Abstract
Objective
To examine white coat adherence over time in children with epilepsy.
Study design
This was a longitudinal prospective study to examine medication adherence prior to and following consecutive, clinic visits over a 13-month period in 120 children with newly-diagnosed epilepsy (Mage=7.2±2.9 years; 38% female) and their caregivers. Electronic monitors were used to assess adherence and ordinal logistic regression models were employed.
Results
Results demonstrated white coat adherence, with adherence increasing during the three days preceding clinic visits. Data also revealed a significant interaction, whereby adherence increased following initial clinic visits, but decreased following the last clinic visit.
Conclusions
White coat adherence occurs for children with newly-diagnosed epilepsy. Increased awareness of white coat adherence has important implications for clinical decision-making and should be examined in other pediatric populations. Increased monitoring of medication patterns can help clinicians avoid unnecessary changes to the treatment regimen. Interventions targeting improved communication around adherence behaviors are necessary to maximize therapy benefits.
Keywords: seizures, antiepileptic drug therapy, children, compliance, clinic appointments
Non-adherence to medical treatment is common (50–60%), limits treatment efficacy, increases morbidity and mortality, reduces quality of life and increases healthcare costs across pediatric illnesses (1). Within pediatric epilepsy, a condition characterized by recurrent unprovoked seizures, 58% of children exhibited non-adherence to antiepileptic drugs in the first six-months of therapy (2). The consequences of non-adherence for children and adults with epilepsy are multi-fold, including increased seizures (3–8), mortality (9, 10), and high healthcare costs (11, 12). Several factors have been associated with poor adherence in pediatric epilepsy, including sociodemographic (e.g., socioeconomic status, child age), disease and treatment (e.g., seizure type, antiepileptic drug dosing frequency), and patient-related factors (e.g., psychological comorbidities, illness beliefs, cognitive functioning)(13); however, the impact of the clinic visit itself on adherence behaviors has received little attention (14, 15).
White coat adherence, defined as improved patient adherence to treatment around clinic visits (16), can erroneously suggest greater patient adherence than is otherwise demonstrated. Such patient behavior may negatively impact patient-provider decision-making, treatment planning, and, as a result, disease outcomes. For example, white coat adherence may artificially inflate blood serum levels leading a provider to mistakenly conclude that a patient is taking a therapeutic dose. With evidence of continued symptoms and without adequate understanding of patient adherence, providers may subsequently make unnecessary changes to the medication regimen (17) and thus, adversely impact both disease control and medication side effects.
Cramer et al demonstrated adherence rates of 88% for the five days before and after a clinic visit compared with 73% one month after the visit in adults with epilepsy (16). In contrast, although our own research indicated significant non-adherence in children with epilepsy (2, 15, 18), we did not detect white coat adherence in the 1, 3, or 5 days preceding the first clinic visit following diagnosis (15). Limitations of these prior white coat adherence studies include cross-sectional designs, small cohorts, and/or analysis of group means rather than trajectories of adherence to specifically determine white coat adherence. Examination of adherence using group means over longer periods of time could provide misleading results (e.g., dilution or artificial inflation of effects). In contrast, use of daily adherence data allows for more precise understanding of the timing of white coat adherence behaviors and the degree to which adherence changes in proximity to the clinic visits over time.
The primary aim of the current prospective, longitudinal study was to investigate the presence of white coat adherence using daily adherence data in children with epilepsy over the first year of antiepileptic drug therapy. It was hypothesized that children would exhibit white coat adherence over time, such that their adherence would improve prior to and decrease following their consecutive scheduled clinic visits.
Methods
Potential participants were recruited from the New Onset Seizure Clinic at Cincinnati Children’s Hospital Medical Center (CCHMC). Inclusion/exclusion criteria included: 1) new diagnosis of epilepsy; 2) aged 2–12 years; 3) no parent-reported comorbid chronic illnesses requiring routine medications (e.g., diabetes) or developmental disorders (e.g., autism), 4) no prior antiepileptic drug treatment, and 5) initiation of carbamazepine or valproic acid monotherapy (standard clinical practice). Parental consent/child assent was obtained during the first scheduled clinic visit (i.e., day of diagnosis). After obtaining consent, parents completed a demographic questionnaire and received the Medication Event Monitoring Systems (MEMS) TrackCap to begin electronically monitoring adherence to their prescribed antiepileptic drug treatment. Study personnel informed parents that the bottle monitored medication taking; however, data from the MEMS TrackCaps were not shared with the healthcare team nor parents/children. As part of routine clinical care, patients returned to clinic approximately one-month following diagnosis and every three months thereafter (i.e., 4, 7, 10, 13 months following diagnosis). During each follow-up appointment, MEMS TrackCaps were downloaded. Parents and children also completed several questionnaires as part of the larger, longitudinal natural-history study of adherence behaviors in children with newly diagnosed epilepsy (National Institutes of Health- K23HD057333). Participants received a $10 gift card for completion of the questionnaires and an additional $10 gift card for bringing back the MEMS TrackCaps. The study was approved by the CCHMC Institutional Review Board.
Basic demographic information, including child age, sex, family composition, and caregiver occupation and education were obtained at the initial clinic visit. A Revised Duncan (TSEI2 (19)), an occupation-based measure of SES (20, 21), was calculated based on parent occupation. Scores range from 15 to 97 with higher scores representing greater occupational attainment. For two-caregiver households, the higher Duncan score was used, and served as a proxy for socioeconomic status. Information regarding seizure type, seizure activity, and initiation of antiepileptic drug therapy was obtained from medical chart review and parent interview at each clinic visit.
The Medication Event Monitoring Systems (MEMS® 6 TrackCap; AARDEX Corporation), an electronic monitoring system that measures the daily adherence to prescribed oral medications, was used to monitor antiepileptic drug adherence. Compared with other measures (e.g., self-report, pharmacy refill), electronic monitoring is currently considered the “gold standard” for adherence measurement (22, 23). The MEMS TrackCap stores times and dates of bottle openings for approximately 36 months and data can be transferred to a Windows-based computer. Data from the MEMS TrackCap were downloaded at each follow-up clinic visit. Truncated adherence rates (maximum of 100%) were used in analyses to reduce inflation as a result of overuse or extra openings that may have occurred due to prescription refills. This method has been used successfully in studies assessing electronically-monitored adherence (5, 6).
Statistical Analyses
Given that all children were prescribed a twice daily medication regimen, daily adherence could take on one of three possible values: 0, 1, or 2 (0, 50%, or 100% adherent to two prescribed doses). As such, an ordinal logistic regression model based on a cumulative logit link function was employed to detect white coat adherence. The model simultaneously examined changes in daily adherence three days before and three days after each clinic visit. Three days was chosen as a benchmark based on the half-lives of the primary antiepileptic drugs prescribed for children with epilepsy within our clinic; it is a long enough timeframe that a change in antiepileptic drug serum concentrations could occur if adherence dramatically changed prior to the clinic visit. The three days before and after the clinic visit were each coded such that the corresponding slope from the statistical model (as opposed to an overall mean score) corresponded to the entire three day period. The day of a given clinic visit was coded 0 for both the before and after clinic visit variables. A variable corresponding to the order of the consecutive clinic visits (e.g., 2nd = 1-month post-diagnosis, 3rd = 4-months post-diagnosis, 4th = 7-months post-diagnosis, 5th = 10-months post-diagnosis, and 6th = 13 months post-diagnosis) was also included in the model to account for longitudinal trends in adherence and was coded so that the intercept in the statistical model corresponded to the second clinic visit (i.e., 1-month clinic visit following diagnosis). Interactions between change in daily adherence before/after the clinic visit and the order of the clinic visits were investigated to determine if white coat adherence changed over time. Generalized estimating equations were used to estimate the model and account for repeated measurements nested within participants. Study participants were part of a larger longitudinal study examining factors underlying variability in antiepileptic drug adherence in pediatric epilepsy; the larger study was determined to be adequately powered with a sample size of at least 93. As a result, power and sample size calculations for this study were not performed. All analyses were conducted using the GENMOD procedure in SAS version 9.2.
Results
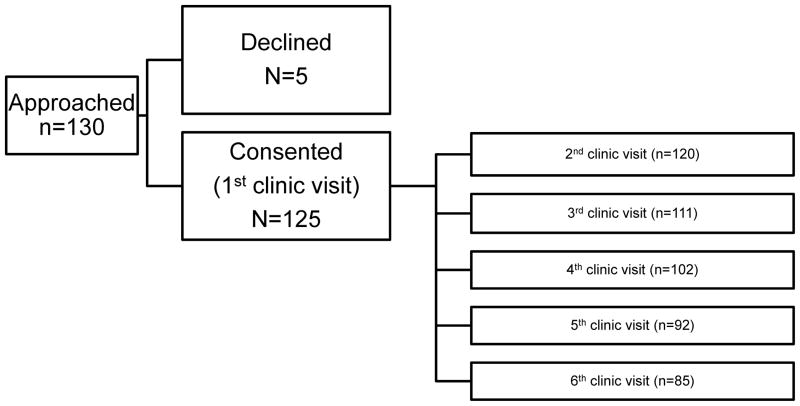
Consecutive children with new-onset epilepsy (n=130)and their parents met inclusion/exclusion criteria for the study. Five eligible participants declined participation due to busy schedules and lack of interest. One participant was found to be ineligible for the study following consent and was disenrolled, and four participants consented but never returned to clinic. Thus, the final sample size included 120 children with new-onset epilepsy and their caregivers. No significant differences were noted between the final sample and the four participants who did not return to clinic on child age, sex, race and ethnicity, seizure type, parent marital status, or family socioeconomic status. Missing data for the remaining study period are illustrated in Figure 1 (available at www.jpeds.com). Reasons for missingness are as follows: moved, missed clinic appointments/no-showed, withdrew from the study, non-monitored MEMS adherence data (e.g., lost bottle, did not use bottle, medication temporarily discontinued), and did not return to clinic for follow-up. Demographic characteristics of the sample are summarized in the Table.
Figure 1.
Flow diagram of the number of participants at each time point.
The intercept in the ordinal logistic regression model corresponding to the probability of taking two of the two daily doses (100% adherence) versus taking less than two daily doses (0 or 50% adherence) was 1.08 (z = 7.50, p < .0001, 95% CI: 0.80–1.36), an estimated probability of 0.75. The intercept corresponding to 50 or 100% adherence versus 0% adherence was 2.04 (z = 10.50, p < .0001, 95% CI: 1.66–2.42), an estimated probability of 0.88.
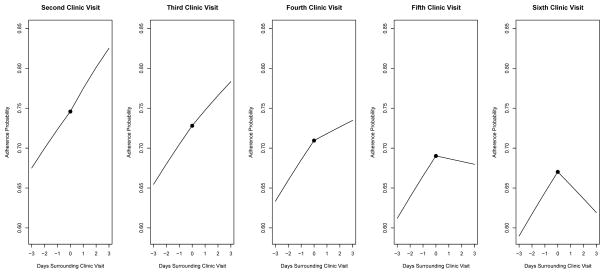
The quadratic effect for the order of the clinic visits (e.g., 1st, 2nd, 3rd visit after diagnosis) was not statistically significant (p > .05); consequently only the linear effect for the order of the clinic visits was retained in the final models. The interaction between the order of the clinic visits and the three days before the clinic visit was not statistically significant (p = .29); however, as hypothesized, the main effect for change in adherence across the three days before the clinic visit was statistically significant (β = 0.35, z = 4.40, p < .0001, 95% CI: 0.19–0.50) with a corresponding odds ratio of 1.42 (95% CI: 1.21–1.65). The interaction between the order of the clinic visits and the three days after the clinic visit was statistically significant (β = −0.17, z = −2.59, p < .01, 95% CI: −0.31-−0.04) with the following main effects: the order of the clinic visits (β = −0.09, z = −1.79, p = .07, 95% CI: −0.19-0.01) and three days after the clinic visit (β = 0.48, z = 2.66, p < .01, 95% CI: 0.13–0.83). To provide a better understanding of this interaction, these estimates imply an odds ratio of 1.62 (i.e., increasing adherence) for the change in adherence three days after the clinic visit at the 2nd visit compared with an odds ratio of 0.82 (i.e., decreasing adherence) for the change in adherence three days after the clinic visit at the 6th visit. To facilitate interpretation, Figure 2 illustrates the probability of 100% versus 0 or 50% adherence for the three days before and after the clinic visit (where clinic visit = 0 on the x-axis) across five consecutive clinic visits following diagnosis. There was no statistically significant relationship between white coat adherence behaviors and seizure activity.
Figure 2.
Model-based probability of 100% versus 0 or 50% adherence for the three days before and after the clinic visit (where clinic visit = 0 on the x-axis) across five consecutive clinic visits following diagnosis (i.e., approximately 1, 4, 7, 10, and 13-months post-diagnosis). The solid circle represents the adherence probability on the day of the clinic visit.
Discussion
This longitudinal study used innovative statistical methods and objective adherence measurement to document white coat adherence over time in children with epilepsy and their caregivers. Supporting our hypothesis, results demonstrated that, over time, adherence increased immediately preceding clinic visits. In contrast, our hypothesis that adherence would decrease following clinic appointments was only partially supported by the data. Results demonstrated variable effects over time following the clinic visits, with increased adherence at the second, third, and fourth clinic visits and decreased adherence following the fifth and sixth clinic visits.
Taken as a whole and consistent with the larger behavioral literature, our data indicated that the clinic visit, which incorporates routine blood draws every 3 months and/or face-to-face contact with health care providers, likely served as a “positive reinforcer” (i.e., a stimulus that serves to increase a specific behavior) on a “fixed interval” schedule of reinforcement for adherence behavior initially. Unlike the current study, prior cross-sectional research that utilized mean adherence data 3 to 5 days before and after clinic visits (15, 16) cannot demonstrate immediate changes in adherence behaviors over time. Our longitudinal models extend and more fully explain adherence behaviors relative to these prior studies, including our pilot study of 35 patients with epilepsy at their first clinic visit (15), which used group means of adherence. Specifically, examination of changes before and after clinic visits enabled us to identify differential white coat adherence patterns, with the clinic visit serving as an anchor point. This is extremely salient because during the first three clinic visits, adherence increased both before and after the clinic visits. However, during the sixth clinic visit, adherence increased prior to the visit and decreased following the clinic visit, which demonstrated inconsistencies in white coat adherence around clinic visits over time. It may be that as clinic visits became routine and families adjusted to the child’s diagnosis, contact with the health care provider was viewed as a less effective reinforcer for adherence behavior. This phenomena, coined “habituation” is well documented in the behavioral literature (24) and further illustrated by our declining adherence rates over time. Future research warrants identification of factors that reduce the likelihood of white coat adherence and/or implementation of more robust or varied reinforcers to maintain adherence behaviors over time. For example, if the existing, regularly scheduled blood draw is a potential reinforcer for white coat adherence, asking families to have blood draws at random intervals (i.e., “variable ratio” reinforcement schedule) may decrease white coat adherence.
Longitudinal modeling also enabled us to illustrate white coat adherence increases over time in a newly diagnosed cohort preceding clinic visits, such that adherence increased between 7.0 and 8.0 %. Following clinic visits, adherence rates increased from 2.5 to 7.9% over the first three clinic visits and decreased 5.1 % at the last clinic visit. Due to social desirability, families may be motivated to “look good” or appear more adherent for their provider and thus engage in white coat adherence prior to the clinic visit. Conversely, caregivers may have become savvier over time to avoid negative health care provider interactions (e.g., reprimands around nonadherence). Although the reasons for white coat adherence are currently unknown, this behavior has significant implications for clinical decision making and family-provider communication.
Overall, our findings have a number of implications for clinical practice in managing pediatric epilepsy. These data highlight that health care providers may be making clinical decisions based on misinformation, such as serum levels which do not accurately reflect adherence behaviors. The exception to this is serum levels in the non-therapeutic or non-existent range, which clearly signifies that the patient is non-adherent. However, if children have drug serum levels in the therapeutic levels due to white coat adherence but exhibit poor seizure control, health care providers are likely to increase antiepileptic drug doses or switch antiepileptic drugs. This could lead to toxicities if the patient begins taking their medication more routinely. Furthermore, variable adherence can potentially increase the risk of breakthrough seizures. Open communication between the patient, family, and provider surrounding adherence behaviors is critical. Given the significant ramifications of white coat adherence, it is beneficial to monitor adherence with valid and reliable indicators, such as electronic monitors. Electronic monitors provide the most objective information about daily adherence behaviors (22, 23). Although the use of electronic monitors in clinical practice may be cost prohibitive, other more cost-effective measures (i.e., reliable self-report (18), pill counts) can also be used. Such data can promote frank discussions between providers and families regarding strategies to promote adherence and reduce barriers to consistent adherence over time.
Several limitations of the current study must be noted with subsequent directions for future research. First, although MEMS is an empirically-supported measure of medication adherence (23), the device only records bottle openings. As such, it is limited by its inability to account for the number of capsules taken each day (i.e., multiple doses removed at one time) and actual ingestion of the medication. However, until new technologies are developed, MEMS remains the preferred assessment measure for oral medications. Second, our sample was limited in size and only represents one disease population. Non-adherence is a universal issue across populations (1); however, future studies are needed to confirm the generalizability of our findings to other disease groups. Third, the current study design does not allow us to identify the motivation for white coat adherence in that it remains unclear whether the clinic visit itself or the blood draw served as the reinforcer in this model. One method to distinguish the actual reinforcer would be to ask families to have their blood drawn between clinic visits and assess whether adherence increases prior to the blood draw. Fourth, adherence is a complex issue. White coat adherence is only one factor in understanding adherence behaviors; however, other factors (e.g., disease education, parent and patient attitudes toward medication, access to care, transition of responsibility from parents to children for disease management) also likely influence overall adherence and need to be studied. Finally, the relation between white coat adherence and disease symptoms is an important next step in the field. Little is known about how white coat adherence affects seizure control. For example, if adherence is higher prior to a clinic visit and then immediately declines after clinic visits, does it place children at higher risk for break-through seizures?
Documentation and awareness of white coat adherence is integral to improving clinical care. Similar to recently published data in pediatric diabetes (14), our data demonstrated that white coat adherence exists, with increased adherence prior to and following clinic visits for several clinic visits after diagnosis and then decreased adherence following the clinic visit one-year post-diagnosis. In contrast to other white coat adherence studies, this data is the first to demonstrate that clinic visits/blood draws/interactions with health care providers may serve as a positive reinforcer initially. Thus, adherence interventions and improving patient-provider communication around adherence behaviors is necessary to maximize the benefits of therapy.
Table.
Child Participant Demographics
| M (SD) or % | |
|---|---|
| Age (yrs) | 7.2 (2.9); range = 2.0–12.9 |
|
| |
| Sex | 38% female |
|
| |
| Race | White = 75% |
| Black = 17.5% | |
| Bi/multi-racial = 6.7% | |
| Asian = 0.8% | |
|
| |
| Ethnicity | Hispanic = 3% |
|
| |
| Seizure Type | Partial = 59% |
| Generalized = 25% | |
| Unclassified = 16% | |
|
| |
| Initial AED | Carbamazepine = 60% |
| Valproate = 40% | |
|
| |
| AED at 16-months post-diagnosis* | Carbamazepine = 40% |
| Valproate = 29% | |
| Levetiracetam = 18% | |
| Topiramate = 7% | |
| Oxcarbazepine = 3% | |
| Ethosuximide = 3% | |
| Lamotrigine = 3% | |
| Gabapentin = 1% | |
|
| |
| % of participants on polytherapy at 16-months post-diagnosis | 4% |
|
| |
| Parent Marital Status | Married = 64% |
|
| |
| Participating Caregiver | Mother/Stepmother = 84.2% |
| Father = 13.3% | |
| Other guardian (e.g. aunt) = 2.4% | |
|
| |
| Caregiver Age (yrs) | 34.9 (6.9); range = 20.9–60.0 |
|
| |
| Family Duncan Score** | 52.7 (20.2) |
Percentage is above 100% because 4% of the sample were on two AEDs.
The mean Duncan score reflects occupations such as physician’s assistants, artists, performers, and firefighters.
Acknowledgments
Funded by the National Institutes of Health awarded to the first author (grant K23HD057333).
Abbreviations
- CCHMC
Cincinnati Children’s Hospital Medical Center
- MEMS
Medication Event Monitoring Systems
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Avani C. Modi, Cincinnati Children’s Hospital Medical Center, Division of Behavioral Medicine and Clinical Psychology
Lisa M. Ingerski, Cincinnati Children’s Hospital Medical Center, Division of Behavioral Medicine and Clinical Psychology
Joseph R. Rausch, Cincinnati Children’s Hospital Medical Center, Division of Behavioral Medicine and Clinical Psychology
Tracy A. Glauser, Cincinnati Children’s Hospital Medical Center, Division of Neurology
Dennis Drotar, Cincinnati Children’s Hospital Medical Center, Division of Behavioral Medicine and Clinical Psychology
References
- 1.Rapoff M, editor. Adherence to pediatric medical regimens. 2. New York: Springer Science+Business Media; 2010. [Google Scholar]
- 2.Modi AC, Rausch JR, Glauser TA. Patterns of non-adherence to antiepileptic drug therapy in children with newly diagnosed epilepsy. JAMA. 2011;305:1669–76. doi: 10.1001/jama.2011.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kyngas H. Compliance with health regimens of adolescents with epilepsy. Seizure. 2000 Dec;9:598–604. doi: 10.1053/seiz.2000.0470. [DOI] [PubMed] [Google Scholar]
- 4.Bassili A, Omar T, Zaki A, Abdel-Fattah M, Tognoni G. Pattern of diagnostic and therapeutic care of childhood epilepsy in Alexandria, Egypt. Int J Qual Health Care. 2002 Aug;14:277–84. doi: 10.1093/intqhc/14.4.277. [DOI] [PubMed] [Google Scholar]
- 5.Cramer JA, Glassman M, Rienzi V. The relationship between poor medication compliance and seizures. Epilepsy Behav. 2002 Aug;3:338–42. doi: 10.1016/s1525-5050(02)00037-9. [DOI] [PubMed] [Google Scholar]
- 6.Jones RM, Butler JA, Thomas VA, Peveler RC, Prevett M. Adherence to treatment in patients with epilepsy: Associations with seizure control and illness beliefs. Seizure. 2006 Oct;15:504–8. doi: 10.1016/j.seizure.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Gopinath B, Radhakrishnan K, Sarma PS, Jayachandran D, Alexander A. A questionnaire survey about doctor-patient communication, compliance and locus of control among south Indian people with epilepsy. Epilepsy Res. 2000 Mar;39:73–82. doi: 10.1016/s0920-1211(99)00112-6. [DOI] [PubMed] [Google Scholar]
- 8.Manjunath R, Davis KL, Candrilli SD, Ettinger AB. Association of antiepileptic drug nonadherence with risk of seizures in adults with epilepsy. Epilepsy Behav. 2009 Feb;14:372–8. doi: 10.1016/j.yebeh.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Faught E, Duh MS, Weiner JR, Guerin A, Cunnington MC. Nonadherence to antiepileptic drugs and increased mortality: findings from the RANSOM Study. Neurology. 2008 Nov 11;71:1572–8. doi: 10.1212/01.wnl.0000319693.10338.b9. [DOI] [PubMed] [Google Scholar]
- 10.Sillanpaa M, Shinnar S. Long-term mortality in childhood-onset epilepsy. N Engl J Med. 2010 Dec 23;363:2522–9. doi: 10.1056/NEJMoa0911610. [DOI] [PubMed] [Google Scholar]
- 11.Ettinger AB, Manjunath R, Candrilli SD, Davis KL. Prevalence and cost of nonadherence to antiepileptic drugs in elderly patients with epilepsy. Epilepsy Behav. 2009 Feb;14:324–9. doi: 10.1016/j.yebeh.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 12.Faught RE, Weiner JR, Guerin A, Cunnington MC, Duh MS. Impact of nonadherence to antiepileptic drugs on health care utilization and costs: findings from the RANSOM study. Epilepsia. 2009 Mar;50:501–9. doi: 10.1111/j.1528-1167.2008.01794.x. [DOI] [PubMed] [Google Scholar]
- 13.Modi AC, Guilfoyle SM. Adherence to Antiepileptic Drug Therapy Across the Developmental Life-span. In: Pinikahana J, Walker C, editors. Society, Behaviour and Epilepsy. New York: Nova Science Publishers Inc; 2011. pp. 175–205. [Google Scholar]
- 14.Driscoll KA, Bennett Johnson S, Yang F, Deeb L, Silverstein J. Does Blood Glucose Monitoring Increase Prior to Clinic Visits in Children with Type 1 Diabetes? Diabetes Care. doi: 10.2337/dc11-0388. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Modi AC, Morita DA, Glauser TA. One-month adherence in children with new-onset epilepsy: white-coat compliance does not occur. Pediatrics. 2008 Apr;121:e961–6. doi: 10.1542/peds.2007-1690. [DOI] [PubMed] [Google Scholar]
- 16.Cramer JA, Scheyer RD, Mattson RH. Compliance declines between clinic visits. Arch Intern Med. 1990 Jul;150:1509–10. [PubMed] [Google Scholar]
- 17.Koumoutsos JE, Modi AC, Morita DA, Monahan SR, Glauser TA. The dual clinical impact of non-adherence: Seizures and “avoidable” AED dosage increases [Abstract] Epilepsia. 2007;48:56–7. [Google Scholar]
- 18.Modi AC, Guilfoyle SM, Morita DA, Glauser TA. Development and reliability of a correction factor for parent-reported adherence to pediatric antiepileptic drug therapy. Epilepsia. 2011;52:370–6. doi: 10.1111/j.1528-1167.2010.02789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevens G, Featherman DL. A revised socioeconomic index of occupational status. Soc Sci Res. 1981;10:364–95. [Google Scholar]
- 20.Nakao K, Treas J. The 1989 Socioeconomic Index of Occupations: construction from the 1989 Occupational Prestige Scores. Chicago: University of Chicago, National Opinion Research Center; 1992. [Google Scholar]
- 21.Hauser RM. Measuring socioeconomic status in studies of child development. Child Dev. 1994 Dec;65:1541–5. doi: 10.1111/j.1467-8624.1994.tb00834.x. [DOI] [PubMed] [Google Scholar]
- 22.Ingerski LM, Hente EA, Modi AC, Hommel KA. Electronic measurement of medication adherence in pediatric chronic illness: A review of measures. Journal of Pediatrics. 2011;159:528–34. doi: 10.1016/j.jpeds.2011.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quittner AL, Modi AC, Lemanek KL, Ievers-Landis CE, Rapoff MA. Evidence-based assessment of adherence to medical treatments in pediatric psychology. Journal of pediatric psychology. 2008 Oct;33:916–36. doi: 10.1093/jpepsy/jsm064. discussion 37–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy ES, Sweeney FK, Smith RG, McComas JJ. Dynamic changes in reinforcer effectiveness: theoretical, methodological, and practical implications for applied research. J Appl Behav Anal. 2003;36:421–38. doi: 10.1901/jaba.2003.36-421. [DOI] [PMC free article] [PubMed] [Google Scholar]