Abstract
Background and Objectives
Oxytocin (OXY) is a neuropeptide that has recently been recognized as an important component of descending analgesic systems. The present study sought to determine if OXY produces antinociception to noxious visceral stimulation.
Methods
Urethane-anesthetized female rats had intrathecal catheters placed acutely, and the effect of intrathecal OXY on visceromotor reflexes (VMRs; abdominal muscular contractions quantified using electromyograms) to urinary bladder distension (UBD; 10-60 mm Hg, 20 s; transurethral intravesical catheter) was determined. The effect of OXY applied to the surface of exposed spinal cord was determined in lumbosacral dorsal horn neurons excited by UBD using extracellular recordings.
Results
OXY doses of 0.15 μg or 1.5 μg inhibited VMRs to UBD by 37 ± 8% and 68 ± 10%, respectively. Peak inhibition occurred within 30 minutes and was sustained for at least 60 minutes. The effect of OXY was both reversed and prevented by the intrathecal administration of an OXY receptor antagonist. Application of 0.5 mM OXY to the dorsum of the spinal cord inhibited UBD-evoked action potentials by 76 ± 12%. Consistent with the VMR studies, peak inhibition occurred within 30 minutes and was sustained for greater than 60 minutes.
Conclusions
These results argue that intrathecal OXY produces an OXY receptor specific antinociception to noxious UBD, with part of this effect due to inhibition of spinal dorsal horn neurons. To our knowledge, these studies provide the first evidence that intrathecal OXY may be an effective pharmacological treatment for visceral pain.
Introduction
Oxytocin (OXY) is an endogenous nonapeptide that is produced in the supraoptic and paraventricular nuclei (PVN) of the hypothalamus. Best known for its role in parturition and lactation, OXY is also increasingly recognized for its role in social interaction, learning, and antinociception.1-3 Previous work has demonstrated that oxytocinergic neurons from the PVN send axonal projections to the superficial and central lamina of the spinal cord,4,5 which closely correlates with OXY receptor expression as determined by autoradiographic binding.6 Interestingly, supraspinal sites appear to be the only sources of OXY in the spinal cord because neither dorsal horn neurons nor primary afferent neurons express significant levels of OXY.7 Together, these data indicate that oxytocinergic axonal projections from the PVN are appropriately situated to modulate synaptic transmission of nociceptive inputs at the level of the spinal cord.
The ability of intrathecal OXY to produce somatic antinociception or analgesia has been demonstrated in naïve rats8-10 following acute inflammation10 and neuropathic injury.11 Further, stimulation of the PVN increases OXY levels in the cerebrospinal fluid and produces antinociception.11,12 Finally, in humans, intrathecal injection of OXY in patients with both acute and chronic low back pain produced significant analgesia,8 demonstrating the likely translational significance of these findings.
Although OXY has been demonstrated to produce somatic analgesia, little is known about its role in visceral antinociception. Recent work from this lab provided the first evidence that large doses of intraperitoneal OXY can produce antinociception to acute noxious distention of the bladder.13 However, it was not determined whether this effect was due to actions on the central or peripheral nervous system. Intravenous infusions OXY are capable of raising OXY CSF concentrations if the plasma concentration remains high,14 so it is possible that the antinociceptive effect of systemic OXY was due to action at a central site.
The present study was undertaken to evaluate the ability of intrathecal OXY to inhibit the nociceptive responses to acute noxious bladder distention. To our knowledge, this is the first reported study to evaluate the role of intrathecal OXY in visceral antinociception. In the first series of experiments, we utilized pseudo-affective and pharmacological methods to test if intrathecal OXY inhibited the well-characterized visceromotor reflex (VMR) to urinary bladder distention (UBD). In addition, we assessed the receptor specificity of this effect through the use of an OXY receptor antagonist (OTA). In the second series of experiments, we utilized extracellular recording of dorsal horn neurons to test whether OXY applied to the dorsum of the spinal cord effectively inhibits neuronal activity evoked by UBD.
Methods
General
Female Sprague-Dawley rats (Harlan Laboratories, Inc., Prattville, Alabama; 230-290 g) were housed in a 12-hour light cycle room with food and water provided ad libitum. Female rats were chosen because disorders of the urinary bladder that are associated with pain are prevalent in and primarily affect the female population. Estrous cycle was not controlled for in the present study.
All procedures were conducted in accordance with the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health, and were approved by the University of Alabama-Birmingham Institutional Animal Care and Use Committee.
Surgical Preparation for VMR Experiments
Rats were anesthetized with 2% to 3% isoflurane for surgical preparation. The atlanto-occipital membrane was exposed and incised, and 7.5 cm of PE-10 tubing was threaded caudally in the intrathecal space. Anesthesia was switched to urethane (1.6-1.8 g/kg s.c.); the isoflurane anesthesia was then discontinued and intactness of neurological function verified by return of bilateral flexion reflexes to hind paw pinch. A 22-gauge angiocather was inserted into the bladder via the urethra and secured, using a tight suture around the distal urethral opening. Platinum wires were inserted into the external oblique musculature immediately superior to the inguinal ligament to record electromyographic (EMG) activity. UBDs were then performed using compressed air as previously described.15 This rat model of bladder nociception has been validated and utilized by this lab and others.15,16 VMRs in response to UBD are reliable, reproducible, and dose-dependently inhibited by analgesics. In addition, these responses are augmented by stress, inflammation, and initially by repeated noxious distentions.13,15,17 EMG activity was differentially amplified (Grass P511 AC Amplifier, Astro-Med, Inc., West Warwick, Rhode Island), digitally converted (CED Micro 1401 processor), and analyzed with Spike-2 software (Cambridge Electronic Design Ltd, Cambridge, United Kingdom) as previously described.14 All VMR data are presented as a percentage of pre-drug measures.
Protocols for VMR Experiments
EMGs were recorded during 3 initial 60 mm Hg, 20 s UBDs administered 3 minutes apart. Three minutes after completion of these initial distentions, responses to graded (10-60 mm Hg, 20 s) distentions were determined with 1 minute between trials. Graded UBDs were repeated at 3, 15, 30, 45, and 60 minutes following delivery of drugs. For the experiment in which intrathecal OXY was injected alone, a 10 μl volume of drug (either 0.15 μg or 1.5 μg total) was injected followed by a 7 μl flush. For the experiment assessing the ability of the OTA to block the effects of OXY, a total volume of 18 μl of OXY (1.5 μg) and OTA (15 μg) was injected followed by a 7 μl flush. For the experiment assessing the effect of OTA alone, a volume of 15 μl (15 μg) was injected followed by 7 μl flush. All intrathecal injections were made slowly over 2 minutes. Injection volumes vary slightly due to solubility of the antagonist.
Extracellular Dorsal Horn Neuron Electrophysiology
Rats were initially anesthetized with isoflurane (2%-3%) for surgical preparation and subsequently given a subcutaneous injection of 1.8 g/kg of urethane, and the isoflurane discontinued, similar to the VMR experiments. A catheter was placed in the jugular vein for intravenous delivery of fluids and drugs. A 22-gauge angiocatheter was placed in the urinary bladder via the urethra, as in the VMR experiments. A tracheal tube was placed and artificial ventilation begun with a volume-cycled respirator and supplemental oxygen. Neuromuscular blockade was established with 0.2 mg/kg of vecuronium. The thoracolumbar vertebrae were stabilized with clamps from which the rat was suspended. Laminectomies were performed to expose the L6-S2 spinal cord segments. The dura mater was carefully removed, using jewelers' forceps and microscissors. Single-unit extracellular recordings were obtained using a tungsten microelectode (Micro Probe, Inc., Gaithersburg, Maryland; 1.4 MOhm) that was inserted, 0 to 1 mm lateral to midline and 0 to 1mm deep to the spinal cord dorsum. Brief, phasic 60 mm Hg UBDs were used as search stimuli. Unit responses were displayed on an oscilloscope for continuous monitoring, discriminated conventionally from background, converted into uniform pulses, counted, and saved to a computer. Individual units consistently excited by UBD on 3 consecutive trials were further characterized. Mapping of convergent cutaneous receptive fields for both noxious and innocuous stimuli was performed, using rat-toothed forceps and light brush, respectively. Responses to graded UBD (20, 40, and 60 mm Hg, 20 s) were determined. Due to natural variability of unit firing, responses were normalized to unit activity in response to a 60 mmHg, 20 s UBD measured prior to the delivery of OXY. After baseline responses were obtained, a solution of 0.5 mM OXY or vehicle was applied to the dorsum of the spinal cord. Responses to graded UBDs were determined at 3, 15, 30, 45, and 60 minutes after drug administration. Only a single neuron was studied in each rat after the initial application of OXY. In some rats, in order to assess the receptor specificity of the effect, OTA (1 mM) was applied to the dorsum of the cord following the 60-minute, post-OXY measurement, and additional responses to graded UBDs obtained 30 and 60 minutes later.
Drugs
OXY was obtained from Bachem (Torrance, California, Catalog number H-2510, CAS 50-56-6). (d(CH2)51,Tyr(Me)2,Orn8)-Oxytocin, a selective OTA, was obtained from Bachem (Torrance, California, Catalog number H-2908, CAS 77327-45-8). Both OXY and OTA were reconstituted in 0.9% saline to concentrations of 0.5 μg/μl (0.5 mM) and 1 μg/μl (1 mM), respectively. The vehicle for all experiments was 0.9% NaCl solution. Urethane was purchased from Sigma-Aldrich Corporation (St. Louis, Missouri), isoflurane from MWI (Meridian, Idaho), and vecuronium from Bedford Laboratories (Bedford, Ohio). Doses of OXY chosen for the present study were selected because they are well below what has been observed to produce non-specific motor effects in rats.9
Statistical Analysis
Statistical data are denoted as mean ± standard error of the mean (SEM) unless otherwise noted. VMR and evoked neuronal activity are expressed as percentages of pre-drug responses. For experiments with greater than 2 groups, a repeated measures 2-way analysis of variance (ANOVA) was performed with Bonferroni's post-hoc test, and comparisons were made to the vehicle control. For the experiments using antagonists, repeated ANOVAs were performed. For the electrophysiology, repeated t-tests with Holm's correction for multiple comparisons were used, and comparisons were made to baseline responses.18 Data from a single rat in the VMR dose-response experiments was excluded as an outlier, using the Grubbs Test. Changes in neuronal activity were limited to 100% for purposes of statistical analysis. All statistics were performed using either GraphPad Prism 5.0b (GraphPad Software, San Diego, California) or SigmaPlot 11.0 (Systat Software Inc., San Jose, California). Statistical significance was defined as P ≤ 0.05.
Results
Visceromotor Reflexes (VMRs) to UBD
Effect of OXY
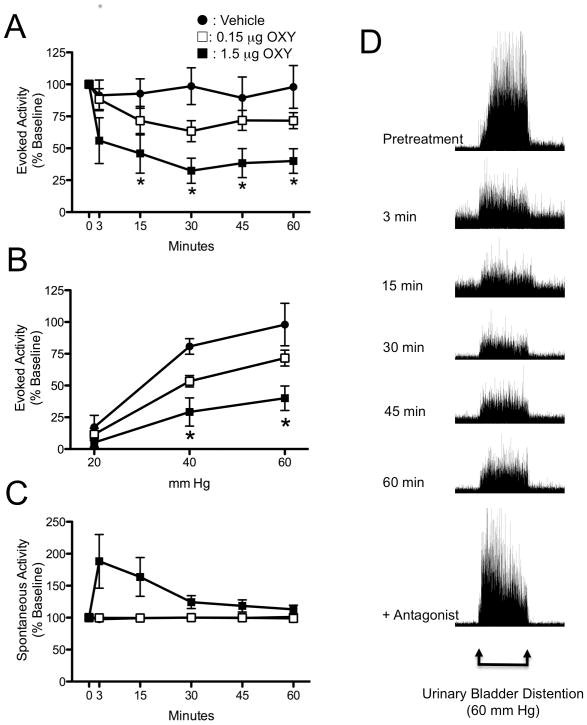
Intrathecal administration of 1.5 μg OXY produced a statistically significant reduction in VMR to a 60-mm Hg, 20 s UBD (Fig. 1A; F = 5.96; P< 0.02). The maximal decrease in VMR was observed within 30 minutes following injection. 0.15 μg OXY produced a 37 ± 8 % reduction in VMR, while 1.5 μg OXY produced a 68 ± 10 % reduction in VMR at the 30-minute time point (Fig. 1A). The reduction was sustained for up to 60 minutes without significant time-related reversal of effect. Formal stimulus-response functions for graded UBD are presented in Figure 1B, and demonstrate that 1.5 μg OXY significantly reduced the VMR response to both 40 mm Hg (mildly noxious, P < 0.01) and 60 mm Hg (noxious, P < 0.001) UBD. The response to the innocuous 20 mm Hg UBD was unchanged. The vehicle control for these experiments was 0.9% NaCl, which did not affect VMR at any time point after injection. Intrathecal delivery of OXY produced a noticeable, but not statistically significant, increase in ongoing EMG activity that generally lasted 15 to 30 minutes (Fig. 1C). This effect was observed following the 1.5 μg OXY dose, but not with either the vehicle or 0.15 μg OXY dose. Comparing Figures 1A and 1C, it is evident that the antinociceptive effects of intrathecal OXY were significantly longer in duration than the increase in spontaneous EMG response.
Figure 1.
Effect of intrathecally (i.th.)-administered oxytocin (OXY) on visceromotor reflex (VMR) to urinary bladder distention (UBD). Evoked activity is expressed as percent of EMG response to 60 mm Hg, 20s UBD prior to delivery of i.th. OXY. A: Time course for the effect of OXY on evoked VMR activity to UBD at 60 mm Hg. * P < 0.05 versus baseline value. B: Stimulus-response curves for the effect of OXY on VMR to graded UBD 60 minutes after i.th. OXY. * P < 0.05 versus vehicle control. C: Time course for the effect of OXY on spontaneous electromyographic (EMG) activity to UBD at 60 mm Hg. Spontaneous activity was determined during the 10 s prior to UBD, and is expressed as percent of baseline prior to delivery of i.th. OXY. Spontaneous activity vehicle data is obscured by the 0.15 ug OXY data. N = 5-6 per group in all panels. D: Representative time course example of EMG activity to 60 mm Hg UBD at pre-treatment (baseline), 3, 15, 30, 45, and 60 min after i.th. oxytocin. The bottom figure is the EMG from the same rat when i.th. OXY antagonist was given after the 60-minute time point.
Effect of OTA
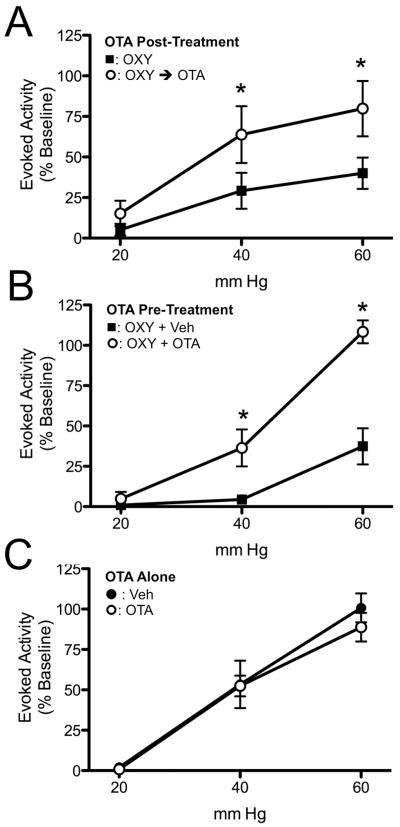
Figure 2A displays data demonstrating the ability of intrathecal OTA to reverse the effects of previously delivered OXY. In this experiment, 1.5 μg of intrathecal OXY resulted in a reduction in the VMR to UBD. After 60 minutes, while inhibition was still at its peak, 15 μg of intrathecal OTA reversed the effect of OXY within 15 to 30 minutes (F = 27.78, P = 0.002). Given that OXY and the OTA used in these experiments have extremely similar molecular weights, the dose of OTA given was a 10-fold molar excess to the OXY given. Testing 60 minutes after OXY, or 30 minutes after OTA, was chosen because pilot experiments demonstrated that these times produced reproducible effects for both drugs. Figure 2B demonstrates the ability of intrathecal OTA to block the effects of OXY when delivered simultaneously. In this experiment, 1.5 μg of intrathecal OXY was either delivered with vehicle or with 15 μg OTA. After 30 minutes, the OTA was able to prevent the effect of intrathecal OXY on VMRs, while vehicle-treated rats continued to be inhibited by intrathecal OXY (F = 33.63, P = 0.0004). Figure 2C demonstrates the effect of intrathecal OTA alone. An effect of OTA alone would strongly argue for the role of endogenous OXY in modulating acute visceral pain. In this experiment, 15 μg OTA or vehicle was given intrathecally, and the VMR to UBD was determined 30 minutes later. OTA alone did not have any effect on the VMR to UBD.
Figure 2.
Effect of oxytocin antagonist (OTA) on the OXY effect on VMR to UBD. Evoked activity is expressed as percent of EMG response to 60 mm Hg, 20s UBD prior to delivery of i.th. OXY. A: Ability of OTA to reverse the effect of previously given OXY. Stimulus-response curves in the same rats 60 minutes after i.th. OXY ■ and then 30 minutes later following i.th. OTA ○. * P < 0.05 versus OXY alone group. N = 5. B: Ability of OTA pre-treatment to block the effect of OXY. Stimulus-response curves in separate rats 30 minutes following i.th. delivery of 1.5 μg OXY plus vehicle ■ or 1.5 μg OXY plus 15 μg OTA ○. * P < 0.05 versus OXY group. C: Effect of i.th. OTA alone on VMR to UBD. Stimulus-response curves in separate rats 30 minutes following i.th. administration of vehicle ● or 15 μg OTA ○. N = 5 per group.
Dorsal Horn Neuronal Responses to UBD
Baseline characteristics
Twelve lumbosacral neurons that were responsive to UBD were identified, using brief 60 mm Hg UBD as a search stimulus. For the neurons in which OXY was studied, 7 were nociceptive specific with receptive fields in either 1 or both hind legs; 1 neuron was a wide-dynamic range neuron with brush activation near the base of the tail, and 1 neuron did not have an identifiable cutaneous receptive field. A comparison of the baseline characteristics of neurons treated with spinally-applied OXY versus those treated with spinally-applied vehicle solution demonstrated no statistically significant differences, although the sample of vehicle-treated neurons was small (n=3): mean depth 250 ± 87 vs 283 ±119 μm below cord dorsum, respectively; spontaneous activity 5.7 ± 2.6 vs. 1.9 ± 1.5 Hz, respectively; evoked activity 225 ± 61 vs. 163 ± 64 action potentials in 20 s, respectively.
Effect on Evoked Activity
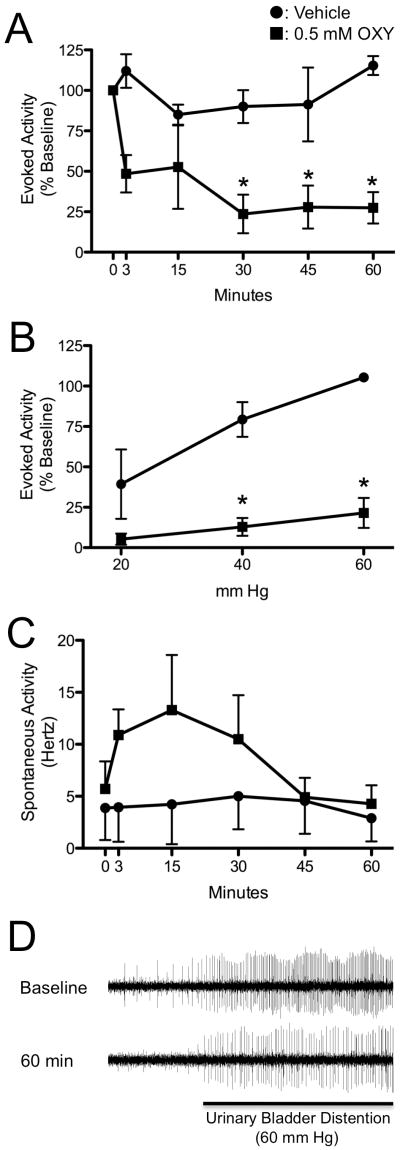
Topical application of a solution of 0.5 mM OXY to the dorsum of the spinal cord resulted in a significant reduction in activity evoked by UBD. Thirty minutes following application of OXY, the evoked activity was reduced by 76 ± 12 %, and this effect was sustained for at least 60 minutes (Fig. 3A). During application of OXY, no visible changes occurred to the color or size of the dorsal spinal arteries. Stimulus-response functions were generated for evoked activity at 60 minutes (shown in Figure 3B). The 3 neurons treated with topical vehicle application to the spinal cord demonstrated little effect of this treatment and maintained increasing responses to graded UBD at 20, 40, and 60 mm Hg at all time points. In contrast, OXY-treated neurons had an almost complete loss of graded responses to UBD. The inhibition of neuronal activity in 7 neurons treated with OXY that were subsequently administered a topical application of OTA at the conclusion of the experiment demonstrated reversal of the OXY effect: post-treatment with OTA increased evoked activity by a median of 57% above the activity 60 minutes after OXY treatment.
Figure 3.
Effect of OXY on the evoked and spontaneous activity of dorsal horn neurons to UBD. A: Time course of effect for 0.5 mM OXY ■ or vehicle ● delivered to the dorsum of the spinal cord on neuronal activity evoked by UBD. Evoked activity is expressed as a percent of pre-drug response to 60 mmHg, 20s UBD. B: Evoked activity stimulus response curves for graded UBD 60 min after OXY or vehicle. P < 0.05 versus vehicle group. C: Time course of effect for 0.5 mM OXY ■ or vehicle ● delivered to the dorsum of the spinal cord on spontaneous neuronal activity. Spontaneous activity was recorded for the 10 s prior to UBD, and is expressed in Hertz (Hz). For graphs A-C, N = 9 for OXY, and N = 3 for vehicle. D: Typical examples of neuronal oscillographic tracings at baseline and 60 minutes after application of 0.5 mM OXY to the dorsum of the spinal cord. Period of evoked activity during UBD to 60 mm Hg designated by black bar. Oscillations in the height of action potentials due to movement from respiration.
Effect on Spontaneous Activity
The topical application of OXY resulted in an increase in the spontaneous activity in 8 of 9 recorded neurons (mean 89% increase). This increase in spontaneous activity started within 3 minutes of application and ended approximately 30 to 45 minutes later (Fig. 3C). This effect was not observed in response to application of the vehicle solution. In addition, during 5 of the 9 neuronal recordings in which OXY was applied, 1 or more additional nearby, previously silent, neurons were activated by the application of OXY. The duration of the spontaneous discharges from nearby neurons was generally limited to less than 30 minutes. Careful discrimination of the original neuron action potential morphology ensured consistent measures were recorded from the originally characterized neuron.
Discussion
The key finding of this study is that spinally-delivered OXY can modulate the transmission of noxious visceral information at the level of the spinal cord. Specifically, we showed that intrathecal OXY inhibits the VMR to UBD, and that this process is mediated by the OXY receptor. Additionally, OXY has a minimal tonic role in modulating nociception, as evidenced by the lack of effect of the OTA alone. Using single-unit extracellular recordings, we demonstrated that spinally-delivered OXY inhibits dorsal horn neuronal responses to UBD. Collectively, these results argue that spinal OXY receptors are a viable drug target for the modulation of visceral pain from the urinary bladder.
The VMR is a pseudo-affective reflex that is valuable in studying antinociceptive and analgesic agents in rodents. Our results demonstrate that OXY is capable of inhibiting the VMR to UBD. This effect is consistent with studies of both mechanical and thermal somatic nociception in rats.8-10 It is likely that this effect is largely due to inhibition of the sensory portion of the reflex circuit, because our own data and other studies have observed OXY-induced inhibition of evoked dorsal horn neuron activity.19,20 Our maximal OXY dose of 1.5 μg is well below the level previously reported to consistently produce nonspecific motor effects in rats.9 One interesting finding was the extended duration of effect with intrathecal OXY, which generally lasted at least 60 minutes for the doses used in these experiments. Previous studies have reported OXY effects for 30 to 45 minutes.9,21,22 Our extended duration is interesting because the half-life of intravenous OXY is generally 5 to 10 minutes clinically. However, a recent study demonstrated that the half-life is dose-dependent in rats, with a half-life of approximately 45 minutes with the dose we used in these experiments.23 As such, the presence of a significant OXY effect past 60 minutes is not surprising. The fact that the OXY effect could readily be antagonized in the VMR experiments 60 minutes after administration further indicates that the sustained OXY effect was both real and OXY-receptor mediated.
The physiological correlate of intrathecal delivery of OXY is activation of the PVN, causing release of OXY in the superficial layers of the spinal cord. Previous work has demonstrated that stimulation of the rat PVN leads to increased OXY in the cerebrospinal fluid and produces somatic analgesia.11,12 In addition, multiple other studies have demonstrated spinal neurons projecting to the hypothalamus.eg,.5 Together with our data showing that intrathecal OXY produces visceral antinociception, these data collectively argue for a spinal-hypothalamic-spinal circuit that is important in the modulation of noxious visceral information at the level of the spinal cord.
One of the more interesting findings in these studies was the increase in baseline EMG activity upon initial injection of intrathecal OXY. Although this increase never reached a level of statistical significance, it does provide limited evidence that, at least for a short period of time, OXY either facilitates the VMR or directly activates motor neurons innervating the abdominal musculature. It is important to note, however, that the duration of antinociception from intrathecal OXY was much longer than the initial increase in myoelectric activity. As such, the decrease in evoked activity was not due simply to a nonspecific increase in the myoelectrical activity, but rather to a true reduction in evoked activity.
Similar to results obtained in the VMR experiments, OXY applied to the dorsum of the spinal cord reliably inhibited evoked neuronal activity for greater than 60 minutes. The fact that no visible changes occurred to the color or size of the dorsal spinal arteries following application of OXY argues that the effect was neuronal receptor mediated, and not due to a change in mean tissue perfusion. The fact that 100% of the neurons recorded following OXY administration were inhibited may be a result of a relatively homogenous population of neurons due to the search criteria. Because the majority of spontaneously active dorsal horn neurons were not responsive to UBD and somatic stimuli were not used as search criteria, the neurons recorded in these experiments are difficult to directly compare with those recorded in previous studies of somatic antinociception. The lesser reversal of OXY effect with OTA during the extracellular recording experiments may be due to the use of a 2-fold molar excess of OTA in those experiments, as opposed to a 10-fold excess in the VMR experiments. Even noting this, the vast majority (78%) of neurons were readily reversed at least, in part, by OTA, demonstrating the OXY-receptor specificity of the effect.
The mechanism by which OXY inhibits dorsal horn neurons is complex and remains to be fully elucidated. Due to the increased density of OXY receptors in the superficial laminae,6 a presumed target of OXY would be the GABAergic neurons in this region. However, after stimulation of the PVN, c-fos expression was localized in superficial lamina neurons that were not glutamic acid decarboxylase (GAD) positive, suggesting that GABAergic neurons of the superficial lamina are not the initially activated neurons.24 Previous extracellular recording work has demonstrated that PVN stimulation leads to inhibition of wide dynamic range neuron responses to Aδ and C-fibers, but not Aβ-fiber stimulation.25 In that study, stimulation of the PVN also appeared to activate a previously silent population of neurons near the originally recorded WDR neuron, and the activity of the WDR neuron and the previously silent neurons were inversely proportional.25 Collectively these data appear to indicate that OXY leads to the indirect activation of a population of GABAergic interneurons that then pre-synaptically inhibit glutamatergic transmission of nociceptive signals from Aδ and C-fibers. The reason that Aβ-fiber information is not also inhibited remains to be elucidated. Consistent with this theory, we noted that 5 of the 9 recorded neurons from our OXY group had at least 1 previously silent nearby neuron that became activated following the application of OXY. A distinct possibility is that these neurons represented inhibitory GABAergic interneurons that are activated by OXY and which, in turn, inhibit the recorded neuron, similar to that seen in the PVN stimulation studies.25
In addition to providing the first documented evidence that OXY can modify noxious visceral information from the urinary bladder at the level of the spinal cord, this study also has significant clinical application. Pain syndromes involving the bladder are common and typically difficult to treat. The data presented here indicate that OXY is now a potentially novel drug treatment for these conditions. Several previous human studies have already documented that peripherally delivered OXY can alter visceral sensation. For instance, intranasal OXY can reduce the pain from chronic constipation,26 and intravenous OXY increases the pain threshold to colonic distention in irritable bowel syndrome.27 In addition, in somatic studies, both chronic and acute low back pain were significantly relieved following the intrathecal administration of OXY.8 While these data indicate that OXY may be an effective analgesic in humans, the report is lacking in a number of important details, including a description of the participants' back pain and the duration of the OXY effect. While the present study used female rats, a number of other studies have demonstrated that OXY increases tail-flick latencies in male rats,8,9 and this effect is similar to what was observed in female rats.28 It is highly likely that OXY would produce antinociception to visceral stimuli in male rats as well. Although more rigorous neurotoxicity studies are required, the present paper indicates that the intrathecal delivery of OXY may be a potential therapeutic regimen appropriate for painful disorders of the urinary bladder in both male and female patients.
Acknowledgments
This work was supported by The University of Alabama-Birmingham Department of Anesthesiology Resident Academic Career Development Award to M.P.E. It was also supported by DK51413 (T.J.N.) and DK080981 (M.T.R).
Supported by: The University of Alabama-Birmingham Department of Anesthesiology Resident Academic Career Development Award to M.P.E., DK51413 (T.J.N.), and DK080981 (M.T.R).
Footnotes
Presented at: The 2011 American Society of Regional Anesthesia and Pain Medicine Meeting and at The 2011 Society for Neuroscience Annual Meeting.
Conflicts of Interest: Timothy J. Ness and Meredith T. Robbins have a patent for the use of oxytocin and derivatives thereof in improving weak bladder function and for the treatment of conditions associated with weak bladder function, such as, but not limited to, incomplete bladder emptying; however no component of this patent is related to bladder pain.
References
- 1.Lee HJ, Macbeth AH, Pagani JH, Young WS., 3rd Oxytocin: the great facilitator of life. Prog Neurobiol. 2009;88(2):127–151. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81(2):629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- 3.Viero C, Shibuya I, Kitamura N, Verkhratsky A, Fujihara H, Katoh A, Ueta Y, Zingg HH, Chvatal A, Sykova E, Dayanithi G. Oxytocin: Crossing the bridge between basic science and pharmacotherapy. CNS Neurosci Ther. 2010;16(5):e138–156. doi: 10.1111/j.1755-5949.2010.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Condes-Lara M, Martinez-Lorenzana G, Rojas-Piloni G, Rodriguez-Jimenez J. Branched oxytocinergic innervations from the paraventricular hypothalamic nuclei to superficial layers in the spinal cord. Brain Res. 2007;1160:20–29. doi: 10.1016/j.brainres.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 5.Rousselot P, Papadopoulos G, Merighi A, Poulain DA, Theodosis DT. Oxytocinergic innervation of the rat spinal cord. An electron microscopic study. Brain Res. 1990;529(1-2):178–184. doi: 10.1016/0006-8993(90)90825-v. [DOI] [PubMed] [Google Scholar]
- 6.Reiter MK, Kremarik P, Freund-Mercier MJ, Stoeckel ME, Desaulles E, Feltz P. Localization of oxytocin binding sites in the thoracic and upper lumbar spinal cord of the adult and postnatal rat: a histoautoradiographic study. Eur J Neurosci. 1994;6(1):98–104. doi: 10.1111/j.1460-9568.1994.tb00251.x. [DOI] [PubMed] [Google Scholar]
- 7.Robinson DA, Wei F, Wang GD, Li P, Kim SJ, Vogt SK, Muglia LJ, Zhuo M. Oxytocin mediates stress-induced analgesia in adult mice. J Physiol. 2002;540(Pt 2):593–606. doi: 10.1113/jphysiol.2001.013492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J. Intrathecal administration of oxytocin induces analgesia in low back pain involving the endogenous opiate peptide system. Spine (Phila Pa 1976) 1994;19(8):867–871. doi: 10.1097/00007632-199404150-00001. [DOI] [PubMed] [Google Scholar]
- 9.Yang J, Yang Y, Chen JM, Liu WY, Wang CH, Lin BC. Central oxytocin enhances antinociception in the rat. Peptides. 2007;28(5):1113–1119. doi: 10.1016/j.peptides.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Yu SQ, Lundeberg T, Yu LC. Involvement of oxytocin in spinal antinociception in rats with inflammation. Brain Res. 2003;983(1-2):13–22. doi: 10.1016/s0006-8993(03)03019-1. [DOI] [PubMed] [Google Scholar]
- 11.Miranda-Cardenas Y, Rojas-Piloni G, Martinez-Lorenzana G, Rodriguez-Jimenez J, Lopez-Hidalgo M, Freund-Mercier MJ, Condes-Lara M. Oxytocin and electrical stimulation of the paraventricular hypothalamic nucleus produce antinociceptive effects that are reversed by an oxytocin antagonist. Pain. 2006;122(1-2):182–189. doi: 10.1016/j.pain.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 12.Martinez-Lorenzana G, Espinosa-Lopez L, Carranza M, Aramburo C, Paz-Tres C, Rojas-Piloni G, Condes-Lara M. PVN electrical stimulation prolongs withdrawal latencies and releases oxytocin in cerebrospinal fluid, plasma, and spinal cord tissue in intact and neuropathic rats. Pain. 2008;140(2):265–273. doi: 10.1016/j.pain.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Black LV, Ness TJ, Robbins MT. Effects of oxytocin and prolactin on stress-induced bladder hypersensitivity in female rats. J Pain. 2009;10(10):1065–1072. doi: 10.1016/j.jpain.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones PM, Robinson IC. Differential clearance of neurophysin and neurohypophysial peptides from the cerebrospinal fluid in conscious guinea pigs. Neuroendocrinol. 1982;34:297–302. doi: 10.1159/000123316. [DOI] [PubMed] [Google Scholar]
- 15.Castroman P, Ness TJ. Vigor of visceromotor responses to urinary bladder distension in rats increases with repeated trials and stimulus intensity. Neurosci Lett. 2001;306(1-2):97–100. doi: 10.1016/s0304-3940(01)01886-9. [DOI] [PubMed] [Google Scholar]
- 16.Blatt LK, Lashinger ES, Laping NJ, Su X. Evaluation of pressor and visceromotor reflex responses to bladder distension in urethane anesthetized rats. Neurourol Urodyn. 2009;28:442–446. doi: 10.1002/nau.20650. [DOI] [PubMed] [Google Scholar]
- 17.Randich A, Uzzell T, Cannon R, Ness TJ. Inflammation and enhanced nociceptive responses to bladder distension produced by intravesical zymosan in the rat. BMC Urology. 2006;6:2. doi: 10.1186/1471-2490-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holm S. A Simple Sequentially Rejective Multiple Test Procedure. Scand J Statist. 1979;6(2):65–70. [Google Scholar]
- 19.Rojas-Piloni G, Lopez-Hidalgo M, Martinez-Lorenzana G, Rodriguez-Jimenez J, Condes-Lara M. GABA-mediated oxytocinergic inhibition in dorsal horn neurons by hypothalamic paraventricular nucleus stimulation. Brain Res. 2007;1137(1):69–77. doi: 10.1016/j.brainres.2006.12.045. [DOI] [PubMed] [Google Scholar]
- 21.Condes-Lara M, Gonzalez NM, Martinez-Lorenzana G, Delgado OL, Freund-Mercier MJ. Actions of oxytocin and interactions with glutamate on spontaneous and evoked dorsal spinal cord neuronal activities. Brain Res. 2003;976(1):75–81. doi: 10.1016/s0006-8993(03)02690-8. [DOI] [PubMed] [Google Scholar]
- 22.DeLaTorre S, Rojas-Piloni G, Martinez-Lorenzana G, Rodriguez-Jimenez J, Villanueva L, Condes-Lara M. Paraventricular oxytocinergic hypothalamic prevention or interruption of long-term potentiation in dorsal horn nociceptive neurons: electrophysiological and behavioral evidence. Pain. 2009;144(3):320–328. doi: 10.1016/j.pain.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Breton JD, Poisbeau P, Darbon P. Antinocicpetive action of oxytocin involves inhibition of potassium channel currents in lamina II neurons of the rat spinal cord. Mol Pain. 2009;5:63. doi: 10.1186/1744-8069-5-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morin V, Del Castillo JR, Authier S, Ybarra N, Otis C, Gauvin D, Gutkowska J, Troncy E. Evidence for non-linear pharmacokinetics of oxytocin in anesthetizetized rat. J Pharm Pharm Sci. 2008;11(4):12–24. doi: 10.18433/j3pk5x. [DOI] [PubMed] [Google Scholar]
- 24.Breton JD, Veinante P, Uhl-Bronner S, Vergnano AM, Freund-Mercier MJ, Schlichter R, Poisbeau P. Oxytocin-induced antinociception in the spinal cord is mediated by a subpopulation of glutamatergic neurons in lamina I-II which amplify GABAergic inhibition. Mol Pain. 2008;4:19. doi: 10.1186/1744-8069-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rojas-Piloni G, Martinez-Lorenzana G, Condes-Lara M, Rodriguez-Jimenez J. Direct sensorimotor corticospinal modulation of dorsal horn neuronal C-fiber responses in the rat. Brain Res. 2010;1351:104–114. doi: 10.1016/j.brainres.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Ohlsson B, Truedsson M, Bengtsson M, Torstenson R, Sjolund K, Bjornsson ES, Simren M. Effects of long-term treatment with oxytocin in chronic constipation; a double blind, placebo-controlled pilot trial. Neurogastroenterol Motil. 2005;17(5):697–704. doi: 10.1111/j.1365-2982.2005.00679.x. [DOI] [PubMed] [Google Scholar]
- 27.Louvel D, Delvaux M, Felez A, Fioramonti J, Bueno L, Lazorthes Y, Frexinos J. Oxytocin increases thresholds of colonic visceral perception in patients with irritable bowel syndrome. Gut. 1996;39(5):741–747. doi: 10.1136/gut.39.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterssen M, Alster A, Lundeberg T, Uvnas-Moberg K. Oxytocin increases nociceptive thresholds in a long term perspective in female and male rats. Neurosci Lett. 1996;212:87–90. doi: 10.1016/0304-3940(96)12773-7. [DOI] [PubMed] [Google Scholar]