Abstract
The effects of exposure to an environment where the background magnetic field has been reduced were studied on wild-type Drosophila melanogaster by measuring its ability to survive a single exposure to ionizing radiation during its larval stage. The experimental design presented shows a timeframe, ionizing radiation dose and background magnetic field parameters that will cause a significant and reproducible reduction of survival on this insect model. These results suggest that background magnetic fields may play a fundamental role in the recovery or harm of a biological system that is exposed to single doses of ionizing radiation.
Keywords: zero field, weakened geomagnetic field, hypogeomagnetic field, low-level fields, magnetic shielding, magnetic vacuum
It has been previously suggested that modifying the static and/or time-varying component of the background magnetic field (BMF) affected Drosophila melanogaster (D. melanogaster) in biologically relevant ways. For instance, Zhang et al. [2004] reported that this insect model can become completely amnesiac after 10 generations in a BMF environment where only the static magnetic field component was reduced by coil-generated magnetic fields when compared to a control where the static component was left unchanged (∼52 μT). Similarly, Graham et al. [2000] reported that the developmental stability of D. melanogaster was enhanced by a coil-generated 1.5 μT magnetic field at 60 Hz when compared to a control where there was no purposely generated time-varying magnetic field. Unfortunately, these studies fall short in providing a complete description of either the time-varying or the static component of the BMF present in their experimental setups. This is important in the sense that these fields may be significantly different between the control and exposed groups. The reason is that laboratories, incubators and experimental apparati are not only immersed in the BMF, but also contribute to it. For example, it is not surprising to find time-varying magnetic fields near the laboratory room's electrical distribution lines or near electrically powered equipment that are of the same order of magnitude as those mentioned here. Likewise, variations in the static component of the BMF between laboratories or even in the same room are also common as magnetically relevant materials are frequently utilized components in buildings and laboratory equipment.
In this study, we report the extent of the previously suggested effects of magnetic fields on D. melanogaster after being exposed for several generations to BMF environments with well-characterized static and time-varying magnetic field components. We cultured D. melanogaster for several generations in three distinct BMF environments with otherwise identical environmental conditions. After one year (more than 10 generations) of continuous exposure to each different BMF, we challenged the D. melanogaster with increasing doses of ionizing radiation (IR) while insects were at the larval stage. IR damages cells and this property underlies its use as a leading anticancer therapy because it can cause DNA damage and induce apoptosis [Jaklevic et al., 2006; Mesicek et al., 2010]. Damage at an early developmental stage can escalate significantly with time, expectedly facilitating the detection of possible small IR-BMF effects at a later stage of development.
Three cylindrical containers were built in our laboratory to isolate the different BMF environments studied. One of the BMF environments was produced by attenuating the static and time-varying component of the existing BMF in our incubator with a double-walled μ-metal container (Engineering Kit, Ad-Vance Magnetics, Rochester, IN, USA). Another BMF environment was produced by attenuating the same incubator's static and time-varying magnetic field component to a lesser degree with a double-walled stainless steel container (Stock No. 6030, K & S Engineering, Chicago, IL, USA). A third container, which provided no BMF attenuation, was made of cardboard and plastic (Quick-Tube, Quikrete Atlanta, GA, USA) and served as a control, allowing no visible light penetration in accordance with the other two. The dimensions of the containers are as follows: height of outer cylinder = 15.5 cm, height of inner cylinder = 14.0 cm, diameter of outer cylinder = 24.0 cm, diameter of inner cylinder = 21.7 cm. All containers were treated with a plastic coating to prevent humidity degradation and allowed to cure for 10 days prior to the start of the experiment. The incubator used to house the containers (Model I-36VL, Percival Scientific, Boone, IA, USA) was maintained at 27 °C ± 0.5 °C and 60% relative humidity. The containers were placed at the same height inside the incubator and were simultaneously fed with air from the same incubator in order to account for any possible temperature and/or humidity differences between containers that were below our measurement capabilities. The magnitudes of the static component of the BMF were measured at the center of each container with a fluxgate magnetometer (Model FGM 4D2N, Walker Scientific, Worcester, MA, USA). The magnetometer was calibrated to ∼0 μT (± 0.5 μT) by using a cylinder (inner diameter = 57.15 mm, length = 343 mm, attenuation = 99.87% or 58.12 dB at the center) made of helicoidally wound μ-metal (5 ft of AD-MU-80, .01′ thick × 4″ wide, Ad-Vance Magnetics). The time-varying magnetic field was measured by induction with a sensor comprised of a set of square, mutually perpendicular coils (diameter 1 = 9.92 ×10-4 m, diameter 2 = 9.70 ×10-4 m, diameter 3 = 7.56 ×10-4 m, each with a thickness of 2×10-3 m and 200 turns of 34 coated AWG copper wires, fed in twisted pairs). The signal from the coils was amplified and filtered (10 KHz low-pass filter) by a high impedance differential amplifier (OSP-1 oscilloscope preamplifier, Advanced Research Instruments, Golden, CO, USA) and the spectrum up to 600 Hz was analyzed. The complete time-varying magnetic field inherent noise of the sensing system was below 2.41 ×10-6 T-Hz root mean square (RMS). The field measurements obtained for each cylindrical container are presented in Table 1.
Table 1.
| Magnetic Field Measured for the Different Environments in the Experiments | |||||||
|---|---|---|---|---|---|---|---|
| Environment | Time-Varying, up to 600 Hz (AC) | Static (DC) | |||||
| Dominant Frequency (Hz) | RMS Magnitude of the Dominant Frequency (T) | Attenuation (dB) | Attenuation | Magnitude (T) | Attenuation (dB) | Attenuation | |
| μ-Metal Cylinder (inside incubator) | 60 | 2.01 × 10-7 | -4.13 | 2.59 | 1.70 × 10-6 | 14.42 | 0.04 |
| Stainless Steel Cylinder (inside incubator) | 60 | 9.51 × 10-7 | -10.88 | 12.26 | 1.82 × 10-5 | 4.12 | 0.39 |
| Plastic Cylinder (inside incubator) | 60 | 1.06 × 10-6 | -11.35 | 13.66 | 4.25 × 10-5 | 0.44 | 0.90 |
| X-ray Generator | 60 | 2.14 × 10-8 | 5.59 | 0.28 | 4.60 × 10-5 | 0.09 | 0.98 |
| Laboratory Bench | 60 | 7.76 × 10-8 | 0 | 1.00 | 4.70 × 10-5 | 0 | 1.00 |
Magnitudes reported for both static (DC) and time-varying (AC) measurements were obtained by vector summation of the fields recorded at the 3 perpendicular axes (x,y,z). The AC measurements reported for each environment correspond to the frequency with maximum amplitude in a spectrum up to 600 Hz. All spectrums had maximums at 60 Hz and were mainly composed harmonics of that frequency. The attenuation values are referenced to the readings made on the laboratory bench. The static magnetic field measurements have an uncertainty of ± 5 × 10-7 T; time-varying magnetic fields have an uncertainty of ± 6 × 10-8 T.
Wild-type (Sevelin stock) D. melanogaster was kept in each container for 12 to 18 months prior to the start of each experiment. Therefore, the data presented here corresponds to a group of experiments performed in a 6-month timeframe. Each experiment comprised the following: embryos were collected from D. melanogaster from each container for up to 5 h in standard 177 ml polypropylene cornmeal-agar bottles. These were then aged to feeding-stage third instar larvae over a 5-day period, post-collection, and subsequently were taken out of the containers, processed in the laboratory's BMF, and then placed in an IR generator to be exposed to doses of X-rays between 0 and 100 Gy (Torrex 120D X-ray inspection system, Astrophysics Research, Long Beach, CA, USA).
The IR exposure was done by dividing the larvae from each container between six polystyrene vials for a total of 18 vials, which were then exposed to doses in increments of 20 Gy (0, 20, 40, 60, 80 and 100) at an exposure rate of 24 mGy/s. This corresponds to 14 min exposures for 20 Gy and 70 min exposures for 100 Gy. The separation of the larvae into the vials to be exposed to IR took 20 min, during which time flies occupied the laboratory bench's BMF environment. After IR exposure, D. melanogaster larvae were incubated in their original containers for 7 days under the same conditions as pre-IR exposure. After this time passed and under normal environmental conditions, larvae should have crawled out of the cornmeal-agar preparation into the walls of the container, encapsulated in a pupal case, undergone metamorphosis, and then emerge from their pupal case into fully matured/viable adults (eclosed). Therefore, the vials were frozen to halt development at day 7, ending the experiment. The pupal cases in the walls of the each container were then counted in terms of their respective eclosion state (eclosed or non-eclosed) from where a survival percentage was obtained and interpreted as an index of IR resistance. There were no detectable differences in the ambient/background IR in all 3 containers or the room, as measured with a Geiger counter (Model 3007A with a 370 end window probe, Dosimeter Corporation of America, Cincinnati, OH, USA).
Data manipulation and statistical analysis were done with Microsoft Office Excel 2007 (Redmond, WA, USA) and MATLAB R2007a (Mathworks, Natick, MA, USA). Nested, two-tailed t-tests and one-way ANOVAs were performed for each IR dose group. Only experiments with no less than 50 and no more than 155 pupae were considered in the final dataset (a total of 10 blinded, independent experiments), reducing the artifacts generated for under- or overcrowding of the vials. Refer to the online supplementary material for further details on the exposure conditions, experimental design and raw datasets.
Our results show that D. melanogaster, which were inside the μ-metal container environment over several generations, have reduced ability to survive an IR exposure of 80 Gy or more than an otherwise identical population exposed to the BMF environment found in the cardboard-plastic container. The stainless steel container-generated BMF environment failed to elicit a decrease in survivability in the insect model (Fig. 1). Both BMF components, the time-varying and the static, were significantly reduced in the μ-metal container but only partially in the stainless steel container (Table 1). This suggests that the threshold in magnetic field magnitude required for obtaining the observed biological effects is below that measured in the stainless steel container.
Figure 1. Survival percentages at increasing IR absorbed doses in different magnetic field environments.
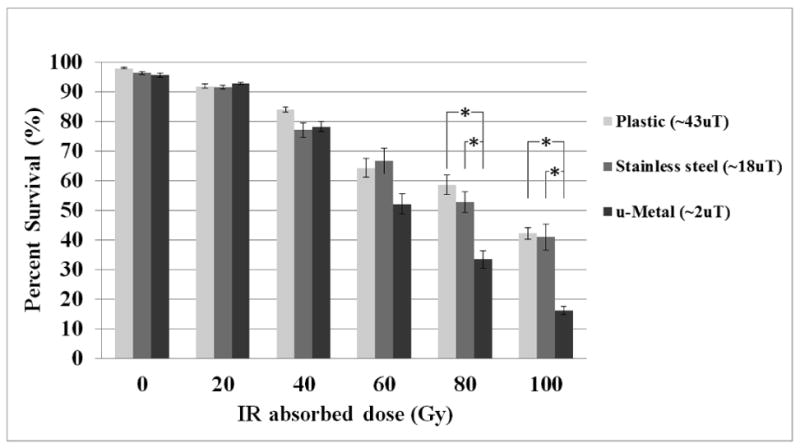
Results for each cylindrical container at each IR absorbed dose are given in terms of surviving pupae percentage. Error bars represent one standard error for each individual experiment (n = 10). See Table 1 for the magnetic field measurements obtained in each environment. Asterisk denotes significance (p-value < 0.01) with a two-tailed t-test and one-way ANOVA. See the online supplementary material for a table of the p-values obtained for each case.
Our experimental design is limited to providing a timeframe, ionizing radiation dose, and magnetic field parameters that will cause a significant and reproducible biological effect on D. melanogaster. Additional refinement of these findings was beyond the scope of this study and therefore a subject of further research. Moreover, the possibility of an adaptation-type effect, which could affect the amount of harm made by the IR exposure at the BMF environmental conditions found in the IR generator, the temporary exposure to the BMF found in the laboratory's bench, or the possibility for an inherent diminished recovery and/or repair ability when D. melanogaster is exposed to the BMF environment in the μ-metal container are also paths subject to further study. A likely important detail is that during preparations for IR exposure, D. melanogaster larvae were suddenly exposed to light after being deprived of light for more than 10 generations as they were inside the three cylindrical containers. This may have significant effects on their unused visual and light detection system and a set of effects due to direct action on their circadian rhythm [Malpel et al., 2004]. This, in turn, will have physiological effects that could contribute to their survival. Complementarily, directional orientation of birds as well as pain sensitivity in mice, both previously reported to be dependent on BMF magnitude, have been also shown to be dependent on certain frequencies in the visible spectrum [Prato et al., 2009; Wiltschko et al., 2010].
Although the identification of the specific mechanism by which the effects shown are made possible is beyond the scope of this work, it is important to point out that extensive theoretical work has been done on this topic. This has yielded a number of possible mechanisms that could explain the systemic effect of magnetic fields of the magnitudes and frequencies observed here. The proposed mechanisms are based on the “ion cyclotron resonance model”, “ion parametric resonance model” [Adair, 1998; Belova and Panchelyuga, 2010], “molecular gyroscope model” [Binhi et al., 2001], “Lorentz models” [Muehsam and Pilla, 2009], “radical pair model” [Timmel and Henbest, 2004; Engström, 2006] and other modifications and combinations of these.
This report contributes to a growing body of evidence that shows the importance of magnetic fields as an environmentally relevant parameter in biological systems. Our results are consistent with selected literature suggesting that strict control, as well as characterization of the magnetic spectrum in different experimental environments, is a basic foundation to resolve the relevant magnetic conditions responsible for the effects observed in specific biological systems.
Supplementary Material
Acknowledgments
The authors wish to thank Tin Tin Su and her team for their outstanding and indispensable guidance on this project. We also wish to thank Adam Sadoff for his excellent comments and revision on the manuscript and Theodore Schomay for his assistance with field measurements and comments on the manuscript.
Grant sponsors: NIH; Grant number: R01EB009115-02; Frank Barnes Gordon Bernard Prize.
Footnotes
Additional supporting material may be found in the online version of this article.
References
- Adair RK. A physical analysis of the ion parametric resonance model. Bioelectromagnetics. 1998;19:181–191. [PubMed] [Google Scholar]
- Belova N, Panchelyuga V. Lednev's model: Theory and experiment. Biophysics. 2010;55:661–674. [PubMed] [Google Scholar]
- Binhi VN, Alipov YD, Belyaev IY. Effect of static magnetic field on E. coli cells and individual rotations of ion–protein complexes. Bioelectromagnetics. 2001;22:79–86. doi: 10.1002/1521-186x(200102)22:2<79::aid-bem1009>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Engström S. Magnetic field effects on free radical reactions in biology. In: Barnes F, Greenebaum B, editors. Handbook of Biological Effects of Electromagnetic Fields: Biological and Medical Aspects of Electromagnetic Fields. 3rd. Boca Raton, FL: CRC Press; 2006. p. 151. [Google Scholar]
- Graham JH, Fletcher D, Tigue J, McDonald M. Growth and developmental stability of Drosophila melanogaster in low frequency magnetic fields. Bioelectromagnetics. 2000;21:465–472. [PubMed] [Google Scholar]
- Jaklevic B, Uyetake L, Lemstra W, Chang J, Leary W, Edwards A, Vidwans S, Sibon O, Su TT. Contribution of growth and cell cycle checkpoints to radiation survival in Drosophila. Genetics. 2006;174:1963–1972. doi: 10.1534/genetics.106.064477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malpel S, Klarsfeld A, Rouyer F. Circadian synchronization and rhythmicity in larval photoperception-defective mutants of Drosophila. Journal of Biological Rhythms. 2004;19:10–21. doi: 10.1177/0748730403260621. [DOI] [PubMed] [Google Scholar]
- Mesicek J, Lee H, Feldman T, Jiang X, Skobeleva A, Berdyshev E, Haimovitz-Friedman A, Fuks Z, Kolesnick R. Ceramide synthases 2, 5, and 6 confer distinct roles in radiation-induced apoptosis in HeLa cells. Cellular Signalling. 2010;22:1300–1307. doi: 10.1016/j.cellsig.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehsam DJ, Pilla AA. A Lorentz model for weak magnetic field bioeffects: Part I–Thermal noise is an essential component of AC/DC effects on bound ion trajectory. Bioelectromagnetics. 2009;30:462–475. doi: 10.1002/bem.20494. [DOI] [PubMed] [Google Scholar]
- Prato FS, Desjardins-Holmes D, Keenliside LD, McKay JC, Robertson JA, Thomas AW. Light alters nociceptive effects of magnetic field shielding in mice: Intensity and wavelength considerations. The Royal Society Interface. 2009;6:17–28. doi: 10.1098/rsif.2008.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmel CR, Henbest KB. A study of spin chemistry in weak magnetic fields. Philosophical Transactions of the Royal Society of London Series A–Mathematical Physical and Engineering Sciences. 2004;365:2573–2589. doi: 10.1098/rsta.2004.1459. [DOI] [PubMed] [Google Scholar]
- Wiltschko R, Stapput K, Thalau P, Wiltschko W. Directional orientation of birds by the magnetic field under different light conditions. Journal of The Royal Society Interface. 2010;7:S163–S177. doi: 10.1098/rsif.2009.0367.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Lu H, Xi W, Zhou X, Xu S, Zhang K, Jiang J, Li Y, Guo A. Exposure to hypomagnetic field space for multiple generations causes amnesia in Drosophila melanogaster. Neuroscience Letters. 2004;371:190–195. doi: 10.1016/j.neulet.2004.08.072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


