsulinemia and mild hyperglycemia during late gestational periods in Gcggfp/gfp females are possible causes. Changes in other peptides/hormones that are involved in regulation of reproduction may be also involved [30]. It is also noteworthy that amino acid metabolism is altered in both Gcggfp/gfp and Gcgr−/− mice and that both show hyperaminoacidemia [31], [32], as hyperaminoacidemia is observed in poorly controlled GDM and is associated with poor outcome [33].
Pups from Gcggfp/gfp dams were lost more frequently within 36 hours after birth than those from control dams, and neonatal survival was not improved in multiparous Gcggfp/gfp dams, in contrast to multiparous control dams. These results suggest that Gcggfp/gfp dams have defects in relation to improving breeding capacity. Given that it has been shown that Glp1r null mice (Glp1r−/−) have defects in learning and memory [34], [35], the lack of GLP-1 might be responsible for the partially impaired breeding capacity of Gcggfp/gfp dams.
In the present study, we analyzed fertility and pregnancy-associated change in pancreatic islet function in the absence of proglucagon-derived peptides. Our data clearly showed that proglucagon-derived peptides, including glucagon and GLP-1, are not required for reproduction and pregnancy-induced β-cell proliferation. Nevertheless, female Gcggfp/gfp mice showed a decreased pregnancy rate, smaller litter size, insufficient insulin secretion during pregnancy and defects in breeding capacity. Multiple bioactive peptides, including glucagon and GLP-1, are produced from proglucagon and it is impossible to ablate one of these peptides selectively. Ablation of the glucagon receptor results in α-cell hyperplasia, markedly increased GLP-1 production and lower blood glucose levels. Future studies that employ multiple genetic models, such as Gcggfp/gfp and Gcgr−/−, should further elucidate the role of proglucagon-derived peptides in pregnancy and reproduction.
Acknowledgments
We are indebted to staff at the animal care unit in the Futuristic Environmental Simulation Center at the Research Institute of Environmental Medicine, Nagoya University for their help with the animal experiments.
Funding Statement
This work was supported in part by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (21659232 to Y. H.) and a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (23122507 to Y. H.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Vanassche FA, Aerts L, Deprins F (1978) Morphological study of endocrine pancreas in human pregnancy. Br J Obstet Gynaecol 85: 818–820. [DOI] [PubMed] [Google Scholar]
- 2. Butler AE, Cao-Minh L, Galasso R, Rizza RA, Corradin A, et al. (2010) Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy. Diabetologia 53: 2167–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Parsons JA, Brelje TC, Sorenson RL (1992) Adaptation of islets of Langerhans to pregnancy: increased islet cell proliferation and insulin secretion correlates with the onset of placental lactogen secretion. Endocrinology 130: 1459–1466. [DOI] [PubMed] [Google Scholar]
- 4. Sorenson RL, Brelje TC (1997) Adaptation of islets of Langerhans to pregnancy: beta-cell growth, enhanced insulin secretion and the role of lactogenic hormones. Horm Metab Res 29: 301–307. [DOI] [PubMed] [Google Scholar]
- 5.Ijas H, Vaarasmaki M, Morin-Papunen L, Keravuo R, Ebeling T, et al. (2010) Metformin should be considered in the treatment of gestational diabetes: a prospective randomised study. BJOG: 880–885. [DOI] [PubMed]
- 6. Valsamakis G, Margeli A, Vitoratos N, Boutsiadis A, Sakkas EG, et al. (2010) The role of maternal gut hormones in normal pregnancy: fasting plasma active glucagon-like peptide 1 level is a negative predictor of fetal abdomen circumference and maternal weight change. Eur J Endocrinol 162: 897–903. [DOI] [PubMed] [Google Scholar]
- 7. Ben-Haroush A, Yogev Y, Hod M (2004) Epidemiology of gestational diabetes mellitus and its association with Type 2 diabetes. Diabet Med 21: 103–113. [DOI] [PubMed] [Google Scholar]
- 8. Kim C, Newton KM, Knopp RH (2002) Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 25: 1862–1868. [DOI] [PubMed] [Google Scholar]
- 9. Brelje TC, Scharp DW, Lacy PE, Ogren L, Talamantes F, et al. (1993) Effect of homologous placental lactogens, prolactins, and growth hormones on islet B-cell division and insulin secretion in rat, mouse, and human islets: implication for placental lactogen regulation of islet function during pregnancy. Endocrinology 132: 879–887. [DOI] [PubMed] [Google Scholar]
- 10. Fielder PJ, Ogren L, Edwards D, Talamantes F (1987) Effects of fasting on serum lactogenic hormone concentrations during mid-and late pregnancy in mice. Am J Physiol 253: E40–44. [DOI] [PubMed] [Google Scholar]
- 11. Huang C, Snider F, Cross JC (2009) Prolactin receptor is required for normal glucose homeostasis and modulation of beta-cell mass during pregnancy. Endocrinology 150: 1618–1626. [DOI] [PubMed] [Google Scholar]
- 12. Kieffer TJ, Habener JL (1999) The glucagon-like peptides. Endocr Rev 20: 876–913. [DOI] [PubMed] [Google Scholar]
- 13. Dey A, Lipkind GM, Rouille Y, Norrbom C, Stein J, et al. (2005) Significance of prohormone convertase 2, PC2, mediated initial cleavage at the proglucagon interdomain site, Lys70-Arg71, to generate glucagon. Endocrinology 146: 713–727. [DOI] [PubMed] [Google Scholar]
- 14. Baggio LL, Drucker DJ (2007) Biology of incretins: GLP-1 and GIP. Gastroenterology 132: 2131–2157. [DOI] [PubMed] [Google Scholar]
- 15. Drucker DJ (2006) The biology of incretin hormones. Cell Metab 3: 153–165. [DOI] [PubMed] [Google Scholar]
- 16. Hayashi Y, Yamamoto M, Mizoguchi H, Watanabe C, Ito R, et al. (2009) Mice deficient for glucagon gene-derived peptides display normoglycemia and hyperplasia of islet alpha-cells but not of intestinal L-cells. Mol Endocrinol 23: 1990–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Miettinen PJ, Ustinov J, Ormio P, Gao R, Palgi J, et al. (2006) Downregulation of EGF receptor signaling in pancreatic islets causes diabetes due to impaired postnatal beta-cell growth. Diabetes 55: 3299–3308. [DOI] [PubMed] [Google Scholar]
- 18. Kim H, Toyofuku Y, Lynn FC, Chak E, Uchida T, et al. (2010) Serotonin regulates pancreatic beta cell mass during pregnancy. Nat Med 16: 804–U106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schraenen A, Lemaire K, de Faudeur G, Hendrickx N, Granvik M, et al. (2010) Placental lactogens induce serotonin biosynthesis in a subset of mouse beta cells during pregnancy. Diabetologia 53: 2589–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vuguin PM, Kedees MH, Cui L, Guz Y, Gelling RW, et al. (2006) Ablation of the glucagon receptor gene increases fetal lethality and produces alterations in islet development and maturation. Endocrinology 147: 3995–4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. ter Braak EW, Evers IM, Erkelens DW, Visser GHA (2002) Maternal hypoglycemia during pregnancy in type 1 diabetes: maternal and fetal consequences. Diabetes Metab Res Rev 18: 96–105. [DOI] [PubMed] [Google Scholar]
- 22. Smoak IW, Sadler TW (1990) Embryopathic effects of short-term exposure to hypoglycemia in mouse embryos in vitro. Am J Obstet Gynecol 163: 619–624. [DOI] [PubMed] [Google Scholar]
- 23. Persson B, Hansson U (1993) Hypoglycemia in pregnancy. Baillieres Clin Endocrinol Metab 7: 731–739. [DOI] [PubMed] [Google Scholar]
- 24. Ali S, Lamont BJ, Charron MJ, Drucker DJ (2011) Dual elimination of the glucagon and GLP-1 receptors in mice reveals plasticity in the incretin axis. J Clin Invest 121: 1917–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nasir I, Kedees MH, Ehrlich ME, Teitelman G (2005) The role of pregnancy hormones in the regulation of Pdx-1 expression. Mol Cell Endocrinol 233: 1–13. [DOI] [PubMed] [Google Scholar]
- 26. Vasavada RC, Gonzalez-Pertusa JA, Fujinaka Y, Fiaschi-Taesch N, Cozar-Castellano I, et al. (2006) Growth factors and beta cell replication. Int J Biochem Cell Biol 38: 931–950. [DOI] [PubMed] [Google Scholar]
- 27. Lindberg K, Ronn SG, Tornehave D, Richter H, Hansen JA, et al. (2005) Regulation of pancreatic beta-cell mass and proliferation by SOCS-3. J Mol Endocrinol 35: 231–243. [DOI] [PubMed] [Google Scholar]
- 28. Ouhilal S, Vuguin P, Cui L, Du XQ, Gelling RW, et al. (2011) Hypoglycemia, hyperglucagonemia and feto-placental defects in glucagon receptor knockout mice: a role for glucagon action in pregnancy maintenance. Am J Physiol Endocrinol Metab 302: E522–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jones HN, Powell TL, Jansson T (2007) Regulation of placental nutrient transport: a review. Placenta 28: 763–774. [DOI] [PubMed] [Google Scholar]
- 30. Mao P, Meshul CK, Thuillier P, Goldberg NR, Reddy PH (2012) CART peptide is a potential endogenous antioxidant and preferentially localized in mitochondria. PLoS One 7: e29343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Watanabe C, Seino Y, Miyahira H, Yamamoto M, Fukami A, et al. (2012) Remodeling of hepatic metabolism and hyperaminoacidemia in mice deficient in proglucagon-derived peptides. Diabetes 61: 74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee Y, Wang M-Y, Du XQ, Charron MJ, Unger RH (2011) Glucagon receptor knockout prevents insulin-deficient type 1 diabetes in mice. Diabetes 60: 391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reece EA, Homko C, Wiznitzer A (1994) Metabolic changes in diabetic and nondiabetic subjects during pregnancy. Obstet Gynecol Surv 49: 64–71. [DOI] [PubMed] [Google Scholar]
- 34. During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, et al. (2003) Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med 9: 1173–1179. [DOI] [PubMed] [Google Scholar]
- 35. Abbas T, Faivre E, Holscher C (2009) Impairment of synaptic plasticity and memory formation in GLP-1 receptor KO mice: Interaction between type 2 diabetes and Alzheimer’s disease. Behav Brain Res 205: 265–271. [DOI] [PubMed] [Google Scholar]