Abstract
The melanocortins (MC) can affect interscapular brown adipose tissue (IBAT) thermogenesis via its sympathetic nervous system (SNS) innervation. We chose a site of high MC4-receptor (MC4-R) mRNA co-localization with SNS outflow neurons to IBAT, the subzona incerta (subZI) to test whether IBAT thermogenesis could be increased or decreased. We first performed immunohistochemical characterization of the subZI and found neurons and/or fibers in this area positive for melanin concentrating hormone, oxytocin, arginine vasopressin, agouti-related protein and alpha-melanocyte stimulating hormone. Functional characterization of the subZI was tested via site-specific microinjections. The MC3/4-R agonist, melanotan II [MTII (0.025, 0.05 and 0.075 nmol)], and specific MC4-R agonist (cyclo [ß-Ala-His-D-Phe-Arg-Trp-Glu]-NH2; 0.024 nmol) both significantly increased IBAT temperature (TIBAT) and pretreatment with the MC4R antagonist, HS024 (0.072 nmol) blocked the MC4-R agonist-induced increased TIBAT in conscious, freely moving Siberian hamsters. Injection of the MC4-R antagonist alone significantly decreased TIBAT up to 3 h post injection. Collectively, these results highlight the identification of a brain area that possesses high concentrations of MC4-R mRNA and SNS outflow neurons to IBAT that has not been previously reported to be involved in the control of TIBAT. These results add to previously identified neural nodes that are components of the central circuits controlling thermogenesis.
Keywords: MTII, Siberian hamster, melanocortin-4 receptor, HS024, interscapular brown adipose tissue
1.0 Introduction
Recent interest in brown adipose tissue (BAT) has arisen from the realization that normal adult humans have functional and fairly substantial amounts of BAT, an inadvertent finding resulting from the use of fluorodeoxyglucose positron emission tomography used to detect tumor growth through glucose uptake [for review see: (Nedergaard, Bengtsson, and Cannon, 2007)]. Thus, the importance of BAT in energy balance has taken on greater significance than when the tissue was largely thought to be confined to rodents and could prove an important target for increases in energy expenditure to thwart or reverse obesity if it can be specifically activated.
BAT is the primary effector tissue for non-shivering thermogenesis in mammals [for review see (Cannon and Nedergaard, 2004)]. BAT, as with white adipose tissue (WAT), is clearly innervated by the sympathetic nervous system [SNS; for review see: (Bartness, Shrestha, Vaughan, Schwartz, and Song, 2010;Bartness, Vaughan, and Song, 2010)]. We first described the origins of the SNS outflow from brain to BAT using the transneuronal viral retrograde tract tracer, pseudorabies virus [PRV; (Bamshad, Song, and Bartness, 1999)]. The chemical neuroanatomy of this outflow to BAT, however, is incompletely known. One of the neurochemicals strongly implicated in the sympathetic control of BAT and WAT is the melanocortins [for review see: (Cone, 2005;Richard, 2007)]. Alpha-melanocyte stimulating hormone (α-MSH) is a naturally-occurring agonist for melanocortin receptors (MCRs), derived from the posttranslational processing of the prepropeptide hormone proopiomelanocortin (POMC) and is produced in the arcuate nucleus (Arc) and the nucleus of the solitary tract (Sol). α-MSH is one of the most important members of the melanocortin family for the control of energy balance (Butler and Cone, 2003). Agouti-related protein (AgRP), also produced in the Arc, is an inverse agonist for MCRs [for review see: (Pritchard, Turnbull, and White, 2002)] and is another important member of the melanocortin family for the control of energy balance. Of the MCRs, the melanocortin 4 receptor (MC4-R) is considered the predominant MCR for the central control of energy balance, with the melanocortin 3 receptor (MC3-R) receiving lesser support [for review see: (Ellacott and Cone, 2004)]. Key evidence implicating the melanocortins in control of energy expenditure, perhaps via BAT, is that intracerebroventricularly administered melanotan II (MTII), a synthetic analogue of α-MSH, triggers decreases in body fat of laboratory rats (Raposinho, White, and Aubert, 2003) that cannot be accounted for by the well-established MTII-induced inhibition of food intake alone [e.g., (Grill, Ginsberg, Seeley, and Kaplan, 1998;Hollopeter, Erickson, Seeley, Marsh, and Palmiter, 1998)].
Using a viral transneuronal retrograde tract tracer, PRV, to label the SNS outflow from brain ultimately innervating interscapular BAT (IBAT), and in situ hybridization to localize MC4-R mRNA, we found significant numbers of double-labeled cells for PRV and MC4-R mRNA across the neuroaxis (∼60% for all sites) suggesting that MC4-Rs are important contributors to the control of BAT thermogenesis (Song, Vaughan, Keen-Rhinehart, Harris, Richard, and Bartness, 2008). Indeed, we found acute injection of MTII into the 3rd ventricle (3V) increases the sympathetic drive to IBAT and that a highly specific MC4-R agonist, cyclo [ß-Ala-His-D-Phe-Arg-Trp-Glu]-NH2 (Bednarek, MacNeil, Kalyani, Tang, Van Der Ploeg, and Weinberg, 2000) increases IBAT temperature (TIBAT), as measured using thermistors implanted under this fat depot (Brito, Brito, Baro, Song, and Bartness, 2007). We found a similar increase in TIBAT with acute parenchymal MTII microinjections into the hypothalamic paraventricular nucleus (PVH) lasting as long as 4 h (Song, Vaughan, Keen-Rhinehart, Harris, Richard, and Bartness, 2008). Thus, central melanocortin receptor agonism can increase the sympathetic drive to BAT thereby increasing its thermogenesis.
One of the sites of high MC4-R mRNA co-localization with SNS outflow neuronal circuitry ultimately innervating IBAT is a brain area located ventral to the zona incerta (ZI) that we have termed the sub zona incerta (subZI; (Song, Vaughan, Keen-Rhinehart, Harris, Richard, and Bartness, 2008)) that has not been previously tested for its role in mediating changes in IBAT thermogenesis. Moreover, it also is a site of high MC4-R mRNA co-localization with the SNS outflow to WAT in Siberian hamsters (Song, Jackson, Harris, Richard, and Bartness, 2005). The subZI appears in all species examined to date (Siberian hamsters, laboratory rats and mice; unpublished observations) and could be important in the control of energy balance. Thus, the purpose of the present experiment was to explore this site in greater detail neuroanatomically and functionally. Therefore, we asked: 1) What are some of the neurochemical phenotypes of neurons found in the subZI?, 2) Does site-specific melanocortin receptor agonism trigger IBAT thermogenesis? and 3) Does site-specific blockade of MC4-Rs diminish or block MC4-R agonist-induced increases in IBAT thermogenesis?
2.0 Results
2.1 Experiment 1: What are some of the neurochemical phenotypes of subZI neurons?
From previous studies (Song, Jackson, Harris, Richard, and Bartness, 2005;Song, Vaughan, Keen-Rhinehart, Harris, Richard, and Bartness, 2008), the subZI was determined to approximately span the rostral-caudal axis from about 0.82 mm to 1.02 mm caudal to bregma (Fig. 1) using the mouse brain atlas (Paxinos and Franklin, 2007). MCH-ir was diffusely distributed in cell bodies of the subZI that formed a cluster lateral to the PVH and ventral to the zona incerta (Fig. 2, A-B). The density of MCH-ir cells and fibers was the same along the rostral to caudal extent of the subZI. There were no TH-ir cell bodies in the subZI, though the A13 population of TH-ir fibers and cell bodies was seen medial to the subZI in the PVH and dorsal to the subZI in the ZI (data not shown), as described by others [e.g., (Sita, Elias, and Bittencourt, 2003)]. There were no fibers or cell bodies positive for DBH in the subZI, but they were present in motor trigeminal, paragigantocellular and medial vestibular nuclei as we previously reported in Siberian hamsters (Shi and Bartness, 2000).
Fig 1.
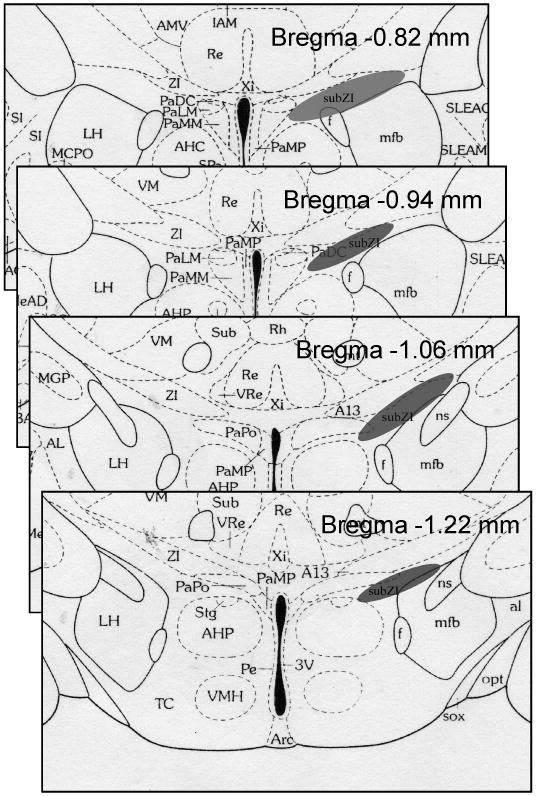
Location of the subZI using the mouse atlas (Paxinos and Franklin, 2007).
Fig 2.
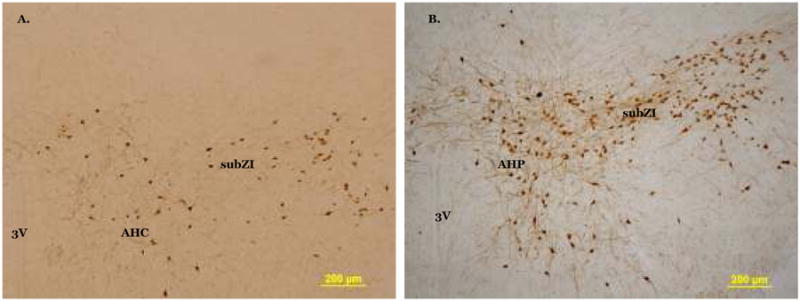
Photomicrographs of melanin concentrating hormone immunoreactivity in the rostral subZI (A) and caudal subZI (B). in the subZI. AHP, anterior hypothalamic nucleus, posterior part; AHC, anterior hypothalamic nucleus, central part; 3V, third ventricle. Scale bar = 200 μm.
There were OXY-ir fibers in the rostral subZI that appear to originate from densely labeled cell bodies in the lateral magnocellular portion of the PVH (Fig 3A). OXY-ir was found in the caudal subZI (Fig 3B) in a horizontal row of fibers and cell bodies, lateral to the posterior PVH (PaPo). In rat brain, OXY-ir is evident in PaPo (Sawchenko and Swanson, 1982) and we saw additional OXY-ir lateral to PVH that suggests the subZI is distinctly different from PaPo. AVP fibers are sparsely present in the rostral subZI (Fig 3C) immediately lateral to the PVH, but not seen ventral to the ZI as with MCH-ir and OXY-ir. There are neither AVP-ir cell bodies nor fibers evident in caudal subZI (Fig 3D). Dense AgRP fibers are present in the PVH, but not in the rostral subZI (Fig 4A). In the caudal subZI (Fig 4B), a moderate density of AgRP-ir fibers is present in contrast to no fiber staining in the anterior hypothalamic nucleus. Sparse α-MSH-ir fibers are present in rostral subZI immediately lateral to the PVH and are more prevalent in the caudal subZI (Fig 4C-D).
Fig 3.
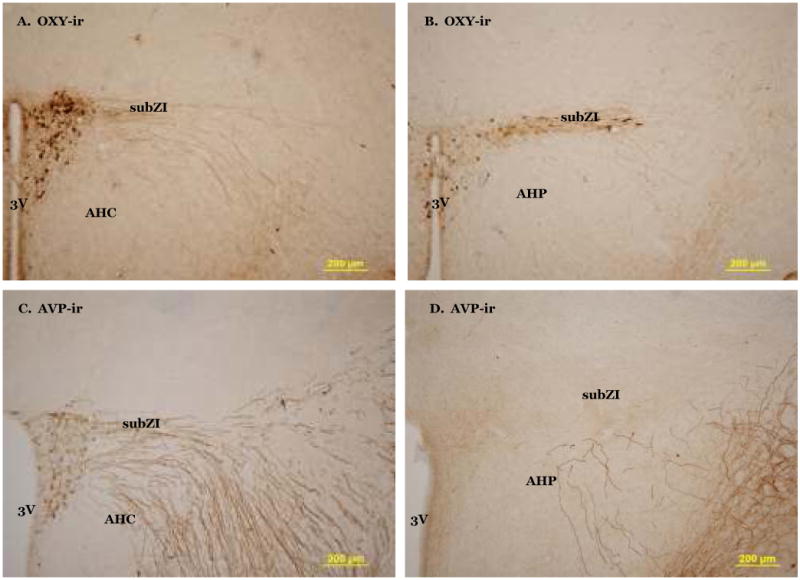
Photomicrographs of oxytocin (OXY)-immunoreactivity in the rostral (A) and caudal (B) subZI. Arginine vasopressin (AVP)-immunoreactivity in the rostral (C) and caudal (D) subZI. AHP, anterior hypothalamic nucleus, posterior part; AHC, anterior hypothalamic nucleus, central part; 3V, third ventricle.
Fig 4.
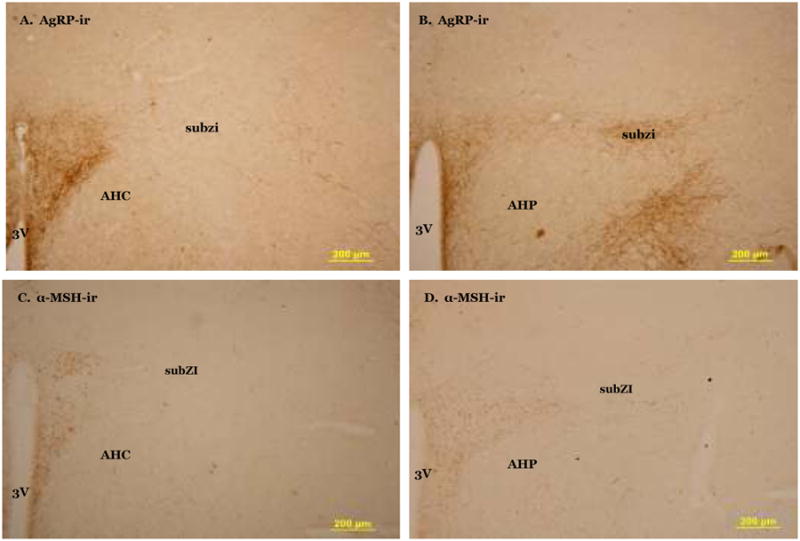
Photomicrograph of agouti related protein (AgRP) fibers in the hypothalamus. No AgRP is present in the rostral subZI (A) but is present in caudal subzi (B). α-MSH fibers are present in rostral (C) and caudal (D) subZI. AHP, anterior hypothalamic nucleus, posterior part; AHC, anterior hypothalamic nucleus, central part; 3V, third ventricle.
2.2 Experiment 2: Does site-specific melanocortin receptor agonism increase TIBAT?
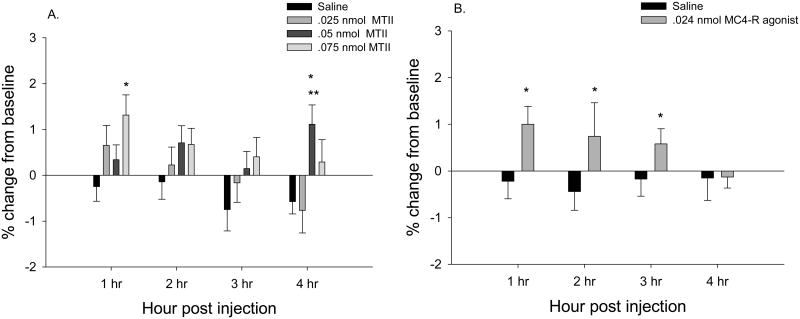
The highest dose of MTII (0.075 nmol) significantly increased TIBAT 1 h post injection (P<0.05; Fig 5A). A lower dose of MTII (0.05 nmol) significantly increased TIBAT only at 4 h post injection (P<0.05; Fig 5A). The MC4-R specific agonist increased TIBAT at 1, 2, and 3 h after injection (Ps<0.05; Fig 5B). Examples of cannula hits and misses are shown in Fig 6. The neuroanatomical specificity of the MC4-R agonist-triggered increased TIBAT was demonstrated by the nearby cannulae placement misses where MTII or the specific MC4-R agonist that did not significantly increase TIBAT (Table 1).
Fig 5.
Percent change in IBAT temperature from baseline. MTII (A) and MC4-R agonist (cyclo ßβ-Ala-His-D-Phe-Arg-Trp-Glu]-NH2) (B) unilaterally into the subZI increases IBAT temperature. P<.05, * greater than saline, ** greater than 0.05 nmol.
Fig 6.
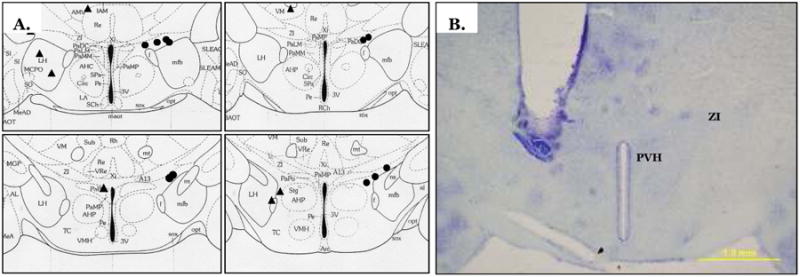
Schematic showing all hits (circles) and misses (triangles) using the mouse atlas (A) (Paxinos and Franklin, 2007). Each circle represents more than one hamster. Example of a correct unilateral cannula placement (B). PVH, paraventricular nucleus of the hypothalamus; ZI, zona incerta.
Table 1.
Absolute values of TIBAT for cannula misses.
| Experiment 2 | ||||
|---|---|---|---|---|
| Treatment | 1 hr | 2 hr | 3 hr | 4 hr |
| Saline | 36.51 ± 0.1 | 36.51 ± 0.1 | 36.42 ± 0.1 | 36.47 ± 0.1 |
| .025 nmol MTII | 36.58 ±0.2 | 36.65 ± 0.2 | 36.56 ± 0.2 | 36.48 ± 0.2 |
| .05 nmol MTII | 36.94 ± 0.1 | 36.88 ± 0.1 | 36.89 ± 0.1 | 36.89 ± 0.1 |
| .075 nmol MTII | 36.99 ± 0.2 | 36.91 ± 0.2 | 36.58 ± 0.2 | 36.58 ± 0.1 |
| Saline | 36.78 ± 0.3 | 37.15 ± 0.2 | 37.19 ± 0.2 | 37.27 ± 0.6 |
| .024 nmol MC4-R agonist | 37.03 ± 0.2 | 37.28 ± 0.2 | 37.18 ± 0.2 | 36.95 ± 0.1 |
| Experiment 3 | ||||
|---|---|---|---|---|
| Treatment | 1 hr | 2 hr | 3 hr | 4 hr |
| Saline + saline | 36.37 ± 0.2 | 36.38 ± 0.2 | 36.55 ± 0.1 | 36.55 ± 0.2 |
|
Saline + MC4-R agonist Without outliers |
37.27 ± 0.2* 37.09 ± 0.2 |
37.15 ± 0.1* 37.09 ± 0.1 |
36.95 ± 0.2 | 36.97 ± 0.2 |
| MC4-R antagonist + saline | 36.50 ± 0.3 | 36.55 ± 0.3 | 36.54 ± 0.2 | 36.78 ± 0.2 |
|
MC4-R agonist + MC4-R antagonist Without outliers |
36.78 ± 0.4 | 37.22 ± 0.3* 37.0 ± 0.3 |
36.75 ± 0.3 | 36.83 ± 0.4 |
p <.05, different from saline.
2.3 Experiment 3: Does site-specific melanocortin receptor antagonism diminish or abolish MC4-R agonist-induced increases in TIBAT?
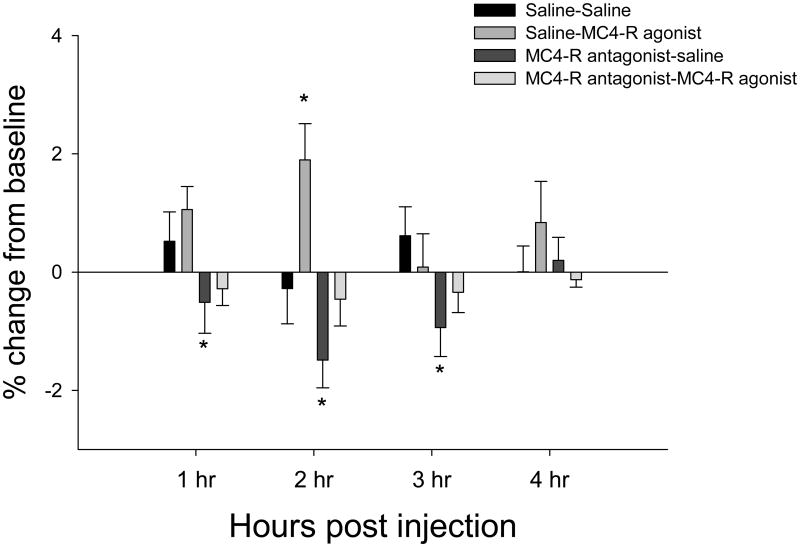
The MC4-R agonist significantly increased TIBAT 2 h post injection (P<0.05; Fig. 7). Injection of HS024 alone, the MC4-R specific antagonist, significantly decreased TIBAT at 1 h, 2 h and 3 h post injection (Ps<0.05; Fig. 7). Prior administration of the MC4-R antagonist blocked the MC4-R agonist-induced increase in TIBAT (Fig 7). Examples of cannulae hits and misses are shown in Fig 8. In the misses group, there were three hamsters that had increases of 2°C and were considered outliers and removed from statistical analyses (Table 1). Two of the three hamsters had increased TIBAT at 1h and 2h after saline + MC4-R agonist injection. In both cases one side the cannula hit the subZI and the other the cannula hit the lateral hypothalamus (LH). One of the three had increased TIBAT at 2h after MC4-R agonist + MC4-R antagonist injection and in this case one side of the cannula hit the subZI and the other hit the PaPo.
Fig 7.
Percent change in IBAT temperature from baseline. MC4-R agonist and MC4-R antagonist alone or together bilaterally into the subZI. P<.05, * different from saline
Fig 8.
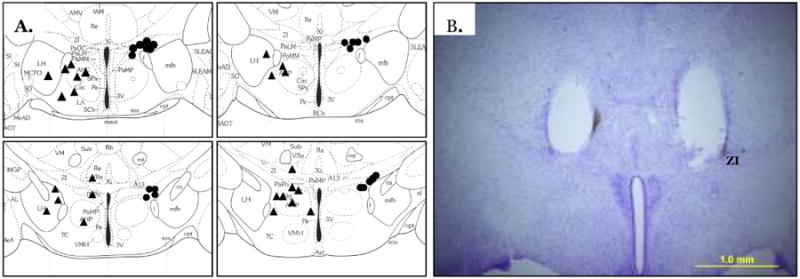
Schematic showing all hits (circles) and misses (triangles) using the mouse atlas (A) (Paxinos and Franklin, 2007). Each circle represents more than one hamster. Example of a correct bilateral cannula placement (B). ZI, zona incerta.
3.0 Discussion
The subZI is an area with a high concentration of colocalized MC4-R mRNA with sympathetic outflow neurons to IBAT (Song, Vaughan, Keen-Rhinehart, Harris, Richard, and Bartness, 2008). Using previous evidence (Song, Jackson, Harris, Richard, and Bartness, 2005;Song, Vaughan, Keen-Rhinehart, Harris, Richard, and Bartness, 2008) of subZI characterization and current histology (Figs. 2-4), we report that the area we term the subZI is laterodorsal to the PVH, ventral to the zona incerta and dorsal and lateral to the anterior hypothalamus. The subZI contains cells and/or fibers immunoreactive for MCH, OXY, AVP, and most importantly for this study, AgRP and α-MSH, both endogenous ligands for the MC4-R. MCH- and OXY-ir is seen throughout the rostral to caudal extent of the subZI. The other peptides seen are localized in the rostral portion (e.g. AVP) or the caudal portion (e.g. AgRP, α-MSH).
We specifically tested the involvement of MC-Rs in the subZI on thermogenesis for the first time by injecting MTII, the MC3/4-R agonist, or a highly selective MC4-R agonist (cyclo [ß-Ala-His-D-Phe-Arg-Trp-Glu]-NH2) and/or a highly selective MC4-R antagonist HS024 to functionally test the effects of their activation /inhibition on IBAT thermogenesis. MTII and the MC4-R specific agonist produced significant increases in TIBAT up to 4 h post injection, and prior injection of HS024, the MC4-R antagonist, blocked the MC4-R specific agonist-induced increases in TIBAT. In addition, injection of HS024 alone significantly decreased basal TIBAT up to 3 h post injection.
The subZI is rostral to the incerto-hypothalamic area (IHy) (Sita, Elias, and Bittencourt, 2007); Elias, 2008 13995 /id}. There is no overlap of IHy and subZI in the rostral portions of the subZI (Fig 1). The IHy begins at the rostral end of the ventromedial hypothalamus (Sita, Elias, and Bittencourt, 2007) which partially coincides with the caudal portion of the subZI; thus, it is possible that at this level some cells in the subZI may also belong to the IHy. A likely phenotype of these cells could be MCH producing neurons considering our current evidence (Fig 2) and MCH-ir in the IHy reported by Elias and colleagues (Elias, Sita, Zambon, Oliveira, Vasconcelos, and Bittencourt, 2008). Biotin dextran amine, an anterograde tracer, injections into the IHY of rats, however, reveal ventral ascending fibers that travel through the PVH, perifornical area and anterior hypothalamus and terminate primarily in an area dorsal to the supraoptic nucleus (Sita, Elias, and Bittencourt, 2007). Therefore, neuroanatomical evidence of MCH-ir in both the IHy and subZI, and lack of evidence of afferent projections from the IHy to the subZI, suggest that these two brain areas are not the same, nor do they appear to interact directly. In addition, the subZI, at all levels, does not show TH-ir, a characteristic marker of cells in the IHy (Bjorklund, Lindvall, and Nobin, 1975) as well as the zona incerta (Chan-Palay, Zaborszky, Kohler, Goldstein, and Palay, 1984;Kolmac and Mitrofanis, 1999). Thus, overall, the majority of the subZI appears to be neuroanatomically distinct from the IHy.
The function and circuitry of the ZI has been described extensively in many species (Mitrofanis, 2002;Mitrofanis, 2005;Mitrofanis, Ashkan, Wallace, and Benabid, 2004;Watanabe and Kawana, 1982). In rats, the ZI is generally thought to be divided into rostral, dorsal, ventral and caudal subareas (Kim, Gregory, and Hall, 1992) and the area in Siberian hamsters that we targeted in the current study is ventral to the anterior extent of the rostral portion of the ZI (rZI) (Fig. 1). Kawana & Watanabe (Kawana and Watanabe, 1981) report the most anterior portion of the ZI in rat appears to be the pars rostro-polaris, which corresponds closely to our subZI. Cells in the pars rostro-polaris are ‘loosely packed’ in comparison to the adjacent parvicellular cells in the PVH (Kawana and Watanabe, 1981) and this also is what we have observed in the subZI. Thus, the subZI does not appear to be cells that are formally an extension of the most lateral portions of the PVH.
Descending fibers from the bed nucleus of the stria terminalis (Dong and Swanson, 2003) travel through the lateral parvicellular part of the PVH (PVHlp) and a forniceal part of the PVH (PVHf); both the PVHlp and PVHf correspond to our caudal subZI. As mentioned in the Results section, OXY-ir is evident in PaPo (Sawchenko and Swanson, 1982) and we saw additional OXY-ir lateral to PVH. Simmons and Swanson (Simmons and Swanson, 2009); however, report neither staining for OXY in the PVHlp nor in the PVHf of laboratory rats. This discrepancy could be due to a species difference or additional evidence that the subZI is a separate brain region/nucleus.
In the current study, we tested two MC4-R agonists, MTII, the MC3/4-R agonist and (cyclo [ß-Ala-His-D-Phe-Arg-Trp-Glu]-NH2, the MC4-R specific agonist, for their ability to increase TIBAT in vivo in freely moving hamsters after an acute injection into the sub ZI. We previously have shown that single injections of MC4-R agonists into the 3V of Siberian hamsters increase TIBAT (Brito, Brito, Baro, Song, and Bartness, 2007), as do MTII injections into the 4V and medullary raphe of laboratory rats (Skibicka and Grill, 2008). In the present study, a single unilateral microinjection of MTII into the novel sympathetic outflow site to BAT, the subZI, also significantly increased TIBAT. MTII injections into structures adjacent to the subZI that also have sympathetic outflow neurons to IBAT possessing high concentrations of MC4-R mRNA such as the PVH (Song, Vaughan, Keen-Rhinehart, Harris, Richard, and Bartness, 2008) and anterior hypothalamic area also increase TIBAT in laboratory rats (Skibicka and Grill, 2009) and Siberian hamsters (Song, Vaughan, Keen-Rhinehart, Harris, Richard, and Bartness, 2008), and core body temperature in laboratory rats (Skibicka and Grill, 2009) suggesting the subZI and MC4-Rs are part of a distributed set of sites controlling sympathetic drive to IBAT (Skibicka and Grill, 2009;Song, Vaughan, Keen-Rhinehart, Harris, Richard, and Bartness, 2008). The significant percent increases in TBAT by MC4-R agonists compared with vehicle injections represent absolute increases in between ∼0.2°C to 2.4°C and as such are of comparable magnitude to those found by others measuring TBAT in response to other centrally acting receptor agonists [e.g., cannabinoid CB1 receptor antagonist rimonabant; (Verty, Allen, and Oldfield, 2009)]. To our knowledge we are the only group to inject the highly specific MC4-R agonist, cyclo[beta-Ala-His-D-Phe-Arg-Trp-Glu]NH2 into brain parenchyma. The increases in TIBAT seen after a single sub ZI injection of this MC4-R agonist (Figs. 5 & 7) further supports the role of the MC4-Rs and the subZI in thermoregulation. Whether MC4-R activation in the subZI or other sites occurs to naturally-occurring stimuli such as cold exposure to increase TIBAT is unknown at this time.
In order to test bilateral blockade and/or stimulation of the subZI, we injected (cyclo [ß-Ala-His-D-Phe-Arg-Trp-Glu]-NH2) and a highly selective MC4-R antagonist, HS024, together and separately. These drugs were used because they have high selectivity for MC4-Rs over MC3-Rs (Bednarek, MacNeil, Tang, Kalyani, Van Der Ploeg, and Weinberg, 2001;Jonsson, Skarphedinsson, Skuladottir, Watanobe, and Schioth, 2002;Kask, Mutulis, Muceniece, Pahkla, Mutule, Wikberg, Rago, and Schioth, 1998). In the current study, prior injection of HS024 blocked the MC4-R specific agonist-induced increases in TIBAT and, when injected alone, HS024 significantly decreased basal TIBAT for 3 h. Chronic central administration of the MC4-R antagonist, HS024, significantly increases food intake and body fat gain (Jonsson, Skarphedinsson, Skuladottir, Watanobe, and Schioth, 2002); however, IBAT function has not been measured after HS024 administration before the present study. A similar MC4-R antagonist, HS014, however, does not affect core body temperature (Sinha, Schioth, and Tatro, 2003), but does decrease IBAT UCP-1 mRNA, a surrogate of BAT thermogenesis (Baran, Preston, Wilks, Cooney, Kraegen, and Sainsbury, 2002;Small, Kim, Stanley, Mitchell, Murphy, Morgan, Ghatei, and Bloom, 2001). This latter report supports the significant decreases in TIBAT after HS024 microinjection into the subZI we observed here, as well as its ability to successfully block MC4-R agonist-induced significant increases in TIBAT in the present study (Fig. 7). Given the high concentrations of IBAT sympathetic outflow neurons possessing MC4-R-mRNA in the subZI (Song, Vaughan, Keen-Rhinehart, Harris, Richard, and Bartness, 2008), it seems likely these receptors were involved in the HS024-induced decreases in TIBAT seen here.
We did observe increases in TIBAT of hamsters with cannulae that missed the subZI being positioned nearby in the LH and PaPo, nuclei that have IBAT SNS outflow neurons that also possess MC4-R mRNA (Song, Vaughan, Keen-Rhinehart, Harris, Richard, and Bartness, 2008). At 1 h and 2 h following injection of the saline + MC4-R agonist, two animals with cannulae in the LH had increased TIBAT (Table 1). The LH has been strongly implicated in the SNS drive to IBAT more generally (Yoshida, Kemnitz, and Bray, 1983), but we are unaware of any attempts to increase TIBAT by parenchymal application of MC3/4-R agonists into the LH. At 2 h following injection of the MC4-R agonist + MC4-R antagonist, one of the misses residing in the PaPo triggered an increase in TIBAT (Table 1). As mentioned above these misses attest to the localization of MC4-Rs on sympathetic outflow neurons contributing to a distributed set of sites controlling sympathetic drive to IBAT (Skibicka and Grill, 2009;Song, Vaughan, Keen-Rhinehart, Harris, Richard, and Bartness, 2008;Williams, Bowers, Bartness, Kaplan, and Grill, 2003). Finally, the overall magnitude of TIBAT changes (Table 1) that occurred in the misses is very low and suggests that the spread of injectate was localized to the subZI.
In conclusion, we report for the first time, the role of the subZI in the control of TIBAT, with MC3/4-R (MTII) and the MC4-R (cyclo [ß-Ala-His-D-Phe-Arg-Trp-Glu]-NH2) agonism triggering prolonged increases in TIBAT that can be blocked by a specific MC4-R antagonist (HS024) and that TIBAT can be decreased by MC4-R antagonism (HS024), effects likely occurring by interaction of these substances with MC4-Rs located on sympathetic outflow circuit neurons ultimately innervating IBAT (Song, Vaughan, Keen-Rhinehart, Harris, Richard, and Bartness, 2008). It is tempting to speculate that a naturally-occurring agonism of these receptors by α-MSH or inverse agonism by AgRP might modulate BAT thermogenesis given the presence of fibers for both melanocortins in the subZI. These basic research data add to the clinical reports of the presence and potential importance of BAT in normal adults (Cypess, Lehman, Williams, Tal, Rodman, Goldfine, Kuo, Palmer, Tseng, Doria, Kolodny, and Kahn, 2009;van Marken Lichtenbelt, Vanhommerig, Smulders, Drossaerts, Kemerink, Bouvy, Schrauwen, and Teule, 2009) and with polymorphisms of the MC4-R being a contributor to alterations in energy expenditure, perhaps via BAT (Rutanen, Pihlajamaki, Karhapaa, Vauhkonen, Kuusisto, Moilanen, and Laakso, 2004)
4.0 Methods and Materials
All procedures were approved by the Georgia State University Institutional Animal Care and Use Committee and are in accordance with Public Health Service and United States Department of Agriculture guidelines.
4.1 Experiment 1: What are some of the neurochemical phenotypes of subZI neurons?
4.1.1 Animals
Male Siberian hamsters (Phodopus sungorus; n=55; ∼4-5 mo old) were single-housed after being selected from our breeding colony (16h:8h light:dark cycle; lights on at 0200 h). Room temperature was maintained at 21 ± 2 °C, with relative humidity at ∼50 ± 10 %. The lineage of this colony has been described previously (Bowers, Festuccia, Song, Shi, Migliorini, and Bartness, 2004). Hamsters were single-housed for 4 d before perfusions.
4.1.2 Histological tissue preparation
Hamsters were overdosed with pentobarbital sodium (300mg/kg) and perfused transcardially with heparinized (0.02 %) saline and 4 % paraformaldehyde in phosphate buffer (0.1 M; pH: 7.4). A subset of hamsters was perfused with 0.9 % saline and a 2 % paraformaldehyde + 3 % formalin solution (pH: 4.15) to enhance cell body fixation for our α-MSH immunoreactivity (ir) staining. Brains were stored in their respective fixatives at 4° C for at least 24 h then transferred to a sucrose (30 %) solution with 0.1 % sodium azide and stored at 4° C until they were sectioned on a freezing stage sliding microtome at 35 μm into 2 sets for each hamster. Sections were stored in 0.1 M PBS with 0.1 % sodium azide until immunohistochemical processing.
4.1.3 Immunohistochemistry (IHC)
All washes and incubations occurred at room temperature, unless otherwise noted. We stained the subZI for melanin concentrating hormone (MCH)-ir to test whether this characteristic neuropeptide of some of the areas surrounding the subZI [e.g. the zona incerta, dorsomedial and lateral hypothalamic areas; (Broberger, 1999)], also was found in the subZI. For the MCH IHC, sections were incubated in 1 % sodium borohydride (20 min), then 2 % H2O2 in PBS with 0.4 % Triton X-100 (PBTx) for 20 min, with rinses before and after incubation. As a blocking step, sections were incubated in 10 % normal goat serum (NGS; Vector Laboratories, Burlingame, CA) in PBTx (30 min). Sections were then incubated for 48 h in the primary antibody for MCH (rabbit anti-MCH, 1:10,000; Sigma Aldrich, St. Louis, MO) in 2 % NGS and 0.1 % sodium azide in PBTx. Sections were rinsed (0.1 M PBS; 3×) and then placed in the secondary antibody, biotinylated goat anti-rabbit (1:500; Vector Laboratories, Burlingame, CA), for 2 h then rinsed.
AgRP- and α-MSH-ir was obtained for the subZI to test for projections to this area as a chemical neuroanatomical compliment to our microinjection studies in Experiments 2 and 3 (below). Procedures used for AgRP IHC have been described previously (Leitner and Bartness, 2008). For the α-MSH IHC, sections were incubated in 2 % H2O2 in 0.3 % PBTx for 30 min, with rinses before and after incubations. Sections were then blocked in 3 % normal donkey serum (Jackson ImmunoResearch Laboratories, West Grove, PA) in PBTx for 30 min. Sections were then incubated at 37° C for 1 h in the primary serum (sheep anti- α-MSH, 1:1000; Millipore, Billerica, MA) in blocking solution. Sections were rinsed in 0.1 M PBS (3×), placed in the secondary antibody, biotinylated donkey anti-sheep (1:500; R & D Systems, Minneapolis, MN), overnight and then rinsed.
We also performed IHC in the subZI for tyrosine hydroxylase (TH), the rate limiting enzyme in synthesizing catecholamines, and dopamine β-hydroxylase (DBH), a marker enzyme for the presence of norepinephrine, both of which label noradrenergic neurons involved in autonomic responses (Sawchenko and Swanson, 1981). We also stained for oxytocin (OXY) and arginine vasopressin (AVP), neurochemicals associated with sympathetic outflow to the brainstem (Sawchenko and Swanson, 1982;Swanson and Sawchenko, 1980). We have described our procedures for TH [mouse anti-TH, 1:10,000; Immunostar], DBH [rabbit anti-DBH, 1:1000; Immunostar], OXY [mouse anti-OXY, 1:5000; Millipore] and AVP [rabbit anti-AVP, 1:40,000; Immunostar] IHC previously (Leitner and Bartness, 2008;Shi and Bartness, 2001). A minor modification was added to the TH IHC to reduce background staining in which sections were incubated in 1 % H2O2 in PBTx and 2 % NGS before primary antibody incubation overnight.
For all IHC procedures, sections were placed in avidin biotin solution (Vector Laboratories, Burlingame, CA), rinsed and a chromagen was generated using 3.3′-diaminobenzidine tetrahydrochloride in presence of H2O2. Finally, the sections were rinsed with 0.1 M PBS (3×) to stop the color reaction, mounted onto microscope slides (Superfrost; Fisher Scientific, Suwanee, GA), and dried overnight. The slides were then dehydrated, delipidated, and coverslipped using Permount (Fisher Scientific).
4.2 Experiment 2: Does site-specific melanocortin receptor agonism increase TIBAT?
4.2.1 Animals
Male hamsters (n=29; ∼2 mo old) weighing 37 ± 1 g were obtained from our breeding colony. For Experiments 2 and 3, after a week of single housing, hamsters were implanted with a cannula into the subZI.
4.2.2 Stereotaxic cannulae implantation
A guide cannula (26-gauge stainless steel; Plastics One, Roanoke, VA) was lowered stereotaxically according to coordinates targeted for placement just above the left subZI (−0.03 mm posterior to bregma, 0.08 mm to the left of midline, and ventral −6.0 mm from the top of the skull). The guide cannula was secured to the skull using cyanoacrylate ester gel, 2 3/16 mm jeweler's screws and dental acrylic. A removable obturator (Plastics One, Roanoke, VA) sealed the opening in the guide cannula throughout the experiment except when it was removed for the injections. Hamsters were given fresh apple bits to encourage food/fluid intake for 2 d post surgery and were allowed at least 1 wk to recover fully in their cages. Hamsters received 0.2mg/kg injections of buprenorphine once daily for two days following surgery to minimize postsurgical discomfort.
4.2.3 Experimental design
Following recovery, hamsters were divided into control and experimental groups matched on their percent change in body mass before and after surgery. Hamsters were handled for two consecutive days before the experimental procedure to acclimate hamsters to handling during injections. Hamsters were separated into four treatment groups (saline, 0.025, 0.05 and 0.075 nmol MTII [Phoenix Pharmaceuticals, Burlingame, CA]) where half received drug or saline (200 nl of each). Following a 24 h washout period, hamsters that previously received drug received saline and vice versa. The doses of MTII used and washout period were based on increases in TIBAT after previous parenchymal injections into the PVH (Song, Vaughan, Keen-Rhinehart, Harris, Richard, and Bartness, 2008)] and nucleus of the solitary tract (Vaughan, Song, Keen-Rhinehart, and Bartness, 2007). The MTII doses used in the current study are within a range previously determined to have no effect on TIBAT when injected into the 3V (Song, Vaughan, Keen-Rhinehart, Harris, Richard, and Bartness, 2008)].
After a ∼2.5 wk washout period hamsters were handled for 2 consecutive d and injected with either 0.024 nmol of a highly selective MC4-R agonist, cyclo[beta-Ala-His-D-Phe-Arg-Trp-Glu]NH2 (Phoenix Pharmaceuticals, Burlingame, CA), or saline in 200 nl. Because immediate effects (4h post injection) of the agonist were being measured and no lingering effects were found (unpublished observations), injections were administered the next day without additional washout time. Hamsters received the opposite treatment and then were monitored for changes in TIBAT (see Test day injection protocol below).The dose of the MC4-R agonist used was chosen because it is approximately a 10-fold decrease of the dose previously found to increase TIBAT after a 3V injection (Brito, Brito, Baro, Song, and Bartness, 2007) and determined in pilot studies for the present study to have no significant effect on TIBAT when injected into the 3V (unpublished observations).
4.3 Experiment 3: Does site-specific melanocortin receptor antagonism diminish or abolish MC4-R agonist-induced increases in TIBAT?
4.3.1 Animals
Male hamsters (n=117; ∼2-3 mo old) weighing 40 ± 0.5 g were obtained from our breeding colony.
4.3.2 Stereotaxic cannulae implantation
Guide cannulae for bilateral injections (26-gauge stainless steel) was stereotaxically lowered according to coordinates (−0.03 mm posterior to bregma, 1.2 mm center to center distance from midline, and ventral −6.25 mm from the top of the skull) targeted for placement just above the subZI. The guide cannulae were secured as described above. The recovery protocol was the same as above.
4.3.3 Experimental design
Hamsters were divided into four treatment groups (saline + saline, saline + MC4-R agonist, MC4-R antagonist + saline, MC4-R antagonist + MC4-R agonist) based on their percent change in body mass between Week 1 and Week 2 after surgery. The purpose of the ‘MC4-R antagonist + MC4-R agonist' group was to test the effect of site-specific melanocortin receptor antagonism on MC4-R agonist-induced increases in TIBAT. In order to block access to the MC4-R, the MC4-R antagonist, HS024 (0.072 nmol; NeoMPS, Strasbourg, France) (Bednarek, MacNeil, Kalyani, Tang, Van Der Ploeg, and Weinberg, 2001;Kask, Mutulis, Muceniece, Pahkla, Mutule, Wikberg, Rago, and Schioth, 1998;Schioth, Bouifrouri, Rudzish, Muceniece, Watanobe, Wikberg, and Larhammar, 2002) was injected immediately (∼5 min) before the MC4-R agonist cyclo[beta-Ala-His-D-Phe-Arg-Trp-Glu]NH2 (0.024 nmol). The dose of HS024 was based on an effective blockade of MC4-Rs using a ∼3:1 antagonist to agonist molar ratio as demonstrated by Sinha and colleagues (Sinha, Schioth, and Tatro, 2003).
The purpose of the ‘saline + MC4-R agonist’ and ‘MC4-R antagonist + saline’ groups was to control for the effects of two injections (∼5 min apart) and to test the effects of the MC4-R agonist and antagonist separately on TIBAT. The purpose of the ‘saline + saline’ group was the control for the two injections (∼5 min apart; see Test Day Injection Protocol below). Total volume for all groups was 100 nl per side.
4.3.4 Temperature transponder implantation
Cannulae and transponder implantation were done in a single surgery as we described previously (Brito, Brito, Baro, Song, and Bartness, 2007;Leitner and Bartness, 2009;Song, Vaughan, Keen-Rhinehart, Harris, Richard, and Bartness, 2008). In brief, hamsters were anesthetized with isoflurane (Baxter Healthcare Corporation, Deerfield, IL) and a midline incision was made along the upper dorsal surface of the body. A temperature transponder (Implantable Programmable Temperature Transponder [IPTT] 300, BioMedic Data Systems, Seaford, DE) was tied to sterile silk sutures and then secured under the left IBAT pad.
4.3.5 Test day injection protocol
A portable hand held reader-programmer (DAS-5002 Notebook system, BioMedic Data Systems, Seaford, DE) was used to scan TIBAT as we described previously (Brito, Brito, Baro, Song, and Bartness, 2007;Leitner and Bartness, 2009;Song, Vaughan, Keen-Rhinehart, Harris, Richard, and Bartness, 2008). Temperatures were taken at baseline adaptation periods (−2 h, −1 h), before parenchymal injection (0 h), and hourly for the next 4 h. Nestlets and food from the home cage & cheek pouches were removed at −2 h, with water available ad libitum throughout the test.
At 0 h, hamsters were injected with 200 nl (Experiment 2) or 100 nl of drug or saline on each side and followed by another injection ∼5 min later of 100 nl of drug or saline (Experiment 3). The injector cannulae (33-gauge stainless steel) were custom cut to extend 0.5 mm beyond the tip of the guide cannula and connected at the other end to a microsyringe via polyethylene tubing that was backfilled with deionized water. Each hamster was tested once. Animals were lightly restrained by hand for 30 s during injection(s), and the injector was kept in place for an additional ∼30 s before removal to minimize reflux. Food and fresh nestlets were returned after the last temperature reading of the test day.
4.3.6 Transponder and cannulae verification
Cannula placement was verified by injecting 200 nl (Experiment 2) or 100 nl on each side (Experiment 3) of 0.1% toluidine blue into the subZI. For Experiments 2 and 3, after anesthetization and before perfusion, the transponders were verified visually by examining the position of the copper portion of the transponder relative to the medial border of the left IBAT pad. Hamsters that had the transponder misplaced were eliminated from the analyses.
4.3.7 Histological tissue preparation
The procedures are the same as in Experiment 1, with the exception that the brains were sliced at 80 μm and sections were counterstained with 2% cresyl violet. Cannula placements were determined by superimposing (Adobe Photoshop (v7.0; San Jose, CA) digital images of brain slices containing the cannulae tracts over corresponding plate images from the mouse brain atlas (Paxinos and Franklin, 2007), as there is no Siberian hamster brain atlas commercially. Moreover, the size and shape of most mouse brain areas are more similar to that of Siberian hamster than Syrian hamster brains. All hamsters with correctly placed transponders and confirmed cannulae placements were included in the analyses.
4.4 Statistical analyses
One way ANOVAs at each time point between treatment groups were conducted. Significance was set at P < 0.05. Exact probabilities and test values were omitted for simplicity and clarity of the presentation of the results.
Acknowledgments
The authors thank Dr. C.K. Song for invaluable input on immunohistochemistry and neuroanatomy and Dr. B.J.W. Teubner for help with the bilateral injections in Experiment 3. We also acknowledge Yang Liu, Bruce Smith, Jr., Daniel Vizcaino, Huyen Do, and Omuwa Braimah for technical assistance. This work was supported by NIH R37 DK35254 to T.J.B. and F32 DK082143 to C.H.V.
Footnotes
This work was funded, in part, by NIH R37 DK36254 to TJB.
Reference List
- Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–E452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Shrestha YB, Vaughan CH, Schwartz GJ, Song CK. Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Mol Cell Endocrinol. 2010;318:34–43. doi: 10.1016/j.mce.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartness TJ, Vaughan CH, Song CK. Sympathetic and sensory innervation of brown adipose tissue. Int J Obes (Lond) 2010;34(1):S36–S42. doi: 10.1038/ijo.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol. 1999;276:R1569–R1578. doi: 10.1152/ajpregu.1999.276.6.R1569. [DOI] [PubMed] [Google Scholar]
- Richard D. Energy expenditure: a critical determinant of energy balance with key hypothalamic controls. Minerva Endocrinol. 2007;32:173–183. [PubMed] [Google Scholar]
- Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- Butler AA, Cone RD. Knockout studies defining different roles for melanocortin receptors in energy homeostasis. Ann N Y Acad Sci. 2003;994:240–245. doi: 10.1111/j.1749-6632.2003.tb03186.x. [DOI] [PubMed] [Google Scholar]
- Pritchard LE, Turnbull AV, White A. Pro-opiomelanocortin processing in the hypothalamus: impact on melanocortin signalling and obesity. J Endocrinol. 2002;172:411–421. doi: 10.1677/joe.0.1720411. [DOI] [PubMed] [Google Scholar]
- Ellacott KL, Cone RD. The central melanocortin system and the integration of short- and long-term regulators of energy homeostasis. Recent Prog Horm Res. 2004;59:395–408. doi: 10.1210/rp.59.1.395. [DOI] [PubMed] [Google Scholar]
- Raposinho PD, White RB, Aubert ML. The melanocortin agonist melanotan-II reduces the orexigenic and adipogenic effects of neuropeptide Y (NPY) but does not affect the NPY-driven suppressive effects on the gonadotropic and somatotropic axes in the male rat. J Neuroendocrinol. 2003;15:173–181. doi: 10.1046/j.1365-2826.2003.00962.x. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Ginsberg AB, Seeley RJ, Kaplan JM. Brainstem application of melanocortin receptor ligands produces long- lasting effects on feeding and body weight. J Neurosci. 1998;18:10128–10135. doi: 10.1523/JNEUROSCI.18-23-10128.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollopeter G, Erickson JC, Seeley RJ, Marsh DJ, Palmiter RD. Response of neuropeptide Y-deficient mice to feeding effectors. Regul Pept. 1998:75–76. 383–389. doi: 10.1016/s0167-0115(98)00092-5. [DOI] [PubMed] [Google Scholar]
- Song CK, Vaughan CH, Keen-Rhinehart E, Harris RB, Richard D, Bartness TJ. Melanocortin-4 receptor mRNA expressed in sympathetic outflow neurons to brown adipose tissue: Neuroanatomical and functional evidence. Am J Physiol. 2008;295:R417–R428. doi: 10.1152/ajpregu.00174.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarek MA, MacNeil T, Kalyani RN, Tang R, Van Der Ploeg LH, Weinberg DH. Analogs of lactam derivatives of alpha-melanotropin with basic and acidic residues. Biochem Biophys Res Commun. 2000;272:23–28. doi: 10.1006/bbrc.2000.2589. [DOI] [PubMed] [Google Scholar]
- Brito MN, Brito NA, Baro DJ, Song CK, Bartness TJ. Differential activation of the sympathetic innervation of adipose tissues by melanocortin receptor stimulation. Endocrinology. 2007;148:5339–53347. doi: 10.1210/en.2007-0621. [DOI] [PubMed] [Google Scholar]
- Song CK, Jackson RM, Harris RB, Richard D, Bartness TJ. Melanocortin-4 receptor mRNA is expressed in sympathetic nervous system outflow neurons to white adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1467–R1476. doi: 10.1152/ajpregu.00348.2005. [DOI] [PubMed] [Google Scholar]
- Bowers RR, Festuccia WTL, Song CK, Shi H, Migliorini RH, Bartness TJ. Sympathetic innervation of white adipose tissue and its regulation of fat cell number. Am J Physiol. 2004;286:R1167–R1175. doi: 10.1152/ajpregu.00558.2003. [DOI] [PubMed] [Google Scholar]
- Broberger C. Hypothalamic cocaine- and amphetamine-regulated transcript (CART) neurons: histochemical relationship to thyrotropin-releasing hormone, mealanin-concentrating hormone, orexin/hypocretin and neuropeptide Y. Brain Res. 1999;27:101–113. doi: 10.1016/s0006-8993(99)01977-0. [DOI] [PubMed] [Google Scholar]
- Leitner C, Bartness TJ. Food deprivation-induced changes in body fat mobilization after neonatal monosodium glutamate treatment. Am J Physiol. 2008;294:R775–R783. doi: 10.1152/ajpregu.00369.2007. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. Central noradrenergic pathways for the integration of hypothalamic neuroendocrine and autonomic responses. Science. 1981;214:685–687. doi: 10.1126/science.7292008. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol. 1982;205:260–272. doi: 10.1002/cne.902050306. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology. 1980;31:410–417. doi: 10.1159/000123111. [DOI] [PubMed] [Google Scholar]
- Shi H, Bartness TJ. Neurochemical phenotype of sympathetic nervous system outflow from brain to white fat. Brain Res Bull. 2001;54:375–385. doi: 10.1016/s0361-9230(00)00455-x. [DOI] [PubMed] [Google Scholar]
- Vaughan CH, Song CK, Keen-Rhinehart E, Bartness TJ. Effects of central melanocortin administration on brown adipose tissue (BAT) thermogenesis. Appetite. 2007;49(1):336. [Google Scholar]
- Bednarek MA, MacNeil T, Kalyani RN, Tang R, Van Der Ploeg LH, Weinberg DH. Selective, high affinity peptide antagonists of alpha-melanotropin action at human melanocortin receptor 4: their synthesis and biological evaluation in vitro. J Med Chem. 2001;44:3665–3672. doi: 10.1021/jm010165y. [DOI] [PubMed] [Google Scholar]
- Kask A, Mutulis F, Muceniece R, Pahkla R, Mutule I, Wikberg JE, Rago L, Schioth HB. Discovery of a novel superpotent and selective melanocortin-4 receptor antagonist (HS024): evaluation in vitro and in vivo. Endocrinology. 1998;139:5006–5014. doi: 10.1210/endo.139.12.6352. [DOI] [PubMed] [Google Scholar]
- Schioth HB, Bouifrouri AA, Rudzish R, Muceniece R, Watanobe H, Wikberg JE, Larhammar D. Pharmacological comparison of rat and human melanocortin 3 and 4 receptors in vitro. Regul Pept. 2002;106:7–12. doi: 10.1016/s0167-0115(02)00025-3. [DOI] [PubMed] [Google Scholar]
- Sinha PS, Schioth HB, Tatro JB. Activation of central melanocortin-4 receptor suppresses lipopolysaccharide-induced fever in rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1595–R1603. doi: 10.1152/ajpregu.00581.2002. [DOI] [PubMed] [Google Scholar]
- Leitner C, Bartness TJ. Acute brown adipose tissue temperature response to cold in monosodium glutamate-treated Siberian hamsters. Brain Res. 2009;1292:38–51. doi: 10.1016/j.brainres.2009.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Academic Press; New York: 2007. [Google Scholar]
- Sita LV, Elias CF, Bittencourt JC. Dopamine and melanin-concentrating hormone neurons are distinct populations in the rat rostromedial zona incerta. Brain Res. 2003;970:232–237. doi: 10.1016/s0006-8993(03)02345-x. [DOI] [PubMed] [Google Scholar]
- Shi H, Bartness TJ. Catecholaminergic enzyme, vasopressin and oxytocin distribution in Siberian hamster brain. Brain Res Bull. 2000;53:833–843. doi: 10.1016/s0361-9230(00)00429-9. [DOI] [PubMed] [Google Scholar]
- Sita LV, Elias CF, Bittencourt JC. Connectivity pattern suggests that incerto-hypothalamic area belongs to the medial hypothalamic system. Neuroscience. 2007;148:949–969. doi: 10.1016/j.neuroscience.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Elias CF, Sita LV, Zambon BK, Oliveira ER, Vasconcelos LA, Bittencourt JC. Melanin-concentrating hormone projections to areas involved in somatomotor responses. J Chem Neuroanat. 2008;35:188–201. doi: 10.1016/j.jchemneu.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Lindvall O, Nobin A. Evidence of an incerto-hypothalamic dopamine neurone system in the rat. Brain Res. 1975;89:29–42. doi: 10.1016/0006-8993(75)90131-6. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V, Zaborszky L, Kohler C, Goldstein M, Palay SL. Distribution of tyrosine-hydroxylase-immunoreactive neurons in the hypothalamus of rats. J Comp Neurol. 1984;227:467–496. doi: 10.1002/cne.902270403. [DOI] [PubMed] [Google Scholar]
- Kolmac C, Mitrofanis J. Distribution of various neurochemicals within the zona incerta: an immunocytochemical and histochemical study. Anat Embryol (Berl) 1999;199:265–280. doi: 10.1007/s004290050227. [DOI] [PubMed] [Google Scholar]
- Mitrofanis J, Ashkan K, Wallace BA, Benabid AL. Chemoarchitectonic heterogeneities in the primate zona incerta: clinical and functional implications. J Neurocytol. 2004;33:429–440. doi: 10.1023/B:NEUR.0000046573.28081.dd. [DOI] [PubMed] [Google Scholar]
- Mitrofanis J. Evidence for an auditory subsector within the zona incerta of rats. Anat Embryol (Berl) 2002;205:453–462. doi: 10.1007/s00429-002-0268-3. [DOI] [PubMed] [Google Scholar]
- Mitrofanis J. Some certainty for the “zone of uncertainty”? Exploring the function of the zona incerta. Neuroscience. 2005;130:1–15. doi: 10.1016/j.neuroscience.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Kawana E. The cells of origin of the incertofugal projections to the tectum, thalamus, tegmentum and spinal cord in the rat: a study using the autoradiographic and horseradish peroxidase methods. Neuroscience. 1982;7:2389–2406. doi: 10.1016/0306-4522(82)90203-2. [DOI] [PubMed] [Google Scholar]
- Kim U, Gregory E, Hall WC. Pathway from the zona incerta to the superior colliculus in the rat. J Comp Neurol. 1992;321:555–575. doi: 10.1002/cne.903210405. [DOI] [PubMed] [Google Scholar]
- Kawana E, Watanabe K. A cytoarchitectonic study of zona incerta in the rat. J Hirnforsch. 1981;22:535–541. [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from the rhomboid nucleus of the bed nuclei of the stria terminalis: implications for cerebral hemisphere regulation of ingestive behaviors. J Comp Neurol. 2003;463:434–472. doi: 10.1002/cne.10758. [DOI] [PubMed] [Google Scholar]
- Simmons DM, Swanson LW. Comparison of the spatial distribution of seven types of neuroendocrine neurons in the rat paraventricular nucleus: toward a global 3D model. J Comp Neurol. 2009;516:423–441. doi: 10.1002/cne.22126. [DOI] [PubMed] [Google Scholar]
- Skibicka KP, Grill HJ. Energetic Responses Are Triggered by Caudal Brainstem Melanocortin Receptor Stimulation and Mediated by Local Sympathetic Effector Circuits. Endocrinology. 2008;7:3605–3616. doi: 10.1210/en.2007-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibicka KP, Grill HJ. Hypothalamic and hindbrain melanocortin receptors contribute to the feeding, thermogenic, and cardiovascular action of melanocortins. Endocrinology. 2009;150:5351–5361. doi: 10.1210/en.2009-0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verty AN, Allen AM, Oldfield BJ. The effects of rimonabant on brown adipose tissue in rat: implications for energy expenditure. Obesity (Silver Spring) 2009;17:254–261. doi: 10.1038/oby.2008.509. [DOI] [PubMed] [Google Scholar]
- Bednarek MA, MacNeil T, Tang R, Kalyani RN, Van Der Ploeg LH, Weinberg DH. Potent and selective peptide agonists of alpha-melanotropin action at human melanocortin receptor 4: their synthesis and biological evaluation in vitro. Biochem Biophys Res Commun. 2001;286:641–645. doi: 10.1006/bbrc.2001.5444. [DOI] [PubMed] [Google Scholar]
- Jonsson L, Skarphedinsson JO, Skuladottir GV, Watanobe H, Schioth HB. Food conversion is transiently affected during 4-week chronic administration of melanocortin agonist and antagonist in rats. J Endocrinol. 2002;173:517–523. doi: 10.1677/joe.0.1730517. [DOI] [PubMed] [Google Scholar]
- Baran K, Preston E, Wilks D, Cooney GJ, Kraegen EW, Sainsbury A. Chronic central melanocortin-4 receptor antagonism and central neuropeptide-Y infusion in rats produce increased adiposity by divergent pathways. Diabetes. 2002;51:152–158. doi: 10.2337/diabetes.51.1.152. [DOI] [PubMed] [Google Scholar]
- Small CJ, Kim MS, Stanley SA, Mitchell JR, Murphy K, Morgan DG, Ghatei MA, Bloom SR. Effects of chronic central nervous system administration of agouti- related protein in pair-fed animals. Diabetes. 2001;50:248–254. doi: 10.2337/diabetes.50.2.248. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Kemnitz JW, Bray GA. Lateral hypothalamic lesions and norepinephrine turnover in rats. J Clin Invest. 1983;72:919–927. doi: 10.1172/JCI111063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL, Bowers RR, Bartness TJ, Kaplan JM, Grill HJ. Brainstem melanocortin 3/4 receptor stimulation increases uncoupling protein gene expression in brown fat. Endocrinology. 2003;144:4692–4697. doi: 10.1210/en.2003-0440. [DOI] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- Rutanen J, Pihlajamaki J, Karhapaa P, Vauhkonen I, Kuusisto J, Moilanen ML, Laakso M. The Val103Ile polymorphism of melanocortin-4 receptor regulates energy expenditure and weight gain. Obes Res. 2004;12:1060–1066. doi: 10.1038/oby.2004.133. [DOI] [PubMed] [Google Scholar]