Abstract
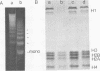
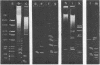
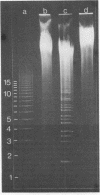
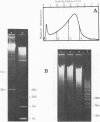
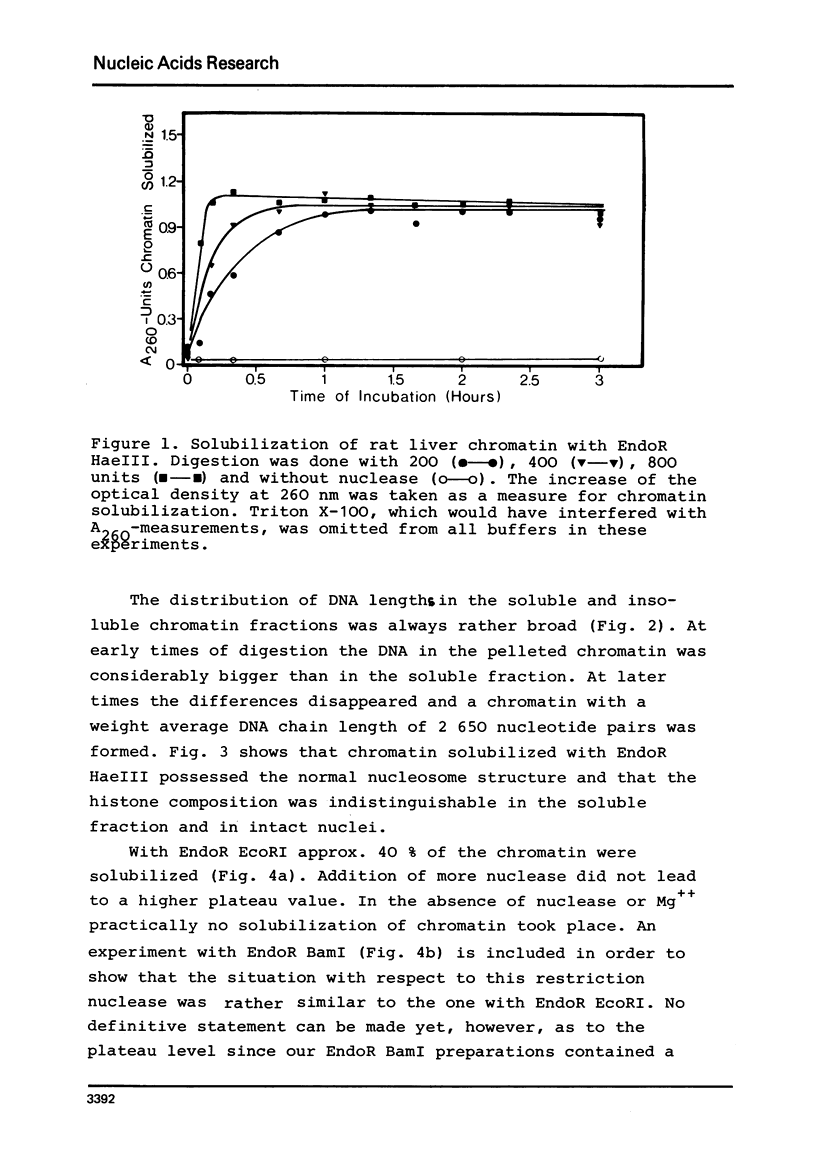
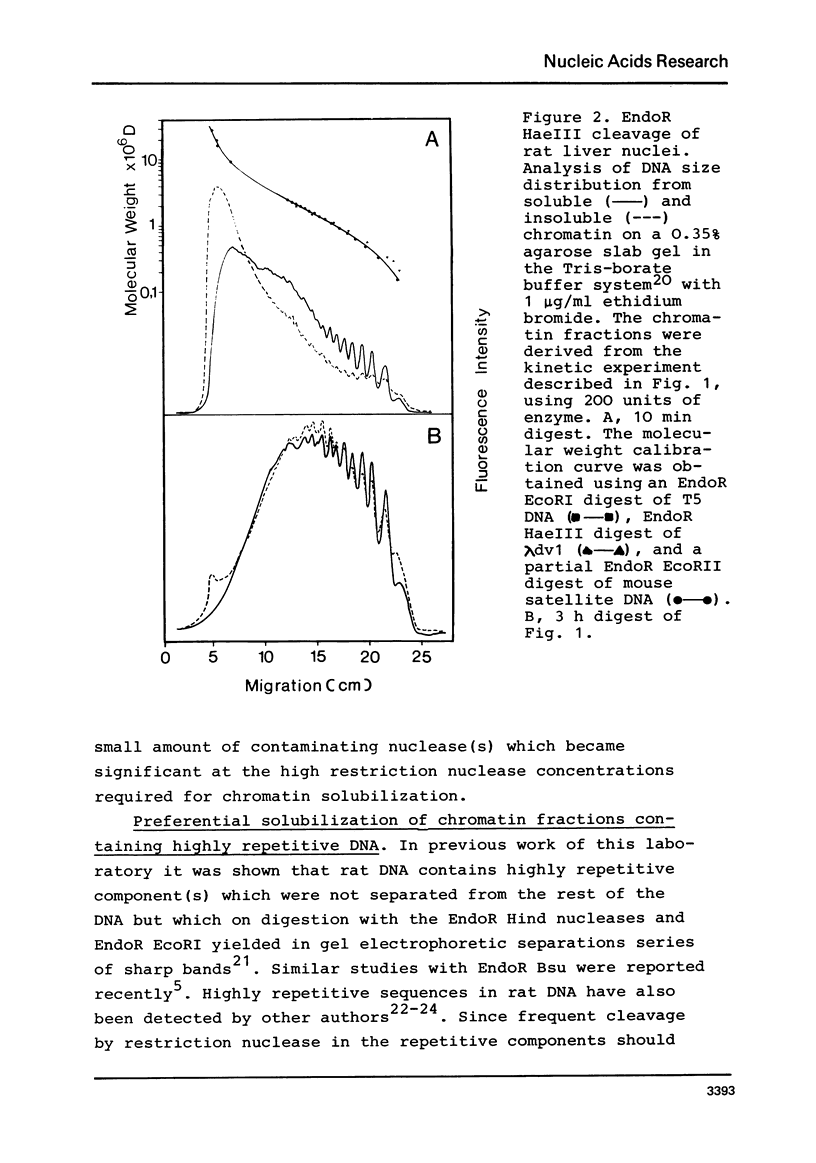
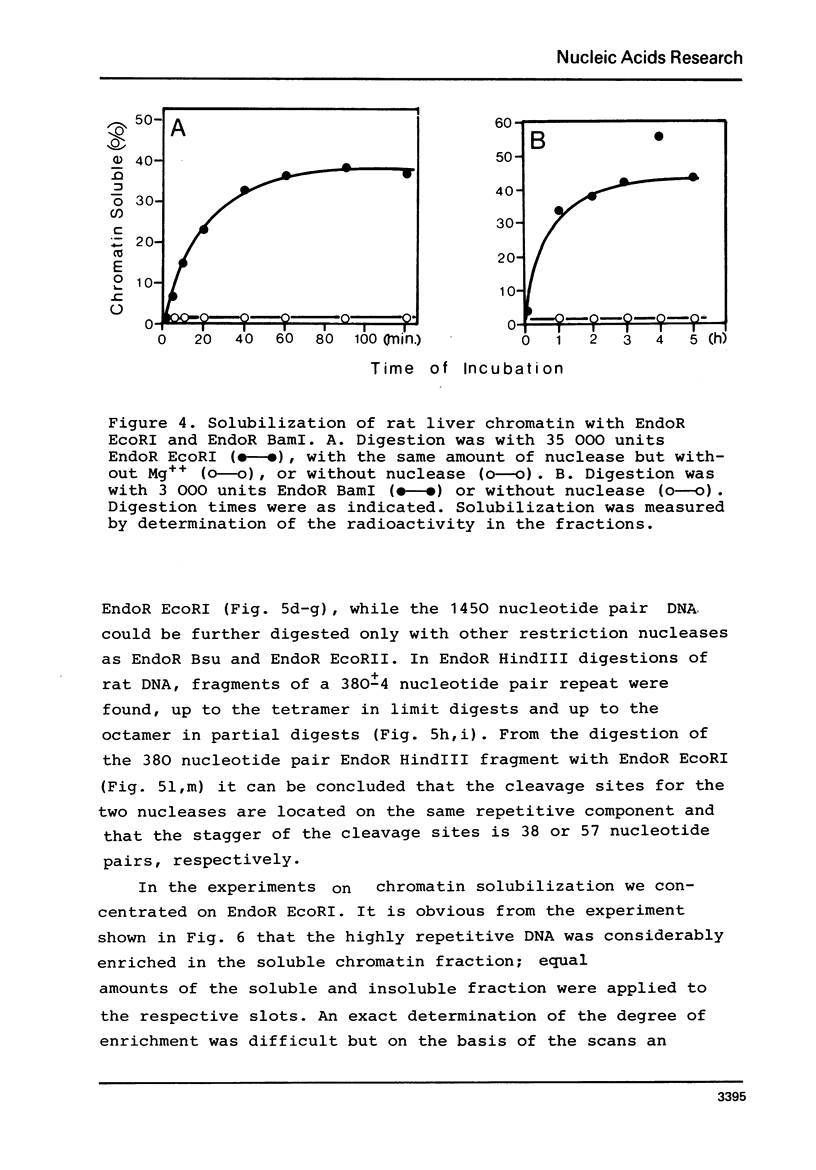
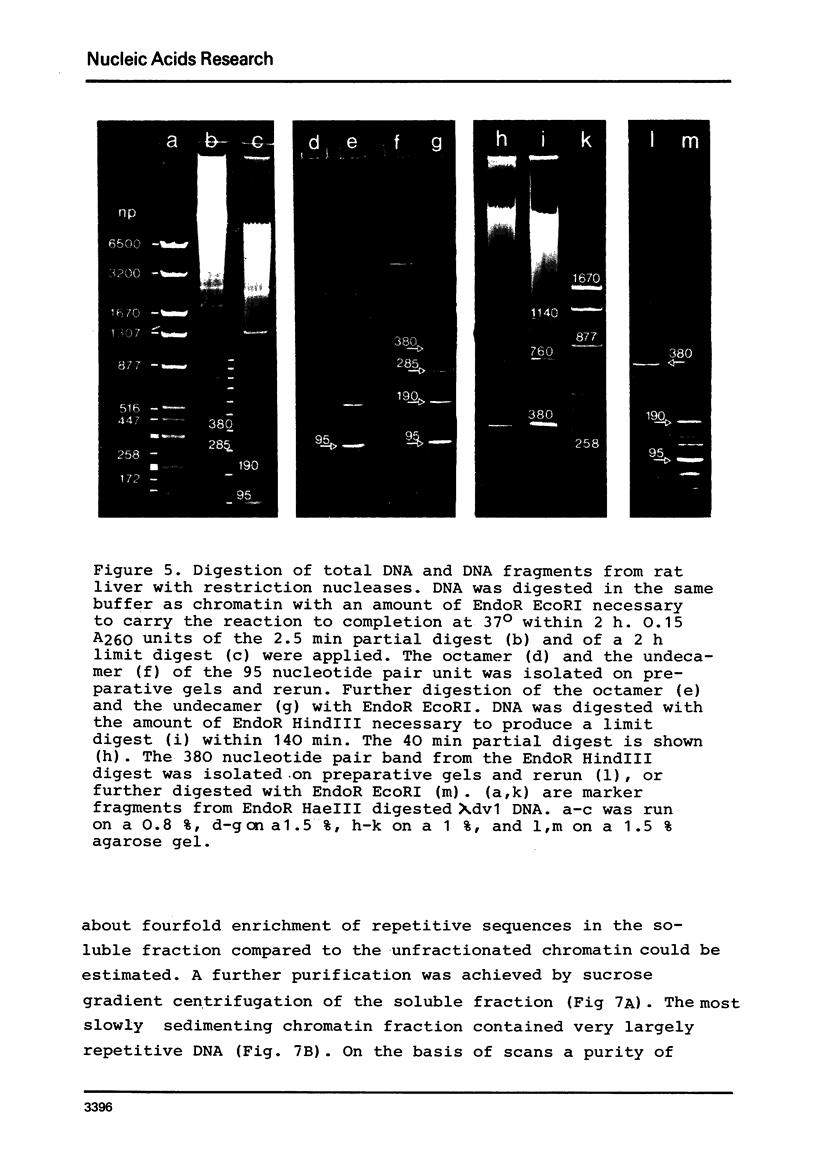
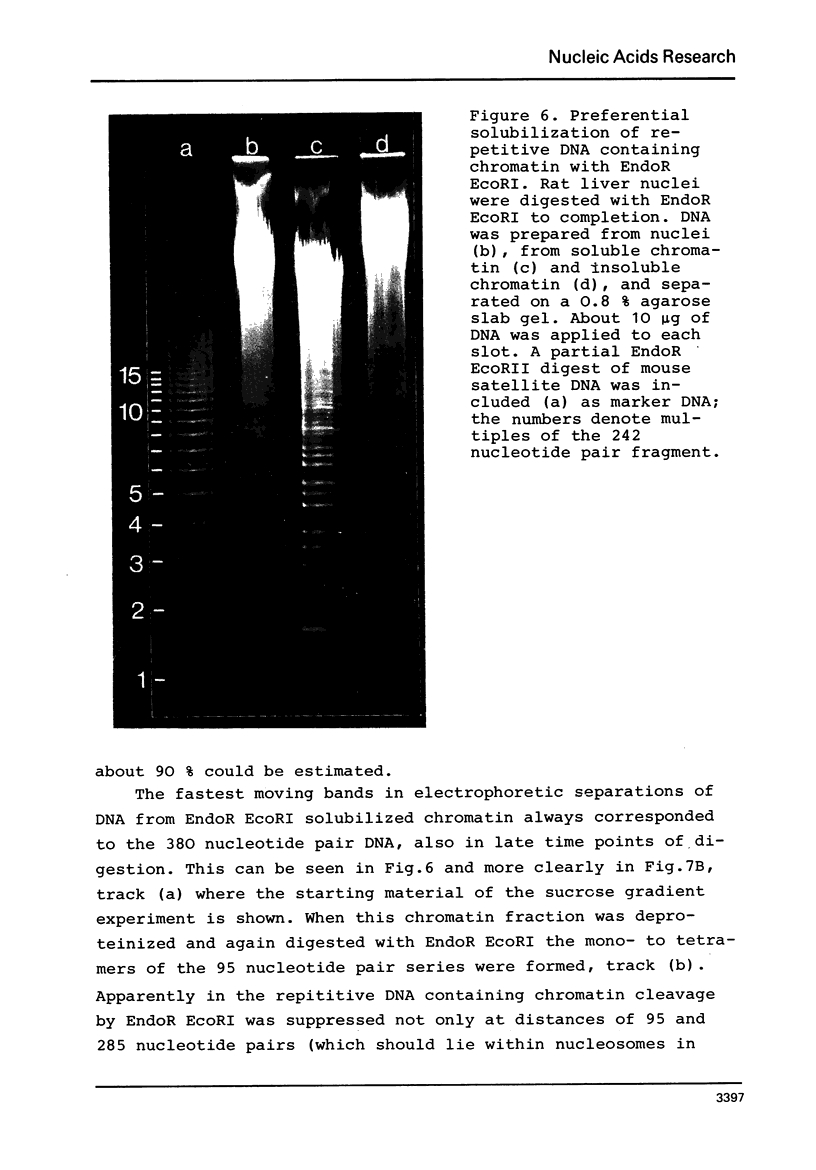
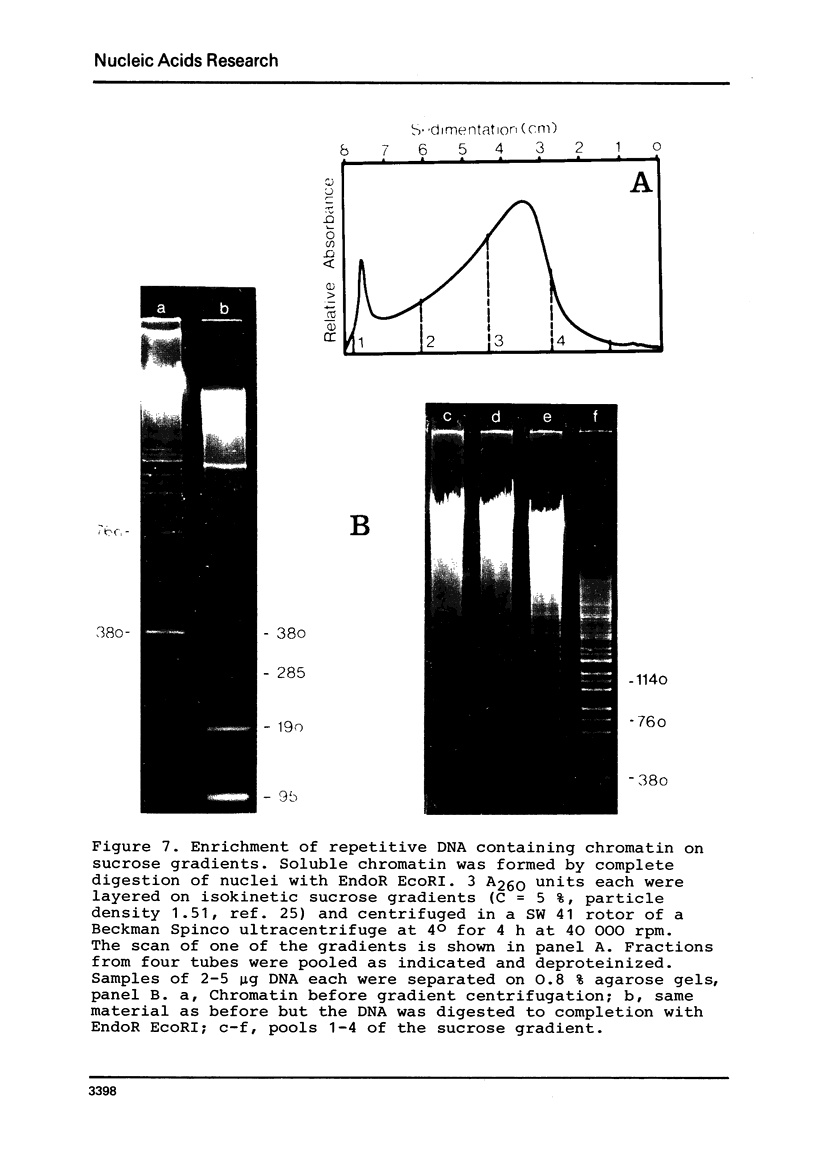
By digestion of rat liver nuclei with EndoR HaeIII, EndoR EcoRI, and EndoR Bam and subsequent lysis of the nuclei approx. 90%, 40%, and 45%, respectively, of the chromatin were solubilized. The plateau values of solubilization are in agreement with a model in which the chromatin strands are crosslinked and/or attached to a supporting structure. The distribution of DNA lengths in the soluble and insoluble chromatin fractions were determined. According to digestion experiments with restriction nucleases rat liver DNA contains highly repetitive sequences, some of which are arranged in tandem repeats of 95 and 380 nucleotide pairs, respectively. With EndoR EcoRI chromatin containing the repetitive RNA was preferentially solubilized and, by subsequent sucrose gradient centrifugation, purified to about 90%. The useful properties of chromatin prepared by the specific action of restriction nucleases are discussed.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradbury E. M., Danby S. E., Rattle H. W., Giancotti V. Studies on the role and mode of operation of the very-lysine-rich histone H1 (F1) in eukaryote chromatin. Histone H1 in chromatin and in H1 - DNA complexes. Eur J Biochem. 1975 Sep 1;57(1):97–105. doi: 10.1111/j.1432-1033.1975.tb02280.x. [DOI] [PubMed] [Google Scholar]
- Elgin S. C., Weintraub H. Chromosomal proteins and chromatin structure. Annu Rev Biochem. 1975;44:725–774. doi: 10.1146/annurev.bi.44.070175.003453. [DOI] [PubMed] [Google Scholar]
- Greil W., Igo-Kemenes T., Zachau H. G. Nuclease digestion in between and within nucleosomes. Nucleic Acids Res. 1976 Oct;3(10):2633–2644. doi: 10.1093/nar/3.10.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttann T., Votavová H., Pivec L. Base composition heterogeneity of mammalian DNAs in CsCl-netropsin density gradient. Nucleic Acids Res. 1976 Mar;3(3):835–845. doi: 10.1093/nar/3.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. Chromatin sub-structure. The digestion of chromatin DNA at regularly spaced sites by a nuclear deoxyribonuclease. Biochem Biophys Res Commun. 1973 May 15;52(2):504–510. doi: 10.1016/0006-291x(73)90740-7. [DOI] [PubMed] [Google Scholar]
- Hörz W., Igo-Kemenes T., Pfeiffer W., Zachau H. G. Specific cleavage of chromatin by restriction nucleases. Nucleic Acids Res. 1976 Nov;3(11):3213–3226. doi: 10.1093/nar/3.11.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg R. D. Structure of chromatin. Annu Rev Biochem. 1977;46:931–954. doi: 10.1146/annurev.bi.46.070177.004435. [DOI] [PubMed] [Google Scholar]
- Leake R. E., Trench M. E., Barry J. M. Effect of cations on the consideration of hen erythrocyte nuclei and its relation to gene activation. Exp Cell Res. 1972 Mar;71(1):17–26. doi: 10.1016/0014-4827(72)90257-1. [DOI] [PubMed] [Google Scholar]
- Lipchitz L., Axel R. Restriction endonuclease cleavage of satellite DNA in intact bovine nuclei. Cell. 1976 Oct;9(2):355–364. doi: 10.1016/0092-8674(76)90125-2. [DOI] [PubMed] [Google Scholar]
- McCarty K. S., Jr, Vollmer R. T., McCarty K. S. Improved computer program data for the resolution and fractionation of macromolecules by isokinetic sucrose density gradient sedimentation. Anal Biochem. 1974 Sep;61(1):165–183. doi: 10.1016/0003-2697(74)90343-1. [DOI] [PubMed] [Google Scholar]
- Noll M., Thomas J. O., Kornberg R. D. Preparation of native chromatin and damage caused by shearing. Science. 1975 Mar 28;187(4182):1203–1206. doi: 10.1126/science.187.4182.1203. [DOI] [PubMed] [Google Scholar]
- Pays E., Ronsse A. Interspersion of repetitive sequences in rat liver DNA. Biochem Biophys Res Commun. 1975 Feb 17;62(4):862–867. doi: 10.1016/0006-291x(75)90402-7. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Resolution of multiple ribonucleic acid species by polyacrylamide gel electrophoresis. Biochemistry. 1967 Jun;6(6):1818–1827. doi: 10.1021/bi00858a033. [DOI] [PubMed] [Google Scholar]
- Pfeiffer W., Horz W., Igo-Kemenes T., Zachau H. G. Restriction nucleases as probes of chromatin structure. Nature. 1975 Dec 4;258(5534):450–452. doi: 10.1038/258450a0. [DOI] [PubMed] [Google Scholar]
- Philippsen P., Streeck R. E., Zachau H. G. Defined fragments of calf, human, and rat DNA produced by restriction nucleases. Eur J Biochem. 1974 Jun 15;45(2):479–488. doi: 10.1111/j.1432-1033.1974.tb03573.x. [DOI] [PubMed] [Google Scholar]
- Rhoades M. Cleavage of T5 DNA by the Escherichia coli R-I restriction endonuclease. Virology. 1975 Mar;64(1):170–179. doi: 10.1016/0042-6822(75)90089-6. [DOI] [PubMed] [Google Scholar]
- Roberts R. J., Breitmeyer J. B., Tabachnik N. F., Myers P. A. A second specific endonuclease from Haemophilus aegyptius. J Mol Biol. 1975 Jan 5;91(1):121–123. doi: 10.1016/0022-2836(75)90375-7. [DOI] [PubMed] [Google Scholar]
- Roizés G. Analysis of eucaryotic DNAs with a restriction endonuclease from H. influenzae: isolation of "hidden" satellite DNAs. Nucleic Acids Res. 1974 Sep;1(9):1099–1120. doi: 10.1093/nar/1.9.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeck R. E., Hobom G. Mapping of cleavage sites for restriction endonucleases in lambdadv plasmids. Eur J Biochem. 1975 Sep 15;57(2):595–606. doi: 10.1111/j.1432-1033.1975.tb02335.x. [DOI] [PubMed] [Google Scholar]
- Takatsuka Y., Kohno M., Higashi K., Hirano H., Sakamoto Y. Redistribution of chromatin containing ribosomal cistrons during liver regeneration. Exp Cell Res. 1976 Nov;103(1):191–199. doi: 10.1016/0014-4827(76)90255-x. [DOI] [PubMed] [Google Scholar]
- Wilson G. A., Young F. E. Isolation of a sequence-specific endonuclease (BamI) from Bacillus amyloliquefaciens H. J Mol Biol. 1975 Sep 5;97(1):123–125. doi: 10.1016/s0022-2836(75)80028-3. [DOI] [PubMed] [Google Scholar]