Abstract
Ubiquitous protein kinase CK2 is a key regulator of cell migration, proliferation and tumor growth. CK2 is abundant in retinal astrocytes, and its inhibition suppresses retinal neovascularization in a mouse retinopathy model. In human astrocytes, CK2 co-distributes with GFAP-containing intermediate filaments, which implies its association with cytoskeleton. Contrary to astrocytes, CK2 is co-localized in microvascular endothelial cells (HBMVEC) with microtubules and actin stress fibers, but not with vimentin-containing intermediate filaments. Specific CK2 inhibitors (TBB, TBI, TBCA and DMAT) and nine novel CK2 inhibiting compounds (TID43, TID46, Quinolone-7, Quinolone-39, FNH28, FNH62, FNH64, FNH68 and FNH74) were tested at 10–200 μM for their ability to induce morphological alterations in cultured human astrocytes (HAST-40), and HBMVEC (For explanation of the inhibitor names, see “Methods” section). CK2 inhibitors caused dramatic changes in shape of cultured cells with effective inhibitor concentrations between 50 and 100 μM. Attached cells retracted, acquired shortened processes, and eventually rounded up and detached. CK2 inhibitor-induced morphological alterations were completely reversible and were not blocked by caspase inhibition. However, longer treatment or higher inhibitor concentration did cause apoptosis. The speed and potency of the CK2 inhibitors effects on cell shape and adhesion were inversely correlated with serum concentration. Western analyses showed that TBB and TBCA elicited a significant (about twofold) increase in the activation of p38 and ERK1/2 MAP kinases that may be involved in cytoskeleton regulation. This novel early biological cell response to CK2 inhibition may underlie the anti-angiogenic effect of CK2 suppression in the retina.
Keywords: Protein kinase CK2, Inhibitors, TBB, TBCA, Cell shape, Cytoskeleton
Introduction
Protein kinase CK2 is a multifunctional regulatory molecule that participates in a wide variety of cellular events by phosphorylating and/or interacting with key signaling molecules, structural proteins, and transcription factors [1, 2]. It is an important mediator of cell proliferation, migration, differentiation, survival, apoptosis, as well as tumor growth [2, 3]. Our previous data showed that CK2 also plays a role in angiogenesis. Many retinal endothelial cell responses important for the angiogenic process could be significantly downregulated by CK2 inhibitors [4, 5]. Additionally, intraperitoneally administered CK2 inhibitors significantly reduced retinal neovascularization in an in vivo model of oxygen-induced proliferative retinopathy (OIR) model (5). Moreover, systemically administered potent and specific CK2 inhibitors including 4,5,6,7-tetra-bromobenzotriazole (TBB) and tetrabromocinnamic acid (TBCA) considerably reduced incorporation of intravitre-ally injected hematopoietic stem cells (HSC) into retinal neovessels in the OIR neonatal mouse model [6]. Therefore, interfering with HSC recruitment during angiogenesis may be an important mechanism of CK2 inhibitor action.
An integral part of retinal angiogenesis is migration of astrocytes that normally lead endothelial precursor cells to areas of ischemia [7]. Cell migration depends on dynamic changes of cell shape and cytoskeletal organization, and is controlled by a complex network of regulatory pathways. CK2 is involved in the regulation of cellular morphology, and the actin and tubulin cytoskeleton networks [8]. Studies have shown that CK2 phosphorylates membrane and cytoskeletal proteins including ankyrin [9], spectrin [10], myosin [11], dystrophin [12], caldesmon [13], and adducin [9], all involved in the regulation of the actin cytoskeleton. Important roles of CK2 in regulation of the acto-myosin contractility and cell shape have been recently demonstrated after siRNA knockdown of CK2 in vascular smooth muscle [14] and human mesenchymal stem cells [15].
In addition, CK2 has been implicated in control of the microtubule cytoskeleton and its dynamics either by associating with or phosphorylating tubulin and the microtubule-associated protein-1B (MAP-1B) [16, 17]. Recently, it was shown that treatment of rat retinas with a CK2 inhibitor led to disruption of their microtubules and to blockage of nuclear migration of retinal progenitor cells during development [18].
These data, together with our observations on close connection of CK2 to the cytoskeleton in cultured astrocytic and vascular endothelial cells, prompted us to investigate a possible involvement of CK2 in the regulation of cytoskeletal organization and cell shape in retinal cells. If established, such a role may account for the suppressing effect of CK2 inhibition on angiogenesis.
Methods
CK2 inhibitor treatment and immunostaining
Human embryonic astrocytes (HAST-40), astrocytic glioblastoma cell line U87MG, and HEK 293 cells, as well as bovine retinal (BREC) and human brain microvascular endothelial cells (HBMVEC) were cultured as described elsewhere [4, 5]. Four highly specific CK2 inhibitors of the brominated benzimidazole/triazole derivatives class TBB (4,5,6,7-tetrabromo-benzotriazole), DMAT (2-dimethyl-amino-4,5,6,7-tetrabromo-1H-benzimidazole), TBCA (3-(2, 3,4,5-tetrabromophenyl)acrylic acid, or tetrabromocinnamic acid) (all from EMD Biosciences, San Diego, CA), and TBI (4,5,6,7-tetrabromobenzimidazole), as well as nine novel compounds TID43 (2-(4,5,6,7-tetraiodo-1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)acetic acid), TID46 (2-(4,5, 6,7-tetraiodo-1,3-dioxo-1,3-dihydro-2H-isoindol-2-yl)propanoic acid), Quinolone-7 (5,6,8-trichloro-4-oxo-1,4-dihy-droquinoline-3-carboxylic acid), Quinolone-39 (7,8-dichloro-4-oxo-1,4-dihydroquinoline-3-carboxylic acid), FNH28 (2-(4-hydroxy-3-methoxy-phenyl)-6,8-dimethyl-chromen-4-one), FNH62 (6-bromo-2-(4-hydroxy-3-methoxy-phenyl)-chromen-4-one), FNH64 (2-(3-chloro-4-hydroxy-5-methoxy-phenyl)-6,8-dimethyl-chromen-4-one), FNH68 (6,8-dichloro-2-(4-hydroxy-3-methoxy-phenyl)-chromen-4-one) and FNH74 (2-(3-bromo-4-hydroxy-phenyl)-6,8-dimethyl-chromen-4-one) (all from Otava Ltd, Kyiv) [19, 20] (Table 1) dissolved in dimethylsulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO, USA) were added 1 day after passage of cultured cells at concentrations of 0.01–0.2 mM to the medium containing either 5, 0.5, or 0.1% fetal bovine serum (FBS). After 1–3 days of treatment, cultured cells were fixed in 4% p-formaldehyde for 10 min, permeabilized in 0.1% Triton X-100 (Sigma-Aldrich), then blocked in 5% normal goat serum and incubated with mouse anti-CK2 antibody (D8E mAb, IgM) [21], rabbit anti-GFAP (Sigma-Aldrich), mouse anti-β-tubulin (clone 2-28-33, Sigma-Aldrich), anti-vimentin antibody (clone V9, Sigma-Aldrich), or phalloidin conjugated with rhodamine (Sigma-Aldrich) for 2 h, followed by cross-species adsorbed secondary antibodies conjugated with either fluorescein or rhodamine (Millipore, Billerica, MA).
Table 1.
Comparative ability of various CK2 inhibitors to induce cell rounding
| Rank | Compound | Class | Minimal effective concentration, μM | IC50, μM |
|---|---|---|---|---|
| 1 | DMAT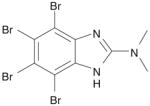
|
A | 50 | 0.13 [50] |
| 2 | TBB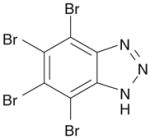
|
A | 75 | 0.15 [50] |
| 3 | TID46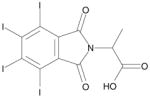
|
B | 75 | 0.15 [20] |
| 4 | TBCA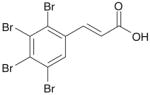
|
A | 75 | 0.11 [23] |
| 5 | Quinolone-7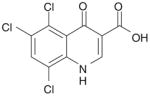
|
C | 75 | 0.3 [19] |
| 6 | Quinolone-39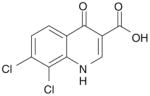
|
C | 75 | 0.8 [19] |
| 7 | TBI
|
A | 100 | 0.60 [50] |
| 8 | FNH68
|
D | 100 | 0.01 |
| 9 | FNH74
|
D | 100 | 0.05 |
| 10 | FNH64
|
D | 100 | 0.09 |
| 11 | TID43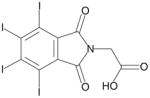
|
B | >150 | 0.3 [20] |
| 12 | FNH28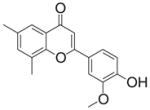
|
D | >150 | 0.1 |
| 13 | FNH62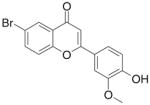
|
D | >150 | 0.12 |
CK2 inhibitors could be graded according to their minimal effective concentrations and ability to induce rapid (within 6 h) or slow (within 24 h) morphological alterations. Three compounds (TID43, FNH28 and FNH62) were graded as inactive, as they failed to induce the cell shape change even after longer (24–48 h) treatment at 150 μM
Class B—4,5,6,7-tetrahalogeno-1H-isoindole-1,3(2H)-diones (20)
Class C—3-carboxy-4(1H)-quinolones [19]
Class D—2-(4-Hydroxy-phenyl)-chromen-4-ones
The images were captured with high-sensitivity 2-megapixel color digital MicroFire camera (Optronics, Goleta, CA, USA) attached to a BX40 Olympus microscope (Olympus USA, Melville, NY, USA) and were combined using MicroFire 2.1c software.
Evaluation of cell shape changes and cell viability
Our initial finding was that CK2 inhibitors caused cell rounding. We focused our experimental observations primarily on rapid (within 6 h) cell shape changes (cell retraction and rounding) upon inhibitor administration, because longer treatments could cause the appearance of dying cells that might also get rounded. Accordingly, the study was directed to determining the concentration of a compound that was required to render the majority of cells conditionally round within 6 h after treatment. Conditionally round cells were defined as approximately round, contracted cells with no more that three remaining short extensions. If the inhibitor could elicit morphological changes only after a longer incubation period (up to 24 h), it was considered as capable of causing slow response, and thus graded as less active compound.
Cell viability was determined after 0.4% Trypan blue (Cellgro®; Mediatech, Inc., Manassas, VA, USA) staining of live cells treated with CK2 inhibitors by counting cells that excluded the dye.
Apoptosis was evaluated using Annexin V-FITC apoptosis detection Kit II (EMD Biosciences) according to the manufacturer’s instructions. A broad-spectrum caspase inhibitor, Z-Val-DL-Asp-fluoromethylketone (ZVD-fmk) (Bachem Americas, Inc., Torrance, CA), was added at 10 μM 1 h prior to TBB treatment [22].
For western blotting, cultured cell extracts were obtained using lysis buffer with proteinase and phosphatase inhibitors (1% SDS, 1% Triton X-100, 10 μg/ml aprotinin, 20 μM leupeptin, 1 mM E-64, 1 mM NaF, 200 μM sodium pervanadate, 1 mM dithiothreitol, 5 mM EDTA, 25 mM Tris, pH 6.8). Proteins were resolved by SDS-PAGE (8–16% gradient gels; Invitrogen, Carlsbad, CA, USA) and transferred to nitrocellulose membranes (Invitrogen) for immunodetection. Gel loading was normalized by β-actin content using a monoclonal antibody (clone AC-74, Sigma-Aldrich). For analyses of protein phosphorylation, monoclonal antibodies (mAb) to phospho-ERK1/2 and phospho-p38 (mAb 4370 and mAb 9215, respectively; Cell Signaling, Danvers, MA, USA) were used, and immune reaction was revealed with alkaline phosphatase-conjugated secondary antibodies (Millipore).
Statistical analysis
Data were expressed as average ± SEM and analyzed by two-tailed paired t test. P value <0.05 was considered significant.
Results
CK2 co-localizes with cytoskeletal structures
In previous work, we have shown that in cultured HAST-40 human astrocytes, CK2 co-localized with the GFAP-containing cytoskeleton [5]. Here, we show by immunofluorescence analysis that in cultured human cells HBMVEC, a major fraction of CK2 appeared to be co-localized with the tubulin-containing cytoskeleton, especially in the perinuclear region (Fig. 1a–c). In these cells, CK2 did not associate with cytoskeletal elements that contained other intermediate filament proteins, vimentin (Fig. 1d–f), and desmin (not shown). Interestingly, in a minor (10–20%) fraction of HBMVEC CK2 co-distributed with filamentous actin (F-actin) in stress fibers (Fig. 2a–c) and in cortical actin ring (Fig. 2d–f), whereas its association with microtubules was not pronounced. To our knowledge, this is the first evidence supporting CK2 association with contractile actin microfilaments, namely F-actin in stress fibers or cortical ring. Stress fiber formation is connected to generation of centripetal tension in cells that are anchoring to the substratum or during migration. It appears that in HBMVEC, CK2 may preferentially associate either with microtubules or acto-myosin stress fibers depending on physiological conditions that dictate what cytoskeletal element is being reorganized. The connection of CK2 with the cytoskeleton in cultured human astrocytes and endothelial cells might implicate CK2 in its regulation and prompted us to examine whether cytoskeleton and cell shape would become altered after treatment of the cells with CK2 inhibitors.
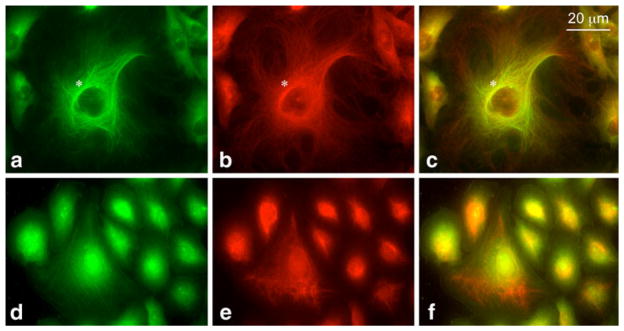
Fig. 1.
CK2 association with the cytoskeleton in HBMVEC as revealed by double immunostaining with anti-CK2 (green) and anti-tubulin (a–c) or anti-vimentin (d–f) antibodies (both red). CK2 co-distributed with the tubulin-containing cytoskeleton (especially in the perinuclear region, marked by the asterisk), but not with cytoskeletal elements that contained vimentin. (Color figure online)
Fig. 2.
Co-localization of CK2 and F-actin in HBMVEC by double staining with anti-CK2 (green) and rhodamine-phalloidin (red). Yellow color (in c and f) demonstrates co-distribution of CK2 with actin microfilaments of stress fibers (a–c, marked by the asterisks) and cortical actin ring (d–f, marked by arrowheads). The results are representative of three experiments. (Color figure online)
CK2 inhibitors cause cell rounding
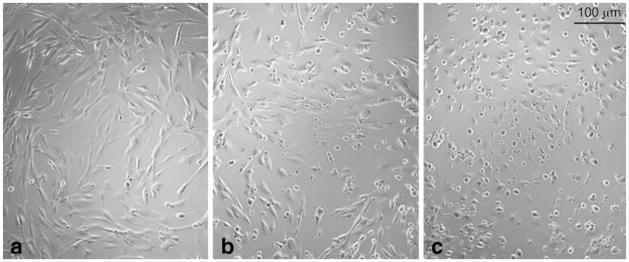
A highly specific CK2 inhibitor TBB (75 μM) caused dramatic changes in cell shape and adhesion of a number of cultured (four human and one bovine) cell lines, though time course of these changes varied depending on the cell type (see below). Typically, we observed a rapid transformation of the attached cells with highly spread elongated or polygonal cell shape to cells with or without shortened processes, and eventually, to round cells (Fig. 3a, b) that later tended to detach from the substratum. Before acquiring a round shape (presumably due to cytoskeleton collapse) and then detaching from the substratum, cells with significantly contracted cytoplasm would still remain attached to the substratum via adhesion sites connected to the shrunk cell body by very thin processes (Fig. 3e, f). Interestingly, when cells were treated with TBB at the time they were plated onto plastic dish (not 24 h after plating, as usual), they failed to attach and spread, and died within a fairly short time. Normally, trypsin-treated round cells would spread out on the substratum and then form adhesions that would allow them to escape entering apoptotic pathway. TBB appeared to block transformation of the cells that became round after trypsin treatment (not known to affect cytoskeleton directly) into attached and spread ones, and this again implicated CK2 into regulation of cell shape and/or cytoskeleton. It also suggests that cell detachment observed at a later stage was, most likely, secondary to the dramatic and rapid cell retraction (caused by CK2 inhibition) that could itself compromise adhesion.
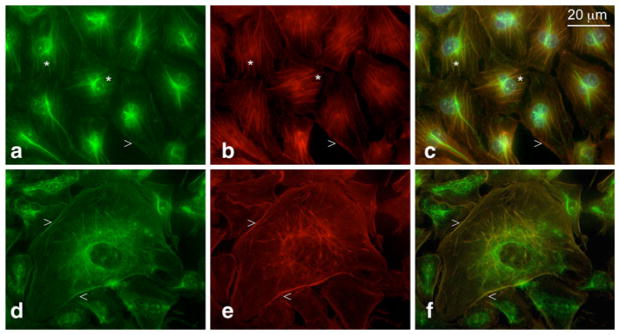
Fig. 3.
Dramatic cell shape changes induced by CK2 inhibitor TBB in HAST-40 cells: a control cells, treated with 0.05% DMSO, b cells treated with 100 μM TBB. The results are representative of five independent experiments. Co-distribution of CK2 (green color) and GFAP (red color) is preserved in HAST-40 cells treated with 0.1 mM TBB. c–e Control DMSO-treated cells, f–h TBB-treated cells. The results are representative of three experiments. (Color figure online)
The co-localization of CK2 and GFAP in HAST-40 cells (Fig. 3c–f), or tubulin in HBMVEC (not shown), was preserved upon treatment by TBB.
Similar results were obtained for other CK2 inhibitors of the same class as TBB, i.e., TBI, and more remote derivatives, DMAT and TBCA, that had effective concentrations between 50 and 100 μM. TBCA is one of the most specific CK2 inhibitors, as it has a 200-fold higher selectivity toward CK2 than toward protein kinase DYRK1a that can be blocked by other inhibitory compounds with affinities comparable to those for CK2 [23]. This result suggests that the observed cell shape changes were indeed induced by inhibition of CK2 rather than other protein kinases, such as DYRK1a.
The concentrations of TBB and other related CK2 inhibitors that induced significant rounding effect (50–100 μM) correspond well to the concentrations of TBB that produced significant suppressing effect on phosphorylation of specific CK2 targets in living cells, such as HS-1 protein [24] or Akt [25] in Jurkat cells.
As similar results were obtained for TBB and other CK2 inhibitors of its class, the data presented in this article will be further referred to as obtained with TBB as a representative of the brominated benzimidazole class of CK2 inhibitors.
The ability of various novel CK2 inhibitors to promote cell shape alterations correlates with their inhibitory activity
The goal of using various inhibitors and cell lines was to demonstrate a universal character of the observed morphological response, and to examine whether there was a relationship between their ability to induce cell shape change and the published activity data. In addition to the well-known CK2 inhibitors (TBB, TBI, DMAT, and TBCA), we tested nine novel compounds of different chemical classes that were previously characterized as effective CK2 inhibitors in the in vitro tests [19, 20], but have not been evaluated for biological effects.
Although an accurate comparison of potencies between compounds is difficult on the basis of morphological changes, such an approach could allow us to obtain a preliminary characterization of various CK2 inhibitors, and to establish a correlation between their inhibitory activity and capacity to cause morphological changes. However, this methodology had certain limitations, as we did not observe a clear linear “dose–effect” relationship for the compounds tested. Instead, they demonstrated a “threshold”-type effect when very little or no changes in cell shape were observed at lower doses, whereas upon reaching a critical (effective) concentration of the compound, the vast majority of cells developed striking changes, i.e., dramatic cell retraction and rounding. Also, the minimal effective concentrations of the compounds varied within a rather narrow range (50–100 μM) further hampering their grading. Nevertheless, we found that active compounds with similar effective concentrations were different with respect to the time required for rounding to develop, presumably, due to variations in their CK2 inhibitory activity. The compounds could be graded according to their ability to induce rapid (within 6 h) or slow (within 24 h) morphological alterations (Table 1) from the most active (ranked 1) to the least active (ranked 10). Three compounds (ranked 11–13) were graded as inactive, as they failed to induce the cell shape change even after longer (24–48 h) treatment at 150 μM. The data presented in Table 1 suggest a possible correlation between the inhibitory activity (IC50) and the biological effect (cell shape change) of various compounds. However, such a correlation is present only within the structural classes of compounds, and differences between the classes is likely to be associated with variations in solubility and permeability, as was previously shown for other kinase inhibitors [26].
TBB-induced changes in cell shape are not due to apoptosis
To address a possibility that the observed early changes in cell shape were due to apoptosis, cells were pre-treated with Z-Val-DL-Asp-fluoromethylketone (zVD-fmk), a potent broad-spectrum caspase inhibitor. ZVD-fmk did not prevent TBB-induced cell shape changes, indicating that apoptosis did not contribute to the observed effects at the early stage of TBB action (up to 24 h). ZVD-fmk, however, appeared to increase survival of the cells treated with 100 μM TBB for a longer time (3–4 days that led to cell death without ZVD-fmk), which agreed with its anti-apoptotic effect. No significant increase in staining for annexin V that is exposed on the surface of apoptotic cells was found within 24 h, the time period when cell shape alterations were fully developed (data not shown).
Cell shape changes caused by CK2 inhibition are reversible
TBB-induced early cell shape changes could be reversed after replacing the medium for the one without TBB (Fig. 4a–d), further indicating that the morphological changes were not caused by apoptosis. The reversal occurred rapidly, e.g., within 30 min after replacing the medium significant changes in cell shape and spreading were detected. This suggests that the cells with normal shape that was observed shortly after replacing TBB were the formerly round cells that rapidly acquired normal phenotype, rather than the progeny of the few TBB-resistant normal-shaped cells that proliferated after removal of TBB. However, longer TBB treatment or higher TBB concentrations did cause cell death, in accordance with well-documented anti-apoptotic effects of CK2. Also, TBB-induced changes in the activation of Akt kinase involved in pro-survival signaling were not consistent (data not shown).
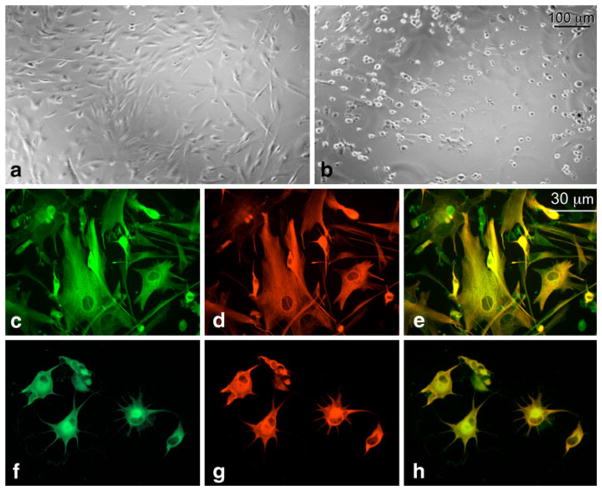
Fig. 4.
The reversal of cell shape changes induced after CK2 inhibition by TBB. The cultured U87MG cells were treated with DMSO (a), or 0.1 mM TBB (b) for 18 h, followed by incubation in medium without TBB for 6 h (c) or 24 h (d). After 6 h of incubation in the medium without TBB, formerly round cells (b) acquired nearly normal polygonal morphology (c) although they remained less spread than the cells after 24-h recovery period (d). The results are representative of three independent experiments
Serum affects TBB-induced cell shape changes
A striking effect of partial serum deprivation on TBB-induced changes was observed (Fig. 5), such that the lower the concentration of FBS, the faster and stronger the effects of TBB on cell shape and adhesion occurred. However, we did not detect a significant influence of serum concentration on the minimal effective TBB concentration, which was determined to be 75 μM. Accordingly, the speed of the reversal to normal morphology after TBB treatment correlated with concentration of FBS, and no recovery was observed when cells were treated with TBB in the presence of 0.05–0.1% FBS. Presumably, CK2 and serum factors promoting actin polymerization and spreading (lysophosphatidic acid, thrombin) act in concert [27, 28], to maintain normal morphological characteristics. Serum deprivation appeared to exacerbate the CK2 inhibitor-induced collapse of the cytoskeleton and cell shape changes.
Fig. 5.
Partial serum deprivation facilitates the development of TBB-induced cell shape changes in HAST-40 cultured cells. Cells were incubated with medium containing 0.5% FBS and DMSO (a), or treated with 0.1 mM TBB in the presence of 5% (b) or 0.5% (c) FBS for 6 h. Virtually all the cells treated with TBB in the presence of 0.5% FBS retracted and turned round, whereas the majority of the cells treated at 5% FBS failed to do so. The results are representative of four experiments
Cell shape alterations induced by CK2 inhibition vary among cell lines
There were significant differences between various cells with respect to the time course of TBB-induced effects. Cultured normal (HAST-40) and transformed (U87MG) human astrocytes showed strong TBB-induced changes within 3 h, whereas it took cultured retinal (BREC) or microvascular brain endothelial cells (HBMVEC) much longer times to develop those changes (20 h and 8 h, respectively). Contrary to this, the reversal to the normal morphology was noticeable just after 2–3 h after medium replacement for BREC, whereas the recovery of HAST-40 cells was significantly delayed (or did not occur at all if the recovery proceeded at low serum concentrations). Presumably, there is a wide range of TBB sensitivity among the cell lines tested with HAST-40 and U87MG cells being the most and BREC, the least susceptible to the action of TBB. This may reflect variations of signaling pathways involved in cell shape regulation and affected by CK2 inhibition.
CK2 is apparently involved in control of MAPK signaling
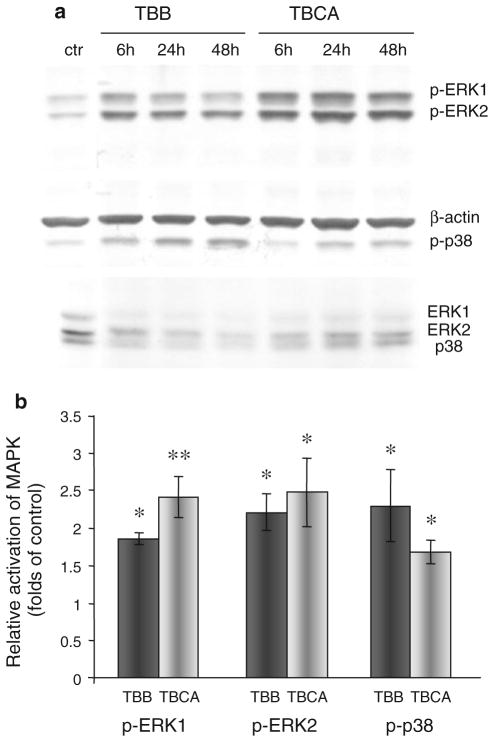
To test the possible role of CK2 in the regulation of major signaling pathways involving p38 MAPK (mitogen-activated protein kinase) and ERK (extracellular signal-regulated kinase) their activation in HBMVEC after CK2 inhibition was examined by western analysis. CK2 inhibitors (TBB and TBCA) elicited a significant (about twofold, P < 0.05) elevation in phosphorylation of p38 and ERK1/2 (Fig. 6). This activation was detectable within 6 h after treatment, roughly coinciding with cell shape transformation, and persisted during fully developed cell shape changes, declining by 48 h. These data were partly corroborated by preliminary analyses of 18 protein kinases using human Phospho-MAPK Array that showed up-regulation in phosphorylation of ERK1, ERK2, and their substrate kinase MSK2, after TBCA treatment of HBM-VEC for 24 h (data not shown). Further studies will be required to elucidate possible roles of p38, and ERK in the cell shape alterations promoted by CK2 inhibition using inhibitors of activated MAP kinases.
Fig. 6.
MAPK activation after CK2 inhibition. a Western analyses of activated signaling molecules ERK1, ERK2, and p38 MAPK after treatment of HBMVEC with CK2 inhibitors TBB and TBCA for 6–48 h. Phosphospecific p38 or ERK1 and ERK2 antibodies were used to visualize phosphorylated p38 MAPK and ERKs. Antibodies to β-actin or unphosphorylated ERKs and p38 were used to ensure equal loading. The typical blot is representative of three independent experiments. ctr, DMSO-treated control. b Quantitation of CK2 inhibitor-induced phosphorylation of MAPK. The bar graph represents average ± SEM of pooled values (n = 5) of densitometric scans. *P < 0.05, **P < 0.01 compared with control values (taken as 1) by paired two-tailed t test
Discussion
Alterations of cell shape and cytoskeletal organization may be important during development and differentiation, and can underlie certain pathological conditions. Possible involvement of CK2 in the regulation of cytoskeleton has been proposed earlier [8] based on its association with and phosphorylation of cytoskeletal proteins obtained mostly in the in vitro experiments, and can now be supported by more recent data on utilizing pharmacological inhibition of CK2 in cells. For example, formation of the axon initial segment (AIS) is an early step in the development of neurons, and it appears to be regulated by AIS-associated CK2. Inhibition of CK2 by DMAT has recently been reported to modify AIS microtubule characteristics and impair the association of ankyrin G with AIS [29].
In a cellular model of α-synucleinopathy based on overexpression of α-synuclein in oligodendroglial cells, α-synuclein aggregation led to microtubule retraction from cell processes. Such aggregation was promoted by the phosphorylation of α-synuclein at Ser129, and the aggregation could be partially reversed by CK2 inhibitors (DMAT, DRB) treatment [30]. This suggested a certain role for CK2 in the development of microtubule retraction that precedes entering the apoptotic pathway and subsequent oligodendroglial degeneration.
Finally, recent findings reported by Wang and Jang [15] showed close similarity of minor alterations in cell morphology after long-term suppression of CK2 and after X-ray exposure, accompanied by reduction in myosin-9 phosphorylation. It was suggested that CK2 might regulate the cytoskeleton reorganization in this peculiar case of ionizing radiation-induced senescence.
Here, we present evidence for possible role of CK2 in the regulation of overall cell morphology and cytoskeleton. We have shown an association of CK2 with various cytoskeletal components (microtubules and GFAP-containing intermediate filaments) and, for the first time, a co-localization of CK2 with the actin microfilaments in stress fibers in cultured human cells. We also presented the first data to document a novel early morphological cell response upon chemical inhibition of CK2 in cells. Our data show that a number of highly specific CK2 inhibitors (including TBB and TBCA) caused dramatic changes in cell shape and adhesion of cultured human astrocytes and microvascular endothelial cells. We observed a rather fast transformation of the attached cells with well-spread polygonal cell shape to contracted cells with shortened processes, and eventually, to round cells that often detached from the substratum. TBB-induced morphological changes could be reversed rapidly after removing CK2 inhibitor from the medium indicating that these alterations in cell shape were not due to apoptosis. A more direct evidence for that was obtained when cells were pre-treated by caspase inhibitor and apoptosis blocker ZVD-fmk that failed to prevent TBB-induced effects on cell shape and adhesion. These data, together with dramatic morphological alterations after CK2 inhibition, may implicate CK2 in the regulation of the cytoskeleton. Interestingly, Lim et al. [16] reported that siRNA knockdown of CK2α/α′ caused marked destabilization of microtubules that was completely compensated by expressing enzymatically inactive mutant of CK2 in the CK2α/α′ knockdown cells. It was assumed that CK2 plays a role as a microtubule-associated protein that exercises its regulatory action on microtubule organization via its physical association with microtubules but not through its enzymatic activity. On the contrary, our data showed that suppression of CK2 enzymatic activity by a specific inhibitor TBB led to dramatic changes in the cytoskeleton organization resulting in cell rounding. Whereas one cannot exclude that TBB, when bound near the ATP-binding site of CK2, may physically interfere with the ability of CK2 to bind and stabilize microtubules, there are no data yet to confirm that possibility.
Phosphorylation status of cytoskeletal and adhesion components may be very important for their function, and the balance of protein kinase and protein phosphatase (PP) activities can play a significant role in the determination of cell shape that might be regulated in different ways. One may suggest that CK2 inhibitors can mimic the effect of PP and thus affect the balance of protein kinase and PP activities. Calyculin A, an inhibitor of protein phosphatases PP1 and PP2A, caused cell retraction and rounding of bovine endothelial cells, and these morphological changes were strongly inhibited by staurosporine, a wide range protein kinase inhibitor. These results suggested that calyculin A unmasked the activities of some protein Ser/Thr kinases that might lead to cell shape changes [31]. A good candidate here may be myosin light chain (MLC) kinase since inhibition of MLC-phosphatase by Calyculin A promotes acto-myosin contractility leading to rounding of myofibroblast cells [32]. We, however, observed that it was inhibition of CK2 kinase activity that led to similar morphological changes, indicating that there may be multiple ways of cell shape regulation. Interestingly, CK2 appears to be involved, as a terminal effector, in a novel pathway of estrogen regulation of the cytoskeleton in epithelial cells via the epidermal growth factor (EGF) and its receptor, and ERK-MAPK cascades. It has been demonstrated that in cervical epithelial cells treated with estrogen, there is an increase in phosphorylation of non-muscle myosin-II mediated by CK2 that can block myosin filamentation and disrupt the actomyosin ring increasing cell deformability [33].
One might suggest that CK2 can either phosphorylate cytoskeletal proteins, or interact with and modulate major signaling networks that control cytoskeletal organization, such as those involving MAP kinases. Various effects of activated MAP kinases with possible implication in the regulation of cytoskeleton include p38 MAPK phosphorylation of microtubule-associated protein Tau [34], or ERK1/2 activation of the protein kinase RSK1 that phosphorylates filamin A [35], a cytoskeleton-associated protein implicated in membrane ruffling. MAPK-activated kinase MK2 has multiple loci of cytoskeleton regulation, as it phosphorylates intermediate filament protein vimentin [36], and controls actin reorganization by phosphorylating both HSP27 [37] and p16-Arc, a component of Arp2-complex [38].
p38 MAPK can interact with CK2 and allosterically regulate its activity [39, 40], whereas ERK2 phosphorylates CK2α and enhances it activity in response to EGF stimulation [41]. Although there is no direct evidence for CK2 phosphorylation of MAP kinases, modulation of CK2 may affect MAPK activation through regulation by feedback loops, or via signaling scaffold structures as was described for p38 MAPK and ERK1/2 [42–44]. CK2 has been identified as a component of the molecular scaffold that facilitated Raf/MEK/ERK signaling, by performing as a Raf kinase N-regional kinase [45]. Conversely, CK2 overexpression was found to inhibit serum-induced activation of ERK2 in NIH 3T3 cells, possibly through CK2 activation of PP2A that can dephosphorylate ERK activator MEK1, and down-regulate the ERK-MAPK pathway [46]. The latter observation is in accord with our data that demonstrated upregulation of activated ERK1/2 after CK2 inhibition by TBB or TBCA in human cultured vascular endothelial cells (Fig. 5). Similarly, another CK2 inhibitor, apigenin, markedly increased ERK1/2 phosphorylation in serum-starved HeLa cells [47]. However, this effect may not be attributed solely to CK2 inhibition, as apigenin has much lower selectivity than TBB or TBCA, and can also inhibit other protein kinases, such as PI3 kinase [48], or protein kinase C [49]. Further studies are required to understand possible ways of CK2 regulation of MAPK and other signaling pathways involved in the control of the cytoskeleton and cell motility that may play an important role during pathological neovascularization.
In conclusion, CK2 inhibition in cultured human cells causes dramatic early changes in cell shape and cytoskeleton organization, which presumably may affect their adhesive properties and migratory ability. One may suggest that similar changes in retinal astrocytes and/or vascular endothelial cells may underlie the previously reported anti-angiogenic effect of CK2 inhibition in the mouse model of oxygen-induced retinopathy.
Acknowledgments
This work was supported by R01 EY13431 (AAK, AVL); M01 RR00425; Winnick Family Foundation (AVL); Department of Surgery, Cedars-Sinai Medical Center (AAK, AVL); OneSight Research Foundation (AVL); Eye Defects Research Foundation (AAK, AVL); grant 0107U004939 from the National Academy of Sciences of Ukraine (AGG, VGB, SMY); NIH grant UO1-CA15062 and V.A. Medical Research Funds (KA); grant from Warsaw University of Technology (MB).
Abbreviations
- CK2
Casein kinase II or 2
- ERK
Extracellular signal-regulated kinase
- GFAP
Glial fibrillar acidic protein
- HBMVEC
Human brain microvascular endothelial cells
- MAPK
Mitogen-activated protein kinase
- TBB
4,5,6,7-Tetrabromobenzotriazole
- ZVD-fmk
Z-Val-DL-Asp-fluoromethylketone
Contributor Information
A. A. Kramerov, Email: kramerova@cshs.org, Ophthalmology Research Laboratories, Cedars-Sinai Medical Center, Room SSB-341, CSMC, 8700 Beverly Boulevard, Los Angeles, CA 90048, USA
A. G. Golub, Institute of Molecular Biology and Genetics, National Academy of Sciences of Ukraine, Kyiv, Ukraine
V. G. Bdzhola, Institute of Molecular Biology and Genetics, National Academy of Sciences of Ukraine, Kyiv, Ukraine
S. M. Yarmoluk, Institute of Molecular Biology and Genetics, National Academy of Sciences of Ukraine, Kyiv, Ukraine
K. Ahmed, Cellular and Molecular Biochemistry Research Laboratory, Veterans Affairs Medical Center, University of Minnesota, Minneapolis, MN, USA. Department of Laboratory Medicine and Pathology, University of Minnesota, Minneapolis, MN, USA
M. Bretner, Faculty of Chemistry, Warsaw University of Technology, Warsaw, Poland. Institute of Biochemistry and Biophysics, Polish Academy of Sciences, Warsaw, Poland
A. V. Ljubimov, Ophthalmology Research Laboratories, Cedars-Sinai Medical Center, Room SSB-341, CSMC, 8700 Beverly Boulevard, Los Angeles, CA 90048, USA. David Geffen School of Medicine at UCLA, Los Angeles, CA, USA
References
- 1.Meggio F, Pinna LA. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 2003;17:349–368. doi: 10.1096/fj.02-0473rev. [DOI] [PubMed] [Google Scholar]
- 2.Guerra B, Issinger O-G. Protein kinase CK2 in human disease. Curr Med Chem. 2008;15:1870–1886. doi: 10.2174/092986708785132933. [DOI] [PubMed] [Google Scholar]
- 3.Trembley JH, Wang G, Unger G, Slaton J, Ahmed K. Protein kinase CK2 in health and disease: CK2: a key player in cancer biology. Cell Mol Life Sci. 2009;66:186–1858. doi: 10.1007/s00018-009-9154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ljubimov AV, Caballero S, Aoki A, Pinna LA, Grant MB, Castellon R. Involvement of protein kinase CK2 in angi-ogenesis and retinal neovascularization. Invest Ophthalmol Vis Sci. 2004;45:4583–4591. doi: 10.1167/iovs.04-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kramerov AA, Saghizadeh M, Pan H, Kabosova A, Montenarh M, Ahmed K, Penn JS, Chan CK, Hinton DR, Grant MB, Ljubimov AV. Expression of protein kinase CK2 in astroglial cells of normal and neovascularized retina. Am J Pathol. 2006;168:1722–1736. doi: 10.2353/ajpath.2006.050533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kramerov AA, Saghizadeh M, Caballero S, Shaw LC, Li Calzi S, Bretner M, Montenarh M, Pinna LA, Grant MB, Ljubimov AV. Inhibition of protein kinase CK2 suppresses angiogenesis and hematopoietic stem cell recruitment to retinal neovascularization sites. Mol Cell Biochem. 2008;316:177–186. doi: 10.1007/s11010-008-9831-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fruttiger M. Development of the mouse retinal vasculature: angiogenesis versus vasculogenesis. Investig Ophthalmol Vis Sci. 2002;43:522–527. [PubMed] [Google Scholar]
- 8.Canton DA, Litchfield DW. The shape of things to come: an emerging role for protein kinase CK2 in the regulation of cell morphology and the cytoskeleton. Cell Signal. 2006;18:267–275. doi: 10.1016/j.cellsig.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Wei TQ, Tao M. Human erythrocyte casein kinase II: characterization and phosphorylation of membrane cytoskeletal proteins. Arch Biochem Biophys. 1993;307:206–216. doi: 10.1006/abbi.1993.1580. [DOI] [PubMed] [Google Scholar]
- 10.Greenquist AC, Wyatt JL, Guatelli JC, Shohet SB. The spectrin phosphorylation reaction in human erythrocytes. Prog Clin Biol Res. 1978;20:1–24. [PubMed] [Google Scholar]
- 11.Singh TJ, Akatsuka A, Blake KR, Huang KP. Phosphorylation of troponin and myosin light chain by cAMP-independent casein kinase-2 from rabbit skeletal muscle. Arch Biochem Biophys. 1983;220:615–622. doi: 10.1016/0003-9861(83)90454-x. [DOI] [PubMed] [Google Scholar]
- 12.Luise M, Presotto C, Senter L, Betto R, Ceoldo S, Furlan S, Salvatori S, Sabbadini RA, Salviati G. Dystrophin is phosphorylated by endogenous protein kinases. Biochem J. 1993;293:243–247. doi: 10.1042/bj2930243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vorotnikov AV, Shirinsky VP, Gusev NB. Phosphorylation of smooth muscle caldesmon by three protein kinases: implication for domain mapping. FEBS Lett. 1988;236:321–324. doi: 10.1016/0014-5793(88)80047-4. [DOI] [PubMed] [Google Scholar]
- 14.Smolock EM, Wang T, Nolt JK, Moreland RS. siRNA knock down of casein kinase 2 increases force and cross-bridge cycling rates in vascular smooth muscle. Am J Physiol Cell Physiol. 2007;292:C876–C885. doi: 10.1152/ajpcell.00343.2006. [DOI] [PubMed] [Google Scholar]
- 15.Wang D, Jiang DJ. Protein kinase CK2 regulates cytoskeletal reorganization during ionizing radiation-induced senescence of human mesenchymal stem cells. Cancer Res. 2009;69:8200–8207. doi: 10.1158/0008-5472.CAN-09-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim AC, Tiu SY, Li Q, Qi RZ. Direct regulation of microtubule dynamics by protein kinase CK2. J Biol Chem. 2004;279:4433–4439. doi: 10.1074/jbc.M310563200. [DOI] [PubMed] [Google Scholar]
- 17.Moreno FJ, Díaz-Nido J, Jiménez JS, Avila J. Distribution of CK2, its substrate MAP1B and phosphatases in neuronal cells. Mol Cell Biochem. 1999;191:201–205. [PubMed] [Google Scholar]
- 18.Carneiro AC, Fragel-Madeira L, Silva-Neto MA, Linden R. A role for CK2 upon interkinetic nuclear migration in the cell cycle of retinal progenitor cells. Dev Neurobiol. 2008;68:620–631. doi: 10.1002/dneu.20613. [DOI] [PubMed] [Google Scholar]
- 19.Golub AG, Yakovenko OY, Bdzhola VG, Sapelkin VM, Zien P, Yarmoluk SM. Evaluation of 3-carboxy-4(1H)-quinolones as inhibitors of human protein kinase CK2. J Med Chem. 2006;49:6443–6450. doi: 10.1021/jm050048t. [DOI] [PubMed] [Google Scholar]
- 20.Golub AG, Yakovenko OY, Prykhod’ko AO, Lukashov SS, Bdzhola VG, Yarmoluk SM. Evaluation of 4,5,6,7-tetrahalogeno-1H-isoindole-1,3(2H)-diones as inhibitors of human protein kinase CK2. Biochim Biophys Acta. 2008;1784:143–149. doi: 10.1016/j.bbapap.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Goueli SA, Davis AT, Arfman E, Vessella R, Ahmed K. Monoclonal antibodies against nuclear casein kinase NII (PK-N2) Hybridoma. 1990;9:609–618. doi: 10.1089/hyb.1990.9.609. [DOI] [PubMed] [Google Scholar]
- 22.Yde CW, Frogne T, Lykkesfeldt AE, Fichtner I, Issinger OG, Stenvang J. Induction of cell death in antiestrogen resistant human breast cancer cells by the protein kinase CK2 inhibitor DMAT. Cancer Lett. 2007;256:229–237. doi: 10.1016/j.canlet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Pagano MA, Poletto G, Di Maira G, Cozza G, Ruzzene M, Sarno S, Bain J, Elliott M, Moro S, Zagotto G, Meggio F, Pinna LA. Tetrabromocinnamic acid (TBCA) and related compounds represent a new class of specific protein kinase CK2 inhibitors. Chembiochem. 2007;8:129–139. doi: 10.1002/cbic.200600293. [DOI] [PubMed] [Google Scholar]
- 24.Sarno S, Reddy H, Meggio F, Ruzzene M, Davies SP, Donella-Deana A, Shugar D, Pinna LA. Selectivity of 4,5,6,7-tetrabromobenzotriazole, an ATP site-directed inhibitor of protein kinase CK2 (‘casein kinase-2’) FEBS Lett. 2001;496:44–48. doi: 10.1016/s0014-5793(01)02404-8. [DOI] [PubMed] [Google Scholar]
- 25.Di Maira G, Salvi M, Arrigoni G, Marin O, Sarno S, Brustolon F, Pinna LA, Ruzzene M. Protein kinase CK2 phosphorylates and upregulates Akt/PKB. Cell Death Differ. 2005;12:668–677. doi: 10.1038/sj.cdd.4401604. [DOI] [PubMed] [Google Scholar]
- 26.Norris JL, Williams KP, Janzen WP, Hodge CN, Blackwell LJ, Popa-Burke IG. Selectivity of SB203580, SB202190 and other commonly used p38 inhibitors: profiling against a multi-enzyme panel. Lett Drug Des Discov. 2005;2:516–521. [Google Scholar]
- 27.Jalink K, Eichholtz T, Postma FR, van Corven EJ, Moolenaar WH. Lysophosphatidic acid induces neuronal shape changes via a novel, receptor-mediated signaling pathway: similarity to thrombin action. Cell Growth Differ. 1993;4:247–255. [PubMed] [Google Scholar]
- 28.Suidan HS, Nobes CD, Hall A, Monard D. Astrocyte spreading in response to thrombin and lysophosphatidic acid is dependent on the Rho GTPase. Glia. 1997;21:244–252. doi: 10.1002/(sici)1098-1136(199710)21:2<244::aid-glia7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 29.Sanchez-Ponce D, Muñoz A, Garrido JJ. Casein kinase 2 and microtubules control axon initial segment formation. Mol Cell Neurosci. 2011 doi: 10.1016/j.mcn.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Kragh CL, Lund LB, Febbraro F, Hansen HD, Gai WP, El-Agnaf O, Richter-Landsberg C, Jensen PH. α-Synuclein aggregation and Ser-129 phosphorylation-dependent cell death in oligodendroglial cells. J Biol Chem. 2009;284:10211–10222. doi: 10.1074/jbc.M809671200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leung YM, Kwan TK, Kwan CY, Daniel EE. Calyculin A-induced endothelial cell shape changes are independent of [Ca2+](i) elevation and may involve actin polymerization. Biochim Biophys Acta. 2002;1589:93–103. doi: 10.1016/s0167-4889(02)00161-1. [DOI] [PubMed] [Google Scholar]
- 32.Parizi M, Howard EW, Tomasek JJ. Regulation of LPA-promoted myofibroblast contraction: role of Rho, myosin light chain kinase, and myosin light chain phosphatase. Exp Cell Res. 2000;254:210–220. doi: 10.1006/excr.1999.4754. [DOI] [PubMed] [Google Scholar]
- 33.Li X, Zhou L, Gorodeski GI. Estrogen regulates epithelial cell deformability by modulation of cortical actomyosin through phosphorylation of nonmuscle myosin heavy-chain II-B filaments. Endocrinology. 2006;147:5236–5248. doi: 10.1210/en.2006-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goedert M, Hasegawa M, Jakes R, Lawler S, Cuenda A, Cohen P. Phosphorylation of microtubule-associated protein tau by stress-activated protein kinases. FEBS Lett. 1997;409:57–62. doi: 10.1016/s0014-5793(97)00483-3. [DOI] [PubMed] [Google Scholar]
- 35.Woo MS, Ohta Y, Rabinovitz I, Stossel TP, Blenis J. Ribosomal S6 kinase (RSK) regulates phosphorylation of filamin A on an important regulatory site. Mol Cell Biol. 2004;24:3025–3035. doi: 10.1128/MCB.24.7.3025-3035.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng TJ, Lai YK. Identification of mitogen-activated protein kinase-activated protein kinase-2 as a vimentin kinase activated by okadaic acid in 9L rat brain tumor cells. J Cell Biochem. 1998;71:169–181. [PubMed] [Google Scholar]
- 37.Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda AR. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 38.Singh S, Powell DW, Rane MJ, Millard TH, Trent JO, Pierce WM, Klein JB, Machesky LM, McLeish KR. Identification of the p16-Arc subunit of the Arp 2/3 complex as a substrate of MAPK-activated protein kinase 2 by proteomic analysis. J Biol Chem. 2003;278:36410–36417. doi: 10.1074/jbc.M306428200. [DOI] [PubMed] [Google Scholar]
- 39.Sayed M, Kim SO, Salh BS, Issinger OG, Pelech SL. Stress-induced activation of protein kinase CK2 by direct interaction with p38 mitogen-activated protein kinase. J Biol Chem. 2000;275:16569–16573. doi: 10.1074/jbc.M000312200. [DOI] [PubMed] [Google Scholar]
- 40.Hildesheim J, Salvador JM, Hollander MC, Fornace AJ., Jr Casein kinase 2- and protein kinase A-regulated adenomatous polyposis coli and beta-catenin cellular localization is dependent on p38 MAPK. J Biol Chem. 2005;280:17221–17226. doi: 10.1074/jbc.M410440200. [DOI] [PubMed] [Google Scholar]
- 41.Ji H, Wang J, Nika H, Hawke D, Keezer S, Ge Q, Fang B, Fang X, Fang D, Litchfield DW, Aldape K, Lu Z. EGF-induced ERK activation promotes CK2-mediated disassociation of alpha-Catenin from beta-Catenin and transactivation of beta-Catenin. Mol Cell. 2009;36:547–559. doi: 10.1016/j.molcel.2009.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang H, Shi X, Hampong M, Blanis L, Pelech S. Stress-induced inhibition of ERK1 and ERK2 by direct interaction with p38 MAP kinase. J Biol Chem. 2001;276:6905–6908. doi: 10.1074/jbc.C000917200. [DOI] [PubMed] [Google Scholar]
- 43.McKay MM, Ritt DA, Morrison DK. Signaling dynamics of the KSR1 scaffold complex. Proc Natl Acad Sci USA. 2009;106:11022–11027. doi: 10.1073/pnas.0901590106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramos JW. The regulation of extracellular signal-regulated kinase (ERK) in mammalian cells. Int J Biochem Cell Biol. 2008;40:2707–2719. doi: 10.1016/j.biocel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 45.Ritt DA, Zhou M, Conrads TP, Veenstra TD, Copeland TD, Morrison DK. CK2 is a component of the KSR1 scaffold complex that contributes to Raf kinase activation. Curr Biol. 2007;17:179–184. doi: 10.1016/j.cub.2006.11.061. [DOI] [PubMed] [Google Scholar]
- 46.Lebrin F, Bianchini L, Rabilloud T, Chambaz EM, Goldberg Y. CK2α-protein phosphatase 2A molecular complex: possible interaction with the MAP kinase pathway. Mol Cell Biochem. 1999;191:207–212. [PubMed] [Google Scholar]
- 47.Llorens F, Miró FA, Casañas A, Roher N, Garcia L, Plana M, Gómez N, Itarte E. Unbalanced activation of ERK1/2 and MEK1/2 in apigenin-induced HeLa cell death. Exp Cell Res. 2004;299:15–26. doi: 10.1016/j.yexcr.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 48.Van Dross RT, Hong X, Pelling JC. Inhibition of TPA-induced cyclooxygenase-2 expression by apigenin through downregulation of Akt signal transduction in human keratinocytes. Mol Carcinog. 2005;44:83–91. doi: 10.1002/mc.20123. [DOI] [PubMed] [Google Scholar]
- 49.Lin JK, Chen YC, Huang YT, Lin-Shiau SY. Suppression of protein kinase C and nuclear oncogene expression as possible molecular mechanisms of cancer chemoprevention by apigenin and curcumin. J Cell Biochem. 1997;28:39–48. [PubMed] [Google Scholar]
- 50.Pagano MA, Bain J, Kazimierczuk Z, Sarno S, Ruzzene M, Di Maira G, Elliott M, Orzeszko A, Cozza G, Meggio F, Pinna LA. The selectivity of inhibitors of protein kinase CK2: an update. Biochem J. 2008;415:353–365. doi: 10.1042/BJ20080309. [DOI] [PubMed] [Google Scholar]