Abstract
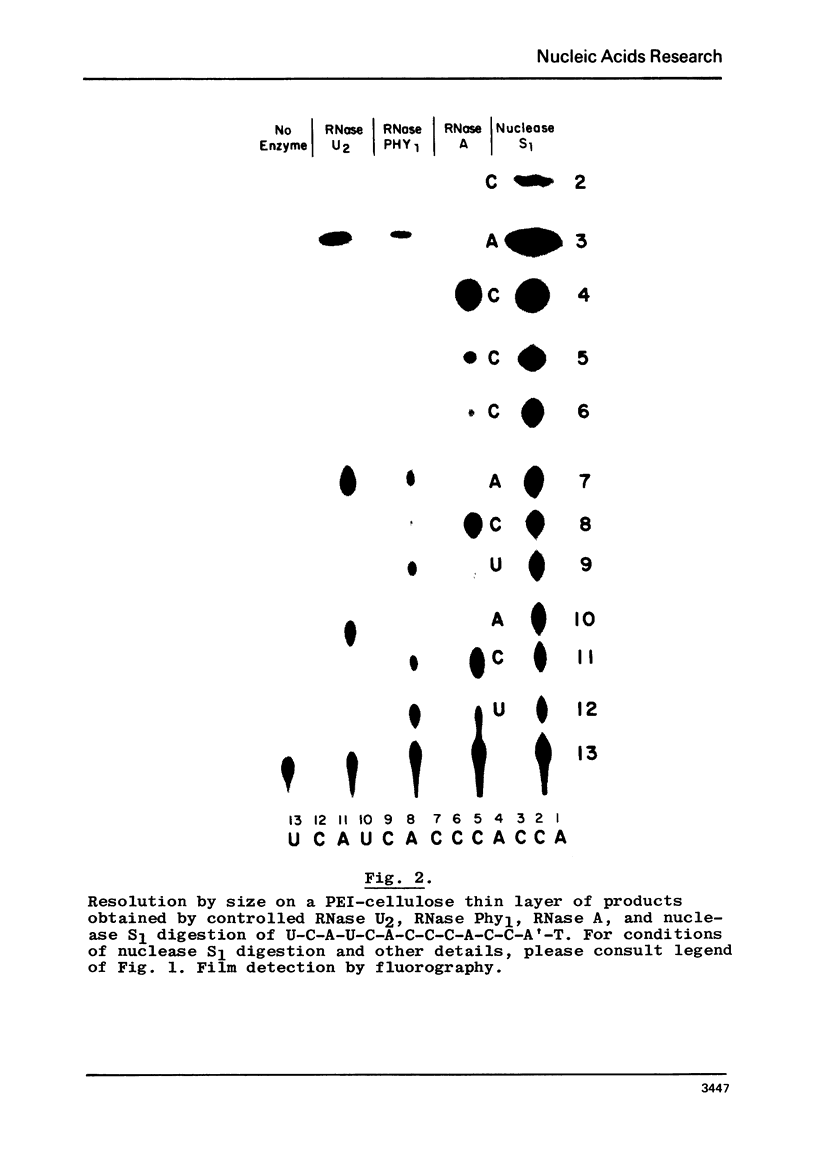
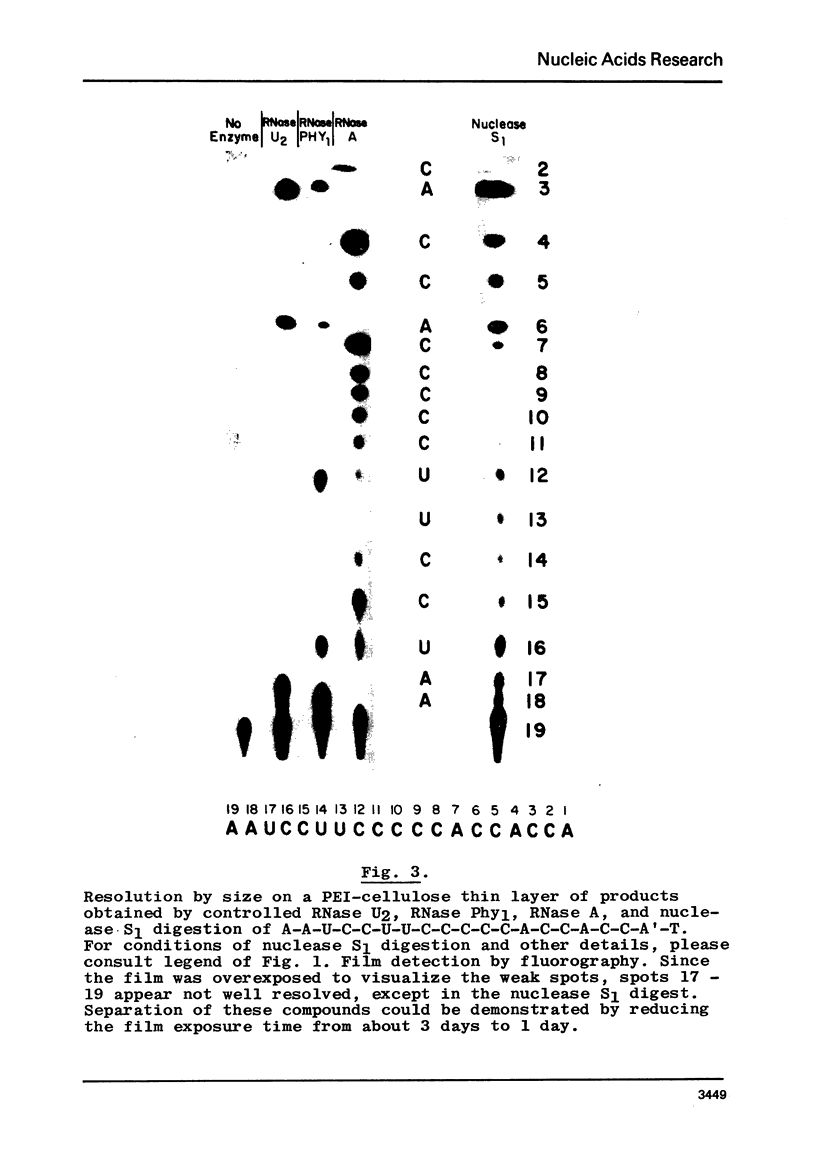
The extracellular ribonuclease I of the common slime mold physarum polycephalum (RNase Phy1), which has recently been purified to homogeneity, has been used to distinguish between C and U residues in 3'-end-labeled oligoribonucleotides. As shown by Bargetzi and coworkers, this enzyme exhibits strong cleavage preference for U-N over C-N and N-C over N-U bonds. In the present paper, conditions are being detailed, which enable one to deduce the sequences of rather large, pyrimidine-rich, terminally labeled oligonucleotides by partial digestion with RNases U2, A, and Phy1, followed by resolution of the cleavage products by size. The techniques described in this and a previous communication provide a direct means for identifying A, G, C, and U residues in end-labeled polyribonucleotides.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun R., Behrens K. A ribonuclease from Physarum. Biochemical properties and synthesis in the mitotic cycle. Biochim Biophys Acta. 1969 Nov 19;195(1):87–98. doi: 10.1016/0005-2787(69)90605-4. [DOI] [PubMed] [Google Scholar]
- Chia L. L., Randerath K., Randerath E. Base analysis of ribopolynucleotides by tritium incorporation following analytical polyacrylamide gel electrophoresis. Anal Biochem. 1973 Sep;55(1):102–113. doi: 10.1016/0003-2697(73)90295-9. [DOI] [PubMed] [Google Scholar]
- Contreras R., Fiers W. A method for the isolation of cytidylate series from ribonuclease T1-oligonucleotides. Anal Biochem. 1975 Jul;67(1):319–326. doi: 10.1016/0003-2697(75)90299-7. [DOI] [PubMed] [Google Scholar]
- Gupta R. C., Randerath E., Randerath K. A double-labeling procedure for sequence analysis of picomole amounts of nonradioactive RNA fragments. Nucleic Acids Res. 1976 Nov;3(11):2895–2914. doi: 10.1093/nar/3.11.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. C., Randerath K. Use of specific endonuclease cleavage in RNA sequencing. Nucleic Acids Res. 1977 Jun;4(6):1957–1978. doi: 10.1093/nar/4.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada F., Kimura F., Nishimura S. Nucleotide sequence of valine tRNA 1 from Escherichia coli B. Biochim Biophys Acta. 1969 Dec 16;195(2):590–592. doi: 10.1016/0005-2787(69)90671-6. [DOI] [PubMed] [Google Scholar]
- Randerath K. An evaluation of film detection methods for weak beta-emitters, particularly tritium. Anal Biochem. 1970 Mar;34:188–205. doi: 10.1016/0003-2697(70)90100-4. [DOI] [PubMed] [Google Scholar]
- Randerath K., Randerath E., Chia L. S., Gupta R. C., Sivarajan M. Sequence analysis of nonradioactive RNA fragments by periodate-phosphatase digestion and chemical tritium labeling: characterization of large oligonucleotides and oligonucleotides containing modified nucleosides. Nucleic Acids Res. 1974 Sep;1(9):1121–1141. doi: 10.1093/nar/1.9.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaniv M., Barrell B. G. Nucleotide sequence of E. coli B tRNA1-Val. Nature. 1969 Apr 19;222(5190):278–279. doi: 10.1038/222278a0. [DOI] [PubMed] [Google Scholar]






