Abstract
Background
Delayed antibiotic prescribing is promoted as a strategy to reduce antibiotic consumption, but its use and its effect on antibiotic consumption in routine care is poorly described.
Aim
To quantify delayed antibiotic prescribing in adults presenting in primary care with acute cough/lower respiratory tract infection (LRTI), duration of advised delay, consumption of delayed antibiotics, and factors associated with consumption.
Design and setting
Prospective observational cohort in general practices in 14 primary care networks in 13 European countries.
Method
GPs recorded clinical features and antibiotic prescribing for adults presenting with an acute infective illness with cough as the dominant symptom. Patients recorded their consumption of antibiotics from any source during the 28-day follow up.
Results
Two hundred and ten (6.3%) of 3368 patients with usable consultation data were prescribed delayed antibiotics. The median recommended delay period was 3 days. Seventy-five (44.4%) of the 169 with consumption data consumed the antibiotic course and a further 18 (10.7%) took another antibiotic during the study period. 50 (29.6%) started their delayed course on the day of prescription. Clinician diagnosis of upper respiratory tract/viral infection and clinician’s perception of patient’s wanting antibiotics were associated with less consumption of the delayed prescription. Patient’s wanting antibiotics was associated with greater consumption.
Conclusion
Delayed antibiotic prescribing was used infrequently for adults presenting in general practice with acute cough/LRTI. When used, the effect on antibiotic consumption was less than found in most trials. There are opportunities for standardising the intervention and promoting wider uptake.
Keywords: anti-bacterial agents, cough, respiratory tract infections, medication adherence, primary health care
INTRODUCTION
Concern about the overuse of antibiotics for self-limiting infections, such as many respiratory tract infections,1 has led some to recommend the use of a delayed or deferred prescribing approach.2 Delayed antibiotic prescribing involves prescribing an antibiotic but advising the patient not to start taking the course unless they deteriorate or fail to improve after a set period. Advocates suggest that in addition to reducing antibiotic consumption, delayed prescribing may increase patient empowerment and satisfaction, avoid medicalisation, reduce reconsultations, and provide a safety net.2–5 Trials and systematic reviews of delayed prescribing strategies have demonstrated reductions in the use of antibiotics for patients with cough,4,6 acute otitis media,7 and sore throat.8 However, delayed prescribing may lead to medicalisation of minor illness, storage of unused medication leading to use at a later date, and lower levels of satisfaction.9
Evidence supporting the use of delayed prescribing and recommendations to adopt this strategy have been widely disseminated since the late 1990’s.8 Reductions in antibiotic use in the UK during the late nineties and early 2000s have been partly ascribed to widespread adoption of delayed prescribing.3,10 However, there is little evidence about how frequently this strategy is used in everyday clinical practice, or what effect it has on actual consumption. Between 24%7 and 45%6 of patients prescribed antibiotics in trials of delayed prescribing collect their prescriptions, but it is not clear how often patients who are prescribed delayed prescriptions actually take them outside of trial conditions.
Describing clinicians’ uptake of this approach, antibiotic consumption by patients receiving a delayed prescription, and factors that are associated with a decision to use a delayed prescription, will help assess the current impact of this approach, and may identify opportunities for improvement.
In this observational study of acute cough in adults in 14 European primary care networks the study set out to quantify delayed antibiotic prescribing in adults presenting in primary care with acute cough, duration of advised delay, consumption of delayed antibiotics, and factors associated with consumption.
METHOD
Study design
A prospective observational study was conducted in 14 primary care networks in 13 European countries. The aim was to describe the presentation, management and outcome of acute cough in primary care, and the variation between different European countries. More details on this observational GRACE (Genomics to combat Resistance against Antibiotics in Community-acquired LRTI in Europe; www.grace-lrti.org) study of acute cough have been reported elsewhere.11–16
How this fits in
Delayed antibiotic prescribing has been shown to reduce antibiotic use for respiratory tract infections in trials of this approach and is recommended in UK NICE guidelines. Little is known about the use of this approach in everyday clinical practice, the effect it has on antibiotic consumption, or the factors that are associated with consumption of a delayed prescription. This study shows that delayed prescribing for acute cough was used infrequently across European primary care networks, and when used, was not being implemented in line with current evidence. More than half of those prescribed delayed antibiotics consumed an antibiotic during the study period.
Participants
Eligible patients were aged ≥18 years consulting with what the clinician judged to be a new infective illness where an acute or worsened cough was the main or dominant symptom, or had a clinical presentation that suggested a lower respiratory tract infection (LRTI), and with a duration of up to and including 28 days. The inclusion criteria were deliberately broad because there is a wide variation in the use of diagnostic terms for respiratory tract infections (RTIs) and the study wanted to capture usual practice. However, clinicians were asked to record their working diagnosis. Participating practices were asked to recruit consecutive eligible patients between October and November 2006 and from Late January to March 2007.
Data collection
Clinicians recorded aspects of patients’ history, symptoms, comorbidities (diabetes, chronic lung disease including chronic obstructive pulmonary disease (COPD) and cardiovascular disease), clinical findings, their working diagnosis, whether they thought the patient wanted antibiotics, and their management including antibiotic prescribing decision on a case report form (CRF) at the time of first presenting in primary care. Clinicians recorded whether they prescribed antibiotics or not, and if they did, the name, dose, and duration of the antibiotic treatment, whether it was for immediate or delayed use, and if delayed, the advised delay period. Patients were asked to complete a daily symptom diary for 28 days, which included weekly details on antibiotic consumption, stating which day and what antibiotic had been taken and whether or not it was prescribed. At the start of the diary they were asked if they were expecting their GP to prescribe antibiotics, if they hoped that their GP would prescribe antibiotics, and if they had asked their GP for antibiotics. Those who had indicated that they either hoped for antibiotics or had asked for antibiotics were classified as wanting antibiotics.
A formal power calculation for the current analyses was not conducted. The original sample size calculation was based on estimating the prevalence of prescribing within each network with a certain degree of confidence assuming a prevalence of 50%.12
Analysis
Descriptives
The proportion of patients prescribed immediate, delayed, and no antibiotics overall and by network was calculated. For those prescribed delayed antibiotics, the median advised delay period was calculated, overall and by network, and the proportion of patients in each network who were advised to delay for ≥7 days.4 Patient reported weekly medication use was categorised as an antibiotic or not by hand based on free text reporting of medicines taken. Ambiguous responses were discussed by the study team and classified or recorded as missing depending on group consensus. The proportion of patients who reported consuming their delayed prescription and the proportion who reported taking another antibiotic at any point in the 4-week follow-up period were then calculated for all participants.
Regression analysis
The study used a two level hierarchical logistic regression model, with patients nested within clinicians, to investigate factors associated with the consumption of a delayed antibiotic prescription. The study tested variables at a univariate level and included those that were significant at the 10% level. Variables in the model included patient characteristics (age; sex; comorbid illness [at least one of chronic obstructive pulmonary disease {COPD}, asthma, other lung disease, heart failure, ischaemic heart disease, other heart disease or diabetes]; and employment status [categorised as: employed, not employed]), advised duration of delay (dichotomised to <7 days and ≥7 days), clinician rated severity of illness,12 patient rated severity of illness at day 1,12 whether the patient wanted antibiotics or not, clinicians’ perception of patient expectation for antibiotics, and clinicians perception that antibiotics would help the patient get better quicker. No individual symptoms (phlegm production, shortness of breath, wheeze, coryza, fever, chest pain, muscle aches, headache, disturbed sleep, feeling generally unwell, interference with normal activities, confusion, and diarrhoea) were associated with consumption of delayed antibiotics at the univariate level and therefore none were included in the model.
RESULTS
Three hundred and eighty-seven GPs included 3398 eligible patients. Case report form (CRF) data from the index consultation was available for 3368 (99%), diary data for 2714 (80%) and both CRF and diary data for 2690 (79%) participants (Figure 1). The symptoms and working diagnoses for those who had a CRF completed are given in Table 1.
Figure 1.
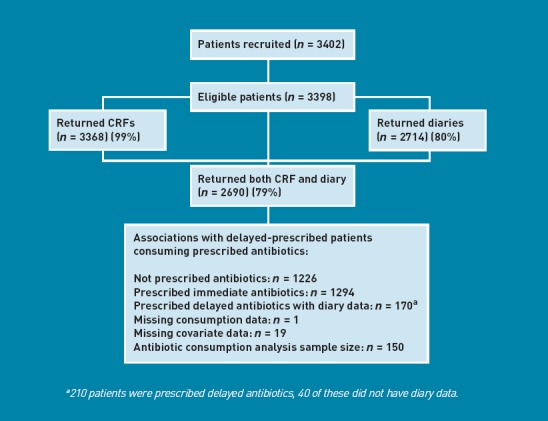
Patient flow diagram.
Table 1.
Symptoms and working diagnosis by antibiotic treatment
| No antibioticsa | Immediate antibioticsa | Delayed antibioticsa | Totala | |
|---|---|---|---|---|
| Symptom | ||||
| Cough | 1587 (99.8) | 1561 (99.8) | 210 (100) | 3358 (99.8) |
| Phlegm production | 1112 (70.0) | 1318 (84.3) | 162 (77.5) | 2592 (77.1) |
| Shortness of breath | 709 (44.6) | 908 (58.1) | 103 (49.3) | 1720 (51.2) |
| Wheeze | 459 (28.9) | 722 (46.1) | 89 (42.6) | 1270 (37.8) |
| Coryza | 1046 (65.8) | 1050 (67.2) | 144 (68.9) | 2240 (66.6) |
| Fever | 637 (40.3) | 934 (59.7) | 136 (65.1) | 1707 (50.9) |
| Chest pain | 608 (38.3) | 784 (50.1) | 91 (43.5) | 1483 (44.1) |
| Muscle aches | 762 (48.0) | 832 (53.2) | 116 (55.5) | 1710 (50.9) |
| Headache | 904 (56.9) | 964 (61.6) | 129 (61.7) | 1997 (59.4) |
| Disturbed sleep | 999 (62.9) | 979 (62.6) | 137 (65.6) | 2115 (62.9) |
| Feeling generally unwell | 1194 (75.2) | 1328 (84.9) | 176 (84.2) | 2698 (80.3) |
| Interference with normal activities | 1052 (66.3) | 1140 (72.9) | 143 (68.4) | 2335 (69.5) |
| Confusion | 50 (3.1) | 75 (4.8) | 10 (4.8) | 135 (4.0) |
| Diarrhoea | 91 (5.7) | 93 (5.9) | 12 (5.7) | 196 (5.8) |
| Working diagnosis | ||||
| LRTI | 406 (25.6) | 963 (61.7) | 91 (44.6) | 1460 (43.5) |
| URTI | 529 (33.3) | 261 (16.7) | 44 (21.6) | 834 (24.9) |
| Asthma/COPD related | 97 (6.1) | 106 (6.8) | 13 (6.4) | 216 (6.4) |
| Non-specific RTI | 184 (11.6) | 53 (3.4) | 15 (7.4) | 252 (7.5) |
| Viral/other RTI | 361 (22.7) | 49 (3.1) | 40 (19.6) | 450 (13.4) |
| Pneumonia | 11 (0.7) | 129 (8.3) | 1 (0.5) | 141 (4.2)a |
COPD = chronic obstructive pulmonary disease. LRTI = lower respiratory tract infection. URTI = upper respiratory tract infection.
Number with symptom or working diagnosis (proportion within antibiotic treatment category — %).
Of the 3368 with CRF data, 210 (6.3%) were prescribed an antibiotic and asked to delay its use, 1566 (46.5%) received an antibiotic prescription for immediate use, and 1592 (47.6%) were not prescribed antibiotics at the index consultation. At the network level, the use of delayed prescribing varied from 0.2% of consultations (Barcelona) to 33.1% (Southampton). Only three networks Łódź Milan, and Southampton) used a delayed prescribing approach in more than 5% of consultations. The proportion of participants receiving delayed, immediate, and no antibiotic prescriptions in each network are shown in Table 2.
Table 2.
Immediate, delayed, and no antibiotic prescribing by network
| Proportion (%) | |||
|---|---|---|---|
| Network | Delayed antibiotics | Immediate antibiotics | No antibiotics |
| Antwerp | 9/216 (4.2) | 47/216 (21.8) | 160/216 (74.1) |
| Balatonfüred | 31/323 (9.6) | 210/323 (65.0) | 82/323 (25.4) |
| Barcelona | 2/277 (0.2) | 55/277 (19.9) | 220/277 (79.4) |
| Bratislava | 11/299 (3.7) | 251/299 (83.9) | 37/299 (12.4) |
| Cardiff | 10/300 (3.3) | 199/300 (66.3) | 91/300 (30.3) |
| Helsinki | 5/103 (4.9) | 38/103 (36.9) | 60/103 (58.3) |
| Jönköping | 8/300 (2.7) | 106/300 (35.3) | 186/300 (62.0) |
| Łódź | 27/301 (9.0) | 188/301 (62.5) | 86/301 (28.6) |
| Mataró | 5/196 (2.6) | 62/196 (31.6) | 129/196 (65.8) |
| Milan | 21/207 (10.1) | 134/207 (64.7) | 52/207 (25.1) |
| Rotenburg | 3/229 (1.3) | 76/229 (33.2) | 150/229 (65.5) |
| Southampton | 71/214 (33.1) | 63/214 (29.4) | 80/214 (37.4) |
| Tromsø | 4/203 (2.0) | 57/203 (28.1) | 142/203 (70.0) |
| Utrecht | 3/200 (1.5) | 80/200 (40.0) | 117/200 (58.5) |
| OVERALL | 210/3368 (6.3) | 1566/3368 (46.5) | 1592/3368 (47.6) |
Advised delay period
For those who were prescribed delayed antibiotics the median advised delay period varied from 2 days (Balatonfüred, Barcelona, Cardiff, Helsinki, Mataró, Rotenburg) to 7 days (Southampton, Utrecht) (Table 3). Only 22% of participants wee advised to delay their prescriptions by >7 days.
Table 3.
Duration of delay advised by networks
| Network | Duration of delay, days, median (IQR) | Advised a delay of at least 7 days, proportion (%) |
|---|---|---|
| Antwerp | 3 (2.5–7) | 3/8 (37.5) |
| Balatonfüred | 2 (2–3) | 0/31 (0.0) |
| Barcelona | 2 (2–2) | 0/2 (0.0) |
| Bratislava | 3 (2–3) | 0/11 (0.0) |
| Cardiff | 2 (2–2) | 0/10 (0.0) |
| Helsinki | 2 (2–2) | 0/5 (0.0) |
| Jönköping | 2.5 (2–3.5) | 1/8 (12.5) |
| Łódź | 3 (2–3) | 1/27 (3.7) |
| Mataró | 2 (2–4) | 0/5 (0.0) |
| Milan | 3 (2–3) | 2/21 (9.5) |
| Rotenburg | 2 (1.5–2) | 0/3 (0.0) |
| Southampton | 7 (4–8.5) | 37/71 (52.1) |
| Tromsø | 3 (2.5–3) | 0/4 (0.0) |
| Utrecht | 7 (5.5–7) | 2/3 (66.7) |
| OVERALL | 3 (2–5) | 46/209 (22.0) |
Antibiotic consumption
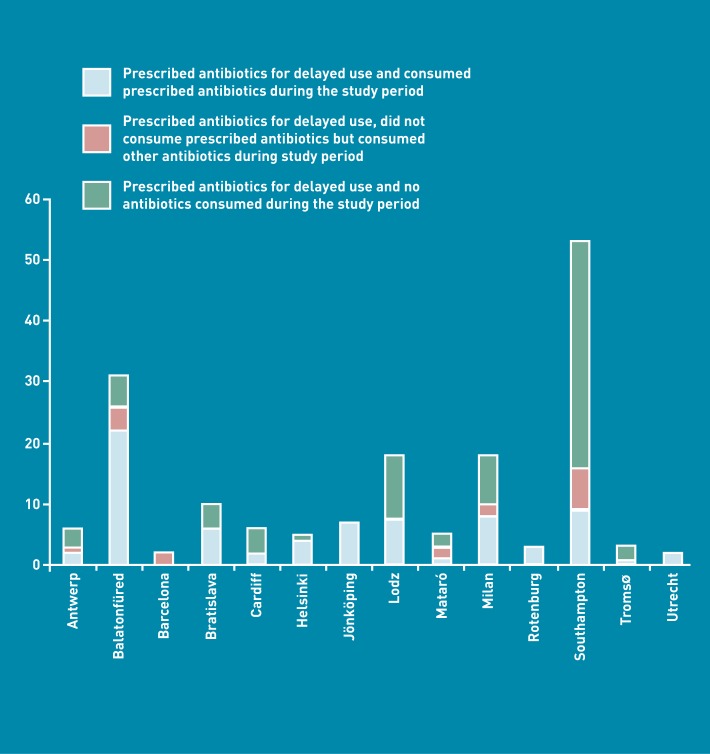
Of the 210 participants who were prescribed delayed antibiotics 169 provided data on antibiotic consumption. Of these, 75 (44.4%) consumed their delayed antibiotic, 18 (10.7%) consumed an antibiotic other than the delayed antibiotic during the follow-up period and 76 (45.0%) did not consume any antibiotics during the study period. Therefore, 93 (55.0%) participants consumed an antibiotic during the study period. In comparison, 924 (71.5%) of those prescribed immediate antibiotics consumed an antibiotic during the study period. 50 (29.6%) started taking their delayed antibiotics on the day that they were prescribed. Antibiotic consumption by network for patients given a delayed prescription is illustrated in Figure 2.
Figure 2.
Antibiotic consumption by network for 265 patients prescribed delayed antibiotics.a
Factors associated with consumption of the delayed antibiotic prescription
No individual symptoms were associated with consumption at a univariate level and therefore none were included in the regression analysis. 150 participants had data on consumption of their delayed antibiotic prescription and data for all included covariables (Figure 1). Having a working diagnosis of ‘upper respiratory tract infection’, ‘non-specific respiratory tract infection’ or ‘viral / other illness’ was associated with a reduction in the odds of consuming a delayed antibiotic prescription (Table 4). After controlling for patient characteristics, patient illness, duration of advised delay and the clinicians views on the likely benefit from antibiotics, patients who wanted an antibiotic had increased odds of consuming their prescribed antibiotic (OR = 2.51, 95% CI = 1.06 to 5.90, P = 0.035) and clinician’s perception that the patient wanted them to prescribe antibiotics was associated with a reduced odds of consuming them (OR = 0.38, 95% CI = 0.15 to 1.00, P = 0.050) (Table 4).
Table 4.
Association between patient/clinical characteristics and consumption of a delayed antibiotic in 150 patients who consulted with 66 cliniciansa with LRTI and were issued with a delayed prescription
| Variable | Odds ratio | 95% CI | P-value |
|---|---|---|---|
| Patient characteristics | |||
| Age (decades) | 1.15 | 0.86 to 1.54 | 0.343 |
| Sex: Female (Ref) Male |
0.55 | 0.22 to 1.38 | 0.204 |
| No comorbidities (Ref) | |||
| At least one comorbidity | 0.55 | 0.17 to 1.82 | 0.326 |
| Employed (Ref) | |||
| Not employed | 1.00 | 0.39 to 2.54 | 1.000 |
| Patient illness information | |||
| Clinician rated symptom severity at consultationb | 1.13 | 0.78 to 1.64 | 0.545 |
| Patient rated symptom severity on day 1b | 0.99 | 0.72 to 1.35 | 0.938 |
| Waited <7 days before consulting (Ref) | |||
| Waited ≥7 days before consulting | 0.86 | 0.35 to 2.13 | 0.746 |
| Clinician working diagnosis | |||
| Lower respiratory tract infection (Ref) | |||
| Asthma/COPD-related illness | 0.35 | 0.05 to 2.60 | 0.308 |
| Upper respiratory tract infection | 0.35 | 0.12 to 1.00 | 0.050 |
| Non-specific respiratory tract infection | 0.21 | 0.05 to 0.97 | 0.046 |
| Viral/other illness | 0.11 | 0.02 to 0.53 | 0.005 |
| Duration of advised delay | |||
| <7 days (Ref) | |||
| ≥7 days | 0.38 | 0.11 to 1.29 | 0.120 |
| Patient behaviour | |||
| Patient did not want antibiotics (Ref) | |||
| Patient wanted antibiotics | 2.51 | 1.06 to 5.90 | 0.035 |
| Clinician perception ‘This patient wanted me to prescribe antibiotics for them’ | |||
| Clinician does not agree (Ref) | |||
| Clinician agrees | 0.38 | 0.15 to 1.00 | 0.050 |
| Clinician perception: ‘Antibiotics will help this patient get better quicker’ | |||
| Clinician does not agree (Ref) | |||
| Clinician agrees | 1.30 | 0.52 to 3.22 | 0.573 |
COPD = chronic obstructive pulmonary disease.
The clinician-level intracluster correlation coefficient (using the standard π2/3 estimator) was 0.10.
OR for a 10 percentage point increase
DISCUSSION
Summary
In this observational study of adults presenting with acute cough, delayed prescribing was used in the minority (6.3%) of consultations, but there was large variation between networks in the frequency with which this approach was used. The median advised delay period was 3 days, with a median advised delay of ≥7 days in only two of the networks. Over half (54.4%) of those prescribed delayed antibiotics took an antibiotic at some point in the 4-week follow-up period, and nearly half (44.4%) took the antibiotic prescribed at the index consultation. Two-thirds of those who took their delayed prescription started it on the day it was prescribed. Patients with a working diagnosis of upper respiratory tract infection (URTI), viral infection or non-specific infection were less likely to consume their delayed antibiotic prescription. Patient’s who indicated that they wanted an antibiotic were more likely to consume their delayed prescription, but patients whose clinician had a perception that they wanted antibiotics were less likely to consume them.
Strengths and limitations
The study described routine care in 14 networks in 13 European countries. The patient eligibility criteria were broad and patients were not randomised. Data on routine prescribing behaviour in everyday clinical practice can only be obtained through observational data. The nature of this study, with clinicians asked to record their usual practice, and patients asked to record their behaviours prospectively, meant that these results are likely to reflect routine care in these practices. The multinational nature of the study also increases the generalisability of the results and allows for comparisons between countries.
Although clinicians were asked to record the use of the delayed prescribing strategy, there are no data on what was actually said to patients in consultations, and it is possible that some clinicians suggested, either overtly or subtly, delaying use of the antibiotics but did not record this advice. As delayed prescribing was used in a minority of cases, the study may not have sufficient power to clearly describe the association of some factors with consumption of a delayed antibiotic. Furthermore, the use of a delayed prescribing approach was dominated by a few networks with only the Southampton and Milan networks using the strategy in more than 10% of consultations and only the Southampton and Balatonfüred networks with 30 or more patients who had received a delayed prescription. Therefore, the results of the regression model may reflect local factors. For the same reason, the description of how frequently the strategy was used and the median advised delay has limited precision in networks where the approach was used infrequently.
Comparison with existing literature
Despite delayed prescribing being widely researched since the late 1990s, and recommended in National Institute for Health and Clinical Excellence (NICE) guidance on antibiotic prescribing for respiratory tract infections in the UK,2 little is known about how frequently this strategy is used in conditions of usual clinical care. This is largely because the advice to delay taking a prescription is generally not coded in general practice electronic records, and therefore not easily accessible in database studies.17 The data shows that delayed antibiotic prescribing has not been widely adopted for acute cough/LRTI throughout Europe. The approach was used in approximately one-third of consultations in the English network (Southampton), and therefore may be used more extensively in England than the rest of Europe. However, this network is linked to a university department that led many locally recruiting studies of delayed prescribing, and therefore may not be representative of the rest of the UK. In the Cardiff network, delayed prescribing was used in only 3.3% of consultations. Sharland and colleagues compared data on prescribing with data on prescriptions issued by pharmacists in England and found evidence of a reduction in the proportion of antibiotic prescriptions taken to pharmacists during the late 1990s and early 2000s following the publication of a trial of delayed prescribing in 1997.10 They hypothesised that this was explained by uptake of delayed prescribing and suggested that at least in the UK use of the approach is likely to be widespread. An observational study of 273 patients with respiratory tract infections presenting in general practices in Germany found that delayed prescribing was not used at all.18 This study’s data therefore describe the largest experience so far on the use of this strategy across Europe.
Possible explanations for the apparent lack of use of delayed prescribing for acute cough across Europe include a lack of inclusion in many national guidelines, a lack of awareness of the strategy and concerns about the approach among primary care clinicians and/or patients. A qualitative interview and focus group study of primary care clinicians in the UK found low reported use of delayed prescribing and concerns about sending out conflicting messages and altering the locus of control from the clinician to the patient.19 However, the latter study provided no details about how clinicians conceived of/operationalised delayed prescribing, and the results are in contrast to the trial evidence, where the change in beliefs and behaviour are similar to no prescribing. A similar study in New Zealand, that also explored the views of patients, found considerable variation in reported use and opinions about the strategy.20 Some patients reported feeling uncomfortable about being given the decision about when to use antibiotics, and others reported taking ‘delayed’ antibiotics immediately. Some clinicians thought that the strategy helped empower patients, provided reassurance, and helped to meet their expectations, while others expressed concerns about patients using them inappropriately, about masking serious illness, and about medicolegal problems.
The study found that just under half of the patients given a delayed prescription took them; a proportion that is higher than in trials of this approach.4,6 The results are not dissimilar to observational studies of this approach in children with acute otitis media in the US, where 31% consumed a delayed prescription,21 and patients with upper respiratory tract infections (URTI) in south-east England, where 53.1% consumed their delayed antibiotics.22 However, both of these studies were conducted in a relatively small group of practices and therefore the results probably provide more accurate data on use in everyday practice.
The finding that two-thirds of those who consumed their delayed prescription did not adhere to any delay (started their antibiotics on the day they were prescribed) is in contrast to the 23.7% who started taking their antibiotics immediately in a study of URTI.22 However, this is consistent with the finding that URTI was associated with reduced odds of consuming a delayed antibiotic prescription compared with LRTI. Only nine of the 53 (17.0%) patients who received a delayed prescription in Southampton consumed their delayed prescription. Many of the Southampton practices had previously participated in trials of delayed prescribing, so this may reflect the benefit of having taken part in these trials or the effects of local opinion leaders.
In trials of delayed prescribing clinicians are usually instructed to use the approach as part of a package that includes; i) advice about the (limited) effectiveness and disadvantages of using antibiotics for their illness, and ii) advice about the likely time-course of their symptoms and how to decided when to take the antibiotic. RTI symptoms commonly last longer than patients expect, and therefore advice about how long to delay the prescription is an important element of this package. Inadequate provision of advice, or provision of inaccurate or inappropriate advice, are possible reasons for the difference in the reported consumption found in trials of this approach and in this study. In particular, the duration of delay advised by clinicians in this study was considerably shorter than the 1 to 2 weeks used in the trials and recommended in guidelines.4,6 The study did not find a significant association between a longer delay and lower consumption. However, the point estimate (0.38) was in the direction of such an association, and a lack of association may have been a Type II error. The study has no data concerning the advice given by clinicians when a delayed prescription was used. However, given the poor adherence of clinicians to the advice about duration of delay, it seems likely that other elements of the delayed prescribing strategy (such as providing advice regarding the limited effectiveness of antibiotics, their disadvantages, and when to consider using the antibiotics) were also poorly adhered to, which is likely to undermine the effectiveness of the strategy.
The method of delivering the delayed prescription to the patient may also have influenced how delayed prescriptions were used. Consumption of delayed antibiotics is likely to be lower if it is left up to the patients to collect the prescription at a later point rather than being given to the patient during the consultation.3 There is no data for this study concerning the method of providing the delayed prescription employed. The finding that the clinician’s perception that the patient wanted an antibiotic was associated with less consumption may seem at odds with the finding that patient’s express hope for antibiotics was associated with greater consumption. The most likely explanation is that clinicians would be more likely to discourage use of the delayed prescription when they had opted for a delayed (rather than immediate) prescribing approach and thought that the patient wanted to take antibiotics.
Implications for practice and research
Delayed prescribing has been shown to be an effective approach to reducing antibiotic prescribing for acute cough in clinical trials. However, it was found that the strategy was used infrequently across research Networks in Europe, and is therefore currently likely to be having little overall effect on antibiotic consumption. Indeed the finding that two-thirds of those who consumed antibiotics prescribed in their delayed prescription did so on the day it was prescribed suggests either poor communication about the delayed prescribing strategy or a degree of resistance among patients to adopt this approach. Opinion leaders may be able to play a role in increasing awareness about the need for clear communication as part of any delayed prescribing package.
Further studies can be used to explore the barriers and opportunities for improving uptake of this approach and understand how the various components of the delayed prescribing ‘package’ affect uptake of the approach and subsequent consumption of antibiotics. Two elements that deserve further clarification are the acceptability and effect of varying the advised delay period, and effect of different methods of delivery on antibiotic consumption and patient satisfaction. Finally, a clearer understanding of the views and behaviours of clinicians and patients around use of this approach is needed; for example, whether clinicians and patients feel happy with advice to delay antibiotics by 1–2 weeks, and what advice they give patients about when to use their prescription (symptoms not resolved, not getting better, getting worse) and about safety netting (when to reconsult).
Acknowledgments
We would like to thank all patients, clinicians, and networks who participated in the GRACE-01 study. We acknowledge the entire GRACE team for their expertise, hard work, and enthusiasm.
Funding
This study was funded by 6th Framework Programme of the European Commission (LSHM-CT-2005-518226). The South East Wales Trials Unit and the Wales School of Primary Care Research are funded by the National Institute for Social Care and Health Research. In Flanders (Belgium) this work was supported by the Research Foundation-Flanders (G.0274.08N).
Ethical approval
Ethical approval was obtained from ethics committees in all participating countries.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article on the Discussion Forum: http://www.rcgp.org.uk/bjgp-discuss
REFERENCES
- 1.Smith SM, Fahey T, Smucny J, et al. Antibiotics for acute bronchitis. Cochrane Database Syst Rev. 2004;(4) doi: 10.1002/14651858.CD000245.pub2. CD000245. [DOI] [PubMed] [Google Scholar]
- 2.National Institute for Health and Clinical Excellence. Prescribing of antibiotics for self-limiting respiratory tract infections in adults and children in primary care. Clinical guideline 69. London: NICE; 2008. wwwniceorguk/CG69 (accessed 17 Jul 2012) [PubMed] [Google Scholar]
- 3.Little P. Delayed prescribing of antibiotics for upper respiratory tract infection. BMJ. 2005;331(7512):301–302. doi: 10.1136/bmj.331.7512.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Little P, Rumsby K, Kelly J, et al. Information leaflet and antibiotic prescribing strategies for acute lower respiratory tract infection: a randomized controlled trial. JAMA. 2005;293(24):3029–3035. doi: 10.1001/jama.293.24.3029. [DOI] [PubMed] [Google Scholar]
- 5.Cates C. Delayed prescriptions in primary care. Br J Gen Pract. 2003;53(496):836–837. [PMC free article] [PubMed] [Google Scholar]
- 6.Dowell J, Pitkethly M, Bain J, Martin S. A randomised controlled trial of delayed antibiotic prescribing as a strategy for managing uncomplicated respiratory tract infection in primary care. Br J Gen Pract. 2001;51(464):200–205. [PMC free article] [PubMed] [Google Scholar]
- 7.Little P, Gould C, Williamson IG, et al. Pragmatic randomised controlled trial of two prescribing strategies for childhood acute otitis media. BMJ. 2001;322(7282):336–342. doi: 10.1136/bmj.322.7282.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Little P, Williamson I, Warner G, et al. Open randomised trial of prescribing strategies in managing sore throat. BMJ. 1997;314(7082):722–727. doi: 10.1136/bmj.314.7082.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spurling GKP, Del Mar CB, Dooley L, Foxlee R. Delayed antibiotics for symptoms and complications of respiratory infections. Cochrane Database Syst Rev. 2007;(2) doi: 10.1002/14651858.CD004417.pub2. CD004417. [DOI] [PubMed] [Google Scholar]
- 10.Sharland M, Kendall H, Yeates D, et al. Antibiotic prescribing in general practice and hospital admissions for peritonsillar abscess, mastoiditis, and rheumatic fever in children: time trend analysis. BMJ. 2005;331(7512):328–329. doi: 10.1136/bmj.38503.706887.AE1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butler CC, Kelly MJ, Hood K, et al. Antibiotic prescribing for discoloured sputum in acute cough/lower respiratory tract infection. Eur Respir J. 2011;38(1):119–125. doi: 10.1183/09031936.00133910. [DOI] [PubMed] [Google Scholar]
- 12.Butler CC, Hood K, Verheij TJ, et al. Variation in antibiotic prescribing and its impact on recovery in patients with acute cough in primary care: prospective study in 13 countries. BMJ. 2009;338:b2242. doi: 10.1136/bmj.b2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanton N, Hood K, Kelly MJ, et al. Are smokers with acute cough in primary care prescribed antibiotics more often, and to what benefit? An observational study in 13 European countries. Eur Respir J. 2010;35(4):761–767. doi: 10.1183/09031936.00168409. [DOI] [PubMed] [Google Scholar]
- 14.Jakobsen KA, Melbye H, Kelly MJ, et al. Influence of CRP testing and clinical findings on antibiotic prescribing in adults presenting with acute cough in primary care. Scand J Prim Health Care. 2010;28(4):229–236. doi: 10.3109/02813432.2010.506995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butler CC, Hood K, Kelly MJ, et al. Treatment of acute cough/lower respiratory tract infection by antibiotic class and associated outcomes: a 13 European country observational study in primary care. J Antimicrob Chemother. 2010;65(11):2472–2478. doi: 10.1093/jac/dkq336. [DOI] [PubMed] [Google Scholar]
- 16.Wood J, Butler CC, Hood K, et al. Antibiotic prescribing for adults with acute cough/lower respiratory tract infection: congruence with guidelines. Eur Respir J. 2011;38(1):112–118. doi: 10.1183/09031936.00145810. [DOI] [PubMed] [Google Scholar]
- 17.Gulliford M, Latinovic R, Charlton J, et al. Selective decrease in consultations and antibiotic prescribing for acute respiratory tract infections in UK primary care up to 2006. J Public Health (Oxf) 2009;31(4):512–520. doi: 10.1093/pubmed/fdp081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer T, Fischer S, Kochen MM, Hummers-Pradier E. Influence of patient symptoms and physical findings on general practitioners' treatment of respiratory tract infections: a direct observation study. BMC Fam Pract. 2005;6(1):6. doi: 10.1186/1471-2296-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters S, Rowbotham S, Chisholm A, et al. Managing self-limiting respiratory tract infections: a qualitative study of the usefulness of the delayed prescribing strategy. Br J Gen Pract. 2011 doi: 10.3399/bjgp11X593866. DOI: 10.3399/bjgp11X593866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arroll B, Goodyear-Smith F, Thomas DR, Kerse N. Delayed antibiotic prescriptions: what are the experiences and attitudes of physicians and patients? J Fam Pract. 2002;51(11):954–959. [PubMed] [Google Scholar]
- 21.Siegel RM, Kiely M, Bien JP, et al. Treatment of otitis media with observation and a safety-net antibiotic prescription. Pediatrics. 2003;112(3 Pt 1):527–531. doi: 10.1542/peds.112.3.527. [DOI] [PubMed] [Google Scholar]
- 22.Edwards M, Dennison J, Sedgwick P. Patients' responses to delayed antibiotic prescription for acute upper respiratory tract infections. Br J Gen Pract. 2003;53(496):845–850. [PMC free article] [PubMed] [Google Scholar]