Abstract
Stroke is the third leading cause of death and the primary cause of disability in the developed world. Experimental and clinical data indicate that stroke is a sexually dimorphic disease, with males demonstrating an enhanced intrinsic sensitivity to ischemic damage throughout most of their lifespan. The neuroprotective role of estrogen in the female brain is well established, however, estrogen exposure can also be deleterious, especially in older women. The mechanisms for this remain unclear. Our current understanding is based on studies examining estrogen as it relates to neuronal injury, yet cerebral ischemia also induces a robust sterile inflammatory response involving local and systemic immune cells. Despite the potent anti-inflammatory effects of estrogen, few studies have investigated the contribution of estrogen to sex differences in the inflammatory response to stroke. This review examines the potential role for estrogen-mediated immunoprotection in ischemic injury.
Keywords: stroke, brain, sex differences, estrogen, cerebral ischemia, gender differences, middle cerebral artery occlusion, hypoxia-ischemia
Stroke
Stroke is now the third leading of cause of death after heart disease and cancer. Stroke affects 15 million people worldwide each year, and is the leading cause of long-term disability, affecting nearly 800,000 in US alone (Lloyd-Jones et al., 2009). Increasing life expectancy together with an expanding aging population will place a profound burden on the economy well beyond the current estimate of $69 billion annually (Lloyd-Jones et al., 2009). The continued inability to effectively treat stroke coupled with our aging population and the projected lack of health care resources will place patients at a higher risk of mortality and long-term disability than currently seen today.
Sex and Stroke
Analysis of the epidemiological data indicates that stroke is a sexually dimorphic disease (Bushnell, 2008; Reeves et al., 2008; Turtzo and McCullough, 2010). Although the overall life-time incidence of stroke in men is approximately 30% higher than in women, females have more severe strokes with poorer recovery and greater long-term disability (Appelros et al., 2009; Fukuda et al., 2009; Niewada et al., 2005; Roquer et al., 2003). Women now account for 61% of all stroke deaths (Rosamond et al., 2008). The risk for incident stroke at 65 years of age has decreased significantly from 19.5% to 14.5% in men but more modestly from 18.0% to 16.1% in women (Roger et al., 2011). The higher mortality in women is in part due to older age and the greater number of co-morbid conditions in women at the time of stroke (Devries et al., 2011). Men experience a higher risk of stroke over most of the lifespan, with several notable exceptions. There is a stroke surge in women between the ages of 19 and 30 years, associated with the peripartum (Pathan and Kittner, 2003; Putaala et al., 2009; Rasura et al., 2006) and a second increase in risk in women between 45 and 54 years of age, in the peri-menopause (Towfighi et al., 2007). While it appears that transitioning to a lower estrogenic state predisposes females to higher stroke risk, it is unlikely that sex hormones fully account for these sex differences. For example, after the age of 50 years, the risk of stroke for both sexes rises dramatically, but the incidence in women does not surpass that of men until after the age of 85, well past menopause (Lloyd-Jones et al., 2009). In addition, childhood ischemic stroke is more common in boys than in girls, despite relatively equivalent hormone levels, possibly due to a greater influence of sex chromosomes (Golomb et al., 2009). However, an emerging contribution from testosterone has added complexity to studies of sex differences in stroke. Exposure to testosterone may contribute to the higher incidence and severity of stroke in boys, as elevated levels correlated with stroke risk (Normann et al., 2009; Siegel et al.; Vannucci and Hurn, 2009), and recent studies in elderly men showed an increased risk in men treated with exogenous testosterone replacement (Basaria et al., 2010).
The concept that genetic sex may also contribute to ischemic sensitivity independently of the actions of gonadal steroids is becoming increasing investigated. Expression of both autosomal and sex chromosome-linked genes in the mouse brain displays regional sexual dimorphism, as well as sex-specific imprinting from the parent of origin (Gregg et al., 2010). Females inherit one X-chromosome from each parent, whereas males inherit a single, maternal X-chromosome. While one of the X chromosomes in females is randomly inactivated during early development, up to 20% of X-linked genes escape X inactivation in humans (Carrel and Willard, 1999). X inactivation can also become unstable with aging (Hatakeyama et al., 2004; Smrt et al., 2011) allowing for re-expression on genes silenced earlier in development. This may be an important contributor to the immunological response to stroke, as clinical data demonstrate that men and women differ in their humoral, innate, and cell-mediated responses to other immunological challenges such as viral vaccines (Klein et al., 2010). However, as both androgen and estrogen response elements are present in the promoters of several innate immunity genes, hormone receptor concentration, and duration of sex steroid exposure may also lead to sex differences in the innate immune responses.
The concept that intrinsic sex differences after ischemic injury exist between males and females also comes from emerging in vitro experiments using cells derived from male (XY) versus female (XX) animals. One critical advantage of studying cells in culture is that sex steroid hormones (and agents that may activate sex steroid responses, such as phenol red) can be eliminated from the cell culture medium. If sex-typed cells are used, any differences in behavior seen between male and female cells must result from inherent sex differences within the cells, from prenatal hormone exposure (ie. “organizational effects”), or a combination of the two. The two most common methods of mimicking an ischemic insult in cell culture are either to subject cells to oxygen-glucose deprivation (OGD) or to expose them to N-methyl-D-aspartic acid (NMDA). In cultures of hippocampal slices, slices from female P7 pups demonstrated intrinsic protection against OGD or NMDA exposure relative to those from male pups (Li et al., 2005). Similarly, primary rat hippocampal neuron male cultures were more sensitive to hypoxic insult than female cultures (Heyer et al., 2005). Sex differences in ischemic sensitivity have also been reported in cultured astrocytes (Liu et al., 2007a) and splenocytes (Du et al., 2004), demonstrating that sex dimorphism is present in multiple cell types (see Table 1). The reader is directed to several recent reviews on this topic (Herson et al., 2009; Manwani and McCullough, 2011).
Table 1.
The anti-inflammatory effects of estrogen on glial cells
| Astrocytes | |||
|---|---|---|---|
| Author | Model | Drug/Treatment/mutation | Downstream Effect |
| (Santos-Galindo et al., 2011) | In vitro Mouse | LPS (sex effect) | Male: ↑IL-1b, IL-6, TNF Female: ↑TPSO, pregnenolone |
| (Liang et al., 2002) | In vitro Human (Alzheimer) | E2 | ↑GLT-1, GLAST |
| (Pawlak et al., 2005) | In vitro Mouse | E2 | ↑GLT-1, GLAST |
| (Barouk et al., 2011) | In vivo Rat | E2 | ↑VEGF |
| (Giraud et al., 2010) | In vivo / In Vitro Mouse | E2 + EAE, TNF stimulation | ↓CCL2 |
| Brain Edema | |||
| (Tomas-Camardiel et al., 2005) | In vivo Rat | E2 + LPS | ↓AQ -4, BBB deterioration |
| (Rutkowsky et al., 2011) | In vitro Rat | E2 + hypoxia | ↓AQ-4, swelling |
| (Liu et al., 2008b) | In vivo Mouse | AQ-4 deletion, MCAO | NO SEX DIFFERENCES in AQ-4 or edema |
| Microglia | |||
| Author | Model | Drug/Treatment/mutation | Downstream Effect |
| (Saijo et al., 2011) | In vitro - Mouse, Human | E2 + LPS | ↓IL-1β, IL-6, IL-23p19, iNOS |
| (Liu et al., 2005) | In vitro - Rat | E2 + LPS | ↑neuroprotection (neuronal co-culture) ↓TNF |
| (Dimayuga et al., 2005) | In vitro - Mouse | E2 + LPS | ↑IL-10 ↓TNF, IFNγ, MHCI/II, CD40, CD86, CD152, Fas/L |
| (Bruce-Keller et al., 2001; Bruce-Keller et al., 2000) | In vitro - Rat, Mouse | E2 + LPS | ↑MAPK-phosphorylation ↓iNOS |
| (Vegeto et al., 2001) | In vitro - Rat | E2 + LPS | ↓iNOS, PGE-2, MMP-9 |
| (Barreto et al., 2007) | In vivo - Rat | E2 + stab wound | ↓MHCII |
| (Vegeto et al., 2006) | In vivo - Mouse | E2 + LPS | ↓CCL2, MIP-2, TNF |
| (Zhang et al., 2004) | In vivo - Rat | E2 + hypoxia-ischemia | ↑bfl -1 |
As epidemiological data and clinical observations suggested that exposure estrogen was responsible for the lower incidence of stroke in pre-menopausal women (Billeci et al., 2008), most early preclinical studies focused on estrogen as the primary mediator of sex differences in ischemic sensitivity. Estrogen’s neuroprotective actions have been well studied in the laboratory using experimental models of stroke in animals (see Table 1), most of which have utilized 17 beta-Estradiol, which binds equally well to both isoforms of the estrogen receptor. Of note, most clinical studies have utilized other estrogen formulations (i.e., Premarin), which may also contribute to conflicting experimental and clinical results. Young female animals are more resistant to ischemic damage than males, an effect that can be reversed in part by ovariectomy (OVX) and rescued with estrogen replacement (McCullough and Hurn, 2003). Estrogen also reduces damage after induced stroke in intact males implying a direct role for sex hormone-mediated neuroprotection. The epidemiology of stroke in aging is also well mirrored in animal models, with older reproductively senescent females showing greater infarct volume than ovary-intact young females and age-matched males (Liu et al., 2009), a reversal of what is observed in young animals. At this point it is unknown why stroke displays such profound sexual dimorphism at different ages, but an enhanced inflammatory response in the aged female brain may be responsible for some of these differences. As levels of gonadal hormones fluctuate throughout the lifespan, interactions between chromosomal factors (XX and XY), sex steroid levels and aging are likely. Adding another layer of complexity is that sex steroid hormones can act via organizational effects which irreversibly commit tissues to a male or female phenotype or through activational effects that are dependent on the continued presence of the hormone (Arnold, 2009b; Becker et al., 2005). One difficulty with studies examining “sex differences” after stroke is that the organizational effects of sex steroids are not reversible by gonadectomy, and cannot be completely eliminated in cell culture models. Current approaches aimed at studying the genetic role of sex in stroke include the evaluation of epigenetic differences, X-chromosomal dosage, and most recently, the analysis of stroke outcomes in the four-core genotype model (Arnold and Chen, 2009; Siegel et al., 2011; Turtzo et al., 2011). This model, in which the testis-determining gene, Sry, has been deleted from the Y chromosome and inserted in an autosome, eliminates many of the activational and organizational effects of gonadal hormones, while maintaining the sex chromosome complement (XX vs. XY).
Sex, Stroke, and Inflammation
Emerging data suggests that stroke-induced inflammation significantly contributes to neuronal injury and clinical outcome (Dirnagl et al., 2003; Han and Yenari, 2003; Ishikawa et al., 2004; Seymour et al., 2003). Local and systemic inflammatory responses following cerebral ischemia have highlighted a pivotal role for the innate immune system in the brain’s response to injury, including activation of glia and myeloid cells (Iadecola and Anrather, 2011; Stoll et al., 1998). Injury also leads to the activation of several transcription factors involved in the inflammatory response, including nuclear factor κB (NF-κB) (Wang et al., 2007) with the subsequent up-regulation of numerous pro-inflammatory genes, such as tumor necrosis factor-α (TNF- α), monocyte chemotactic protein-1 (MCP-1), and interlukin-6 (IL-6), etc. (Altinoz and Korkmaz, 2004; Wang et al., 2007). A growing body of evidence (Kalaitzidis and Gilmore, 2005; Stein and Yang, 1995) has shown that estrogen interacts with many of these inflammatory pathways, but estrogen’s effects on immune cells after ischemic injury has not been as well studied. This review will focus on recent work examining the effect of estrogen on immune cells that could contribute to sex differences in stroke. Potential hormone-independent effects will be highlighted, although few of these studies have been performed to date.
The role of estrogen as a neuroprotectant in stroke is well established, however, much less is known about estrogen’s contribution to the immune response after ischemic injury despite numerous reports suggesting citing its role as a potent anti-inflammatory agent (Straub, 2007; Suzuki et al., 2009). estrogen likely has actions on both resident immune cells as well as on infiltrating peripheral leukocytes but the contribution of each to estrogen’s neuroprotective effects is unknown. Sexual dimorphism in immunity is evidenced by a more robust cellular and humoral response in females, which may partially explain the female bias in developing autoimmune disease (Lleo et al., 2008; McCombe et al., 2009). Females also show an increased resistance to infection and sepsis (Angele et al., 2006). Importantly, these improved outcomes correlate with female reproductive status, with less damage incurred during high estrogen states, suggesting an important contribution of gonadal hormones to these sex differences (Raju et al., 2008). The anti-inflammatory effects of estrogens on immune cells, endothelium, and in the brain are well documented (Kublickiene and Luksha, 2008; Nilsson, 2007; Pozzi et al., 2006; Straub, 2007; Vegeto et al., 2008; Villablanca et al., 2010). Although recent data suggests involvement of an adaptive and local autoimmune component following stroke, this review will focus primarily on the innate response as it relates to local and systemic activation of myeloid cells and how sex and gonadal hormones may influence this response (Becker, 2009; Vogelgesang and Dressel, 2011).
Experimental Evidence of estrogen mediated Anti-inflammatory Effects after Stroke
To date, few studies have detailed the specific role of estrogen in the immunological response to stroke. An early study by Santizo et al. (2000) found that female OVX mice had higher baseline leukocyte adhesion and more sustained adhesion six hours following stroke compared to ovary-intact controls. Subsequent findings have demonstrated that estrogen exposure decreases NF-κB activity, IkB, iNOS, and TNF expression which is associated with ischemic neuroprotection (Iadecola and Ross, 1997; Liao et al., 2002; Nagayama et al., 1999; Wen et al., 2004). Importantly, male rats treated with estrogen prior to MCAO showed a significant reduction in cortical IL-1β and reduced infarct size compared to non-treated males (Chiappetta et al., 2007), suggesting these effects are independent from genetic sex (XX vs. XY). Estrogen can suppress IL-1β-mediated activation of astrocytes, and the induction of COX-2 in cerebral endothelium (Friedman et al., 1996; Ospina et al., 2004). Interestingly, Koerner et al (2008) showed that estrogen-replacement in OVX mice led to a unique temporal pattern of brain cytokine expression following stroke, with an early increase in TNF, IL-1α, IL-1β, and IL-6 levels followed by a delayed reduction in expression of these pro-inflammatory molecules. This suggests that estrogen has temporal anti-inflammatory effects that may reflect a complex, sequential activation and suppression of the cellular immune response. Combination treatment with estrogen and progesterone (Dang et al., 2011) decreased cortical Iba1 (putative microglia) and CD3 (T- /NK cells) expression, and suppressed IL-6 and chemokine (CCL2, CCL5) expression in male rats, suggesting these anti-inflammatory effects of gonadal hormones are independent of chromosomal sex. However, this regimen was also neuroprotective, and the reduced immune response may simply reflect the decreased damage seen in treated males. While it is intriguing to speculate that similar estrogenic effects on cytokine expression might exist in stroke patients, thus far no sex difference has been observed in peripheral mononuclear cells or serum concentration of cytokines (Czlonkowska et al., 2006).
Estrogen and Inflammation: Timing of Therapy
The protective effects of estrogen are well established in the scientific literature, although not without caveats. Efforts to exploit estrogen for its therapeutic value have come up short in clinical trials, as both the Women estrogen Stroke Trial (WEST) and the Women Health Initiative (WHI) trial, reported a surprising increase in stroke incidence in estrogen-treated woman (Viscoli et al., 2001; Wassertheil-Smoller et al., 2003). The explanation for these findings has been debated extensively in the literature (Lisabeth and Bushnell, 2012; Lobo, 2007)). The timing of HRT initiation and the dose used are emerging as key factors in the vascular response to hormonal therapies. In the Nurses’ Health Study (NHS) HRT initiated early after menopause led to a lower stroke risk than if initiated in older cohorts of women. In addition high doses of estrogen were less protective against vascular disease and promoted stroke risk (Grodstein et al., 2000). The proinflammatory effects are also reflected in the serum levels of C-reactive protein (CRP), a marker of vascular risk (Cushman et al., 1999; Lakoski and Herrington, 2005). CRP was elevated 65% in healthy women (>65 years of age) exposed to 12 weeks of a high dose of micronized E2 (1 mg/d), and remained 92% higher than placebo even 12 weeks after treatment was discontinued (Prestwood et al., 2004). Interestingly, in reproductive-age women, endogenous E2 levels negatively correlate with CRP protein levels across the menstrual cycle (Gaskins et al., 2012; Wander et al., 2008), suggesting an overall anti-inflammatory effect with physiological levels of estrogens. In the Women’s Health Initiative (WHI) and the Women’s Estrogen for Stroke Trial (WEST) participants were well beyond menopause when they received HRT/ERT. It has been hypothesized that ERT should be initiated immediately at menopause in order to achieve its protective effects (Suzuki et al., 2007a), an effect recently modeled in preclinical studies (Liu et al., 2011; Selvamani and Sohrabji, 2010). In young animals estrogen treatment immediately following gonadectomy led to a significant reduction in local and plasma concentrations of pro-inflammatory cytokines (MCP-1, IL-6, TNF, GM-CSF) after stroke (Suzuki et al., 2007a). However, this effect was lost in females which had a 10-week delay between ovariectomy and initiation of estrogen-replacement suggesting that prolonged loss of estrogen makes subsequent treatment ineffective, or perhaps even detrimental.
The inflammatory changes seen throughout the lifespan differ in males and females, and may contribute to the sexually dimorphic responses to stroke (Leon et al., 2011; Liu et al., 2009). Importantly, our lab has recently shown that prolonged periods of hypoestrogenicity prior to estrogen replacement therapy (ERT) lead to a dramatically enhanced inflammatory response in aged female mice. Chronic ERT initiated in late middle age, during the estropause (at 14–15 months), markedly decreased nuclear NF-κB translocation, reduced the expression of pro-inflammatory cytokines, and led to a significant decrease in total infarct volume in animals subjected to middle cerebral artery occlusion (MCAO) at the age of 20 months (Liu et al., 2011). However, acute ERT in aged females (initiated 2 weeks prior to stroke) led to enhanced nuclear NF-κB translocation, a pro-inflammatory milieu and poorer behavioral and histological outcomes after injury. Interestingly, aged male mice receiving estrogen treatment, even acutely, demonstrated a significant reduction in pro-inflammatory markers and infarct size following stroke compared to age-matched females. This suggests that there is a poorly understood interaction between sex, hormones, and aging that leads to a sex-specific vulnerability to the detrimental effects of estrogen in aging (Bao et al., 2006; Grodstein et al., 2001; Hao et al., 2007a; Levine and Hewett, 2003; Sunday et al., 2007).
In addition to the initial pro-inflammatory phase, stroke induces a subsequent systemic immunosuppressive response, leading to splenic atrophy, T-lymphopenia and decreased peripheral cytokines levels (Offner et al., 2009; Urra et al., 2009b). Although this may benefit the brain, immunodepression may account for the increased susceptibility to infection in the days following stroke (Klehmet et al., 2009; Vogelgesang et al., 2008; Woiciechowsky et al., 1999). Sex hormones differentially alter this post-stroke immunosuppressive state. Estrogen treatment reduced stroke-induced peripheral immunosuppression in OVX female mice, and restored splenocyte numbers and proliferative capacity compared to untreated OVX mice (Zhang et al., 2010). Both male and female sex steroids seem to have effects on post-stroke immune status, as dihydrotestosterone (DHT)- reduced splenocyte proliferation and increased the number of splenic regulatory T cells following MCAO in male castrates compared to non-treated castrates (Dziennis et al., 2011). Regulatory T cells are a subset of CD4+ Fox3p+ T cells which release TGF-β and IL-10 and are thought to limit inflammation, an effect associated with splenic atrophy and post-stroke immunosuppression (Levings et al., 2006; Offner and Polanczyk, 2006). Somewhat surprisingly, no changes in CD4+ T cells, CD19+ B cells, or CD45+CD11b+ microglia/macrophage populations were seen between treated and non-treated castrates in ischemic brain. Whether these effects are also estrogen mediated via the aromatization of testosterone to estrogen is not yet known. Further studies are needed in both sexes to clarify the hormones vs. non-hormonal contribution to the post-stroke immune response.
CELLULAR COMPONENTS OF THE IMMUNE RESPONSE: HORMONE AND SEX EFFECTS
Astrocytes
Astrocytes are intimately associated with many unique cell types including neurons, endothelial cells, and microglia (Kimelberg and Nedergaard, 2010). It is becoming increasingly evident that astrocytes are endowed with the transcriptional machinery to perform specific immunological functions in the brain which were originally only attributed to central nervous system (CNS) resident myeloidderived microglia and macrophages (Dong and Benveniste, 2001). Inflammatory mediators released by astrocytes have been shown to activate endothelial cells, effect blood brain barrier permeability, regulate activation of microglia, promote B cell survival and differentiation, recruit leukocytes via chemokine signals, and regulate myelination (Farina et al., 2007). The development of neuroprotective agents which target astrocytes may promote neuronal survival and maintain the integrity of the neurovascular unit (Zhao and Rempe, 2010).
The effects of estrogen on astrocytes were first observed over 30 years ago and we direct the reader to excellent reviews by Garcia-Segura and colleagues (Azcoitia et al., 2010). Following stroke, there is an increase in estrogen receptor (ER)-α expression in astrocytes, a protective mechanism induced by the injured brain (Blurton-Jones and Tuszynski, 2001). Cortical ER-α gene expression increases rapidly after stroke as a result of a demethylation of the ER promoter in females, allowing for transcription of estrogen-responsive genes (Dubal et al., 2006; Westberry et al., 2010; Wilson and Westberry, 2009). At this point it is not known if this occurs in neurons, astrocytes, resident immune cells or possibly even infiltrating immune cells, but this does appear to be sexually dimorphic, as the increase in ER-α expression following MCAO was seen only in females (Wilson et al., 2011). In a recent study (Spence et al., 2011) utilizing the Cre-loxP system for ER-α, the neuroprotective actions of ERsignaling were found to be primarily mediated by astrocytes and not neurons in experimental allergic encephomyelitis (EAE) but similar studies have not yet been performed in stroke. In contrast to in vivo data suggesting a beneficial effect of astrocytic ER-signaling, in vitro studies are less consistent. For instance, primary rat astrocyte cultures demonstrated a decrease in ER-α expression after OGD (Al-Bader et al., 2011), and others have implicated ER-β as the receptor mediating the beneficial effects of estrogen after lipopolysaccharide (LPS)-induced injury (Saijo et al., 2011). Interestingly, in mice, females have as many as ~20% more hippocampal astrocytes compared to males, an effect that becomes more pronounced with aging (Mouton et al., 2002). Whether this is secondary to the loss of estrogen with gonadal senescence or due to a true “sex difference” is not yet known. OVX mice were not examined in this study, and even with removal of activational effects of steroids, early organizational effects remain. Estrogen does appear to have a suppressive effect on glial activation and proliferation after stroke (see Figure 1). Real-time imaging of GFAP-luciferase expression demonstrated lower GFAP expression during stages of the estrous cycle with the highest estrogen levels (Cordeau et al., 2008), an effect independent of infarct size.
Figure 1. The potential estrogenic effects on glial cells in the ischemic brain.
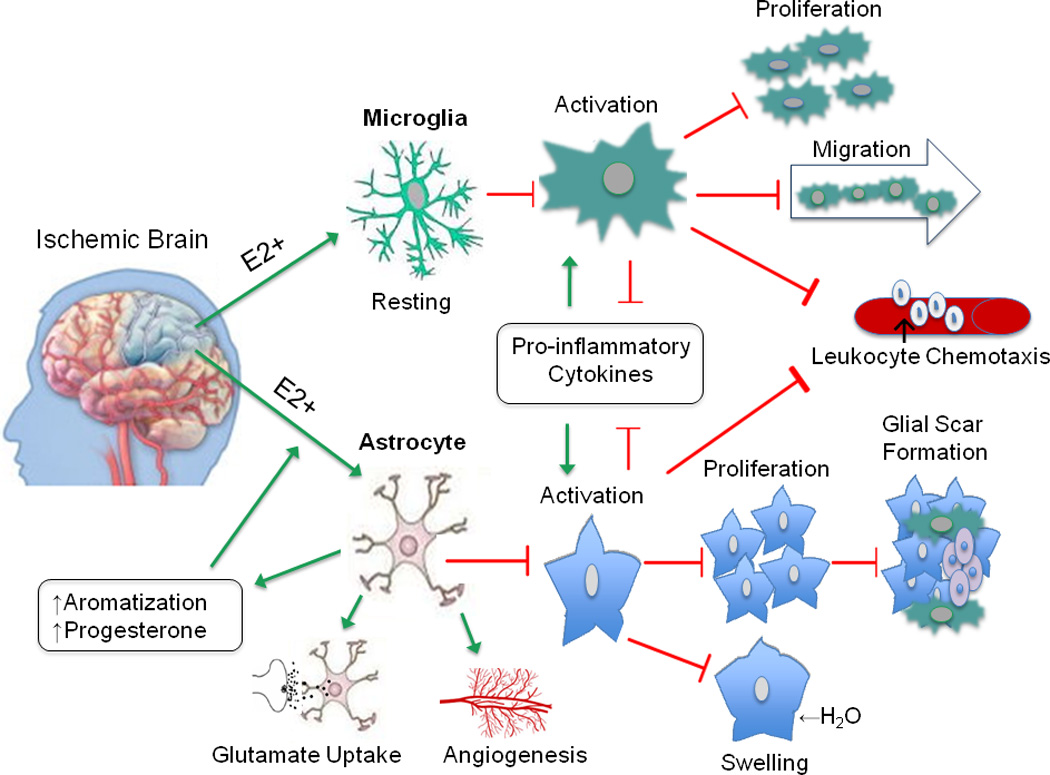
The anti-inflammatory effects of estrogen in the brain are primarily mediated by glial cells. Estrogen promotes a resting phenotype in microglia, as evidenced by increased ramified morphology and decreased MHCII expression. These actions serve to limit microglial activation, proliferation, and migration toward the injured site. Similar changes occur in astrocytes, ultimately leading to decreased glial scar formation. Estrogen-mediated decrease in aquaporin expression prevents osmotic imbalance and may lower risk of edema. Pro-inflammatory cytokines and chemoattractant signals normally released by activated astrocytes and microglia are suppressed in the presence of estrogen, thereby reducing extravasation of infiltrating immune cells. As well, estrogen enhances astrocytic expression of glutamate transporters which facilitates glutamate uptake, mitigating injury due to neuronal excitoxicity. In addition, astrocytes increase synthesis of endogenous steroid production after injury, and secrete growth factors like VEGF, which promote angiogenesis. Together, these actions have a profound impact on curbing neuronal injury due to ischemia by conferring protection via local immunosuppression. The green arrow represents enhancing or promoting action, and the red arrow represents inhibitory action.
Abbreviations: E2 (17 beta-estradiol), MHCII (major histocompatibility complex class II), VEGF (vascular endothelial growth factor)
Although ovarian-derived estrogens lead to neuroprotection in many animal models of brain injury (McCullough and Hurn, 2003), estrogens can also be synthesized by the local conversion of testosterone by aromatase in cells outside the gonads. Brain aromatase expression increases following MCAO in OVX stroke-prone hypertensive rats, consistent with other studies citing increased aromatase activity following CNS injury (Carswell et al., 2005; Garcia-Segura et al., 1999). Increasing local estrogen concentrations by enhancing glial aromatase expression may represent an intrinsic neuronal survival mechanism. Aromatase knockout female mice had significantly more damage after induced stroke compared to wild-type OVX littermates, suggesting that local, extra-gonadal production of estrogen contributes to neuroprotection (McCullough et al., 2003; Saldanha et al., 2009). Work by Liu et al. (2007a) also provided evidence of a sexual dimorphic response to aromatase-mediated neuroprotection. Female-derived astrocytes were resistant to OGD and H2O2-induced cell death compared to astrocytes derived from males, secondary to a female-specific increase in aromatization (Liu et al., 2008a). However, it is unclear if this is secondary to early developmental effects of estrogen (or testosterone) exposure, leading to a life-long increase in aromatase signaling in females, or is secondary to non-hormonal chromosomal effects. Sex steroid binding globulin maintains steroid levels in the low range after birth (Kanova and Bicikova, 2011), but ongoing work in our lab suggests major developmental effects of pre- and early postnatal sex steroid exposure on aromatase expression which can modify the response to stroke in adulthood.
Microglia
Microglia are CNS-resident macrophage-like cells derived from primitive myeloid precursors early in development. The role of microglia in ischemic injury has been extensively studied (see Iadecola and Anrather (2011), Yenari et al. (2010), and Jin et al. (2010)). Evidence suggests that sex differences exist in both absolute microglia number and their responsiveness to injury. Estrogens inhibit microglial proliferation in vitro (Ganter et al., 1992). On average, female mice have 20–40% more Mac-1+ microglia in the (C57Bl/6J) hippocampus than age-matched males (Mouton et al., 2002). Aged females had ~20% more microglia than young females, implicating sex hormones as a causal factor. Follow up studies revealed that estrogen replacement in OVX female mice suppressed this age-induced increase in microglia numbers (Gyenes et al., 2010; Lei et al., 2003a). Estrogen-replacement decreases microglial activation in several other neurodegenerative models (see Table 1). In addition to inhibiting proliferation, estrogen also promotes a resting phenotype, as evidenced by restoration to a ramified morphology and decreased MHCII expression (Barreto et al., 2007; Prat et al., 2011). In our studies, ovariectomy increased microglial numbers, an effect evident in both sham and stroke mice, suggesting that estrogen has a restraining effect on microglial activation (Figure 2). Hippocampal ER-β expression in microglia was selectively upregulated after ischemia in monkey, however, the contribution of ER subtype activation on microglia, at least as it relates to neuroprotection, is still a matter of debate (Baker et al., 2004; Saijo et al., 2011). ER-α knockout mice have spontaneous microglial activation, but subsequent studies have shown larger infarcts in mice with selective loss of neuronal (CamKII-Cre) ER-α compared to myeloid (LysM-Cre) ER-α knockout mice after permanent MCAO (Elzer et al., 2010). This suggests that the myeloid-specific loss of ER-α is not critical, at least to ischemic neuroprotection.
Figure 2. Microglial activation after stroke.
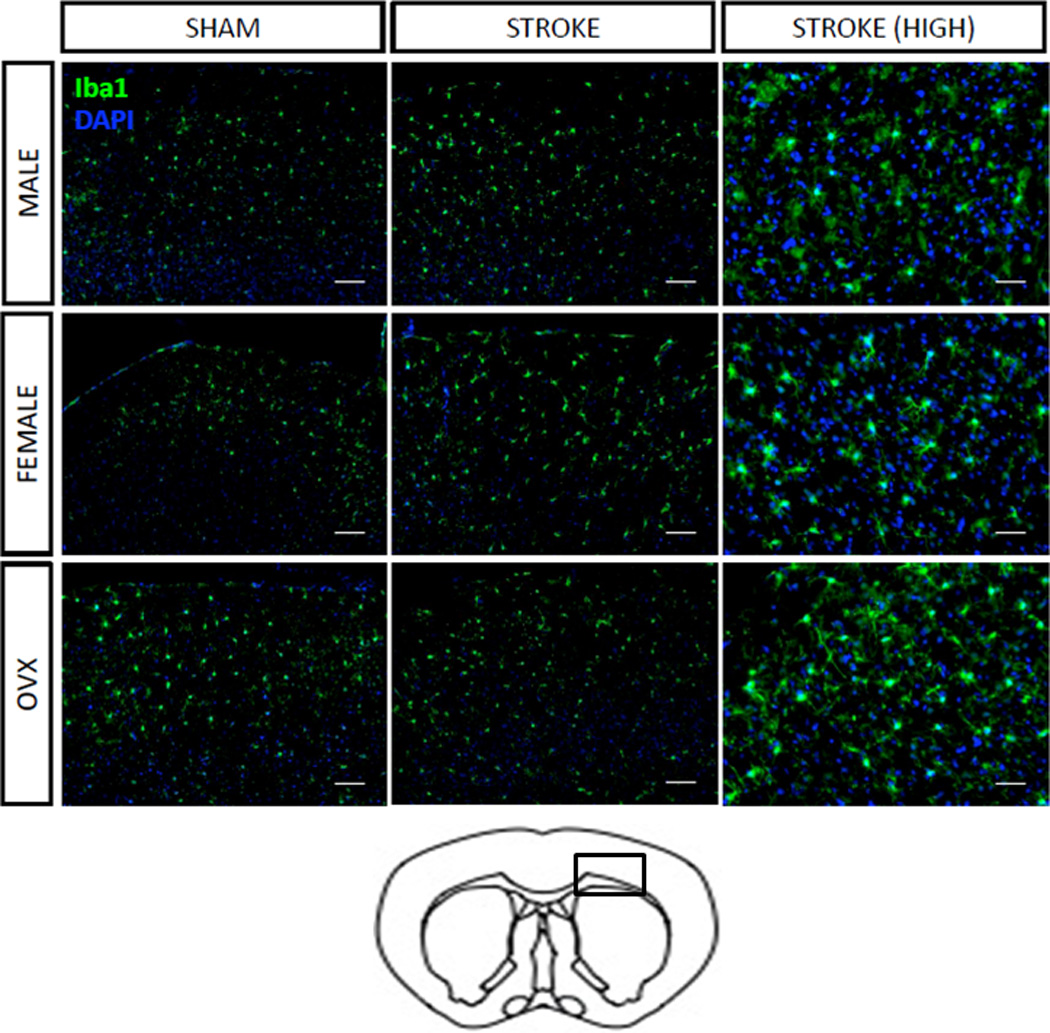
Coronal section of mouse brain at low (10×) and high (20×) power demonstrating increased Iba staining (a marker for microglia) in the penumbral region (indicated by a box in the schematic section) in ovariectomized females both at baseline (sham) and after stroke. DAPI was utilized as a nuclear counter stain.
Monocytes
Monocytes undergo rapid mobilization in response to inflammatory signals released at sites of tissue injury. Monocytes do not proliferate, thus turnover is constitutive, and required for proper renewal of macrophage and dendritic cell populations. Monocytes exhibit a high degree of heterogeneity, based on cell surface molecule expression and functionality. In fact, recent reports describe a subpopulation of ‘inflammatory monocytes’ which contribute to disease (Prinz et al., 2011). While monocytes have been shown to infiltrate the ischemic brain following stroke, their presence may be both detrimental and beneficial to the progression of the sterile inflammatory response (Garcia et al., 1994; Urra et al., 2009a). The number of circulating monocytes in patients following stroke is correlated with the risk of post-stroke infection (Chamorro et al., 2006).
In women, monocyte levels are higher both during ovulation (Maoz et al., 1985), and during gestation as compared to postpartum (Pitkin and Witte, 1979). Physiological estrogen levels have a significant impact on myelopoeisis (Barak et al., 1986; Wang et al., 2006). Evidence also points to a role for estrogen in regulating monocyte migration behavior (Miyagi et al., 1992; Okada et al., 1997) and estrogen treatment has been shown to reduce CXCR2 (interleukin 8 receptor beta), a chemokine receptor important for both monocyte and neutrophil migration (Lei et al., 2003b). Interestingly, the estrogen effect on monocyte adhesion may be temporal, in that it has been observed that estrogen is less effective at reducing monocyte adhesion when endothelial cells are exposed to cytokines (TNF, IL-1β) chronically (Mikkola and St Clair, 2002). Taken together, the ability of estrogen to reduce the migratory and adhesive capacity of monocytes may serve to limit entry and further amplification of the stroke-induced inflammatory response, but a specific role for monocytes in mediating ischemic sexual dimorphism remains to be determined.
Macrophages
In addition to microglia, distinct populations of hematopoietic-born macrophages are resident in the CNS. These include choroid plexus, meningeal, and perivascular macrophages. Because microglia and macrophages share many overlapping features, the contribution of brain macrophages to stroke has been difficult to assess. It is now understood that microglia populate the brain during a finite period in development, while CNS macrophages continually renew via hematopoeisis in the bone marrow. However, the ability to accurately discriminate between local proliferation of microglia and infiltrating monocyte-derived macrophages following CNS injury is still an unresolved issue (Guillemin and Brew, 2004). Thus, current knowledge of brain macrophage function is limited to- and deduced from- studies on peripheral macrophages and cell lines.
In a recent study by Scotland et al. (2011), compartmental sex differences in immune cell compositions and phenotypes in rodents was seen, with higher numbers of resident leukocytes in the peritoneal and pleural cavities in females compared to males. These resident female macrophage populations exhibited greater Toll-like receptor (TLR) expression, phagocytic activity, and NADPH oxidase activity compared to male macrophages, while cytokine production was attenuated, allowing for a dampened (and more controlled) inflammatory cell recruitment and leukocyte influx in response to sepsis. Although this more efficient response may be responsible for the female survival advantage seen in severe sepsis, it is not yet known if these differences are seen in sterile injury models such as stroke. Interestingly no difference in TLR expression was seen in female blood vessels, suggesting sex-specific up-regulation of TLR expression is restricted to female leukocytes. OVX reversed some of the differences, enhancing basal chemokine function to levels seen in males, and equalizing macrophage phenotype, function, and numbers. However, OVX had no significant impact on T-lymphocyte populations, which appeared to be regulated independently of activational levels of gonadal steroids. Sex differences in T cell function, although described for autoimmune diseases, are less well documented in stroke models but are an area of active investigation (Lakhan et al., 2009; Rubtsov et al., 2010).
Neutrophils
In response to infection or injury, neutrophils migrate to sites of inflammation where they function to contain the spread of injury though phagocytic removal of dead cells and release of inflammatory signals which amplify the response and serve to recruit more leukocytes. Historically, the large influx of neutrophils into the brain during a sterile inflammatory event has been viewed as detrimental (McColl et al., 2007). This view has been shaped by observations that neutrophil accumulation is associated with poor outcome after injury (Akopov et al., 1996; Price et al., 2004). However, neutrophil activation is an integral component of the host immune response, thus some studies suggest that neutrophilia may be a beneficial host response following stroke (Beray-Berthat et al., 2003; Hao et al., 2007b; Hayward et al., 1996). Evidence for sex differences in neutrophils came from early studies which described a positive correlation between estrogen levels and neutrophil counts in the blood (Bain and England, 1975; Pitkin and Witte, 1979). Interestingly, several studies have shown that women have comparably higher myeloperoxidase activity in neutrophils than men, which correlated with the menstrual cycle (Jansson, 1991; Kabutomori et al., 1999). Physiological levels of estrogen (10nM) enhanced neutrophil degranulation and promoted release of myeloperoxidase, elastase, and superoxide (Chiang et al., 2004). Interestingly, estrogen levels were positively correlated with ER expression levels, but differed between sexes; as estrogen upregulated both ER-α and ER-β in women, but only ER-α in men (see Table 2). The dynamics of neutrophil infiltration into the female brain and the result of activation following stroke has been yet to be determined.
Table 2.
The anti-inflammatory effects of estrogen on myeloid cells
| Monocytes | |||
|---|---|---|---|
| Author | Model | Drug/Treatment/mutation | Downstream Effect |
| (Kanda and Watanabe, 2002, 2003) | In vitro - Human | E2 | ↑NGF, VEGF |
| (Stefano et al., 1999) | In vitro - Human | E2 | ↑NO release |
| (Pioli et al., 2007) | In vitro - Human | E2 + LPS | ↓CXCL8 |
| (Kramer et al., 2004) | In vitro - Human | E2 | ↓CD16, TNF, IL-1 β, IL-6 |
| (Nathan et al., 1999; Pervin et al., 1998) | In vivo - Rabbit | E2 + hypercholesteremia | ↓VCAM, MCP-1 |
| (Lei et al., 2003b) | In vivo - Rat | E2 + hypercholesteremia | ↓CXCR2 |
| Macrophages | |||
| Author | Model | Drug/Treatment/mutation | Downstream Effect |
| (Ghisletti et al., 2005) | In vitro - Mouse | E2 + LPS | ↓NF-κB translocation |
| (Adamski et al., 2004) | In vitro - Mouse | E2 + IFN-γ | ↓MHCII |
| (Huang et al., 2008) | In vitro - Mouse | E2 + H2O2 | ↓TNF, IL-1 β, MIP-2, MCP-1 |
| (Murphy et al., 2010) | In vitro - Human | E2 + LPS | ↑KappaB-Ras2, miR-125b ↓TNF, let-71 miRNA |
| (Jawaheer et al., 2010; Suzuki et al., 2007c) | In vivo – Mouse, Rat | E2 + trauma-hemorrhage | ↓IL-6, TNF, MIP-1a, MIP-2, TLR4 |
| (Zheng et al., 2006) | In vivo - Mouse | E2 + hypoxia | ↓MyD88, Src, IL-6 |
| (Scotland et al., 2011) | In vivo - Mouse | Physiological E2 | ↑TLR2/3/4, MYd88, CCL2, CX3CR1, CXCL1, CXCL12, CCR1, CCR2, CXCR4, NAPDH oxidase |
| Atherosclerosis | |||
| (Uzui et al., 2011) | In vitro - Human | E2 + Ox-LDL | ↓MMP-9 |
| (Chiba et al., 2011) | In vivo - Mouse | E2 + apoE(−/−) hypercholesteremia | ↑neutral cholesteryl ester hydrolase activity |
| (Rayner et al., 2009) | In vivo - Mouse | E2 + apoE(−/−)/(HSP27o/e) | ↑HSP27 ↓Ox-LDL uptake |
| Dendritic Cells | |||
| Author | Model | Drug/Treatment/mutation | Downstream Effect |
| (Bengtsson et al., 2004) | In vitro - Human | E2 + LPS | ↑IL -6, IL-8, MCP-1, osteoprotegerin, stimulative capacity, migratory activity |
| (Yang et al., 2006a; Yang et al., 2005) | In vitro - Mouse | E2 | ↑MHCII, IL-6, IL-10, CD40, CD54, viability, stimulative capacity ↓NF-kBp65, endocytosis |
| (Polanczyk et al., 2006) | In vitro/In vivo - Mouse | E2 + EAE | ↑PD-1, Treg activity ↓ T-cell proliferation |
| (Papenfuss et al., 2011) | In vitro/In vivo - Mouse | Estriol + EAE, LPS | ↑Th2 response, CD80, CD86, PD-L1/2, B7-H3/4, IL-10, TGF-β ↓IL-6, IL-12, IL-23, T-cell proliferation |
| (Kawasaki et al., 2008) | In vitro/In vivo - Mouse | E2 + trauma-hemmorhage, LPS | ↑MHCII, CD83, Cd40, TNF, IL-6, IL-12p40, antigen-presenting capacity ↓Apoptosis |
| Neutrophils | |||
| Author | Model | Drug/Treatment/mutation | Downstream Effect |
| (Geraldes et al., 2006) | In vitro - Pig | E2 + IFNγ | ↓CD40, CD40L, adhesion |
| (Chiang et al., 2004) | In vitro - Human | E2 + fMLP | ↑Myeloperoxidase, elastase, superoxide, LDL oxidation |
| (Miller et al., 2004) | In vitro/In vivo - Rat | E2 + Vascular Injury | ↓Chemotaxis, IL-1, IL-6, CINC-2α |
| (Doucet et al., 2010) | In vivo - Rat | E2 + trauma-hemorrhage | ↓Respiratory burst activity |
| (Yu et al., 2006) | In vivo - Rat | E2 + trauma-hemorrhage | ↓Myeloperoxidase, CINC-1, CINC-3, ICAM-1 |
| (Garcia-Duran et al., 1999) | In vivo - Human | E2 | ↑nNOS ↓CD18 |
| (Kabutomori et al., 1999) | In vivo - Human | Physiological E2 | ↑Myeloperoxidase activity |
| (Nadkarni et al., 2011) | In vivo - Human | E2 | ↑Annexin A1 ↓Adhesion |
| Platelets | |||
| Author | Model | Drug/Treatment/mutation | Downstream Effect |
| (Bracamonte et al., 2002) | In vivo - Pig | Physiological E2 | ↓PDGF-BB, TGF-β1, TGF-β2 |
| (Jayachandran et al., 2003) | In vivo - Pig | Physiological E2 | ↓Aggregation, ATP secretion, MMP-2 ↑MMP-14 |
| (Jayachandran et al., 2005b) | In vivo - Pig | E2 | ↑Tissue factor ↓CD40, CD40L |
| (Selles et al., 2001) | In vitro - Rat | E2 | ↑NO ↓Aggregation |
| (Leng et al., 2004) | In vitro - Mouse | ADP, collagen-related peptide, agonist-induced fibrinogen (sex effect) | ↑Female platelet aggregation |
Dendritic cells
Known for their remarkable capacity to stimulate memory and naïve T-cells, dendritic cells (DC) are considered to be the only ‘professional’ antigen presenting cell. DC’s are active players in the innate response and participate in phagocytosis, and cytokine and chemokine release. Much consideration has been given to DCs in recent years, for their unique role in bridging the innate and adaptive responses in diseases like atherosclerosis, multiple sclerosis, and more recently, stroke. Evidence that DCs may have a role in ischemic brain injury was first put forth by Reichmann et al. (2002) and Kostulas et al. (2002), whom both showed an increase in DC recruitment to the rodent brain after injury. Later work by Felger et al. (2010) indicates that a local population of DCs exhibits high MHCII and CD80 expression at 72hrs post-reperfusion, a time-point associated with maximal lymphocyte infiltration. These studies parallel the work of many others showing increased accumulation of DCs in the brain in diverse models of neuroinflammation (Newman et al., 2005; Pashenkov et al., 2001; Pashenkov and Link, 2002; Serafini et al., 2000; Suter et al., 2003). Taken together, these findings suggest that the number of DCs in the brain following injury positively correlate with the degree of injury. Estrogen-treated EAE mice had improved outcome and reduced levels of brain DCs, and decreased expression of pro-inflammatory cytokines in DCs in the spleen. Several recent studies (see Table 2) have confirmed the effects of estrogen on DC’s.
Platelets
In the initial response to vascular injury, platelets bind to collagen and extracellular matrix proteins and become activated, signaling the release of inflammatory mediators, attracting additional platelets, and inducing thrombus formation. Adherence of platelets to leukocytes induces a proinflammatory response potentiating thrombus formation and increases the risk of embolization (Cerletti et al., 2011). Antiplatelet therapy has become a mainstay in both primary and secondary stroke prevention, and interestingly, there has been some recent clinical evidence that women may benefit more from therapy than men, at least for the prevention of stroke (Ridker et al., 2005).
E2 reduces both platelet aggregation and aggregate size (Haque et al., 2001; Nakano et al., 1998; Selles et al., 2001). Platelet aggregation and secretion of ATP, PDGF, and MMP-2 are increased in OVX pigs, an effect which is mitigated by estrogen replacement (Jayachandran et al., 2005a). Yet, the findings of a potentially anti-thrombotic role for HRT are challenged by clinical data showing that both exposure to oral contraceptives in younger patients (DeLoughery, 2011) and HRT in post-menopausal women (Norris et al., 2002) increases thrombotic risk. Age, the overall health of the endothelium, and the route of hormone administration (as transdermal ERT is much less thrombogenic) are probably key factors in determining the overall effect of E2 on platelet and fibrinolytic function (see Figure 3) (Canonico et al., 2007; Scarabin et al., 1997). Several conflicting studies have reported enhanced reactivity in female compared to male-derived platelets, indicating a pro-aggregating role of sex or hormonal exposure (Jayachandran et al., 2005b; Johnson et al., 1975; Kurrelmeyer et al., 2003; Leng et al., 2004; Moro et al., 2005). To date only one study has directly examined the effects of E2 selectively on platelet activation following experimental stroke. OVX rats had increased platelet reactivity as evidenced by increased P-selectin expression, which was abrogated by estrogen-treatment in an eNOS-independent manner (Littleton-Kearney et al., 2005). However, because inflammation is also prothrombotic, the MCAO model of arterial occlusion by ligation does not accurately mimic the atherogenic/thrombogenic setting in humans, and thus, future studies examining the effects of estrogen on platelet responses in spontaneous or embolic models of stroke may prove more insightful.
Figure 3. The atheroprotective effects of estrogen.
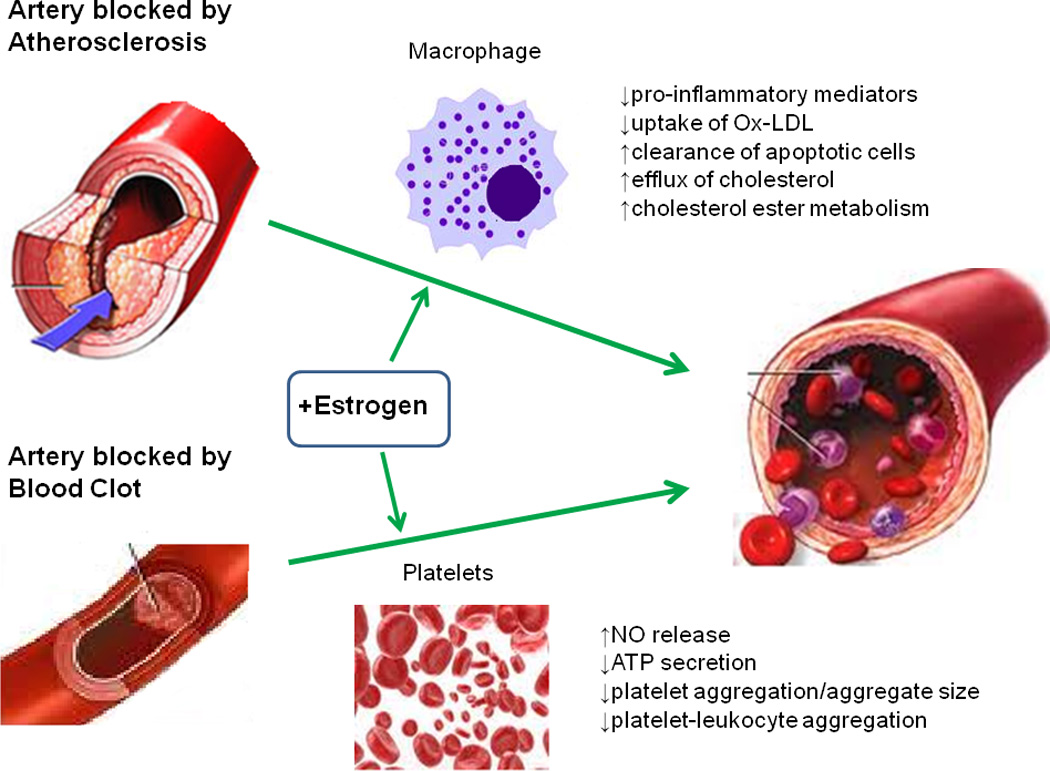
Primary and secondary stroke prevention centers around vascular dysfunction associated with atherosclerosis and blood clotting, respectively. Macrophages are key players in atherogenesis, the development of arterial plaques. Estrogen acts to suppress pro-inflammatory cytokine production, and enhance removal of dead cells from the atherosclerotic tissue. Decreased uptake of modified lipoproteins prevents foam cell formation. Estrogen also prevents lipid buildup by increasing cholesterol ester metabolism and enhancing efflux transport of cholesterol out of the cell. Excessive blood clotting in response to vascular injury following stroke can impede reperfusion of blood flow back to ischemic tissue. Platelets are major contributors in the formation of blood clots. Estrogen has been shown to increase platelet- and endothelial derived NO levels, which plays an important role in reducing adhesion, aggregation, and recruitment. Decreased platelet-leukocyte aggregation due to estrogen exposure prevents lesion progression, plaque rupture, thrombus formation, and the risk of embolization. In sum, these changes prevent clotting and re-establish blood flow back to the ischemic site. The green arrow represents enhancing or promoting action.
Abbreviations: Ox-LDL (oxidized low-density lipoprotein), NO (nitric oxide), ATP (adenosine triphosphate)
In addition, many of the pro-thrombotic effects of hormone therapy are related to changes in circulating plasma levels of several endogenous anticoagulants (antithrombin, protein S, and plasminogen activator inhibitor) which decrease in response to estrogen, and are regulated by the menstrual cycle (Mendelsohn and Karas, 1999), rather than direct effects on platelets. As most strokes occur in post-menopausal women, understanding the effect of loss of E2 with menopause on both platelet function and coagulation factors is critical. Sex differences are present in the response to thrombolytic as well as antiplatelet therapy (Meyer et al., 2011). In a pooled analysis of thrombolytic trials (Hill et al., 2006; Kent et al., 2005), women had a greater margin of benefit compared to men when treated for stroke with thrombolytic drugs compared with placebo. Using noninvasive vascular imaging to assess vessel patency within 72 hours of IV tissue plasminogen activator (tPA) treatment, recanalization (TIMI score 1–3) was more likely in women after thrombolysis (Savitz et al., 2005). Aging and female sex have significant procoagulant effects (Roeloffzen et al.). For example, plasminogen activator inhibitor (PAI)-1 (a procoagulant) increases post-menopause (Koh et al., 1997) and higher plasma levels of PAI-1 are associated with thrombolysis failure (Kim et al., 2005). Future studies are urgently needed in this area as use of reperfusion therapies increases.
The potential influence of genetic sex on immunity and the immune response to stroke
Sex differences in X-linked and Y-linked gene expression have been widely documented (Xu et al., 2002; Xu and Disteche, 2006) and sex differences in X-chromosome gene expression in the blood leukocytes of ischemic stroke patients has been recently reported (Stamova et al., 2011). Female-specific expression of X-chromosome genes involved in natural killer cell signaling, TNFR1 signaling and axon guidance, transforming growth factor-signaling, and IL17 signaling (tissue inhibitor of matrix metalloproteinase-1) were seen. Male-specific changes in expression involved genes associated with development, cell-trafficking, and cellular movement, suggestive of a more robust inflammatory response in men.
Evidence for the potential role of genetics underlying sex differences in the neuroinflammatory response may be found in studies which observe an escape from X-chromosomal inactivation by certain X-linked genes that lack a Y counterpart (Libert et al., 2010). This phenomenon not only increases allelic diversity in females which could alter the immune response, but the presence of two functional copies increases gene dosage relative to males. X-chromosome genes which have been shown to escape inactivation and may be relevant to the stroke response include tissue inhibitor of metalloproteinase 1 (TIMP1), NF-kB activating protein (NKAP), and IL-1R-associated kinase (IRAK1) (Carrel and Willard, 2005).
The contribution of X chromosome dosage (XX vs. XO) to experimental stroke outcome was recently evaluated in our laboratory. Female XX or XO gonadally intact, OVX, and ovariectomized females supplemented with estrogen were subjected to temporary MCAO (Turtzo et al., 2011). Infarct sizes were equivalent between ovariectomized XX and XO mice, between intact XX and XO mice, and between estrogen-supplemented ovariectomized XX and XO mice. Estrogen was neuroprotective in both XX and XO mice. While this investigation showed no overall effect of X chromosome dosage on stroke, as with all negative studies, potential for type 1 error exists. Individual X-linked genes may be more important in humans, as up to 20% of X-linked genes escape X inactivation in humans (Carrel and Willard, 1999). In contrast, a much smaller percentage of X-inactivation escapees exist in mouse (Disteche et al., 2002; Tsuchiya and Willard, 2000). In addition, homologs of some genes present on the human X chromosome are located on autosomes in mouse (Carrel and Willard, 1999; Disteche et al., 2002; Tsuchiya and Willard, 2000). For these genes, escape from X inactivation may be crucial for dosage in humans, but irrelevant in mouse. In addition, which genes escape from X-inactivation can vary in a tissue-specific and age-dependent manner (Lopes et al., 2010). As this study only examined young mice, and X-linked contributions to the inflammatory response are probably more relevant in the aging brain, future studies are needed. Whether a stressor, such as cerebral ischemia, can trigger expression of X-inactivation escapees in humans or in mice has yet to be investigated. Sex differences could also be secondary to the male-limited expression of genes in the non-recombining region (NRY) of the Y chromosome (Sekido and Lovell-Badge, 2009; Skaletsky et al., 2003; Teuscher et al., 2006) or in the Sry gene itself.
In 2004, Bourdeau and colleagues identified 12,515 ERE (estrogen-responsive element) sites in the human genome, 11,810 in the mouse genome, and 660 gene-proximal EREs that are conserved between mouse and human (Bourdeau et al., 2004). In addition to estrogen-responsive autosomal genes, many sex chromosomal genes are also regulated by E2. Indeed, the X chromosome contains a high number of ERE sequences, although few have been validated to date. A list of ERE-containing genes on the X chromosome is provided in Table 3. The clinical significance of estrogen-responsive X-chromosomal genes is poorly understood, however, post-menopausal loss of estrogen could potentially alter gene expression, provided one allele is X-inactivated (or an X-escapee) and the other is constitutively regulated by estrogen. In such a scenario, it is plausible that X-chromosomal gene dosage could become severely disturbed following menopause. In a comprehensive analysis of male and female gene expression differences between sexes, Yang and company (2006b) screened over 300 mice and revealed more than 10,000 genes with sex-biased expression in four different somatic tissues. Approximately 13% of actively transcribed genes in the brain showed a sexual dimorphism, with 355 genes more highly expressed in females and 257 genes more highly expressed in males. Given the heterogeneity of gene expression in specific brain regions, these numbers likely underestimate the degree of sexual dimorphism in the brain (Arnold, 2004; Yang et al., 2006b). In addition to tissue-specific enrichment of sexually dimorphic genes on autosomes, the authors reported significant enrichment on the X-chromosome, which contained more female-biased genes. While the organizational and activational effects of endogenous sex hormones likely contributed to these differences, the epigenetic regulation of autosomal gene expression by sex chromosomes has also been widely reported, as evidenced by differences in chromatin and gene dosage in X and Y chromosomes (see review by(Wijchers and Festenstein, 2011).
Table 3.
Inflammation-associated ERE-containing X-chromosomal genes and X-encoded MicroRNAs.
| Author | X-linked genes containing ERE sites |
|---|---|
| (Bourdeau et al., 2004) | tlr8 |
| tnfsf5 | |
| cxcr3 | |
| cldn2 | |
| c6 | |
| IL-2rg | |
| IL-13ra | |
| TLR-7 | |
| TIMP-1 | |
| Factor VIII | |
| Factor IX | |
| IL-1Rap12 | |
| IKBKG | |
| IRAK1 | |
| Author | X-linked MicroRNAs |
| (Pinheiro et al., 2011) | miR-18b |
| miR-98 | |
| miR-106 | |
| miR-223 | |
Sex Differences in MicroRNA Expression and Implications for Stroke
X-linked differences in the expression of X-encoded miRNAs may also alter regulation of the immune response and provide a basis for sex-linked disease (Pinheiro et al., 2011). The reader is referred to an excellent recent review for details on the miRNA’s located on the X chromosome that have been implicated in immunity and cancer (Pinheiro et al., 2011). Analysis of the genomic distribution of miRNAs found higher densities of miRNAs positioned on the X chromosome (Guo et al., 2009) and remarkably, to date, no miRNAs have been identified on the Y chromosome. Many of these X-linked microRNAs escape meiotic sex chromosome inactivation (Song et al., 2009). Although evidence is still scant, specific X-located miRNAs have been implicated in multiple sclerosis and in the regulation of both the innate immune response and monocytopoiesis (Otaegui et al., 2009; Pinheiro et al., 2011). Recent work in our lab has also provided evidence for miRNA in the etiology of sex differences in stroke. In males cell death is triggered by the mitochondrial release of apoptosis-inducing factor, a mechanism that is caspase-independent. In contrast, females are exquisitely sensitive to cell death induced by caspase activation (Siegel et al., 2010). As X-linked inhibitor of apoptosis (XIAP) is the primary endogenous inhibitor of caspases, we explored its regulation in the response to injury in females. Stroke induced a significant decrease in XIAP mRNA in females, whereas no changes were seen in the male brain. However, XIAP protein levels decreased in both sexes after injury. This was secondary to expression of miR-23a, a microRNA that directly bound the 3' UTR of XIAP. This sex-specific miRNA regulation was independent of activational levels of E2 (Siegel et al., 2011), and may explain the sensitivity of females to caspase-induced cell death. Furthermore, miR-23 is among other miRNAs found to be differentially regulated in the blood of ischemic stroke patients (Kulshreshtha et al., 2008; Tan et al., 2009). More recently, Selvamani and colleagues (2012) describe the neuroprotective effects of anti-Let7f treatment which regulates microglial expression of insulin-like growth factor 1 (IGF-1), a protein known to possess both neurotrophic and angiogenic properties. Interestingly anti-Let7f only reduced injury in gonadally intact females, with no effects in males or ovariectomized females, suggesting that the actions of miRNAs are influenced by local steroid levels. Rapidly evolving clusters of miRNA have been documented on the X chromosome in primates (Zhang et al., 2007) which are preferentially expressed in testis (Bentwich et al., 2005). However, the functional significance of X-linked miRNA expansion is unknown.
To specifically dissociate the organizational effects from sex chromosome effects, several investigators have capitalized on the recently developed “four core genotype” (FCG) mouse model (Arnold, 2009a; Arnold and Chen, 2009). Several studies utilizing the FCG mice have successfully demonstrated the importance of sex chromosome complement to male sexual behaviors, pain responses, addiction behavior and neuroinflammation (Bonthuis et al., 2012; Gioiosa et al., 2008; Smith-Bouvier et al., 2008). Recent work has found that mean arterial pressure was greater in XX mice compared with XY mice in the GDX state, suggesting that sex chromosome effects encoded within the XX sex chromosome complement contribute to hypertension in post-menopausal women, the major modifiable risk factor for stroke (Caeiro et al., 2011; Ji et al., 2010). Stroke studies on these mice should help us dissect out the contribution of genetics and hormones to ischemic stroke sensitivity.
Therapeutic Potential of Hormone Therapy in Ischemic Brain Injury
Despite the promise of early animal studies and observational human data indicating the potential benefits of HRT, the failure to translate in clinical trials in both the primary (WHI) and secondary (HERS; WEST) prevention of stroke has been disappointing (Hulley et al., 1998; Simon et al., 2001; Viscoli et al., 2001; Wassertheil-Smoller et al., 2003). While reports have been mixed, many studies suggest an increased risk of stroke occurrence following HRT or ER treatment (Anderson et al., 2004; Barrett-Connor et al., 2006; Fisher et al., 1998; Hendrix et al., 2006; Tannen et al., 2007; Wassertheil-Smoller et al., 2003). Moreover, a meta-analysis of 28 clinical trials found that hormone replacement therapy was associated with an increased risk of ischemic stroke (not hemorrhagic) and an increased risk of fatal stroke (Bath and Gray, 2005). The importance of these trials is reflected in the national guidelines which note that HRT provides no benefit for stroke risk reduction and should be reserved for the acute treatment of peri-menopausal symptoms (Lisabeth and Bushnell, 2012; Sacco et al., 2006). The timing, duration, dosage, and route of administration (ie., transvaginal, oral, transdermal) are critical parameters in determining a HRT regimen and must individualized to minimize risk (Shoupe, 2011). Postmenopausal or disease-related changes in estrogen receptor expression patterns and genetic variation in ERE binding sites may also influence the actions of estrogen (Higaki et al., 2012; Wang et al., 2012; Yu et al., 2011). The higher incidence of ischemic stroke following HRT, suggests a link between estrogen, inflammation, and thrombogenesis. In fact, evidence from several clinical trials indicates an increased risk of occurrence of venous thrombosis following HRT (Brass, 2004; Canonico et al., 2007). Such risks are offset when estrogen is administered via transdermal delivery as opposed to the more conventional oral route (Olie et al., 2010; Speroff, 2010). In particular, low dose, short duration, transdermal estrogen (with or without progestogen) is not associated with an increased risk of stroke, and may present a safer alternative therapeutic option (Renoux et al., 2010; Renoux and Suissa, 2011). The transdermal approach avoids the first-pass effect in the liver associated with the oral route, which is known to increase hepatic protein synthesis of clotting factors, inflammation markers, and sex hormone binding globulin (SHBG) (Goodman, 2012).
The potential for HRT or other hormone based therapies to be developed for stroke treatment remains unclear as existing studies enrolled patients that were at high risk for stroke. To definitively address the potential context-dependent role for estrogen, we must await the results of the Kronos Early Estrogen Prevention Study (KEEPS), a four-year, controlled, randomized, clinical trial designed to answer whether initiating HT (low dose estrogen and progestin) either as an oral or transdermal formulation in recently menopausal women slows the rate of atherosclerosis as measured by carotid intima-media thickness (IMT) (Harman et al., 2005). Importantly, the vast majority of experimental trials have demonstrated the efficacy of estrogens as neuroprotective agents in acute injury paradigms (induced stroke, oxygen-glucose deprivation etc.). Ongoing trials of hormone-based therapy (both progestins and E2) for acute traumatic brain injury (TBI) may help pave the way for future stroke trials (Stein and Wright, 2010). One caveat is that TBI patients are traditionally much younger than stroke patients. The efficacy and safety of therapy will have to be confirmed in older individuals prior to any potential trial, especially with recent preclinical data demonstrating a pro-inflammatory effect of acute estradiol in aged female animals (Liu et al., 2012). However in that study, “acute” exposure was a two week pre-treatment paradigm that was given to mimic prior HRT exposure before an induced stroke. Studies that give acute doses of estrogen post-stroke have shown clear efficacy with a prolonged therapeutic window (6 hours) in animal models but have yet to be performed in aged animals (Liu et al., 2007b). Pilot trials utilizing acute transdermal estrogen treatment to reduce injury in stroke patients should be considered. This may avoid potential the detrimental changes in coagulation and inflammation seen with chronic oral therapy, and could be tested in both sexes. Rationale trial design with measurement of perfusion status by MRI or CT, and concomitant measurements of serum biomarkers (i.e., CRP) may improve patient selection. Emerging pre-clinical studies demonstrating that estrogen treatment enhances stroke-induced neurogenesis may also be of relevance for the development of future therapeutic trials examining chronic functional recovery after stroke (Li et al., 2011; Suzuki et al., 2007b).
Summary
While the host response to sterile inflammatory events is generally viewed as a beneficial response, overactive or dysregulated signaling can lead to homeostatic imbalances which give rise to chronic inflammation. Given that prolonged inflammation can be detrimental to outcome and that stroke-induced immunosuppression can increase susceptibility to infection, recent efforts have been aimed at targeting the immune system to develop novel neuroprotective therapies. Inflammation is an attractive target for therapy due to its wide therapeutic window. The current FDA-approved drug, tPA, is only effective in treating stroke within four and a half hours after the onset of symptoms (Carpenter et al., 2011; Hacke and Lichy, 2008). Because patients may not recognize symptoms, or arrive to the hospital too late to receive treatment, the need to develop new treatments with wider treatment windows is paramount.
In conclusion, factors beyond the activational effects of hormones contribute to ischemic sexual dimorphism. The contribution of sex chromosome dosage, early prenatal organizational effects of hormones, and epigenetic factors, or the interaction between these in stroke related inflammatory signaling promises to be a complex but rewarding area for future researchers in both the clinic and in the laboratory.
Highlights.
A literature review on potential sex differences in the immune response to stroke
The underlying basis of sex differences in stroke: hormones versus genetics
Discussion of hormone and sex effects of the cellular components of the immune response
Abbreviations
- OGD
oxygen-glucose deprivation
- NMDA
N-methyl-D-aspartic acid
- estrogen
17 beta-Estradiol
- OVX
ovariectomy
- TNF-α
tumor necrosis factor-α
- MCP-1
monocyte chemotactic protein-1
- ERT
estrogen replacement therapy
- MCAO
middle cerebral artery occlusion
- DHT
dihydrotestosterone
- CNS
central nervous system
- LPS
lipopolysaccharide
- EAE
experimental allergic encephomyelitis
- ER
estrogen receptor
- TLR
Toll-like receptor
- DC
dendritic cell
- tPA
tissue plasminogen activator
- NRY
non-recombining region
- XIAP
X-linked inhibitor of apoptosis
- FCG
four core genotype
- NHS
Nurses’ Health Study
- WHI
Women’s Health Initiative
- WEST
Women’s Estrogen for Stroke Trial
- HERS
Heart and Estrogen/progestin Replacement Study
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamski J, Ma Z, Nozell S, Benveniste EN. 17beta-Estradiol inhibits class II major histocompatibility complex (MHC) expression: influence on histone modifications and cbp recruitment to the class II MHC promoter. Mol Endocrinol. 2004;18:1963–1974. doi: 10.1210/me.2004-0098. [DOI] [PubMed] [Google Scholar]
- Akopov SE, Simonian NA, Grigorian GS. Dynamics of polymorphonuclear leukocyte accumulation in acute cerebral infarction and their correlation with brain tissue damage. Stroke; a journal of cerebral circulation. 1996;27:1739–1743. doi: 10.1161/01.str.27.10.1739. [DOI] [PubMed] [Google Scholar]
- Al-Bader MD, Malatiali SA, Redzic ZB. Expression of estrogen receptor alpha and beta in rat astrocytes in primary culture: effects of hypoxia and glucose deprivation. Physiological research/Academia Scientiarum Bohemoslovaca. 2011 doi: 10.33549/physiolres.932167. [DOI] [PubMed] [Google Scholar]
- Altinoz MA, Korkmaz R. NF-kappaB, macrophage migration inhibitory factor and cyclooxygenase-inhibitions as likely mechanisms behind the acetaminophen- and NSAID-prevention of the ovarian cancer. Neoplasma. 2004;51:239–247. [PubMed] [Google Scholar]
- Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O'Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA : the journal of the American Medical Association. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- Angele MK, Frantz MC, Chaudry IH. Gender and sex hormones influence the response to trauma and sepsis: potential therapeutic approaches. Clinics (Sao Paulo) 2006;61:479–488. doi: 10.1590/s1807-59322006000500017. [DOI] [PubMed] [Google Scholar]
- Appelros P, Stegmayr B, Terent A. Sex differences in stroke epidemiology: a systematic review. Stroke; a journal of cerebral circulation. 2009;40:1082–1090. doi: 10.1161/STROKEAHA.108.540781. [DOI] [PubMed] [Google Scholar]
- Arnold AP. Sex chromosomes and brain gender. Nature reviews. Neuroscience. 2004;5:701–708. doi: 10.1038/nrn1494. [DOI] [PubMed] [Google Scholar]
- Arnold AP. Mouse models for evaluating sex chromosome effects that cause sex differences in non-gonadal tissues. Journal of neuroendocrinology. 2009a;21:377–386. doi: 10.1111/j.1365-2826.2009.01831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Hormones and behavior. 2009b;55:570–578. doi: 10.1016/j.yhbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Chen X. What does the"four core genotypes" mouse model tell us about sex differences in the brain and other tissues? Frontiers in neuroendocrinology. 2009;30:1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azcoitia I, Santos-Galindo M, Arevalo MA, Garcia-Segura LM. Role of astroglia in the neuroplastic and neuroprotective actions of estradiol. The European journal of neuroscience. 2010;32:1995–2002. doi: 10.1111/j.1460-9568.2010.07516.x. [DOI] [PubMed] [Google Scholar]
- Bain BJ, England JM. Variations in leucocyte count during menstrual cycle. British medical journal. 1975;2:473–475. doi: 10.1136/bmj.2.5969.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker AE, Brautigam VM, Watters JJ. Estrogen modulates microglial inflammatory mediator production via interactions with estrogen receptor beta. Endocrinology. 2004;145:5021–5032. doi: 10.1210/en.2004-0619. [DOI] [PubMed] [Google Scholar]
- Bao JZ, Ni CR, Zheng WQ. Age-related effects of estrogen on the expression of estrogen receptor alpha and beta mRNA in the ovariectomized monkey hypothalamus. Neuroscience bulletin. 2006;22:97–102. [PubMed] [Google Scholar]
- Barak V, Biran S, Halimi M, Treves AJ. The effect of estradiol on human myelomonocytic cells. II. Mechanism of enhancing activity of colony formation. Journal of reproductive immunology. 1986;9:355–363. doi: 10.1016/0165-0378(86)90035-5. [DOI] [PubMed] [Google Scholar]
- Barouk S, Hintz T, Li P, Duffy AM, MacLusky NJ, Scharfman HE. 17beta-estradiol increases astrocytic vascular endothelial growth factor (VEGF) in adult female rat hippocampus. Endocrinology. 2011;152:1745–1751. doi: 10.1210/en.2010-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreto G, Veiga S, Azcoitia I, Garcia-Segura LM, Garcia-Ovejero D. Testosterone decreases reactive astroglia and reactive microglia after brain injury in male rats: role of its metabolites, oestradiol and dihydrotestosterone. The European journal of neuroscience. 2007;25:3039–3046. doi: 10.1111/j.1460-9568.2007.05563.x. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Mosca L, Collins P, Geiger MJ, Grady D, Kornitzer M, McNabb MA, Wenger NK. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. The New England journal of medicine. 2006;355:125–137. doi: 10.1056/NEJMoa062462. [DOI] [PubMed] [Google Scholar]
- Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, Eder R, Tennstedt S, Ulloor J, Zhang A, Choong K, Lakshman KM, Mazer NA, Miciek R, Krasnoff J, Elmi A, Knapp PE, Brooks B, Appleman E, Aggarwal S, Bhasin G, Hede-Brierley L, Bhatia A, Collins L, LeBrasseur N, Fiore LD, Bhasin S. Adverse events associated with testosterone administration. The New England journal of medicine. 2010;363:109–122. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath PM, Gray LJ. Association between hormone replacement therapy and subsequent stroke: a meta-analysis. BMJ. 2005;330:342. doi: 10.1136/bmj.38331.655347.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- Becker KJ. Sensitization and tolerization to brain antigens in stroke. Neuroscience. 2009;158:1090–1097. doi: 10.1016/j.neuroscience.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengtsson AK, Ryan EJ, Giordano D, Magaletti DM, Clark EA. 17beta-estradiol (E2) modulates cytokine and chemokine expression in human monocyte-derived dendritic cells. Blood. 2004;104:1404–1410. doi: 10.1182/blood-2003-10-3380. [DOI] [PubMed] [Google Scholar]
- Bentwich I, Avniel A, Karov Y, Aharonov R, Gilad S, Barad O, Barzilai A, Einat P, Einav U, Meiri E, Sharon E, Spector Y, Bentwich Z. Identification of hundreds of conserved and nonconserved human microRNAs. Nature genetics. 2005;37:766–770. doi: 10.1038/ng1590. [DOI] [PubMed] [Google Scholar]
- Beray-Berthat V, Palmier B, Plotkine M, Margaill I. Neutrophils do not contribute to infarction, oxidative stress, and NO synthase activity in severe brain ischemia. Experimental neurology. 2003;182:446–454. doi: 10.1016/s0014-4886(03)00106-7. [DOI] [PubMed] [Google Scholar]
- Billeci AM, Paciaroni M, Caso V, Agnelli G. Hormone replacement therapy and stroke. Current vascular pharmacology. 2008;6:112–123. doi: 10.2174/157016108783955338. [DOI] [PubMed] [Google Scholar]
- Blurton-Jones M, Tuszynski MH. Reactive astrocytes express estrogen receptors in the injured primate brain. The Journal of comparative neurology. 2001;433:115–123. doi: 10.1002/cne.1129. [DOI] [PubMed] [Google Scholar]
- Bonthuis PJ, Cox KH, Rissman EF. X-chromosome dosage affects male sexual behavior. Hormones and behavior. 2012;61:565–572. doi: 10.1016/j.yhbeh.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdeau V, Deschenes J, Metivier R, Nagai Y, Nguyen D, Bretschneider N, Gannon F, White JH, Mader S. Genome-wide identification of high-affinity estrogen response elements in human and mouse. Mol Endocrinol. 2004;18:1411–1427. doi: 10.1210/me.2003-0441. [DOI] [PubMed] [Google Scholar]
- Bracamonte MP, Rud KS, Owen WG, Miller VM. Ovariectomy increases mitogens and platelet-induced proliferation of arterial smooth muscle. American journal of physiology. Heart and circulatory physiology. 2002;283:H853–H860. doi: 10.1152/ajpheart.00201.2002. [DOI] [PubMed] [Google Scholar]
- Brass LM. Hormone replacement therapy and stroke: clinical trials review. Stroke; a journal of cerebral circulation. 2004;35:2644–2647. doi: 10.1161/01.STR.0000143218.20061.ac. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Barger SW, Moss NI, Pham JT, Keller JN, Nath A. Pro-inflammatory and pro-oxidant properties of the HIV protein Tat in a microglial cell line: attenuation by 17 beta-estradiol. Journal of neurochemistry. 2001;78:1315–1324. doi: 10.1046/j.1471-4159.2001.00511.x. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Keeling JL, Keller JN, Huang FF, Camondola S, Mattson MP. Antiinflammatory effects of estrogen on microglial activation. Endocrinology. 2000;141:3646–3656. doi: 10.1210/endo.141.10.7693. [DOI] [PubMed] [Google Scholar]
- Bushnell CD. Stroke and the female brain. Nature clinical practice. Neurology. 2008;4:22–33. doi: 10.1038/ncpneuro0686. [DOI] [PubMed] [Google Scholar]
- Caeiro XE, Mir FR, Vivas LM, Carrer HF, Cambiasso MJ. Sex chromosome complement contributes to sex differences in bradycardic baroreflex response. Hypertension. 2011;58:505–511. doi: 10.1161/HYPERTENSIONAHA.111.175661. [DOI] [PubMed] [Google Scholar]
- Canonico M, Oger E, Plu-Bureau G, Conard J, Meyer G, Levesque H, Trillot N, Barrellier MT, Wahl D, Emmerich J, Scarabin PY. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation. 2007;115:840–845. doi: 10.1161/CIRCULATIONAHA.106.642280. [DOI] [PubMed] [Google Scholar]
- Carpenter CR, Keim SM, Milne WK, Meurer WJ, Barsan WG. Thrombolytic therapy for acute ischemic stroke beyond three hours. The Journal of emergency medicine. 2011;40:82–92. doi: 10.1016/j.jemermed.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrel L, Willard HF. Heterogeneous gene expression from the inactive X chromosome: an X-linked gene that escapes X inactivation in some human cell lines but is inactivated in others. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7364–7369. doi: 10.1073/pnas.96.13.7364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–404. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- Carswell HV, Dominiczak AF, Garcia-Segura LM, Harada N, Hutchison JB, Macrae IM. Brain aromatase expression after experimental stroke: topography and time course. The Journal of steroid biochemistry and molecular biology. 2005;96:89–91. doi: 10.1016/j.jsbmb.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Cerletti C, Tamburrelli C, Izzi B, Gianfagna F, de Gaetano G. Platelet-leukocyte interactions in thrombosis. Thrombosis research. 2011 doi: 10.1016/j.thromres.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Chamorro A, Amaro S, Vargas M, Obach V, Cervera A, Torres F, Planas AM. Interleukin 10, monocytes and increased risk of early infection in ischaemic stroke. Journal of neurology, neurosurgery, and psychiatry. 2006;77:1279–1281. doi: 10.1136/jnnp.2006.100800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang K, Parthasarathy S, Santanam N. Estrogen, neutrophils and oxidation. Life sciences. 2004;75:2425–2438. doi: 10.1016/j.lfs.2004.04.035. [DOI] [PubMed] [Google Scholar]
- Chiappetta O, Gliozzi M, Siviglia E, Amantea D, Morrone LA, Berliocchi L, Bagetta G, Corasaniti MT. Evidence to implicate early modulation of interleukin-1beta expression in the neuroprotection afforded by 17beta-estradiol in male rats undergone transient middle cerebral artery occlusion. International review of neurobiology. 2007;82:357–372. doi: 10.1016/S0074-7742(07)82019-8. [DOI] [PubMed] [Google Scholar]
- Chiba T, Ikeda M, Umegaki K, Tomita T. Estrogen-dependent activation of neutral cholesterol ester hydrolase underlying gender difference of atherogenesis in apoE(−/−) mice. Atherosclerosis. 2011;219:545–551. doi: 10.1016/j.atherosclerosis.2011.08.051. [DOI] [PubMed] [Google Scholar]
- Cordeau P, Jr, Lalancette-Hebert M, Weng YC, Kriz J. Live imaging of neuroinflammation reveals sex and estrogen effects on astrocyte response to ischemic injury. Stroke; a journal of cerebral circulation. 2008;39:935–942. doi: 10.1161/STROKEAHA.107.501460. [DOI] [PubMed] [Google Scholar]
- Cushman M, Legault C, Barrett-Connor E, Stefanick ML, Kessler C, Judd HL, Sakkinen PA, Tracy RP. Effect of postmenopausal hormones on inflammation-sensitive proteins: the Postmenopausal Estrogen/Progestin Interventions (PEPI) Study. Circulation. 1999;100:717–722. doi: 10.1161/01.cir.100.7.717. [DOI] [PubMed] [Google Scholar]
- Czlonkowska A, Ciesielska A, Gromadzka G, Kurkowska-Jastrzebska I. Gender differences in neurological disease: role of estrogens and cytokines. Endocrine. 2006;29:243–256. doi: 10.1385/ENDO:29:2:243. [DOI] [PubMed] [Google Scholar]
- Dang J, Mitkari B, Kipp M, Beyer C. Gonadal steroids prevent cell damage and stimulate behavioral recovery after transient middle cerebral artery occlusion in male and female rats. Brain, behavior, and immunity. 2011;25:715–726. doi: 10.1016/j.bbi.2011.01.013. [DOI] [PubMed] [Google Scholar]
- DeLoughery TG. Estrogen and thrombosis: controversies and common sense. Reviews in endocrine & metabolic disorders. 2011;12:77–84. doi: 10.1007/s11154-011-9178-0. [DOI] [PubMed] [Google Scholar]
- Devries D, Zhang Y, Qu M, Ma J, Lin G. Gender Difference in Stroke Case Fatality: An Integrated Study of Hospitalization and Mortality. Journal of stroke and cerebrovascular diseases : the official journal of National Stroke Association. 2011 doi: 10.1016/j.jstrokecerebrovasdis.2011.10.011. [DOI] [PubMed] [Google Scholar]
- Dimayuga FO, Reed JL, Carnero GA, Wang C, Dimayuga ER, Dimayuga VM, Perger A, Wilson ME, Keller JN, Bruce-Keller AJ. Estrogen and brain inflammation: effects on microglial expression of MHC, costimulatory molecules and cytokines. Journal of neuroimmunology. 2005;161:123–136. doi: 10.1016/j.jneuroim.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends in neurosciences. 2003;26:248–254. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- Disteche CM, Filippova GN, Tsuchiya KD. Escape from X inactivation. Cytogenetic and genome research. 2002;99:36–43. doi: 10.1159/000071572. [DOI] [PubMed] [Google Scholar]
- Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–190. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- Doucet DR, Bonitz RP, Feinman R, Colorado I, Ramanathan M, Feketeova E, Condon M, Machiedo GW, Hauser CJ, Xu DZ, Deitch EA. Estrogenic hormone modulation abrogates changes in red blood cell deformability and neutrophil activation in trauma hemorrhagic shock. The Journal of trauma. 2010;68:35–41. doi: 10.1097/TA.0b013e3181bbbddb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L, Bayir H, Lai Y, Zhang X, Kochanek PM, Watkins SC, Graham SH, Clark RS. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J Biol Chem. 2004;279:38563–38570. doi: 10.1074/jbc.M405461200. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Rau SW, Shughrue PJ, Zhu H, Yu J, Cashion AB, Suzuki S, Gerhold LM, Bottner MB, Dubal SB, Merchanthaler I, Kindy MS, Wise PM. Differential modulation of estrogen receptors (ERs) in ischemic brain injury: a role for ERalpha in estradiol-mediated protection against delayed cell death. Endocrinology. 2006;147:3076–3084. doi: 10.1210/en.2005-1177. [DOI] [PubMed] [Google Scholar]
- Dziennis S, Akiyoshi K, Subramanian S, Offner H, Hurn PD. Role of dihydrotestosterone in post-stroke peripheral immunosuppression after cerebral ischemia. Brain, behavior, and immunity. 2011;25:685–695. doi: 10.1016/j.bbi.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzer JG, Muhammad S, Wintermantel TM, Regnier-Vigouroux A, Ludwig J, Schutz G, Schwaninger M. Neuronal estrogen receptor-alpha mediates neuroprotection by 17beta-estradiol. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2010;30:935–942. doi: 10.1038/jcbfm.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends in immunology. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Felger JC, Abe T, Kaunzner UW, Gottfried-Blackmore A, Gal-Toth J, McEwen BS, Iadecola C, Bulloch K. Brain dendritic cells in ischemic stroke: time course, activation state, and origin. Brain, behavior, and immunity. 2010;24:724–737. doi: 10.1016/j.bbi.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. Journal of the National Cancer Institute. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- Friedman WJ, Thakur S, Seidman L, Rabson AB. Regulation of nerve growth factor mRNA by interleukin-1 in rat hippocampal astrocytes is mediated by NFkappaB. The Journal of biological chemistry. 1996;271:31115–31120. doi: 10.1074/jbc.271.49.31115. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Kanda T, Kamide N, Akutsu T, Sakai F. Gender differences in long-term functional outcome after first-ever ischemic stroke. Intern Med. 2009;48:967–973. doi: 10.2169/internalmedicine.48.1757. [DOI] [PubMed] [Google Scholar]
- Ganter S, Northoff H, Mannel D, Gebicke-Harter PJ. Growth control of cultured microglia. Journal of neuroscience research. 1992;33:218–230. doi: 10.1002/jnr.490330205. [DOI] [PubMed] [Google Scholar]
- Garcia-Duran M, de Frutos T, Diaz-Recasens J, Garcia-Galvez G, Jimenez A, Monton M, Farre J, Sanchez de Miguel L, Gonzalez-Fernandez F, Arriero MD, Rico L, Garcia R, Casado S, Lopez-Farre A. Estrogen stimulates neuronal nitric oxide synthase protein expression in human neutrophils. Circulation research. 1999;85:1020–1026. doi: 10.1161/01.res.85.11.1020. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura LM, Wozniak A, Azcoitia I, Rodriguez JR, Hutchison RE, Hutchison JB. Aromatase expression by astrocytes after brain injury: implications for local estrogen formation in brain repair. Neuroscience. 1999;89:567–578. doi: 10.1016/s0306-4522(98)00340-6. [DOI] [PubMed] [Google Scholar]
- Garcia JH, Liu KF, Yoshida Y, Lian J, Chen S, del Zoppo GJ. Influx of leukocytes and platelets in an evolving brain infarct (Wistar rat) The American journal of pathology. 1994;144:188–199. [PMC free article] [PubMed] [Google Scholar]
- Gaskins AJ, Wilchesky M, Mumford SL, Whitcomb BW, Browne RW, Wactawski-Wende J, Perkins NJ, Schisterman EF. Endogenous reproductive hormones and C-reactive protein across the menstrual cycle: the BioCycle Study. American journal of epidemiology. 2012;175:423–431. doi: 10.1093/aje/kwr343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geraldes P, Gagnon S, Hadjadj S, Merhi Y, Sirois MG, Cloutier I, Tanguay JF. Estradiol blocks the induction of CD40 and CD40L expression on endothelial cells and prevents neutrophil adhesion: an ERalpha-mediated pathway. Cardiovascular research. 2006;71:566–573. doi: 10.1016/j.cardiores.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Ghisletti S, Meda C, Maggi A, Vegeto E. 17beta-estradiol inhibits inflammatory gene expression by controlling NF-kappaB intracellular localization. Molecular and cellular biology. 2005;25:2957–2968. doi: 10.1128/MCB.25.8.2957-2968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioiosa L, Chen X, Watkins R, Klanfer N, Bryant CD, Evans CJ, Arnold AP. Sex chromosome complement affects nociception in tests of acute and chronic exposure to morphine in mice. Horm Behav. 2008;53:124–130. doi: 10.1016/j.yhbeh.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraud SN, Caron CM, Pham-Dinh D, Kitabgi P, Nicot AB. Estradiol inhibits ongoing autoimmune neuroinflammation and NFkappaB-dependent CCL2 expression in reactive astrocytes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:8416–8421. doi: 10.1073/pnas.0910627107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb MR, Fullerton HJ, Nowak-Gottl U, Deveber G. Male predominance in childhood ischemic stroke: findings from the international pediatric stroke study. Stroke; a journal of cerebral circulation. 2009;40:52–57. doi: 10.1161/STROKEAHA.108.521203. [DOI] [PubMed] [Google Scholar]
- Goodman MP. Are all estrogens created equal? A review of oral vs. transdermal therapy. J Womens Health (Larchmt) 2012;21:161–169. doi: 10.1089/jwh.2011.2839. [DOI] [PubMed] [Google Scholar]
- Gregg C, Zhang J, Butler JE, Haig D, Dulac C. Sex-specific parent-of-origin allelic expression in the mouse brain. Science. 2010;329:682–685. doi: 10.1126/science.1190831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Annals of internal medicine. 2000;133:933–941. doi: 10.7326/0003-4819-133-12-200012190-00008. [DOI] [PubMed] [Google Scholar]
- Grodstein F, Manson JE, Stampfer MJ. Postmenopausal hormone use and secondary prevention of coronary events in the nurses' health study. a prospective, observational study. Annals of internal medicine. 2001;135:1–8. doi: 10.7326/0003-4819-135-1-200107030-00003. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Brew BJ. Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. Journal of leukocyte biology. 2004;75:388–397. doi: 10.1189/jlb.0303114. [DOI] [PubMed] [Google Scholar]
- Guo X, Su B, Zhou Z, Sha J. Rapid evolution of mammalian X-linked testis microRNAs. BMC genomics. 2009;10:97. doi: 10.1186/1471-2164-10-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyenes A, Hoyk Z, Csakvari E, Siklos L, Parducz A. 17beta-estradiol attenuates injury-induced microglia activation in the oculomotor nucleus. Neuroscience. 2010;171:677–682. doi: 10.1016/j.neuroscience.2010.09.033. [DOI] [PubMed] [Google Scholar]
- Hacke W, Lichy C. Thrombolysis for acute stroke under antiplatelet therapy: safe enough to be beneficial? Nature clinical practice. Neurology. 2008;4:474–475. doi: 10.1038/ncpneuro0867. [DOI] [PubMed] [Google Scholar]
- Han HS, Yenari MA. Cellular targets of brain inflammation in stroke. Curr Opin Investig Drugs. 2003;4:522–529. [PubMed] [Google Scholar]
- Hao J, Rapp PR, Janssen WG, Lou W, Lasley BL, Hof PR, Morrison JH. Interactive effects of age and estrogen on cognition and pyramidal neurons in monkey prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2007a;104:11465–11470. doi: 10.1073/pnas.0704757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Q, Chen Y, Zhu Y, Fan Y, Palmer D, Su H, Young WL, Yang GY. Neutrophil depletion decreases VEGF-induced focal angiogenesis in the mature mouse brain. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2007b;27:1853–1860. doi: 10.1038/sj.jcbfm.9600485. [DOI] [PubMed] [Google Scholar]
- Haque SF, Matsubayashi H, Izumi S, Sugi T, Arai T, Kondo A, Makino T. Sex difference in platelet aggregation detected by new aggregometry using light scattering. Endocrine journal. 2001;48:33–41. doi: 10.1507/endocrj.48.33. [DOI] [PubMed] [Google Scholar]
- Harman SM, Brinton EA, Cedars M, Lobo R, Manson JE, Merriam GR, Miller VM, Naftolin F, Santoro N. KEEPS: The Kronos Early Estrogen Prevention Study. Climacteric : the journal of the International Menopause Society. 2005;8:3–12. doi: 10.1080/13697130500042417. [DOI] [PubMed] [Google Scholar]
- Hatakeyama C, Anderson CL, Beever CL, Penaherrera MS, Brown CJ, Robinson WP. The dynamics of X-inactivation skewing as women age. Clinical genetics. 2004;66:327–332. doi: 10.1111/j.1399-0004.2004.00310.x. [DOI] [PubMed] [Google Scholar]
- Hayward NJ, Elliott PJ, Sawyer SD, Bronson RT, Bartus RT. Lack of evidence for neutrophil participation during infarct formation following focal cerebral ischemia in the rat. Experimental neurology. 1996;139:188–202. doi: 10.1006/exnr.1996.0093. [DOI] [PubMed] [Google Scholar]
- Hendrix SL, Wassertheil-Smoller S, Johnson KC, Howard BV, Kooperberg C, Rossouw JE, Trevisan M, Aragaki A, Baird AE, Bray PF, Buring JE, Criqui MH, Herrington D, Lynch JK, Rapp SR, Torner J. Effects of conjugated equine estrogen on stroke in the Women's Health Initiative. Circulation. 2006;113:2425–2434. doi: 10.1161/CIRCULATIONAHA.105.594077. [DOI] [PubMed] [Google Scholar]
- Herson PS, Koerner IP, Hurn PD. Sex, sex steroids, and brain injury. Seminars in reproductive medicine. 2009;27:229–239. doi: 10.1055/s-0029-1216276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer A, Hasselblatt M, von Ahsen N, Hafner H, Siren AL, Ehrenreich H. In vitro gender differences in neuronal survival on hypoxia and in 17beta-estradiol-mediated neuroprotection. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2005;25:427–430. doi: 10.1038/sj.jcbfm.9600056. [DOI] [PubMed] [Google Scholar]
- Higaki S, Takumi K, Itoh M, Watanabe G, Taya K, Shimizu K, Hayashi M, Oishi T. Response of ERbeta and aromatase expression in the monkey hippocampal formation to ovariectomy and menopause. Neuroscience research. 2012;72:148–154. doi: 10.1016/j.neures.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Hill MD, Kent DM, Hinchey J, Rowley H, Buchan AM, Wechsler LR, Higashida RT, Fischbein NJ, Dillon WP, Gent M, Firszt CM, Schulz GA, Furlan AJ. Sex-based differences in the effect of intra-arterial treatment of stroke: analysis of the PROACT-2 study. Stroke. 2006;37:2322–2325. doi: 10.1161/01.STR.0000237060.21472.47. [DOI] [PubMed] [Google Scholar]
- Huang H, He J, Yuan Y, Aoyagi E, Takenaka H, Itagaki T, Sannomiya K, Tamaki K, Harada N, Shono M, Shimizu I, Takayama T. Opposing effects of estradiol and progesterone on the oxidative stress-induced production of chemokine and proinflammatory cytokines in murine peritoneal macrophages. The journal of medical investigation : JMI. 2008;55:133–141. doi: 10.2152/jmi.55.133. [DOI] [PubMed] [Google Scholar]
- Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA : the journal of the American Medical Association. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nature medicine. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Ross ME. Molecular pathology of cerebral ischemia: delayed gene expression and strategies for neuroprotection. Annals of the New York Academy of Sciences. 1997;835:203–217. doi: 10.1111/j.1749-6632.1997.tb48631.x. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Cooper D, Arumugam TV, Zhang JH, Nanda A, Granger DN. Platelet-leukocyte-endothelial cell interactions after middle cerebral artery occlusion and reperfusion. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2004;24:907–915. doi: 10.1097/01.WCB.0000132690.96836.7F. [DOI] [PubMed] [Google Scholar]
- Jansson G. Oestrogen-induced enhancement of myeloperoxidase activity in human polymorphonuclear leukocytes--a possible cause of oxidative stress in inflammatory cells. Free radical research communications. 1991;14:195–208. doi: 10.3109/10715769109088949. [DOI] [PubMed] [Google Scholar]
- Jawaheer D, Olsen J, Lahiff M, Forsberg S, Lahteenmaki J, da Silveira IG, Rocha FA, Magalhaes Laurindo IM, Henrique da Mota LM, Drosos AA, Murphy E, Sheehy C, Quirke E, Cutolo M, Rexhepi S, Dadoniene J, Verstappen SM, Sokka T. Gender, body mass index and rheumatoid arthritis disease activity: results from the QUEST-RA Study. Clinical and experimental rheumatology. 2010;28:454–461. [PMC free article] [PubMed] [Google Scholar]
- Jayachandran M, Mukherjee R, Steinkamp T, LaBreche P, Bracamonte MP, Okano H, Owen WG, Miller VM. Differential effects of 17beta-estradiol, conjugated equine estrogen, and raloxifene on mRNA expression, aggregation, and secretion in platelets. American journal of physiology. Heart and circulatory physiology. 2005a;288:H2355–H2362. doi: 10.1152/ajpheart.01108.2004. [DOI] [PubMed] [Google Scholar]
- Jayachandran M, Owen WG, Miller VM. Effects of ovariectomy on aggregation, secretion, and metalloproteinases in porcine platelets. American journal of physiology. Heart and circulatory physiology. 2003;284:H1679–H1685. doi: 10.1152/ajpheart.00958.2002. [DOI] [PubMed] [Google Scholar]
- Jayachandran M, Sanzo A, Owen WG, Miller VM. Estrogenic regulation of tissue factor and tissue factor pathway inhibitor in platelets. American journal of physiology. Heart and circulatory physiology. 2005b;289:H1908–H1916. doi: 10.1152/ajpheart.01292.2004. [DOI] [PubMed] [Google Scholar]
- Ji H, Zheng W, Wu X, Liu J, Ecelbarger CM, Watkins R, Arnold AP, Sandberg K. Sex chromosome effects unmasked in angiotensin II-induced hypertension. Hypertension. 2010;55:1275–1282. doi: 10.1161/HYPERTENSIONAHA.109.144949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. Journal of leukocyte biology. 2010;87:779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M, Ramey E, Ramwell PW. Sex and age differences in human platelet aggregation. Nature. 1975;253:355–357. doi: 10.1038/253355a0. [DOI] [PubMed] [Google Scholar]
- Kabutomori O, Yanagihara T, Iwatani Y, Kawarazaki A, Kabutomori M. Sex difference in myeloperoxidase activity of neutrophils. American journal of hematology. 1999;60:312–313. doi: 10.1002/(sici)1096-8652(199904)60:4<312::aid-ajh13>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Kalaitzidis D, Gilmore TD. Transcription factor cross-talk: the estrogen receptor and NF-kappaB. Trends Endocrinol Metab. 2005;16:46–52. doi: 10.1016/j.tem.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Kanda N, Watanabe S. 17beta-estradiol enhances vascular endothelial growth factor production and dihydrotestosterone antagonizes the enhancement via the regulation of adenylate cyclase in differentiated THP-1 cells. The Journal of investigative dermatology. 2002;118:519–529. doi: 10.1046/j.0022-202x.2002.01672.x. [DOI] [PubMed] [Google Scholar]
- Kanda N, Watanabe S. 17Beta-estradiol enhances the production of nerve growth factor in THP-1-derived macrophages or peripheral blood monocyte-derived macrophages. The Journal of investigative dermatology. 2003;121:771–780. doi: 10.1046/j.1523-1747.2003.12487.x. [DOI] [PubMed] [Google Scholar]
- Kanova N, Bicikova M. Hyperandrogenic states in pregnancy. Physiological research/Academia Scientiarum Bohemoslovaca. 2011;60:243–252. doi: 10.33549/physiolres.932078. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Choudhry MA, Suzuki T, Schwacha MG, Bland KI, Chaudry IH. 17beta-Estradiol's salutary effects on splenic dendritic cell functions following trauma-hemorrhage are mediated via estrogen receptor-alpha. Molecular immunology. 2008;45:376–385. doi: 10.1016/j.molimm.2007.06.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent DM, Price LL, Ringleb P, Hill MD, Selker HP. Sex-based differences in response to recombinant tissue plasminogen activator in acute ischemic stroke: a pooled analysis of randomized clinical trials. Stroke; a journal of cerebral circulation. 2005;36:62–65. doi: 10.1161/01.STR.0000150515.15576.29. [DOI] [PubMed] [Google Scholar]
- Kim SH, Han SW, Kim EH, Kim DJ, Lee KY, Kim DI, Heo JH. Plasma fibrinolysis inhibitor levels in acute stroke patients with thrombolysis failure. J Clin Neurol. 2005;1:142–147. doi: 10.3988/jcn.2005.1.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelberg HK, Nedergaard M. Functions of astrocytes and their potential as therapeutic targets. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2010;7:338–353. doi: 10.1016/j.nurt.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klehmet J, Harms H, Richter M, Prass K, Volk HD, Dirnagl U, Meisel A, Meisel C. Stroke-induced immunodepression and post-stroke infections: lessons from the preventive antibacterial therapy in stroke trial. Neuroscience. 2009;158:1184–1193. doi: 10.1016/j.neuroscience.2008.07.044. [DOI] [PubMed] [Google Scholar]
- Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. The Lancet infectious diseases. 2010;10:338–349. doi: 10.1016/S1473-3099(10)70049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerner IP, Zhang W, Cheng J, Parker S, Hurn PD, Alkayed NJ. Soluble epoxide hydrolase: regulation by estrogen and role in the inflammatory response to cerebral ischemia. Frontiers in bioscience : a journal and virtual library. 2008;13:2833–2841. doi: 10.2741/2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh KK, Mincemoyer R, Bui MN, Csako G, Pucino F, Guetta V, Waclawiw M, Cannon RO., 3rd Effects of hormone-replacement therapy on fibrinolysis in postmenopausal women. The New England journal of medicine. 1997;336:683–690. doi: 10.1056/NEJM199703063361002. [DOI] [PubMed] [Google Scholar]
- Kostulas N, Li HL, Xiao BG, Huang YM, Kostulas V, Link H. Dendritic cells are present in ischemic brain after permanent middle cerebral artery occlusion in the rat. Stroke; a journal of cerebral circulation. 2002;33:1129–1134. doi: 10.1161/hs0402.105379. [DOI] [PubMed] [Google Scholar]
- Kramer PR, Kramer SF, Guan G. 17 beta-estradiol regulates cytokine release through modulation of CD16 expression in monocytes and monocyte-derived macrophages. Arthritis and rheumatism. 2004;50:1967–1975. doi: 10.1002/art.20309. [DOI] [PubMed] [Google Scholar]
- Kublickiene K, Luksha L. Gender and the endothelium. Pharmacological reports : PR. 2008;60:49–60. [PubMed] [Google Scholar]
- Kulshreshtha R, Davuluri RV, Calin GA, Ivan M. A microRNA component of the hypoxic response. Cell death and differentiation. 2008;15:667–671. doi: 10.1038/sj.cdd.4402310. [DOI] [PubMed] [Google Scholar]
- Kurrelmeyer K, Becker L, Becker D, Yanek L, Goldschmidt-Clermont P, Bray PF. Platelet hyperreactivity in women from families with premature atherosclerosis. J Am Med Womens Assoc. 2003;58:272–277. [PubMed] [Google Scholar]
- Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. Journal of translational medicine. 2009;7:97. doi: 10.1186/1479-5876-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakoski SG, Herrington DM. Effects of hormone therapy on C-reactive protein and IL-6 in postmenopausal women: a review article. Climacteric : the journal of the International Menopause Society. 2005;8:317–326. doi: 10.1080/13697130500345109. [DOI] [PubMed] [Google Scholar]
- Lei DL, Long JM, Hengemihle J, O'Neill J, Manaye KF, Ingram DK, Mouton PR. Effects of estrogen and raloxifene on neuroglia number and morphology in the hippocampus of aged female mice. Neuroscience. 2003a;121:659–666. doi: 10.1016/s0306-4522(03)00245-8. [DOI] [PubMed] [Google Scholar]
- Lei ZB, Fu XJ, Lu ZT, Wang BC, Liu XL, You NZ. Effect of estradiol on chemokine receptor CXCR2 expression in rats: implications for atherosclerosis. Acta pharmacologica Sinica. 2003b;24:670–674. [PubMed] [Google Scholar]
- Leng XH, Hong SY, Larrucea S, Zhang W, Li TT, Lopez JA, Bray PF. Platelets of female mice are intrinsically more sensitive to agonists than are platelets of males. Arteriosclerosis, thrombosis, and vascular biology. 2004;24:376–381. doi: 10.1161/01.ATV.0000110445.95304.91. [DOI] [PubMed] [Google Scholar]
- Leon RL, Huber JD, Rosen CL. Potential age-dependent effects of estrogen on neural injury. The American journal of pathology. 2011;178:2450–2460. doi: 10.1016/j.ajpath.2011.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AJ, Hewett L. Estrogen replacement therapy and frontotemporal dementia. Maturitas. 2003;45:83–88. doi: 10.1016/s0378-5122(03)00142-7. [DOI] [PubMed] [Google Scholar]
- Levings MK, Allan S, d'Hennezel E, Piccirillo CA. Functional dynamics of naturally occurring regulatory T cells in health and autoimmunity. Advances in immunology. 2006;92:119–155. doi: 10.1016/S0065-2776(06)92003-3. [DOI] [PubMed] [Google Scholar]
- Li H, Pin S, Zeng Z, Wang MM, Andreasson KA, McCullough LD. Sex differences in cell death. Annals of neurology. 2005;58:317–321. doi: 10.1002/ana.20538. [DOI] [PubMed] [Google Scholar]
- Li J, Siegel M, Yuan M, Zeng Z, Finnucan L, Persky R, Hurn PD, McCullough LD. Estrogen enhances neurogenesis and behavioral recovery after stroke. J Cereb Blood Flow Metab. doi: 10.1038/jcbfm.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Siegel M, Yuan M, Zeng Z, Finnucan L, Persky R, Hurn PD, McCullough LD. Estrogen enhances neurogenesis and behavioral recovery after stroke. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2011;31:413–425. doi: 10.1038/jcbfm.2010.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, Valla J, Sefidvash-Hockley S, Rogers J, Li R. Effects of estrogen treatment on glutamate uptake in cultured human astrocytes derived from cortex of Alzheimer's disease patients. Journal of neurochemistry. 2002;80:807–814. doi: 10.1046/j.0022-3042.2002.00779.x. [DOI] [PubMed] [Google Scholar]
- Liao SL, Chen WY, Chen CJ. Estrogen attenuates tumor necrosis factor-alpha expression to provide ischemic neuroprotection in female rats. Neuroscience letters. 2002;330:159–162. doi: 10.1016/s0304-3940(02)00754-1. [DOI] [PubMed] [Google Scholar]
- Libert C, Dejager L, Pinheiro I. The X chromosome in immune functions: when a chromosome makes the difference. Nature reviews. Immunology. 2010;10:594–604. doi: 10.1038/nri2815. [DOI] [PubMed] [Google Scholar]
- Lisabeth L, Bushnell C. Stroke risk in women: the role of menopause and hormone therapy. Lancet neurology. 2012;11:82–91. doi: 10.1016/S1474-4422(11)70269-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littleton-Kearney MT, Gaines JM, Callahan KP, Murphy SJ, Hurn PD. Effects of estrogen on platelet reactivity after transient forebrain ischemia in rats. Biological research for nursing. 2005;7:135–145. doi: 10.1177/1099800405276832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Benashski SE, Xu Y, Siegel M, McCullough LD. Effects of Chronic and Acute Oestrogen Replacement Therapy in Aged Animals after Experimental Stroke. Journal of neuroendocrinology. 2011 doi: 10.1111/j.1365-2826.2011.02248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Benashski SE, Xu Y, Siegel M, McCullough LD. Effects of chronic and acute oestrogen replacement therapy in aged animals after experimental stroke. Journal of neuroendocrinology. 2012;24:319–330. doi: 10.1111/j.1365-2826.2011.02248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Yuan R, Benashski SE, McCullough LD. Changes in experimental stroke outcome across the life span. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2009;29:792–802. doi: 10.1038/jcbfm.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Hurn PD, Roselli CE, Alkayed NJ. Role of P450 aromatase in sex-specific astrocytic cell death. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2007a;27:135–141. doi: 10.1038/sj.jcbfm.9600331. [DOI] [PubMed] [Google Scholar]
- Liu M, Oyarzabal EA, Yang R, Murphy SJ, Hurn PD. A novel method for assessing sex-specific and genotype-specific response to injury in astrocyte culture. Journal of neuroscience methods. 2008a;171:214–217. doi: 10.1016/j.jneumeth.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Wang X, Liu Q, Yang SH, Simpkins JW. Dose dependence and therapeutic window for the neuroprotective effects of 17beta-estradiol when administered after cerebral ischemia. Neuroscience letters. 2007b;415:237–241. doi: 10.1016/j.neulet.2007.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Fan XL, Zhao Y, Luo GR, Li XP, Li R, Le WD. Estrogen provides neuroprotection against activated microglia-induced dopaminergic neuronal injury through both estrogen receptor-alpha and estrogen receptor-beta in microglia. Journal of neuroscience research. 2005;81:653–665. doi: 10.1002/jnr.20583. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhang W, Alkayed NJ, Froehner SC, Adams ME, Amiry-Moghaddam M, Ottersen OP, Hurn PD, Bhardwaj A. Lack of sex-linked differences in cerebral edema and aquaporin-4 expression after experimental stroke. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2008b;28:1898–1906. doi: 10.1038/jcbfm.2008.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lleo A, Battezzati PM, Selmi C, Gershwin ME, Podda M. Is autoimmunity a matter of sex? Autoimmunity reviews. 2008;7:626–630. doi: 10.1016/j.autrev.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- Lobo RA. Menopause and stroke and the effects of hormonal therapy. Climacteric : the journal of the International Menopause Society. 2007;10(Suppl 2):27–31. doi: 10.1080/13697130701550903. [DOI] [PubMed] [Google Scholar]
- Lopes AM, Burgoyne PS, Ojarikre A, Bauer J, Sargent CA, Amorim A, Affara NA. Transcriptional changes in response to X chromosome dosage in the mouse: implications for X inactivation and the molecular basis of Turner Syndrome. BMC genomics. 2010;11:82. doi: 10.1186/1471-2164-11-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manwani B, McCullough LD. Sexual dimorphism in ischemic stroke: lessons from the laboratory. Womens Health (Lond Engl) 2011;7:319–339. doi: 10.2217/whe.11.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maoz H, Kaiser N, Halimi M, Barak V, Haimovitz A, Weinstein D, Simon A, Yagel S, Biran S, Treves AJ. The effect of estradiol on human myelomonocytic cells. 1. Journal of reproductive immunology. 1985;7:325–335. doi: 10.1016/0165-0378(85)90027-0. [DOI] [PubMed] [Google Scholar]
- McColl BW, Rothwell NJ, Allan SM. Systemic inflammatory stimulus potentiates the acute phase and CXC chemokine responses to experimental stroke and exacerbates brain damage via interleukin-1- and neutrophil-dependent mechanisms. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:4403–4412. doi: 10.1523/JNEUROSCI.5376-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCombe PA, Greer JM, Mackay IR. Sexual dimorphism in autoimmune disease. Current molecular medicine. 2009;9:1058–1079. doi: 10.2174/156652409789839116. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Blizzard K, Simpson ER, Oz OK, Hurn PD. Aromatase cytochrome P450 and extragonadal estrogen play a role in ischemic neuroprotection. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:8701–8705. doi: 10.1523/JNEUROSCI.23-25-08701.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough LD, Hurn PD. Estrogen and ischemic neuroprotection: an integrated view. Trends in endocrinology and metabolism: TEM. 2003;14:228–235. doi: 10.1016/s1043-2760(03)00076-6. [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. The New England journal of medicine. 1999;340:1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- Meyer DM, Eastwood JA, Compton MP, Gylys K, Zivin JA, Ovbiagele B. Sex differences in antiplatelet response in ischemic stroke. Womens Health (Lond Engl) 2011;7:465–474. doi: 10.2217/whe.11.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkola TS, St Clair RW. Estradiol reduces basal and cytokine induced monocyte adhesion to endothelial cells. Maturitas. 2002;41:313–319. doi: 10.1016/s0378-5122(01)00301-2. [DOI] [PubMed] [Google Scholar]
- Miller AP, Feng W, Xing D, Weathington NM, Blalock JE, Chen YF, Oparil S. Estrogen modulates inflammatory mediator expression and neutrophil chemotaxis in injured arteries. Circulation. 2004;110:1664–1669. doi: 10.1161/01.CIR.0000142050.19488.C7. [DOI] [PubMed] [Google Scholar]
- Miyagi M, Aoyama H, Morishita M, Iwamoto Y. Effects of sex hormones on chemotaxis of human peripheral polymorphonuclear leukocytes and monocytes. Journal of periodontology. 1992;63:28–32. doi: 10.1902/jop.1992.63.1.28. [DOI] [PubMed] [Google Scholar]
- Moro L, Reineri S, Piranda D, Pietrapiana D, Lova P, Bertoni A, Graziani A, Defilippi P, Canobbio I, Torti M, Sinigaglia F. Nongenomic effects of 17beta-estradiol in human platelets: potentiation of thrombin-induced aggregation through estrogen receptor beta and Src kinase. Blood. 2005;105:115–121. doi: 10.1182/blood-2003-11-3840. [DOI] [PubMed] [Google Scholar]
- Mouton PR, Long JM, Lei DL, Howard V, Jucker M, Calhoun ME, Ingram DK. Age and gender effects on microglia and astrocyte numbers in brains of mice. Brain research. 2002;956:30–35. doi: 10.1016/s0006-8993(02)03475-3. [DOI] [PubMed] [Google Scholar]
- Murphy AJ, Guyre PM, Pioli PA. Estradiol suppresses NF-kappa B activation through coordinated regulation of let-7a and miR-125b in primary human macrophages. J Immunol. 2010;184:5029–5037. doi: 10.4049/jimmunol.0903463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadkarni S, Cooper D, Brancaleone V, Bena S, Perretti M. Activation of the annexin A1 pathway underlies the protective effects exerted by estrogen in polymorphonuclear leukocytes. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:2749–2759. doi: 10.1161/ATVBAHA.111.235176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayama M, Aber T, Nagayama T, Ross ME, Iadecola C. Age-dependent increase in ischemic brain injury in wild-type mice and in mice lacking the inducible nitric oxide synthase gene. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1999;19:661–666. doi: 10.1097/00004647-199906000-00009. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Oshima T, Matsuura H, Kajiyama G, Kambe M. Effect of 17beta-estradiol on inhibition of platelet aggregation in vitro is mediated by an increase in NO synthesis. Arteriosclerosis, thrombosis, and vascular biology. 1998;18:961–967. doi: 10.1161/01.atv.18.6.961. [DOI] [PubMed] [Google Scholar]
- Nathan L, Pervin S, Singh R, Rosenfeld M, Chaudhuri G. Estradiol inhibits leukocyte adhesion and transendothelial migration in rabbits in vivo : possible mechanisms for gender differences in atherosclerosis. Circulation research. 1999;85:377–385. doi: 10.1161/01.res.85.4.377. [DOI] [PubMed] [Google Scholar]
- Newman TA, Galea I, van Rooijen N, Perry VH. Blood-derived dendritic cells in an acute brain injury. Journal of neuroimmunology. 2005;166:167–172. doi: 10.1016/j.jneuroim.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Niewada M, Kobayashi A, Sandercock PA, Kaminski B, Czlonkowska A. Influence of gender on baseline features and clinical outcomes among 17,370 patients with confirmed ischaemic stroke in the international stroke trial. Neuroepidemiology. 2005;24:123–128. doi: 10.1159/000082999. [DOI] [PubMed] [Google Scholar]
- Nilsson BO, et al. Modulation of the inflammatory response by estrogens with focus on the endothelium and its interactions with leukocytes. Inflammation research : official journal of the European Histamine Research Society. 2007;56:269–273. doi: 10.1007/s00011-007-6198-z. [DOI] [PubMed] [Google Scholar]
- Normann S, de Veber G, Fobker M, Langer C, Kenet G, Bernard TJ, Fiedler B, Strater R, Goldenberg NA, Nowak-Gottl U. Role of endogenous testosterone concentration in pediatric stroke. Annals of neurology. 2009;66:754–758. doi: 10.1002/ana.21840. [DOI] [PubMed] [Google Scholar]
- Norris LA, Joyce M, O'Keeffe N, Sheppard BL, Bonnar J. Haemostatic risk factors in healthy postmenopausal women taking hormone replacement therapy. Maturitas. 2002;43:125–133. doi: 10.1016/s0378-5122(02)00202-5. [DOI] [PubMed] [Google Scholar]
- Offner H, Polanczyk M. A potential role for estrogen in experimental autoimmune encephalomyelitis and multiple sclerosis. Annals of the New York Academy of Sciences. 2006;1089:343–372. doi: 10.1196/annals.1386.021. [DOI] [PubMed] [Google Scholar]
- Offner H, Vandenbark AA, Hurn PD. Effect of experimental stroke on peripheral immunity: CNS ischemia induces profound immunosuppression. Neuroscience. 2009;158:1098–1111. doi: 10.1016/j.neuroscience.2008.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada M, Suzuki A, Mizuno K, Asada Y, Ino Y, Kuwayama T, Tamakoshi K, Mizutani S, Tomoda Y. Effects of 17 beta-estradiol and progesterone on migration of human monocytic THP-1 cells stimulated by minimally oxidized low-density lipoprotein in vitro. Cardiovascular research. 1997;34:529–535. doi: 10.1016/s0008-6363(97)00060-6. [DOI] [PubMed] [Google Scholar]
- Olie V, Canonico M, Scarabin PY. Risk of venous thrombosis with oral versus transdermal estrogen therapy among postmenopausal women. Current opinion in hematology. 2010;17:457–463. doi: 10.1097/MOH.0b013e32833c07bc. [DOI] [PubMed] [Google Scholar]
- Ospina JA, Brevig HN, Krause DN, Duckles SP. Estrogen suppresses IL-1beta-mediated induction of COX-2 pathway in rat cerebral blood vessels. American journal of physiology. Heart and circulatory physiology. 2004;286:H2010–H2019. doi: 10.1152/ajpheart.00481.2003. [DOI] [PubMed] [Google Scholar]
- Otaegui D, Baranzini SE, Armananzas R, Calvo B, Munoz-Culla M, Khankhanian P, Inza I, Lozano JA, Castillo-Trivino T, Asensio A, Olaskoaga J, Lopez de Munain A. Differential micro RNA expression in PBMC from multiple sclerosis patients. PloS one. 2009;4:e6309. doi: 10.1371/journal.pone.0006309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papenfuss TL, Powell ND, McClain MA, Bedarf A, Singh A, Gienapp IE, Shawler T, Whitacre CC. Estriol generates tolerogenic dendritic cells in vivo that protect against autoimmunity. J Immunol. 2011;186:3346–3355. doi: 10.4049/jimmunol.1001322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashenkov M, Huang YM, Kostulas V, Haglund M, Soderstrom M, Link H. Two subsets of dendritic cells are present in human cerebrospinal fluid. Brain : a journal of neurology. 2001;124:480–492. doi: 10.1093/brain/124.3.480. [DOI] [PubMed] [Google Scholar]
- Pashenkov M, Link H. Dendritic cells and immune responses in the central nervous system. Trends in immunology. 2002;23:69–70. doi: 10.1016/s1471-4906(01)02114-7. author reply 70. [DOI] [PubMed] [Google Scholar]
- Pathan M, Kittner SJ. Pregnancy and stroke. Current neurology and neuroscience reports. 2003;3:27–31. doi: 10.1007/s11910-003-0033-x. [DOI] [PubMed] [Google Scholar]
- Pawlak J, Brito V, Kuppers E, Beyer C. Regulation of glutamate transporter GLAST and GLT-1 expression in astrocytes by estrogen. Brain research. Molecular brain research. 2005;138:1–7. doi: 10.1016/j.molbrainres.2004.10.043. [DOI] [PubMed] [Google Scholar]
- Pervin S, Singh R, Rosenfeld ME, Navab M, Chaudhuri G, Nathan L. Estradiol suppresses MCP-1 expression In vivo : implications for atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 1998;18:1575–1582. doi: 10.1161/01.atv.18.10.1575. [DOI] [PubMed] [Google Scholar]
- Pinheiro I, Dejager L, Libert C. X-chromosome-located microRNAs in immunity: might they explain male/female differences? The X chromosome-genomic context may affect X-located miRNAs and downstream signaling, thereby contributing to the enhanced immune response of females. BioEssays : news and reviews in molecular, cellular and developmental biology. 2011;33:791–802. doi: 10.1002/bies.201100047. [DOI] [PubMed] [Google Scholar]
- Pioli PA, Jensen AL, Weaver LK, Amiel E, Shen Z, Shen L, Wira CR, Guyre PM. Estradiol attenuates lipopolysaccharide-induced CXC chemokine ligand 8 production by human peripheral blood monocytes. J Immunol. 2007;179:6284–6290. doi: 10.4049/jimmunol.179.9.6284. [DOI] [PubMed] [Google Scholar]
- Pitkin RM, Witte DL. Platelet and leukocyte counts in pregnancy. JAMA : the journal of the American Medical Association. 1979;242:2696–2698. [PubMed] [Google Scholar]
- Polanczyk MJ, Hopke C, Vandenbark AA, Offner H. Estrogen-mediated immunomodulation involves reduced activation of effector T cells, potentiation of Treg cells, and enhanced expression of the PD-1 costimulatory pathway. Journal of neuroscience research. 2006;84:370–378. doi: 10.1002/jnr.20881. [DOI] [PubMed] [Google Scholar]
- Pozzi S, Benedusi V, Maggi A, Vegeto E. Estrogen action in neuroprotection and brain inflammation. Annals of the New York Academy of Sciences. 2006;1089:302–323. doi: 10.1196/annals.1386.035. [DOI] [PubMed] [Google Scholar]
- Prat A, Behrendt M, Marcinkiewicz E, Boridy S, Sairam RM, Seidah NG, Maysinger D. A novel mouse model of Alzheimer's disease with chronic estrogen deficiency leads to glial cell activation and hypertrophy. Journal of aging research. 2011;2011:251517. doi: 10.4061/2011/251517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestwood KM, Unson C, Kulldorff M, Cushman M. The effect of different doses of micronized 17beta-estradiol on C-reactive protein, interleukin-6, and lipids in older women. The journals of gerontology. Series A, Biological sciences and medical sciences. 2004;59:827–832. doi: 10.1093/gerona/59.8.m827. [DOI] [PubMed] [Google Scholar]
- Price CJ, Menon DK, Peters AM, Ballinger JR, Barber RW, Balan KK, Lynch A, Xuereb JH, Fryer T, Guadagno JV, Warburton EA. Cerebral neutrophil recruitment, histology, and outcome in acute ischemic stroke: an imaging-based study. Stroke; a journal of cerebral circulation. 2004;35:1659–1664. doi: 10.1161/01.STR.0000130592.71028.92. [DOI] [PubMed] [Google Scholar]
- Prinz M, Priller J, Sisodia SS, Ransohoff RM. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nature neuroscience. 2011;14:1227–1235. doi: 10.1038/nn.2923. [DOI] [PubMed] [Google Scholar]
- Putaala J, Curtze S, Hiltunen S, Tolppanen H, Kaste M, Tatlisumak T. Causes of death and predictors of 5-year mortality in young adults after first-ever ischemic stroke: the Helsinki Young Stroke Registry. Stroke; a journal of cerebral circulation. 2009;40:2698–2703. doi: 10.1161/STROKEAHA.109.554998. [DOI] [PubMed] [Google Scholar]
- Raju R, Bland KI, Chaudry IH. Estrogen: a novel therapeutic adjunct for the treatment of trauma-hemorrhage-induced immunological alterations. Mol Med. 2008;14:213–221. doi: 10.2119/2008-00001.Raju. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasura M, Spalloni A, Ferrari M, De Castro S, Patella R, Lisi F, Beccia M. A case series of young stroke in Rome. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2006;13:146–152. doi: 10.1111/j.1468-1331.2006.01159.x. [DOI] [PubMed] [Google Scholar]
- Rayner K, Sun J, Chen YX, McNulty M, Simard T, Zhao X, Wells DJ, de Belleroche J, O'Brien ER. Heat shock protein 27 protects against atherogenesis via an estrogen-dependent mechanism: role of selective estrogen receptor beta modulation. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:1751–1756. doi: 10.1161/ATVBAHA.109.193656. [DOI] [PubMed] [Google Scholar]
- Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, Khatiwoda A, Lisabeth L. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet neurology. 2008;7:915–926. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmann G, Schroeter M, Jander S, Fischer HG. Dendritic cells and dendritic-like microglia in focal cortical ischemia of the mouse brain. Journal of neuroimmunology. 2002;129:125–132. doi: 10.1016/s0165-5728(02)00184-4. [DOI] [PubMed] [Google Scholar]
- Renoux C, Dell'aniello S, Garbe E, Suissa S. Transdermal and oral hormone replacement therapy and the risk of stroke: a nested case-control study. BMJ. 2010;340:c2519. doi: 10.1136/bmj.c2519. [DOI] [PubMed] [Google Scholar]
- Renoux C, Suissa S. Hormone therapy administration in postmenopausal women and risk of stroke. Womens Health (Lond Engl) 2011;7:355–361. doi: 10.2217/whe.11.28. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. The New England journal of medicine. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- Roeloffzen WW, Kluin-Nelemans HC, Mulder AB, Veeger NJ, Bosman L, de Wolf JT. In normal controls, both age and gender affect coagulability as measured by thrombelastography. Anesth Analg. 110:987–994. doi: 10.1213/ANE.0b013e3181d31e91. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roquer J, Campello AR, Gomis M. Sex differences in first-ever acute stroke. Stroke; a journal of cerebral circulation. 2003;34:1581–1585. doi: 10.1161/01.STR.0000078562.82918.F6. [DOI] [PubMed] [Google Scholar]
- Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- Rubtsov AV, Rubtsova K, Kappler JW, Marrack P. Genetic and hormonal factors in female-biased autoimmunity. Autoimmunity reviews. 2010;9:494–498. doi: 10.1016/j.autrev.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutkowsky JM, Wallace BK, Wise PM, O'Donnell ME. Effects of estradiol on ischemic factor-induced astrocyte swelling and AQP4 protein abundance. American journal of physiology. Cell physiology. 2011;301:C204–C212. doi: 10.1152/ajpcell.00399.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, Goldstein LB, Gorelick P, Halperin J, Harbaugh R, Johnston SC, Katzan I, Kelly-Hayes M, Kenton EJ, Marks M, Schwamm LH, Tomsick T. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Stroke; a journal of cerebral circulation. 2006;37:577–617. doi: 10.1161/01.STR.0000199147.30016.74. [DOI] [PubMed] [Google Scholar]
- Saijo K, Collier JG, Li AC, Katzenellenbogen JA, Glass CK. An ADIOL-ERbeta-CtBP transrepression pathway negatively regulates microglia-mediated inflammation. Cell. 2011;145:584–595. doi: 10.1016/j.cell.2011.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha CJ, Duncan KA, Walters BJ. Neuroprotective actions of brain aromatase. Frontiers in neuroendocrinology. 2009;30:106–118. doi: 10.1016/j.yfrne.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santizo RA, Anderson S, Ye S, Koenig HM, Pelligrino DA. Effects of estrogen on leukocyte adhesion after transient forebrain ischemia. Stroke; a journal of cerebral circulation. 2000;31:2231–2235. doi: 10.1161/01.str.31.9.2231. [DOI] [PubMed] [Google Scholar]
- Santos-Galindo M, Acaz-Fonseca E, Bellini MJ, Garcia-Segura LM. Sex differences in the inflammatory response of primary astrocytes to lipopolysaccharide. Biology of sex differences. 2011;2:7. doi: 10.1186/2042-6410-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz SI, Schlaug G, Caplan L, Selim M. Arterial occlusive lesions recanalize more frequently in women than in men after intravenous tissue plasminogen activator administration for acute stroke. Stroke; a journal of cerebral circulation. 2005;36:1447–1451. doi: 10.1161/01.STR.0000170647.42126.a8. [DOI] [PubMed] [Google Scholar]
- Scarabin PY, Alhenc-Gelas M, Plu-Bureau G, Taisne P, Agher R, Aiach M. Effects of oral and transdermal estrogen/progesterone regimens on blood coagulation and fibrinolysis in postmenopausal women. A randomized controlled trial. Arteriosclerosis, thrombosis, and vascular biology. 1997;17:3071–3078. doi: 10.1161/01.atv.17.11.3071. [DOI] [PubMed] [Google Scholar]
- Scotland RS, Stables MJ, Madalli S, Watson P, Gilroy DW. Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood. 2011;118:5918–5927. doi: 10.1182/blood-2011-03-340281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekido R, Lovell-Badge R. Sex determination and SRY: down to a wink and a nudge? Trends Genet. 2009;25:19–29. doi: 10.1016/j.tig.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Selles J, Polini N, Alvarez C, Massheimer V. Progesterone and 17 beta-estradiol acutely stimulate nitric oxide synthase activity in rat aorta and inhibit platelet aggregation. Life sciences. 2001;69:815–827. doi: 10.1016/s0024-3205(01)01174-2. [DOI] [PubMed] [Google Scholar]
- Selvamani A, Sathyan P, Miranda RC, Sohrabji F. An antagomir to microRNA Let7f promotes neuroprotection in an ischemic stroke model. PloS one. 2012;7:e32662. doi: 10.1371/journal.pone.0032662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvamani A, Sohrabji F. Reproductive age modulates the impact of focal ischemia on the forebrain as well as the effects of estrogen treatment in female rats. Neurobiology of aging. 2010;31:1618–1628. doi: 10.1016/j.neurobiolaging.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini B, Columba-Cabezas S, Di Rosa F, Aloisi F. Intracerebral recruitment and maturation of dendritic cells in the onset and progression of experimental autoimmune encephalomyelitis. The American journal of pathology. 2000;157:1991–2002. doi: 10.1016/S0002-9440(10)64838-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour JF, Pro B, Fuller LM, Manning JT, Hagemeister FB, Romaguera J, Rodriguez MA, Ha CS, Smith TL, Ayala A, Hess M, Cox JD, Cabanillas F, McLaughlin P. Long-term follow-up of a prospective study of combined modality therapy for stage I–II indolent non-Hodgkin's lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21:2115–2122. doi: 10.1200/JCO.2003.07.111. [DOI] [PubMed] [Google Scholar]
- Shoupe D. Individualizing hormone therapy to minimize risk: accurate assessment of risks and benefits. Womens Health (Lond Engl) 2011;7:475–485. doi: 10.2217/whe.11.42. [DOI] [PubMed] [Google Scholar]
- Siegel C, Li J, Liu F, Benashski SE, McCullough LD. miR-23a regulation of X-linked inhibitor of apoptosis (XIAP) contributes to sex differences in the response to cerebral ischemia. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:11662–11667. doi: 10.1073/pnas.1102635108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel C, Turtzo C, McCullough LD. Sex differences in cerebral ischemia: possible molecular mechanisms. J Neurosci Res. 88:2765–2774. doi: 10.1002/jnr.22406. [DOI] [PubMed] [Google Scholar]
- Siegel C, Turtzo C, McCullough LD. Sex differences in cerebral ischemia: possible molecular mechanisms. Journal of neuroscience research. 2010;88:2765–2774. doi: 10.1002/jnr.22406. [DOI] [PubMed] [Google Scholar]
- Simon JA, Hsia J, Cauley JA, Richards C, Harris F, Fong J, Barrett-Connor E, Hulley SB. Postmenopausal hormone therapy and risk of stroke: The Heart and Estrogen-progestin Replacement Study (HERS) Circulation. 2001;103:638–642. doi: 10.1161/01.cir.103.5.638. [DOI] [PubMed] [Google Scholar]
- Skaletsky H, Kuroda-Kawaguchi T, Minx PJ, Cordum HS, Hillier L, Brown LG, Repping S, Pyntikova T, Ali J, Bieri T, Chinwalla A, Delehaunty A, Delehaunty K, Du H, Fewell G, Fulton L, Fulton R, Graves T, Hou SF, Latrielle P, Leonard S, Mardis E, Maupin R, McPherson J, Miner T, Nash W, Nguyen C, Ozersky P, Pepin K, Rock S, Rohlfing T, Scott K, Schultz B, Strong C, Tin-Wollam A, Yang SP, Waterston RH, Wilson RK, Rozen S, Page DC. The male-specific region of the human Y chromosome is a mosaic of discrete sequence classes. Nature. 2003;423:825–837. doi: 10.1038/nature01722. [DOI] [PubMed] [Google Scholar]
- Smith-Bouvier DL, Divekar AA, Sasidhar M, Du S, Tiwari-Woodruff SK, King JK, Arnold AP, Singh RR, Voskuhl RR. A role for sex chromosome complement in the female bias in autoimmune disease. J Exp Med. 2008;205:1099–1108. doi: 10.1084/jem.20070850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smrt RD, Pfeiffer RL, Zhao X. Age-dependent expression of MeCP2 in a heterozygous mosaic mouse model. Human molecular genetics. 2011;20:1834–1843. doi: 10.1093/hmg/ddr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, Ro S, Michaels JD, Park C, McCarrey JR, Yan W. Many X-linked microRNAs escape meiotic sex chromosome inactivation. Nature genetics. 2009;41:488–493. doi: 10.1038/ng.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence RD, Hamby ME, Umeda E, Itoh N, Du S, Wisdom AJ, Cao Y, Bondar G, Lam J, Ao Y, Sandoval F, Suriany S, Sofroniew MV, Voskuhl RR. Neuroprotection mediated through estrogen receptor-alpha in astrocytes. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:8867–8872. doi: 10.1073/pnas.1103833108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speroff L. Transdermal hormone therapy and the risk of stroke and venous thrombosis. Climacteric : the journal of the International Menopause Society. 2010;13:429–432. doi: 10.3109/13697137.2010.507111. [DOI] [PubMed] [Google Scholar]
- Stamova B, Tian Y, Jickling G, Bushnell C, Zhan X, Liu D, Ander BP, Verro P, Patel V, Pevec WC, Hedayati N, Dawson DL, Jauch EC, Pancioli A, Broderick JP, Sharp FR. The X-Chromosome Has a Different Pattern of Gene Expression in Women Compared With Men With Ischemic Stroke. Stroke; a journal of cerebral circulation. 2011 doi: 10.1161/STROKEAHA.111.629337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefano GB, Prevot V, Beauvillain JC, Fimiani C, Welters I, Cadet P, Breton C, Pestel J, Salzet M, Bilfinger TV. Estradiol coupling to human monocyte nitric oxide release is dependent on intracellular calcium transients: evidence for an estrogen surface receptor. J Immunol. 1999;163:3758–3763. [PubMed] [Google Scholar]
- Stein B, Yang MX. Repression of the interleukin-6 promoter by estrogen receptor is mediated by NF-kappa B and C/EBP beta. Mol Cell Biol. 1995;15:4971–4979. doi: 10.1128/mcb.15.9.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DG, Wright DW. Progesterone in the clinical treatment of acute traumatic brain injury. Expert opinion on investigational drugs. 2010;19:847–857. doi: 10.1517/13543784.2010.489549. [DOI] [PubMed] [Google Scholar]
- Stoll G, Jander S, Schroeter M. Inflammation and glial responses in ischemic brain lesions. Progress in neurobiology. 1998;56:149–171. doi: 10.1016/s0301-0082(98)00034-3. [DOI] [PubMed] [Google Scholar]
- Straub RH. The complex role of estrogens in inflammation. Endocrine reviews. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- Sunday L, Osuna C, Krause DN, Duckles SP. Age alters cerebrovascular inflammation and effects of estrogen. American journal of physiology. 2007;292:H2333–H2340. doi: 10.1152/ajpheart.01057.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter T, Biollaz G, Gatto D, Bernasconi L, Herren T, Reith W, Fontana A. The brain as an immune privileged site: dendritic cells of the central nervous system inhibit T cell activation. European journal of immunology. 2003;33:2998–3006. doi: 10.1002/eji.200323611. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Brown CM, Dela Cruz CD, Yang E, Bridwell DA, Wise PM. Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and antiinflammatory actions. Proceedings of the National Academy of Sciences of the United States of America. 2007a;104:6013–6018. doi: 10.1073/pnas.0610394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Brown CM, Wise PM. Neuroprotective effects of estrogens following ischemic stroke. Frontiers in neuroendocrinology. 2009;30:201–211. doi: 10.1016/j.yfrne.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Gerhold LM, Bottner M, Rau SW, Dela Cruz C, Yang E, Zhu H, Yu J, Cashion AB, Kindy MS, Merchenthaler I, Gage FH, Wise PM. Estradiol enhances neurogenesis following ischemic stroke through estrogen receptors alpha and beta. J Comp Neurol. 2007b;500:1064–1075. doi: 10.1002/cne.21240. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Shimizu T, Yu HP, Hsieh YC, Choudhry MA, Bland KI, Chaudry IH. Estrogen receptor-alpha predominantly mediates the salutary effects of 17beta-estradiol on splenic macrophages following trauma-hemorrhage. American journal of physiology. Cell physiology. 2007c;293:C978–C984. doi: 10.1152/ajpcell.00092.2007. [DOI] [PubMed] [Google Scholar]
- Tan KS, Armugam A, Sepramaniam S, Lim KY, Setyowati KD, Wang CW, Jeyaseelan K. Expression profile of MicroRNAs in young stroke patients. PloS one. 2009;4:e7689. doi: 10.1371/journal.pone.0007689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannen RL, Weiner MG, Xie D, Barnhart K. A simulation using data from a primary care practice database closely replicated the women's health initiative trial. Journal of clinical epidemiology. 2007;60:686–695. doi: 10.1016/j.jclinepi.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Teuscher C, Noubade R, Spach K, McElvany B, Bunn JY, Fillmore PD, Zachary JF, Blankenhorn EP. Evidence that the Y chromosome influences autoimmune disease in male and female mice. Proc Natl Acad Sci U S A. 2006;103:8024–8029. doi: 10.1073/pnas.0600536103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas-Camardiel M, Venero JL, Herrera AJ, De Pablos RM, Pintor-Toro JA, Machado A, Cano J. Blood-brain barrier disruption highly induces aquaporin-4 mRNA and protein in perivascular and parenchymal astrocytes: protective effect by estradiol treatment in ovariectomized animals. Journal of neuroscience research. 2005;80:235–246. doi: 10.1002/jnr.20443. [DOI] [PubMed] [Google Scholar]
- Towfighi A, Saver JL, Engelhardt R, Ovbiagele B. A midlife stroke surge among women in the United States. Neurology. 2007;69:1898–1904. doi: 10.1212/01.wnl.0000268491.89956.c2. [DOI] [PubMed] [Google Scholar]
- Tsuchiya KD, Willard HF. Chromosomal domains and escape from X inactivation: comparative X inactivation analysis in mouse and human. Mammalian genome : official journal of the International Mammalian Genome Society. 2000;11:849–854. doi: 10.1007/s003350010175. [DOI] [PubMed] [Google Scholar]
- Turtzo LC, McCullough LD. Sex-specific responses to stroke. Future neurology. 2010;5:47–59. doi: 10.2217/fnl.09.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turtzo LC, Siegel C, McCullough LD. X chromosome dosage and the response to cerebral ischemia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:13255–13259. doi: 10.1523/JNEUROSCI.0621-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urra X, Cervera A, Villamor N, Planas AM, Chamorro A. Harms and benefits of lymphocyte subpopulations in patients with acute stroke. Neuroscience. 2009a;158:1174–1183. doi: 10.1016/j.neuroscience.2008.06.014. [DOI] [PubMed] [Google Scholar]
- Urra X, Obach V, Chamorro A. Stroke induced immunodepression syndrome: from bench to bedside. Current molecular medicine. 2009b;9:195–202. doi: 10.2174/156652409787581574. [DOI] [PubMed] [Google Scholar]
- Uzui H, Sinha SK, Rajavashisth TB. 17beta-estradiol inhibits oxidized low-density lipoprotein-induced increase in matrix metalloproteinase-9 expression in human macrophages. Journal of investigative medicine : the official publication of the American Federation for Clinical Research. 2011;59:1104–1108. doi: 10.2310/JIM.0b013e3182279e4b. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Hurn PD. Gender differences in pediatric stroke: is elevated testosterone a risk factor for boys? Annals of neurology. 2009;66:713–714. doi: 10.1002/ana.21925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegeto E, Belcredito S, Ghisletti S, Meda C, Etteri S, Maggi A. The endogenous estrogen status regulates microglia reactivity in animal models of neuroinflammation. Endocrinology. 2006;147:2263–2272. doi: 10.1210/en.2005-1330. [DOI] [PubMed] [Google Scholar]
- Vegeto E, Benedusi V, Maggi A. Estrogen anti-inflammatory activity in brain: a therapeutic opportunity for menopause and neurodegenerative diseases. Frontiers in neuroendocrinology. 2008;29:507–519. doi: 10.1016/j.yfrne.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegeto E, Bonincontro C, Pollio G, Sala A, Viappiani S, Nardi F, Brusadelli A, Viviani B, Ciana P, Maggi A. Estrogen prevents the lipopolysaccharide-induced inflammatory response in microglia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:1809–1818. doi: 10.1523/JNEUROSCI.21-06-01809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villablanca AC, Jayachandran M, Banka C. Atherosclerosis and sex hormones: current concepts. Clin Sci (Lond) 2010;119:493–513. doi: 10.1042/CS20100248. [DOI] [PubMed] [Google Scholar]
- Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI. A clinical trial of estrogen-replacement therapy after ischemic stroke. The New England journal of medicine. 2001;345:1243–1249. doi: 10.1056/NEJMoa010534. [DOI] [PubMed] [Google Scholar]
- Vogelgesang A, Dressel A. Immunological consequences of ischemic stroke: immunosuppression and autoimmunity. Journal of neuroimmunology. 2011;231:105–110. doi: 10.1016/j.jneuroim.2010.09.023. [DOI] [PubMed] [Google Scholar]
- Vogelgesang A, Grunwald U, Langner S, Jack R, Broker BM, Kessler C, Dressel A. Analysis of lymphocyte subsets in patients with stroke and their influence on infection after stroke. Stroke; a journal of cerebral circulation. 2008;39:237–241. doi: 10.1161/STROKEAHA.107.493635. [DOI] [PubMed] [Google Scholar]
- Wander K, Brindle E, O'Connor KA. C-reactive protein across the menstrual cycle. American journal of physical anthropology. 2008;136:138–146. doi: 10.1002/ajpa.20785. [DOI] [PubMed] [Google Scholar]
- Wang D, D'Costa J, Civin CI, Friedman AD. C/EBPalpha directs monocytic commitment of primary myeloid progenitors. Blood. 2006;108:1223–1229. doi: 10.1182/blood-2005-12-008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JM, Hou X, Adeosun S, Hill R, Henry S, Paul I, Irwin RW, Ou XM, Bigler S, Stockmeier C, Brinton RD, Gomez-Sanchez E. A Dominant Negative ERbeta Splice Variant Determines the Effectiveness of Early or Late Estrogen Therapy after Ovariectomy in Rats. PloS one. 2012;7:e33493. doi: 10.1371/journal.pone.0033493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. Journal of neuroimmunology. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, Baird A, Kotchen T, Curb JD, Black H, Rossouw JE, Aragaki A, Safford M, Stein E, Laowattana S, Mysiw WJ. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women's Health Initiative: a randomized trial. JAMA : the journal of the American Medical Association. 2003;289:2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- Wen Y, Yang S, Liu R, Perez E, Yi KD, Koulen P, Simpkins JW. Estrogen attenuates nuclear factor-kappa B activation induced by transient cerebral ischemia. Brain research. 2004;1008:147–154. doi: 10.1016/j.brainres.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Westberry JM, Trout AL, Wilson ME. Epigenetic regulation of estrogen receptor alpha gene expression in the mouse cortex during early postnatal development. Endocrinology. 2010;151:731–740. doi: 10.1210/en.2009-0955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijchers PJ, Festenstein RJ. Epigenetic regulation of autosomal gene expression by sex chromosomes. Trends in genetics : TIG. 2011;27:132–140. doi: 10.1016/j.tig.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Westberry JM. Regulation of oestrogen receptor gene expression: new insights and novel mechanisms. Journal of neuroendocrinology. 2009;21:238–242. doi: 10.1111/j.1365-2826.2009.01830.x. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Westberry JM, Trout AL. Estrogen receptor-alpha gene expression in the cortex: sex differences during development and in adulthood. Hormones and behavior. 2011;59:353–357. doi: 10.1016/j.yhbeh.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woiciechowsky C, Schoning B, Lanksch WR, Volk HD, Docke WD. Mechanisms of brain-mediated systemic anti-inflammatory syndrome causing immunodepression. J Mol Med (Berl) 1999;77:769–780. doi: 10.1007/s001099900051. [DOI] [PubMed] [Google Scholar]
- Xu J, Burgoyne PS, Arnold AP. Sex differences in sex chromosome gene expression in mouse brain. Human molecular genetics. 2002;11:1409–1419. doi: 10.1093/hmg/11.12.1409. [DOI] [PubMed] [Google Scholar]
- Xu J, Disteche CM. Sex differences in brain expression of X- and Y-linked genes. Brain research. 2006;1126:50–55. doi: 10.1016/j.brainres.2006.08.049. [DOI] [PubMed] [Google Scholar]
- Yang L, Hu Y, Hou Y. Effects of 17beta-estradiol on the maturation, nuclear factor kappa B p65 and functions of murine spleen CD11c-positive dendritic cells. Molecular immunology. 2006a;43:357–366. doi: 10.1016/j.molimm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Yang L, Liang J, Yao G, Chen P, Hou Y. 17beta-estradiol regulates the numbers, endocytosis, stimulative capacity and IL-10 secretion of mouse spleen dendritic cells. Toxicology letters. 2005;155:239–246. doi: 10.1016/j.toxlet.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Yang X, Schadt EE, Wang S, Wang H, Arnold AP, Ingram-Drake L, Drake TA, Lusis AJ. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome research. 2006b;16:995–1004. doi: 10.1101/gr.5217506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yenari MA, Kauppinen TM, Swanson RA. Microglial activation in stroke: therapeutic targets. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2010;7:378–391. doi: 10.1016/j.nurt.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HP, Shimizu T, Hsieh YC, Suzuki T, Choudhry MA, Schwacha MG, Chaudry IH. Tissue-specific expression of estrogen receptors and their role in the regulation of neutrophil infiltration in various organs following trauma-hemorrhage. Journal of leukocyte biology. 2006;79:963–970. doi: 10.1189/jlb.1005596. [DOI] [PubMed] [Google Scholar]
- Yu JC, Hsiung CN, Hsu HM, Bao BY, Chen ST, Hsu GC, Chou WC, Hu LY, Ding SL, Cheng CW, Wu PE, Shen CY. Genetic variation in the genome-wide predicted estrogen response element-related sequences is associated with breast cancer development. Breast cancer research : BCR. 2011;13:R13. doi: 10.1186/bcr2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Subramanian S, Dziennis S, Jia J, Uchida M, Akiyoshi K, Migliati E, Lewis AD, Vandenbark AA, Offner H, Hurn PD. Estradiol and G1 reduce infarct size and improve immunosuppression after experimental stroke. J Immunol. 2010;184:4087–4094. doi: 10.4049/jimmunol.0902339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Nair A, Krady K, Corpe C, Bonneau RH, Simpson IA, Vannucci SJ. Estrogen stimulates microglia and brain recovery from hypoxia-ischemia in normoglycemic but not diabetic female mice. The Journal of clinical investigation. 2004;113:85–95. doi: 10.1172/JCI200418336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Peng Y, Wang W, Su B. Rapid evolution of an X-linked microRNA cluster in primates. Genome research. 2007;17:612–617. doi: 10.1101/gr.6146507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Rempe DA. Targeting astrocytes for stroke therapy. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2010;7:439–451. doi: 10.1016/j.nurt.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng R, Pan G, Thobe BM, Choudhry MA, Matsutani T, Samy TS, Kang SC, Bland KI, Chaudry IH. MyD88 and Src are differentially regulated in Kupffer cells of males and proestrus females following hypoxia. Mol Med. 2006;12:65–73. doi: 10.2119/2006-00030.Zheng. [DOI] [PMC free article] [PubMed] [Google Scholar]


