Abstract
Shear stresses are powerful regulators of cellular function and potent mediators of the development of vascular disease. We have designed and optimized a system allowing the application of flow to cultured cells in a multichannel format. By using a multichannel peristaltic pump, flow can be driven continuously in the system for long-term studies in multiple isolated flow loops. A key component of the system is a dual-chamber pulse dampener that removes the pulsatility of the flow without the need for having an open system or elevated reservoir. We optimized the design parameters of the pulse dampening chambers for the maximum reduction in flow pulsation while minimizing the fluid needed for each isolated flow channel. Human umbilical vein endothelial cells (HUVECs) were exposed to steady and pulsatile shear stress using the system. We found that cells under steady flow had a marked increased production of eNOS and formation of actin stress fibers in comparison to those under pulsatile flow conditions. Overall, the results confirm the utility of the device as a practical means to apply shear stress to cultured cells in the multichannel format and provide steady, long term flow to microfluidic devices.
Keywords: Endothelial Cells, Shear Stress, Vascular Mechanotransduction, Multichannel Flow Chambers, Endothelial Nitric Oxide Synthase (eNOS), Nitric Oxide
Introduction
The past decades of cardiovascular research have revealed a variety of shear stress-mediated processes that are powerful regulators of vascular homeostasis and disease progression.1 These mechanical force-mediated mechanisms are integral in controlling a diverse set of pathophysiological processes including thrombosis, atherosclerosis and the response to arterial injury.2 Fluid shear forces have direct effects on the endothelium and mediate circulating cell interactions.3 In addition, shear forces can also have a direct mechanical effect on the vessel wall and can mediate the interactions of blood proteins and circulating cells with the luminal surface.4 As shear forces have been found to be integral in regulating many aspects of vascular biology, a number of systems have been created to examine the effects of shear forces on cultured cells in a controlled manner.5–11
Existing devices have utilized two broad categories of driving mechanisms for creating flow over cultured cells.12 The first is the parallel-plate or channel configuration.5–7 These systems consist of a culture chamber coated to allow cell culture on one or more of the surfaces. Fluid flow through the chamber is typically driven by either a syringe or peristaltic pump with an elevated reservoir to control pressure and create steady flow. Microfluidic platforms using this principle have also been adapted to serve to provide shear stress to cells in culture.13–15 An alternative type of flow system involves the use of a low-angle cone that is rotated at a constant velocity over the cells.9–11 This has the advantage of being able to create very defined fluid stresses and has been used to create both steady flow and complex, time-varying flow profiles.11
In this work we sought to create a flow system that was capable of creating steady flow in cells cultured in multichannel flow plates. A limitation of many of the current systems for creating fluid flow on cultured cells is the low throughput of the system. This is a critical parameter for studies that aim to examine the interactions of multiple pathways or to understand systems-level behavior in mechanotransduction. Another limiting factor is the length of experiments that can be performed using the system. Understanding the chronic, long-term response to changes in fluid shear stress is critical to understanding the mechanisms that mediate chronic vascular diseases. Consequently, long term experiments on cellular adaptation to shear stresses are clearly of interest. In addition, shear stresses also mediate the interactions of the various cell types that are suspended in the blood flow. Thus, in-vitro assays of cells in suspension interacting with adherent cell types are critical in studying the mechanisms of important pathophysiological processes including immune cell recruitment16, cancer cell metastasis17 and thrombosis18.
Our aim was to create a system that could overcome the limitations of previous designs by allowing higher and expandable throughput with steady flow and a closed-loop configuration to allow cells to be cultured indefinitely under flow in isolation from other flow channels. The system presented here consists of a multichannel peristaltic pump connected with a novel pulse dampening system to allow steady flow in a closed loop. We performed a detailed optimization of this pulse dampener to minimize volume and flow variation from pumping. Finally, we compared the cellular response to undamped, partially-damped and fully-damped flow in terms of shear stress induced changes in endothelial cell alignment/cytoskeletal arrangement, and production of eNOS.
Experimental Methods
Flow Loop Components and Pulsatility Measurements
A multichannel peristaltic pump (MCP Standard, Ismatec) with a 24 channel pump head was used to create flow in the system. The pumping heads were calibrated for average flow rates using pumped volume measurements to an accuracy of less than ±0.5%. The recorded flow velocities were measured using an in-line ultrasonic flowsensor (Model ME3PXN, Transonic USA) connected to a flowmeter (TS410 Transit Time Flowmeter, Transonic USA) with an accuracy of ±4%. The output signal from the flow was processed using Powerlab 4/30 and LabChart Pro software (ADInstruments). Post-processing was performed with Matlab (Mathworks). Shear stress in the flow chambers at the cell culture surface was calculated based on the mean flow rate from the flowsensor using the following relation for laminar flow in a rectangular channel:
where ,
2h is the height of the channel, 2b is the width of the channel, η is viscosity, and Φ is the flow rate through the channel19.
Cell Culture and Immunocytochemical Staining
Human umbilical vein endothelial cells (HUVECs) were used between passages 4 and 6. These cells were grown in MCDB-131 media with growth supplements (Lonza) with a total of 7.5% fetal bovine serum (FBS; Invitrogen). HUVECs were seeded into the flow chambers (μ-Slide VI0.4; ibidi, LLC) at 7×105 cells/mL and grown to confluence. During the application of flow, the entire system was placed in an incubator and kept at 37°C and 5% CO2. The cells were then washed with PBS and fixed in 4% paraformaldehyde for 20 min. The cells were then permeabilized in 0.1% Triton X-100 and blocked with 5% FBS for 1 hour. Next, the cells were exposed to a 1:50 dilution of primary antibodies for paxillin and eNOS (Santa Cruz Biotechnology) in 1% bovine serum albumin (BSA) overnight at 4°C. The cells were then washed three times in PBS/1% BSA and treated with a 1:1000 dilution of fluorescently-labeled secondary antibodies for 1 hour at room temperature. The actin cytoskeleton was also labeled by treating the cells for 1 hour in a 1:500 solution of Alexa 594-labeled phalloidin (Invitrogen). Finally the cells were mounted in a DAPI-containing mounting medium and imaged using an epifluorescent microscope (Carl Zeiss). Morphometric analysis of the cells was performed using Metamorph software (Molecular Devices).
Statistical Analysis
All results are shown as mean ± standard error of the mean. Comparisons between only two groups were performed using a 2-tailed Student’s t-test. Differences were considered significant at p<0.05. Multiple comparisons between groups were analyzed by 2-way ANOVA followed by a Tukey post-hoc test. A 2-tailed probability value <0.05 was considered statistically significant.
Results
Characterization of baseline pulsation in the multichannel peristaltic pump
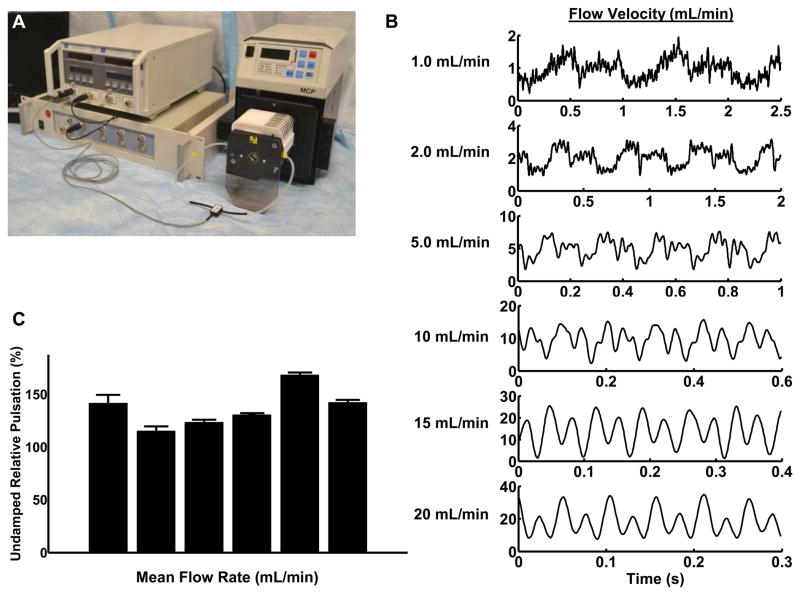
Peristaltic pumping works through the rhythmic constriction of flexible tubing to create a local pressure gradient that drives flow. This mode of pumping is advantageous in that it does not require flow-driving elements (impeller or similar components) to interact with the flow directly. We first characterized the temporal profiles of flow in the closed flow loop with a peristaltic pump. Baseline measurements of the fluid velocity are shown in Figure 1. These demonstrate that the pump produced significant pulsation in a closed-loop system with the peristaltic pump. The magnitude of the variation (from minimum trough to maximum peak) was quantified and expressed as relative to the mean velocity (Figure 1). At all pumping speeds tested, the variation in velocity was greater than 100% of the mean flow.
Figure 1. Baseline characterization of flow loop velocity profiles at different mean flow rates.
(A) Experimental set-up including an in-line ultrasonic flow probe with an analog-to-digital converter and software recording program was used to measure flow within the loop. (B) The magnitude and form of the flow from the peristaltic pump varied with the mean flow rate. (C) Pulsation of the pump varied with mean flow velocity. Quantitative measurement of relative pulsation was calculated as a ratio of minimum to maximum flow to the overall mean flow rate.
Two dampening chambers are required to effectively limit flow pulsation in a closed flow loop
We designed a prototype system for a pulsation dampener that consisted of a simple chamber having inflow and outflow ports (Supplemental Figure S1). We first incorporated this single pulse dampener into our system between the outflow of the peristaltic pump and the inlet of the parallel-plate flow channel. The pulsation dampening chamber was partially filled with culture media while it was open to the surrounding atmosphere. The lid was then closed to trap a volume of air within the system. This mode of initiating the system eliminated trapped bubbles and allowed us to adjust the ratio between air and culture media. The tubing in the non-pumping region of the system was gas permeable to allow for gas exchange.
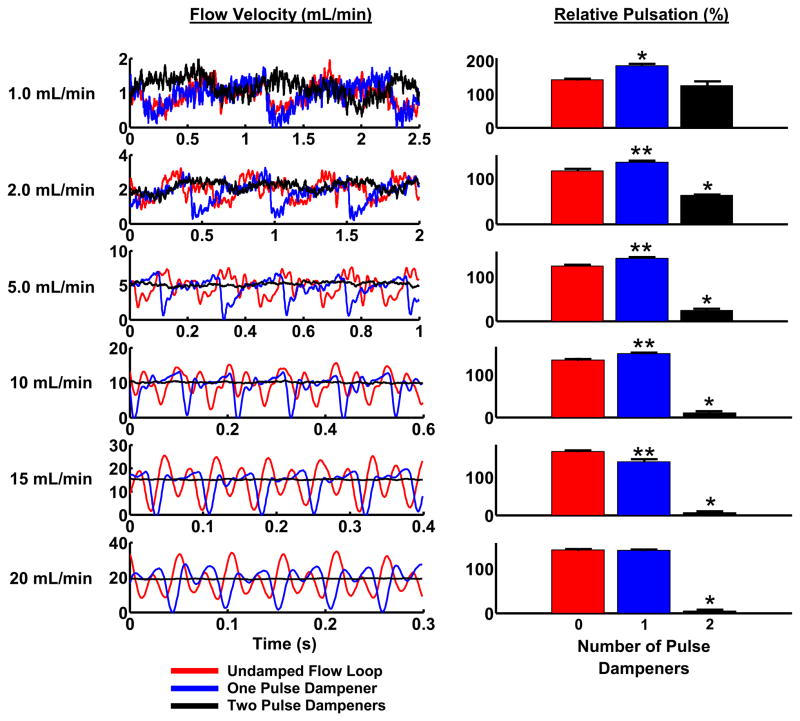
We found that a single pulse dampener in the outflow of the pump had little effect on the mean variation in flow (Figure 2). The single dampener essentially shifted the flow pulsation to a lower magnitude, lowering the maximum flow but maintaining the same mean flow variation. This effect is likely due to the closed-loop configuration of the system we were using. In a closed system, the input and output pressure for the peristaltic pump is a complex function of the type and mechanism of pumping.20 We added a second pulse dampener at the output side of the parallel-plate flow chamber and the input side of the pump. The addition of the second pulse dampener dramatically decreased the mean flow variation (Figure 2). The effectiveness of the dual pulse dampener system increased with increasing mean velocity, correlating also with increased frequency of pulsation (Figure 2).
Figure 2. Two pulse dampening units are needed to reduce pulsatility from a peristaltic pump in a closed flow loop.
Flow waveforms were recorded the undamped system, with a single pulse dampener upstream of the flow probe, and with two pulse dampeners (one on each the inlet and outlet sides of the peristaltic pump). For these recordings, the inlet and outlet of the dampening chambers were placed at the middle and lower positions, respectively, and the liquid to volume ratio was set to 0.40. *Significantly different from the other two conditions (p<0.05). **The single-damped system is significantly different than the undamped system (p<0.05).
Effect of total volume on pulse dampening effectiveness
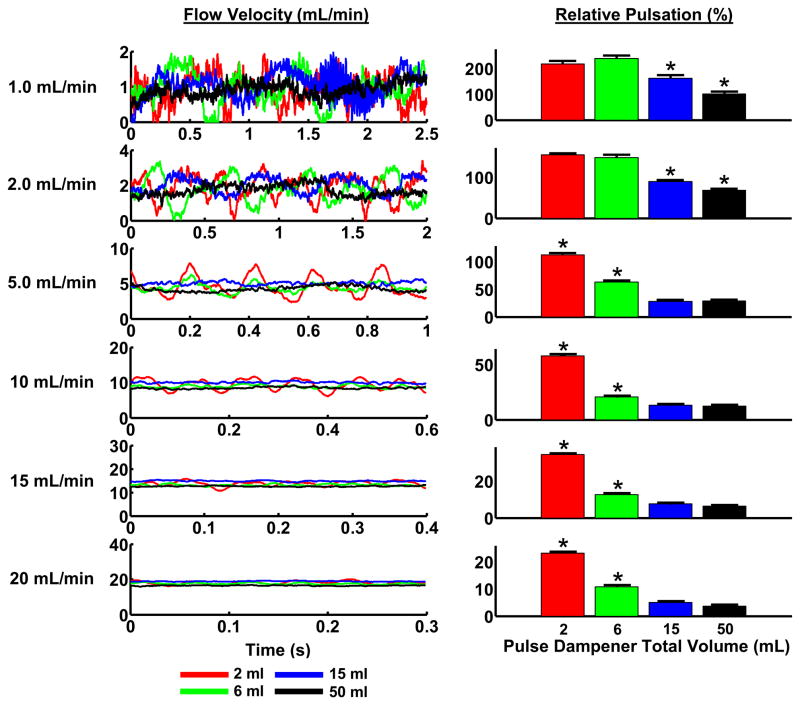
After demonstrating the need for a two pulse dampener system to reduce flow pulsation, we sought to further optimize other design parameters to maximize reduction of pulsation while minimizing the fluidic volume needed per flow loop. While a larger volume of fluid protects the cells from rapid changes in temperature, pH and the buildup of metabolic products, a compromise is needed between these factors and the economic burden of high volumes of media. To test the effects of altering the total overall volume of the pulse dampener on damping the flow pulsations, we chose a fixed intermediate liquid volume to total volume ratio of 0.4 within the various chambers. Our experimental tests of chambers of differing total volume demonstrated a relation between mean velocity of pumping, chamber size and the mean flow variation (Figure 3). Larger chambers reduced the amount of pulsatility but with diminishing returns as the chamber size increased. From the sizes tested we selected the total volume size of 15 mL as this captured the majority of the dampening effect while minimizing the media volume and maintaining an overall size that was compatible with culture incubators.
Figure 3. Increased pulse dampener volume reduces pulsation in a peristaltic, closed-loop system.
Flow waveforms were recorded using pulse dampeners of varying total volumes. The inlets and outlets were placed at the middle and lower positions, respectively, and the liquid to volume ratio was set to 0.40. Relative pulsation was calculated as the peak-to-peak measurement divided by the mean flow rate. *Significantly different values from the other three conditions (p <0.05).
Effect of liquid volume ratio on pulse dampening of flow system
We next investigated the effects of altering the liquid to total volume ratio within the pulse dampener chamber. The gas component in the dampener provides compliance through its compressibility while the liquid component provides compliance through changes in fluid height. We found that maximizing the air in the chamber (a liquid to total volume ratio of 0.25) led to close to a 100% decrease in pulsation in comparison to a ratio of 0.85 (Supplemental Figure S2).
Inlet-to-outlet height had a limited effect on pulse dampening effectiveness
The transition between small diameter tubing and a larger chamber induces a small region of complex flow that introduces resistance. In addition, this transition creates a reflected wave similar to those formed in arterial system at bifurcations.21 Flow within the chamber is complex and may contain circulation currents that depend on flow velocity. Flow circulation itself can be used as a means of pulse dampening and flow circulation pulse dampeners have been designed to work on large scale, industrial hydraulic systems.22 To examine whether these effects altered the effectiveness of the pulse dampening system, we explored the effect of altering the ratio between the inlet and outlet height on the pulse dampener effectiveness. The rationale was that the positioning of the inlet and outlet may lead to changes in flow within the dampener. We found that at most of the mean flow velocities, the location of the inlet and outlet had only a weak effect on the damping that occurred. However, in the high flow case (20 mL/min) having the flow in a high-inlet/mid-outlet configuration caused a small decrease in damping capacity (Supplemental Figure S3). This presumably was a consequence of the internal flow created within the chamber (having a greater effect as flow velocities increased). Due to its high damping capacity and compatibility with other optimized parameters, the mid-inlet/low-outlet configuration was chosen.
Alterations in fluid viscosity over the physiological range for blood had a minimal effect on pulse dampener effectiveness
While standard culture media is a common and simple way to create fluid shear over cells in culture, many groups have also explored using fluids that have a viscosity similar to blood.23, 24 In addition, studies on thrombosis have used blood in a flow system to examine the mechanisms of thrombosis.25 We examined the ability of the flow system to dampen flow pulsation while varying the viscosity of the flowing liquid to match that of culture media, blood and twice the value of blood viscosity. Over this range of viscosities there was little change in the overall pulse dampening (Supplemental Figure S4).
Final design and validation of high-throughput pulse dampener
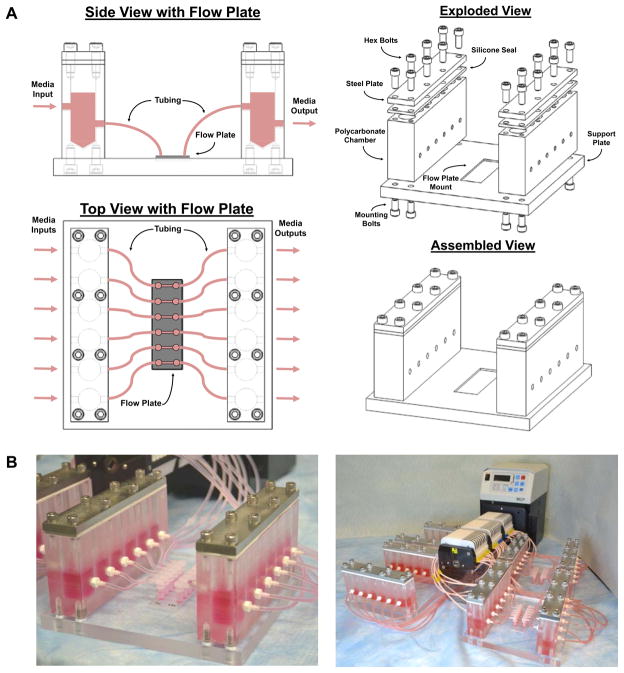
After optimizing the prototype system, we created a final version of the device that has two polycarbonate dampeners with six 15mL chambers each mounted on a support plate. (Figure 4). Two ports have been machined into each chamber to allow the mounting of a tubing connector for interfacing the pulse dampeners with the flow loop. These two multichamber blocks are made air tight with a steel plate that compresses a silicone seal. These multichamber blocks are attached to an underlying mounting plate with an indentation for mounting a multichamber flow slide. The system was designed to be modular, allowing each six-well flow plate to be used in isolation as experimental demands change. In addition, the support plate immobilizes the flow plate and simplifies the logistics of performing higher-throughput experiments with flow. Overall this system provides flow profiles for each isolated flow loop that were identical to those of the optimized prototype system.
Figure 4. Design of low-volume pulse dampener for multichannel flow.
(A) The dampener consists of two compliance chambers containing culture media and air. A flow plate with six parallel plate flow chambers was placed between the pairs of compliance chambers and connected with tubing and elbow connectors. (B) Photographs of the assembled pulse dampener with up to 24 parallel channels.
Comparison of pulsating flow versus steady flow in controlling endothelial cell response to shear stress
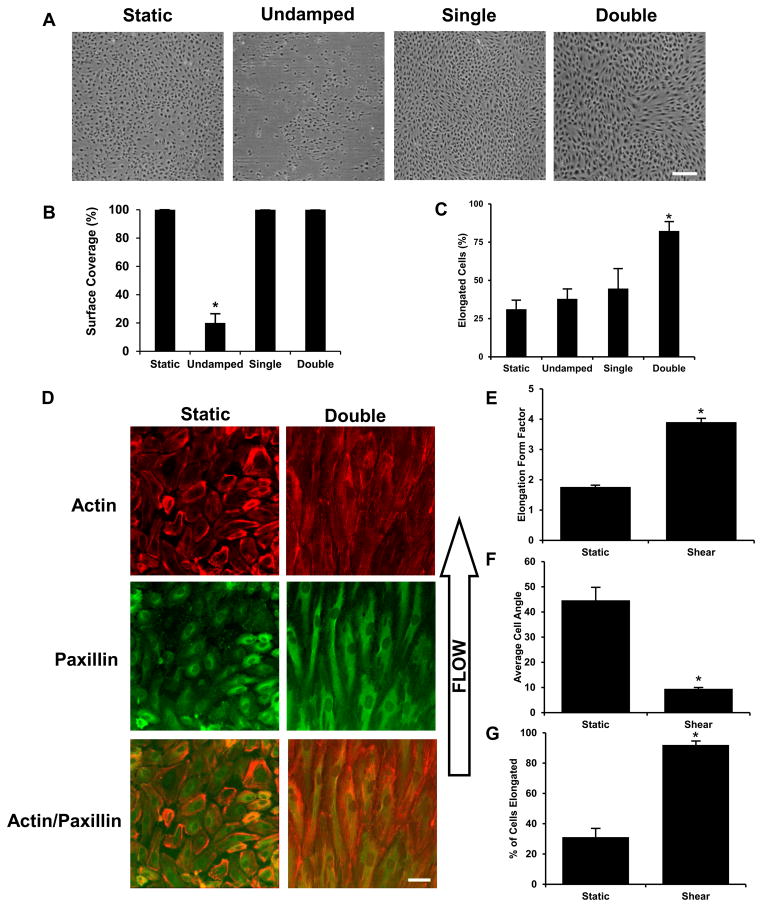
To examine the biocompatibility and utility of our multichannel flow system we examined the viability and responsiveness of cultured endothelial cells to shear flow. We applied 20 dynes/cm2 of shear stress to HUVECs for 12 hours in the flow system without pulse dampeners (undamped), with a single pulse dampener or with full system with two dampeners. Cells exposed to the undampened flow loop were found to detach and had 80% less surface coverage of cells in comparison to those in the system with a single or dual pulse dampening unit (Figure 5A and 5B).
Figure 5. Cell coverage and elongation in response to steady and pulsatile shear stress.
Mean flow of 16.3 mL/min (20 dynes/cm2) was applied to endothelial for 12 hours using the undamped, single-damped and double-damped systems. (A) Phase contrast images of the cells after each of the flow conditions: static, undamped, single-damped, and double damped. (B) Cell coverage is the percent area covered by the cells in the monolayer. The undamped system shows a significant loss in cell coverage during flow. (C) Cell elongation was defined as a major to minor axis greater than two. Bar = 200μm. (D) Mean flow of 16.3 mL/min (20 dynes/cm2) was applied to endothelial for 38 hours using the double-damped system. Bar = 50 μm. (E) Cell elongation was defined as the major to minor axis ratio. (F) Cell angle was relative to direction of flow and was calculated for wells with an elongation form factor > 2. (G) Cell elongation was defined as a major to minor axis greater than two. *Statistically different from all other groups (p < 0.05).
Shear stress induced alterations in cellular elongation and cytoskeletal organization is a well-characterized response in endothelial cells. We examined the effect of treating cells with 20 dynes/cm2 of mean shear stress using the undamped, single-damped and double-damped systems. Both the undamped and single-damped system applied pulsatile flow while the double-damped system applied steady flow. Elongation by flow was seen in 80% of cells exposed to steady flow in the double-damped system while only 40–50% of cells elongated that were exposed to pulsatile flow after 12 hours (Figure 5C). To examine the long-term compatibility of the system cell culture we applied steady flow using the pulse dampened system for 38 hours and found no signs of cell loss (Figure 5D). These cells had increased alignment and elongation in comparison to cells at the shorter time points (Figure 5E–G).
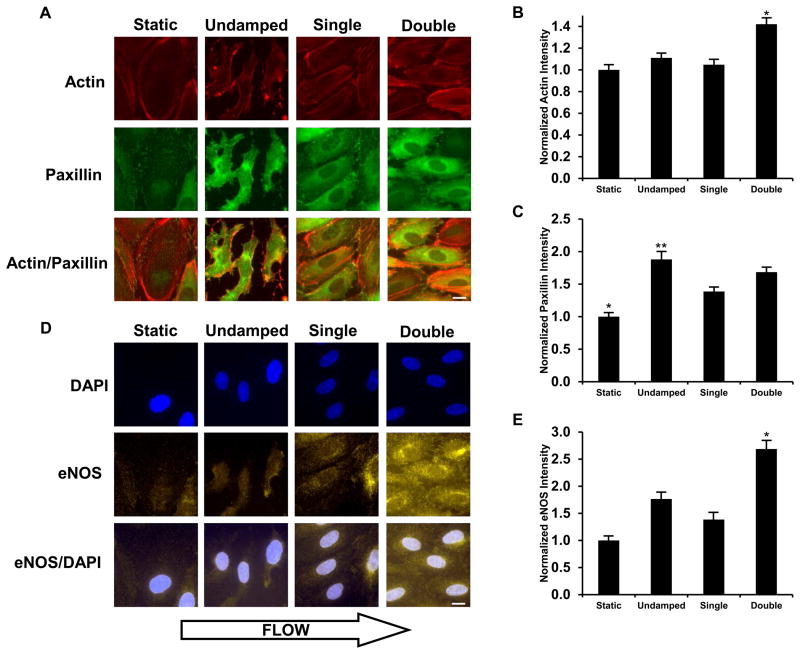
We next examined the ability of the shear stress in the system to stimulate two well-characterized endothelial cell responses to shear stress including cytoskeletal remodeling and an enhancement in the production of expression of eNOS and production of NO.25 After 12 hours of exposure to steady flow endothelial cells in the system had enhanced formation of actin stress fibers and overall actin intensity (Figure 6A and 6B). Focal adhesion formation was also enhanced under steady flow as evidenced by paxillin staining with most but most prominently in the undamped system (Figure 6C). Cells treated with 20 dynes/cm2 mean shear stress in the double-damped system also had enhancement in eNOS expression of around 2.5 fold over the static control after 12 hours of flow and at least 50% greater than that of the undamped and single-damped systems (Figure 6D and 6E).
Figure 6. Biological validation of the double-damped flow system versus undamped and single-damped flow systems.
(A) Fluorescence images were stained for actin and paxillin in each of the flow conditions: static, undamped, single-damped, and double-damped. Cells were treated with 20 dyn/cm2 shear stress for 12 hours. (B) Average actin intensity per cell was normalized to static culture levels. The double-damped system shows a significant increase in actin levels compared to the static, undamped, and single-damped conditions. (C) Average paxillin intensity per cell was normalized to static culture levels. *Significantly lower values than the other three conditions (p<0.05). **Significantly lower than the undamped system (p<0.05). (D) Fluorescence images were labeled with DAPI and an antibody against eNOS in each of the flow conditions: static, undamped, single-damped, and double-damped flow. (E) Steady flow increases eNOS to a greater extent than pulsatile flow. Average eNOS intensity per cell was normalized to static culture levels. *The double-damped system shows a significant increase in eNOS levels over the other conditions (p < 0.05). **The undamped system is significantly higher than the static system (p < 0.05). Bar = 25 μm.
Discussion
Systems for studying vascular mechanotransduction in cultured cells have shown the importance of mechanical environment in controlling many aspects of vascular biology.2 In this work, we sought to develop a simple, high-throughput device to allow the application of fluid shear stress to cultured cells that was accessible to many laboratories. Several main approaches have been used in the past to create flow over cells in culture. These include parallel-plate flow chambers,5–7 flow tubes,26 and cone-and-plate devices.9, 10 We chose to pursue the parallel-plate flow chamber configuration as it creates flow in a format highly amenable to live cell imaging, immunofluorescent staining and other common assays used in studying mechanotransduction. Parallel-plate flow chambers compatible with our system are available commercially and are also easily custom manufactured. Recently a number of microfluidic platforms have been developed to provide devices to study cultured cells under flow conditions.13–15 The system developed in this work presents a very accessible means to apply steady flow in a multiwell format. This capability allows it to interface with existing commercially available flow chambers and would allow the application of long term multichannel flow to microfluidic chips.
In our system, we found that at least one pulse damper was essential for maintaining cell viability and attachment in the closed flow loop. The likely reason for this finding is the reduction in maximum flow rate from the undampened system versus the system with a single pulse dampener. The addition of a single pulse dampener at a mean flow rate of 15 mL/min reduces the maximum flow rate of the system from 25 mL/min to around 18 mL/min. This corresponds to shift from 31 dyn/cm2 to 22 dyn/cm2 in maximum shear stress. Thus, cell detachment with this high shear rate is the likely the cause of cell loss in the undampened system at physiological flows of 18 mL/min.
We optimized our system to effectively remove pulsation in the flow loop at physiologic arterial flow rates. The compliance added into a flow system by adding a volume of trapped compressible gas acts low-pass filter, passing lower frequencies with greater magnitude than high frequencies.27 In a peristaltic pump, the flowrate is increased by accelerating the rotation of the rollers and, thus, the frequency of the rhythmic occlusion of the tubing. Consequently, as we move to higher flow rates the frequency of pulsation increases. A major consequence of this pump behavior and the use of a capacitance as a pulse dampener is that as the mean flow rate decreases and frequency of pumping decreases, the pulse damping system becomes less effective. Therefore, for slower flow rates/pulsatility a larger compliance is needed to fully dampen the unsteady nature of the flow as observed in our studies. While we concentrated on exploring a two-dampener system, one could potentially use additional dampeners in series; however, we feel that this would not have a substantial advantage over increasing the gas volume in the compliance chamber.
We tested the biocompatibility of our system by examining several shear-mediated behaviors in endothelial cells. Previous studies have shown that shear stress can activate the eNOS pathway in endothelial cells.28–32 This activity is an essential property of the healthy endothelium and is lost when endothelial cells become dysfunctional in disease.33 In our study, we compared the effects of pulsatile flow versus steady flow in activating eNOS under the identical mean flow conditions. Our results demonstrated that steady flow induces a more robust increase in the eNOS expression in comparison to the high-frequency pulsatile flow produced by the system with a single dampener. These findings are consistent with those of other groups that compared eNOS mRNA expression and found reduced production of nitric oxide and eNOS mRNA with flow simulating human arterial pulsatile flow versus continuous flow.28 In our study we used a pulsation cycle that was about 10–15 fold faster than human arterial pulsation. While this is beyond the physiological heart rate of humans, the rate of pulsation from the pump corresponds to a heart rate of 600–900 beats per minute, a rate similar to the average or elevated heart rate of mice. In addition to validating the biocompatibility of our system, these findings add suggest that endothelium is capable of sensing high frequency pulsations and can markedly alter the endothelial cell production of eNOS.
In summary, the system developed here allows the application of fluid flow to cells in culture in a multichannel format which is amenable to use in providing steady flow to macroscale and microscale fluidic devices. The device is relatively simple to manufacture and we hope will enhance the rate and quality of studies in many fields examining shear stress-mediated mechanotransduction. The overall design would be compatible to many types of studies involving fluorescence imaging and in examining interaction of cells in suspension culture with adherent cell types. The device could also be applied to in vitro experiments utilizing suspended cell interactions, including leukocyte rolling and adhesion, circulating tumor cell metastasis, and bacterial biofilm formation. Additionally, the system has potential applications in driving simultaneous continuous-flow microfluidics such as diagnostic assays and other lab-on-a-chip devices.
Supplementary Material
Acknowledgments
The authors would like to acknowledge support through the American Heart Association (10SDG2630139) and through the NIH Director’s New Innovator Grant (1DP2 OD008716-01).
Footnotes
Disclosures. None.
References
- 1.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nature clinical practice Cardiovascular medicine. 2009;6:16–26. doi: 10.1038/ncpcardio1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: Pathophysiological basis and clinical perspectives. Physiological reviews. 2011;91:327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stroka KM, Aranda-Espinoza H. A biophysical view of the interplay between mechanical forces and signaling pathways during transendothelial cell migration. The FEBS journal. 2010;277:1145–1158. doi: 10.1111/j.1742-4658.2009.07545.x. [DOI] [PubMed] [Google Scholar]
- 4.Deng X, Marois Y, Guidoin R. Fluid filtration across the arterial wall under flow conditions: Is wall shear rate another factor affecting filtration rate? Annals of the New York Academy of Sciences. 1998;858:105–115. doi: 10.1111/j.1749-6632.1998.tb10145.x. [DOI] [PubMed] [Google Scholar]
- 5.Chien S, Li S, Shyy YJ. Effects of mechanical forces on signal transduction and gene expression in endothelial cells. Hypertension. 1998;31:162–169. doi: 10.1161/01.hyp.31.1.162. [DOI] [PubMed] [Google Scholar]
- 6.Frangos JA, Eskin SG, McIntire LV, Ives CL. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985;227:1477–1479. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- 7.Geiger RV, Berk BC, Alexander RW, Nerem RM. Flow-induced calcium transients in single endothelial cells: Spatial and temporal analysis. The American journal of physiology. 1992;262:C1411–1417. doi: 10.1152/ajpcell.1992.262.6.C1411. [DOI] [PubMed] [Google Scholar]
- 8.Levitan I, Helmke BP, Davies PF. A chamber to permit invasive manipulation of adherent cells in laminar flow with minimal disturbance of the flow field. Annals of biomedical engineering. 2000;28:1184–1193. doi: 10.1114/1.1317529. [DOI] [PubMed] [Google Scholar]
- 9.Dewey CF, Jr, Bussolari SR, Gimbrone MA, Jr, Davies PF. The dynamic response of vascular endothelial cells to fluid shear stress. Journal of biomechanical engineering. 1981;103:177–185. doi: 10.1115/1.3138276. [DOI] [PubMed] [Google Scholar]
- 10.Franke RP, Grafe M, Schnittler H, Seiffge D, Mittermayer C, Drenckhahn D. Induction of human vascular endothelial stress fibres by fluid shear stress. Nature. 1984;307:648–649. doi: 10.1038/307648a0. [DOI] [PubMed] [Google Scholar]
- 11.Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vaughn S, Blackman BR, Kamm RD, Garcia-Cardena G, Gimbrone MA., Jr Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:14871–14876. doi: 10.1073/pnas.0406073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young EW, Simmons CA. Macro- and microscale fluid flow systems for endothelial cell biology. Lab on a chip. 2010;10:143–160. doi: 10.1039/b913390a. [DOI] [PubMed] [Google Scholar]
- 13.Kim J, Taylor D, Agrawal N, Wang H, Kim H, Han A, Rege K, Jayaraman A. A programmable microfluidic cell array for combinatorial drug screening. Lab on a chip. 2012 doi: 10.1039/c2lc21202a. [DOI] [PubMed] [Google Scholar]
- 14.Liu L, Loutherback K, Liao D, Yeater D, Lambert G, Estevez-Torres A, Sturm JC, Getzenberg RH, Austin RH. A microfluidic device for continuous cancer cell culture and passage with hydrodynamic forces. Lab on a chip. 2010;10:1807–1813. doi: 10.1039/c003509b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chau L, Doran M, Cooper-White J. A novel multishear microdevice for studying cell mechanics. Lab on a chip. 2009;9:1897–1902. doi: 10.1039/b823180j. [DOI] [PubMed] [Google Scholar]
- 16.Lawson C, Rose M, Wolf S. Leucocyte adhesion under haemodynamic flow conditions. Methods Mol Biol. 2010;616:31–47. doi: 10.1007/978-1-60761-461-6_3. [DOI] [PubMed] [Google Scholar]
- 17.Liang S, Slattery MJ, Dong C. Shear stress and shear rate differentially affect the multi-step process of leukocyte-facilitated melanoma adhesion. Experimental cell research. 2005;310:282–292. doi: 10.1016/j.yexcr.2005.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolandaivelu K, Swaminathan R, Gibson WJ, Kolachalama VB, Nguyen-Ehrenreich KL, Giddings VL, Coleman L, Wong GK, Edelman ER. Stent thrombogenicity early in high-risk interventional settings is driven by stent design and deployment and protected by polymer-drug coatings. Circulation. 2011;123:1400–1409. doi: 10.1161/CIRCULATIONAHA.110.003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cornish RJ. Flow in a pipe of rectangular cross-section. Proc R Soc A. 1928;120:691–700. [Google Scholar]
- 20.Moscato F, Colacino FM, Arabia M, Danieli GA. Pressure pulsation in roller pumps: A validated lumped parameter model. Medical engineering & physics. 2008;30:1149–1158. doi: 10.1016/j.medengphy.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 21.van de Vosse FNSN. Pulse wave propagation in the arterial tree. Ann Rev Fluid Mech. 2011;43:467–499. [Google Scholar]
- 22.Glover RC. US Patent 3,731,709 Liquid pulsation dampener. 1971
- 23.Slattery MJ, Liang S, Dong C. Distinct role of hydrodynamic shear in leukocyte-facilitated tumor cell extravasation. American journal of physiology Cell physiology. 2005;288:C831–839. doi: 10.1152/ajpcell.00439.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malek A, Izumo S. Physiological fluid shear stress causes downregulation of endothelin-1 mrna in bovine aortic endothelium. The American journal of physiology. 1992;263:C389–396. doi: 10.1152/ajpcell.1992.263.2.C389. [DOI] [PubMed] [Google Scholar]
- 25.Kolandaivelu K, Edelman ER. Low background, pulsatile, in vitro flow circuit for modeling coronary implant thrombosis. Journal of biomechanical engineering. 2002;124:662–668. doi: 10.1115/1.1517062. [DOI] [PubMed] [Google Scholar]
- 26.Olesen SP, Clapham DE, Davies PF. Haemodynamic shear stress activates a k+ current in vascular endothelial cells. Nature. 1988;331:168–170. doi: 10.1038/331168a0. [DOI] [PubMed] [Google Scholar]
- 27.Doebelin E. System dynamics. CRC Pres; 1998. [Google Scholar]
- 28.Blackman BR, Garcia-Cardena G, Gimbrone MA., Jr A new in vitro model to evaluate differential responses of endothelial cells to simulated arterial shear stress waveforms. Journal of biomechanical engineering. 2002;124:397–407. doi: 10.1115/1.1486468. [DOI] [PubMed] [Google Scholar]
- 29.Rubanyi GM, Romero JC, Vanhoutte PM. Flow-induced release of endothelium-derived relaxing factor. The American journal of physiology. 1986;250:H1145–1149. doi: 10.1152/ajpheart.1986.250.6.H1145. [DOI] [PubMed] [Google Scholar]
- 30.Nishida K, Harrison DG, Navas JP, Fisher AA, Dockery SP, Uematsu M, Nerem RM, Alexander RW, Murphy TJ. Molecular cloning and characterization of the constitutive bovine aortic endothelial cell nitric oxide synthase. The Journal of clinical investigation. 1992;90:2092–2096. doi: 10.1172/JCI116092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sessa WC, Pritchard K, Seyedi N, Wang J, Hintze TH. Chronic exercise in dogs increases coronary vascular nitric oxide production and endothelial cell nitric oxide synthase gene expression. Circulation research. 1994;74:349–353. doi: 10.1161/01.res.74.2.349. [DOI] [PubMed] [Google Scholar]
- 32.Davis ME, Cai H, Drummond GR, Harrison DG. Shear stress regulates endothelial nitric oxide synthase expression through c-src by divergent signaling pathways. Circulation research. 2001;89:1073–1080. doi: 10.1161/hh2301.100806. [DOI] [PubMed] [Google Scholar]
- 33.Endemann DH, Schiffrin EL. Endothelial dysfunction. Journal of the American Society of Nephrology: JASN. 2004;15:1983–1992. doi: 10.1097/01.ASN.0000132474.50966.DA. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.